Abstract
Background
Recent publications reached conflicting conclusions about the cost‐effectiveness of left atrial appendage closure (LAAC) with the Watchman device (Boston Scientific, Marlborough, MA) for stroke risk reduction in nonvalvular atrial fibrillation (AF). This analysis sought to assess the cost‐effectiveness of LAAC relative to both warfarin and nonwarfarin oral anticoagulants (NOACs) using pooled, long‐term data from the randomized PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long‐Term Warfarin) trials.
Methods and Results
A Markov model was constructed from a US payer perspective with a lifetime (20‐year) horizon. LAAC clinical event rates and stroke outcomes were from pooled PROTECT AF and PREVAIL trial 5‐year data. Warfarin and NOAC inputs were derived from published meta‐analyses. The model was populated with a cohort of 10 000 patients, aged 70 years, at moderate stroke and bleeding risk. Sensitivity analyses were performed. LAAC was cost‐effective relative to warfarin by year 7 ($48 674/quality‐adjusted life‐year) and dominant (more effective and less costly) by year 10. LAAC became cost‐effective and dominant compared with NOACs by year 5. Over a lifetime, LAAC provided 0.60 more quality‐adjusted life‐years than warfarin and 0.29 more than NOACs. In sensitivity analyses, LAAC was cost‐effective relative to warfarin and NOACs in 98% and 95% of simulations, respectively.
Conclusions
Using pooled, 5‐year PROTECT AF and PREVAIL trial data, LAAC proved to be not only cost‐effective, but cost saving relative to warfarin and NOACs. LAAC with the Watchman device is an economically viable stroke risk reduction strategy for patients with AF seeking an alternative to lifelong anticoagulation.
Keywords: anticoagulant, atrial fibrillation, cost‐effectiveness, left atrial appendage closure, nonwarfarin oral anticoagulants, Watchman
Subject Categories: Atrial Fibrillation, Catheter-Based Coronary and Valvular Interventions, Cost-Effectiveness, Intracranial Hemorrhage, Ischemic Stroke
Clinical Perspective
What Is New?
This present analysis uses the complete, 5‐year pooled analysis of PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long‐Term Warfarin) randomized controlled trial data to explore the cost‐effectiveness of left atrial appendage closure relative to warfarin and nonwarfarin oral anticoagulants, whereas previously published US economic analyses have used interim data from the individual trials.
What Are the Clinical Implications?
Despite the increased risk of ischemic stroke observed in the PREVAIL trial, left atrial appendage closure is cost‐effective and cost saving relative to nonwarfarin oral anticoagulants and warfarin when the full body of randomized controlled trial data is taken into consideration.
Left atrial appendage closure with the Watchman device is an economically viable stroke risk reduction strategy for patients with atrial fibrillation seeking an alternative to lifelong anticoagulation.
Introduction
Atrial fibrillation (AF) has a substantial impact on patient lives and medical costs. It is estimated that treating patients with AF adds $26 billion to US healthcare costs, predominantly caused by AF‐related stroke.1, 2 Several stroke prevention strategies now exist for patients with nonvalvular AF, including pharmacotherapies, such as warfarin and the nonwarfarin oral anticoagulants (NOACs), and the device‐based strategy of percutaneous left atrial appendage closure (LAAC). Warfarin has been used to treat AF for >50 years and is effective at reducing ischemic stroke risk, but has a narrow therapeutic window in which to achieve maximal risk reduction. It is associated with drug and food interactions and high nonadherence to therapy.3 NOACs have demonstrated similar efficacy to warfarin for stroke prevention without the need for routine monitoring; however, NOAC‐related complications, such as increased risk for gastrointestinal tract bleeding, have been observed in various patient subpopulations.4, 5, 6, 7 And more important, any drug‐based therapy is dependent on patient adherence to treatment.
Percutaneous LAAC with the Watchman device (Boston Scientific, Marlborough, MA) was approved for use in the United States on the basis of 2 pivotal, randomized controlled trials (RCTs): PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long‐Term Warfarin).8, 9, 10 Patients in both trials were followed up for 5 years, and the long‐term outcomes from these studies have demonstrated LAAC is: as effective as warfarin in preventing all‐cause strokes; similar to warfarin in preventing ischemic strokes; superior in preventing hemorrhagic stroke; superior in preventing disabling/fatal stroke; superior in preventing postprocedure major bleeding; and superior in reduction of all‐cause mortality.10 Finally, US real‐world clinical experience after US Food and Drug Administration (FDA) approval has been favorable.11 In 3822 consecutive patients, Watchman implantation was successful in 95.6% of patients and was associated with a favorable safety profile; compared with PROTECT AF/PREVAIL trials, pericardial effusion rates decreased from 3.69% to 1.02% and procedure‐related stroke decreased from 0.82% to 0.05%.10, 11
To ensure patient access to novel therapies, it is becoming increasingly important to demonstrate both clinical and economic value. Published US economic analyses to date have explored the cost‐effectiveness of LAAC using either the PROTECT AF or PREVAIL trial data sets separately.12, 13 These analyses yielded conflicting results, thereby leading to confusion in the clinical and policy community. To further inform the economics of LAAC, this present analysis uses the complete, 5‐year RCT evidence in its totality: a patient‐level pooled analysis of both PROTECT AF and PREVAIL trials.10 Herein, we estimate the cost‐effectiveness of LAAC with Watchman relative to both warfarin and NOACs for stroke risk reduction in nonvalvular AF. This comprehensive assessment uses the most complete body of clinical evidence for LAAC in an attempt to more definitively evaluate the therapy's economic value and provide guidance for future analyses.
