Abstract
Background
This study aimed to explore whether statins reduce radiation‐induced vascular complications in cancer patients postradiotherapy to the thorax, head, and neck.
Methods and Results
We conducted a retrospective cohort study within a provincial linked database of 5718 cardiac patients with thorax and head or neck cancer having undergone radiotherapy between 2000 and 2011. One thousand five hundred fifty‐two patients were identified as nonstatin users and 4166 as statin users. The primary outcome of interest was the composite of cerebrovascular (transient ischemic attack, and fatal or nonfatal stroke) or cardiovascular events (fatal or nonfatal myocardial infarction). Time‐dependent Cox proportional hazard analyses were performed. The crude event rate was 10.31% for nonusers and 9.03% for statin users (hazard ratio of 0.92 [95% CI 0.76–1.10, P=0.3451]), over a mean time to event/censoring of 534±687 days for nonusers and 594±706 days for the statin users. After adjusting for age, sex, prior history of stroke/transient ischemic attack or myocardial infarction, diabetes mellitus, dyslipidemia, atrial fibrillation, chronic kidney disease, heart failure, and hypertension, statin use postradiotherapy was associated with a nonsignificant 15% relative risk reduction, but a strong trend toward reducing the primary outcome (hazard ratio=0.85 95% CI 0.69–1.04, P=0.0811). The use of statins was associated with a significant reduction of 32% for the outcome of stroke alone (hazard ratio=0.68, 95% CI 0.48–0.98, P=0.0368).
Conclusions
Statin use post radiation therapy was associated with a significant reduction in stroke, with a trend toward significantly reducing cardiovascular and cerebrovascular events.
Keywords: cancer and stroke, myocardial infarction, radiotherapy, statin
Subject Categories: Cardiovascular Disease, Risk Factors, Vascular Disease, Lipids and Cholesterol, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
Radiation‐induced atherosclerosis is a risk factor for cardiovascular and cerebrovascular events.
There are no studies exploring whether statins reduce vascular complications in cancer patients post thorax, head, and neck radiotherapy.
In this retrospective cohort study of cardiac patients post radiotherapy, we demonstrate that statin use was associated with a significant 32% reduction in stroke, and a strong trend toward reducing the composite outcome of cardiovascular and cerebrovascular events.
What Are the Clinical Implications?
This raises the need for prospective randomized controlled trials to definitively establish the benefit of statins in this at‐risk population and to define guidelines on the management of radiation‐induced vascular disease.
Radiation therapy (RT) remains an effective treatment for many different types of cancers. However, the net clinical benefit of RT may be attenuated by an increased risk of cardiovascular and cerebrovascular disease (CVD) associated with RT exposure. Cardiovascular disease is the leading cause of morbidity and mortality among cancer survivors.1 In one of the larger observational studies of childhood cancer survivors, the relative risk for cardiac death was 3.1 times that expected for the general population (CI, 2.4–3.7).2 In addition to cardiovascular death, RT has also been associated with increased risk of ischemic stroke, transient ischemic attack, carotid stenosis, and general carotid injury post cervical irradiation.3, 4, 5, 6 The mechanism of RT‐induced vascular disease is incompletely understood, but appear to be independent of conventional vascular risk factors with data suggesting a key role for inflammation and accelerated atherosclerosis.7, 8 Furthermore, the pattern of plaque generation within affected arteries (ie, carotid and coronary arteries) appears to be distinct from that generated solely by traditional atherosclerosis.3, 8, 9, 10 There is extensive literature illustrating the vascular impact of statin therapy in both primary and secondary prevention of cardiovascular diseases and CVD among the general population.11 Additionally, statins reduce inflammation. However, despite this plausibility, no study has been conducted in humans addressing whether statin therapy reduces the risk of vascular events after RT, particularly among patients with thorax, head, and neck cancers. Therefore, the aim of this observational study was to test whether statins are protective against vascular events among patients who have received RT to the thorax, head, and neck.
Methods
Data Sources
The data, analytic methods, or study materials will not be available to other researchers, given that the government agencies who released the database to us do not allow data sharing, even for this anonymized information.
The administrative, computerized health insurance databases of Quebec constituted the primary data source. All Quebec residents have access to universal care, and the medical and drug services database was merged with the vital status and hospitalization databases by means of a unique anonymous identifier. These databases are now widely used for research purposes and provide suitable clinical granularity (with unlimited procedural codes) to allow for substantial control of confounding variables. The pharmaceutical database has been evaluated and its validity was confirmed.12 Moreover, validity studies have been done for several medical services claims.13, 14 Approval for the construction and use of the database was granted by the McGill University Health Center ethics board and the Quebec government agency responsible for privacy of access. Given that patient data were collected and tracked anonymously, individual informed consent was not required.
