Abstract
Reductions in animal body size over recent decades are often interpreted as an adaptive evolutionary response to climate warming. However, for reductions in size to reflect adaptive evolution, directional selection on body size within populations must have become negative, or where already negative, to have become more so, as temperatures increased. To test this hypothesis, we performed traditional and phylogenetic meta-analyses of the association between annual estimates of directional selection on body size from wild populations and annual mean temperatures from 39 longitudinal studies. We found no evidence that warmer environments were associated with selection for smaller size. Instead, selection consistently favoured larger individuals, and was invariant to temperature. These patterns were similar in ectotherms and endotherms. An analysis using year rather than temperature revealed similar patterns, suggesting no evidence that selection has changed over time, and also indicating that the lack of association with annual temperature was not an artefact of choosing an erroneous time window for aggregating the temperature data. Although phenotypic trends in size will be driven by a combination of genetic and environmental factors, our results suggest little evidence for a necessary ingredient—negative directional selection—for declines in body size to be considered an adaptive evolutionary response to changing selection pressures.
Keywords: adaptation, Bergmann's rule, body size, climate change, natural selection, temperature
1. Introduction
It has been suggested that reductions in adult animal body size may be a general response of animal populations to global warming [1–3], and hence that ‘shrinking’ body size may be considered a third general response to climate change equivalent to changes in species distributions and phenology [4,5]. Although the evidence for consistent declines is not unequivocal [1,6], and other analyses have suggested no such general pattern exists [7,8], declines in body size have been reported in both ectotherms and endotherms, and across aquatic and terrestrial systems [1,2]. These declines in size are also apparent over both contemporary (recent decades [1,2]) and longer timescales (past tens of thousands of years; [9]).
Whether or not declines in body size are a general phenomenon, declines in size with warmer temperatures are often interpreted as an adaptive evolutionary response to a warming climate, presumably generated by changing selection pressures [6,10]. This inference typically stems from extension of the biogeographic pattern known as Bergmann's rule [11], by which, within a given clade, smaller species tend to be found in warmer climates. For endotherms that maintain a constant body temperature, this pattern may be an energetically based adaptive response to temperature because smaller size results in a higher surface area to volume ratio, improving heat loss in warmer conditions [11]. However, the coupling of body size and temperature associated with Bergmann's rule has been described for ectotherms as well as endotherms [1,3]. Furthermore, numerous studies of ectotherms have demonstrated genetic differentiation in adult size among populations or congeneric species along latitudinal and elevational gradients [12–16], suggesting that the pattern may be the result of adaptive evolution for ectotherms as well.
Phenotypic plasticity may also contribute to the biogeographic pattern of smaller body sizes being found at warmer temperatures. In ectotherms, for example, higher temperatures during development typically lead to smaller adult body size, an empirical pattern known as the temperature–size rule [17]. Recent work has also found that warmer temperatures during development can lead to smaller body size in endotherms, suggesting that temperature-dependent developmental plasticity may be a general response to warmer temperatures (e.g. [18]). The reasons for the observed thermal plasticity in size, and its potential adaptive value, remain poorly understood [19–23]. However, if smaller body size has fitness advantages at higher temperatures, temperature–size plasticity may represent adaptive variation [22,24–28]. As a result, in general, it follows that for both endotherms and ectotherms that thermal plasticity in body size may be adaptive if smaller size is favoured at warmer temperatures.
Any observed declines in adult size during recent climate warming could therefore be due to adaptive evolution, to adaptive or non-adaptive phenotypic plasticity or to a combination thereof, but the contributions of these mechanisms to observed trends are currently unknown [6,10,29]. One difficulty in invoking adaptive evolution as an explanation is that few studies have both estimated selection and documented that changes in body size through time are genetically based [6,30,31]. Critically, however, if adaptive evolution in response to warming environmental temperature is the mechanism resulting in reductions in body size, selection on body size must become negative, or, where already negative, it must have become more negative with increased temperatures. The general applicability of this explanation can be tested by comparing selection analyses across many populations. This test is now possible due to the availability of numerous estimates of natural selection in the wild from contemporary populations [32–34], and the hard work of quantifying selection and reporting these data in a standardized format has made possible attempts at generalizing patterns of selection [35–37]. Previous synthetic analyses have shown that larger body size is associated with higher fitness components in many organisms [38,39], but did not consider whether environmental factors modulated this relationship. Another analysis investigated whether climate could explain spatial and temporal variation in selection and found that while precipitation consistently explained most variation in selection, variation in temperature did explain variation in selection for some organisms, particularly invertebrates [40]. However, that analysis did not investigate the specific predictions concerning selection on body size and temperature as outlined above.
Here, we tested the hypothesis that the direction and magnitude of selection on body size in a population has changed consistently with the temperature experienced by that population. Specifically, we examined whether warmer temperatures are associated with selection for smaller body sizes, which would constitute a necessary—if far from sufficient—component of the argument that adaptive evolutionary responses underlie declines in body size. We also considered whether this relationship differs between endothermic and ectothermic animals, and whether selection on body size has simply changed over time, by considering the relationship between estimates of selection and year. We did so by combining published databases of estimates of selection in the wild collated from longitudinal studies where selection has been measured across two or more years within a population, with annual temperature data (as detailed in [40]). From these datasets, we built a meta-analytical model that estimated the association between temperature and selection on body size, while controlling for the effects of sampling error and variation among species and different studies.
2. Methods
(a). Selection database
We used a previously assembled database of temporally replicated studies of phenotypic selection on quantitative traits from wild populations, using studies where two or more annual estimates of selection were available from a given population. Full methods describing how the database was assembled are available in [40] and we refer the reader to that paper for additional details. In brief, this database is from an exhaustive literature review using a keyword search to identify studies (published up until December 2012) that reported temporally replicated estimates of natural selection from wild populations. We then supplemented this database with results from a literature search, using the same methodologies employed before, for additional studies meeting the above criteria published up until December 2018. No evidence of publication bias was detected through inspection of funnel plots (electronic supplementary material, figure S1).
The database consists of standardized measures of selection coefficients: gradients and differentials. Selection gradients identify the strength and direction of direct selection acting on body size after accounting for indirect selection via trait correlations, whereas selection differentials reflect total selection (direct and indirect). We do note that although selection gradients in principle reflect the direct targets of selection, this is only the case when all relevant traits have been included in the original analyses. Thus, there is still likely an indirect component of selection that exists in the estimated selection gradients, and hence, the extent to which they predict the evolution of body size. These standardized selection coefficients represent selection on size in terms of the relationship between relative fitness and variation in size measured in standard deviation units, and are desirable because they allow for cross-study comparisons, irrespective of study organism or fitness measure [32,41].
