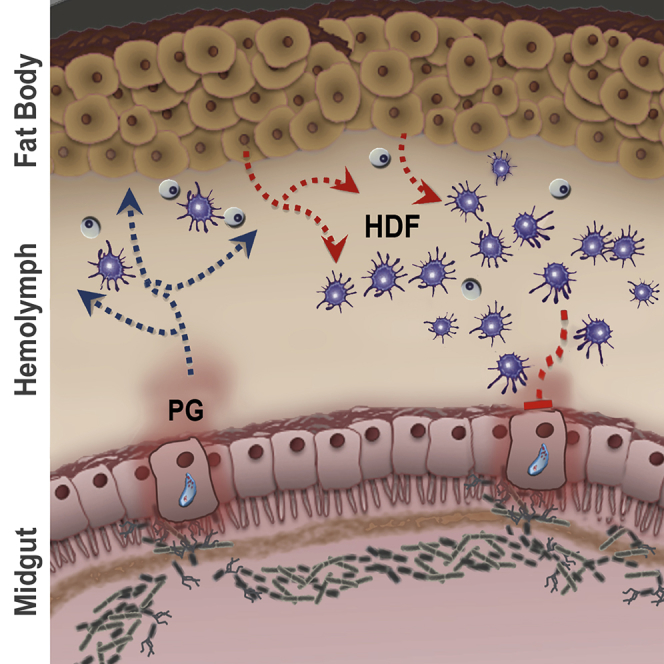
Summary
Anopheles gambiae mosquitoes that have been infected with Plasmodium mount a more effective immune response to a subsequent infection. Priming is established when Plasmodium invasion of the mosquito midgut allows contact of the gut microbiota with epithelial cells. This event is followed by a systemic release of a hemocyte differentiation factor (HDF) consisting of Lipoxin A4 bound to Evokin, a lipocalin carrier, which increases the proportion of circulating hemocytes. We show that mosquito midgut cells produce and release prostaglandin E2 (PGE2), which attracts hemocytes to the midgut surface and enhances their patrolling activity. Systemic injection of prostaglandins (PGs) recapitulates the priming response and enhances antiplasmodial immunity by triggering HDF production. Although insects lack cyclooxygenases, two heme peroxidases, HPX7 and HPX8, catalyze essential steps in PG biosynthesis in mosquitoes. Mosquito midgut PGE2 release attracts hemocytes and establishes a long-lasting enhanced systemic cellular immune response to Plasmodium infection.
Subject Areas: Biological Sciences, Immunology, Immune System, Microbiology, Oral Microbiology, Microbial Interactions
Graphical Abstract
Highlights
-
•
Plasmodium invasion or bacterial exposure triggers midgut prostaglandin synthesis
-
•
Prostaglandins attract mosquito hemocytes and increase their patrolling activity
-
•
Two midgut peroxidases, HPX7 and HPX8, catalyze midgut prostaglandin synthesis
-
•
Systemic release of midgut prostaglandins is essential to establish immune priming
Biological Sciences; Immunology; Immune System; Microbiology; Oral Microbiology; Microbial Interactions
Introduction
Anopheles gambiae mosquitoes can mount an effective antiplasmodial immune response that involves the coordinated activation of epithelial (Oliveira Gde et al., 2012), cellular (Castillo et al., 2017) and complement-like immune defenses (Blandin et al., 2004, Povelones et al., 2009, Fraiture et al., 2009). Plasmodium ookinete midgut invasion triggers immune priming by bringing the gut microbiota into direct contact with midgut cells (Rodrigues et al., 2010). Primed mosquitoes release a hemocyte differentiation factor (HDF) into their hemolymph and mount a more effective immune response to subsequent Plasmodium infections (Rodrigues et al., 2010). HDF release is a life-long response and results in a permanent increase in the proportion of circulating granulocytes and a state of enhanced immunity (Rodrigues et al., 2010). HDF is a complex of Lipoxin A4, a signaling eicosanoid, and Evokin, a lipid carrier of the lipocalin protein family that is essential for biological activity (Ramirez et al., 2015). Although the interaction of bacteria with epithelial cells takes place in the midgut lumen, the priming response is systemic and sustained, suggesting that the midgut epithelium releases a signal that elicits broad changes in immune function. In this study, we provide direct experimental evidence for the role of prostaglandin E2 (PGE2) as a chemotactic signal that attracts hemocytes to the basal surface of An. gambiae midgut cells and as the midgut signaling molecule that establishes immune priming. Furthermore, we identified two mosquito heme peroxidases that mediate midgut PGE2 synthesis and are essential for hemocyte chemotaxis and immune priming.