Methods
We evaluated the cost‐effectiveness of 3 treatment strategies: (1) LAAC with the Watchman device; (2) NOACs as a class; and (3) adjusted‐dose warfarin. This analysis was conducted using an Excel‐based (Microsoft, Redmond, WA) Markov model developed to assess the cost‐effectiveness of LAAC using pooled PROTECT AF and PREVAIL 5‐year, pivotal clinical trial data. Patients were assumed to be 70 years of age and have a mean Congestive Heart Failure, Hypertension, Age≥75 years, Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack, Vascular Disease, Age 65–74 years, Sex Category (CHA2DS2‐VASc) score of 4.0 (annual stroke risk of 4.8%) and a Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol (HAS‐BLED) score of 1.98 (annual risk of bleeding of 1.88%).8, 9, 10 The model was constructed from the perspective of the Centers for Medicare and Medicaid Services with a lifetime horizon (defined as 20 years) and 3‐month cycle length. Within each cycle, patients could experience clinical events leading to death, disability, and/or therapy discontinuation and incur associated costs and quality‐of‐life (QoL) adjustments. Model parameters are available for other researchers on request to the corresponding author. Institutional review board review was not required for this analysis.
Cost‐effectiveness was evaluated using the US commonly accepted willingness to pay threshold of $50 000 per quality‐adjusted life‐year (QALY) gained and reported as the incremental cost‐effectiveness ratio (ICER).14 The ICER provides a standardized approach to measure cost per unit of health improvement in and across health states. Cost‐effectiveness was assessed annually to determine at which point in time treatment options achieved accepted levels of cost‐effectiveness.
Model Structure and Clinical Pathways
The model began with patients being assigned to LAAC, NOACs, or warfarin (Figure 1). For the base‐case analysis, patients in the LAAC arm faced one‐time, procedure‐related risk of ischemic stroke caused by air embolism (0.82%), major bleeding (0.55%), pericardial effusion (3.69%), and device embolization (0.68%).15 Patients undergoing LAAC could experience a successful or failed implantation procedure. Successfully implanted patients (92.5%) were assumed to receive warfarin for 45 days, aspirin plus clopidogrel from 46 days to 6 months, and aspirin thereafter in accordance with the treatment algorithms in the LAAC trials.8, 9, 15 After a failed procedure, patients were assumed to return to warfarin therapy.8, 9, 10, 15 Patients in the warfarin and NOAC arms could discontinue therapy after a bleeding event or for nonclinical reasons. Patients who discontinued primary drug therapy were assumed to switch to aspirin.4, 6, 16, 17, 18, 19 Discontinuation of second‐line therapy was assumed to result in no treatment.
Figure 1.
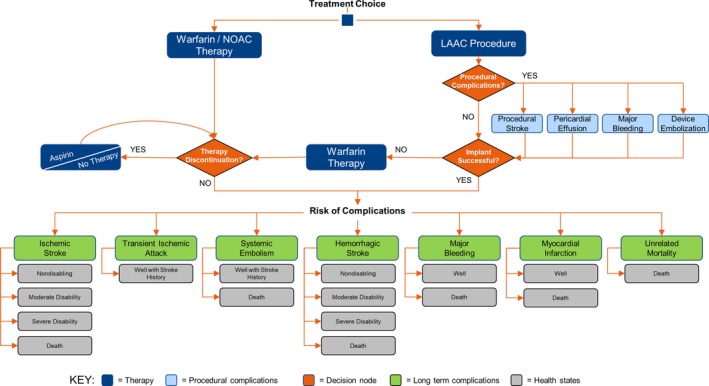
Model schematic depicting left atrial appendage closure (LAAC) and nonwarfarin oral anticoagulant (NOAC) patient pathways. Patient pathways for LAAC and NOAC.
On entering the model, patients were assumed to be “Well” or in normal, good health for an average‐aged patient diagnosed with nonvalvular AF. Patients transitioned from Well to different health states after a clinical event or death. Each health state corresponded to an event‐specific QoL decrement and cost. Only ischemic and hemorrhagic stroke impacted disability outcomes. Patients undergoing a second stroke could either remain in the same health state or worsen to greater disability. Transient ischemic attack and systemic embolism led to patients being well with a history of stroke, which increased their risk of subsequent stroke. All events, except transient ischemic attack, could lead to death.
Clinical Inputs
Clinical inputs are presented in Table 1. LAAC procedural complications and clinical event probabilities were derived from pooled PROTECT AF and PREVAIL clinical trial data at 5 years of follow‐up.10, 15 Relative risks for postprocedural stroke and bleeding were used to apply a standard efficacy estimate to the derived baseline risks.10 Warfarin and NOAC clinical inputs were derived primarily from meta‐analyses and AF stroke prevention clinical trials.4, 6, 16, 17, 18, 19, 20, 21, 22 Event probabilities were extrapolated over the lifetime horizon for all treatment strategies. Last, a scenario analysis was conducted to compare LAAC trial‐based procedural outcomes to post‐FDA, real‐world procedural outcomes.11
Table 1.
