Abstract
Adolescent’s consumption of caffeine and caffeinated beverage is increasing, yet little is known about the consequences of chronic caffeine exposure during the critical development period of adolescence. In the present study, we investigated the effect of beginning chronic caffeine consumption in adolescence on locomotor, mood, sensorimotor gating, and reward seeking behaviors through adolescence and in adulthood. During the light cycle, caffeine exposed mice exhibited hypoactivity in a novel open-field box and increased anxiety-like and depressive-like behaviors, while maintaining normal home cage locomotor activity. In contrast, during the dark cycle caffeine exposed mice displayed normal locomotor activity in a novel open-field box with hyperactive home cage activity. Interestingly, we found that caffeine exposed mice also showed enhanced prepulse inhibition during the light cycle whereas they displayed a deficit of prepulse inhibition during the dark cycle. Reward seeking for sucrose was higher in caffeine exposed than control mice during the light cycle. Additionally, when granted 24-hour access to ethanol as adults, caffeine exposed mice consumed more ethanol in the absence of acute caffeine use. Altogether, mice that consumed chronic caffeine beginning in adolescence had increased reward seeking and exhibited a circadian-dependent pattern of mood fluctuations in adulthood.
Keywords: Adolescence, Caffeine, Mania, Depression, Prepulse inhibition, Circadian, Diurnal, Reward
1. Introduction
Caffeine is the most commonly used psychoactive substance in the world. Importantly, approximately 73% of adolescents consume caffeine daily [1]. While caffeine has several beneficial effects in adults [2], excessive caffeine consumption during adolescence corresponds to several adverse effects. Adolescent caffeine use is associated with attention deficit and hyperactivity disorder, tobacco, marijuana, and other illicit drug use, increased anxiety, aggressive behaviors [3], sleep disturbances [4], and in rodents it has been shown to enhance cocaine sensitivity [5]. Despite the correlations between adolescent behavior and caffeine use, a recent systematic review of caffeine use identified a paucity of available data for the ways in which caffeine impacts children and adolescents [6].
Pharmacologically, caffeine acts as an adenosine A1 and A2A receptor antagonist. Adenosine is an essential regulator of the sleep/wake cycle, accumulates in the basal forebrain during wakefulness [7, 8], and is a key player in circadian timing [9, 10]. Importantly, astrocytic release of adenosine contributes to sleep homeostasis in a mechanism downstream of the adenosine A1 receptor [11]. Notably, disruptions in circadian rhythms potentiate mood-related episodes while people with mood disorders commonly have disruptions in circadian rhythms and experience sleep disturbances [12]. Caffeine has also been associated with psychotic and mood disorder symptoms and psychiatric patients consume more caffeine than healthy control subjects [13, 14].
Both increased advertising and its availability in various forms promote caffeine intake beginning at younger ages. Caffeine is an ingredient in coffee, tea, soft drinks, and is often added to energy drinks, chocolate, bottled water, chewing gum and medications [15]. In fact, energy drinks, which on average contain 70 to 80 mg caffeine per 8-oz serving, are frequently consumed by up to 50% of adolescents (12–18 years old) and young adults (19–25 years old) [16]. Moreover, according to a recent study, 75% of children consume caffeine daily, with children aged 5 to 7 years old consuming approximately 52 mg while children 8 to 12 years old consumed approximately 110 mg of caffeine per day [17]. It is common for adolescents to use caffeine to enhance their energy or alertness, especially in order to improve school performance [18]. Understanding the implications of increased adolescent caffeine use given these known behavioral associations has therefore become a pressing need. In this study, we sought to determine whether chronic, high-dose caffeine consumption throughout adolescence can cause behavioral changes that extend into adulthood. We therefore performed a battery of behavioral tests in order to assess how beginning caffeine consumption during adolescence impacts adult activity, mood, sensorimotor gating, and reward seeking behavior.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice (3 weeks old, Jackson Laboratories, Bar Harbor, ME) were grouped housed (4-5 mice per group) in standard Plexiglas cages under a 12 h light (>500 lux)/dark (<0.5 lux) cycle with lights on at 6:00 AM. Food and water were provided ad libitum for 1 week. Mice were singly housed when they reached 4 weeks of age, at which time caffeine exposed mice (CAF) were provided with a caffeine solution, while control animals continued ad libitum water access. Male mice only were used as male and female mice respond differently to adolescent social isolation for behaviors we tested, including acoustic startle and anxiety [19]. Food was provided ad libitum unless otherwise stated. Behavioral testing began at 18 days of caffeine drinking and two cohorts, one for light cycle (9AM) and one for dark cycle (8PM) experiments, were used sequentially for: open field test (OFT), forced swim test (FST), home cage activity, prepulse inhibition (PPI), elevated plus maze (EPM), and sucrose reward seeking. As previous work has demonstrated a connection between caffeine and ethanol consumption, caffeine use was discontinued while we assessed ethanol consumption, allowing us to determine how a history of caffeine use during adolescence effects adult ethanol consumption without interference from acute caffeine effects [20]. Taste preference was assessed after ethanol consumption assessment and both CAF and control animals were provided with ad libitum water without caffeine (Fig. 1). Mice were brought to the test room 30 minutes before testing to allow them to acclimate; this acclimation occurred in the light for animals tested during the light cycle and in the dark for animals tested during the dark cycle. A second cohort of mice was used to measure plasma corticosterone. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with NIH guidelines.
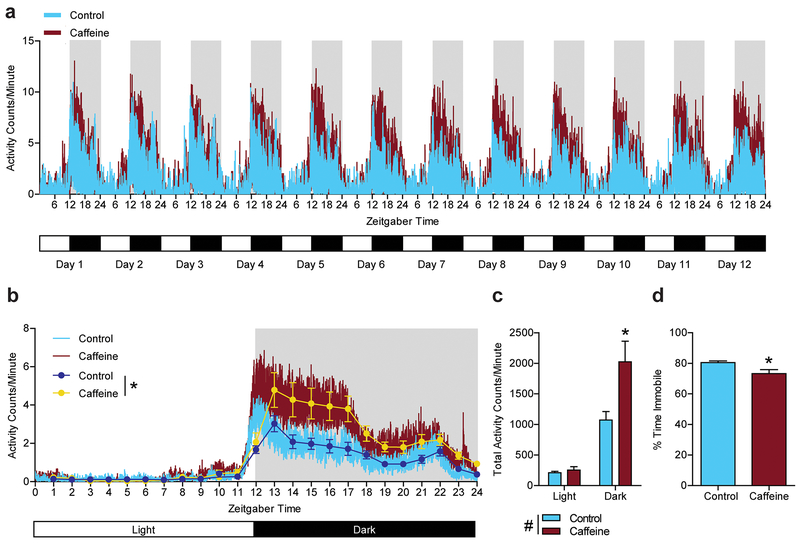
Fig. 1.
