Supplemental Digital Content is available in the text.
Keywords: acute coronary syndrome, alirocumab, cholesterol, mortality, PCSK9 protein
Abstract
Background:
Previous trials of PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitors demonstrated reductions in major adverse cardiovascular events, but not death. We assessed the effects of alirocumab on death after index acute coronary syndrome.
Methods:
ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) was a double-blind, randomized comparison of alirocumab or placebo in 18 924 patients who had an ACS 1 to 12 months previously and elevated atherogenic lipoproteins despite intensive statin therapy. Alirocumab dose was blindly titrated to target achieved low-density lipoprotein cholesterol (LDL-C) between 25 and 50 mg/dL. We examined the effects of treatment on all-cause death and its components, cardiovascular and noncardiovascular death, with log-rank testing. Joint semiparametric models tested associations between nonfatal cardiovascular events and cardiovascular or noncardiovascular death.
Results:
Median follow-up was 2.8 years. Death occurred in 334 (3.5%) and 392 (4.1%) patients, respectively, in the alirocumab and placebo groups (hazard ratio [HR], 0.85; 95% CI, 0.73 to 0.98; P=0.03, nominal P value). This resulted from nonsignificantly fewer cardiovascular (240 [2.5%] vs 271 [2.9%]; HR, 0.88; 95% CI, 0.74 to 1.05; P=0.15) and noncardiovascular (94 [1.0%] vs 121 [1.3%]; HR, 0.77; 95% CI, 0.59 to 1.01; P=0.06) deaths with alirocumab. In a prespecified analysis of 8242 patients eligible for ≥3 years follow-up, alirocumab reduced death (HR, 0.78; 95% CI, 0.65 to 0.94; P=0.01). Patients with nonfatal cardiovascular events were at increased risk for cardiovascular and noncardiovascular deaths (P<0.0001 for the associations). Alirocumab reduced total nonfatal cardiovascular events (P<0.001) and thereby may have attenuated the number of cardiovascular and noncardiovascular deaths. A post hoc analysis found that, compared to patients with lower LDL-C, patients with baseline LDL-C ≥100 mg/dL (2.59 mmol/L) had a greater absolute risk of death and a larger mortality benefit from alirocumab (HR, 0.71; 95% CI, 0.56 to 0.90; Pinteraction=0.007). In the alirocumab group, all-cause death declined with achieved LDL-C at 4 months of treatment, to a level of approximately 30 mg/dL (adjusted P=0.017 for linear trend).
Conclusions:
Alirocumab added to intensive statin therapy has the potential to reduce death after acute coronary syndrome, particularly if treatment is maintained for ≥3 years, if baseline LDL-C is ≥100 mg/dL, or if achieved LDL-C is low.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01663402.
Clinical Perspective.
What Is New?
In a randomized, double-blind trial in 18 924 patients with acute coronary syndrome and elevated atherogenic lipoproteins despite intensive statin treatment, fewer deaths occurred with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor alirocumab (334, 3.5%) than with placebo (392, 4.1%; P=0.03, nominal P value).
In 8242 patients eligible for at least 3 years of follow-up, alirocumab reduced mortality (P=0.01).
What Are the Clinical Implications?
Alirocumab added to intensive statin therapy has the potential to reduce death after acute coronary syndromes, particularly if treatment is maintained for ≥3 years.
Editorial, see p 113
Monoclonal antibodies targeting PCSK9 (proprotein convertase subtilisin-kexin type 9) produce substantial and sustained reductions in low-density lipoprotein cholesterol (LDL-C), alone or when added to statin therapy.1–4 In the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk)5 and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events-2)6 trials, treatment with the PCSK9 antibodies evolocumab or bococizumab reduced nonfatal cardiovascular events in patients with high cardiovascular risk or stable atherosclerotic cardiovascular disease, but did not demonstrate a statistically significant reduction in death (hazard ratio [HR], 1.04; 95% CI, 0.91 to 1.19 for FOURIER and HR, 0.91; 95% CI, 0.63 to 1.32 for SPIRE-2).
The ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trial7 compared the PCSK9 human monoclonal antibody alirocumab with placebo in patients with recent acute coronary syndrome (ACS) and elevated atherogenic lipoproteins despite intensive or maximum tolerated statin therapy. Alirocumab reduced the risk of the primary composite outcome of coronary heart disease (CHD) death, ischemic stroke, myocardial infarction, or unstable angina requiring hospital admission.8 Fewer deaths were observed in patients randomized to alirocumab.
To better understand the effects of alirocumab on death, this report describes all-cause, cardiovascular, and noncardiovascular death in participants eligible for ≥3 years of follow-up, the interaction of treatment and baseline LDL-C on death, and the association between nonfatal cardiovascular events and noncardiovascular death.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
Details of the study have been published.7,8 Patients were aged ≥40 years, had provided written informed consent, had been hospitalized with ACS 1 to 12 months before randomization, and had LDL-C levels ≥70 mg/dL (1.81 mmol/L), or non–high-density lipoprotein cholesterol levels ≥100 mg/dL (2.59 mmol/L), or apolipoprotein B levels ≥80 mg/dL after ≥2 weeks of stable treatment with atorvastatin 40 to 80 mg daily, rosuvastatin 20 to 40 mg daily, or the maximum tolerated dose of one of these statins.