In order to test the implications of changing temperature for patterns of selection on size, we only considered directional selection. Thus, we only included those studies that quantified selection on size-based traits, which included selection on overall body size or mass, but also on components of size such as principal component scores. These estimates came from studies conducted between 1965 and 2016, the majority of which involve long-term studies of endotherms, particularly birds and mammals, and resulted in a total of 1595 estimates of selection gradients (from 23 studies) and 1181 estimates of selection differentials (from 16 studies; table 1) from a total of 32 species (electronic supplementary material, table S1).
Table 1.
Characteristics of the data analysed: number of datasets and number of estimates; mean and standard deviation (s.d.); median and range of the duration of studies; and the number of datasets, studies, species and number of estimates of selection coefficients for different taxonomic groups. (Note that a ‘dataset’ is defined as the set of selection estimates for a given phenotypic trait and fitness component for a given study, so a study may contain multiple datasets.) See also electronic supplementary material, table S1.
| selection gradients | selection differentials | |
|---|---|---|
| number of datasets (total no. estimates) | 109 (1595) | 83 (1181) |
| mean (s.d.) of study duration, in years | 13.6 (9.5) | 13.9 (9.9) |
| median (range) of study duration, in years | 12 (2–33) | 12 (2–36) |
| taxonomic groups: no. of datasets, studies, species and estimates | ||
| amphibian | 1, 1, 1, 3 | 1, 1, 1, 3 |
| bird | 59, 7, 7, 1333 | 43, 6, 6, 979 |
| fish | 12, 5, 4, 56 | 10, 3, 3, 54 |
| insect | 11, 3, 3, 39 | 15, 3, 3, 63 |
| mammal | 10, 4, 3, 124 | 10, 2, 2, 74 |
| reptile | 17, 4, 4, 40 | 4, 1, 1, 8 |
(b). Temperature database
To relate the annual estimates of selection to temperature in the same year in the same geographical location for each study, we used a previously assembled climate database [40]. This climate database was subsequently updated to include temperature data for the additional selection studies uncovered in the new literature search. In brief, assembly of this database involved obtaining local temperature data at a resolution of 0.5 × 0.5° cells from the CRU-TS 3.1 (CRU-TS 4.02 for updated studies) Climate Database [42]. This is one of the finest scale climate databases available at a global scale and has been used for similar analyses [43,44]. From these data, we generated grid files containing annual mean temperature. To obtain appropriate temporal climatic information for each study location, we performed a spatial overlay of study site coordinates over each climatic grid using the function over in the R package sp [45]. We acknowledge that there may be very fine-scale local temperature variation within a 0.5° grid cell [46,47], but given the scale of the geographical and temporal variation in our datasets, these 0.5° grid cell annual estimates provide a manageable means of testing for broad-scale general patterns which will be relevant even if only indirectly linked [48]; we return to this point in the Discussion. All analyses used temperature values that were mean-centred within each study, so as to assess the impact of relative changes in temperature at each site.
We first verified that there was an increase in annual mean temperature over time within populations. We assessed this by using a mixed model, regressing temperature on year
| 2.1 |
where tij was the (mean-centred) temperature in interval i of study j. C and D are the fixed effect intercept and slope of the regression of temperature on year, yij is the year for interval i of study j, centred on the year 1990 (an arbitrarily defined mid-time point over the period of study in the database), cj and dj are study-specific random effects for the intercept and slope, respectively, and eij are residuals. This model estimates the variances of, and covariances between, the cj and dj terms, as well as the residual variance.
Results from this model showed that across studies reporting selection gradients, temperature has increased by 0.043 °C yr−1 (95% CI: 0.031, 0.057), and for studies reporting differentials, temperature has increased by 0.022 °C yr−1 (95% CI: 0.016, 0.027). Both the temporal and spatial span of studies presenting gradients or differentials differ, which we suspect explains the observed differences in the slopes. Regardless, annual mean temperature of the specific study sites associated with our selection estimates has increased through time, matching global patterns [49,50].
(c). Meta-analytical model
Though we also implemented a phylogenetically informed approach (see below and electronic supplementary material), we started with the simpler approach taken in [40], using a random effects hierarchical meta-analysis to estimate an overall slope of the relationship between the estimated selection coefficients and the respective temperature measure for each site at each time point, taking into account sampling error, and variation between species, between studies and within studies (as random effects). We also included a random regression term of temperature within each study to model any variation between populations in the effect of temperature. This hierarchical model separates the observation process (i.e. modelling statistical noise in inference of individual selection coefficients stemming from sampling error) from a process model (i.e. modelling variation in the underlying (latent) selection coefficients in response to temperature, which is the biological process in which we are ultimately interested). Because sampling error can lead to erroneous inferences regarding variation in selection [35,36], we used only those estimates from the database that had associated standard errors. Additionally, even though the overall regressions in which we are interested are not strictly biased if sampling error is not accounted for [36], such regressions could be very sensitive to influential outliers [51]. Indeed, such outliers are common in databases of selection estimates (including those in the present analyses; cf. figures 1–3; electronic supplementary material, figures S2–S4), and it is almost always the case that outliers result from extremely low-powered studies (e.g. where n is especially small).