Results and Discussion
Effect of Plasmodium Infection and Bacterial Exposure on Prostaglandin Synthesis and Systemic Release
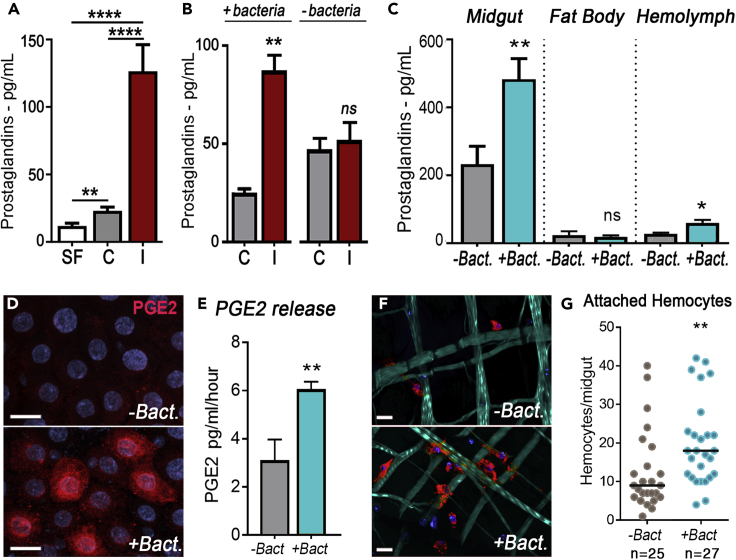
PGE2 has been detected in cultured Anopheles albimanus mosquito midguts in the presence of gut bacteria (Garcia Gil de Muñoz et al., 2008), and several studies have shown that blood feeding leads to bacterial proliferation in the mosquito midgut (Dong et al., 2009). Therefore, we investigated the potential role of prostaglandins (PGs) on mosquito responses to blood feeding and Plasmodium infection. We observed a significant increase in mosquito hemolymph PG levels at 24 h post blood feeding (PF) on a healthy control mouse (Figure 1A, p = 0.0062, Unpaired t test, Table S1). In uninfected mosquitoes, the gut microbiota is kept from coming in direct contact with the midgut epithelium by the peritrophic matrix (PM), a chitinous network that surrounds the blood meal. Ookinete midgut invasion disrupts the PM, allowing direct contact between bacteria and midgut epithelial cells. PG levels were 6-fold higher in females fed on a P. berghei-infected mouse than those fed on a healthy mouse at 24 h PF, when ookinete midgut invasion is taking place (Figure 1A, p < 0.0001, Unpaired t test, Table S1). We investigated the role of the gut microbiota on Plasmodium-induced production of PG and confirmed that, in the presence of the gut microbiota, ookinete invasion increased PG release (Figures 1A and 1B, p = 0.0043, Unpaired t test, Tables S1 and S2). However, this effect was no longer observed when the gut microbiota was eliminated by pre-treating mosquitoes with oral antibiotics (Figure 1B, p = 0.6943, Unpaired t test, Table S2).
Figure 1.
Effect of Plasmodium Infection and Bacterial Exposure on Prostaglandin Synthesis and Systemic Release
(A) Hemolymph prostaglandin levels in sugar fed (SF), blood-fed control (C), and P. berghei infected (I).
(B) Hemolymph prostaglandin levels in response to gut microbiota in blood-fed control (C) and P. berghei-infected (I) mosquitoes 26 h post infection.
(C) Prostaglandin levels in different tissues 24 h after bacterial feeding.
(D) PGE2 midgut immunostaining 6 h after bacterial feeding. Nuclei, blue; PGE2, red. Scale bar: 10 μm.
(E) In vitro PGE2 release by midguts dissected 6 h after bacterial feeding.
(F) Hemocytes recruitment to the basal surface of the midgut in response to bacterial feeding. Actin (phalloidin), cyan; Hemocytes (stained with Vybrant CM-DiI), red; Nuclei, blue. Scale bar: 15 μm.
(G) Number of hemocytes attached to the midgut 6 h post bacterial feeding.
Error bars in A–C and E represent mean ± SEM. ANOVA Tukey's multiple comparison test was used in A. Unpaired t test was used in B, C, and E. *p ≤ 0.05; **p ≤ 0.01; ****p ≤ 0.0001; NS, p > 0.05. Prostaglandin levels were measured in pools of hemolymph. Each treatment had at least two biological replicates, and the results were confirmed in at least two independent experiments. In G, hemocyte numbers were determined for each individual midgut and the median are indicated by the line. Hemocytes were counted in 5–12 mosquitoes for each treatment, and the results were confirmed in three independent experiments. Mann-Whitney test, **p ≤ 0.01. Detailed information on biological replicates and exact p values are listed in Tables S1–S5.