Increased home cage locomotor activity and reduced sleep during the dark cycle in caffeine drinking mice, (a) Activity per minute for 12 days in the home cage, (b) Representative single day activity; CAF treated mice (light brown = activity counts per minute; dark brown = activity counts per minute averaged every hour) were hyperactive during the dark cycle compared with control mice (light blue = activity counts per minute; dark blue = activity counts per minute averaged every hour), particularly between zeitgeber time 13 and 17. (#P <0.05 for main effect of treatment by two-way repeated measures ANOVA). (c) Total activity counts for dark cycle and light cycle. CAF treated mice exhibited significantly increased activity counts during the dark cycle compared to controls (#P <0.05 for main effect of treatment by two-way ANOVA; *P < 0.05 compared to control by Tukey’s post hoc test). (d) Percent time immobile was reduced in CAF treated mice (*P < 0.05 by student’s t-test). (n = 9 per treatment group; all data are presented as mean ± standard error of the mean (SEM).
2.2. Chronic caffeine consumption
At 4 weeks of age mice were individually housed and provided either a single water bottle for control subjects or a single bottle with 1mg/ml caffeine in tap water (CAF), which is considered a high caffeine condition in rodent studies [21]. Caffeine consumption was assessed by weighing bottles and mice weekly, at which time cages were also changed. In our primary cohort, caffeine treatment continued through behavioral testing for OFT, FST, home cage activity, PPI, EPM, and sucrose reward seeking tests. The first behavioral test occurred after 18 days of caffeine treatment. Caffeine treatment was discontinued for two-bottle choice to avoid acute effects of caffeine on ethanol consumption and mice remained untreated during taste preference assessment [19]. Therefore, for ethanol consumption and taste preference CAF animals have a history of caffeine consumption without acute caffeine effects. A second cohort of mice was treated with caffeine in the manner described above for four weeks before blood was collected for corticosterone measurement.
2.3. Home cage activity
Full time home cage activity was monitored for 12 consecutive days using overhead passive infrared [22] sensors connected to a Clocklab system (Coulbourn Instruments, Whitehall, PA) as previously described [23]. Immobile time was used as a proxy for sleep [24] and was defined as spending 1 min completely immobile, which is highly correlated with sleep in mice [25].
2.4. Open-field locomotor activity
Spontaneous locomotor activity was measured for one hour in Plexiglas chambers (26 cm × 26 cm) [26]. The house light (500 lux) was on during light cycle and off during dark cycle experiments. Center zone activity was determined by post hoc division into a central zone (13 × 13 cm; center equidistant from all four walls of the chamber) and a peripheral zone (the remaining area of the floor). Time spent in each area was calculated from the locomotor activity data.
2.5. Elevated plus maze
The elevated plus maze was used to assess anxiety-like behavior during the light cycle in mice as previously described [27]. Mice were placed in the center of the elevated plus maze, facing an open arm and facing away from the investigator. Mice were allowed to explore the elevated plus maze for 5 minutes. X–Y ambulatory movements were monitored using a video-tracking system (Viewpoint, Champagne-au-Mont-d’Or, France).
2.6. Corticosterone levels in serum
To determine relative stress levels due to caffeine treatment, corticosterone concentrations were measured using a Corticosteroid ELISA kit (Enzo Life Science, Inc.) during the light cycle. After 4 weeks of caffeine or water consumption serum samples from a separate group of mice that did not undergo behavioral testing were collected and analyzed per manufactures protocol.
2.7. Forced swim test
To assess depression-like behavior, we performed the forced swim test [28]. Briefly, each mouse was placed in a 2000 ml beaker filled with water (depth = 30 cm) for a total of 5 minutes. The duration of time spent swimming versus immobile (floating) was recorded by a condition blind observer. Lights were on for mice tested during the light cycle, while during the dark cycle overhead light was off and red light was used to enable video recording. The water was adjusted to 30°C to avoid causing stress or hypothermia.
2.8. Prepulse inhibition
Acoustic startle response was measured in sound attenuating chambers (SR-LAB; San Diego Instruments, San Diego, CA) as previously described [29]. Chambers were equipped with a loudspeaker and house lights (500 lux) that were on during light and off during dark cycle experiments. The percentage of acoustic PPI was calculated as: % PPI = 1 − [(prepulse + startle pulse)/(startle pulse alone)] × 100.
2.9. Sucrose seeking test
Sucrose reward seeking was examined in the light cycle only. Mice underwent food restriction to maintain their body weight at 85% of their free-feeding weight and exposed to a modified Pavlovian conditioning procedure (magazine training [30] where mice were allowed to retrieve a 20% sucrose liquid reward (sucrose seeking). Sucrose seeking was recorded in computer-controlled mouse operant chambers (Model ENV-307W; Med Associates Inc.) equipped with a rear 3W house light. Opposite the house light were a left and a right nose poke response holes, each of which was separated by a solution-dispensing magazine trough. The left and right nose poke response holes were equipped with cue lights, and responses to these apertures were recorded but did not have programmed consequences. For conditioning during baseline magazine training, a random time inter trial interval (on average 30 s in duration) preceded presentation of a tone (65dB, 1 s duration; ENV-323HAM, 4500Hz Sonalert, Med-Associates) was paired with a 1 s illumination of the left nose poke response hole cue light as well as simultaneous syringe pump activation which delivered10 μl/reward at the magazine trough. Responses on the non-illuminated, inactive right nose poke response hole were recorded but resulted in no consequences. On the following day, the sucrose seeking test was performed. This test session was similar to the baseline magazine training described above, with the exception that delivery of reward was now controlled via a differential reward of low rate (DRL) of behavior schedule [28]. Briefly, for DRL-controlled magazine training, detection of a premature head entry (ENV-302HD, Med-Associates) at the magazine trough during the ITI (on average 30 s in duration) and prior to signaled reward delivery (concurrent tone and illumination of the left nose poke hole), restarted the random time ITI and delayed reward delivery. For both baseline testing and the sucrose-seeking test, we quantified the number of total trough entries and this served as our measurement of reward seeking. For both baseline testing and the sucrose-seeking test, session termination occurred after 50 reward deliveries or 30 min, whichever occurred first. The magazine training program, nose poke hole cue lights, and syringe pump activations (Model PHM-100; Med Associates) were controlled by Med PC-IV (Med Associates).
