Abstract
Over 1.4 million Americans have been diagnosed with Inflammatory Bowel Disease (IBD), and ulcerative colitis (UC) makes up approximately half of those diagnoses. As a disease, UC cycles between periods of remission and flare, which is characterized by intense abdominal pain, increased weight loss, intestinal inflammation, rectal bleeding, and dehydration. Interestingly, a widespread recommendation to IBD patients for avoidance of a flare period is ‘Don’t Drink Alcohol’ as recent work correlated alcohol consumption with increased GI symptoms in patients with IBD. Alcohol alone not only induces a systemic pro-inflammatory response, but can also be directly harmful to gut barrier integrity. However, how alcohol could result in the exacerbation of UC in both patients and murine models of colitis has yet to be elucidated. Therefore, we conducted a retrospective analysis of patients admitted for IBD with a documented history of alcohol use in conjunction with a newly developed mouse model of binge alcohol consumption following DSS-induced colitis. We found that alcohol negatively impacts clinical outcomes of patients with IBD, specifically increased intestinal infections, antibiotic injections, abdomen CT scans, and large intestine biopsies. Furthermore, in our mouse model of binge alcohol consumption following an induced colitis flare we found alcohol exacerbates weight loss, clinical scores, colonic shortening and inflammation, and propensity to infection. These findings highlight alcohol’s ability to potentiate symptoms and susceptibility to infection in UC and suggest alcohol as an underlying factor in perpetuating symptoms of IBD.
Summary Sentence:
This study builds on previous findings that alcohol has adverse effects in IBD and establishes these effects in a mouse model of colitis.
Introduction
Inflammatory Bowel Disease (IBD) is diagnosed in over 1.4 million Americans each year. Yet, the exact mechanisms behind disease onset remain elusive1–3. IBD is subdivided into two clinical categories of either Crohn’s disease (CD) or Ulcerative Colitis (UC), characterized by inflammation of the entire gastrointestinal tract in CD or the large intestine only in UC2,4. UC accounts for over 700,000 IBD diagnoses and is most prevalent in patients under thirty; the age range where an estimated 70% of binge alcohol drinking episodes occur1,3,5. UC is a life-long disease characterized by cycles of asymptomatic remission and active disease flares. UC patients are subject to symptoms of bloody stool and subsequent anemia, bowel incontinence, and weight loss2.
The precise etiology of UC is still unknown, but research has focused on genetic factors leading to over activation of the immune system with a break in tolerance to commensal intestinal bacteria, environmental factors resulting in dysbiosis of the bacterial population residing in the gut, or some combination thereof that could potentially be contributing to disease onset. As there is no cure for UC, patients are forced into maintenance regimens obtaining symptomatic relief through the use of immunosuppressive drugs, antibiotics, or surgical therapies2,6–8. Although these treatments aid in relieving symptoms of UC, the resulting immunosuppression could allow for the invasion of pathogenic bacteria that would have normally been defended against. Furthermore, changes in bacterial populations from antibiotic treatment could open specific niches more favorable to invading pathogens, which then gives rise to the increased susceptibility to bacterial infections seen in IBD/UC patients.
Maintenance of UC requires patients to avoid stress, certain foods, and alcohol, as all three can potentially induce flare periods of UC2,9. Specifically, physicians recommend UC patients maintain a sober lifestyle9, but there exists a gap in knowledge as to how alcohol intoxication affects UC flare periods.
Alcohol consumption is well known to be both pro-inflammatory and directly harmful to gut barrier function as it breaks down the normal physical and immunological barrier provided by intestinal epithelial cells and gut associated lymphoid tissue (GALT), respectively10–13. Alcohol alone is known to induce intestinal erosion, which can impair intestinal absorption leading to increases in diarrhea and intestinal permeability, allowing for leakage of bacteria or bacterial endotoxins into the circulation12,14,15. Despite this, only a handful of studies have explored the impact of alcohol in the setting of UC. Some research has shown that alcohol has a deleterious role in UC by increasing gastrointestinal symptoms16, inducing a flare17 and promoting disease onset18, while one study describes that alcohol has no effect in the onset of UC19. The inconsistency of evidence in either support or contradiction of alcohol’s role in exacerbating UC flare and/or onset prompted us firstly to elucidate whether alcohol is a contributing factor in UC and UC research.
To further address this question, we conducted a retrospective analysis of patient databases to assess clinical outcomes of patients admitted with diagnoses for IBD, UC, and CD that either have or do not have documented history of alcohol use. The results of this analysis suggested that patients with documented history of alcohol use may have an increased risk of infections as well as require more diagnostic and therapeutic procedures.
These findings led us to generate two models that focus on the impact of alcohol in one form of IBD, UC with the hypothesis that not only does alcohol further exacerbate UC flares, but also increases susceptibility of UC patients to bacterial infections. In one model, mice were treated with DSS and a subsequent binge alcohol paradigm, allowing us to examine the ability of alcohol to exacerbate a flare period. In the other model, mice received the combined DSS-induced colitis and binge-alcohol paradigm followed by an inoculation of Citrobacter rodentium (C. rod), which allowed us to understand how alcohol increases susceptibility of UC patients to bacterial infections.
Methods
Patient database analysis
We obtained data from the Healthcare Cost and Utilization Project (HCUP) State in Patient Databases (SID) for New York and Florida from 2009–2013. Using the International Classification of Diseases, 9th Revision, Clinical Modification, (ICD-9-CM) diagnosis codes, we defined three study groups: IBD, UC, and CD. All patient admission data was retained if the patient had a primary diagnosis of IBD. All of the ICD-9-CM codes used in this study can be found in Table S1. In addition, we utilized the HCUP cost-to-charge ratio files, which provide a ratio of the actual cost relative to the total amount charged to insurance for each separate hospital. These files are used to estimate the actual cost for individual patient admissions.
Comorbidities were assigned to patients on the basis of the Agency for Healthcare Research and Quality (AHRQ) comorbidity measures. For each individual patient, if the presence of a comorbidity was indicated, then the comorbidity was applied to all of that patient’s admissions. Patients were classified as smokers based on whether any admission contained ICD-9-CM codes for tobacco use disorder or personal history of tobacco use. To evaluate the impact of alcohol on outcomes in IBD, patients were identified as having a documented history of alcohol use (+A) based on whether any admission contained ICD-9-CM codes relating to alcohol use or the AHRQ comorbidity measure for alcohol abuse, otherwise the patients were classified as not having a documented history of alcohol use (−A).
Following this, only admissions for which the primary cause was IBD, UC, or CD were retained and all other admissions were excluded. Patients aged 18 to 90 years of age were included for study. Admissions missing data elements for the median household income national quartile for patient ZIP code, unique VisitLink patient identifier, age, length of stay, total charges and patient race, were excluded. The −A and +A patients from each respective study group of IBD, UC or CD, were propensity score matched 1:1 at their first admission based on age, sex, race, primary expected payer, median household income national quartile for patient ZIP code, smoking status and various comorbidities (AIDS, congestive heart failure, chronic pulmonary disease, coagulopathy, depression, diabetes mellitus, drug abuse, hypertension (combined complicated and uncomplicated), neurological disorders, obesity, psychoses, renal failure). Subsequent admissions of propensity matched patients following their first recorded hospital admission were collected. The complete admission history of matched patients within each study group was used for data analysis, allowing for a five year perspective of what occurs when −A and +A patients are admitted for IBD, UC, or CD. The patient selection workflow is detailed in Figure S1.