After a prerandomization run-in phase, patients were randomly assigned to blinded treatment with alirocumab 75 mg or matching placebo given by subcutaneous injection every 2 weeks (see Methods in the online-only Data Supplement). All patients provided written informed consent. All sites obtained institutional review board approval as per local and national guidelines.
Study End Points
The primary end point was the composite of death caused by CHD, nonfatal myocardial infarction, ischemic stroke, or unstable angina requiring hospitalization. To control for multiplicity, secondary end points were analyzed in the following prespecified hierarchical sequence to preserve type I error8: any CHD event; major CHD event; any cardiovascular event; the composite of all-cause death, nonfatal myocardial infarction, or ischemic stroke; CHD death; cardiovascular death; and all-cause death. Initially the protocol only had all-cause death in the hierarchy7; a protocol amendment (10/11/2017) inserted CHD death and cardiovascular death before all-cause death. A Clinical Events Committee, blinded to treatment assignment and lipid levels, adjudicated all primary and secondary end points and categorized each death as cardiovascular or noncardiovascular, and subcategorized causes of death within each category. Noncardiovascular death considered alone was a post hoc end point. Deaths of indeterminate cause were analyzed as cardiovascular deaths.
Statistical Analysis
Efficacy was determined by time to first occurrence of any component of a given end point by intention-to-treat. Design assumptions were a primary end point incidence of 11.4% at 4 years in the placebo group and a median baseline LDL-C level of 90 mg/dL (2.33 mmol/L), reduced by 50% with alirocumab treatment, resulting in an expected 15% hazard reduction. At a 5% significance level, the trial had 90% power with 1613 primary end point events occurring in 18 000 patients with a median follow-up of approximately 3 years. In China, 614 patients were randomized after the main trial cohort. The protocol specified that the trial continue until at least 1613 primary end point events occurred and all evaluable patients had been followed for ≥2 years (except those from China), ensuring a minimum observation time to assess safety and efficacy. There were no study-powering assumptions around death. Treatment HRs and 95% CIs were estimated by Cox proportional hazards models, stratified by geographic region; P values were determined by stratified log-rank tests. Heterogeneity of treatment effects in subgroups based on incidences in the absolute scale were compared with the Gail-Simon test.9
A prespecified analysis (outside of the hierarchical analysis of efficacy) evaluated treatment effect on death among patients eligible for ≥3 years of follow-up (ie, randomized ≥3 years before the common study end date) on an intention-to-treat basis. Another prespecified analysis determined whether treatment effect on death was different before and after 1 year of follow-up, by comparing Cox proportional hazard models that allowed the treatment HR to vary before and after 1 year (stratified by region) to models where the treatment HR was assumed constant over time, and was done to test whether the former or latter analysis provided a better fit to the observed data.
To explore the association between the risks of nonfatal cardiovascular events and cardiovascular or noncardiovascular death, hazard functions for total (first and subsequent) nonfatal cardiovascular events (myocardial infarction, stroke [including hemorrhagic], or unstable angina requiring hospitalization) and cardiovascular or noncardiovascular death were estimated by general joint semiparametric models.10 The model includes 2 independent association parameters that represent the strength of within-patient association between nonfatal event times and within-patient association of nonfatal and fatal event times. If the association parameter between nonfatal events is 0, nonfatal event times for a given patient are independent, whereas an association parameter >0 indicates association between nonfatal event times. Likewise, if the association parameter for nonfatal and fatal events is 0, nonfatal and fatal event times for a given patient are independent, whereas an association parameter >0 indicates that nonfatal and fatal event times are associated. Treatment effects on nonfatal and fatal events are summarized separately by HRs and corresponding 95% CIs. Point estimates and corresponding 95% CIs are also generated for association parameters. Additional details of the model are provided in the Methods in the online-only Data Supplement.
To determine the association between baseline LDL-C and death, patients were classified according to baseline levels of LDL-C in prespecified categories of <80 mg/dL (2.07 mmol/L), 80 to <100 mg/dL (2.07 to <2.59 mmol/L), and ≥100 mg/dL (2.59 mmol/L). To assess the relationship between LDL-C achieved on alirocumab at month 4 and the subsequent risk of death, a spline plot was created, with an HR of 1 set to the median value of achieved LDL-C in that treatment group. The latter analysis was performed post hoc and was adjusted for age, region, diabetes mellitus status, and baseline LDL-C.
Results
A total of 18 924 patients were randomized at 1315 sites in 57 countries (see Figure in the Data Supplement). Median follow-up was 2.8 (interquartile range, 2.3–3.4) years. Premature treatment discontinuation for reasons other than death or blind switch to placebo because of low LDL-C levels occurred in 1343 (14.2%) patients receiving alirocumab and 1496 (15.8%) patients receiving placebo. Ascertainment for vital status was complete in 99.8% of potential patient-years of follow-up.
