Abstract
Functional neural circuits of mature animals are shaped during postnatal development by eliminating early-formed redundant synapses and strengthening of necessary connections. In the nervous system of newborn animals, redundant synapses are only transient features of the circuit. During subsequent postnatal development, some synapses are strengthened whereas other redundant connections are weakened and eventually eliminated. In this review, we introduce recent studies on the mechanisms of developmental remodeling of climbing fiber–to–Purkinje cell synapses in the cerebellum and synapses from the retina to neurons in the dorsal lateral geniculate nucleus of the visual thalamus (retinogeniculate synapses). These are the two representative models of developmental synapse remodeling in the brain and they share basic principles, including dependency on neural activity. However, recent studies have disclosed that, in several respects, the two models use different molecules and strategies to establish mature synaptic connectivity. We describe similarities and differences between the two models and discuss remaining issues to be tackled in the future in order to understand the general schemes of developmental synapse remodeling.
Keywords: cerebellum, climbing fiber, Purkinje cell, dorsal lateral geniculate nucleus, retinal ganglion cell, development, synapse remodeling
Introduction
In the developing nervous system of neonatal animals, redundant synaptic connections are formed and are present only transiently. Subsequently, some synapses are strengthened whereas other redundant connections are weakened and eventually eliminated 1– 5. Such synapse remodeling is widely thought to be essential for shaping functionally mature neural circuits during postnatal development 4, 5. Developmental synapse remodeling has been studied in several regions of the brain 6, 7 in addition to the neuromuscular junction and autonomic ganglia of the periphery 8, 9. Among them, postnatal development of the cerebellar climbing fiber (CF)–to–Purkinje cell (PC) synapse and the retinogeniculate synapse in the dorsal lateral geniculate nucleus (dLGN), the visual thalamus that relays visual information from the retina to the primary visual cortex, are two representative experimental models 10– 13. In these two models, postsynaptic neurons receive excitatory synaptic inputs from a limited number of presynaptic axons, and activation of each axon generates relatively large and discernible postsynaptic response. During postnatal development, the majority of the presynaptic axons innervating each neuron are pruned while one or a few axons remain and expand their innervation territories over the postsynaptic neuron. These developmental changes are analogous to those seen in the neuromuscular junction and autonomic ganglia during postnatal development. The CF-to-PC synapse and the retinogeniculate synapse provide simple experimental models of developmental synapse elimination that can be examined quantitatively. Therefore, to gain insight into basic principles of synapse remodeling in the developing brain, we focus on recent advances on the cellular and molecular mechanisms of synapse remodeling in the cerebellum and dLGN.
Multiple phases of developmental CF-to-PC synapse remodeling in the cerebellum
In the cerebellum of neonatal rodents, PCs are initially innervated by more than five CFs with similar strengths of synaptic inputs. During the first three postnatal weeks, redundant CFs are eliminated and most PCs become innervated by single CFs 10, 11, 13. This developmental process, termed CF synapse elimination, consists of at least four distinct phases in mice 1, 3, 14: (1) selective strengthening of a single CF out of multiple CFs innervating each PC from postnatal day 3 (P3) to around P7 (termed “functional differentiation”) 15, (2) translocation and expansion of innervation territory of the strongest CF (“winner” CF) to PC dendrites from P9 (termed “dendritic translocation”) 16, (3) elimination of somatic synapses of the “winner” CF and those of weaker CFs (“loser” CFs) from P7 to around P11 (termed “early phase of CF elimination”) 17, 18, and (4) elimination of the remaining somatic CF synapses from around P12 to P17 in a manner dependent on excitatory synapse formation from parallel fibers (PFs) onto PC dendrites (termed “late phase of CF elimination”) 18, 19 ( Figure 1).
Figure 1. Remodeling of CF and PF synaptic connections onto PCs during postnatal cerebellar development.
(Upper panel) Schematics depicting developmental changes in CF and PF synaptic connections to PCs at ~P3, ~P7, ~P15, and ~P30. (Lower panel) Key events related to postnatal development of CF-to-PC and PF-to-PC synapses from birth to ~P30. BC, basket cell; CF, climbing fiber; GrC, granule cell; PC, Purkinje cell; PF, parallel fiber; SC, stellate cell.
Previous studies indicate that neural activity plays crucial roles in CF synapse elimination. In a transgenic mouse line that expressed a chloride channel–YFP fusion specifically in PCs, burst firing of PC was significantly reduced and multiple CF innervation of individual PCs persisted until three months of age 20. Andjus et al. administered harmaline, which induced synchronous activation of neurons in the inferior olive, to rats from P9 to P12 and altered the normal activity pattern of CFs 21. This treatment impaired CF synapse elimination, and PCs remained multiply innervated by CFs up to P87. These results clearly indicate that normal activity levels and firings of postsynaptic PCs and normal activity patterns of presynaptic CFs are both prerequisites for CF synapse elimination.
In regard to the molecules that mediate activities of PCs, previous studies have shown that the P/Q-type voltage-dependent Ca 2+ channel (VDCC), the major VDCC in PCs, is essential for all four phases of CF synapse remodeling during postnatal cerebellar development 17, 22, 23 ( Figure 2). In addition, a study using in vivo whole-cell recording from single PCs 24 and a recent report of two-photon calcium imaging of PC population activities 25 strongly support that P/Q-VDCC is crucial for strengthening of single “winner” CF inputs ( Figure 2). On the other hand, the late phase of CF elimination has been shown to require the metabotropic glutamate receptor subtype 1 (mGluR1)–to–protein kinase Cγ (PKCγ) cascade in PCs 26– 31, involves activation of the immediate early gene Arc 22, 32, and is regulated by GABAergic inhibition of the PC soma by basket cells 33 ( Figure 2).