Secondary diagnoses occurring during admission that were identified by ICD-9- CM codes, included Clostridium difficile infection, poorly defined intestinal infection, and all other intestinal infections, inclusive of C. difficile and poorly defined intestinal infection. Procedures during admission were identified by ICD-9-CM volume 3 and included CT scan of abdomen, small intestine biopsy, small intestine resection, colectomy, large intestine biopsy, rectal biopsy, and antibiotic injection.
Since the patient data within the HCUP SID is de-identified and publically available, the database analysis was exempt from institutional review board approval.
Induction of DSS Colitis
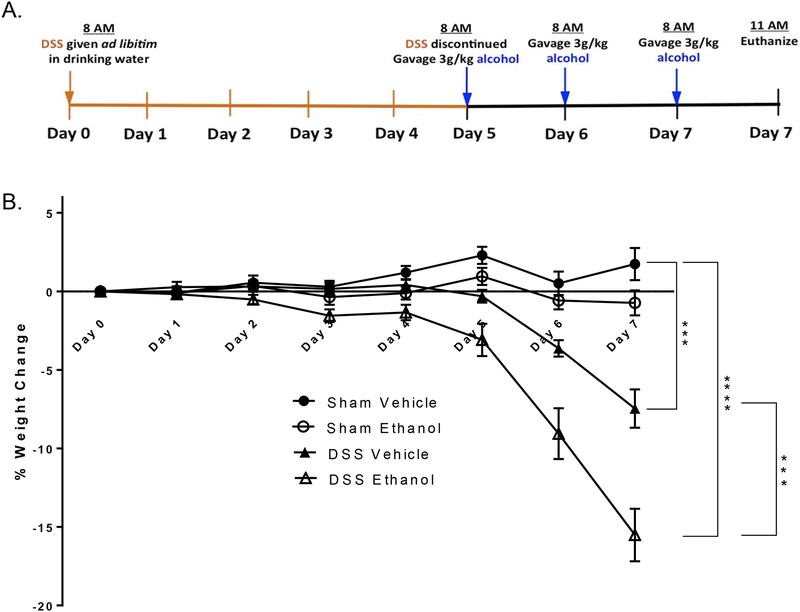
Male 8–9 week old (~23–25g body weight) C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA). Briefly, as can be seen in Figure 1A, mice were randomly separated into four experimental groups: Sham Vehicle, Sham Ethanol, DSS Vehicle, and DSS Ethanol. DSS treated mice received 2% (wt/vol) DSS (40,000 kDa; MP Biomedicals), ad libitum, in their drinking water for five days. Mice in the Sham group received water only for 5 days acting as a control. On day 5, DSS was discontinued and replaced with normal drinking water in both the DSS and Sham/control groups. On day 5, mice in both the DSS and Sham/control group were further subdivided into two subgroups: mice gavaged with alcohol (~3g/kg) or mice gavaged with water on days 5, 6, and 7 to mimic a binge alcohol abuse pattern. The amount of DSS water drank per animal was recorded and no differences in intake between groups were observed. Mice were weighed every day for the determination of percent weight change. This was calculated as: % weight change = (weight at day X- weight at day 0/weight at day 0) × 100. Animals were monitored clinically for rectal bleeding, diarrhea, and general signs of morbidity, including hunched posture and failure to groom.
Figure 1.).
A. Murine model of DSS-induced colitis and ethanol. A 2% DSS concentration was administered ad libitum in drinking water for 5 days to mimic symptoms of UC. On day 5, DSS was discontinued to allow entrance into UC remission. A binge alcohol paradigm was employed where mice were gavaged with alcohol or water on days 5, 6, and 7. Mice were euthanized 3 hours post last gavage on day 7. B. Ethanol Increased DSS-induced weight loss. Percent weight change of animals was determined by the following equation: % weight change = (weight at day X- weight at day 0/weight at day 0)*100. Values are mean ± SEM, n=8–14 animals/group. ***p<0.001 DSS Ethanol compared to DSS Vehicle, ***p<0.001 DSS Vehicle compared to Sham Vehicle; ****p<0.001 DSS Ethanol compared to Sham Vehicle by ANOVA on day 7.
Citrobacter rodentium (C. rod) infection
Mice were subjected to the DSS-induced colitis and binge alcohol paradigm as described above. However, after the last gavage on day 7, mice were further divided into two subgroups: mice gavaged with 1 × 105 CFUs of C. rod suspended in 300 μL of PBS or 300 μL of PBS alone. This resulted in four experimental groups: DSS Vehicle, DSS Ethanol, DSS Vehicle + C. rod, and DSS Ethanol + C. rod.
The experiments described here were carried out in adherence with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and are approved by the Institutional Animal Care and Use Committee, Loyola University Chicago Health Sciences Division, Maywood IL.
Tissue Staining
For hematoxylin and eosin (H&E) staining, 2cm of colon tissue closest to the rectum was taken from each mouse and saved in 10% formalin. Tissue was fixed with 10% phosphate buffered formalin, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin and eosin by AML laboratories (Saint Augustine, Florida). Images were taken on an Olympus BX43 Microscope using an Olympus DP26 camera.
Histopathology Scoring
H&E stained sections were analyzed and scored in a blinded manner by Dr. Xianzhong Ding. Dr. Ding is a trained pathologist at Loyola University Chicago and author on this study. Scoring was based on a modified 0–4 point scale examining exudate, epithelial damage, polymorphonuclear leukocyte invasion, and submucosal edema20. The values from each of the 4 categories were added to produce a combined histopathology score for each animal.
Colon Length and Average Clinical Scores
Immediately following euthanasia, colons were excised and length measured. Baseline clinical scores were determined using a modified protocol from Siegmund et al.21,22. Briefly, no weight loss was registered as 0, weight loss of 1–5% from baseline was assigned 1 point, 6–10% 2 points, 11–20% 3 points, and more than 20% 4 points. For stool consistency, 0 points were assigned for well-formed pellets, 2 points for pasty and semiformed stools that did not adhere to the anus, and 4 points for liquid stools that did adhere to the anus. For bleeding, 0 was assigned for no blood by using hemoccult (Beckman Coulter), 2 points for positive hemoccult, and 4 points for gross bleeding.
ELISA
Mice in all four groups were sacrificed three hours after the last gavage on day 7, as seen in Figure 1A. Large intestines were harvested and homogenized. The homogenates were analyzed for IL-18 (eBioscience), IL-1B (R&D Systems), IL-6 (R&D Systems), TNFα (eBioscience), and KC (BD Biosciences) using their respective ELISAs per the manufacturer’s instructions. The cytokine levels were expressed per milligram of total protein in the homogenates.