Study Patient Population
The study population was derived from a sample of the total Quebec population. Specifically, we had a data set of 380 000 patients >45 years old who had coronary angiography, acute coronary syndrome, or undergone a coronary revascularization procedure—either percutaneous angioplasty or coronary artery bypass surgery—between August 2000 and March 2011. Only patients >65 years old were included in this study as only this subset has access to universal drug coverage and hence, documentation of a prescribed medication list. From this study population, all patients with thorax and head or neck cancer having undergone radiotherapy were identified using International Classification of Diseases (ICD), Ninth and Tenth Revision codes and were included in the present study (Table S1). Baseline demographic data were collected, including diagnosis, medical comorbidities, and age on entry into the cohort (Table S2).
Ascertainment of Radiation and Statin Exposure
For each patient in the cohort, all healthcare medical visits and exposure to head, neck, and mediastinal radiotherapy were identified (Table S1).15, 16, 17 Because this database does not include treatments administered in the hospital, RT exposure was also derived from physicians’ billing codes, by identifying corresponding procedure codes for RT. Exposure to statins was measured as the first statin prescription starting from 1 year before time zero (radiation exposure) to event or censure. The subjects were considered not exposed to statins until exposed. For sake of fluidity, patients prescribed statin were considered statin users.
Ascertainment of Outcomes and Comorbidities
The primary outcome of interest was the occurrence of any of cerebrovascular events (including transient ischemic attack, stroke, carotid revascularization, or stroke death) or cardiovascular events (including myocardial infarction [MI] or fatal MI). The secondary outcome of interest was the occurrence of stroke alone. Multivariate Cox proportional hazard model analyses were conducted to identify predictors of worst outcome. Potential predictors analyzed included age at first RT exposure, sex, prior history of hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, heart failure, chronic kidney disease, peripheral vascular disease, coronary artery disease (CAD), and CVD. Age was analyzed both as a continuous and a categorical variable. Age was treated as a categorical variable: 66 to 70 (reference group), 71 to 75 years old, 76 to 80 years old, and >81 years old.
Diagnostic codes for cardiovascular outcomes adhered to the ICD‐9 (before 2005) and 10th revisions. For these codes, high positive predictive values have been reported in the general population.18, 19, 20 Survival time was calculated from the index date, the first occurrence to RT until the date of study outcomes, or end of follow‐up, whichever occurred first.
Comorbidities were ascertained based on ICD‐9 and ‐10 codes from both inpatient and outpatient medical databases. Statin‐associated side effects (hepatitis, transaminitis, myopathy, myositis, myalgia, rhabdomyolysis, and sleep disorders) were identified based on ICD‐9 and ICD‐10 codes from both inpatient and outpatient medical databases (Table S3).
Statistical Analyses
Baseline characteristics according to treatment assignment were compared using χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables.
Cox proportional hazards models were used to calculate the hazard ratio and 95% CI for the comparison of event rates in the 2 groups. In the Cox proportional hazards model, time zero was taken as the first occurrence of the exposure (RT). Exposure to statins was measured as the first statin prescription starting from 1 year before time zero to event or censure. Initial statin exposure was analyzed in a time‐dependent manner to avoid immortal time bias. Once initial exposure was ascertained, it was analyzed in a time‐invariant fashion. Proportional hazards assumption was tested statistically and verified.
Statin‐associated side effects were assessed using logistic regression where time zero was the time of radiotherapy exposure. Side effects were categorized by whether they occurred after or before statin exposure to verify a temporal association with exposure. Statistical analyses were performed using SAS (SAS Proprietary Software 9.4 [TS1M1] Institute, Inc, Cary, NC).
Results
Baseline and Clinical Characteristics
We identified a total of 5718 patients with thorax, head, or neck cancer who had exposure to RT. The patient cohort was divided into 2 groups according to statin use (Figure 1). There were 1552 subjects in the nonuser group and 4166 subjects in the statin‐user group. Table 1 summarizes the baseline characteristics and comorbidities for statin‐users and nonusers. Overall the mean age for the statin‐user group was 75±5.8 years and 76±6.8 for the nonusers (P<0.0001), with females comprising 43% and 51% of the 2 cohorts, respectively (P<0.0001). Mean time to event/censoring was 534±687 days for nonusers and 594±706 days for the statin‐users.
Figure 1.
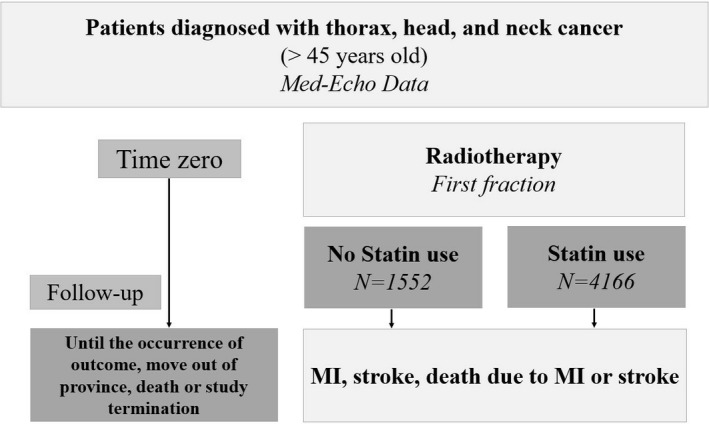
Study design—retrospective data collection. MI indicates myocardial infarction.
Table 1.