We investigated the production of PGs by different tissues in response to bacterial feeding. Adult females were fed a sterile BSA solution supplemented with arachidonic acid (BSA + AA), an essential precursor in PG synthesis normally present in vertebrate blood, and dissected 24 hours after feeding. PG levels were low in the fat body and hemolymph, close to the lowest limit of detection of the ELISA assay. In contrast, PG levels were about 10-fold higher and clearly detectable in the midgut (Figure 1C). Midgut PG levels increased by 2-fold when live bacteria were included in the BSA + AA solution (Figure 1C, p = 0.0042, Unpaired t test, Table S3), accompanied by a modest but significant increase in the hemolymph (Figure 1C, p = 0.0376, Unpaired t test, Table S3). However, the presence of bacteria had no effect on PG levels in the fat body (Figure 1C, p = 0.5705, Unpaired t test, Table S3). The absence of PG in the bacteria + BSA + AA suspension was confirmed, ruling it out as the source of PG (Figure S1). PGE2 immunofluorescence staining 6 h post-feeding was strong in the midguts of females fed bacteria (Figure 1D). Some cells stained stronger than others, and overall, PGE2 staining was most prominent in the perinuclear region (Figure 1D). Furthermore, when midguts of females fed sterile or bacteria-containing BSA were dissected and cultured in vitro, the rate of PGE2 secretion was significantly higher in the presence of bacteria (Figure 1E, p = 0.0079, Unpaired t test, Table S4). While investigating the effect of ingestion of bacteria on the interaction of hemocytes with the mosquito midgut, we observed that phagocytosed bacteria in hemocytes were associated with the midgut basal lamina in females that received BSA with bacteria (Figure S2). Additionally, a significant increase in the number of hemocytes attached to the basal surface of the midgut was observed 6 h after females were fed a BSA-bacterial suspension (Figures 1F and 1G, p = 0.0028, Mann-Whitney U test, Table S5), suggesting that PG release by the midgut attracts hemocytes.
Effect of PGE2 on Mosquito Hemocyte Chemotaxis and Patrolling Activity
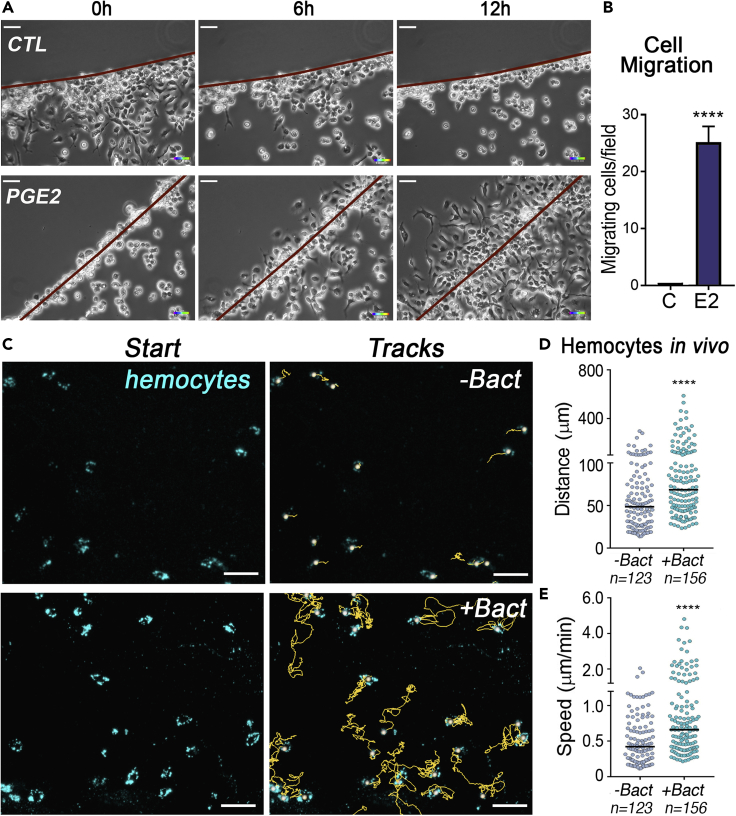
We tested this hypothesis in vitro by placing An. gambiae hemocyte-like Sua 5.1 cells in a culture dish with an agarose spot containing either PGE2 or a buffer control. Cells were allowed to settle for 4 h, and their movements were followed in real time (Figure 2A and Videos S1 and S2). After 12 h, an average of 25 cells/field migrated under the agarose spots containing PGE2, whereas no migration was observed under the control spots (Figure 2B, p < 0.0001, Mann-Whitney U test, Table S6). In similar experiments with PGF2, significant chemotaxis was also observed (Figure S3, p = 0.0104, Mann-Whitney U test, Table S7), but the rate of migration was significantly lower than with PGE2 (6.8 cells/field; p < 0.0001, Mann-Whitney U test, Table S6). The effect of bacterial feeding on hemocyte dynamics was evaluated by imaging midgut-associated hemocytes in live females fed on sterile BSA or BSA containing bacteria. Hemocytes were labeled by systemic injection of a lipophilic dye (Vybrant CM-DiD) and imaged for 2 h, starting at 4 h post-feeding. Only hemocytes that remained associated with the midgut surface for at least 1 hour were included in the analysis. Midgut-associated hemocytes from females fed BSA with bacteria traveled a longer distance and moved faster than those from females fed sterile BSA (Figure 2C, Videos S3 and S4). The distance traveled (track length) during the first hour of observation was significantly longer (Figure 2D, p < 0.0001, Mann-Whitney U test, Table S8), and the speed (total distance/total time of observation) was significantly higher (Figure 2E, p < 0.0001, Mann-Whitney U test, Table S9) in females that ingested BSA containing bacteria. The positive chemotactic response of hemocytes to PGE2 released by the midgut when cells come in direct contact with bacteria could limit potential systemic bacterial infections, and would be particularly important when ookinetes traverse the PM and disrupt the midgut epithelium.