Figure 2. Mechanisms for neural activity-mediated remodeling of CF-to-PC synapses during postnatal cerebellar development.
( A) Ca 2+ influx through P/Q-VDCC into PCs triggers strengthening of a single CF at ~P3 to ~P7 (functional differentiation). ( B) Ca 2+ influx through P/Q-VDCC promotes translocation of the single strong CF to the PC dendrite and at the same time eliminates CF synapses from the PC soma at ~P7 to ~P11 (early phase of CF elimination). GABAergic inhibition from BC to PC inhibits Ca 2+ influx and thereby regulates elimination of somatic CFs from ~P10. ( C) mGluR1 to PKCγ signaling and Arc activated by Ca 2+ influx through P/Q-VDCC promotes elimination of somatic CF synapses at ~P12 to ~P17 (late phase of CF elimination). ( D) mGluR1 and its downstream signaling in PCs promote elimination of PF synapses from proximal portions of PC dendrites from ~P15 to ~P30 (PF synapse elimination). BC, basket cell; CF, climbing fiber; mGluR1, metabotropic glutamate receptor subtype 1; PC, Purkinje cell; PF, parallel fiber; PKCγ, protein kinase Cγ; VDCC, voltage-dependent Ca 2+ channel.
A 2016 study by Ichikawa et al. 34 revealed that, besides the aforementioned four phases of CF synapse remodeling, massive elimination of PF synapses occurs on PC dendrites from around P15 to P30 ( Figure 1). The authors showed that the domain of PC proximal dendrites with mixed CF and PF innervation expanded vigorously from P9 to P15 because of translocation of winner CFs from the soma to growing dendrites and simultaneous expansion of dendritic territories of PF innervation. Then, from around P15, PF synapses were massively eliminated from the dendritic domain of mixed CF and PF innervation. At P30, CF and PF innervation territories of PC dendrites became segregated such that a single winner CF monopolizes the proximal dendrites and about 100,000 PFs innervate the distal dendrites of each PC. Importantly, PF synapse elimination from P15 to P30 did not occur in mGluR1 or PKCγ knockout mice. Thus, mGluR1-to-PKCγ signaling in PCs is essential for establishing CF mono-innervation of PCs by eliminating redundant CF synapses from the soma and segregating CF and PF innervation territories on PC dendrites by eliminating PF synapses from the proximal dendrites of PCs 34 ( Figure 2).
Retrograde and anterograde signaling molecules for CF-to-PC synapse remodeling
Uesaka et al. profiled genes that are expressed in PCs during the period of CF-to-PC remodeling, and they focused on genes that encode secreted or membrane-associated molecules which may function as retrograde signaling molecules from PC to CF 35. These candidate genes were screened by performing RNA interference (RNAi) knockdown in PCs 35. The authors injected lentivirus carrying microRNA against the candidate gene together with enhanced green fluorescent protein (EGFP) cDNA in the cerebellum of neonatal mice and performed PC-specific knockdown of the candidate gene in vivo. Then acute cerebellar slices were prepared at various postnatal days and CF innervation was examined by recording CF-mediated excitatory postsynaptic currents (EPSCs) from PCs with knockdown of the candidate gene and from control PCs in the same slices.
Uesaka et al. found that PC-specific knockdown of Sema7A, a membrane-anchored semaphorin, specifically impaired the late phase of CF elimination 35. Furthermore, double knockdown of Sema7A and mGluR1 impaired CF synapse elimination to the same extent as single knockdown of mGluR1. By contrast, the effect of Sema7A knockdown and that of either P/Q-VDCC or the glutamate receptor GluD2 knockdown were additive 35. These results suggest that Sema7A facilitates the late phase of CF elimination downstream of mGluR1 signaling ( Figure 3). Next, to identify receptors in CFs to which Sema7A acts, Uesaka et al. injected lentivirus carrying microRNA against a candidate gene together with EGFP cDNA into the inferior olive, the brainstem nuclei from which CFs originate, of neonatal mice 35. Then the authors prepared cerebellar slices at various postnatal ages and examined CF innervation of PCs surrounded by EGFP-positive CFs with knockdown of a candidate gene. Plexin C1 (PlxnC1) and integrin B1 (ItgB1) in CFs were found to function as receptors for Sema7A derived from PCs ( Figure 3). Moreover, focal adhesion kinase (FAK) was found to mediate ItgB1 signaling in CFs 35, 36.
Figure 3. Molecular and cellular mechanisms for transcellular interaction underlying elimination of loser CFs and strengthening/maintenance of winner CFs.
Bai3, brain-specific angiogenesis inhibitor 3; BC, basket cell; BDNF, brain-derived neurotrophic factor; BG, Bergmann glia; CF, climbing fiber; GLAST, L-glutamate/L-aspartate transporter; ItgB1, integrin B1; mGluR1, metabotropic glutamate receptor subtype 1; PC, Purkinje cell; PF, parallel fiber; PlxnA4, Plexin A4; PlxnC1, Plexin C1; Sema3A, Semaphorin 3A; Sema7A, Semaphorin 7A; Sort1, Sortilin 1; TrkB, tropomyosin receptor kinase B.