Effect on the Primary End Point
As previously reported,8 the primary end point occurred in 903 (9.5%) patients in the alirocumab group and 1052 (11.1%) patients in the placebo group, with 4-year Kaplan-Meier estimates of 12.5% and 14.5%, respectively (HR stratified by region, 0.85; 95% CI, 0.78 to 0.93; P<0.001). The effect of alirocumab was less pronounced during the first year (HR, 0.94; 95% CI, 0.83 to 1.08; P=0.38), and was greater after the first year (HR, 0.77; 95% CI, 0.69 to 0.87; P<0.001). A prespecified analysis showed that a model allowing the treatment effect to differ before and after the first year of follow-up fit the data better than a model with a constant treatment effect (P=0.03 for model fit improvement).
Effect on All-Cause Death
A total of 726 (3.8%) patients died during the trial. The baseline characteristics of the total cohort, survivors, and patients who died are summarized in Table 1. Fewer deaths occurred in the alirocumab group versus the placebo group (334 [3.5%] vs 392 [4.1%], respectively; HR, 0.85; 95% CI, 0.73 to 0.98; P=0.03; Figure 1). Because all-cause death followed CHD death and cardiovascular death in the prespecified hierarchy of main secondary end points, the P value for all-cause death was considered nominal. Based on Kaplan-Meier estimates of death at 4 years of 5.3% and 6.4%, respectively, in the alirocumab and placebo groups, the absolute risk reduction was 1.1%, and the number of patients needed to treat for 4 years to prevent 1 death was 87.
Table 1.
Baseline Characteristics
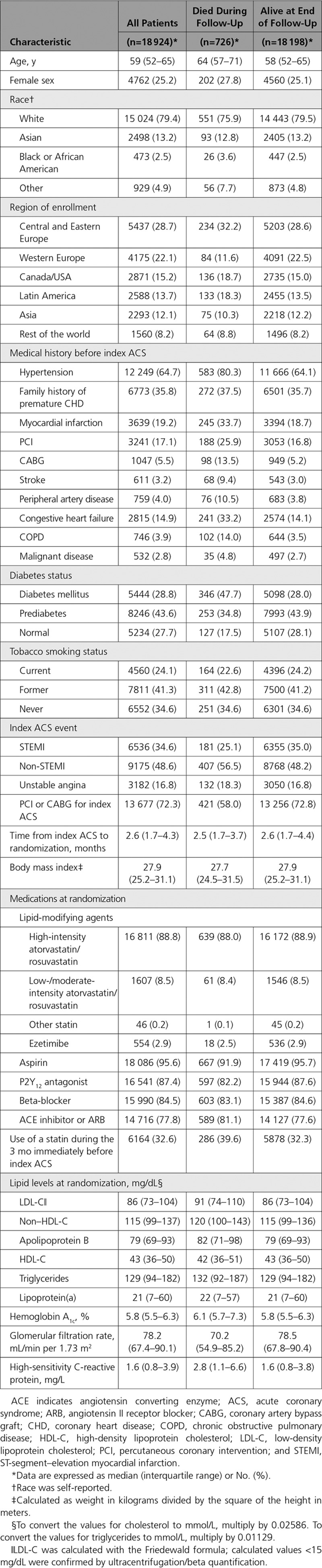
Figure 1.
All-cause, cardiovascular, and noncardiovascular death (intention-to-treat population) shown as Kaplan-Meier curves (left panel) and in a forest plot (right panel). CV indicates cardiovascular; and HR, hazard ratio.
Similar to the primary end point, allowing a nonconstant treatment HR on all-cause death suggested a lag in the treatment effect of alirocumab; treatment benefit was absent during the first year (HR, 1.01; 95% CI, 0.77 to 1.32; P=0.95), but was evident after the first year (HR, 0.79; 95% CI, 0.66 to 0.94; P=0.0073; P=0.13 for improvement in model fit versus the constant hazard model [with an HR of 0.85]).
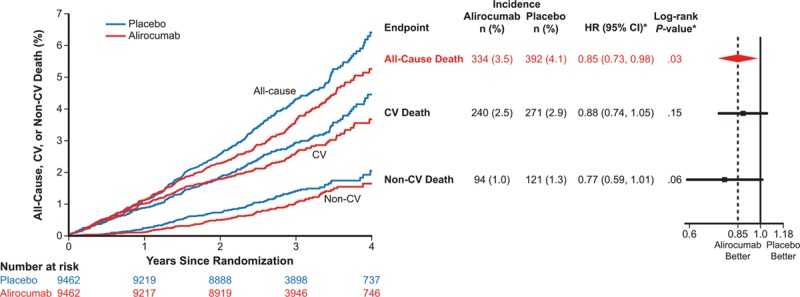
Given prior evidence for a delayed benefit of lipid lowering in many statin trials, a prespecified analysis examined whether lower mortality with alirocumab was related to the potential duration of treatment. We examined death among 8242 patients (44% of the full study cohort) eligible for ≥3 years of follow-up. The baseline characteristics of this subset and of patients enrolled for <3 years before the common study end date are described in Table I in the online-only Data Supplement. Among patients eligible for ≥3 years of follow-up, the benefit of alirocumab on all-cause death (HR, 0.78; 95% CI, 0.65 to 0.94; P=0.01; Figure 2) appeared more pronounced than in the overall trial population. For patients eligible for <3 years of follow-up, the treatment HR for all-cause death was 0.96 (95% CI, 0.76 to 1.21; P=0.71; interaction P=0.19).