2.10. Alcohol self-administration and taste preference
As illustrated in Fig. 1b, after mice were exposed to caffeine, alcohol self-administration and preference were examined using a two-bottle choice experiment [31]. Mice were given 24 h access to two bottles, one containing tap water and the other containing an ethanol solution. The concentration of ethanol was raised every fourth day, increasing from 3 to 6 to 10 to 15 to 20% (v/v) ethanol. One week after the ethanol self-administration experiment, the same mice were tested for saccharin sodium salt (sweet) and quinine hemisulphate salt (bitter) (Sigma, St. Louis, Missouri) solutions. The concentration of saccharin (0.03 and 0.06%) and quinine (30 and 60 μM) were raised every fourth day. The positions of the bottles were changed every 2 days to control for position preference. Average consumption per day was obtained for each ethanol concentration and taste solution. Evaporation and leakage were accounted for using 3 control cages with ethanol and water containing bottles but no mouse.
2.11. Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). Statistical testing was done using GraphPad Prism version 7 for OSx (La Jolla, CA) using unpaired two-tailed Student’s t-test, two-way repeated measures ANOVA followed by Tukey’s post hoc test, or two-way ANOVA followed by Tukey’s post hoc test as appropriate for the particular data. Criterion for statistical significance was P < 0.05.
3. Results
3.1. Increased home cage locomotor activity during the dark cycle in chronically caffeinated mice
Because caffeine is known to regulate sleep and circadian rhythm [32, 33], we first examined the effect of chronic caffeine on full time home cage locomotor activity and both treatment groups exhibited circadian-based locomotor activity across 12 days (Fig. 1a). When activity of mice was averaged every hour, CAF mice exhibited significantly increased activity counts during the dark cycle compared to controls, in particular between 13 and 17 zeitgeber time (ZT) (Fig. 1b; interaction, F(23,368) = 6.05; P < 0.0001, effect of treatment, F(1,16) = 7.55; P=0.029, effect of L/D cycle, F(23,16) = 55.96; P < 0.0001). When total activity counts per minute were averaged for light and dark cycles throughout the test, we found no significant effect of treatment during the light cycle, but during the dark cycle CAF mice were significantly more active than control mice and both CAF and control mice were significantly more active during the dark cycle than during the light cycle (Fig. 1c; interaction, F(1,16) = 6.30; P = 0.011, effect of treatment, F(1,16)= 7.55; P = 0.029, effect of L/D cycle, F(1,16)= 52.80; P < 0.0001). We also used duration of immobility (≥ 1 min) as a proxy to evaluate sleep disturbance with caffeine exposure, as immobility is highly correlated with EEG/EMG assessed sleep in mice [23]. Although we didn’t observe a difference in light cycle activity (Fig. 1c), CAF mice had a significant reduction in percent time immobile during home cage monitoring and therefore likely had reduced sleep (Fig. 1d; t(16)= 2.63; P = 0.018).
Before monitoring home cage activity, we examined liquid drinking to determine caffeine consumption. During their 4th week of caffeine, the presence of caffeine in the water of CAF mice did not inhibit drinking; CAF mice consumed the same amount of fluid as mice drinking water (control), but they weighed less (Fig. 2). With a concentration of 1mg/ml caffeine in drinking water, CAF mice consumed approximately 150 mg/kg/day of caffeine, which has been associated with approximately 10-13 μM plasma caffeine concentration [34], which is similar to plasma caffeine concentrations following a single 160 mg dose in adult humans [35].
Fig. 2.
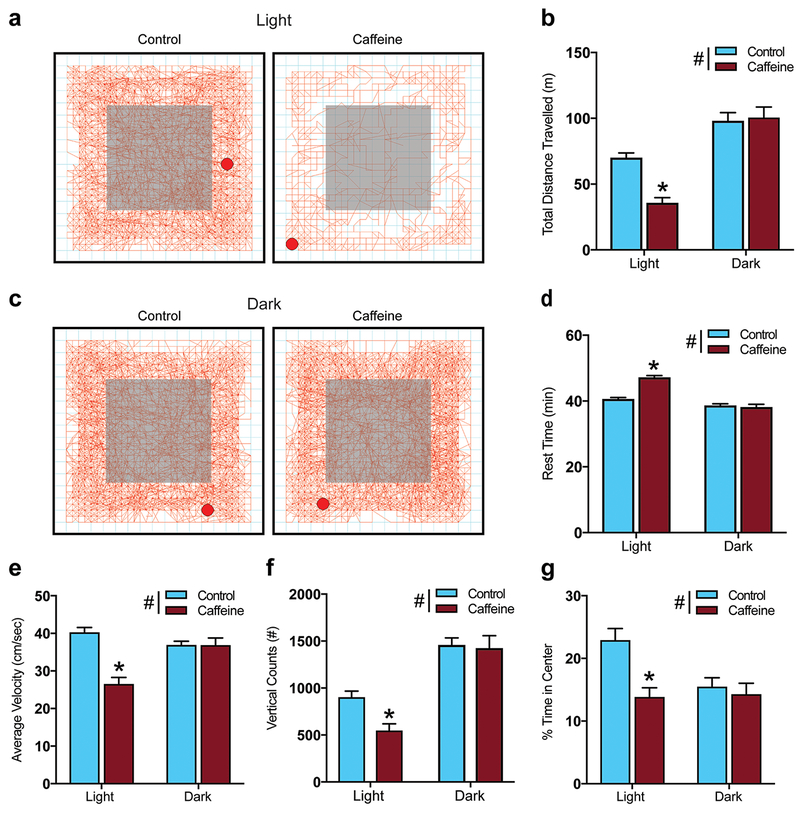
Impaired locomotor activity and increased anxiety during the light cycle, but not dark cycle, following chronic caffeine treatment. (a) Representative activity traces in an open-field chamber during the light cycle with center zone shaded in grey. (b) In an open field test CAF treated mice had decreased total distance traveled in the light cycle, but no difference in the dark cycle. (c) Representative activity traces in an open-field chamber during the dark cycle with center zone shaded in grey. (d) The amount of time spent not moving (rest time) was not affected by CAF treatment. (e) Average velocity in the open-field test showed that CAF treated mice moved at a significantly lower average velocity during the light cycle with no difference during the dark cycle. (f) The number of vertical counts, which is a measure of exploratory behavior, showed a significant decrease with CAF treatment during the light, but not the dark cycle. (g) CAF treated mice spent significantly less time in the center zone of the open field chamber than control mice, an anxiety-like behavior, during the light cycle with no difference during the dark cycle. (Light cycle, control n=15, CAF n=14; for the dark cycle, control n=7, CAF n=8. #P<0.05 for main effect of treatment by two-way ANOVA; *P < 0.05 compared to control by Tukey’s post hoc test; all data are presented as mean ± standard error of the mean (SEM).