Previous studies showed that mice deficient in TrkB, a high-affinity receptor for brain-derived neurotrophic factor (BDNF), were impaired in CF synapse elimination 37, 38. Choo et al. therefore tested the possibility that BDNF derived from PCs mediates a retrograde signal for CF synapse elimination 39 ( Figure 3). They generated PC-specific BDNF knockout mice and also performed lentivirus-mediated BDNF knockdown in PCs of wild-type mice. PC-specific deletion of BDNF was found to impair the late phase of CF elimination. Knockdown of mGluR1 in PCs of PC-specific BDNF knockout mice had no additive effect, whereas knockdown of P/Q-VDCC or GluD2 caused additive impairment of CF synapse elimination in PC-specific BDNF knockout mice. Furthermore, knockdown of TrkB in CFs impaired CF synapse elimination, but the additive effect was not observed in PC-specific BDNF knockout mice. These results suggest that BDNF is released from PCs downstream of mGluR1, retrogradely acts on TrkB in CFs, and facilitates CF synapse elimination during the late phase 39 ( Figure 3). To check whether BDNF-to-TrkB signaling interacts with Sema7A-to-PlxnC1/ItgB1 signaling, the effect of Sema7A knockdown in PCs of PC-specific BDNF knockout mice was examined. The effect of Sema7A knockdown was occluded in PCs lacking BDNF, suggesting that the two retrograde signaling pathways converge presumably within CFs 39 ( Figure 3).
Besides molecules that facilitate CF synapse elimination, a new category of molecules that strengthen CFs and counteract CF synapse elimination has been identified ( Figure 3). PC-specific knockdown of Sema3A, a secreted semaphorin, accelerated CF synapse elimination from P8 to P18 35. Knockdown of Plexin A4 (PlxnA4), a putative receptor for Sema3A, in CFs also accelerated CF synapse elimination, which was occluded in PCs with Sema3A knockdown 35, 36. These results suggest that Sema3A derived from PCs maintains/strengthens CF-to-PC synapses by acting retrogradely via PlxnA4 35, 36 ( Figure 3).
Recently, another set of molecules with a function similar to that of Sema3A and PlxnA4 was identified. Progranulin is a multi-functional growth factor and is known to be implicated in the pathogenesis of certain forms of frontotemporal dementia 40, 41. PC-specific deletion of progranulin resulted in acceleration of CF synapse elimination from P11 to P16 42. Knockdown of a putative progranulin receptor, Sort1, in CFs caused acceleration of CF synapse elimination, which was occluded in progranulin-deleted PCs 42. The effect of progranulin deletion and that of Sema3A deletion were additive in PCs, suggesting that progranulin and Sema3A maintain/strengthen CF synapses through independent pathways 42 ( Figure 3).
Kakegawa et al. demonstrated that, in contrast to Sema3A and progranulin, C1ql1, a member of the C1q family protein that is specifically expressed in CFs, strengthened and maintained a single winner CF by anterogradely acting on PCs 43 ( Figure 3). This effect was found to be mediated by brain-specific angiogenesis inhibitor 3 (Bai3) in PCs 43. Importantly, C1ql1-to-Bai3 anterograde signaling facilitates the elimination of weaker CFs after P9, whereas the same signaling strengthens/maintains the strongest CF and facilitates dendritic translocation of the winner CF 43. Thus, the effects of C1ql1-to-Bai3 anterograde signaling are different from those of Sema3A 35 and progranulin 42 that strengthen/maintain both strong and weaker CFs and therefore counteract the elimination of redundant CFs ( Figure 3).
Roles of microglia and astrocytes in developmental CF synapse remodeling
In retinogeniculate synapses in the developing dLGN, microglia, which are resident immune cells in the brain, and astrocytes are known to play crucial roles in synapse remodeling by actively removing redundant synapses, which will be discussed later. In contrast, much less was known about the roles of microglia and astrocytes in CF synapse remodeling in the developing cerebellum. Recently, Nakayama et al. demonstrated that microglia facilitate CF synapse elimination from P10 44, which largely corresponds to the late phase of CF elimination. Interestingly, the role of microglia in CF synapse remodeling is not phagocytosis of redundant CF synapses but facilitation of inhibitory synapse maturation onto PCs 44 ( Figure 3). Nakayama et al. found that CF synapse elimination after P10 was impaired in mice whose microglia were ablated by microglia-selective deletion of colony-stimulating factor 1 receptor (Csf1r), a molecule critical for differentiation and survival of microglia in the brain. However, in wild-type mice, the authors found no evidence for engulfment of CFs in microglia. In conditional Csf1r knockout mice, GABAergic inhibitory synaptic transmission was impaired. Importantly, enhancing GABA A receptor sensitivity by daily intraperitoneal administration of diazepam from P9 to P12 restored CF synapse elimination. Because maturation of GABAergic inhibition to PCs is known to be required for CF synapse elimination, Nakayama et al. conclude that microglia promote maturation of GABAergic inhibition and thereby facilitate CF synapse elimination 44 ( Figure 3).