Baseline Characteristics of Statin‐Users and Nonusers
| Total (n=5718) | Statin Users (n=4166) N (%) | Nonusers (n=1552) N (%) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
|
Age (median) (y) Mean age (SD) |
74 75 (6.1) |
74 75 (5.8) |
75 76 (6.8) |
<0.0001 |
| Age categories (y) | ||||
| 66–70 | 1610 (28) | 1223 (29) | 387 (25) | <0.0001 |
| 71–75 | 1619 (28) | 1201 (29) | 418 (27) | |
| 76–80 | 1375 (24) | 1027 (25) | 348 (22) | |
| 81 and over | 1114 (19) | 715 (17) | 399 (26) | |
| Sex (female) | 2595 (45) | 1807 (43) | 788 (51) | <0.0001 |
| Cardiovascular risk factors | ||||
| Hypertension | 4062 (71) | 3075 (74) | 987 (64) | <0.0001 |
| Diabetes mellitus | 1702 (30) | 1322 (32) | 380 (24) | <0.0001 |
| Dyslipidemia | 3572 (62) | 3079 (74) | 493 (32) | <0.0001 |
| Carotid artery disease | 286 (5) | 237 (6) | 49 (3) | <0.0001 |
| CAD | 4942 (86) | 3696 (89) | 1246 (80) | <0.0001 |
| PVD | 388 (7) | 316 (8) | 72 (5) | <0.0001 |
| Heart failure | 1261 (22) | 925 (22) | 336 (22) | 0.65 |
| CKD | 836 (15) | 632 (15) | 204 (13) | 0.0538 |
| Atrial fibrillation | 1195 (21) | 846 (20) | 349 (22) | 0.07 |
| Prior stroke | 597 (10) | 466 (11) | 131 (8) | 0.002 |
| Prior MI | 1773 (31) | 1397 (34) | 376 (24) | <0.0001 |
| Prior MI/CAD | 5199 (91) | 3855 (93) | 1344 (87) | <0.0001 |
| Cancer type | 0.10 | |||
| Head and neck | 689 (12) | 520 (12) | 169 (11) | |
| Chest and mediastinal | 5029 (88) | 3646 (88) | 1383 (89) | |
| Cancer therapy | ||||
| Chemotherapy | 1138 (20) | 840 (20) | 298 (19) | 0.42 |
| medication | ||||
| Aspirin | 3280 (57) | 2754 (66) | 526 (34) | <0.0001 |
| ACEI | 2735 (48) | 2300 (55) | 435 (28) | <0.0001 |
| ARB | 1130 (20) | 948 (23) | 182 (12) | <0.0001 |
| BB | 1563 (27) | 1298 (31) | 265 (17) | <0.0001 |
| CCB | 1565 (27) | 1316 (32) | 249 (16) | <0.0001 |
| Clopidogrel | 827 (14) | 751 (18) | 76 (5) | <0.0001 |
| Warfarin | 859 (15) | 654 (16) | 205 (13) | 0.02 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; BB, β‐blocker; CAD, coronary artery disease; CCB, calcium channel blocker; CKD, chronic kidney disease; MI, myocardial infarction; PVD, peripheral vascular disease.
The burden of baseline cardiovascular risk factors (Table 1) and most comorbidities (carotid artery disease, CAD, prior MI, peripheral vascular disease, diabetes mellitus, dyslipidemia, and hypertension) was higher in the statin‐user group than the nonusers group (P<0.0001). Of the statin‐users, 89% had known prior CAD versus 80% of nonusers (P<0.0001). The additional use of chemotherapy was similar in both statin‐users and nonusers (P=0.42). Overall, statin use was reported in 73% of the total study cohort. The statin‐user group was also more commonly using other medications such as aspirin, clopidogrel, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, calcium‐channel blocker (P<0.0001 for all), and warfarin (P=0.02).
Predictors of Cardiovascular and Cerebrovascular Outcomes
The hazard ratios (HR) of stroke, MI, and death caused by MI or stroke for statin use among cancer patients post RT is shown is Table 2. The crude event rate of the primary outcome was 10.3% in nonusers and 9.0% in statin‐users, with a univariable HR of 0.80 (95% CI 0.67–0.96, P=0.019). The annualized event incidence of the primary outcome was 5.74% among statin‐users and 6.41% among nonusers (P=0.24). The annualized event incidence of stroke alone was 1.62% among statin‐users and 2.19% among nonusers (P=0.07). In a multivariable model with statin use as a time‐dependent variable, after adjusting for age, sex, prior history of transient ischemic attack or MI, diabetes mellitus, dyslipidemia, atrial fibrillation, chronic kidney disease, heart failure, and hypertension, statin‐use at the time of RT was associated with a nonsignificant 15% relative risk reduction in the subsequent stroke, MI, and death (HR=0.85, 95% CI 0.69–1.04). The crude and adjusted HR for the secondary outcome of stroke alone were 0.75 (95% CI 0.55–1.04, P=0.0860) and 0.68 (95% CI 0.48–0.95, P=0.0368), respectively. Figures 2 and 3 depict the Kaplan–Meier event‐free survival curves following RT, according to statin use, for the primary composite outcome of MI, stroke, and death caused by MI and stroke (Figure 2), as well as for secondary outcome of stroke alone (Figure 3). The Cox proportional hazard model for the multivariate MI‐free survival analysis is shown in Table S4. The Kaplan–Meier event‐free survival curve for the secondary outcome of MI alone is shown in Figure S1. The overall all‐cause mortality was lower in the statin group (P<0.0001, Figure 4) compared with the nonstatin group.