3.2. Reduced locomotor activity and exploratory behavior in a novel environment during the light cycle, but not the dark cycle in mice chronically consuming caffeine in adolescence
To examine if caffeine exposure during adolescence altered locomotor activity in a novel environment after 18 days of treatment mice were subjected to an open-field chamber for 1 hour during either the dark cycle or light cycle. We found that CAF mice had significantly shorter total distance traveled than control mice during the light cycle (P < 0.0001), but during the dark cycle there was no significant effect of treatment; both control and CAF mice had significantly greater total distance traveled during the dark cycle than in the light cycle (control, P = 0.0049; CAF, P < 0.0001) (Fig. 2b; interaction, F(1, 40) = 10.73; P = 0.0021, effect of treatment, F(1,40) = 7.937; P = 0.0074, effect of L/D cycle, F(1,40) = 67.83; P < 0.0001). This corresponded to lower average velocity in CAF than control mice during the light cycle (P < 0.0001), with no difference with treatment during the dark cycle. CAF (P = 0.001) had lower velocity during the light cycle than the dark cycle, while control mice did not differ in velocity by light dark cycle (Fig. 2e; interaction, F(1, 40) = 14.53; P = 0.0005, effect of treatment, F(1, 40) = 14.8; P = 0.0004, effect of L/D cycle, F(1,40) = 3.753; P = 0.0598). Furthermore, during the light cycle CAF mice spent significantly more time at rest than control mice (P < 0.0001), whereas during the dark cycle there was no significant effect of treatment on rest time (Fig. 2d; interaction, F(1, 40) = 19.97; P < 0.0001, effect of treatment, F(1, 40) = 14.85; P = 0.0004, effect of L/D cycle, F(1, 40) = 47.94; P < 0.0001). Rearing (vertical counts) were used as a measure of exploratory behavior in the novel environment of the OFT [36]. We found significant main effects of treatment and light dark cycle on vertical counts, without a significant interaction. CAF mice had significantly fewer vertical counts than controls in the light cycle (P = 0.0109), with no significant difference during the dark cycle and both control (P = 0.0009) and CAF (P < 0.0001) mice had significantly more vertical counts during the dark (Fig. 2f; interaction, F(1,40) = 2.994; P = 0.0913, effect of treatment, F(1, 40) = 4.424; P = 0.0415, effect of L/D cycle, F(1, 40) = 59.38; P < 0.0001). As 18 days into treatment animals are still in their adolescence, a second naive cohort of mice who consumed caffeine or water control for 20 weeks beginning at 4 weeks of age underwent OFT. At that time, there were no differences with caffeine treatment on total distance traveled, average velocity, or rest time (Fig. 4a–c). However, there was still a significant main effect of treatment on vertical counts (Fig. 4d; effect of treatment, F(1, 16) = 9.76; P = 0.0065). The maintained inhibition of exploratory behavior in animals after 20 weeks of caffeine, despite the development of tolerance to the locomotor effects of caffeine, suggests that the observed difference in locomotor activity in our main cohort is not entirely due to reduced exploratory behavior.
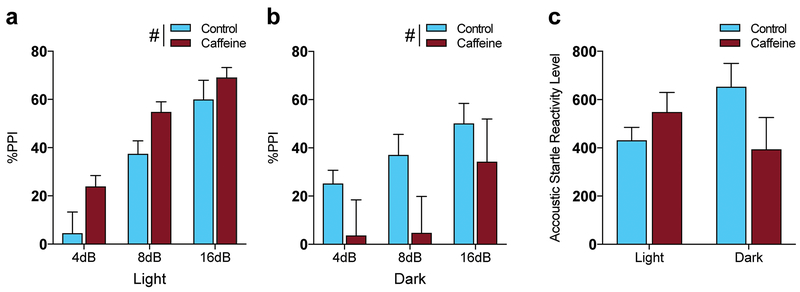
Fig. 4.
Circadian dependent effects of caffeine on prepulse inhibition, (a) During the light cycle %PPI increased as the volume in decibels increased and was significantly higher in CAF than control mice (#P <0.05 for main effect of treatment by two-way ANOVA; n=15 per treatment), (b) During the dark cycle %PPI was significantly reduced in CAF mice (#P < 0.05 for main effect of treatment by two-way ANOVA: control n=9, CAF n=8). (c) Acoustic startle reactivity was not significantly different with CAF treatment or between light and dark cycle.
3.3. Increased anxiety-like behavior during the light cycle in mice chronically consuming caffeine during the light cycle
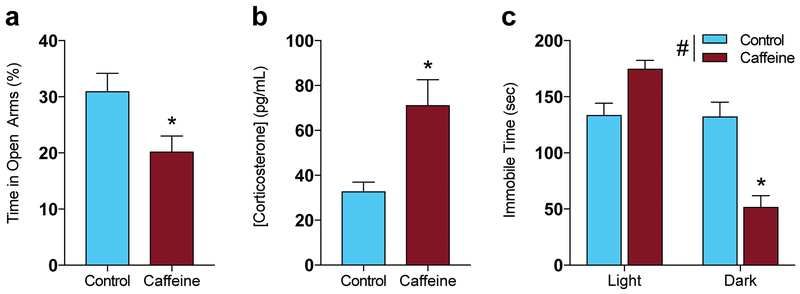
Anxiety-like behavior was assessed by quantifying the percent time that mice spent in the center zone during the open field test. CAF mice spent significantly less time in the center zone than controls during the light cycle (Fig. 2a, Fig. 2g; P = 0.0013) without a significant difference during the dark cycle (Fig. 2b, Fig. 2g; interaction, F(1, 40) = 4.205; P = 0.0469, effect of treatment, F(1,40) = 7.141; P = 0.0108, F(1, 40) = 3.318; P = 0.076). This suggests that CAF mice have higher anxiety than controls during the light cycle. We therefore used the elevated plus maze to confirm this light cycle anxiety-like behavior. CAF mice spent a significantly lower percent of their time in the open arms than controls (Fig. 3a; t(26) = 2.495; P = 0.0193), further suggesting that CAF mice are more anxious than controls during the light cycle. CAF mice also exhibited higher plasma corticosterone concentration during the light cycle than control mice (Fig. 3b; t(8) = 3.186; P = 0.0129).