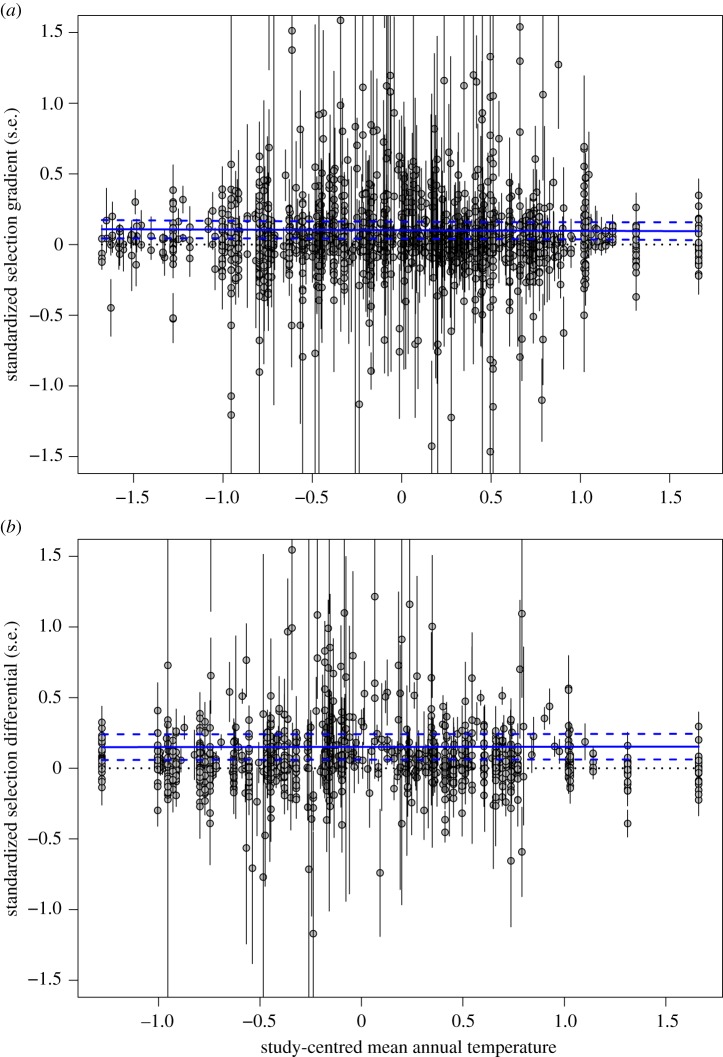
Figure 1.
Warmer temperatures are not associated with selection for smaller body size in animals. The figure shows the relationship between standardized selection (a) gradients or (b) differentials (with error bars of s.e. for each estimate) on animal body size and study-centred annual mean temperature. The blue lines show the overall regression (solid blue line) from models 1 and 2 in table 2 along with the 95% prediction interval (dotted blue lines). The panels do not show outliers (estimated selection coefficients greater than 3.0, which were included in the analysis), which affects the scale on the figure; but see electronic supplementary material, figure S2 for the equivalent figure with all the data (note that all analyses used the full dataset). (Online version in colour.)
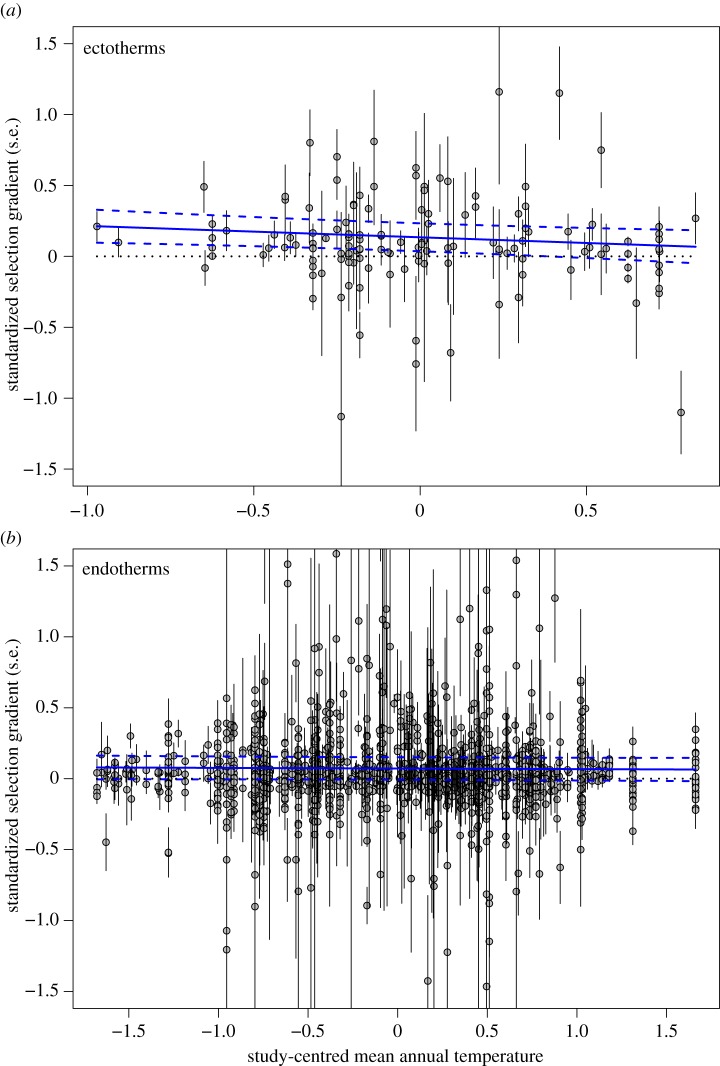
Figure 2.
Warmer temperatures are not associated with selection for smaller body size in either (a) ectotherm or (b) endotherm animals. The figure shows the relationship between standardized selection gradients for (a) ectotherms or (b) endotherms (with error bars of s.e. for each estimate) and study-centred annual mean temperature. The blue lines show the overall regression (solid blue line) from models 3 and 4 in table 2 along with the 95% prediction interval (dotted blue lines). The panels do not show outliers (estimated selection coefficients greater than 3.0, which were included in the analysis), which affects the scale on the figure; but see electronic supplementary material, figure S3 for the equivalent figure with all the data (note that all analyses used the full dataset). (Online version in colour.)