Table 2.
Effect of Statin Use on Outcomes
| Statin Users (n=4166) N (%) | Nonusers (n=1552) N (%) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| MI/stroke/death | ||||
| Crude | 376 (9.0) | 160 (10.3) | 0.92 (0.76–1.10) | 0.3451 |
| Adjusteda | ··· | ··· | 0.85 (0.69–1.04) | 0.1068 |
|
Statin Users (n=4186) N (%) |
Nonusers (n=1532) N (%) |
Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Stroke | ||||
| Crude | 110 (2.6) | 56 (3.7) | 0.75 (0.55–1.04) | 0.0860 |
| Adjusteda | ··· | ··· | 0.68 (0.48–0.98) | 0.0368 |
MI indicates myocardial infarction.
Adjusted for age, sex, atrial fibrillation, coronary artery disease, previous myocardial infarction, chronic kidney disease, cerebrovascular disease, diabetes mellitus, dyslipidemia, heart failure, and hypertension.
Figure 2.
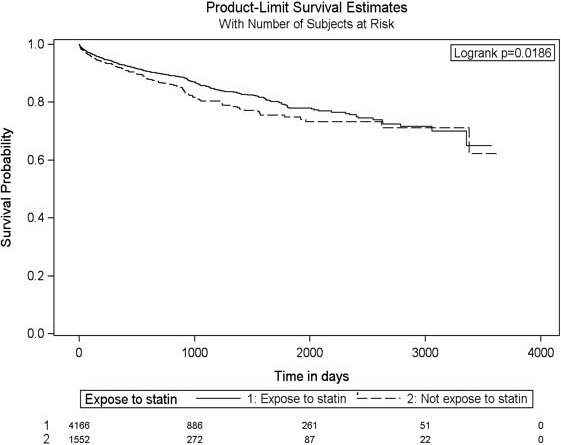
Adjusted Kaplan–Meier survival curves for the composite outcome of MI, stroke, and death from MI or stroke for statin users and nonusers. MI indicates myocardial infarction.
Figure 3.
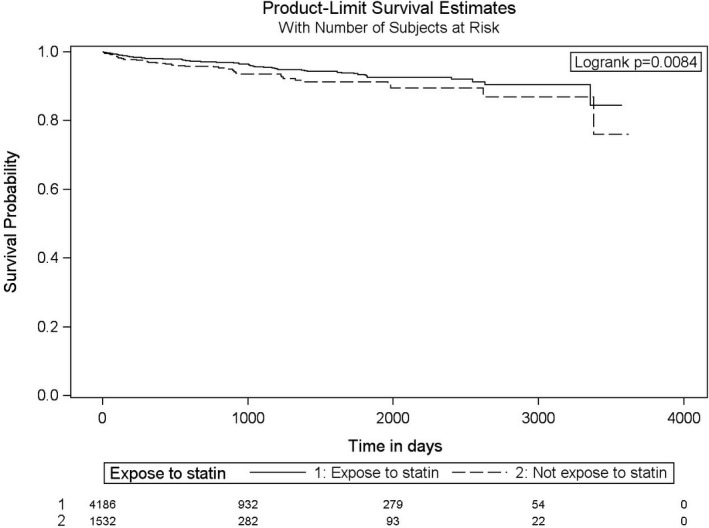
Adjusted Kaplan–Meier survival curves for the outcome of stroke for statin users and nonusers.
Figure 4.
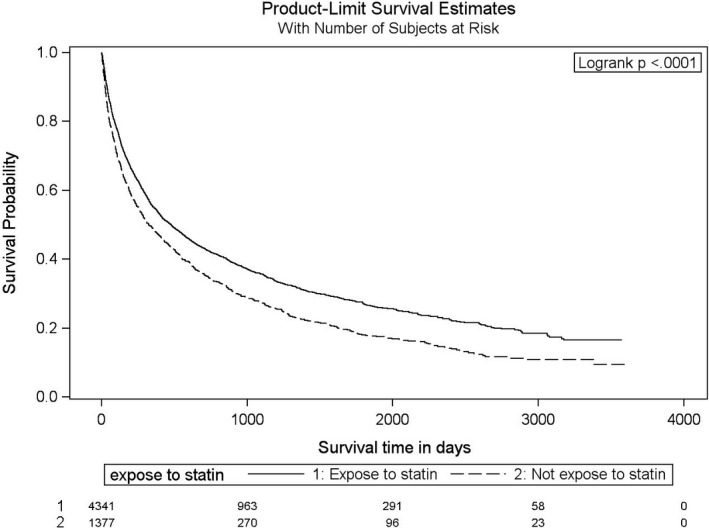
Adjusted Kaplan–Meier survival curves for all‐cause mortality for statin users and nonusers. Age: continuous variable×reference category is from ages 66 to 70 inclusively.