A recent study also shed light on the role of Bergmann glia, specialized astrocytes in the cerebellar cortex, in developmental CF synapse remodeling 45. Bergmann glia thoroughly enwrap PCs by extensive lamellate glial processes 46 that strongly express the L-glutamate/L-aspartate transporter GLAST 47, 48. A previous study demonstrated that PCs of GLAST knockout mice had persistent multiple CF innervation in adulthood 49. However, a later study claimed that apparent multiple CF innervation of PCs in GLAST knockout mice resulted mostly from glutamate spillover from CFs innervating neighboring PCs 50. A recent detailed study by Miyazaki et al. presented clear morphological evidence for multiple CF innervation of PCs in GLAST knockout mice 45. The authors found that Bergmann glial processes were retracted from PC dendrites and synapses in GLAST knockout mice. Furthermore, the main ascending CF branch was weakened, whereas the transverse CF branch, which is normally thin and devoid of synaptic terminals, became thick and contained synaptic terminals. Both types of CF branches frequently formed aberrant synaptic connection onto the proximal and distal dendrites of neighboring PCs, which resulted in massive multiple CF innervation in individual PCs. A previous study reported similar phenotypes in mice with virus-mediated expression of the AMPA receptor GluA2 subunit in Bergmann glia 51. AMPA receptors in normal Bergmann glia lack GluA2 and are highly permeable to calcium. Although virus-mediated overexpression of GluA2 to Bergmann glia is non-physiological, Bergmann glia with GluA2-containing AMPA receptors were calcium-impermeable, their processes were retracted, and PCs had multiple CF innervation 51. These results indicate that the extension of Bergmann glial processes and their complete enwrapping of PCs are prerequisites for establishing CF mono-innervation of PCs by preventing aberrant innervation from CFs innervating neighboring PCs ( Figure 3).
Multiple phases of developmental retinogeniculate synapse remodeling in the dLGN
In the dLGN, mature retinogeniculate synapses formed by retinal ganglion cell (RGC) axons onto thalamo-cortical (TC) relay neurons are established through at least three distinct phases of postnatal development 12, 52, 53 ( Figure 4). First, RGC axons projecting to the dLGN are segregated into eye-specific projection zones by about P10 12, 54 before eye opening at around P12. Each TC neuron receives exuberant weak retinogeniculate synapses at this stage. During the second phase, the average number of RGC axons innervating each TC neuron decreases drastically, whereas a subset of RGC axons per TC neuron become stronger by around P20 55, 56. The first and second phases depend critically on retinal spontaneous activity but not on visual experience 53. During the third phase, which spans from about P20 to P30, retinogeniculate synapses are maintained in a visual experience–dependent manner ( Figure 4). Dark rearing of mice from P20 for 1 week (late dark rearing) causes about a twofold increase in the number and about 50% weakening of retinogeniculate afferents to TC neurons 53, 57.
Figure 4. Remodeling of retinogeniculate synapses during postnatal development of the dorsal lateral geniculate nucleus (dLGN).
(Upper panel) Schematics depicting developmental changes in retinogeniculate synaptic connections to PCs at ~P5, ~P12, ~P20, ~P30, and ~P60. (Lower panel) Key events related to remodeling of retinogeniculate synaptic connections during postnatal development of the dLGN. RGC, retinal ganglion cell.
Hong et al. 58 demonstrated that, unlike developmental synapse elimination in the peripheral nervous system 8, 9, axon retraction or pruning did not occur during the second and third phases of retinogeniculate synapse remodeling. The authors found that, instead, changes in the size and distribution of presynaptic terminal boutons underlay the functional remodeling of retinogeniculate circuits 58 ( Figure 4 and Figure 5). Presynaptic boutons became larger and clustered from around P10 to P20 and underwent dynamic spatial redistribution from around P20 to P30 in response to visual experience 58. Furthermore, Hong et al. found a fourth phase of retinogeniculate circuit development that follows the experience-dependent refinement phase 58. During the fourth phase, significant axon pruning occurred without significant changes in bouton clustering and single fiber strength of retinogeniculate EPSC 58 ( Figure 4).
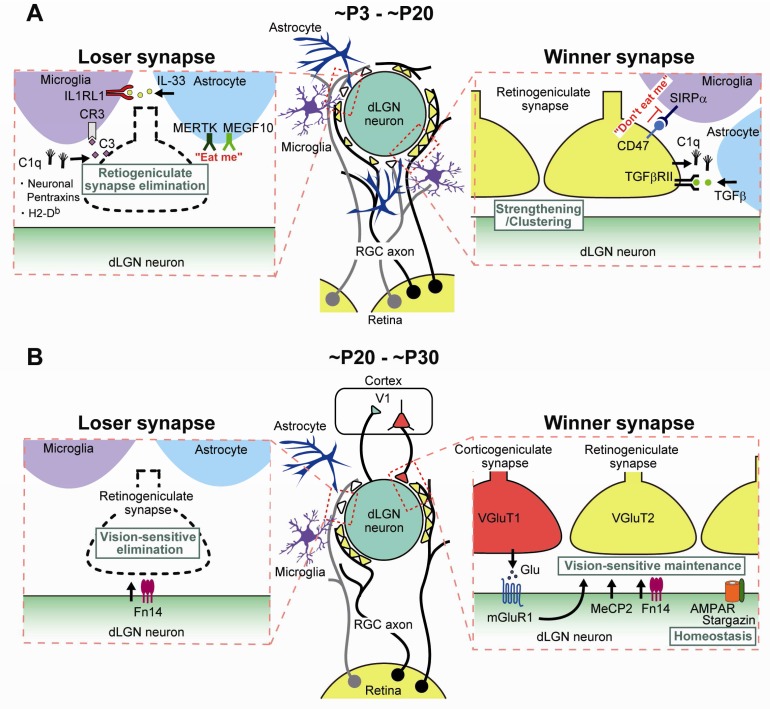
Figure 5. Molecular and cellular mechanisms underlying developmental remodeling of retinogeniculate synapses.