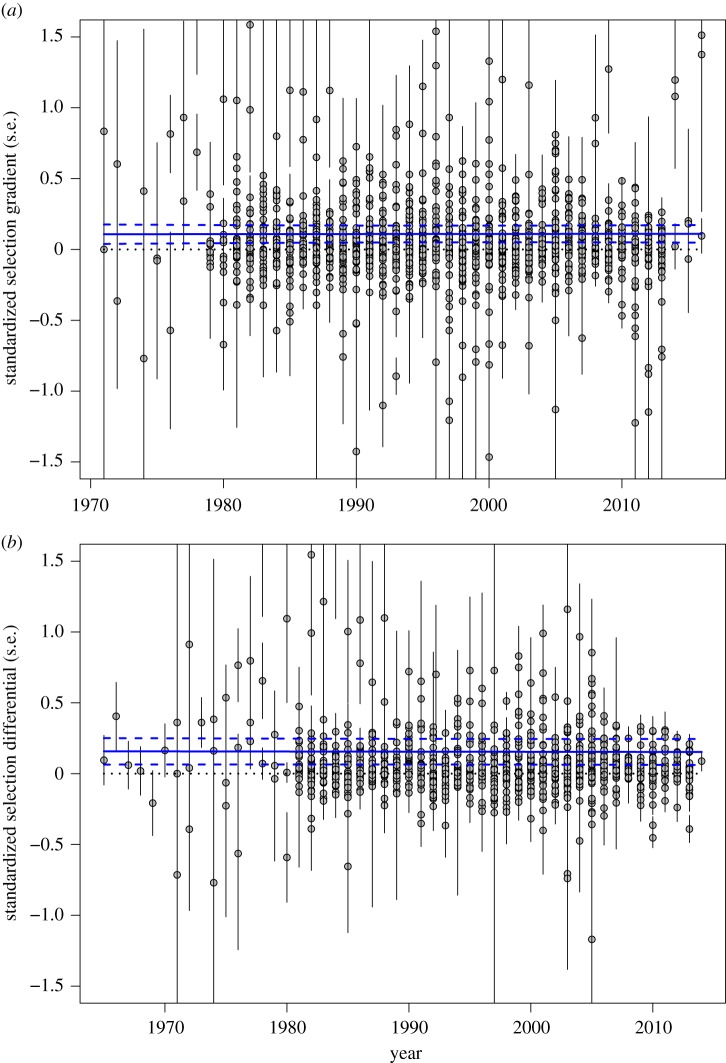
Figure 3.
Directional selection on body size did not consistently change over the 46-year period of record. The figures show the relationship between standardized selection (a) gradients or (b) differentials (with error bars of s.e. for each estimate) and the year selection was estimated. The blue lines show the overall regression (solid blue line) from models 5 and 6 in table 2 along with the 95% prediction interval (dotted blue lines). The fixed slope in this random regression model simply reflects the average within-study slope, and thus reflects a hypothetical study spanning the entire time range. The panels do not show outliers (estimated selection coefficients greater than 3.0, which were included in the analysis), which affects the scale on the figure; but see electronic supplementary material, figure S4 for the equivalent figure with all the data (note that all analyses used the full dataset). (Online version in colour.)
We modelled the distribution of estimated selection coefficients (linear gradients and differentials) according to
| 2.2 |
where is the ith selection coefficient estimate for dataset j (that is, a given combination of phenotypic trait and/or fitness component and/or species, etc. for a given study; hereafter ‘dataset’) in species k. A and B are the fixed effect intercept and slope, respectively, of the regression of selection coefficients on (dataset centred) annual temperature values, (note that these are the same as in equation (2.1), but for completeness here, we also index by k).
The model in (2.2) is a random effects meta-analytic model, but with a greatly elaborated treatment of heterogeneity, including a random effects structure to allow for variation among datasets in the relationship between selection coefficients and temperature. Thus, is the intercept of dataset j and the slope of the effect of temperature within dataset j, with variances and covariance modelled according to
are species-specific random intercepts distributed according to , are measurement errors, with variances described by the reported standard errors for each estimate, , and are residuals distributed as . Note that the variances of measurement errors are defined by the published standard errors of the estimates, while the variances (, ) and covariance associated with the random slopes and intercepts, as well as the variances of species effects and residuals , are estimated by the model.
This model structure is closely related to that used in [40], though the parameters of direct interest here are the fixed effects, and in particular, the overall regression coefficient B, and the model is presented in a more standard mixed-model framework. The models were fitted to different subsets of the database using the R package MCMCglmm [52], using diffuse Gaussian priors for fixed effects, diffuse inverse gamma priors for and and parameter expanded priors [53,54] for the covariances in the random regression component of the model. All statistics reported are means of the posterior distribution, with associated 95% credible intervals (CIs). We note that considering modes rather than means gave identical conclusions. The models were run on the dataset of gradients (model 1 in Results) and differentials (model 2 in Results). As an index of heterogeneity, the variance components associated with the random effect terms are presented in electronic supplementary material, table S2.
In addition, for this main analysis of interest, we also performed a full phylogenetic random regression meta-analytical model (see electronic supplementary material). Because we did not detect a phylogenetic signal in either the intercepts or slopes from these models, we present the simpler models in the main text and place these more complicated phylogenetically informed models in the electronic supplementary material (table S3). The conclusions regarding the fixed effects remained the same across all models.
To explore potential differences in selection between ectotherms and endotherms, we also built models (models 3 and 4) that evaluated whether or not there were differences in the mean selection coefficients in the two groups, and in their relationship with temperature. We did so by including a two-level factor for group (endotherm versus ectotherm), and its interaction with the effect of mean-centred temperature in model 1. We restricted this analysis to selection gradients, because sample sizes of selection differentials for endotherms were insufficient.
Finally, the above analyses modelled the relationship between estimates of directional selection on size and annual mean temperature in their respective year, and as such represent the most general test of selection–temperature relationships. However, it is plausible that annual mean temperature may not be the most critical thermal window shaping selection, assuming such a window exists. For example, an intra-annual component of temperature, such as variance in temperature, or a measure of extremes such as mean daily maximum temperature, may be more relevant (although our previous analyses show that annual mean temperature and maximal temperature are positively correlated across the study sites [40]). Because of these issues, we also performed an analysis that tested for evidence that selection simply changes over time. This was accomplished by investigating the relationship between estimates of selection and year of study (models 5 and 6). We fit models using the year of the study rather than temperature in models 1 and 2 outlined above (i.e. we replace tijk with year terms yijk; where year values were mean-centred on an arbitrary time point of 1990). Of course, any of a number of factors that might drive selection could vary with time in such an analysis. However, this analysis should be able to detect whether selective regimes have changed over time, regardless of the relevant thermal window.
3. Results
Our meta-analysis of patterns of selection on body size showed overall positive directional selection on body size, both for selection gradients (figure 1a) and selection differentials (figure 1b). There was no evidence for change in either selection gradients or selection differentials with increasing temperature (figure 1 and table 2: models 1 and 2, respectively; electronic supplementary material, table S2), and in particular no indication that selection favoured smaller body sizes at higher temperatures (i.e. no evidence that selection became negative or more negative; figure 1). Including phylogeny in this model did not qualitatively change these findings: there was no indication of an effect of temperature on selection coefficients in the phylogenetic models, nor was there support for a phylogenetic component to the species effect (electronic supplementary material, table S3).