Predictors of vascular outcomes and death independent of statin use in patients’ medical regimen were explored. Results of the multivariable Cox regression analysis for post RT cardiovascular and CVD events are shown in Tables 3, 4, 5 through 6. Forest plots of hazard ratios according to baseline covariates for the combined primary outcome and for the outcome of stroke alone are shown in Figure S2–S3.
Table 3.
Multivariate Disease‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model for the Composite Outcome of Stroke, MI, and Death From MI or Stroke With Age as a Continuous Variable
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age (continuous) | 1.04 | 1.03–1.06 | <0.0001 |
| Sexa | 1.56 | 1.30–1.86 | <0.0001 |
| Atrial fibrillation | 1.00 | 0.81–1.24 | 0.9989 |
| CAD/MI | 1.52 | 1.03–2.24 | 0.0357 |
| CKD | 1.29 | 1.02–1.63 | 0.0353 |
| CVD | 1.71 | 1.36–2.17 | <0.0001 |
| Diabetes mellitus | 1.22 | 1.01–1.47 | 0.0421 |
| Dyslipidemia | 1.10 | 0.90–1.34 | 0.3656 |
| Heart failure | 1.44 | 1.18–1.76 | 0.0005 |
| Hypertension | 0.88 | 0.72–1.08 | 0.2186 |
| Statin use | 0.85 | 0.69–1.08 | 0.1068 |
CAD indicates coronary artery disease; CKD, chronic kidney disease; CVD, cerebrovascular disease; MI, myocardial infarction.
Reference female sex.
Table 4.
Multivariate Disease‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model for the Composite Outcome of Stroke, MI, and Death From MI or Stroke With Age as a Categorical Variable
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age (categories) (y)a | |||
| 71–75 | 1.37 | 1.07–1.76 | 0.0124 |
| 76–80 | 1.54 | 1.19–1.98 | 0.001 |
| 81 and over | 2.04 | 1.57–2.65 | <0.0001 |
| Sexb | 1.53 | 1.28–1.83 | <0.0001 |
| Atrial fibrillation | 1.01 | 0.81–1.25 | 0.9522 |
| CAD/MI | 1.52 | 1.03–2.24 | 0.0348 |
| CKD | 1.30 | 1.02–1.65 | 0.0305 |
| CVD | 1.70 | 1.35–2.15 | <0.0001 |
| Diabetes mellitus | 1.21 | 1.00–1.46 | 0.047 |
| Dyslipidemia | 1.09 | 0.89–1.33 | 0.399 |
| Heart failure | 1.44 | 1.17–1.76 | 0.0005 |
| Hypertension | 0.89 | 0.73–1.08 | 0.2498 |
| Statin use | 0.84 | 0.68–1.02 | 0.0811 |
CAD indicates coronary artery disease; CKD, chronic kidney disease; CVD, cerebrovascular disease; MI, myocardial infarction.
Reference category ages 66 to 70 inclusively.
Reference female sex.
Table 5.
Multivariate Stroke‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model With Age as a Continuous Variable
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age (continuous) | 1.03 | 1.01–1.06 | 0.0101 |
| Sexa | 1.30 | 0.94–1.78 | 0.1086 |
| Atrial fibrillation | 1.42 | 0.999–2.03 | 0.0506 |
| CAD/MI | 1.17 | 0.62–2.20 | 0.6212 |
| CKD | 1.63 | 1.09–2.44 | 0.0168 |
| CVD | 3.76 | 2.66–5.33 | <0.0001 |
| Diabetes mellitus | 1.06 | 0.75–1.50 | 0.7308 |
| Dyslipidemia | 1.07 | 0.75–1.53 | 0.7034 |
| Heart failure | 1.15 | 0.79–1.67 | 0.4618 |
| Hypertension | 0.83 | 0.58–1.18 | 0.4618 |
| Statin use | 0.68 | 0.48–0.98 | 0.0368 |
CAD indicates coronary artery disease; CKD, chronic kidney disease; CVD, cerebrovascular disease; MI, myocardial infarction.
Reference female sex.
Table 6.
Multivariate Stroke‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model With Age as a Categorical Variable
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age (categories) (y)a | |||
| 71–75 | 1.430 | 0.920–2.221 | 0.1116 |
| 76–80 | 1.207 | 0.751–1.939 | 0.4375 |
| 81 and over | 1.854 | 1.161–2.960 | 0.0097 |
| Sexb | 1.266 | 0.923–1.737 | 0.1432 |
| Atrial fibrillation | 1.447 | 1.014–2.064 | 0.0418 |
| CAD/MI | 1.165 | 0.622–2.183 | 0.6334 |
| CKD | 1.660 | 1.109–2.483 | 0.0137 |
| CVD | 3.730 | 2.634–5.282 | <0.0001 |
| Diabetes mellitus | 1.050 | 0.745–1.482 | 0.7794 |
| Dyslipidemia | 1.071 | 0.750–1.530 | 0.7052 |
| Heart failure | 1.135 | 0.780–1.650 | 0.5078 |
| Hypertension | 0.842 | 0.589–1.203 | 0.3434 |
| Statin use | 0.67 | 0.472–0.962 | 0.0296 |
CAD indicates coronary artery disease; CKD, chronic kidney disease; CVD, cerebrovascular disease; MI, myocardial infarction.