Statistics
For patient database analysis, we utilized a student’s t-test for mean cost, Mann–Whitney U test for median length of stay with interquartile ranges (IQRs), and χ2 test for categorical variables. Statistical analysis and propensity score matching were performed using STATA version 12 (StataCorp LP, College Station, TX). For the mouse model experiments, comparisons within groups were analyzed using a two-Way ANOVA with a Tukey post-hoc test. Analysis was done using GraphPad Prism software. A confidence level of p < 0.05 was considered statistically significant. Significance is represented throughout the manuscript as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Online Supplemental Material
Figure S1 is a schematic of the patient data selection process. Table S1 lists ICD-9 diagnosis codes used to define study populations and the variables examined in the study. Table S2 and S3 provide patient demographic and comorbidity statistics
Results
Within the patient database study groups, there were 41,810 IBD patients, 18,695 UC patients, and 24,059 CD patients that met the inclusion criteria. The baseline demographics of these groups are shown in Table 1, 2, and 3, respectively. Additional demographics are shown in Table S2. Before matching, the age, race, primary expected payer, and median household income, were nearly all significantly different between the −A and +A patients, notwithstanding the age of patients in the UC study group. Males, African Americans, and smokers were overrepresented within the +A patients. With regard to the primary expected payer, the +A patients were more likely to carry Medicaid and self-pay, while being less likely to carry private insurance. The lowest quartile for median household income contains more +A patients then −A patients, while the inverse can be seen for the highest income quartile. The +A patients had significantly increased comorbidities across all measures compared to the −A patients (Table S3).
Table 1.
IBD Patient Demographics and Comorbidities Pre and Post Match
| Pre match | Post match | |||||
|---|---|---|---|---|---|---|
| IBD-A (n=39,730) | IBD+A (n=2,080) | p value | IBD-A (n=2,021) | IBD+A (n=2,021) | p value | |
| 47.05 | 48.21 | 0.0009 | 48.19 | 48.3 | 0.8251 | |
| Sex | < 0.001 | 0.948 | ||||
| Male | 17,658 (44.4%) | 1,343 (64.6%) | 1,289 (63.8%) | 1,287 (63.7%) | ||
| Female | 22,072 (55.6%) | 737 (35.4%) | 732 (36.2%) | 734 (36.3%) | ||
| Race | < 0.001 | 0.728 | ||||
| White | 28,440 (71.6%) | 1,435 (69.0%) | 1,409 (69.7%) | 1,398 (69.2%) | ||
| African American | 4,112 (10.3%) | 315 (15.1%) | 302 (14.9%) | 303 (15.0%) | ||
| Hispanic | 4,715 (11.9%) | 221 (10.6%) | 218 (10.8%) | 214 (10.6%) | ||
| Asian or Pacific Islander | 518 (1.3%) | 19 (0.9%) | 12 (0.6%) | 19 (0.9%) | ||
| Native American | 74 (0.2%) | <10 (%)* | <10 (%)* | <10 (%)* | ||
| Other | 1,871 (4.7%) | 87 (4.2%) | 79 (3.9%) | 84 (4.2%) | ||
| Smoking Status | < 0.001 | 0.139 | ||||
| Non smoker | 26,956 (67.8%) | 466 (22.4%) | 427 (21.1%) | 466 (23.1%) | ||
| Smoker | 12,774 (32.2%) | 1,614 (77.6%) | 1,594 (78.9%) | 1,555 (76.9%) | ||
AHRQ restricts reporting of values when n<10
Table 2.
UC Patient Demographics and Comorbidities Pre and Post Match
| Pre match | Post match | |||||
|---|---|---|---|---|---|---|
| UC-A (n=17,653) | UC+A (n=1,042) | p value | UC-A (n=992) | UC+A (n=992) | p value | |
| 50.48 | 50.45 | 0.9519 | 50.68 | 50.72 | 0.9515 | |
| Sex | < 0.001 | 0.569 | ||||
| Male | 7,712 (43.7%) | 706 (67.8%) | 649 (65.4%) | 661 (66.6%) | ||
| Female | 9,941 (56.3%) | 336 (32.2%) | 343 (34.6%) | 331 (33.4%) | ||
| Race | < 0.001 | 0.698 | ||||
| White | 12,039 (68.2%) | 665 (63.8%) | 647 (65.2%) | 636 (64.1%) | ||
| African American | 1,926 (10.9%) | 172 (16.5%) | 152 (15.3%) | 162 (16.3%) | ||
| Hispanic | 2,443 (13.8%) | 136 (13.1%) | 143 (14.4%) | 130 (13.1%) | ||
| Asian or Pacific Islander | 293 (1.7%) | 11 (1.1%) | 10 (1.0%) | 11 (1.1%) | ||
| Native American | 40 (0.2%) | <10 (%)* | <10 (%)* | <10 (%)* | ||
| Other | 912 (5.2%) | 56 (5.4%) | 39 (3.9%) | 51 (5.1%) | ||
| Smoking Status | < 0.001 | 0.502 | ||||
| Non smoker | 12,546 (71.1%) | 258 (24.8%) | 245 (24.7%) | 258 (26.0%) | ||
| Smoker | 5107 (28.9%) | 784 (75.2%) | 747 (75.3%) | 734 (74.0%) | ||
AHRQ restricts reporting of values when n<10
Table 3.
CD Patient Demographics and Comorbidities Pre and Post Match
| Pre match | Post match | |||||
|---|---|---|---|---|---|---|
| CD-A (n=22,956) | CD+A (n=1,103) | p value | CD-A (n=1,083) | CD+A (n=1,083) | p value | |
| 44.26 | 45.88 | 0.0005 | 45.35 | 45.82 | 0.4906 | |
| Sex | < 0.001 | 0.826 | ||||
| Male | 10,332 (45.0%) | 682 (61.8%) | 657 (60.7%) | 662 (61.1%) | ||
| Female | 12,624 (55.0%) | 421 (38.2%) | 426 (39.3%) | 421 (38.9%) | ||
| Race | < 0.001 | 0.666 | ||||
| White | 16,994 (74.0%) | 804 (72.9%) | 792 (73.1%) | 790 (72.9%) | ||
| African American | 2,298 (10.0%) | 163 (14.8%) | 166 (15.3%) | 159 (14.7%) | ||
| Hispanic | 2,392 (10.4%) | 91 (8.3%) | 94 (8.7%) | 89 (8.2%) | ||
| Asian or Pacific Islander | 236 (1.0%) | <10 (%)* | <10 (%)* | <10 (%)* | ||
| Native American | 35 (0.2%) | <10 (%)* | <10 (%)* | <10 (%)* | ||
| Other | 1,001 (4.4%) | 36 (3.3%) | 23 (2.1%) | 36 (3.3%) | ||
| Smoking Status | < 0.001 | 0.664 | ||||
| Non smoker | 14,935 (65.1%) | 215 (19.5%) | 207 (19.1%) | 215 (19.9%) | ||
| Smoker | 8,021 (34.9%) | 888 (80.5%) | 876 (80.9%) | 868 (80.1%) | ||
AHRQ restricts reporting of values when n<10
Post propensity matching, the differences observed for age, sex, race, smoker status, primary expected payer, median household income, and comorbidities between the −A and +A patients within the three study groups were no longer statistically significant (Tables 1, S2 and S3). For the IBD study group, there were 2,021 matched patients (Table 1), that encompassed 4,965 IBD−A admissions and 4,013 IBD+A admissions (Table 4). For the UC study group, there were 992 matched patients (Table 2), that encompassed 1,602 UC−A admissions and 1,437 UC+A admissions (Table 4). For the CD study group, there were 1,083 matched patients (Table 3), that encompassed 3,089 CD−A admissions and 2,548 CD+A admissions (Table 4).