Clinical Inputs Derived From Meta‐Analyses and Pivotal Trials
| Variable | Value | Range | Distribution | Source |
|---|---|---|---|---|
| LAAC: clinical trial‐based procedural events | ||||
| Implantation success | 92.50% | 85.00%–100.00% | β | 15 |
| Procedural risk of ischemic stroke | 0.82% | 0.66%–0.98% | β | 15 |
| Procedural risk of major bleeding | 0.55% | 0.44%–0.66% | β | 15 |
| Procedural risk of pericardial effusion requiring intervention | 3.69% | 2.95%–4.43% | β | 15 |
| Procedural risk of device embolization | 0.68% | 0.54%–0.82% | β | 15 |
| LAAC: post‐FDA, real‐world procedural events | ||||
| Implantation success | 95.60% | 85.00%–100.00% | β | 11 |
| Procedural risk of ischemic stroke | 0.05% | 0.04%–0.06% | β | 11 |
| Procedural risk of hemorrhagic stroke | 0.03% | 0.02%–0.03% | β | 11 |
| Procedural risk of major bleedinga | 0.55% | 0.44%–0.66% | β | 15 |
| Procedural risk of pericardial effusion | 1.02% | 0.82%–1.22% | β | 11 |
| Procedural risk of device embolization | 0.24% | 0.19%–0.29% | β | 11 |
| Procedural risk of death | 0.10% | 0.08%–0.13% | β | 11 |
| LAAC: postprocedural events | ||||
| Relative risk of postprocedure ischemic stroke (relative to warfarin) | 1.29 | 1.03–1.54 | Lognormal | 10 |
| Relative risk of hemorrhagic stroke (relative to warfarin) | 0.16 | 0.12–0.19 | Lognormal | 10 |
| Relative risk of postprocedure major bleeding (relative to warfarin) | 0.60 | 0.48–0.72 | Lognormal | 10 |
| Annual risk of systemic embolism | 0.10% | 0.08%–0.12% | β | 10 |
| Relative risk of myocardial infarction (relative to warfarin) | 0.50 | 0.40–0.60 | Lognormal | 8 |
| Risk of minor bleeding | Based on concomitant drug therapy | |||
| Warfarin | ||||
| Relative risk of ischemic stroke (relative to no therapy) | 0.33 | 0.23–0.46 | Lognormal | 20 |
| Relative risk of major bleeding (relative to HAS‐BLED) | 1.00 | 0.80–1.20 | Lognormal | 21 |
| Percentage of major bleeding that is hemorrhagic stroke | 41.80% | 33.40%–50.20% | β | 22 |
| Annual risk of systemic embolism | 0.11% | 0.90%–0.11% | β | 4, 6 |
| Annual risk of myocardial infarction | 1.47% | 0.53%–1.47% | β | 22 |
| Annual risk of minor bleeding | 7.70% | 0.80%–16.40% | β | 20, 21 |
| Nonclinical discontinuation rate | 4.33% | 3.46%–5.19% | Uniform | 16, 17, 18, 19 |
| NOACs | ||||
| Relative risk of ischemic stroke (relative to warfarin) | 0.92 | 0.83–1.02 | Lognormal | 22 |
| Relative risk of hemorrhagic stroke (relative to warfarin) | 0.48 | 0.39–0.59 | Lognormal | 22 |
| Relative risk of extracranial hemorrhage (relative to warfarin) | 1.25 | 1.01–1.55 | Lognormal | 22 |
| Relative risk of systemic embolism | 0.92 | 0.83–1.02 | Lognormal | 22 |
| Relative risk of myocardial infarction (relative to warfarin) | 0.97 | 0.78–1.20 | Lognormal | 22 |
| Annual risk of minor bleeding | 8.70% | 7.00%–10.40% | β | 20, 21 |
| Nonclinical discontinuation rate | 4.18% | 3.34%–5.01% | Uniform | 4, 6 |
| All treatment arms | ||||
| Discount rate | 3.00% | 2.00%–4.00% | β | 39 |
FDA indicates US Food and Drug Administration; LAAC, left atrial appendage closure; NOAC, nonwarfarin oral anticoagulant; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol.
Major bleeding was not an end point in the post‐FDA real‐world data study, so we have used the trial‐based event rate for this input.
Baseline risk of stroke was assigned on the basis of Congestive Heart Failure, Hypertension, Age≥75 years, Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack, Vascular Disease, Age 65–74 years, Sex Category (CHA2DS2‐VASc) scores and risk of bleeds on the basis of HAS‐BLED scores.21, 23 Stroke risk was estimated by converting Congestive Heart Failure, Hypertension, Age≥65 years, Diabetes Mellitus, Stroke or Transient Ischemic Attack (CHADS2) scores that were prospectively collected during PROTECT AF and PREVAIL trials to Congestive Heart Failure, Hypertension, Age≥75 years, Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack, Vascular Disease, Age 65–74 years, Sex Category (CHA2DS2‐VASc).8, 9, 10, 23, 24 A modified HAS‐BLED score, calculated using the available data on 5 of the 7 criteria, was estimated as a weighted mean from PROTECT AF and PREVAIL trials.8, 9, 10, 21 To account for increasing risk with age, rates of ischemic and hemorrhagic stroke were increased by 1.4 and 1.97 times per decade, respectively.25, 26 In addition, the impact of advanced age was explored via a scenario analysis with starting ages of 75 and 80 years. Patients experiencing a transient ischemic attack or systemic embolism were assumed to have a 2.6 times increased risk of experiencing a second ischemic event.25 Healthcare Utilization Project mortality rates were used to inform probabilities of death after systemic embolism, extracranial hemorrhage, or myocardial infarction.27 Risk of death from unrelated causes was obtained from US life tables, with disabled patients facing a 2.3 times greater risk of death.28, 29
Health State Utilities and Stroke Outcomes
Patient QoL was captured in the model as health utility. Health utility values were based on a scale of 0 to 1, with 1 representing perfect health and 0 representing death. The baseline utility values used for Well with warfarin (0.987), Well with NOAC (0.994), and Well with aspirin (0.998) are consistent with values used in previously published analyses.12, 30, 31, 32, 33, 34 As QoL data were not collected as part of the PREVAIL trial, the utility value for Well with LAAC (0.999) was calculated by applying the Nichol ordinary least squares algorithm to 12‐Item Short Form Survey (SF‐12) data collected during the PROTECT AF trial.35, 36 The utility weights for all Well‐based health states were applied as a multiplying factor to an underlying baseline utility of 0.82, representing QoL at the age of 70 years.31 The baseline utility was decremented by 2% per decade to account for general decline in QoL with advancing age.37
In addition, a series of disutilities were applied in the model for acute clinical events, representing a one‐time decrement to QoL experienced for a finite length of time. Utility decrements were assessed for stroke (−0.139), extracranial hemorrhage (−0.181), transient ischemic attack (−0.103), systemic embolism (−0.120), and myocardial infarction (−0.125).38 A value of −0.0315 was used for the LAAC procedure itself, which is based on a 2‐week disruption to healthy life.