Table 2.
Results from the meta-analytical models relating annual selection coefficients to temperature or year. Shown are the posterior means for the intercepts (A) and slopes (B) and their 95% credible intervals when selection on body size was regressed on mean annual temperature (models 1–4) or year (models 5 and 6). Separate models for temperature–selection gradient relationships are presented for ectotherms (model 3) and endotherms (model 4). Details of the variance components for models 1 and 2 are given in electronic supplementary material, table S2.
| model | intercept |
slope |
||
|---|---|---|---|---|
| 95% CI | 95% CI | |||
| temperature as predictor | ||||
| model 1: gradients | 0.108 | 0.051, 0.168 | −0.004 | −0.020, 0.009 |
| model 2: differentials | 0.156 | 0.073, 0.254 | −0.0002 | −0.009, 0.008 |
| model 3: gradients, ectotherms | 0.147 | 0.044, 0.238 | −0.060 | −0.130, 0.011 |
| model 4: gradients, endotherms | 0.070 | −0.005, 0.152 | −0.0008 | −0.014, 0.013 |
| year as predictor | ||||
| model 5: gradients | 0.108 | 0.04, 0.17 | 0.00007 | −0.0011, 0.0011 |
| model 6: differentials | 0.157 | 0.072, 0.247 | −0.0002 | −0.0008, 0.0005 |
We next considered a model that allowed different intercepts and slopes for ectothermic and endothermic taxonomic groups to the basic model specified by equation (2.2). The average selection gradient did not differ between ectotherms and endotherms (endotherm intercept − ectotherm intercept = −0.083, 95% CI: −0.192, 0.026), and there was no difference between the two groups in the slope of the relationship between selection gradients and temperature (endotherm slope − ectotherm slope: 0.064; 95% CI: −0.018, 0.131). For completeness, we subsequently ran two separate models for ectotherms and endotherms (table 2: models 3 and 4, respectively), to generate group-specific slopes and intercepts, rather than contrasts, and again detected no relationship between selection on size and temperature among ectotherms (figure 2a) or endotherms (figure 2b and table 2).
Finally, we found no evidence of any association between directional selection on body size and year for either selection gradients (figure 3a and table 2: model 5) or selection differentials (figure 3b and table 2: model 6). That is, there was no evidence that the strength or direction of selection on body size changed over time.
4. Discussion
Our analyses of contemporary patterns of selection on animal body size documented over the past several decades showed no evidence of any association between temperature increases and selection for reductions in body size. In fact, selection on body size has remained consistently positive. These patterns suggest that selection for smaller body size is not a general phenomenon, and hence that adaptive evolutionary responses to changed selection pressures imposed by a warming climate, as would have been expected under Bergmann's rule, is unlikely to be a general explanation for recent declines in body size where they have occurred. While it is tantalizing to consider that large-scale biogeographic rules might explain contemporary patterns of body size change (e.g. a temporal Bergmann cline; [7]), our results suggest caution is warranted in deriving contemporary predictions from such rules. Our results are consistent with a recent qualitative review that found little evidence for adaptive body size reductions with climate warming [6], as well as a recent analysis of 952 bird and mammal species that found little support for consistent declines in body size among populations that varied in temperature [7]. Although evidence for adaptive evolutionary responses will ultimately require genetic as well as phenotypic data, we can still infer that these evolutionary responses are unlikely to be a ubiquitous pressure towards shrinking body size, because adaptive evolution requires both selection in the appropriate direction and genetic variation in body size.
Of course, the expression of genetic variation is also not necessarily fixed and may vary with environmental conditions through time. However, few studies have assessed how temperature may affect the expression of genetic variance underlying traits. The majority of these studies are laboratory-based, and have suggested that temperature can generate differences in expressed genetic variation—although these generalities are not specific to body size [55]. Moreover, to date, evidence for genotype–environment interactions affecting heritable traits in wild populations is mixed (see review in [52,53]). Most importantly, changes in the expression of genetic variation in response to environmental variation are unlikely to affect the direction of adaptive evolution through time, at least over shorter-term timescales considered here, underlining the requirement for selection pressures to act in the appropriate direction if adaptive reductions in body size are to be considered a general phenomenon.
Overall, the modest slopes of the regressions of selection coefficients on temperature, relative to the positive intercepts, mean that directional selection on body size was on average positive. This result is consistent with previous comparative analyses of directional selection on body size in animals [19,39]. We also found selection to be invariant in response to temperature or year. Extrapolating (excessively) from the meta-analytical model, the trend present in the fixed regression for selection coefficients predicts an eventual shift towards selection for smaller body sizes (i.e. negative values), particularly for ectotherms. Thus, with continued temperature increases, there may be an eventual reduction in the strength of directional selection that currently, on average, favours larger-bodied individuals. However, as with any statistical model, an abundance of caution is warranted in extrapolating beyond the available data. Nevertheless, the predicted increases in temperature over the next several decades [49], along with sustained efforts to maintain long-term studies of selection in the wild [54], will provide an opportunity to evaluate this hypothesis.
The interaction between temperature, body size and fitness that would underlie Bergmann's rule requires that the slope of the relationship between expected absolute fitness and body size is more negative at high temperatures, but positive at low temperatures. However, it is worth noting that the summary statistics by which the form of directional selection is typically reported—selection gradients and differentials—can change in value between different replicate estimates of selection, because of changes to various aspects of the trait-fitness relationship other than its slope; e.g. changes to the distribution of phenotypes [56,57]. Consider a population at a selective optimum, such that its mean body size is at an optimum in the body size–fitness function. Imagine that, due to phenotypic plasticity, an increase in temperature causes a decrease in mean body size (e.g. according to the temperature–size rule for ectotherms, or as recently found in endotherms [18]). In the absence of any effects of temperature on the fitness function, the population will now experience directional selection for larger body size, because most phenotypes will now be in an area of the fitness function that is positively sloped, on average. Such a mechanism would mask, or reduce in magnitude, any pattern of interaction between temperature, body size and fitness that would generate a temporal Bergmann pattern. Unfortunately, most studies of phenotypic selection on body size do not report mean size, so we are unable to evaluate this possibility with the available database. Future studies addressing biogeographic patterns of body size and selection would benefit greatly if summary statistics about trait distributions and absolute fitness were regularly reported.