Fig. 3.
Circadian dependent changes in mood with caffeine treatment, (a) CAF mice spend a lower percent of their time in the open arms of the elevated plus maze than water mice during the light cycle, suggesting increased anxiety (*P < 0.05 by student’s t-test; control n=15, CAF n=13). (b) Plasma corticosterone measured during the light cycle was significantly higher in CAF than in control mice (*P < 0.05 by student’s t-test; n=5 per group), (c) Immobility time in the forced swim test. Mice who received CAF treatment are not significantly different from control mice during the light cycle, but spend significantly less time immobile during the dark cycle (#P <0.05 for main effect of treatment; *P < 0.05 compared to control by Tukey’s post hoc test; control n=10 and CAF n=9 for light and dark cycle).
3.4. Reduced depressive-like behavior only during the dark cycle in mice exposed to chronic caffeine
To examine depression-like behavior, we conducted the forced swim test. Interestingly, there was no difference with treatment during the light cycle, but CAF mice spent significantly less time immobile than control mice during the dark cycle(P = 0.0005), indicating that CAF mice have reduced depressive-like behavior during the dark cycle (Fig. 3c; interaction, F(1,34) = 15.52; P = 0.0004, effect of treatment, F(1,34) = 5.698; P = 0.0227, effect of L/D cycle, F(1,34) = 21.11; P < 0.0001).
3.5. Reduced prepulse inhibition of the startle response during the dark cycle in mice chronically consuming caffeine
Next, we evaluated sensorimotor gating during the light and dark cycle in response to chronic caffeine using prepulse inhibition (PPI) [21]. During the light cycle, there were significant main effects of treatment and stimulus decibel, with no significant interaction; CAF mice had significantly higher %PPI than controls with no one decibel at which the difference between CAF and control was significant by post hoc analysis. Both CAF (P = 0.008) and control (P = 0.0038) mice exhibited significantly higher %PPI at 8dB than at 4dB (Fig. 4a; effect of treatment, F(1, 84) = 9.282; P = 0.0031, effect of decibel, F(2, 84) = 34.26; P < 0.0001). During the dark cycle, CAF mice exhibited a deficit in %PPI compared to control mice, with a significant main effect of treatment, without significant main effect of decibel, or interaction (Fig. 4b; effect of treatment, F(1, 45) = 5.608; P = 0.0222). There were no significant differences in baseline acoustic startle reactivity and therefore this did not contribute to observed differences in %PPI (Fig. 4c).
3.6. Chronic caffeine increases reward seeking behavior while mice are consuming caffeine
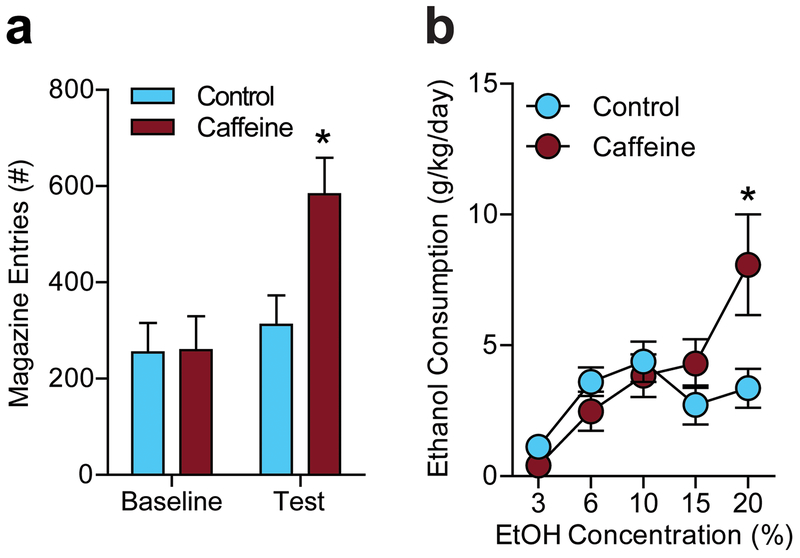
We assessed the effect of chronic caffeine use beginning in adolescence on reward seeking behavior (magazine entries) during the light cycle. CAF mice exhibited significantly more magazine entries during the test session (P = 0.0318) than control mice, with no difference during the baseline session (Fig. 5a; interaction, F(1,28) = 10.39; P = 0.0032, effect of treatment, F(1,28) = 2.547; P = 0.1217, effect of session, F(1,28) = 21.32; P < 0.0001). This indicates increased reward seeking for a sucrose reward in CAF mice compared with controls.
Fig. 5.
Caffeine treated mice display enhanced reward responses, (a) CAF mice had significantly more trough entries during a test session (*P < 0.05 compared to control by Tukey’s post hoc test; n=15 per treatment), (b) CAF treated subjects consumed significantly more ethanol than control mice, which was pronounced at higher ethanol (EtOH) concentrations (#P <0.05 for main effect of treatment by two-way ANOVA; *P < 0.05 compared to control by Tukey’s post hoc test; control n=8, CAF n=7).
3.7. Chronic caffeine exposure increases ethanol consumption in the absence of caffeine
After 34 weeks (241 days) of caffeine treatment, as depicted in Fig. 1b, we sought to assess ethanol drinking in CAF and control mice. Because acute caffeine is known to promote ethanol consumption in mice and humans, caffeine access was removed during this behavioral test [20, 37]. Using a two-bottle choice experiment [26, 38], we found no significant main effect of treatment, but significant interaction and effect of ethanol concentration. CAF mice drank more 20% ethanol than control mice (P < 0.05) in the absence of acute caffeine (Fig. 5b; interaction, F(4,52) = 13.88; P = 0.002, effect of ethanol concentration, F(4,13) = 30.19; P < 0.0001). It is unlikely that taste preference accounts for this difference in consumption, as CAF and control mice did not display differences in preference for sweet or bitter tasting solutions (Fig. 3).