Figure 2.
Effect of PGE2 on Mosquito Hemocyte Chemotaxis and Patrolling Activity
(A) Time-lapse imaging of Sua5.1 hemocyte-like cells migrating in an agarose spot assay in response to PBS (CTL) or 7 μg of PGE2 (PGE2). Scale bar: 30 μm.
(B and C) (B) Quantification of motile cells per field in the PBS control (C) and PGE2 (E2) agarose spots. Error bars in (B) represent mean ± SEM. Unpaired t test, ****p ≤ 0.0001. Quantification of cell migration was performed in at least two independent experiments with two biological replicates for each treatment. Detailed information on biological replicates and exact p values are listed in Table S6 (see Videos S1 and S2). (C) Snapshot of live mosquito hemocytes (cyan) in their initial position. The yellow lines represent hemocyte movement tracked for a minimum of 1 h.
(D) Hemocyte distance traveled in 1 h. Each dot represents the distance traveled by individual hemocytes during the first hour of video imaging.
(E) Hemocyte speed. Each dot represents the mean speed of individual hemocytes during the whole duration of the video imaging. Medians are indicated by the lines. Four midguts were analyzed for each condition and a total of 123 hemocytes in the -Bact and 156 hemocytes in the +Bact. Mann-Whitney test, ****p ≤ 0.0001. Scale bar: 20 μm. Detailed information on biological replicates and exact p values are listed in Tables S8 and S9 (see Videos S3 and S4).
Effect of Bacterial Immune Elicitors on Prostaglandin Synthesis and Immune Priming
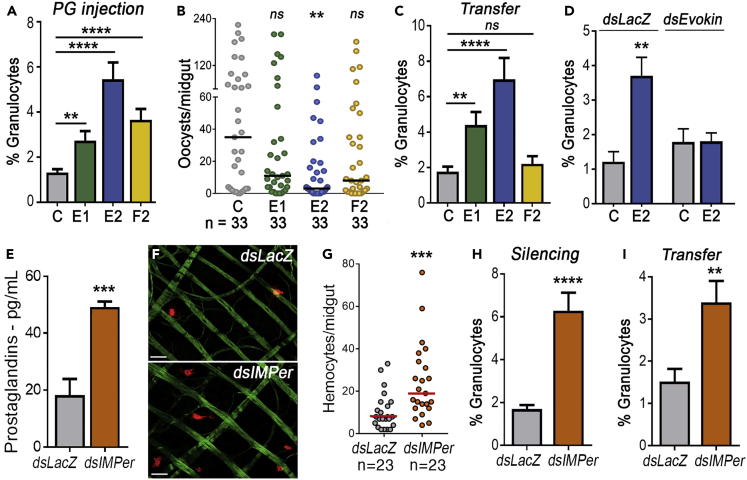
Direct contact between bacteria and midgut cells is necessary to establish immune priming (Rodrigues et al., 2010), suggesting that midgut PG release might be involved in this response. Therefore, we investigated whether systemic injection of PGs previously detected in insects (PGE1, PGE2, and PGF2) (Garcia Gil de Muñoz et al., 2008, Stanley-Samuelson and Ogg, 1994, Ramos et al., 2014) could recapitulate the priming response. Injection of PGE1, PGE2, or PGF2 significantly increased the proportion of circulating granulocytes (Figure 3A, p = 0.0015, <0.0001, and <0.0001, respectively, Mann-Whitney U test, Table S10), with PGE2 having the strongest effect. Systemic injection of PGE2 also enhanced antiplasmodial immunity, significantly reducing the number of oocysts (Figure 3B, p = 0.0016, Mann-Whitney U test, Table S11), whereas the effects of PGE1 and PGF2 on Plasmodium infection were not statistically significant (Figure 3B, p = 0.1842 and p = 0.0582, respectively, Mann-Whitney U test, Table S11).
Figure 3.
Effect of Bacterial Immune Elicitors on Prostaglandin Synthesis and Immune Priming
(A) Effect of systemic prostaglandin injection on the proportion of circulating granulocytes.
(B) Mosquito susceptibility to P. berghei infection after systemic injection of different prostaglandins (PGE1, PGE2, and PGF2).
(C) Effect of hemolymph transfer from mosquito donors shown in (A), collected 4 days after PG injection, on the proportion of circulating granulocytes in the recipients.
(D) Effect of Evokin silencing on the biological activity of hemolymph of females transferred 4 days after PGE2 injection, measured as the effect on the proportion of circulating hemocytes in the recipients.
(E) Effect of IMPer silencing on hemolymph prostaglandin levels 48 h post blood feeding.