Although the emerging general pattern may be for no decline in animal body size [6–8], and our analyses found no evidence that selection on body size has changed over the past approximately five decades, there are examples where body size has declined through time as environments have warmed; some of these responses may have been adaptive [1,6]. However, for reasons that frequently hamper attempts to infer adaptive evolutionary responses, it is often unclear if these changes necessarily represent adaptive evolution. To our knowledge, only four longer-term studies have directly investigated the role of adaptive evolution as an explanation for contemporary declines in body size [29,58–60]. These studies all used breeding values (i.e. estimates of individuals’ additive genetic merit for body size): three of the four found no evidence of selection for smaller body sizes, nor of a genetic basis for observed size changes [29,58,59], whereas Bonnet et al. [60] found that, despite a positive phenotypic association between body size in snow voles and fitness, there was evidence of a genetic change towards smaller body size. The latter indicates an adaptive evolutionary response to viability selection, perhaps driven by changes in the snow-free season favouring juveniles that become small adults [60]. Importantly, these latter results show that the adaptive evolutionary response towards smaller size and the selection pressures driving it were not apparent from phenotypic data alone, because they were masked by plasticity and a non-genetic positive association between body size and viability, respectively [60]. Understanding both the environmental factors generating selection and the potential for evolutionary responses to changing climate remains an important endeavour.
Such findings suggest that explorations of alternative explanations for temporal trends in body size are warranted [31]. For instance, it may be that the observed declines in body size in some populations, especially among endotherms, might be driven by phenotypic plasticity in response to altered environmental conditions that affect growth rates, such as food availability [1,6,29]. As noted by Teplitsky et al. [29], such reductions in body size might therefore be a foreboding signal of populations experiencing increasingly deteriorating conditions, rather than examples of adaptive evolutionary rescue in response to a changing climate. Recent experimental work has also shown that reductions in body size in birds in warmer environments can be explained by phenotypic plasticity in growth (e.g. climate-dependent developmental plasticity) in response to temperature during development, even in the absence of changes in food availability [18]. Clearly, a better understanding of how temperature–size patterns emerge and the genetic basis for them are necessary, especially studies investigating the mechanistic basis of these associations [28]. Finally, changes in body size in relation to temperature might also vary among taxonomic groups, and therefore may not be a universal pattern [1]. For instance, Teplitsky & Millien [6] found that although declines in body size with temperature in birds were common, this pattern was rare among mammals. While it would be interesting to examine if associations between selection and temperature differ among these two groups, there are currently too few data for a robust analysis.
Despite using the most comprehensive datasets of selection and climate available, our study is not without limitations. Two caveats in particular may affect our ability to detect a possible association between selection on body size and temperature. First, annual mean temperatures may not always represent the critical thermal window that could generate an association between selection on body size and temperature. For example, selection on body size might occur via extreme thermal events that occur over a brief time window [61–63], even if they do not substantially shift annual mean temperature. A population might be reasonably well adapted to a given temperature regime, such that even a single episode of extreme temperatures might generate very strong selection [62]. These potential episodic bouts of selection may be major drivers of evolutionary change [63,64], but may be difficult to observe, even among longer-term datasets [8,65,66]. Importantly, however, our analyses demonstrating stasis in selection coefficients across years (figure 3) suggest that there was no hidden temperature–selection relationship that would be revealed by some alternate choice of a thermal window. Indeed, identifying more precisely how climate might influence the dynamics of natural selection in wild populations remains a challenge [6,40]. This is one issue with meta-analytical studies such as ours that are conducted across disparate studies where it is unclear what the critical thermal window may be [40,47,48]. To the best of our knowledge, none of the studies in our database were designed specifically to examine any possible association between selection and temperature. On the one hand, this is a limitation, as above. On the other hand, it also reduces bias in our dataset, in that there should be no ‘file-drawer effect’ (that is, underrepresentation of studies exhibiting no relationship between temperature and selection).
A second caveat arises from the fact that most studies of selection on body size typically extend over a few years, whereas many of the studies of body size trends span several decades [1,6,8]. Because changes in average temperature through time are subtle, it may be that longer-term datasets are the most informative for detecting the overall magnitude of these associations. However, even relatively short-term datasets remain valuable in detecting temperature–selection couplings, because the general expectation is simply that declines in body size through time are linear. Thus, even short-term data sets from temporally replicated studies of selection in wild populations should not preclude our ability to detect selection–climate associations, as we have previously found [40].
5. Conclusion
Overall, based on (i) our analyses finding that selection on body size has not consistently changed with temperature or time and (ii) recent comprehensive analyses of body size in relation to temperature and time showing that body sizes are not uniformly declining [6–8], we find little support for the idea that increasing global temperatures are resulting in widespread adaptive evolutionary shifts towards smaller body size. Whether and how the long-term impacts of a warming climate—and changes in other climate components such as precipitation regimes—will generate adaptive responses thus remains unclear. Studies of selection and the genetic underpinnings of adaptive evolutionary responses in wild populations will continue to be of considerable value in understanding how adaptive evolution may play a role in allowing populations to persist or perish in the face of a changing climate.
Supplementary Material
Acknowledgements
We thank the hundreds of field biologists that are engaged in the study of natural selection in wild populations. We thank Jennifer A. Lau and Nancy C. Emery for discussion and ideas, Jenna M. McCullough for phylogenetic advice and Benjamin P. Gerstner for assembling the phylogeny. We also thank all members of our original NESCent working group who helped with the assembly of the databases: Mathieu Buoro, Christina M. Caruso, Sonya M. Clegg, Tim Coulson, Joseph DiBattista, Kiyoko M. Gotanda, Clinton D. Francis, Joe Hereford, Kate E. Augustine, Ryan A. Martin, Ben C. Sheldon, Nina Sletvold, Erik I. Svensson, Michael J. Wade and Andrew D.C. MacColl. Comments from Erik Postma, Shinichi Nakagawa and an anonymous reviewer were particularly helpful in revising our manuscript and clarifying the ideas presented here.
Data accessibility
All data and code used in analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7md1755 [67].
Authors' contributions
L.E.B.K. and K.D.W. came up with the original idea followed by refinement by all authors, especially J.G.K.; M.B.M. developed analytical approaches and M.B.M., A.M.S. and L.E.B.K. analysed the data; C.D.F. assembled climate data; K.D.W. directed assembly of the phylogeny; A.M.S. and S.M.C. lead development of the selection database; A.M.S. drafted the manuscript and all authors contributed to the writing.