4. Discussion
In the present study, we found that chronic caffeine exposure during adolescence promotes diurnal mood-cycling in adulthood. To our knowledge, this is the first study to show that beginning chronic caffeine consumption in adolescences results in diurnal, biphasic mood-cycling. During the light cycle CAF mice exhibited reduced exploratory behavior and increased anxiety-like behavior as adults. Corticosterone was also elevated in CAF mice during the light cycle, corresponding to increased anxiety-like behavior in both the OFT and EPM. Social isolation stress from early separation to single housing alone has been shown to decrease plasma corticosterone at this point in the light-dark cycle [39] and therefore our observed increase is likely due to caffeine consumption. Notably, CAF mice showed a set of behaviors during the dark cycle that are considered manic-like in rodents: hyperactivity, reduced sleep, reduced depressive-like behavior, reduced sensitivity to prepulse inhibition of the startle response and increased alcohol consumption after cessation of caffeine use [40].
This study focused on the effect of chronic caffeine beginning in adolescence alters adult behavior, but how do these findings compare with adult caffeine consumption? Caffeine’s disruptive influence on sleep, especially its influence on sleep duration and total sleep time, are well established [41]. In our study, we observed this disruption as a decrease in home cage percent time spent immobile (sleeping) for caffeine exposed animals (Fig. 1D). Reduced exploratory behavior and locomotor activity in a novel environment during the light cycle in mice that began caffeine consumption at four weeks is mirrored by previous work with adult mice, in both a time and dose dependent fashion. Mice infused with 97 mg/kg/day caffeine for six days showed reduced horizontal activity counts and reduced vertical counts, but no change in total distance traveled during the light cycle [42]. However, a study on oral caffeine consumption beginning in adolescence in rats found no effect of caffeine consumption (1mg/ml solution) on locomotor activity during the open field test [21]. The absence of a locomotor effect of caffeine in this study could be due to experimental differences, such as housing conditions [43]. Additionally, we must bear in mind that isolation rearing decreases exploratory behavior when comparing these data to studies of caffeine consumption in adult mice [44].
The effect of caffeine on mood has been studied extensively. Caffeine consumption has a well-known anxiogenic effect, while its role in depression is less well understood [41]. Consistent with previous evidence in both adolescent rats [21] and adult mice [45], we found that chronic oral caffeine administration increases anxiety-like behavior during the light cycle. We found reduced depressive-like behavior in caffeine exposed mice during the dark cycle, but not the light cycle. Interestingly, others have reported that caffeine has an antidepressant effect during the light cycle [46–48]. Additional research will disentangle the contributions of acute and chronic caffeine intake on depressive-like behavior in a circadian dependent manner.
Although acute caffeine treatment is known to either attenuate PPI at low doses or potentiate PPI at a high dose, chronic caffeine does not alter sensorimotor gating [49]. In contrast, we found that beginning chronic caffeine in adolescence altered sensorimotor gating in adulthood, with a significant increase in PPI during the light cycle and a significant decrease during the dark cycle. Therefore, our finding of a biphasic effect of caffeine on PPI, with enhanced PPI during the light cycle and attenuated PPI in the dark cycle, may be attributable to a combination of delayed tolerance to caffeine in adolescent mice as observed in OFT (Fig. 2 and Fig. 4) and diurnal variation in caffeine drinking. Future studies are needed to parse out the role of plasma caffeine level and duration of treatment on sensorimotor gating in animals that begin caffeine use during adolescence.
With the introduction of energy drinks and the prevalence of mixing energy drinks and alcohol, there was significant interest in the question of how caffeine consumption influences ethanol consumption [37]. We found a significant effect of a history of adolescent caffeine use on ethanol consumption in a two-bottle choice experiment in the absence of caffeine. While a previous study found no effect of previous adolescent chronic caffeine administration on ethanol consumption in two-bottle choice when caffeine was not provided during two-bottle choice, this study used 10 day caffeine access beginning on post natal day 40, while we provided extended caffeine exposure beginning at post natal day 28 [50]. This distinction may indicate either the importance of continued caffeine use throughout adolescence or a key role of exposure during an earlier developmental period with highly active dendritic spine development for future effects on ethanol consumption [51]. Further study is warranted to investigate whether the timing of adolescent caffeine exposure, the duration of use, or an interaction between the two are critical for increased ethanol consumption at a later time.
Although this diurnal mood-cycling does not recapitulate human bipolar disorders, these apparent circadian mood fluctuations in mice could be developed as a model for rapid mood-cycling or personality disorders. Interestingly, a study using a mouse model with mutated Clock gene (ClockΔ19) found disruptions in circadian rhythms that resulted in mood-cycling with a manic-like phenotype emerging during the light cycle and an euthymic phenotype during the dark cycle [52]. This is opposite to our finding with chronic caffeine, as our model presents with behaviors associated with a manic-like state during the dark cycle and depressive-like and euthymic behavior during the light cycle. In adulthood, CAF mice had increased reward seeking behavior as well as increased alcohol consumption in the absence of acute caffeine use. Caffeine exerts most of its neuropsychotic function through A2AR-regulated signaling [53, 54]. It is also known to increase dopamine and glutamate levels by inhibiting presynaptic adenosine A1R in the shell region of the nucleus accumbens [55]]. Consistently, a genetic variant of A2AR (rs5751876) is associated with caffeine response [56]. Interestingly, caffeine increases dopamine release and enhances D2R function [57]. Since hyper-dopaminergic activity is one of most commonly known neurobiological factors for psychosis [58]. our data showing the fluctuation of prepulse inhibition in response to caffeine may explain diurnal mood swing in mice or provide a possible link between adolescent caffeine abuse and psychosis [59].
In summary, our findings demonstrate that adolescent mice exposed to chronic caffeine beginning at four weeks of age exhibit diurnal mood fluctuations, with caffeine altering locomotor activity, exploratory behavior, anxiety-like behavior, depressive-like behavior, and sensorimotor gating in a light dark cycle dependent manner. Beginning caffeine in adolescence also increased reward seeking for a sucrose reward as adults and increased ethanol drinking in the absence of acute caffeine.