QALYs were calculated by multiplying the health state utility value of each health state by the mean time spent in the health state. Future QALYs were discounted at an annual rate of 3%.39
Stroke outcomes and resulting disability (Table 2) were assigned using the modified Rankin score (MRS) and characterized as nondisabling (MRS 0–2), moderately disabling (MRS 3), severely disabling (MRS 4–5), and fatal (MRS 6). LAAC stroke outcomes were from pooled PROTECT AF and PREVAIL 5‐year trial data.10 Warfarin stroke outcomes were estimated using a weighted average of outcomes from 4 warfarin trials.16, 17, 40, 41 For NOACs, the rate of nondisabling strokes was derived from 2 of the 4 pivotal trials.4, 6 As this was the only stroke outcome reported in the NOAC trials, the inverse represented disabling and fatal strokes, with the distribution of moderately disabling, severely disabling, and fatal strokes assumed to be the same as for warfarin.
Table 2.
Stroke Outcomes and Health State Utilities
| Stroke Outcome | LAAC, % | Warfarin, % | NOAC, % | Utility Value |
|---|---|---|---|---|
| Nondisabling stroke (MRS 0–2) | 75.010 | 24.016, 17, 40, 41 | 44.04, 6 | 0.76035 |
| Moderately disabling stroke (MRS 3) | 2.810 | 29.016, 17, 40, 41 | 21.4a | 0.39035 |
| Severely disabling stroke (MRS 4–5) | 16.710 | 35.016, 17, 40, 41 | 25.8a | 0.11035 |
| Fatal stroke (MRS 6) | 5.610 | 12.016, 17, 40, 41 | 8.8a | 0.000 |
LAAC indicates left atrial appendage closure; MRS, modified Rankin score; NOAC, nonwarfarin oral anticoagulant.
NOAC stroke outcomes were assumed to have the same distribution as warfarin across MRS 3 to 6.
Costs
The model incorporated all direct healthcare costs for the therapies and treatment of associated acute events, as well as costs for long‐term care after a disabling stroke. Costs for acute events were taken from US 2017 diagnosis‐related group (DRG) national average values, and costs for poststroke inpatient rehabilitation represent 2017 case‐mix group (CMG) reimbursement rates.12, 42, 43 Long‐term stroke disability costs were from published literature and inflated to 2017 dollars using the Consumer Price Index for medical care published by the US Bureau of Labor Statistics.32, 33, 34, 44, 45, 46, 47 The cost of the LAAC procedure was calculated as a weighted average of 2 diagnosis‐related groups for percutaneous intracardiac procedures (273 and 274) plus the cost of 2 transesophageal echocardiograms.42, 48 The annual cost of warfarin therapy was also applied for patients receiving LAAC who were unable to discontinue warfarin. Warfarin costs were from US pharmaceutical wholesale acquisition cost in combination with reimbursement rates for Current Procedural Terminology (CPT (R)) codes related to international normalized ratio monitoring.48, 49 NOAC costs were calculated as an average of US wholesale acquisition costs for the first 3 approved drugs: dabigatran, rivaroxaban, and apixaban.49 All costs are in 2017 US dollars and discounted at an annual rate of 3% (Table 3).
Table 3.
Cost Inputs
| Acute Events | Costs, $ | Code | Reference |
|---|---|---|---|
| LAAC procedure+2 transesophageal echocardiogramsa | 16 741 | DRG 273/274 | 42, 43 |
| Fatal ischemic stroke | 11 250 | DRG 063 | 42 |
| Severe ischemic stroke | 48 593 | DRG 061/CMG 108‐110 | 42, 43 |
| Moderate ischemic stroke | 33 613 | DRG 062/CMG 105‐107 | 42, 43 |
| Minor ischemic stroke | 23 951 | DRG 063/CMG 101‐104 | 42, 43 |
| TIA | 4396 | DRG 069 | 42 |
| Systemic embolism (nonfatal) | 5163 | DRG 068 | 42 |
| Systemic embolism (fatal) | 7975 | DRG 067 | 42 |
| Fatal hemorrhagic stroke | 10 446 | DRG 064 | 42 |
| Severe hemorrhagic stroke | 42 721 | DRG 064/CMG 108‐110 | 42, 43 |
| Moderate hemorrhagic stroke | 28 583 | DRG 065/CMG 105‐107 | 42, 43 |
| Minor hemorrhagic stroke | 19 001 | DRG 066/CMG 101‐104 | 42, 43 |
| Major bleeding (nonfatal) | 5879 | DRG 377 | 42 |
| Major bleeding (fatal) | 10 572 | DRG 378 | 42 |
| Minor bleeding | 423 | CPT 42970 | 48 |
| Myocardial infarction (nonfatal) | 5944 | DRG 280, 281, and 282 | 42 |
| Myocardial infarction (fatal) | 8821 | DRG 283, 284, and 285 | 42 |
| Quarterly costs | |||
| Warfarin+INR monitoring | 118 | CPT 85610 and 99211 | 48, 49 |
| NOAC | 1147 | … | 49 |
| Independent after stroke | 109 | CPT 99214 | 48 |
| Moderately disabled after stroke | 9483 | … | 44, 45, 46, 47 |
| Severely disabled after stroke | 15 441 | … | 44, 45, 46, 47 |
CMG indicates case‐mix group; CPT, Current Procedural Terminology; DRG, diagnosis‐related group; INR, international normalized ratio; LAAC, left atrial appendage closure; NOAC, nonwarfarin oral anticoagulant; TIA, transient ischemic attack.