(F and G) Effect of IMPer silencing on the number of hemocytes attached to the midgut basal surface 12 h after blood feeding. Actin (phalloidin), cyan; Hemocytes (stained with Vybrant CM-DiI), red. Scale bar: 15 μm.
(H) Effect of IMPer silencing on the proportion of circulating granulocytes after blood feeding.
(I) Effect of hemolymph transfer from IMPer-silenced donor, collected 4 days after blood feeding, on the proportion of circulating hemocytes in the recipients.
Error bars in A, C–E, H, and I represent mean ± SEM. Mann- Whitney U test and Unpaired t test, **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. Hemocyte counting in C, D, H, and I were obtained from at least 5–10 individual mosquitoes for each treatment in at least two independent experiments. Each dot in B and G represent the number of oocysts or hemocytes, respectively, for individual midguts. The median is indicated by the line. Mann-Whitney U test, **p ≤ 0.01; ***p ≤ 0.001, NS, p > 0.05. Detailed information on biological replicates and exact p values are listed in Tables S10, S11, and S13–S18.
We further investigated the effects of PG injections by exploring the hypothesis that they trigger long-lasting HDF activity in hemolymph. As expected, owing to the short half-life of PGs (Samuelsson et al., 1975), hemolymph collected 4 days after female mosquitoes were injected with PGs contained low levels of PGs that were not significantly different from those of control mosquitoes injected with buffer (Figure S4, p = 0.7693, ANOVA Dunn's multiple comparison test, Table S12). Transfer of this hemolymph from females pre-treated with PGE1 or PGE2 into naive females increased the proportion of granulocytes relative to untreated controls (Figure 3C, p = 0.0025 and p < 0.0001, respectively, Mann-Whitney U test, Table S13). PGE2 elicited the strongest response, whereas PGF2 pre-treatment had no effect (Figure 3C, p = 0.6075, Mann-Whitney U test, Table S13). Furthermore, silencing Evokin, the carrier protein component of HDF in the donor, before PGE2 injection, eliminated the effect of this eicosanoid on hemocyte differentiation in recipient mosquitoes (Figure 3D, dsLacZ, p = 0.0015 and dsEvokin, p = 0.5101, Mann-Whitney U test, Table S14). Taken together, these observations indicate that PGE2 injection triggers long-lasting HDF release into the mosquito hemolymph, prompting the immune priming response.
Next, we investigated whether increased contact with immune elicitors from the endogenous gut flora was sufficient to induce priming. The diffusion of bacteria-derived immune elicitors out of the mosquito midgut lumen is tightly regulated by an Immunomodulatory Peroxidase (IMPer) (Kumar et al., 2010). IMPer is secreted into the ectoperitrophic space, between the PM and the midgut epithelium, and cross-links the proteins present in this space (Kumar et al., 2010). IMPer silencing increases the permeability of the ectoperitrophic space, allowing soluble immune elicitors to interact with the midgut epithelium (Kumar et al., 2010). Silencing IMPer significantly enhanced PG levels in the hemolymph 24 h after ingestion of a blood meal (Figure 3E, p = 0.0001, Unpaired t test, Table S15), a time when the gut microbiota undergoes extensive proliferation. Reducing IMPer expression also significantly increased the number of hemocytes associated with basal surface of the midgut (Figures 3F and 3G, p = 0.0005, Mann-Whitney U test, Table S16) and increased the proportion of circulating granulocytes (Figure 3H, p < 0.0001, Mann-Whitney U test, Table S17). Furthermore, transfer of hemolymph collected 4 days after blood feeding from IMPer-silenced mosquitoes to naive recipients also increased the proportion of granulocytes (Figure 3I, p = 0.003, Mann- Whitney U test, Table S18).