Figure 2.
Prespecified all-cause death subgroup analysis. A, All patients. B, Patients eligible for ≥3 years of follow-up. HR indicates hazard ratio.
Effect on Cardiovascular and Noncardiovascular Death
As previously reported, CVD death occurred in 240 (2.5%) patients in the alirocumab group and 271 (2.9%) patients in the placebo group (HR, 0.88; 95% CI, 0.74 to 1.05; P=0.15), and represented 511 (70.4%) of all-cause deaths. Noncardiovascular death was also numerically lower with alirocumab (94 [1.0%] vs 121 [1.3%]; HR, 0.77; 95% CI, 0.59 to 1.01; P=0.06; Figure 1). Adjudicated cardiovascular and noncardiovascular causes of death are summarized in Tables II and III in the online-only Data Supplement. There was a low proportion of undetermined causes of death. Among 31 fewer cardiovascular deaths in the alirocumab group than in the placebo group, the largest differences between groups were for fatal myocardial infarction (n=10) and sudden cardiac death (n=8). Among 27 fewer noncardiovascular causes of death in the alirocumab group versus the placebo group, the largest difference was for death by pulmonary (n=14) causes.
Relationship Between Nonfatal Cardiovascular Events and Cardiovascular or Noncardiovascular Death
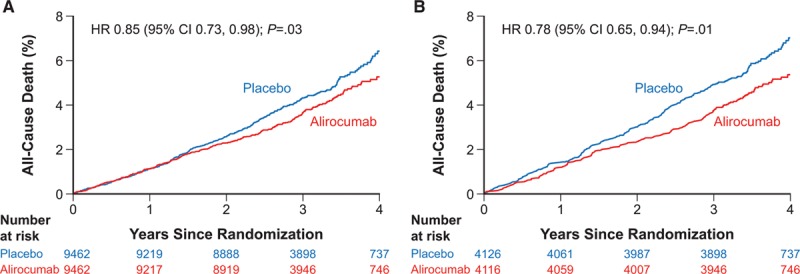
Overall, there were 206 fewer nonfatal cardiovascular events in the alirocumab group than in the placebo group. When nonfatal cardiovascular events and cardiovascular or noncardiovascular death are described as ordinal functions (Table 2), there were numerically fewer of all types of events with alirocumab than with placebo as first events, second events, and third or more events, suggesting a linkage between the nonfatal events and cause-specific death.
Table 2.
Number and Timing of Cases of Cardiovascular or Noncardiovascular Death and Nonfatal Myocardial Infarction, Stroke, or Unstable Angina Requiring Hospitalization According to Ordinal Event Number
Furthermore, successive nonfatal cardiovascular events were associated with a progressively greater risk of subsequent cardiovascular and noncardiovascular deaths. Among patients at risk for a first event in the alirocumab and placebo groups, noncardiovascular death occurred as a first event in 0.8% and 0.9%, while cardiovascular death occurred as a first event in 1.7% and 1.8%, respectively. After a first nonfatal cardiovascular event occurring an overall median of 1.1 (0.4, 1.9) years after randomization, noncardiovascular death occurred as a second event in 1.8% and 2.0%, while cardiovascular death occurred as a second event in 7.9% and 8.0%, respectively, of the patients in the alirocumab and placebo groups. Similarly, after a second nonfatal cardiovascular event occurring an overall median of 1.5 (0.9, 2.3) years after randomization, noncardiovascular death subsequently occurred in 3.7% and 6.2%, while cardiovascular death subsequently occurred in 11.1% and 14.8%, respectively, of the patients in the alirocumab and placebo groups. Qualitatively, these data indicate that each successive prior nonfatal cardiovascular event is associated with an increased subsequent risk for both cardiovascular and noncardiovascular death, and that the rate of subsequent cardiovascular death is four times higher than the rate of subsequent noncardiovascular death.
To provide a quantitative exploration of an association between nonfatal cardiovascular events and cardiovascular death, general joint semiparametric models were constructed for cause-specific death and nonfatal cardiovascular events with treatment assignment in the hazard functions. When the fatal event was noncardiovascular death (see Table IV in the online-only Data Supplement), alirocumab reduced total nonfatal cardiovascular events (HR, 0.82; 95% CI, 0.74 to 0.91; P=0.0002) and was a predictor of reduced noncardiovascular mortality (P=0.052). The association parameter between nonfatal cardiovascular events and noncardiovascular death of 2.35 (95% CI, 1.98 to 2.73) indicates patients at higher risk for noncardiovascular death were also at higher risk for nonfatal cardiovascular events (P<0.0001). Thus, a dependency between antecedent nonfatal cardiovascular events and subsequent noncardiovascular death, coupled with a reduction in the former with alirocumab (described in the primary analysis of the trial8 and in Table V in the online-only Data Supplement), indicates that prevention of nonfatal cardiovascular events may have contributed to the lower number of noncardiovascular deaths with alirocumab.