Procedure cost reflects inclusion of 2 transesophageal echocardiograms and is weighted between 2 DRGs to be consistent with previous analysis.12
Sensitivity Analysis
One‐way sensitivity analysis and probabilistic sensitivity analysis were undertaken to assess the impact of parameter uncertainty on model results. Clinical inputs were varied within 95% CIs, where available, and by ±20% where CIs were not published. The ranges and distributions used for clinical inputs are shown in Table 1. Health state utilities were varied by ±5% and assumed a β distribution. Stroke outcomes were varied by ±20% and assumed a Dirichlet distribution. All costs were varied ±20% and assumed a γ distribution. The probabilistic sensitivity analysis followed a standard Monte Carlo approach based on 5000 randomly drawn simulations of parameter values.
Results
LAAC Versus Warfarin
As expected, LAAC is more costly than warfarin in the early years after the procedure (Figure 2). By year 3, patients undergoing LAAC had more QALYs than patients receiving warfarin (2.185 versus 2.170); this trend continued over the lifetime analysis, with patients undergoing LAAC having 0.60 more QALYs than their warfarin counterparts at 20 years (Table 4). LAAC became cost‐effective relative to warfarin at year 7 ($48 674/QALY) and less costly than warfarin at year 10 (Figure 2). LAAC was dominant (more effective and less costly) relative to warfarin by year 10. Once achieved, LAAC remained cost‐effective and dominant relative to warfarin over the 20‐year lifetime horizon (Table 4).
Figure 2.
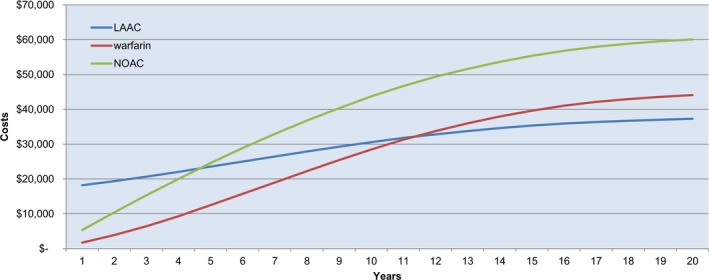
Cumulative cost curve: left atrial appendage closure (LAAC) vs warfarin vs nonwarfarin oral anticoagulant (NOACs). Cumulative costs by year for LAAC, warfarin, and NOACs.
Table 4.
QALYs, Cost, and ICER Results at 10 and 20 Years for LAAC Versus Warfarin and LAAC Versus NOACs
| Time | Total QALYs | Incremental QALYs (Relative to OACs) | Total Costs, $ | Incremental Costs (Relative to OACs), $ | ICER Versus OACs |
|---|---|---|---|---|---|
| 10 Years | |||||
| LAAC | 5.77 | ··· | 32 769 | ··· | ··· |
| Warfarin | 5.52 | 0.25 | 33 286 | −517 | Dominant |
| NOAC | 5.68 | 0.09 | 48 803 | −16 034 | Dominant |
| 20 Years | |||||
| LAAC | 7.77 | ··· | 44 894 | ··· | ··· |
| Warfarin | 7.17 | 0.60 | 61 623 | −16 729 | Dominant |
| NOAC | 7.48 | 0.29 | 77 023 | −32 129 | Dominant |
ICER indicates incremental cost‐effectiveness ratio; LAAC, left atrial appendage closure; NOAC, nonwarfarin OAC; OAC, oral anticoagulant; QALY, quality‐adjusted life‐year.
LAAC Versus NOACs
Over the lifetime analysis, patients undergoing LAAC had more QALYs than patients receiving NOACs (7.772 versus 7.481) (Table 4). LAAC had lower costs than NOACs by year 5 ($23 960 versus $25 691) (Figure 2). LAAC became cost‐effective and dominant relative to NOACs by year 5 and remained so over the lifetime analysis (Table 4).
Scenario Analysis: Post‐FDA, Real‐World Procedure Data
When using the real‐world procedure data, LAAC provided an additional 0.65 QALYs at year 20 relative to warfarin. This represents a slight increase in QALYs relative to the RCT‐based analysis. As in the RCT analysis, LAAC became less costly than warfarin at year 10 (−$2202 versus −$517). LAAC became cost‐effective relative to warfarin at year 7 ($35 051/QALY) and dominant at year 10, and it remained dominant over the 20‐year lifetime horizon.
One‐Way Sensitivity Analysis
Tornado diagrams illustrating the 10 most impactful variables in descending order of influence at 20 years are depicted in Figure S1. One‐way sensitivity analyses of LAAC versus warfarin demonstrated that the health utility value used for Well with LAAC had a significant impact on model results. The baseline risk of stroke and bleeding also influenced the ICER, with higher risks resulting in more favorable cost‐effectiveness for LAAC. When comparing LAAC with NOACs, the 20‐year results were most sensitive to the utility values for Well with LAAC and Well with NOACs, along with the percentage of nondisabling ischemic strokes experienced by patients with LAAC and baseline risk of bleeding; only varying Well with LAAC increased the ICER beyond the $50 000 threshold. Additional results can be found in Data S1.