Role of Two Midgut Peroxidases on Prostaglandin Synthesis and Immune Priming
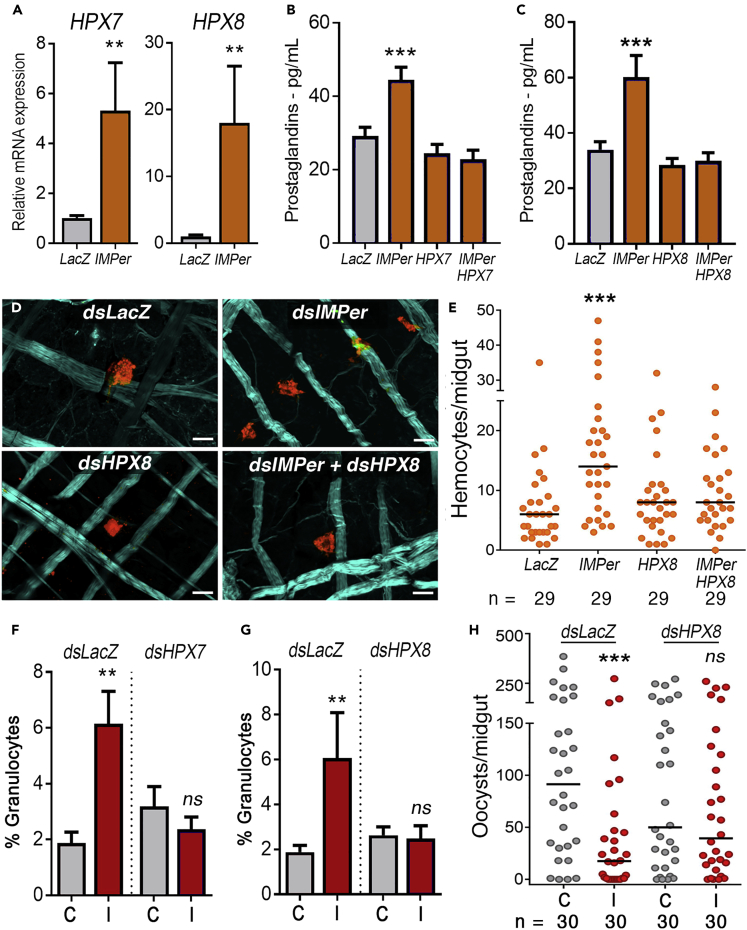
Although PGs have been detected in insects, whole-genome sequencing of multiple species indicates that they lack an ortholog of vertebrate cyclooxygenase (Varvas et al., 2009), suggesting that PG synthesis is mediated by some other enzyme(s) (Garcia Gil de Muñoz et al., 2008, Stanley-Samuelson and Ogg, 1994, Chotiwan et al., 2018). We confirmed our previous observation (Kumar et al., 2010) that feeding mosquito with a BSA solution containing bacteria induces expression of two heme peroxidases, HPX7 and HPX8 (Figure S5, p = 0.0003 and = 0.0002, respectively, Mann-Whitney U test, Table S19). IMPer silencing also induces the expression of these two enzymes 24 h PF (Figure 4A, p = 0.0022 and p = 0.004, respectively, Mann- Whitney U test, Table S20). Furthermore, co-silencing IMPer and HPX7 (Figure 4B, p = 0.7701, ANOVA Dunn's multiple comparison, Table S21) or IMPer and HPX8 (Figure 4C, p = 0.8520, ANOVA Dunn's multiple comparison, Table S22) prevents the increase in PG release after blood feeding that is observed when IMPer alone is silenced (Figure 4B, p = 0.0003 and 4C p = 0.0002, ANOVA Dunn's multiple comparison, Tables S21 and S22). Similarly, the increase in hemocyte binding to the basal surface of the midgut in IMPer-silenced females (Figures 4D and 4E, p = 0.0005, ANOVA Kruskal-Wallis test, Table S23) is no longer observed when HPX8 is co-silenced with IMPer (Figures 4D and 4E, p = 0.1289, ANOVA Kruskal-Wallis test, Table S23).
Figure 4.
Role of Two Midgut Peroxidases on Prostaglandin Synthesis and Immune priming
(A) Relative mRNA expression of HPX7 and HPX8 in the midgut, 48 h post blood-meal, in LacZ (control) and IMPer-silenced mosquitoes.
(B) Effect of IMPer and HPX7 co-silencing on hemolymph prostaglandin levels 48 h after blood feeding.
(C) Effect of IMPer and HPX8 co-silencing on hemolymph prostaglandin levels 48 h after blood feeding.
(D and E) Effect of IMPer, HPX8, or IMPer + HPX8 co-silencing on the number of hemocytes attached to the basal surface of the midgut 12 h post blood feeding. Actin (phalloidin), cyan; Hemocytes (stained with Vybrant CM-DiI), red. Scale bar: 15 μm.
(F) Effect of hemolymph transfer from LacZ controls and HPX7-silenced donors, collected 4 days after feeding on a control (C) or a P.berghei infected (I) mouse, on the proportion of circulating hemocytes in the recipients.
(G) Effect of hemolymph transfer from LacZ controls and HPX8-silenced donors, collected 4 days after feeding on a control (C) or a P.berghei-infected (I) mouse, on the proportion of circulating hemocytes in the recipients.
(H) Effect of hemolymph transfer from LacZ controls and HPX8-silenced donors on the antiplasmodial immunity of the recipients. Hemolymph was collected 4 days post-feeding on control (C) or a P.berghei-infected (I) mouse.
Error bars in A–C, F, and G represent mean ± SEM. Mann-Whitney U test and Unpaired t test, **p ≤ 0.01; ***p ≤ 0.001, NS, p > 0.05. In A–C, 2–3 pools per treatment were used in at least two independent experiments. In F and G, granulocyte numbers were counted in at least 5–10 individual mosquitoes. Each dot in E and H represent the number of hemocytes or oocysts, respectively, for each individual midgut. The median is indicated by the line. Detailed information on biological replicates and exact p values are listed in Tables S20–S26.