Table 4.
Outcomes of Patients During IBD, UC, or CD Admission
| IBD admissions | UC admissions | CD admissions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IBD-A (n=4,965) |
IBD+A (n=4,013) |
p value | UC-A (n=1,602) |
UC+A (n=1,437) |
p value | CD-A (n=3,089) |
CD+A (n=2,548) |
p value | |
| C. difficile intestinal infection | 98 (2.0%) | 105 (2.6%) | 0.042 | 61 (3.8%) | 47 (3.3%) | 0.427 | 52 (1.7%) | 57 (2.2%) | 0.133 |
| Poorly defined intestinal infection | 18 (0.4%) | 31 (0.8%) | 0.009 | 10 (0.6%) | 21 (1.5%) | 0.022 | <10 (%)* | 10 (0.4%) | 0.514 |
| All intestinal infection | 131 (2.6%) | 158 (3.9%) | 0.001 | 75 (4.7%) | 79 (5.5%) | 0.304 | 65 (2.1%) | 78 (3.1%) | 0.023 |
| Procedures/Surgery | |||||||||
| Abdomen CT Scan | 338 (6.8%) | 386 (9.6%) | < 0.001 | 110 (6.9%) | 130 (9.0%) | 0.026 | 226 (7.3%) | 252 (9.9%) | 0.001 |
| Biopsy Small Intestine | 432 (8.7%) | 384 (9.6%) | 0.155 | 158 (9.9%) | 134 (9.3%) | 0.616 | 306 (9.9%) | 246 (9.7%) | 0.752 |
| Resection Small Intestine | 92 (1.9%) | 44 (1.1%) | 0.004 | 12 (0.7%) | 5 (0.3%) | 0.139 | 73 (2.4%) | 39 (1.5%) | 0.026 |
| Biopsy large intestine | 897 (18.1%) | 872 (21.7%) | < 0.001 | 492 (30.7%) | 468 (32.6%) | 0.272 | 475 (15.4%) | 399 (15.7%) | 0.771 |
| Colectomy | 79 (1.6%) | 62 (1.5%) | 0.861 | 75 (4.7%) | 49 (3.4%) | 0.077 | 11 (0.4%) | 13 (0.5%) | 0.376 |
| Biopsy Rectum | 156 (3.1%) | 173 (4.3%) | 0.003 | 133 (8.3%) | 125 (8.7%) | 0.695 | 59 (1.9%) | 46 (1.8%) | 0.772 |
| Antibiotic injection | 154 (3.1%) | 234 (5.8%) | < 0.001 | 61 (3.8%) | 79 (5.5%) | 0.027 | 105 (3.4%) | 148 (5.8%) | < 0.001 |
| Median Length of Stay (Days) | 4 (IQR 2–7) | 4 (IQR 2–6) | 0.0091 | 4 (IQR 3–7) | 4 (IQR 3–7) | 0.1797 | 4 (IQR 2–7) | 4 (IQR 2–6) | 0.0139 |
| Mean Total Cost ($) | 13,880.65 | 12,262.51 | < 0.0001 | 14,564.63 | 13,217.54 | 0.0293 | 13,480.92 | 11,794.42 | 0.0001 |
AHRQ restricts reporting of values when n<10
Examination of intestinal infection diagnoses during the admission for either the IBD, UC, or CD study groups revealed that +A patients had significantly increased intestinal infections (Table 4). The IBD+A patients had increased Clostridium difficile intestinal infection (2% vs 2.6%, p < 0.05), poorly defined intestinal infection (0.4% vs 0.8%, p < 0.01), and overall intestinal infections (2.6% vs 3.9%, p < 0.01). The UC+A patients had increased poorly defined intestinal infections (0.6% vs 1.5%, p < 0.05), while the CD+A patients had increased intestinal infections of all types (2.1% vs 3.1%, p < 0.05).
With regard to procedures during the admission (Table 4), all +A patients had significant increases of abdominal CT scans (IBD: 6.8% vs 9.6%, p < 0.001; UC: 6.9% vs 9.0%, p < 0.05; CD: 7.3% vs 9.9%, p < 0.01) and antibiotic injections (IBD: 3.1% vs 5.8%, p < 0.001; UC: 3.8% vs 5.5%, p < 0.05; CD: 3.4% vs 5.8%, p < 0.001). Large intestine and rectal biopsies were significantly increased in +A patients compared to −A patients within the IBD study group (18.1% vs 21.7%, p < 0.001; 3.1% vs 4.3%, p < 0.01). Within the UC and CD study group, large intestine and rectal biopsies were not significantly changed between the −A and +A patients, although UC+A patients did have increased large intestine biopsies (30.7% vs 32.6%, p = .272). Small intestine biopsies were unchanged between the −A and +A patients. Resection of the small intestine was significantly increased in −A patients with IBD (1.9% vs 1.1%, p < 0.01) and CD (2.4% vs 1.5%, p < 0.05). For the UC study group, −A patients displayed a trend towards increased colectomy (4.7% vs 3.4%, p = 0.077). The differences in percentages of colectomy and small intestine resection procedures for the UC and CD study groups support the appropriate separation of these patients into their respective groups.
The median length of stay was unchanged between −A and +A patients in all study groups (4 days), however the interquartile range was significantly increased for the −A patients in the IBD (2–7 days vs 2–6 days, p < 0.01) and CD (2–7 days vs 2–6 days, p < 0.05) study groups. In terms of total cost, the −A patients had increased total cost during hospitalization for all study groups compared to the +A patients (IBD: $13,880.65 vs $12,262.51, p < 0.0001; UC: $14,564.63 vs $13,217.54, p < 0.05; CD: $13,480.92 vs $11,794.42, p < 0.001) (Table 4).
Having found that alcohol affects the clinical outcomes of IBD patients, we established a novel model of DSS-induced colitis in the context of a binge alcohol paradigm. DSS-induced colitis is a well-established model for studying an UC flare period23, yet no model currently exists to study how alcohol intoxication could exacerbate a UC flare. Hence, our lab developed a murine model as seen in Figure 1. Previous studies on DSS-induced colitis have used concentrations of DSS from 1–3%24–27. In order to parse out minute differences between DSS-induced intestinal damage alone and DSS and alcohol induced intestinal damage, we utilized a 2% concentration of DSS.
As can be seen in Figure 1A, DSS treated mice received DSS ad libitum in their drinking water for 5 days to induce the UC disease state. Mice in sham/control group received water. On day 5, DSS was discontinued to allow entrance into UC remission. A binge alcohol paradigm was then employed where mice were gavaged with alcohol (~3g/kg) or gavaged with water on days 5, 6, and 7. This resulted in four overall experimental groups - Sham Vehicle, Sham Ethanol, DSS Vehicle, and DSS Ethanol.