Reference category ages 66 to 70 inclusively.
Reference female sex.
Overall, the prevalence of potential statin‐associated side effects (Table 7) was low in both study groups (308 [7%] in statin users and 34 [2%] in nonusers). There was no significant difference between the 2 study groups regarding the rate of hepatitis, transaminitis, myositis, or myalgia. The rate of myopathy and sleep disorder was higher in the statin group.
Table 7.
Side Effect Profile Between Users (From First Statin Exposure) and Nonusers of Statins (From Time Zero of Radiotherapy)
| Side effects | Statin Users (N=4332) | Non‐Statin Users (N=1386) | Odds Ratio | CI |
|---|---|---|---|---|
| N (%) 308 (7) | N (%) 34 (2) | |||
| Hepatitis | 20 (0.46) | 2 (0.14) | 3.2 | 0.7–13.7 |
| Transaminitis | 16 (0.37) | 1 (0.07) | 5.1 | 0.7–38.5 |
| Myopathy | 75 (1.73) | 12 (0.86) | 2.0 | 1.1–3.7 |
| Myositis/myalgia | 23 (0.53) | 4 (0.29) | 1.8 | 0.6–5.3 |
| Sleep disorders | 173 (4.01) | 15 (1.06) | 3.9 | 2.3–6.6 |
| Rhabdomyolysis | 1 (0.02) | 0 (0) | NA | NA |
NA indicates quasi‐complete separation.
Discussion
In the largest study to date assessing the impact of statin use on vascular outcomes in a cohort of 5718 cardiac patients who had undergone radiotherapy to the thorax, head, or neck, we have shown that statins were associated with a nonsignificant but strong trend in reducing the composite end point of stroke, MI, or death caused by stroke or MI. After adjusting for comorbidities, statin use was associated with a significant reduction in the secondary outcome of stroke. Most of this cohort population were patients with underlying CAD, for which the benefits of statins persisted mostly for the prevention of cerebrovascular events, even with the added risk of RT. This study demonstrated that statin therapy could be favorable even with the competing risks of cancer and cancer‐associated mortality.
There is a growing body of evidence showing increasing burden of vascular events post RT to the thorax, head, or neck. Evidence of CAD was seen in Hodgkin's lymphoma patients in their 20s post mediastinal RT, with higher risks observed in patients treated at younger ages.21 Studies have demonstrated up to a 5‐fold increase in ischemic stroke post cervical irradiation at 5 years in cancer survivors.3 In patients >65 years of age treated with RT for head and neck squamous cell carcinoma, the HR for ischemic stroke was 1.5,22 similar to what was found in this cohort of patients.
With improvement in cancer therapies, patient survival has significantly increased post cancer diagnoses. This has left a dearth of evidence in the management of the longer‐term complications of cancer therapies. Despite the presence of data showing the association between RT and vascular disease, be it additive or multiplicative to traditional cardiovascular risk factors, there currently exist no guidelines for the treatment or prevention of RIA (radiation‐induced atherosclerosis). Current revascularization strategies are particularly challenging in this population, given that RIA tends to cause more ostial coronary artery lesions including the left anterior descending artery with involvement of proximal artery segments compared with age‐associated atherosclerosis.7, 10, 23, 24 In CVD, carotid lesions have been described as being more extensive and involving longer arterial segments compared with traditional atherosclerosis.9 Endarterectomies are often complicated by increased scar tissue overlying diseased arterial segments.25 This draws attention to the need for better primary and secondary preventive therapies for RIA.
Multiple studies have revealed the beneficial effects of statin therapy on reducing the risk of vascular mortality and morbidity in a variety of different populations. For instance, intensive statin therapy in stable CAD patients with dyslipidemia yielded a HR for major adverse cardiac events of 0.78.26 In a cohort of patients with stroke/ transient ischemic attack, high‐dose statins were associated with HRs of 0.84 and 0.80 for nonfatal/fatal stroke and major cardiovascular events, respectively.27 In addition, evidence has also demonstrated the benefit of statins in the primary prevention in subjects without known underlying CAD. In the CTT (Cholesterol Treatment Trialists’) Collaboration study,28 primary prevention with statins was associated with the proportional reductions per 1.0 mmol/L reduction in low‐density lipoprotein cholesterol in major vascular events for both women (rate ratio 0.84, 99% CI 0.78–0.91) and men (rate ratio 0.78, 99% CI 0.75–0.81). The growing body of literature on cardiovascular prevention and lipid‐lowering strategies now favors intensive statin therapy with lower low‐density lipoprotein targets. In most trials, elderly patients tend to be underrepresented and are at increased risk of statin‐associated side effects. Despite the mean age for our study population being >70 years old, the total number of side effects was small overall in both groups (Table 7). There was no significant difference observed between the 2 groups for most common side effects (myalgia, myositis, and myopathy). Sleep disorders and hepatitis were reported more frequently in the statin‐user group. Keeping in mind the unsuitability of our database for quality of life and medication compliance assessment, these results are in keeping with current guidelines suggesting that statin therapy may be suitable for older adults.