Competing interests
The authors declare no competing interests.
Funding
A.M.S. was supported by NSF (DEB1748945). K.D.W. was supported by NSF (DEB1257965). A NESCent working group supported the development of the databases used in this analysis (NSF grant EF-0905606).
References
- 1.Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 2.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406. ( 10.1038/nclimate1259) [DOI] [Google Scholar]
- 3.Daufresne M, Lengfellner K, Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793. ( 10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 5.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 6.Teplitsky C, Millien V. 2014. Climate warming and Bergmann's rule through time: is there any evidence? Evol. Appl. 7, 156–168. ( 10.1111/eva.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riemer K, Guralnick RP, White EP. 2018. No general relationship between mass and temperature in endothermic species. Elife 7, e27166 ( 10.7554/eLife.27166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotanda KM, Correa C, Turcotte MM, Rolshausen G, Hendry AP. 2015. Linking macrotrends and microrates: re-evaluating microevolutionary support for Cope's rule. Evolution 69, 1345–1354. ( 10.1111/evo.12653) [DOI] [PubMed] [Google Scholar]
- 9.Millien V, Kathleen Lyons S, Olson L, Smith FA, Wilson AB, Yom-Tov Y. 2006. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 9, 853–869. ( 10.1111/j.1461-0248.2006.00928.x) [DOI] [PubMed] [Google Scholar]
- 10.Gardner JL, Heinsohn R, Joseph L. 2009. Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proc. R. Soc. Lond. B 276, 3845–3852. ( 10.1098/rspb.2009.1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann C. 1847. About the relationships between heat conservation and body size of animals. Goett. Stud. 1, 595–708. [Google Scholar]
- 12.Gilchrist GW, Huey RB, Balanya J, Pascual M, Serra L. 2004. A time series of evolution in action: a latitudinal cline in wing size in South American Drosophila subobscura. Evolution 58, 768–780. ( 10.1111/j.0014-3820.2004.tb00410.x) [DOI] [PubMed] [Google Scholar]
- 13.Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. 2000. Rapid evolution of a geographic cline in size in an introduced fly. Science 287, 308–309. ( 10.1126/science.287.5451.308) [DOI] [PubMed] [Google Scholar]
- 14.Imasheva AG, Bubli OA, Lazebny OE. 1994. Variation in wing length in Eurasian natural populations of Drosophila melanogaster. Heredity 72, 508–514. ( 10.1038/hdy.1994.68) [DOI] [PubMed] [Google Scholar]
- 15.Anderson WW. 1966. Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura 3. Divergence in body size. Genet. Res. 7, 255–266. ( 10.1017/S0016672300009666) [DOI] [Google Scholar]
- 16.Stillwell RC, Morse GE, Fox CW. 2007. Geographic variation in body size and sexual size dimorphism of a seed-feeding beetle. Am. Nat. 170, 358–369. ( 10.1086/520118) [DOI] [PubMed] [Google Scholar]
- 17.Atkinson D. 1994. Temperature and organism size: a biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. ( 10.1016/S0065-2504(08)60212-3) [DOI] [Google Scholar]
- 18.Andrew S, Hurley L, Mariette M, Griffith S. 2017. Higher temperatures during development reduce body size in the zebra finch in the laboratory and in the wild. J. Evol. Biol. 30, 2156–2164. ( 10.1111/jeb.13181) [DOI] [PubMed] [Google Scholar]
- 19.Kingsolver JG, Huey RB. 2008. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268. [Google Scholar]
- 20.Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am. Nat. 158, 204–210. ( 10.1086/321314) [DOI] [PubMed] [Google Scholar]
- 21.Berrigan D, Charnov EL. 1994. Reaction norms for age and size at maturity in response to temperature: a puzzle for life historians. Oikos 70, 474–478. ( 10.2307/3545787) [DOI] [Google Scholar]
- 22.Partridge L, Coyne JA. 1997. Bergmann's rule in ectotherms: is it adaptive? Evolution 51, 632–635. ( 10.2307/2411139) [DOI] [PubMed] [Google Scholar]
- 23.Mousseau TA. 1997. Ectotherms follow the converse to Bergmann's Rule. Evolution 51, 630–632. ( 10.2307/2411138) [DOI] [PubMed] [Google Scholar]
- 24.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 25.Kingsolver JG, Huey RB. 1998. Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 38, 545–560. ( 10.1093/icb/38.3.545) [DOI] [Google Scholar]
- 26.Huey RB, Berrigan D, Gilchrist GW, Herron JC. 1999. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 39, 323–336. ( 10.1093/icb/39.2.323) [DOI] [Google Scholar]
- 27.Angilletta MJ Jr, Steury TD, Sears MW. 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509. ( 10.1093/icb/44.6.498) [DOI] [PubMed] [Google Scholar]
- 28.Lafuente E, Duneau D, Beldade P. 2018. Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PLoS Genet. 14, e100786 ( 10.1371/journal.pgen.1007686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merilä J. 2008. Bergmann's rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc. Natl Acad. Sci. USA 105, 13 492–13 496. ( 10.1073/pnas.0800999105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merila J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gienapp P, Teplitsky C, Alho J, Mills J, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 32.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 33.Siepielski AM, DiBattista JD, Carlson SM. 2009. It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol. Lett. 12, 1261–1276. ( 10.1111/j.1461-0248.2009.01381.x) [DOI] [PubMed] [Google Scholar]
- 34.Siepielski AM, Gotanda KM, Morrissey MB, Diamond SE, DiBattista JD, Carlson SM. 2013. The spatial patterns of directional phenotypic selection. Ecol. Lett. 16, 1382–1392. ( 10.1111/ele.12174) [DOI] [PubMed] [Google Scholar]
- 35.Morrissey MB, Hadfield JD. 2011. Directional selection in temporally replicated studies is remarkably consistent. Evolution 66, 435–442. ( 10.1111/j.1558-5646.2011.01444.x) [DOI] [PubMed] [Google Scholar]
- 36.Morrissey MB. 2016. Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J. Evol. Biol. 29, 1882–1904. ( 10.1111/jeb.12950) [DOI] [PubMed] [Google Scholar]