Molecular and cellular mechanisms underlying retinogeniculate synapse remodeling ( A) from ~P3 to ~P20 (eye-specific segregation and synapse elimination) and ( B) from ~P20 to ~P30 (vision-dependent synapse maintenance). dLGN, dorsal lateral geniculate nucleus; IL, interleukin; MeCP2, methyl-CpG-binding protein 2; mGluR1, metabotropic glutamate receptor subtype 1; RGC, retinal ganglion cell; SIRPα, signal regulatory protein alpha; TGF-β, transforming growth factor-beta; V1, primary visual cortex.
How developmental refinement of retinogeniculate synapse contributes to the maturation of functional properties of visual responses in the dLGN was unknown. Tschetter et al. found that spatial receptive fields at the time of eye opening were more than twice as large as in adulthood and decreased in size during the second and third phases of retinogeniculate synapse refinement 59. In a slice preparation, excitatory retinogeniculate input decreased and feedforward inhibition increased in TC relay neurons during the period of spatial receptive field refinement 59. These results suggest that retinogeniculate synapse remodeling and the resultant change in excitatory/inhibitory balance may underlie the spatial receptive field refinement.
Roles of the immune system-related molecules in developmental retinogeniculate synapse remodeling
Several molecules related to the immune system have been shown to be involved in eye-specific segregation and subsequent synapse elimination/strengthening of RGC projections in the dLGN ( Figure 5). Huh et al. reported that mice deficient in class I major histocompatibility complex (MHC) signaling exhibited incomplete eye-specific segregation 60. A later study, by Lee et al., demonstrated that mice deficient in the class I MHC molecules H2-K b and H2-D b (K bD b−/−) were impaired in eye-specific segregation and synapse elimination/strengthening that were dependent on spontaneous activity of the retina 61. The authors also showed that, in dLGN slices from K bD b−/− mice, long-term depression (LTD) was deficient but long-term potentiation was intact at retinogeniculate synapses 61. The impaired synapse refinement and the deficient LTD were both rescued by genetic expression of just H2-D b in neurons of K bD b−/− mice 61, suggesting that H2-D b in neurons is necessary and sufficient for structural synapse refinement and functional synaptic plasticity in the dLGN ( Figure 5).
Neuronal pentraxins (NPs), which are homologous to C-reactive and acute-phase proteins in the immune system, were reported to mediate eye-specific segregation of RGC projections 62. In mice deficient in both neuronal pentraxin 1 and 2 (NP1 and NP2), eye-specific segregation (examined at P10) was impaired despite relatively normal retinal waves 62. Delayed functional maturation of glutamatergic synapses was observed in cultured RGCs 62. These results suggest that NPs are required for functional synapse maturation in RGCs and morphological refinement of retinogeniculate synaptic connections.
Another class of immune molecules involved in retinogeniculate synapse refinement is C1q and the downstream molecules of the classic complement cascade, which is part of the innate immune system. Stevens et al. found that C1q was expressed in RGCs in response to astrocytes and was localized to synapses during early postnatal development with its peak at around P5 63. The authors demonstrated that eye-specific segregation of RGC projections and subsequent synapse elimination/strengthening were impaired in mice deficient in C1q or its downstream protein, complement component 3 (C3). These results suggest the possibility that redundant synapses are tagged by complement and subsequently eliminated in the developing retinogeniculate system ( Figure 5).
Roles of microglia and astrocytes in developmental retinogeniculate synapse remodeling
Accumulating evidence suggests that microglia play an important role in synaptic plasticity and remodeling in the healthy brain 64– 68. These studies suggest that microglia may phagocytose dendritic spines to shape functionally mature neural circuits. The first experimental evidence for the role of microglia in retinogeniculate synapse remodeling was provided by Schafer et al. 69. They demonstrated that microglia engulfed presynaptic retinal inputs during peak retinogeniculate synapse pruning at P5. The engulfment by microglia was dependent on neural activity as blockade of retinal activity by injecting tetrodotoxin into the eye reduced the extent of engulfment 69. Furthermore, the microglial engulfment of presynaptic inputs required a phagocytic receptor, complement receptor 3 (CR3), on the surface of microglia, and its ligand, C3, localized to regions with enriched synapses 69. Disruption of microglia-specific CR3/C3 signaling impaired eye-specific segregation and caused the persistent presence of redundant retinogeniculate synapses 69. Thus, C1q and C3 are considered to function as an “eat me” signal to eliminate unwanted weak retinogeniculate synapses by promoting microglial engulfment through CR3 ( Figure 5).