There was a similar strong dependency between antecedent nonfatal cardiovascular events and subsequent cardiovascular death (see Table VI in the online-only Data Supplement), with association parameter 3.49 (95% CI, 3.03 to 3.95; P<0.0001).
To explore whether the strength of the relationship between nonfatal cardiovascular events and noncardiovascular death was affected by adjustment for factors that were prognostic for one or both of nonfatal cardiovascular events or noncardiovascular death, a multivariable general joint semiparametric model was fit with 13 baseline patient characteristics expected to be predictive of these events (see Methods in the online-only Data Supplement). The resulting association parameter between nonfatal and fatal events of 1.03 (95% CI, 0.77 to 1.28) remained significant (P<0.0001), indicating a persistent, strong relationship between nonfatal cardiovascular events and noncardiovascular death.
Relationship of Death to Baseline and Achieved LDL-C Levels
In a post hoc analysis, death was examined in three predefined subgroups of baseline LDL-C level (<80 mg/dL [2.07 mmol/L], 80 to <100 mg/dL [2.07 to <2.59 mmol/L], and ≥100 mg/dL [2.59 mmol/L]; Figure 3). The HR for death was numerically lowest in the subgroup with baseline LDL-C ≥100 mg/dL (0.71; 95% CI, 0.56 to 0.90), but there was no significant heterogeneity of the effect of alirocumab on relative risk of death across categories of baseline LDL-C (Pinteraction=0.12). In contrast, there were significant gradients of the absolute risk of death in the placebo group and the absolute difference in death between alirocumab and placebo groups across baseline LDL-C subgroups, with greatest risk in the placebo group and greatest absolute risk reduction with alirocumab in the subgroup with baseline LDL-C ≥100 mg/dL (Pinteraction<0.005). Using the Kaplan-Meier estimates of all-cause death at 4 years for patients with a baseline LDL-C value ≥100 mg/dL (5.8% vs 9.6%), the absolute risk reduction was 3.8%, and the number needed to treat for 4 years to avoid 1 death in that patient subset was 26.
Figure 3.
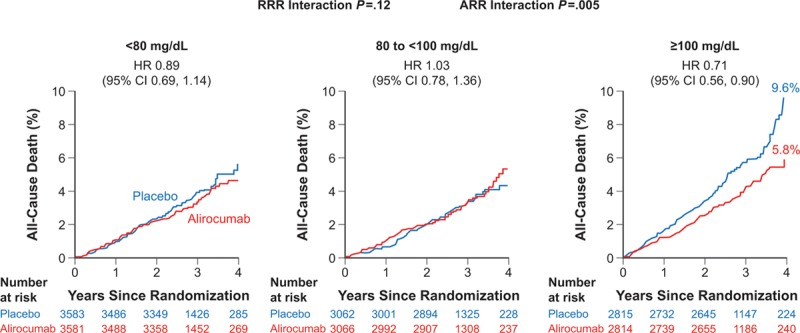
All-cause death by baseline low-density lipoprotein cholesterol subgroup. ARR indicates absolute risk reduction; HR, hazard ratio; and RRR, relative risk reduction.
Spline analysis related continuous values of achieved LDL-C at 4 months to the subsequent risk of death. In the alirocumab group (median achieved LDL-C, 31 mg/dL), the risk of death declined with lower achieved LDL-C, down to an LDL-C level of approximately 30 mg/dL (adjusted P value for model = 0.017 for linear trend) (Figure 4).
Figure 4.
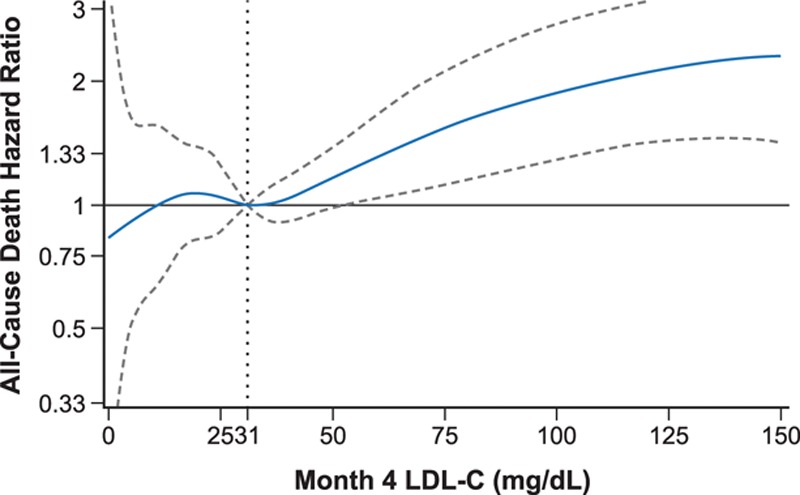
All-cause death spline analysis of continuous intention-to-treat month-4 LDL-C for alirocumab-treated* patients. *Hazard ratio (dashed lines indicate upper and lower bounds of the 95% confidence interval) is relative to median month-4 LDL-C (31 mg/dL [0.80 mmol/L]), adjusted for age, sex, geographic region, diabetes status, and baseline LDL-C. Degree = 3, 3 knots located at month-4 LDL-C quartiles (0.52, 0.80, and 1.27 mmol/L [20, 31, and 49 mg/dL]), P value for month 4 spline effect = 0.0169. LDL-C indicates low-density lipoprotein cholesterol.