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis simulations (Figure 3) demonstrated that at 20 years, LAAC had lower average total costs than warfarin and NOACs: $44 768 (95% CI, $26 009–$70 566) versus $61 585 (95% CI, $35 999–$98 329) versus $76 985 (95% CI, $44 888–$119 604), closely in line with the deterministic estimates. Relative to warfarin, there was a 97% probability that LAAC provided more QALYs and a 94% probability that LAAC was cost saving over the lifetime analysis. The overall probability of cost‐effectiveness was 98%, using a willingness‐to‐pay threshold of $50 000/QALY. At 20 years, there was a 95% probability that LAAC was cost‐effective relative to NOACs.
Figure 3.
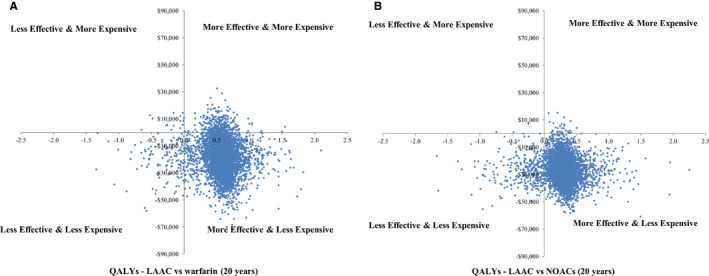
A, Scatter plots of incremental costs and incremental quality‐adjusted life‐years (QALYs) at 20 years for left atrial appendage closure (LAAC) vs warfarin. Probabilistic sensitivity analysis (PSA) results reflect 5000 model simulations to estimate the effect of uncertainty on model results. B, Scatter plots of incremental costs and incremental QALYs at 20 years for LAAC vs nonwarfarin oral anticoagulants (NOACs). PSA results reflect 5000 model simulations to estimate the effect of uncertainty on model results.
Discussion
This analysis demonstrates that LAAC with the Watchman device is a cost‐effective solution relative to both warfarin and NOACs for stroke risk reduction in patients with nonvalvular AF. Using pooled PROTECT AF and PREVAIL 5‐year RCT data, LAAC became cost‐effective and dominant (more effective and less costly) relative to NOACs at 5 years. LAAC achieved cost‐effectiveness relative to warfarin at 7 years, with an ICER of $35 051, and dominance at 10 years. Despite the increase in the risk of ischemic stroke observed in the pooled data, LAAC proved to be the most cost‐effective treatment strategy.
Sensitivity analyses indicate that model results are robust and not overly sensitive to variation in individual parameters. Results of the one‐way sensitivity analysis demonstrated that health state utilities (Well with LAAC, Well with NOACs), baseline risk of stroke and/or bleeding, and stroke outcomes had the greatest impact on model results. These findings confirmed the importance of QoL as well as the need to consider the differential stroke outcomes and costs associated with the treatment modalities. Probabilistic sensitivity analysis results confirmed that when using pooled, long‐term data, there is a high probability that LAAC is cost‐effective relative to warfarin and NOACs at 20 years (98% and 95%, respectively).
In addition to our primary analysis, we conducted a scenario analysis to understand the impact of post‐FDA, real‐world LAAC procedural data on cost‐effectiveness.11 LAAC procedure‐related complications with the Watchman device have continued to decrease since the PROTECT AF trial. Despite 71% of implanting physicians being first‐time operators and performing 50% of the procedures in this cohort, the implantation success rate and complication rate both improved. The combination of increased procedural success rates and lower complication rates in the real‐world data led to slightly higher QALYs than observed with the trial data. As would be expected, time to cost‐effectiveness remained consistent with the trial‐based analysis relative to both warfarin and NOACs. This relatively minimal improvement in cost‐effectiveness is related to the fact that postprocedural clinical events, such as stroke and bleeds, constituted the majority of costs accrued over time.
To ensure the generalizability of our findings, we further explored the impact of age on modeled results. The primary analysis considered a baseline patient 70 years of age at moderate risk of stroke and bleeds. Holding all other model inputs constant, we repeated the analysis using a baseline age of 75 and 80 years and found that LAAC remained cost‐effective relative to warfarin over the lifetime horizon.
The findings from this pooled analysis are consistent with our previously published data using only PROTECT AF 4‐year, follow‐up trial data.12 As expected, given the increased risk of ischemic stroke relative to warfarin in the pooled data, patients receiving LAAC had a modest reduction in QALYs and a slight increase in total costs over the lifetime analysis (7.77 versus 8.03 and $44 894 versus $31 198, respectively). Despite these changes, LAAC time to cost‐effectiveness remained the same: LAAC achieved cost‐effectiveness relative to warfarin and NOACs by years 7 and 5, respectively. Once achieved, LAAC cost‐effectiveness and cost savings were maintained over the lifetime analyses in both studies.
In addition to our PROTECT AF trial 4‐year cost‐effectiveness analysis, one other recent publication assessed the cost‐effectiveness of LAAC compared with warfarin and NOACs from a US payer perspective.13 Freeman et al13 examined the cost‐effectiveness of LAAC versus OACs 2 ways: first using PROTECT AF 4‐year trial data alone and then using PREVAIL 1‐year trial data alone. Consistent with our original analysis, the authors found that LAAC was cost‐effective relative to warfarin and NOACs when using the clinical results from the PROTECT AF trial ($20 486/QALY and $23 422/QALY, respectively). However, when using the PREVAIL trial, LAAC was dominated by warfarin and NOACs at 20 years. This finding was not unexpected given the PREVAIL trial was primarily designed to assess procedural safety and ultimately achieved noninferiority to warfarin for only 1 of its 2 efficacy end points. However, it must be emphasized that warfarin performance in the PREVAIL trial was atypical, with a substantially lower rate of ischemic stroke (0.7%) than ever reported for warfarin in any other stroke prevention trial.4, 5, 6 This is consistent with the wide CIs attendant with the PREVAIL trial's smaller sample size, which, again, was never intended to be analyzed in isolation. Furthermore, the mean follow‐up was much shorter in the PREVAIL trial analysis compared with the PROTECT AF trial analysis, which could account for the lower rate of long‐term complications. Last, and more important, the analysis by Freeman et al13 assumed equivalent QoL and stroke outcomes for all treatments, whereas we have used treatment‐specific data for these outcomes.