A recent study identified “don’t eat me” signals that appear to protect more active retinogeniculate synapses from microglial phagocytosis 70. Lehrman et al. demonstrated that CD47-to-SIRPα signaling prevented excess pruning of retinogeniculate synapses through microglial phagocytosis 70. CD47 is an immunoglobulin superfamily protein on the cell surface and inhibits phagocytosis by binding to its receptor, SIRPα, on professional phagocytes 71, 72. Mice deficient in CD47 or SIRPα showed enhanced microglial engulfment and CD47 knockout mice had a persistently reduced synapse number because of enhanced synapse pruning 70. CD47 was preferentially localized to more active synapses, whereas SIRPα was highly expressed by microglia during peak synapse pruning 70. Thus, these results suggest that CD47-to-SIRPα signaling is regulated by neuronal activity and specifically protects active retinogeniculate synapses from microglial phagocytosis ( Figure 5).
Astrocytes are also shown to play a key role in phagocytosis and pruning of retinogeniculate synapses during postnatal development 73. MEGF10 and MERTK are phagocytic receptors that recognize “eat me” signals on cell debris 74, 75. Chung et al. demonstrated that both MEGF10 and MERTK were localized in astrocytes and they engulfed synapses of retinogeniculate inputs during peak synapse pruning 73 ( Figure 5). Mice deficient in both phagocytotic receptors are impaired in eye-specific segregation and synapse elimination/strengthening in the dLGN 73. This astrocyte-mediated synapse pruning was promoted by retinal neural activity. Importantly, the authors quantitatively estimated the relative contribution of astrocytes and microglia to phagocytosis of synapses in the developing dLGN. The amount of engulfed retinogeniculate synaptic debris per imaging field by astrocytes was about 4- to 10-fold larger than that by microglia from P3 to P9, suggesting that the total amount of synapse pruning by astrocytes may exceed that by microglia 73.
Besides directly phagocytosing synapses, astrocytes regulate or influence functions of microglia. During peak synapse pruning in the dLGN, astrocytes have been reported to release transforming growth factor-beta (TGF-β) that is sensed by TGF-β receptor II expressed in RGCs 76. The astrocyte-derived TGF-β regulated C1q expression in RGCs and thereby controlled the degree of microglial engulfment of RGC inputs 76 ( Figure 5). Furthermore, a recent study in the developing spinal cord and somatosensory thalamus showed that the interleukin 1 (IL-1) family cytokine IL-33 derived from astrocytes promoted synapse engulfment by microglia 77. Thus, astrocytes appear to control microglial phagocytosis of synapses in the developing brain by releasing TGF-β and IL-33 ( Figure 5).
Mechanisms for visual experience–dependent maintenance of refined retinogeniculate synapses
Studies in the past several years have disclosed how retinogeniculate synapses that have undergone eye-specific segregation and synapse elimination/strengthening are maintained during the third phase in a visual experience–dependent manner. In mice deficient in methyl-CpG-binding protein 2 (MeCP2) whose mutations underlie the neurodevelopmental disorder Rett syndrome 78, 79, initial synapse formation, eye-specific segregation, and synapse elimination/strengthening up to P21 were normal 80 ( Figure 5). However, during the experience-dependent maintenance phase, the number of RGC inputs per relay neurons increased, retinal inputs did not strengthen further, and the late dark rearing had no further effect in MeCP2 knockout mice 80. Furthermore, specific impairment of the experience-dependent phase of synapse refinement has been reported in mice lacking the AMPA receptor auxiliary subunit stargazin 81 ( Figure 5). Visual deprivation increased stargazin expression and phosphorylation, which caused reduced rectification of AMPAR-mediated EPSCs 81. Furthermore, stargazin phosphorylation was found to be essential for synaptic scaling 81.
Thompson et al. reported that feedback inputs from the primary visual cortex (V1) to the dLGN regulated the visual experience–dependent phase of retinogeniculate synapse refinement 82. Pharmacological and chemogenetic suppression of cortical feedback activity from the V1 during P20 to P27 increased the number of RGCs innervating each thalamic relay neuron 82. Importantly, chemogenetic enhancement of V1 activity from P20 to P27 induced similar synaptic rewiring, suggesting that maintenance of a mature pattern of retinogeniculate connectivity from P20 to P27 requires proper levels or patterns of cortical feedback activity or both 82 ( Figure 5).
The mGluR1 is richly expressed in the thalamic nuclei including the dLGN 83, 84. Narushima et al. demonstrated that, in mGluR1 knockout mice, visual experience–dependent maintenance of retinogeniculate synapses was specifically impaired 85. Similar impairment was observed by RNAi-mediated knockdown and pharmacological blockade of mGluR1 in the dLGN 85. Late dark rearing had no effect in mGluR1 knockout mice, and the effect of late dark rearing in wild-type mice was rescued by pharmacological activation of mGluR1 in the dLGN 85. Quantitative immune-electron microscopic examination revealed that mGluR1 was preferentially expressed at postsynaptic sites of cortico-geniculate synapses 85. Therefore, it is legitimate to assume that mGluR1 mediates cortical feedback activity for the maintenance of retinogeniculate synapses ( Figure 5).
Recently, single-cell RNA sequencing was employed to obtain a whole transcriptome database of gene expression induced by visual experience in excitatory and inhibitory neurons in the developing dLGN 86. Among hundreds of such genes, the cytokine receptor Fn14 was the most inducible molecule in excitatory neurons. Mice deficient in Fn14 exhibited normal retinogeniculate synapse refinement mediated by spontaneous retinal activity but impaired synapse maintenance during the visual experience–dependent phase 86. Fn14-deficient mice have functionally weaker and morphologically smaller retinogeniculate synapses compared with wild-type mice 86. Thus, Fn14 appears to be a molecule that links visual experience and synapse refinement in the dLGN ( Figure 5).