Discussion
In ODYSSEY OUTCOMES, alirocumab, when added to intensive statin treatment, was associated with fewer all-cause deaths than placebo in patients with recent ACS and elevated atherogenic lipoproteins. This finding is considered nominally significant based on the prespecified hierarchical analysis of secondary end points. In an additional prespecified analysis, the treatment effect on death was particularly prominent among patients with a potential follow-up of ≥3 years. Patients starting at a higher baseline LDL-C level had a particularly prominent reduction in death with alirocumab, and patients achieving lower LDL-C levels at 4 months of treatment with alirocumab experienced a reduced risk of death.
A lower mortality was observed with alirocumab in the ODYSSEY OUTCOMES trial,8 whereas no difference in death was found with evolocumab or bococizumab, compared with placebo, in the FOURIER5 and SPIRE-26 trials. This may be because of differences in the drugs and in the trial populations: the different findings in ODYSSEY OUTCOMES versus the FOURIER and SPIRE trials may be related to a population of patients with recent ACS in the former, whereas FOURIER5 enrolled stable patients with established atherosclerotic cardiovascular disease, and SPIRE-26 enrolled a mixture of patients at high cardiovascular risk or with a previous cardiovascular event. Another possible explanation for different mortality findings is the duration of follow-up, which was longer in ODYSSEY OUTCOMES (median, 2.8 years) than in FOURIER5 (2.2 years) or SPIRE-26 (12 months). Notably, ODYSSEY OUTCOMES had 8242 patients eligible for ≥3 years of follow-up, with some patients followed for as long as 5 years. In ODYSSEY OUTCOMES, a stronger treatment effect was seen beyond year 1 (albeit statistically significantly for only the primary outcome). These observations suggest that longer treatment with alirocumab resulted in greater clinical benefit that was eventually discerned as a reduction in death.
Supporting the mortality reduction with alirocumab is the observation that patients in the highest predefined category of baseline LDL-C (≥100 mg/dL [2.59 mmol/L]) not only were at the highest absolute risk for major adverse cardiovascular events or death (as demonstrated by the event rates in the placebo arm), but also achieved the greatest absolute treatment benefit on major adverse cardiovascular events and death with alirocumab. Importantly, this subset of patients is one that would have been predicted to derive a mortality benefit based upon meta-analysis of previous lipid-lowering trials11 that demonstrated the greatest reductions in total and cardiovascular deaths in patients with high baseline LDL-C levels, particularly those with baseline LDL-C ≥100 mg/dL.
An important observation in this report is that fewer all-cause deaths with alirocumab resulted from numerically fewer noncardiovascular as well as cardiovascular deaths. While the latter effect has an obvious mechanistic linkage to lipid lowering, the former effect may be less intuitive. It may appear to run counter to the concept of competing mortality risks, whereby a reduction in cardiovascular risk leaves more opportunity for death from noncardiovascular causes.12 It might reflect a play of chance or a consequence of an imperfect classification of death because of limited information in some cases. However, there is also evidence to suggest that the observation of fewer noncardiovascular deaths with alirocumab has a pathophysiological basis. First, the time to noncardiovascular death was significantly longer in patients treated with alirocumab than with placebo. Second, joint semiparametric modeling demonstrated that the risks of both noncardiovascular and cardiovascular death were linked to the risk of nonfatal cardiovascular events, and alirocumab had a strong favorable effect on the latter. Thus, the reduction in nonfatal cardiovascular events with alirocumab may have contributed to effects on both noncardiovascular and cardiovascular death, and in turn on all-cause death.13 Clinically, the prevention of nonfatal cardiovascular events may prevent cumulative disability, frailty, and susceptibility to the mechanisms of noncardiovascular illness and death. In that respect, the majority of the difference in noncardiovascular deaths between groups (27 deaths) was related to a reduction in pulmonary deaths (14 fewer with alirocumab; see Table III in the online-only Data Supplement).
Finally, a greater effect of alirocumab to reduce death was associated both with higher baseline LDL-C and with lower on-treatment levels of LDL-C, as previously observed in statin trials.14 This finding suggests that patients with ACS who fail to achieve adequate reduction of LDL-C on conventional lipid-lowering therapy, particularly those whose LDL-C levels remain ≥100 mg/dL, may achieve a survival benefit with alirocumab treatment. Moreover, patients in the alirocumab group had a significant graded relationship of achieved LDL-C level to subsequent mortality, apparent to an achieved LDL-C level of approximately 30 mg/dL.