Weight loss, histopathology score, colonic shortening, and average clinical score are the most common experimental observations used to assess UC disease severity. Therefore, we first monitored weight loss or gain in our newly adapted model of UC and binge alcohol. Consistent with previous reports, mice treated with DSS began losing body weight on day 5 after treatment as calculated by percent weight change from day 0. On day 6, mice receiving a combined insult of DSS Ethanol lost twice as much weight compared to those mice in the DSS Vehicle group, ~10% vs. ~5% respectively. By day 7, the weight loss in mice receiving DSS Ethanol reached 17% to that of their original body weight as compared to only 12% in DSS vehicle mice, Figure 1B.
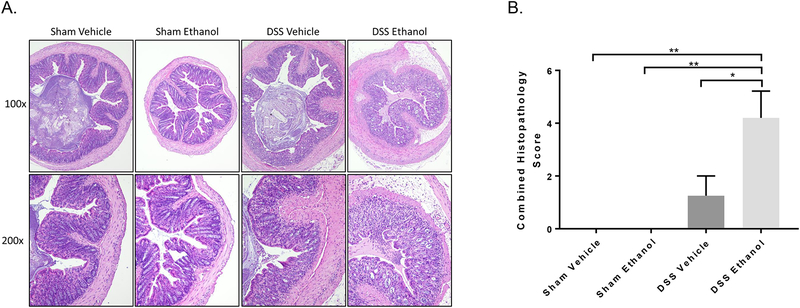
To address differences in histopathology between the four experimental groups, sections of colon were taken closest to the rectum, stained via H&E, blinded, and scored by a pathologist. Figure 2A shows gross differences in large intestine morphology after DSS Ethanol treatment compared to all other groups. Inflammatory infiltrate, epithelial damage, and crypt damage are severely increased in the DSS Ethanol mice compared to that of the DSS Vehicle. Furthermore, combined histopathology scores in Figure 2B, show significant increases in DSS Ethanol compared to DSS Vehicle.
Figure 2.). Gross histological pathologies increased following combined ethanol and DSS treatment.
A. Representative H&E stained sections of the colon on day 7 (Top row x100, Bottom row x200). B. Combined Histopathology Score following blinded histological scoring as described in detail in Methods section above. Values are mean ± SEM, n=4–5 animals/group. **p<0.01 DSS Ethanol compared to Sham Vehicle and Sham Ethanol,; *p<0.05 DSS Ethanol compared to DSS Vehicle by ANOVA.
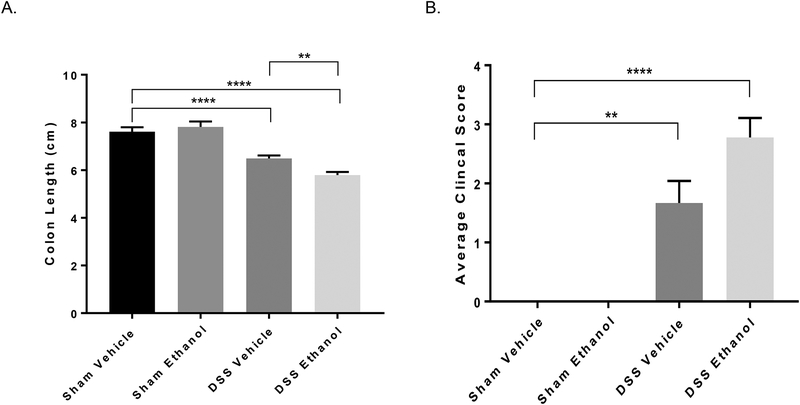
Increased weight loss and histopathology scores accompanied a significant decrease in colon length in our novel model of UC and binge alcohol. As has been observed by many others in the past23, DSS Vehicle treated mice experienced a decrease in colon length compared to that of Sham Vehicle mice. Interestingly, the addition of alcohol to the DSS treated mice resulted in a more severe decrease in colon length compared to DSS Vehicle treated mice, Figure 3A. Clinical scores were obtained by combining weight loss, stool consistency, and blood in stool as described in the methods section above. Data in Figure 3B show that the addition of alcohol to DSS-induced colitis trends toward an increase in the average clinical score compared to DSS Vehicle, highlighting alcohol’s detrimental effect on UC flares.
Figure 3.). Increased colon length shortening and average clinical scores following ethanol and DSS-induced colitis.
A. Colon length measured in centimeters (cm) on day 7. Values are mean ± SEM, n=8–14 animals/group. **p<0.01 DSS Ethanol compared to DSS Vehicle, ****p<0.0001 DSS Vehicle compared to Sham Vehicle; ****p<0.0001 DSS Ethanol compared to Sham Vehicle by Two-way ANOVA. B. Clinical scores as described above. Briefly scores were determined by the average of % body weight loss, stool consistency, and presence of blood in the stool. Values are mean ± SEM, n=4–6 animals/group. **p<0.01 DSS Vehicle compared to Sham Vehicle; ***p<0.001 DSS Ethanol compared to Sham Vehicle by ANOVA.
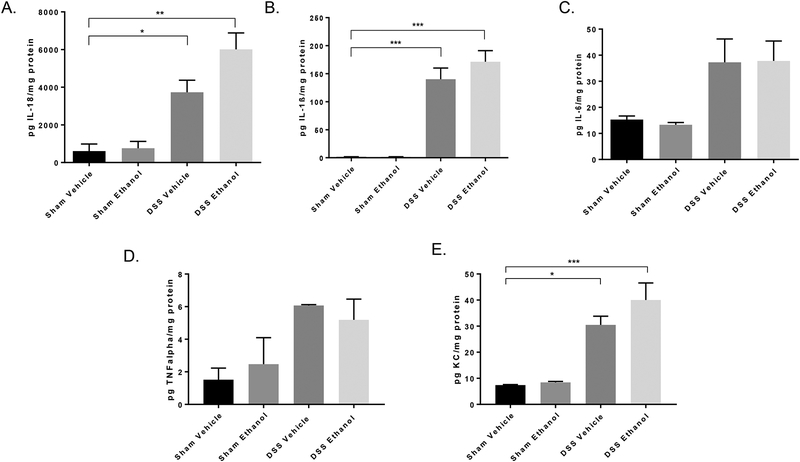
Colonic inflammation is a hallmark symptom of UC. To further delineate how alcohol could be exacerbating an UC flare, levels of large intestine pro-inflammatory cytokines were determined. We hypothesized that alcohol would further increase levels of large intestine pro-inflammatory cytokines following the addition of our alcohol binge paradigm. Our results revealed that in mice receiving DSS Ethanol, IL-18 (Figure 4A), IL-1β (Figure 4B), and KC (Figure 4E) trended to increase compared to DSS Vehicle treated mice. However, the cytokines, IL-6 (Figure 4C) and TNFα (Figure 4D) were not found to be increased in the colons of DSS Ethanol treated mice compared to mice receiving DSS vehicle. Although increases in inflammation following DSS Ethanol did not reach statistical significance, total large intestine homogenates were used for all ELISAs, and we anticipate specifically isolating inflamed areas in contrast to diluting inflamed areas with non-inflamed areas (as commonly occurs in the intestines of UC patients) will yield statistical significance. However, future work will focus on this and the specific cell types in the intestine responsible for the increases in inflammation that we do see in the total homogenates.
Figure 4.). Increased colonic inflammation after DSS-induced colitis and ethanol treatment.