Taking the limitations of the PREVAIL trial into account but recognizing the importance of assessing the full body of RCT evidence, our analysis sought to provide greater certainty as to the cost‐effectiveness of LAAC by using pooled PROTECT AF and PREVAIL trial data over as long a period of follow‐up as possible. This approach is in line with best practices recommended by the International Society for Pharmacoeconomics and Outcomes Research.50 In addition, our analysis differentiated QoL and stroke outcomes by treatment. Patients undergoing LAAC have been shown to have higher QoL and experience fewer disabling strokes compared with patients receiving warfarin.10, 16, 17, 40, 41 Disability after a stroke has substantial implications for patient QoL and costs of care. Furthermore, sensitivity analyses reveal that these variables have notable impact on model results. Given this, one should not understate the importance of using therapy‐specific QoL and stroke outcomes data in economic evaluations of stroke prevention strategies.
Limitations
Clinical inputs were derived from different sources, including pivotal trials and meta‐analyses. No RCT directly comparing LAAC with NOACs exists; indirect comparison techniques were leveraged by necessity. The clinical studies used had different lengths of follow‐up, and data were extrapolated to 20 years; however, 5 years of follow‐up in the PROTECT AF and PREVAIL clinical trials is substantial by stroke prevention RCT standards. In addition, treatments administered in clinical practice may vary in effectiveness compared with the results observed in RCTs. Clinical inputs reflect the results of the intent‐to‐treat analyses, but the model allowed for therapy change. The model allowed for OAC switching and discontinuation through 2 years but did not account for patients restarting anticoagulation after discontinuation because of the lack of long‐term data on clinical outcomes in these treatment scenarios. Switching, discontinuing, and restarting anticoagulation may impact the stroke and bleeding risk and relative effectiveness of treatment for the warfarin and NOAC treatment arms. In the absence of such data, the results would be speculative at best; restarting OAC use may improve outcomes but allowing for further discontinuation beyond 2 years may lead to worse outcomes. Further research is needed to understand long‐term OAC patient adherence and outcomes. The baseline risks of stroke and bleeds were based on modified Congestive Heart Failure, Hypertension, Age≥75 years, Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack, Vascular Disease, Age 65‐74 years, Sex Category (CHA2DS2‐VASc) and HAS‐BLED scores, as not all score components were available in the baseline patient‐level data.8, 9, 10 Although we have presented some post‐FDA real‐world evidence as part of this analysis, these data were limited to procedural outcomes without long‐term follow‐up. Finally, we used a 3‐month cycle length with the chance for one event per cycle, although in real‐world clinical practice, patients may experience >1 event in 3 months.
Conclusion
Despite the increased risk of ischemic stroke observed in the PREVAIL trial, LAAC is cost‐effective and cost saving relative to NOACs and warfarin when the full body of RCT data is taken into consideration. LAAC with the Watchman device is an economically viable stroke risk reduction strategy for patients with AF seeking an alternative to lifelong anticoagulation. These findings confirm those of the earlier PROTECT AF trial–based analyses and should be considered when formulating policy and practice guidelines for stroke prevention in AF.
Sources of Funding
This work was supported by funding from Boston Scientific, which manufactures the Watchman device.
Disclosures
Reddy, Akehurst, Gavaghan, and Holmes are paid consultants to Boston Scientific. Amorosi is a full‐time employee of Boston Scientific.
Supporting information
Data S1. Supplemental Results
Figure S1. Tornado plots of one‐way sensitivity analyses at 20 years of LAAC vs warfarin and LAAC vs NOACs.
Acknowledgments
The authors want to acknowledge the contributions of Yue Zhong, MBBS, PhD, Nicole Gordon, and Kenneth Stein, MD, to this article. Dr Zhong, Ms Gordon, and Dr Stein served as reviewers to the article; and Dr Zhong served as reviewer to the health economic model.
(J Am Heart Assoc. 2019;8:e011577 DOI: 10.1161/JAHA.118.011577.)
References
- 1. Go AS, Mozaffarian D, Roger VL; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 3. Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, Brensinger CM, Newcomb CW, Samaha FF, Gross R. The influence of patient adherence on anticoagulation control with warfarin results from the International Normalized Ratio adherence and genetics (IN‐RANGE) study. Arch Intern Med. 2007;167:229–235. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; on behalf of RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; on behalf of ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; on behalf of ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 7. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; on behalf of ENGAGE AF‐TIMI 48 Investigators . Once‐daily edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D; on behalf of PROTECT AF Steering Committee and Investigators . Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation, a randomized clinical trial. JAMA. 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 9. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long‐term warfarin therapy—the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 10. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, Holmes DR Jr; on behalf of PREVAIL and PROTECT AF Investigators . 5‐Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 11. Reddy VY, Gibson DN, Kar S, O'Neill W, Doshi SK, Horton RP, Buchbinder M, Gordon NT, Holmes DR. Post‐approval US experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–261. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR Jr. Time to cost‐effectiveness following stroke reduction strategies in AF: warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66:2728–2739. [DOI] [PubMed] [Google Scholar]
- 13. Freeman JV, Hutton DW, Barnes GD, Zhu RP, Owens DK, Garber AM, Go AS, Hlatky MA, Heidenreich PA, Wang PJ, Al‐Ahmad A, Turakhia MP. Cost‐effectiveness of percutaneous closure of the left atrial appendage in atrial fibrillation based on results from PROTECT AF versus PREVAIL. Circ Arrhythm Electrophysiol. 2016;9:e003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosse SD. Assessing cost‐effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. [DOI] [PubMed] [Google Scholar]
- 15. Boston Scientific . WATCHMAN [package insert]. http://www.bostonscientific.com/content/dam/Manuals/us/current-rev-en/90746221-01C_Watchman%20Device_DFU_en-US_s.pdf. Accessed May 7, 2019.