We also evaluated the potential participation of these two heme peroxidases in mosquito immune priming after P. berghei infection. When either HPX7 (Figure 4F, p = 0.0022, Mann-Whitney U test, Table S24) or HPX8 (Figure 4G, p = 0.0036, Mann-Whitney U test, Table S25) was silenced, the increase in the proportion of granulocytes after infection (a hallmark of immune priming) was no longer observed (Figures 4F and 4G, p = 0.3930 and p = 0.5795, respectively, Mann-Whitney U test, Tables S24 and S25). Furthermore, exposure of HPX8-silenced females to Plasmodium infection no longer enhanced antiplasmodial immunity when their hemolymph was transferred to naive mosquitoes (Figure 4H, p = 0.0009 and p = 0.5154, Mann-Whitney U test, Table S26), in agreement with the lack of HDF activity in the donors (Figure 4G).
Disruption of the peroxidase pxt gene in Drosophila resulted in sterile female flies, but follicle maturation was rescued in vitro by treatment with PG (Tootle and Spradling, 2008) and fertility was restored in vivo by expressing the mammalian COX-1 protein (Tootle and Spradling, 2008). This study suggests that PGs are necessary for Drosophila follicle maturation and that vertebrate COX1 can complement the biological function of pxt by restoring PG synthesis. However, direct evidence of PG synthesis by pxt was not provided. Our findings agree with these observations and indicate that, besides their role in development, PGs also participate in antimicrobial responses. Additional studies indicate that indomethacin inhibits vertebrate thyroid peroxidase and lactoperoxidase (Van Zyl and Louw, 1979) in addition to cyclooxygenase, suggesting that peroxidases may be the targets of this drug in insects. This could explain the pharmacological effect of indomethacin on antibacterial response in lepidoptera (Downer et al., 1997) and Plasmodium sporozoite infection in mosquitoes (Ramos et al., 2014). Furthermore, there are reports that vertebrate peroxidases can catalyze PG synthesis in vitro using arachidonic acid as a substrate (Zilletti et al., 1989, Panganamala et al., 1974, Egan et al., 1979). Our findings suggest that HPX7 and HPX8 assume the role of cyclooxygenase in converting arachidonic acid to intermediate endoperoxides, such as PGH2, whereas the Prostaglandin E enzymes that convert PGH2 to PGE2 are conserved.
PGs differ in their biological activity in mosquitoes. Although injection of PGE1, PGE2, and PGF2 all increased the proportion of circulating granulocytes, PGE2 elicited the strongest response and significantly enhanced antiplasmodial immunity. Although both PGE1 and PGF2 also reduced Plasmodium infection, their effect was more modest and did not reach statistical significance. PGE2 also evoked a stronger chemotactic response from hemocyte-like cells (Sua 5.1 cell line) than did PGF2. Furthermore, synthesis of PGE2 by midgut cells in mosquitoes fed a BSA solution containing bacteria was documented using a PGE2-specific monoclonal antibody. Taken together, our results indicate that An. gambiae mosquitoes can synthesize PGE2 and that this PG has the strongest biological activity. The precise mechanism by which HPX7 and HPX8 are involved in PGE2 synthesis remains to be established.
Based on our findings, we propose the following mechanism for the establishment of mosquito immune priming in response to Plasmodium infection. When ookinetes invade the midgut, bacteria come in contact with epithelial cells and induce expression of HPX7 and HPX8. The induction of these two enzymes mediates PGE2 synthesis by midgut epithelial cells. PG release, in turn, attracts hemocytes, enhances their patrolling activity, and establishes immune priming. The initial PGE2 release by midgut epithelial cells has long-lasting effects on the mosquito immune system and is a key signal for the establishment of a life-long state of enhanced immunity. In vertebrates, lipopolysaccarides (LPS) has been shown to stimulate PGE2 synthesis and release by alveolar epithelial cells (Speth et al., 2016). PGE2, in turn, modulates local inflammation by triggering secretion of SOCS3-containing microparticles by alveolar macrophages. We conclude that the release of PGE2 by epithelial cells is an ancient innate immunomodulatory response that is conserved from insects to vertebrates.
Limitations of the Study
Although we observe that midgut PG attracts hemocytes, we do not have specific markers available to determine which hemocyte type is being recruited. RNA knockdown of HPX7 and HPX8 decreased the amount of PG in the hemolymph, but since silencing is transient and depends on the protein turnover, we were not able to altogether abolish PG production. To fully characterize the role of HPX7 and HPX8 in PG synthesis, we must develop knockout mosquitoes for those enzymes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research Z01AI000947, NIAID, National Institutes of Health, and by NIH Grant P01GM095467. We thank André Laughinghouse and Kevin Lee for insectary support and Roxanne Withers for editing help. A.B.F.B. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Author Contributions
Experiments were designed by A.B.F.B., N.T., J.L.R, and C.B.-M.; carried out by A.B.F.B., J.L.R., and N.T.; and analyzed by A.B.F.B., N.T., J.L.R, and A.B.F.B. C.B.-M. wrote the paper.
Declaration of Interests
The authors declare no competing financial interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.012.