However, there have been no studies assessing the effect of statin therapy in subjects post RT on combined cardiovascular and cerebrovascular morbidity and mortality. In our study, statin therapy post RT was associated with a nonsignificant 15% reduction in stroke, MI, or death caused by stroke or MI. Although there was a strong trend, the result was not statistically significant, likely because of the high‐risk population of patients, with a crude event incidence of 9.03% and 10.31% over a 1‐ to 2‐year follow‐up. The secondary outcome of stroke alone post RT to the thorax, head, or neck was found to be reduced by 32% in patients on statin therapy. The results of our analysis complement the findings by Addison and colleagues, who demonstrated the salutary effect of statins on CVD in a younger cohort of patients with head and neck cancers and fewer comorbidities.29 Within a group of relatively low vascular risk patients undergoing head and neck radiation, statin use may lower risk of cerebrovascular accidents. Within a much higher vascular risk group of patients, as in our study, statins may also lower the risk of cerebrovascular accidents but the effect size is blunted by the much higher baseline risk of disease.
The 2018 American College of Cardiology and American Heart Association cardiovascular disease prevention guidelines recommend preventive therapy with statins in patients with high risk of atherosclerotic cardiovascular disease (≥20%, Class I indication).30 There are also new recommendations for primary prevention in patients with intermediate (≥7.5% to <20%, Class I) and borderline risks (5% to <7.5%), guided by risk enhancers to facilitate the decision to initiate statin therapy. These risk‐enhancing factors carry a greater lifetime risk of atherosclerotic cardiovascular disease, but do not yet include the exposure to RT to the thorax, head, and neck. The recent US Preventive Services Task Force recommendation sets this threshold at 10%.30 These data support the continuation of statins among those having received RT and who have an indication for statins. Our data act as hypothesis generating as to whether individuals who do not meet Class I indications for statin therapy would benefit from it through risk enhancement from radiotherapy, and whether prospective randomized controlled trials are needed to strongly support statin therapy in the population who do not meet current criteria for statins.
The results of this study should be considered in the context of its inherent limitations. Importantly, this study is retrospective and observational in design, making it impossible to rule out residual and unmeasured confounding between nonrandomized groups as an explanation for observed results. The statin‐user and nonuser groups were largely different from each other. The statin subgroup of patients had significantly more underlying comorbidities, such as CAD, with the added risk factor of RT, which could also explain why they had higher overall all‐cause mortality compared with the nonstatin group. This would be expected to bias the statin group toward having higher risk of vascular events, although the contrary was observed for the outcome of stroke alone (Table 2 and Figure 3). While there was a greater burden of cardiovascular risk factors among statin users who underwent RT, there was greater use of other cardiac medications in this group as well. Despite rigorous attempts to adjust for identified confounders, differences in statin users versus nonusers because of a nonrandomized design may have contributed to the noted beneficial effect of statins in patients post RT. There were also limitations imposed by the database used in this study. While it offered access to a large group of patients, having access to information on smoking status, low‐density lipoprotein levels, statin dosing, and radiation dosing would have greatly added to the analysis but was unavailable. Despite these limitations, this study offers a promising insight into the potential benefits of lipid‐lowering therapies post RT.
Conclusion
This retrospective observational study demonstrated that in a large cohort of patients, with a heavy burden of CAD and having undergone radiation to the thorax, head, or neck, the use of statins was associated with a trend for a 15% reduction in the composite outcome of cardiovascular and cerebrovascular events. The use of statins was associated with a significant reduction of 32% for the outcome of stroke alone. This study supports a benefit of statin therapy in prevention of vascular events. There is a need for prospective randomized control trials to explore the role of statins post RT in less selected populations and to establish more definitive guidelines on the management of radiation‐induced vascular disease.
Disclosures
The views expressed are those of the author and do not reflect official policy of Fort Belvoir Community Hospital, the Defense Health Agency, the Department of Defense, or the US Government.
Supporting information
Table S1. Definitions of Covariates‐Interventions
Table S2. Definitions of Covariates‐Comorbidities and Outcomes
Table S3. Definition of Statin Side Effects
Table S4. Multivariate MI‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model (Age Continuous)
Figure S1. Adjusted Kaplan–Meier survival curves for the outcome of myocardial infarction between statin users and nonstatin users.
Figure S2. Forest plot of hazard ratio for the combined outcome of stroke and myocardial infarction according to baseline covariates.
Figure S3. Forest plot of hazard ratio for the outcome of stroke according to baseline covariates.
(J Am Heart Assoc. 2019;8:e005996 DOI: 10.1161/JAHA.117.005996.)
Research performed at McGill University, Montreal, Quebec, Canada.