There are some limitations to our analysis. First, the classification of deaths as cardiovascular or noncardiovascular is unavoidably imperfect. Thus, total mortality may be a more reliable indicator of efficacy in long-term cardiovascular outcomes trials, and many deaths of unknown cause may in fact be cardiovascular. Indeed, in the Cholesterol Treatment Trialist Collaborative meta-analysis of 26 randomized statin trials,14 there were numerically fewer deaths of unknown cause with statins than with placebo (HR, 0.87; 95% CI, 0.73 to 1.03). In addition, the larger number of total deaths than either cardiovascular (~70% of total) or noncardiovascular deaths (~30% of total) provides more power to discern an effect on death from any cause. Second, the ODYSSEY OUTCOMES trial was designed to test a treat-to-target approach to lowering LDL-C levels after ACS. We cannot determine the extent, if any, to which intentional avoidance of very low LDL-C levels with a blinded dose-titration scheme influenced the mortality findings in the trial. Third, the mortality reduction with alirocumab is considered nominal given the position of all-cause death after end points that were not significantly reduced in the prespecified hierarchy of efficacy end points. Fourth, there are no head-to-head comparisons of the effects of PCSK9 inhibitors on clinical outcomes, and thus there are no data to prove that one agent is more effective than another on any specific clinical outcome or in any specific type of patient. Differences among trials with PCSK9 inhibitors regarding outcomes including death may be caused by differences in trial design (eg, duration of follow-up). Finally, as in any clinical trial, the effects observed in carefully selected participants may not fully reflect those that may be observed when the treatment is applied in a broader, less selective population treated in routine clinical practice.
Conclusions
This report provides evidence that alirocumab, added to intensive statin treatment, may reduce all-cause death after ACS in patients with elevated atherogenic lipoproteins. Greater treatment benefit occurs beyond the first year, in patients with longer follow-up, and in patients with higher baseline LDL-C values (≥100 mg/dL). Patients achieving lower LDL-C values at 4 months, down to approximately 30 mg/dL, appear to be at lower risk of subsequent death. The findings indicate that long-term treatment with alirocumab in appropriately selected patients may result in prolongation of life.
Acknowledgments
We thank the patients, study coordinators, and investigators who participated in this trial. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications, London), provided editorial assistance in the preparation of the manuscript (limited to editing for style, referencing, and figure and table editing) and was funded by Fondation Assistance Publique−Hôpitaux de Paris, France.
The protocol and statistical analysis plan were conceived by the first three authors, developed in conjunction with the other members of the executive steering committee and sponsors, and approved by responsible regulatory authorities and ethics committees. The sponsors participated in study site selection, monitoring, and supervision of data collection. Duke Clinical Research Institute led blinded end point adjudication. An independent data monitoring committee monitored safety and efficacy data. Analyses were performed independently by the academic statistician in parallel with the sponsors. The manuscript was drafted by the first author with input from all authors. The executive steering committee decided to publish the paper and takes responsibility for the completeness and accuracy of the data and the fidelity of the trial to the protocol.
The first author had full access to the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and had final responsibility for the decision to submit for publication.
Additional author contributions are as follows: Concept and design: P.G.S., M.S., D.L.B., V.A.B., R.D., S.G.G., R.A.H., J.W.J., K.W.M., M.T.R., H.D.W., A.M.Z., G.G.S. Acquisition of the data: P.G.S., M.S., D.L.B., V.A.B., A.J.D., R.D., S.G.G., R.A.H., J.W.J., K.W.M., P.O., A.P., M.T.R., R.V., H.D.W., A.M.Z., G.G.S. Analysis or interpretation of the data: P.G.S., M.S., G.L., P.L.T., G.G.S. Drafting of the manuscript: P.G.S. Critical revision of the manuscript: all. Statistical analysis: M.S., G.L. Obtained funding: P.G.S., G.G.S. Administrative, technical or material support: M.F.B., J.M.E., C.H., A.M., R.P. Supervision: P.G.S. and G.G.S.