Data and Code Availability
The raw data and detailed information on individual experiments and number of replicates are available in the Supplementary tables file.
Supplemental Information
References
- Blandin S., Shiao S.H., Moita L.F., Janse C.J., Waters A.P., Kafatos F.C., Levashina E.A. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Castillo J.C., Ferreira A.B.B., Trisnadi N., Barillas-Mury C. Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci. Immunol. 2017;2:eaal1505. doi: 10.1126/sciimmunol.aal1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiwan N., Andre B.G., Sanchez-Vargas I., Islam M.N., Grabowski J.M., Hopf-Jannasch A., Gough E., Nakayasu E., Blair C.D., Belisle J.T. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog. 2018;14:e1006853. doi: 10.1371/journal.ppat.1006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Manfredini F., Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer R.G., Moore S.J., L Diehl-Jones W., Mandato C.A. The effects of eicosanoid biosynthesis inhibitors on prophenoloxidase activation, phagocytosis and cell spreading in Galleria mellonella. J. Insect Physiol. 1997;43:1–8. doi: 10.1016/s0022-1910(96)00100-x. [DOI] [PubMed] [Google Scholar]
- Egan R.W., Gale P.H., Kuehl F.A., Jr. Reduction of hydroperoxides in the prostaglandin biosynthetic pathway by a microsomal peroxidase. J. Biol. Chem. 1979;254:3295–3302. [PubMed] [Google Scholar]
- Fraiture M., Baxter R.H., Steinert S., Chelliah Y., Frolet C., Quispe-Tintaya W., Hoffmann J.A., Blandin S.A., Levashina E.A. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Garcia Gil de Muñoz F.L., Martinez-Barnetche J., Lanz-Mendoza H., Rodriguez M.H., Hernandez-Hernandez F.C. Prostaglandin E2 modulates the expression of antimicrobial peptides in the fat body and midgut of Anopheles albimanus. Arch. Insect Biochem. Physiol. 2008;68:14–25. doi: 10.1002/arch.20232. [DOI] [PubMed] [Google Scholar]
- Kumar S., Molina-Cruz A., Gupta L., Rodrigues J., Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Gde A., Lieberman J., Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilletti L., Ciuffi M., Moneti G., Franchi-Micheli S., Valoti M., Sgaragli G.P. Peroxidase catalysed formation of prostaglandins from arachidonic acid. Biochem. Pharmacol. 1989;38:2429–2439. doi: 10.1016/0006-2952(89)90086-5. [DOI] [PubMed] [Google Scholar]
- Panganamala R.V., Sharma H.M., Sprecher H., Geer J.C., Cornwell D.G. A suggested role for hydrogen peroxide in the biosynthesis of prostaglandins. Prostaglandins. 1974;8:3–11. doi: 10.1016/0090-6980(74)90031-8. [DOI] [PubMed] [Google Scholar]
- Povelones M., Waterhouse R.M., Kafatos F.C., Christophides G.K. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.L., de Almeida Oliveira G., Calvo E., Dalli J., Colas R.A., Serhan C.N., Ribeiro J.M., Barillas-Mury C. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat. Commun. 2015;6:7403. doi: 10.1038/ncomms8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Custodio A., Silveira H. Anopheles gambiae eicosanoids modulate Plasmodium berghei survival from oocyst to salivary gland invasion. Mem. Inst. Oswaldo Cruz. 2014;109:668–671. doi: 10.1590/0074-0276140098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J., Brayner F.A., Alves L.C., Dixit R., Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Granstrom E., Green K., Hamberg M., Hammarstrom S. Prostaglandins. Annu. Rev. Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Speth J.M., Bourdonnay E., Penke L.R., Mancuso P., Moore B.B., Weinberg J.B., Peters-Golden M. Alveolar epithelial cell-derived prostaglandin E2 serves as a request signal for macrophage secretion of suppressor of cytokine signaling 3 during innate inflammation. J. Immunol. 2016;196:5112–5120. doi: 10.4049/jimmunol.1502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley-Samuelson D.W., Ogg C.L. Prostaglandin biosynthesis by fat body from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 1994;24:481–491. doi: 10.1016/0965-1748(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Tootle T.L., Spradling A.C. Drosophila Pxt: a cyclooxygenase-like facilitator of follicle maturation. Development. 2008;135:839–847. doi: 10.1242/dev.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvas K., Kurg R., Hansen K., Jarving R., Jarving I., Valmsen K., Lohelaid H., Samel N. Direct evidence of the cyclooxygenase pathway of prostaglandin synthesis in arthropods: genetic and biochemical characterization of two crustacean cyclooxygenases. Insect Biochem. Mol. Biol. 2009;39:851–860. doi: 10.1016/j.ibmb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Van Zyl A., Louw A. Inhibition of peroxidase activity by some non-steroidal anti-inflammatory drugs. Biochem. Pharmacol. 1979;28:2753–2759. doi: 10.1016/0006-2952(79)90559-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data and detailed information on individual experiments and number of replicates are available in the Supplementary tables file.