Conclusions
Recent studies have disclosed new molecules and cellular interactions that are involved in developmental synapse remodeling in the cerebellum and dLGN. By reviewing synapse remodeling in the two brain regions, similarities and differences have come to light (as summarized below). In the dLGN, bouton clustering but not axon pruning contributes to synapse remodeling during the three phases until around P30 58. In contrast, it is not clear whether similar bouton clustering occurs and contributes to CF-to-PC synapse remodeling in the developing cerebellum. In both the cerebellum and the dLGN, neural activity is essential for developmental remodeling of CF-to-PC synapses 17, 20, 22, 24, 32, 87, 88 and retinogeniculate synapses 53, 89, 90, respectively. In the dLGN, spontaneous retinal activity is necessary for the first and second phases until P20, whereas visual inputs are crucial for the third phase 53. In contrast, it is not clear whether spontaneous activity or some external drive such as somatosensory input is required for any of the four phases of CF-to-PC synapse remodeling. Nevertheless, normal activity levels and firing patterns of postsynaptic PCs 20 and normal activity patterns of presynaptic CFs 21 are both required for CF synapse elimination. As for the molecules mediating neural activity, P/Q-VDCC 17 and mGluR1 in PCs 26, 28, 31 have been identified for CF-to-PC synapse remodeling whereas data for the molecules mediating neural activity for retinogeniculate synapse remodeling are limited. Whereas mGluR1 appears to mediate cortical feedback inputs from V1 to TC relay neurons during the third phase 85, molecules involved in spontaneous retinal activity–dependent synapse remodeling during the first and second phases are not clear. In regard to trans-synaptic molecules involved in pruning or strengthening of synaptic connections, several key molecules, including Sema7A 35, Sema3A 35, BDNF 39, progranulin 42, and C1ql1 43, have been identified for CF-to-PC synapse remodeling whereas little is known for retinogeniculate synapse remodeling. On the other hand, several immune system–related molecules, including H2-D b 61, NPs 62, and C1q, C3, and CR3 63, 69, play important roles in the first and second phases of retinogeniculate synapse remodeling. However, contributions of such immune system–related molecules to CF-to-PC synapse remodeling have not been reported. Furthermore, both microglia and astrocytes directly phagocytose unwanted retinogeniculate synapses 69, 73, and molecules controlling the synapse phagocytosis, including C3 and CR3 69, MEGF10 and MERTK 73, and CD47 and SIRPα 70 as well as TGF-β 76 and IL-33 77, have been reported. In contrast, microglia do not engulf CF-to-PC synapses during the postnatal period but promote the development of inhibitory synapses and thereby facilitate CF synapse elimination 44.
Future studies should further investigate similarities and differences between the two models of developmental synapse remodeling. The molecules that have been identified as involved in one model should be tested in the other provided that the same or similar molecules are also expressed. Furthermore, the molecules that are required for the two models should be tested in synapses in other brain regions such as the hippocampus and cerebral cortex. Such studies will highlight the uniqueness and commonality of synapse remodeling in different brain regions and may ultimately lead to the uncovering of the common principle of functional neural circuit formation during postnatal development.
Abbreviations
Bai3, brain-specific angiogenesis inhibitor 3; BDNF, brain-derived neurotrophic factor; C3, complement component 3; CF, climbing fiber; CR3, complement receptor 3; Csf1r, colony-stimulating factor 1 receptor; dLGN, dorsal lateral geniculate nucleus; EGFP, enhanced green fluorescent protein; EPSC, excitatory postsynaptic current; GLAST, L-glutamate/L-aspartate transporter; IL, interleukin; ItgB1, integrin B1; LTD, long-term depression; MeCP2, methyl-CpG-binding protein 2; mGluR1, metabotropic glutamate receptor subtype 1; MHC, major histocompatibility complex; NP, neuronal pentraxin; P, postnatal day; PC, Purkinje cell; PF, parallel fiber; PKCγ, protein kinase Cγ; PlxnA4, Plexin A4; PlxnC1, Plexin C1; RGC, retinal ganglion cell; RNAi, RNA interference; TC, thalamo-cortical; TGF-β, transforming growth factor-beta; VDCC, voltage-dependent Ca 2+ channel; V1, primary visual cortex
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Roy V. Sillitoe, Department of Pathology and Immunology, Baylor College of Medicine, Houston, USA
Richard Hawkes, Department of Cell Biology & Anatomy and Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Funding Statement
This work was supported in part by Grants-in-Aid for Scientific Research (18H04012 to MK) from Japan Society for the Promotion of Science (JSPS), Japan; by Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and Development (AMED), Japan; and by Strategic Research Program for Brain Sciences (SRPBS) from AMED, Japan.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Hashimoto K, Kano M: Synapse elimination in the developing cerebellum. Cell Mol Life Sci. 2013;70(24):4667–80. 10.1007/s00018-013-1405-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kano M, Hashimoto K: Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19(2):154–61. 10.1016/j.conb.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 3. Kano M, Watanabe T, Uesaka N, et al. : Multiple phases of climbing fiber synapse elimination in the developing cerebellum. Cerebellum. 2018;17(6):722–34. 10.1007/s12311-018-0964-z [DOI] [PubMed] [Google Scholar]