Colons were harvested, homogenized, and processed on day 7 for the analysis of inflammatory mediators using respective ELISAs. A. IL-18, B. IL-1β, C. IL-6, TNFα, E. KC by ELISA. Values are mean ± SEM 3–6 animals per group *p<0.05; **p<0.01; ***p<0.001 all groups compared to Sham Vehicle by ANOVA.
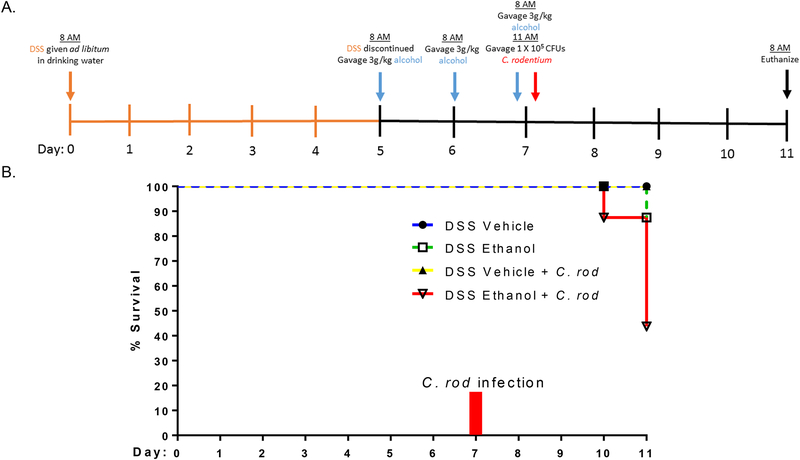
UC patients are at higher risk of developing bacterial infections28,29. To understand whether consumption of alcohol not only impacts UC patient’s increased susceptibility to infection but also increases severity of symptoms related to bacterial infection, we utilized our model of DSS-induced colitis and binge alcohol, as described above, along with a single inoculation of C. rod, a well-known Gram negative enteropathogen associated with colonic infection, at 1 × 105 CFUs three hours after the last gavage on day 7 (Figure 5A). As can be seen in Figure 5B, the percent survival of DSS Ethanol treated mice following C. rod infection fell to 50% by day 11 compared to 100% survival in the DSS Vehicle group with C. rod infection. Interestingly, DSS Ethanol mice with no C. rod infection also experienced a 20% reduction in survival compared to the 100% survival in the DSS Vehicle group with and without C. rod infection.
Figure 5.).
A. Murine model of DSS-induced colitis and ethanol. A 2% DSS concentration was administered ad libitum in drinking water for 5 days to mimic symptoms of UC. On day 5, DSS was discontinued to allow entrance into UC remission. A binge alcohol paradigm was employed where mice were gavaged with alcohol or water on days 5, 6, and 7. Mice were further subdivided and were gavaged with either 1 × 105 CFUs C. rodentium or water 3 hours post last gavage on day 7. Mice were euthanized on day 11.
B. Ethanol decreased % survival following C. rodentium infection. n=7–8 animals/group.
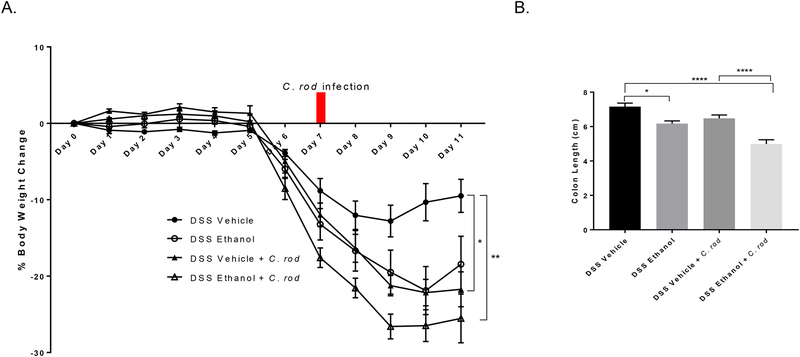
Weight loss and gain was also monitored as described previously by percent change from day 0 up till day 11 as only 50% of the mice in the DSS Ethanol + C. rod infection group survived till day 11. By day 11 following C. rod infection, mice in the DSS Ethanol group experienced a ~27% decrease from their original body weight compared to ~22% in the DSS Vehicle group (Figure 6A) giving evidence to our hypothesis of alcohol not only increasing susceptibility to infection with UC, but also increasing severity of symptoms associated with UC and infection. Increased weight loss accompanied increases in colonic shortening in the DSS Ethanol + C. rod group compared to mice in the DSS Vehicle + C. rod group, Figure 6B.
Figure 6.).
A. Alcohol consumption increases weight loss following C. rodentium infection in DSS-induced colitis. Values are calculated as average % weight change, *p<0.05 DSS Vehicle + C. rod compared to DSS Vehicle; **p<0.01 DSS Ethanol + C. rod compared to DSS Vehicle by Two-way ANOVA, n=7–8 animals/group. B. Increased colonic shortening with C. rodentium infection after alcohol consumption and DSS-induced colitis. Values are means ± SEM, n=7–8 animals/group. *p<0.05 DSS Ethanol compared to DSS Vehicle; ****p<0.0001 DSS Ethanol + C. rod compared to DSS Vehicle and DSS Vehicle + C. rod ANOVA.
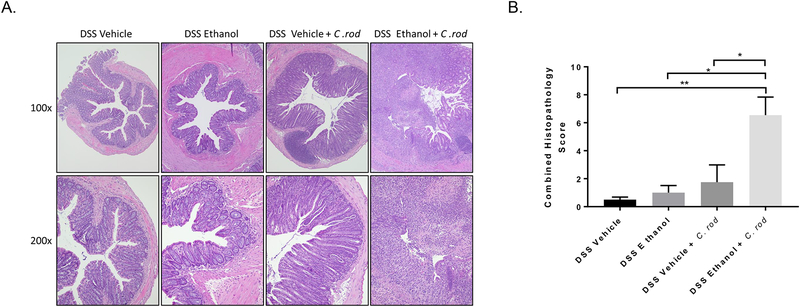
Again, to understand differences in histopathology following infection with C. rod, sections of colon were taken closest to the rectum, stained via H&E, blinded, and scored by a pathologist. Figure 7A shows gross differences in large intestine morphology after DSS Ethanol + C. rod treatment compared to all other groups. As in Figure 2B, inflammatory infiltrate and epithelial damage were assessed and were severely increased in the DSS Ethanol + C. rod mice compared to that of the DSS Vehicle + C. rod group. The combined histopathology scores in DSS Ethanol + C. rod treated mice were significantly increased compared to mice in the DSS Vehicle + C. rod group, Figure 7B.
Figure 7.).
A. Increased colonic damage and inflammatory infiltrate with C. rodentium infection after alcohol consumption and DSS-induced colitis. Representative H&E images, n=6–12 animals/group. B. Combined Histopathology Score following blinded histological scoring as described in detail in Methods section above. Values are mean ± SEM, n=6–12 animals/group. **p<0.01 DSS Ethanol + C. rod compared to DSS Vehicle; *p<0.05 DSS Ethanol + C. rod compared to DSS Vehicle + C. rod and DSS Ethanol by ANOVA.