- 16. Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, Krause‐Steinrauf H, Kurtzke JF, Nazarian SM, Radford MJ, Rickles FR, Shabetai R, Deykin D; on behalf of the Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators . Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. N Engl J Med. 1992;327:1406–1412. [DOI] [PubMed] [Google Scholar]
- 17. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators, Singer DE, Hughes RA, Gress DR, Sheehan MA, Oertel LB, Maraventano SW, Blewett DR, Rosner B, Kistler JP . The effect of low‐dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–1511. [DOI] [PubMed] [Google Scholar]
- 18. w?>The ACTIVE Writing Group on behalf of the ACTIVE Investigators . Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 19. Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol. 1991;18:349–355. [DOI] [PubMed] [Google Scholar]
- 20. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 21. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 22. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 23. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 24. Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 25. Atrial Fibrillation Investigators . Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 26. Ariesen MJ, Claus SP, Rinkel GJE, Algra A. Risk factors for intracerebral hemorrhage in the general population. Stroke. 2003;34:2060–2066. [DOI] [PubMed] [Google Scholar]
- 27. Healthcare Cost and Utilization Project . National inpatient sample. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed May 7, 2019. [DOI] [PMC free article] [PubMed]
- 28. Social Security Administration . Actuarial life table, period life table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed March 16, 2018.
- 29. Dennis MS, Burn JPS, Sandercock PAG, Bamford JM, Wade DT, Warlow C. Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. [DOI] [PubMed] [Google Scholar]
- 30. Gage BF, Scott JD, Owens DK. Marginal utility of warfarin and aspirin in elderly patients with non‐valvular atrial fibrillation. Med Decis Making. 1993;13:386. [Google Scholar]
- 31. O'Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293:699–706. [DOI] [PubMed] [Google Scholar]
- 32. Shah SV, Gage BF. Cost‐effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123:2562–2570. [DOI] [PubMed] [Google Scholar]
- 33. Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost‐effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA. 1995;274:1839–1845. [PubMed] [Google Scholar]
- 34. Lee S, Anglade M, Pham D, Pisacane R, Kluger J, Coleman CI. Cost‐effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845–851. [DOI] [PubMed] [Google Scholar]
- 35. Nichol MB, Sengupta N, Globe DR. Evaluating quality‐adjusted life years: estimation of the health utility index (HU12) from the SF‐36. Med Decis Making. 2001;21:105–112. [DOI] [PubMed] [Google Scholar]
- 36. Alli O, Doshi S, Kar S, Reddy V, Sievert H, Mullin C, Swarup V, Whisenant B, Holmes D Jr. Quality of life assessment in the randomized PROTECT AF trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013;61:1790–1798. [DOI] [PubMed] [Google Scholar]
- 37. Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, Herrington SA, Hays RD, Kaplan RM, Ganiats TG, Feeny D, Kind P. US norms for six generic health‐related quality of life indexes from the national health measurement survey. Med Care. 2007;45:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24:1021–1033. [DOI] [PubMed] [Google Scholar]
- 39. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 40. Stroke Prevention in Atrial Fibrillation Investigators . Stroke Prevention in Atrial Fibrillation Study: final results. Circulation. 1991;84:527–539. [DOI] [PubMed] [Google Scholar]
- 41. Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo‐controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation, the Copenhagen AFASAK study. Lancet. 1989;8631:175–178. [DOI] [PubMed] [Google Scholar]
- 42. Centers for Medicare and Medicaid Services . FY 2018 IPPS final rule home page. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page.html. Accessed May 7, 2019.
- 43. Centers for Medicare and Medicaid Services . Inpatient rehabilitation facility PPS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/index.html. Accessed May 7, 2019.
- 44. Cipriano LE, Steinberg ML, Gazelle GS, Gonzalez RG. Comparing and predicting the costs and outcomes of patients with major and minor stroke using the Boston Acute Stroke Imaging Scale neuroimaging classification system. Am J Neuroradiol. 2009;30:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mercaldi CJ, Siu K, Sander SD, Walker DR, Wu Y, Li Q, Wu N. Long‐term costs of ischemic stroke and major bleeding events among Medicare patients with nonvalvular atrial fibrillation. Cardiol Res Pract. 2012;2012:645469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caro JJ, Huybrechts FK. Stroke treatment economic model (STEM): predicting long‐term costs from functional status. Stroke. 1999;30:2574–2579. [DOI] [PubMed] [Google Scholar]
- 47. U.S. Bureau of Labor Statistics—Division of Consumer Prices and Price Indexes. http://www.bls.gov/CPI. Accessed May 7, 2019.
- 48. The Coding Institute . CPT® code lookup. https://www.supercoder.com/cpt-codes-range. Accessed May 7, 2019.
- 49. DMD America . Analysource—active NDCs. https://www.analysource.com/qry/as_products.taf?_purgefilter=Y. Accessed March 2018.
- 50. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; on behalf of ISPOR‐SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty analysis: a report of the ISPOR‐SMDM modeling good research practices task force‐6. Value Health. 2012;15:835–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Results
Figure S1. Tornado plots of one‐way sensitivity analyses at 20 years of LAAC vs warfarin and LAAC vs NOACs.