Acknowledgements
This work was supported by the Samuel C. Johnson for Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, the Godby Foundation, David Lehr Research Award from American Society for Pharmacology and Experimental Therapeutics, and National Institute on Alcohol Abuse and Alcoholism (AA018779).
Footnotes
Disclosures
Dr. DS Choi is a scientific advisory board member to Peptron Inc. and the Peptron had no role in preparation, review, or approval of the manuscript; nor decision to submit the manuscript for publication. All the other authors declare no biomedical financial interests or potential conflicts of interest.
References
- [1].Branum AM, Rossen LM, Schoendorf KC, Trends in caffeine intake among U.S. children and adolescents, Pediatrics 133(3) (2014) 386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Muller CE, Rodrigues AL, Porciuncula LO, Chen JF, Tome AR, Agostinho P, Canas PM, Cunha RA, Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress, Proc Natl Acad Sci U S A 112(25) (2015) 7833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kristjansson AL, Sigfusdottir ID, Frost SS, James JE, Adolescent caffeine consumption and self-reported violence and conduct disorder, J Youth Adolesc 42(7) (2013) 1053–62. [DOI] [PubMed] [Google Scholar]
- [4].Pollak CP, Bright D, Caffeine consumption and weekly sleep patterns in US seventh-, eighth-, and ninth-graders, Pediatrics 111(1) (2003) 42–6. [DOI] [PubMed] [Google Scholar]
- [5].O’Neill CE, Levis SC, Schreiner DC, Amat J, Maier SF, Bachtell RK, Effects of adolescent caffeine consumption on cocaine sensitivity, Neuropsychopharmacology 40(4) (2015) 813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wikoff D, Welsh BT, Henderson R, Brorby GP, Britt J, Myers E, Goldberger J, Lieberman HR, O’Brien C, Peck J, Tenenbein M, Weaver C, Harvey S, Urban J, Doepker C, Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children, Food Chem Toxicol (2017). [DOI] [PubMed] [Google Scholar]
- [7].Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ, The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats, Neuroscience 123(2) (2004) 361–70. [DOI] [PubMed] [Google Scholar]
- [8].Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW, Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness, Science 276(5316) (1997) 1265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Antle MC, Steen NM, Mistlberger RE, Adenosine and caffeine modulate circadian rhythms in the Syrian hamster, Neuroreport 12(13) (2001) 2901–5. [DOI] [PubMed] [Google Scholar]
- [10].Sigworth LA, Rea MA, Adenosine A1 receptors regulate the response of the mouse circadian clock to light, Brain Res 960(1 −2) (2003) 246–51. [DOI] [PubMed] [Google Scholar]
- [11].Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG, Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss, Neuron 61(2) (2009) 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McClung CA, How might circadian rhythms control mood? Let me count the ways, Biol Psychiatry 74(4) (2013) 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang HR, Woo YS, Bahk WM, Caffeine-induced psychiatric manifestations: a review, Int Clin Psychopharmacol 30(4) (2015) 179–82. [DOI] [PubMed] [Google Scholar]
- [14].Ciapparelli A, Paggini R, Carmassi C, Taponecco C, Consoli G, Ciampa G, Ramacciotti CE, Marazziti D, Dell’Osso L, Patterns of caffeine consumption in psychiatric patients. An Italian study, Eur Psychiatry 25(4) (2010) 230–5. [DOI] [PubMed] [Google Scholar]
- [15].Persad LA, Energy drinks and the neurophysiological impact of caffeine, Front Neurosci 5 (2011) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE, Health effects of energy drinks on children, adolescents, and young adults, Pediatrics 127(3) (2011) 511–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Warzak WJ, Evans S, Floress MT, Gross AC, Stoolman S, Caffeine consumption in young children, J Pediatr 158(3) (2011) 508–9. [DOI] [PubMed] [Google Scholar]
- [18].Turton P, Piche L, Battram DS, Adolescent Attitudes and Beliefs Regarding Caffeine and the Consumption of Caffeinated Beverages, J Nutr Educ Behav 48(3) (2016) 181–9 e1. [DOI] [PubMed] [Google Scholar]
- [19].Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J, Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat, Behav Brain Res 152(2) (2004) 279–95. [DOI] [PubMed] [Google Scholar]
- [20].Fritz BM, Quoilin C, Kasten CR, Smoker M, Boehm SL 2nd, Concomitant Caffeine Increases Binge Consumption of Ethanol in Adolescent and Adult Mice, But Produces Additive Motor Stimulation Only in Adolescent Animals, Alcohol Clin Exp Res 40(6) (2016) 1351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ardais AP, Borges MF, Rocha AS, Sallaberry C, Cunha RA, Porciuncula LO, Caffeine triggers behavioral and neurochemical alterations in adolescent rats, Neuroscience 270 (2014) 27–39. [DOI] [PubMed] [Google Scholar]
- [22].Greenberg BD, Gabriels LA, Malone DA Jr., Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ, Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience, Mol Psychiatry 15(1) (2010) 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vadnie CA, Hinton DJ, Choi S, Choi Y, Ruby CL, Oliveros A, Prieto ML, Park JH, Choi DS, Activation of neurotensin receptor type 1 attenuates locomotor activity, Neuropharmacology 85 (2014) 482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fisher SP, Godinho SI, Pothecary CA, Hankins MW, Foster RG, Peirson SN, Rapid assessment of sleep-wake behavior in mice, J Biol Rhythms 27(1) (2012) 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, Svenson K, Peters LL, Novel method for high-throughput phenotyping of sleep in mice, Physiol Genomics 28(2) (2007) 232–8. [DOI] [PubMed] [Google Scholar]