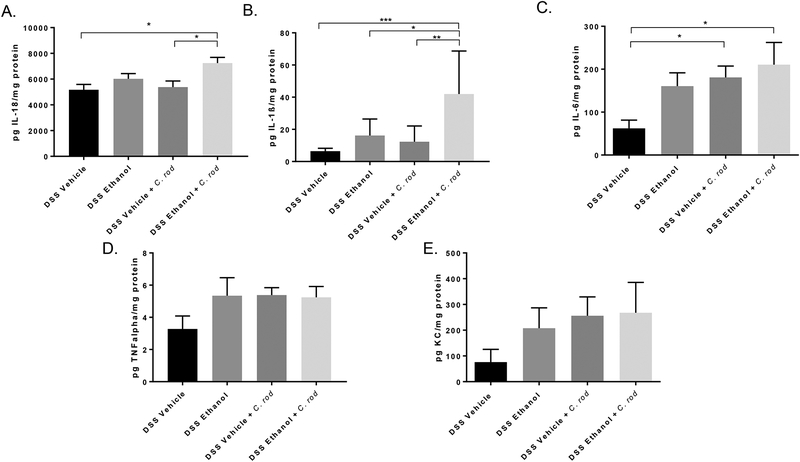
As in Figure 4, we assessed colonic inflammation under the hypothesis that C. rod infection would further increase inflammation in mice receiving DSS Ethanol treatment, which could perpetuate increased colonization of C. rod We found that mice in the DSS Ethanol + C. rod group had increased levels of IL-18 (Figure 8A) and IL-1β (Figure 8B) compared to DSS Vehicle + C. rod. Furthermore, the pro-inflammatory cytokine IL-6 was increased in both the DSS Ethanol + C. rod and DSS Vehicle + C. rod compared to mice treated with DSS alone (Figure 8C). However, both TNFα and KC were not further increased following C. rod infection after DSS Ethanol treatment (Figure 8D and 8E).
Figure 8.). C. rodentium further increases colonic inflammation after DSS-induced colitis and ethanol treatment.
Colons were harvested, homogenized, and processed on day 11 for the analysis of inflammatory mediators using respective ELISAs. A. IL-18 *p<0.05 DSS Ethanol + C. rod compared to DSS Vehicle + C. rod and DSS Vehicle by ANOVA, B. IL-1β *p<0.05; **p<0.01; ***p<0.001 DSS Ethanol + C. rod compared to all other groups by ANOVA, C. IL-6 *p<0.05 DSS Ethanol + C. rod compared to DSS Vehicle and DSS Vehicle + C. rod compared to DSS Vehicle by ANOVA, TNFα, E. KC by ELISA. Values are mean ± SEM 7–8 animals per group.
Discussion
Alcohol is known to have numerous deleterious effects in a variety of settings, yet its effects in IBD are not well understood. In this study, we described a potential role for alcohol affecting the inpatient care of IBD patients as well as prolonging an inflammatory flare period and increasing pathogenic infection in a mouse model of UC.
Research has shown that IBD patients and alcoholic patients carry an intestinal bacterial dysbiosis30,31. A dysbiosis is believed to provide pathogens an opportunity to colonize and proliferate32. Indeed, studies have shown IBD patients and alcoholics are at risk for increased infections33,34. Thus, the combination of alcohol use in a patient with IBD would likely lead to an increase in the amount of intestinal infections, which is exactly what we observe in our patient database analysis. Furthermore, the +A patients required increased antibiotic injections as well as increased diagnostic procedures. Despite these increases, the +A patients had decreased total costs compared to −A patients. This can likely be explained by the decrease in surgical procedures (colectomy and small intestine resection) observed in the +A patients. These patients might be less than ideal candidates for surgery, which is often more expensive. In addition, while the median length of stay was unchanged between the −A and +A patients, the interquartile range for the length of stay was increased for the −A patients, which may also be related to the increase in surgical procedures since surgical patients require longer hospitalization periods post-surgery. How this decrease in surgical interventions affects the clinical course of IBD patients with alcohol use remains to be further elucidated.
We would like to note that to be coded for an ICD-9 diagnosis for an alcohol related disorder, the patient must have had some history of significant alcohol intake, thus our +A study group likely comprises mostly heavy drinkers. Unfortunately, the databases do not contain information regarding the amount of alcohol a patient had prior to an admission. Furthermore, the databases do not contain the exact cause of the IBD admission, thus we cannot discern whether a patient was admitted for a flare or other complication of IBD. Therefore, the findings of this analysis warrant additional research of IBD patients that could provide more detailed information beyond what is available through ICD-9 codes.
Overall, the results of this patient database analysis further support the suggestion that alcohol has some negative impacts on the clinical outcomes of patients with IBD13, which led us to examine and characterize the mechanism by which alcohol exacerbates the flare period and infective capacity in a mouse model of DSS-induced colitis.
To our knowledge, this is the first time a murine model of UC and alcohol has been developed to allow a better understanding of how drinking alcohol could affect a patient with UC. Understanding potential environmental factors that could contribute to disease flares, either as a trigger or an exacerbation of symptoms, is critical to improving the quality of life of UC patients stuck in the maintenance of their disease hoping to avoid a flare or a worsening of symptoms during a flare period.
We were able to show that mice undergoing a binge alcohol paradigm following DSS-induced colitis had exacerbated symptoms of UC as shown by increases in weight loss, colon shortening, histopathology and clinical scores, and inflammation, all of which are standard assessment of UC severity in mouse models. Besides symptoms associated with UC itself, UC patients have a higher propensity to other co-morbidities such as infection. Hence, we adapted our model of UC and binge alcohol to include a low inoculate of C. rodentium in order to understand whether UC patients that drink alcohol would also have increased susceptibility to infection. Our results showed mice receiving C. rodentium following alcohol and DSS-induced colitis had decreased survival and increased weight loss, colon shortening, histopathology and clinical scores, and inflammation.
Reappearance of UC symptoms during a flare stem from increases in intestinal inflammatory cytokines such as IFN-γ, IL-1, IL-6, and TNF-α35–40, which can directly lead to mucosal ulcerations, damage to the colonic epithelium, and crypt micro-abscesses41. Coupled with the knowledge that alcohol itself induces increases in inflammation it follows that alcohol consumption could either perpetuate a current UC flare, such as we’ve provided evidence for above, and/or trigger entrance into UC flare via initiation of the inflammatory cascade, which requires further investigation.
This inflammation induced damage to the intestine and thus intestinal defense mechanisms could have dramatic consequences to UC patients, especially in combination with alcohol. Recent studies found that alcohol could cause a dysbiosis of the intestinal microbiome, which, in turn, could alter the intestinal microenvironment making it more favorable to opportunistic pathogens42. Therefore, it was imperative to understand the susceptibility to intestinal pathogens specifically after alcohol and DSS-induced colitis. With our low inoculate C. rodentium model in combination with alcohol and DSS-induced colitis, we were able to show increases in susceptibility to infection in DSS Ethanol treated mice. We recognize the burden of utilizing the model pathogen, C. rodentium, which itself is used as a model of IBD, on top of our binge alcohol and DSS-induced colitis model. Yet, our adaptation of using a much lower inoculate, 1 × 105 CFUs vs. 1 × 1011 CFUs to induce true IBD symptoms, allowed us to shed light on the increased propensity for +A IBD patients to acquire intestinal infections. However, more analysis of patient data, both prospective and retrospective, is needed to understand the molecular implications of alcohol in UC patient infections.