References
- 1. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, Robison LL, Yasui Y. Cause‐specific late mortality among 5‐year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dabaja B, Cox JD, Buchholz TA. Radiation therapy can still be used safely in combined modality approaches in patients with Hodgkin's lymphoma. J Clin Oncol. 2007;25:3–5. [DOI] [PubMed] [Google Scholar]
- 3. Russell NS, Hoving S, Heeneman S, Hage JJ, Woerdeman LA, de Bree R, Lohuis PJ, Smeele L, Cleutjens J, Valenkamp A, Dorresteijn LD, Dalesio O, Daemen MJ, Stewart FA. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol. 2009;92:477–483. [DOI] [PubMed] [Google Scholar]
- 4. Andratschke N, Maurer J, Molls M, Trott KR. Late radiation‐induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. 2011;100:160–166. [DOI] [PubMed] [Google Scholar]
- 5. Denham JW, Hauer‐Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol. 2002;63:129–145. [DOI] [PubMed] [Google Scholar]
- 6. Hopewell JW, Calvo W, Jaenke R, Reinhold HS, Robbins ME, Whitehouse EM. Microvasculature and radiation damage. Recent Results Cancer Res. 1993;130:1–16. [DOI] [PubMed] [Google Scholar]
- 7. Mousavi N, Nohria A. Radiation‐induced cardiovascular disease. Curr Treat Options Cardiovasc Med. 2013;15:507–517. [DOI] [PubMed] [Google Scholar]
- 8. Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation‐induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng SW, Ting AC, Lam LK, Wei WI. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:517–521. [DOI] [PubMed] [Google Scholar]
- 10. Veinot JP, Edwards WD. Pathology of radiation‐induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. [DOI] [PubMed] [Google Scholar]
- 11. Ebrahim S, Taylor FC, Brindle P. Statins for the primary prevention of cardiovascular disease. BMJ. 2014;348:g280. [DOI] [PubMed] [Google Scholar]
- 12. Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. [DOI] [PubMed] [Google Scholar]
- 13. Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. [DOI] [PubMed] [Google Scholar]
- 14. Tamblyn R, Reid T, Mayo N, McLeod P, Churchill‐Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–194. [DOI] [PubMed] [Google Scholar]
- 15. Liste de médicaments assurés. Régie de l'assurance maladie du québec (RAMQ). 2019. Quebec Minister of Health and Social Services, Quebec, Canada. [Google Scholar]
- 16. Manuel des médecins spécialistes services de laboratoire. Régie de l'assurance maladie du québec (RAMQ). 2016. Quebec Minister of Health and Social Services, Quebec, Canada. [Google Scholar]
- 17. Manuel des médecins spécialistes. Régie de l'assurance maladie du québec (RAMQ). 2016. Quebec Minister of Health and Social Services, Quebec, Canada. [Google Scholar]
- 18. Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCormick N, Lacaille D, Bhole V, Avina‐Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pladevall M, Goff DC, Nichaman MZ, Chan F, Ramsey D, Ortiz C, Labarthe DR. An assessment of the validity of ICD code 410 to identify hospital admissions for myocardial infarction: the Corpus Christi Heart Project. Int J Epidemiol. 1996;25:948–952. [DOI] [PubMed] [Google Scholar]
- 21. Aleman BM, van den Belt‐Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, Hart AA, Klokman WJ, Kuenen MA, Ouwens GM, Bartelink H, van Leeuwen FE. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 22. Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, Krumholz HM, Weber RS, Ang KK, Rosenthal DI. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26:5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brosius FC III, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–530. [DOI] [PubMed] [Google Scholar]
- 24. Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 1993;69:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kasivisvanathan V, Thapar A, Davies KJ, Dharmarajah B, Shalhoub J, Davies AH. Periprocedural outcomes after surgical revascularization and stenting for postradiotherapy carotid stenosis. J Vasc Surg. 2012;56:1143–1152.e1142. [DOI] [PubMed] [Google Scholar]
- 26. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets I . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 27. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels I . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 28. Cholesterol Treatment Trialists C , Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Addison D, Lawler PR, Emami H, Janjua SA, Staziaki PV, Hallett TR, Hennessy O, Lee H, Szilveszter B, Lu M, Mousavi N, Nayor MG, Delling FN, Romero JM, Wirth LJ, Chan AW, Hoffmann U, Neilan TG. Incidental statin use and the risk of stroke or transient ischemic attack after radiotherapy for head and neck cancer. J Stroke. 2018;20:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Force USPST . Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of Covariates‐Interventions
Table S2. Definitions of Covariates‐Comorbidities and Outcomes
Table S3. Definition of Statin Side Effects
Table S4. Multivariate MI‐Free Survival Analysis of Patients With Thorax, Head, or Neck Cancer Treated With Radiotherapy by the Cox Proportional Hazard Model (Age Continuous)
Figure S1. Adjusted Kaplan–Meier survival curves for the outcome of myocardial infarction between statin users and nonstatin users.
Figure S2. Forest plot of hazard ratio for the combined outcome of stroke and myocardial infarction according to baseline covariates.
Figure S3. Forest plot of hazard ratio for the outcome of stroke according to baseline covariates.