- [26].Lee MR, Hinton DJ, Unal SS, Richelson E, Choi DS, Increased ethanol consumption and preference in mice lacking neurotensin receptor type 2, Alcohol Clin Exp Res 35(1) (2011) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, Choi DS, The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice, Genes Brain Behav 6(8) (2007) 776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ruby CL, Walker DL, An J, Kim J, Choi DS, Sex-Specific Regulation of Depression, Anxiety-Like Behaviors and Alcohol Drinking in Mice Lacking ENT1, J Addict Res Ther S4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vadnie CA, Ayers-Ringler J, Oliveros A, Abulseoud OA, Choi S, Hitschfeld MJ, Choi DS, Antipsychotic-like effects of a neurotensin receptor type 1 agonist, Behav Brain Res 305 (2016) 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oliveros A, Cho CH, Cui A, Choi S, Lindberg D, Hinton D, Jang MH, Choi DS, Adenosine A2A receptor and ERK-driven impulsivity potentiates hippocampal neuroblast proliferation, Transl Psychiatry 7(4) (2017) e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee MR, Hinton DJ, Wu J, Mishra PK, Port JD, Macura SI, Choi DS, Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1, Neurosci Lett 490(2) (2011) 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brezinova V, Effect of caffeine on sleep: EEG study in late middle age people, Br J Clin Pharmacol 1(3) (1974) 203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, Achermann P, Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans, Neuropsychopharmacology 29(10) (2004) 1933–9. [DOI] [PubMed] [Google Scholar]
- [34].Kuzmin A, Johansson B, Semenova S, Fredholm BB, Differences in the effect of chronic and acute caffeine on self-administration of cocaine in mice, Eur J Neurosci 12(8) (2000) 3026–32. [DOI] [PubMed] [Google Scholar]
- [35].White JR Jr., Padowski JM, Zhong Y, Chen G, Luo S, Lazarus P, Layton ME, McPherson S, Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults, Clin Toxicol (Phila) 54(4) (2016) 308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lever C, Burton S, O’Keefe J, Rearing on hind legs, environmental novelty, and the hippocampal formation, Rev Neurosci 17(1-2) (2006) 111–33. [DOI] [PubMed] [Google Scholar]
- [37].Ferre S, O’Brien MC, Alcohol and Caffeine: The Perfect Storm, J Caffeine Res 1(3) (2011) 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi DS, Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice, Pharmacol Biochem Behav 95(2) (2010) 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harvey BH, Regenass W, Dreyer W, Möller M, Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: Role of corticosterone, oxytocin, and vasopressin, J Psychopharmacol (2019) 269881119826783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Young JW, Henry BL, Geyer MA, Predictive animal models of mania: hits, misses and future directions, Br J Pharmacol 164(4) (2011) 1263–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE, Actions of caffeine in the brain with special reference to factors that contribute to its widespread use, Pharmacol Rev 51 (1) (1999) 83–133. [PubMed] [Google Scholar]
- [42].Kaplan GB, Greenblatt DJ, Kent MA, Cotreau-Bibbo MM, Caffeine treatment and withdrawal in mice: relationships between dosage, concentrations, locomotor activity and A1 adenosine receptor binding, J Pharmacol Exp Ther 266(3) (1993) 1563–72. [PubMed] [Google Scholar]
- [43].Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA, Adolescent social isolation increases cocaine seeking in male and female mice, Behav Brain Res 359 (2019) 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arakawa H, Interaction between isolation rearing and social development on exploratory behavior in male rats, Behav Processes 70(3) (2005) 223–34. [DOI] [PubMed] [Google Scholar]
- [45].El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM, The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists, Psychopharmacology (Berl) 148(2) (2000) 153–63. [DOI] [PubMed] [Google Scholar]
- [46].Kale PP, Addepalli V, Augmentation of antidepressant effects of duloxetine and bupropion by caffeine in mice, Pharmacol Biochem Behav 124 (2014) 238–44. [DOI] [PubMed] [Google Scholar]
- [47].Poleszak E, Szopa A, Wyska E, Kukula-Koch W, Serefko A, Wosko S, Bogatko K, Wrobel A, Wlaz P, Caffeine augments the antidepressant-like activity of mianserin and agomelatine in forced swim and tail suspension tests in mice, Pharmacol Rep 68(1) (2016) 56–61. [DOI] [PubMed] [Google Scholar]
- [48].Szopa A, Doboszewska U, Herbet M, Wosko S, Wyska E, Swiader K, Serefko A, Korga A, Wlaz A, Wrobel A, Ostrowska M, Terlecka J, Kanadys A, Poleszak E, Dudka J, Wlaz P, Chronic treatment with caffeine and its withdrawal modify the antidepressant-like activity of selective serotonin reuptake inhibitors in the forced swim and tail suspension tests in mice. Effects on Comt, Slc6a15 and Adora1 gene expression, Toxicol Appl Pharmacol 337 (2017) 95–103. [DOI] [PubMed] [Google Scholar]
- [49].Dubroqua S, Yee BK, Singer P, Sensorimotor gating is disrupted by acute but not chronic systemic exposure to caffeine in mice, Psychopharmacology (Berl) 231(21) (2014) 4087–98. [DOI] [PubMed] [Google Scholar]
- [50].Robins MT, DeFriel JN, van Rijn RM, Adolescent intake of caffeinated energy drinks does not affect adult alcohol consumption in C57BL/6 and BALB/c mice, Alcohol 54 (2016) 1–9. [DOI] [PubMed] [Google Scholar]
- [51].Mallya AP, Wang HD, Lee HNR, Deutch AY, Microglial Pruning of Synapses in the Prefrontal Cortex During Adolescence, Cereb Cortex (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF 3rd, Jacobsen JP, Kumar S, Remillard EM, Caron MG, Deisseroth K, McClung CA, Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice, Mol Psychiatry 20(11) (2015) 1406–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Holtzman SG, Caffeine as a model drug of abuse, Trends Pharmacol Sci 11(9) (1990) 355–6. [DOI] [PubMed] [Google Scholar]
- [54].Alsene K, Deckert J, Sand P, de Wit H, Association between A2a receptor gene polymorphisms and caffeine-induced anxiety, Neuropsychopharmacology 28(9) (2003) 1694–702. [DOI] [PubMed] [Google Scholar]
- [55].Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR, Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens, J Neurosci 22(15) (2002) 6321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, Evershed RP, Deckert J, Nutt DJ, Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35(9) (2010) 1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Volkow ND, Wang GJ, Logan J, Alexoff D, Fowler JS, Thanos PK, Wong C, Casado V, Ferre S, Tomasi D, Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain, Transl Psychiatry 5 (2015) e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kesby JP, Eyles DW, McGrath JJ, Scott JG, Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience, Transl Psychiatry 8(1) (2018) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Winston A, Hardwick E, Jaberi N, Neuropsychiatric effects of caffeine, Advances in Psychiatric Treatment 11(6) (2005) 432–439. [Google Scholar]