The idea that alcohol could act in such a way to worsen UC flare periods provides evidence to the clinician’s warning to UC patients to avoid drinking alcohol. However, future work will focus on elucidating the mechanism behind alcohol intensifying UC flare symptoms in order to potentially open doors to patients wanting to participate in social situations that involving drinking alcohol. One potential pathway that upon its activation could act in the amelioration of DSS-induced colitis is the aryl hydrocarbon receptor (AhR) pathway. Studies have shown that activation of the AhR pathway not only through chemical activation via TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) but also by specific probiotics is able to inhibit DSS-induced colitis43,44. Additionally, cytokines known to be induced upon AhR activation, specifically IL-22, are known to be upregulated during remission periods of UC45,46. In the intestine IL-22 can stimulate proliferation, mucous protection, and AMP secretion, which could drive entrance into the remission period from a UC flare47–52. Understanding the interplay between these pathways, UC flare, the gut microbiome, and intestinal inflammation in the context of binge alcohol is critical to the development of a therapeutic intervention that would allow improvements to UC patient’s lifestyles especially those stuck in lifelong maintenance therapy.
Supplementary Material
Figure S1.) Patient data collection workflow. We obtained data from the Healthcare Cost and Utilization Project (HCUP) State in Patient Databases (SID) for New York and Florida from 2009-2013. Patients without a history of primary diagnosis for IBD were excluded from the study. Procedure codes were assigned separately for each state due to differences in the number of procedures coded by each state. The two datasets were then merged, followed by assignment of comorbidities, smoking status, and documented history of alcohol use. The analysis was then subdivided based on whether patients had primary admissions for IBD, UC, or CD. Patients were excluded based on age and missing data elements. Propensity matching was performed to obtain matched patients, followed by collection of all IBD, UC, or CD admissions for each respective study group of matched patients.
Conflicts of Interest and Funding Sources:
The authors have no conflicts of interest to declare
Supported by R21AA022324, T32AA013527, and F31AA025536–01.
References
- 1.U.S. Department of Health and Human Services. Centers for disease control and prevention. http://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm. Updated October, 2015March, 2016.
- 2.Crohn’s and Colitis Foundation. Managing flares and other IBD symptoms. http://www.ccfa.org/assets/pdfs/flares_brochure_final.pdfMarch, 2016.
- 3.Crohn’s and colitis foundation of america. http://www.ccfa.org/resources/facts-about-inflammatory.html. Updated May, 2011March, 2016.
- 4.Hanauer SB. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3–9. [DOI] [PubMed] [Google Scholar]
- 5.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289(1):70–75. [DOI] [PubMed] [Google Scholar]
- 6.Dignass A, Lindsay JO, Sturm A, et al. Second european evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J Crohns Colitis. 2012;6(10):991–1030. [DOI] [PubMed] [Google Scholar]
- 7.Loghman-Adham M Medication noncompliance in patients with chronic disease: Issues in dialysis and renal transplantation. Am J Manag Care. 2003;9(2):155–171. [PubMed] [Google Scholar]
- 8.Higgins PD, Rubin DT, Kaulback K, Schoenfield PS, Kane SV. Systematic review: Impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther. 2009;29(3):247–257. [DOI] [PubMed] [Google Scholar]
- 9.Brown AC, Rampertab SD, Mullin GE. Existing dietary guidelines for crohn’s disease and ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2011;5(3):411–425. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21(7):698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrier L, Berard F, Debrauwer L, et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168(4):1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33(10):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol. 2010;44(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17(4):575–592. [DOI] [PubMed] [Google Scholar]
- 15.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol. 2010;44(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut. 2004;53(10):1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu TY, Shih HM, Wang YC, et al. Effect of alcoholic intoxication on the risk of inflammatory bowel disease: A nationwide retrospective cohort study. PLoS One. 2016;11(11):e0165411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann MM, Hernandez V, Bernigau W, et al. No association of alcohol use and the risk of ulcerative colitis or crohn’s disease: Data from a european prospective cohort study (EPIC). Eur J Clin Nutr. 2017;71(4):566. [DOI] [PubMed] [Google Scholar]
- 20.Hughes ER, Winter MG, Duerkop BA, et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe. 2017;21(2):208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci U S A. 2001;98(23):13249–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. [DOI] [PubMed] [Google Scholar]
- 23.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. [DOI] [PubMed] [Google Scholar]
- 24.Ito R, Kita M, Shin-Ya M, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377(1):12–16. [DOI] [PubMed] [Google Scholar]
- 25.Vlantis K, Polykratis A, Welz PS, van Loo G, Pasparakis M, Wullaert A. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut. 2016;65(6):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. [DOI] [PubMed] [Google Scholar]
- 27.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15(3):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–936. [DOI] [PubMed] [Google Scholar]
- 29.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. Incidence of clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(3):339–344. [DOI] [PubMed] [Google Scholar]
- 30.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and clostridium difficile infection: Is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol. 2013;6(1):53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaram V, May FP, Manne V, Saab S. Effects of clostridium difficile infection in patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2014;12(10):1745–52.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57(2):205–210. [DOI] [PubMed] [Google Scholar]
- 35.Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat Med. 2013;19(7):822–824. [DOI] [PubMed] [Google Scholar]
- 36.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. [DOI] [PubMed] [Google Scholar]
- 37.Masuda H, Iwai S, Tanaka T, Hayakawa S. Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J Clin Lab Immunol. 1995;46(3):111–123. [PubMed] [Google Scholar]
- 38.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154(5):2434–2440. [PubMed] [Google Scholar]
- 39.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21(7):698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. [DOI] [PubMed] [Google Scholar]
- 42.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: Alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37(2):223–236. [PMC free article] [PubMed] [Google Scholar]
- 43.Takamura T, Harama D, Matsuoka S, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88(6):685–689. [DOI] [PubMed] [Google Scholar]
- 44.Takamura T, Harama D, Fukumoto S, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89(7):817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: Linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28(4):310–313. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto-Furusho JK, Miranda-Perez E, Fonseca-Camarillo G, Sanchez-Munoz F, Dominguez-Lopez A, Barreto-Zuniga R. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16(11):1823. [DOI] [PubMed] [Google Scholar]
- 47.Zindl CL, Lai JF, Lee YK, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A. 2013;110(31):12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–1323. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4(5):679–691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.) Patient data collection workflow. We obtained data from the Healthcare Cost and Utilization Project (HCUP) State in Patient Databases (SID) for New York and Florida from 2009-2013. Patients without a history of primary diagnosis for IBD were excluded from the study. Procedure codes were assigned separately for each state due to differences in the number of procedures coded by each state. The two datasets were then merged, followed by assignment of comorbidities, smoking status, and documented history of alcohol use. The analysis was then subdivided based on whether patients had primary admissions for IBD, UC, or CD. Patients were excluded based on age and missing data elements. Propensity matching was performed to obtain matched patients, followed by collection of all IBD, UC, or CD admissions for each respective study group of matched patients.