- 37.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118. ( 10.1007/S10682-012-9563-5) [DOI] [Google Scholar]
- 38.Kingsolver JG, Diamond SE. 2011. Phenotypic selection in natural populations: what limits directional selection? Am. Nat. 177, 346–357. ( 10.1086/658341) [DOI] [PubMed] [Google Scholar]
- 39.Kingsolver JG, Pfennig DW. 2004. Individual-level selection as a cause of Cope's rule of phyletic size increase. Evolution 58, 1608–1612. ( 10.1111/j.0014-3820.2004.tb01740.x) [DOI] [PubMed] [Google Scholar]
- 40.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]
- 41.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 42.Harris I, Jones P, Osborn T, Lister D. 2014. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 dataset. Int. J. Climatol. 34, 623–642. ( 10.1002/joc.3711) [DOI] [Google Scholar]
- 43.Garcia RA, Cabeza M, Rahbek C, Araújo MB. 2014. Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579 ( 10.1126/science.1247579) [DOI] [PubMed] [Google Scholar]
- 44.Stephens PA, et al. 2016. Consistent response of bird populations to climate change on two continents. Science 352, 84–87. ( 10.1126/science.aac4858) [DOI] [PubMed] [Google Scholar]
- 45.Pebesma EJ, Bivand RS. 2005. Classes and methods for spatial data in R. R News 5, 9–13. [Google Scholar]
- 46.Macias-Fauria M, Seddon AW, Benz D, Long PR, Willis K. 2014. Spatiotemporal patterns of warming. Nat. Clim. Change 4, 845–846. ( 10.1038/nclimate2372) [DOI] [Google Scholar]
- 47.Myers-Smith IH, Myers JH. 2018. Comment on ‘Precipitation drives global variation in natural selection’. Science 359, eaan5028 ( 10.1126/science.aan5028) [DOI] [PubMed] [Google Scholar]
- 48.Siepielski AM, et al. 2018. Response to comment on ‘Precipitation drives global variation in natural selection’. Science 359, eaan5760 ( 10.1126/science.aan5760) [DOI] [PubMed] [Google Scholar]
- 49.Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M. 2006. Global temperature change. Proc. Natl Acad. Sci. USA 103, 14 288–14 293. ( 10.1073/pnas.0606291103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blunden J, Arndt DS. 2016. State of the climate in 2016. Bull. Am. Meteorol. Soc. 98, Si–S280. ( 10.1175/2017BAMSStateoftheClimate.1) [DOI] [Google Scholar]
- 51.Fox J. 1991. Regression diagnostics: an introduction. Beverley Hills, CA: Sage. [Google Scholar]
- 52.Hayward AD, Pemberton JM, Berenos C, Wilson AJ, Pilkington JG, Kruuk LE. 2018. Evidence for selection-by-environment but not genotype-by-environment interactions for fitness-related traits in a wild mammal population. Genetics 208, 349–364. ( 10.1534/genetics.117.300498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakers JJ, Culina A, Visser ME, Gienapp P. 2018. Environmental coupling of heritability and selection is rare and of minor evolutionary significance in wild populations. Nat. Ecol. Evol. 2, 1093–1103. ( 10.1038/s41559-018-0577-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 55.Wood CW, Brodie ED. 2016. Evolutionary response when selection and genetic variation covary across environments. Ecol. Lett. 19, 1189–1200. ( 10.1111/ele.12662) [DOI] [PubMed] [Google Scholar]
- 56.Hunter DC, Pemberton JM, Pilkington JG, Morrissey MB. 2018. Quantification and decomposition of environment–selection relationships. Evolution 72, 851–866. ( 10.1111/evo.13461) [DOI] [PubMed] [Google Scholar]
- 57.Steele DB, Siepielski AM, McPeek MA. 2011. Sexual selection and temporal phenotypic variation in a damselfly population. J. Evol. Biol. 24, 1517–1532. ( 10.1111/J.1420-9101.2011.02284.X) [DOI] [PubMed] [Google Scholar]
- 58.Husby A, Hille SM, Visser ME. 2011. Testing mechanisms of Bergmann's rule: phenotypic decline but no genetic change in body size in three passerine bird populations. Am. Nat. 178, 202–213. ( 10.1086/660834) [DOI] [PubMed] [Google Scholar]
- 59.Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St Kilda. Science 325, 464–467. ( 10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonnet T, Wandeler P, Camenisch G, Postma E. 2017. Bigger is fitter? Quantitative genetic decomposition of selection reveals an adaptive evolutionary decline of body mass in a wild rodent population. PLoS Biol. 15, e1002592 ( 10.1371/journal.pbio.1002592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell-Staton SC, Cheviron ZA, Rochette N, Catchen J, Losos JB, Edwards SV. 2017. Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science 357, 495–498. ( 10.1126/science.aam5512) [DOI] [PubMed] [Google Scholar]
- 63.Grant PR, Grant BR, Huey RB, Johnson MT, Knoll AH, Schmitt J. 2017. Evolution caused by extreme events. Phil. Trans. R. Soc. B 372, 20160146 ( 10.1098/rstb.2016.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haney SD, Siepielski AM. 2018. Tipping points in resource abundance drive irreversible changes in community structure. Am. Nat. 191, 668–675. ( 10.1086/697045) [DOI] [PubMed] [Google Scholar]
- 65.Clegg SM, Frentiu FD, Kikkawa J, Tavecchia G, Owens IP. 2008. 4000 years of phenotypic change in an island bird: heterogeneity of selection over three microevolutionary timescales. Evolution 62, 2393–2410. ( 10.1111/j.1558-5646.2008.00437.x) [DOI] [PubMed] [Google Scholar]
- 66.Uyeda JC, Hansen TF, Arnold SJ, Pienaar J. 2011. The million-year wait for macroevolutionary bursts. Proc. Natl Acad. Sci. USA 108, 15 908–15 913. ( 10.1073/pnas.1014503108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siepielski A, Morrissey M, Carlson S, Francis C, Kingsolver J, Whitney K, Kruuk L. 2019. Data from: No evidence that warmer temperatures are associated with selection for smaller body sizes Dryad Digital Repository. ( 10.5061/dryad.7md1755) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Siepielski A, Morrissey M, Carlson S, Francis C, Kingsolver J, Whitney K, Kruuk L. 2019. Data from: No evidence that warmer temperatures are associated with selection for smaller body sizes Dryad Digital Repository. ( 10.5061/dryad.7md1755) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data and code used in analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7md1755 [67].