Sources of Funding
The trial was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosures
Dr Steg: research grants from Bayer, Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Merck, Novartis, Novo-Nordisk, Pfizer, Regeneron Pharmaceuticals, Inc, Sanofi, and Servier. Dr Szarek: consultant/advisory board for CiVi, Resverlogix, Baxter, and Regeneron Pharmaceuticals, Inc. Dr Bhatt: advisory board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Biotronik, Boston Scientific, St Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. Dr Bittner: research grants from Amgen, DalCor, Esperion, Sanofi, AstraZeneca, Bayer Healthcare; honoraria from American College of Cardiology, American Heart Association, National Lipid Association; consultant/advisory board for Sanofi. Dr Brégeault: employee of Sanofi. Dr Dalby: travel grants from Sanofi, Bayer Pharma, Zydus; South African advisory boards: Amgen, Sanofi, Bayer Pharma; speaker’s honorarium: PharmaDynamics. Dr Diaz: research grants from Sanofi, Amgen, Bayer, Dalcor, PHRI, DCRI; honoraria from Sanofi. Dr Edelberg: employee of Sanofi. Dr Goodman: research grants from Daiichi-Sankyo, Luitpold Pharmaceuticals, Merck, Novartis, Servier, Regeneron Pharmaceuticals Inc, Sanofi, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Eli Lilly, Pfizer, Tenax Therapeutics; honoraria from Bristol-Myers Squibb, Eli Lilly, Fenix Group International, Ferring Pharmaceuticals, Merck, Novartis, Pfizer, Servier, Regeneron Pharmaceuticals Inc, Sanofi, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim; consultant/advisory board for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Pfizer, Servier, Tenax Therapeutics, Sanofi, Amgen, Bayer. Dr Hanotin: employee of Sanofi. Dr Harrington: research grants from Apple, CSL, Sanofi, Astra, Portola, Janssen, BMS, Novartis, The Medicines Company; consultant/advisory board for Amgen, Bayer, Gilead, MyoKardia, WebMD; other: board of directors (unpaid) for the American Heart Association and Stanford HealthCare. Dr Jukema: research grants from the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, the European Community Framework KP7 Program; other research support from Amgen, Astellas, AstraZeneca, Daiichi Sankyo, Lilly, Merck/Schering-Plough, Pfizer, Roche, Sanofi-Aventis. Dr Lecorps: employee of Sanofi. Dr Mahaffey: research grants from Afferent, Amgen, Apple, Inc, AstraZeneca, Cardiva Medical, Inc, Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, Novartis, Sanofi, St Jude, Tenax; ownership interest in BioPrint Fitness; consultant/advisory board for Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol-Myers Squibb, Cardiometabolic Health Congress, Elsevier, GlaxoSmithKline, Johnson & Johnson, Medergy, Medscape, Merck, Mitsubishi, Myokardia, Novartis, Oculeve, Portola, Radiometer, Springer Publishing, Theravance, UCSF, WebMd. Dr Moryusef: employee of Sanofi. Dr Ostadal: research grants from Czech Ministry of Health, Xenios; consultant/advisory board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Maquet, Xenios, Sanofi. Dr Parkhomenko: research grants from Bayer/Janssen, Pfizer, Sanofi, Amgen. Dr Pordy: employee of Regeneron Pharmaceuticals, Inc. Dr Roe: research grants from American College of Cardiology, American Heart Association, Familial Hypercholesterolemia Foundation, Ferring Pharmaceuticals, Myokardia, Patient Centered Outcomes Research Institute, Sanofi-Aventis; consultant/advisory board for Amgen, Ardea Biosciences, AstraZeneca, Eli Lilly, Merck; other: Flatiron, Janssen Pharmaceuticals, Novartis, Novo Nordisk, Regeneron Pharmaceuticals, Roche-Genentech. Dr Tricoci: research grant from Merck; consultant/advisory board for Merck. Dr White: research grants from Eli Lilly and Company, National Institutes of Health; other: AstraZeneca, Omthera Pharmaceuticals, Pfizer, Eisai Inc, Eli Lilly and Company, DalCor Pharma UK Inc, Sirtex, Acetelion, CSL Behring LLC, Luitpold Pharmaceuticals Ltd, Sanofi Aventis. Dr Schwartz: coinventor of pending US patent 14/657192 “Method for Reducing Cardiovascular Risk,” assigned in full to University of Colorado; research grants to University of Colorado from Resverlogix, Sanofi, Roche. The other authors report no conflicts.
Supplementary Material
Footnotes
Drs Steg and Schwartz contributed equally.
A full list of the ODYSSEY OUTCOMES Committee and Investigators is given in the online-only Data Supplement (see page 111).
Sources of Funding, see page 111
Guest editor for this article was Christie Ballantyne, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.118.038840.
References
- 1.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 2.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 3.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. doi: 10.1056/NEJMoa1701488. doi: 10.1056/NEJMoa1701488. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 9.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 10.Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, Brouste V, Soubeyran P. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics. 2007;8:708–721. doi: 10.1093/biostatistics/kxl043. doi: 10.1093/biostatistics/kxl043. [DOI] [PubMed] [Google Scholar]
- 11.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319:1566–1579. doi: 10.1001/jama.2018.2525. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanaroff AC, Roe MT, Clare RM, Lokhnygina Y, Navar AM, Giugliano RP, Wiviott SD, Tershakovec AM, Braunwald E, Blazing MA. Competing risks of cardiovascular versus noncardiovascular death during long-term follow-up after acute coronary syndromes. J Am Heart Assoc. 2017;6:1–10. doi: 10.1161/JAHA.117.005840. doi: 10.1161/JAHA.117.005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, Chiang CE, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Kimura T, Kiss RG, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Tricoci P, Xavier D, Zeiher AM, Steg PG ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387–396. doi: 10.1016/j.jacc.2018.10.039. doi: 10.1016/j.jacc.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R Cholesterol Treatment Trialists (CTsT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