- 4. Katz LC, Shatz CJ: Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–8. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- 5. Lichtman JW, Colman H: Synapse elimination and indelible memory. Neuron. 2000;25(2):269–78. 10.1016/S0896-6273(00)80893-4 [DOI] [PubMed] [Google Scholar]
- 6. Riccomagno MM, Kolodkin AL: Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol. 2015;31:779–805. 10.1146/annurev-cellbio-100913-013038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Stein IS, Zito K: Dendritic spine elimination: molecular mechanisms and implications. Neuroscientist. 2019;25(1):27–47. 10.1177/1073858418769644 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Gan WB, Kwon E, Feng G, et al. : Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nat Neurosci. 2003;6(9):956–60. 10.1038/nn1115 [DOI] [PubMed] [Google Scholar]
- 9. Walsh MK, Lichtman JW: In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37(1):67–73. 10.1016/S0896-6273(02)01142-X [DOI] [PubMed] [Google Scholar]
- 10. Crepel F: Regression of functional synapses in the immature mammalian cerebellum. Trends Neurosci. 1982;5:266–9. 10.1016/0166-2236(82)90168-0 [DOI] [Google Scholar]
- 11. Hashimoto K, Kano M: Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res. 2005;53(3):221–8. 10.1016/j.neures.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 12. Hong YK, Chen C: Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21(2):228–37. 10.1016/j.conb.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lohof AM, Delhaye-Bouchard N, Mariani J: Synapse elimination in the central nervous system: functional significance and cellular mechanisms. Rev Neurosci. 1996;7(2):85–101. 10.1515/REVNEURO.1996.7.2.85 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe M, Kano M: Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34(10):1697–710. 10.1111/j.1460-9568.2011.07894.x [DOI] [PubMed] [Google Scholar]
- 15. Hashimoto K, Kano M: Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38(5):785–96. 10.1016/S0896-6273(03)00298-8 [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto K, Ichikawa R, Kitamura K, et al. : Translocation of a "winner" climbing fiber to the Purkinje cell dendrite and subsequent elimination of "losers" from the soma in developing cerebellum. Neuron. 2009;63(1):106–18. 10.1016/j.neuron.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto K, Tsujita M, Miyazaki T, et al. : Postsynaptic P/Q-type Ca 2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2011;108(24):9987–92. 10.1073/pnas.1101488108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashimoto K, Yoshida T, Sakimura K, et al. : Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009;162(3):601–11. 10.1016/j.neuroscience.2008.12.037 [DOI] [PubMed] [Google Scholar]
- 19. Hashimoto K, Ichikawa R, Takechi H, et al. : Roles of glutamate receptor δ 2 subunit (GluRδ 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21(24):9701–12. 10.1523/JNEUROSCI.21-24-09701.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lorenzetto E, Caselli L, Feng G, et al. : Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2009;106(38):16475–80. 10.1073/pnas.0907298106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andjus PR, Zhu L, Cesa R, et al. : A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience. 2003;121(3):563–72. 10.1016/s0306-4522(03)00556-6 [DOI] [PubMed] [Google Scholar]
- 22. Mikuni T, Uesaka N, Okuno H, et al. : Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron. 2013;78(6):1024–35. 10.1016/j.neuron.2013.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyazaki T, Hashimoto K, Shin HS, et al. : P/Q-type Ca 2+ channel α1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24(7):1734–43. 10.1523/JNEUROSCI.4208-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawamura Y, Nakayama H, Hashimoto K, et al. : Spike timing-dependent selective strengthening of single climbing fibre inputs to Purkinje cells during cerebellar development. Nat Commun. 2013;4:2732. 10.1038/ncomms3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Good JM, Mahoney M, Miyazaki T, et al. : Maturation of cerebellar Purkinje cell population activity during postnatal refinement of climbing fiber network. Cell Rep. 2017;21(8):2066–73. 10.1016/j.celrep.2017.10.101 [DOI] [PubMed] [Google Scholar]
- 26. Ichise T, Kano M, Hashimoto K, et al. : mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288(5472):1832–5. 10.1126/science.288.5472.1832 [DOI] [PubMed] [Google Scholar]
- 27. Kano M, Hashimoto K, Chen C, et al. : Impaired synapse elimination during cerebellar development in PKC γ mutant mice. Cell. 1995;83(7):1223–31. 10.1016/0092-8674(95)90147-7 [DOI] [PubMed] [Google Scholar]
- 28. Kano M, Hashimoto K, Kurihara H, et al. : Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18(1):71–9. 10.1016/S0896-6273(01)80047-7 [DOI] [PubMed] [Google Scholar]
- 29. Kano M, Hashimoto K, Watanabe M, et al. : Phospholipase cβ4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci U S A. 1998;95(26):15724–9. 10.1073/pnas.95.26.15724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Offermanns S, Hashimoto K, Watanabe M, et al. : Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq. Proc Natl Acad Sci U S A. 1997;94(25):14089–94. 10.1073/pnas.94.25.14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohtani Y, Miyata M, Hashimoto K, et al. : The synaptic targeting of mGluR1 by its carboxyl-terminal domain is crucial for cerebellar function. J Neurosci. 2014;34(7):2702–12. 10.1523/JNEUROSCI.3542-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawata S, Miyazaki T, Yamazaki M, et al. : Global scaling down of excitatory postsynaptic responses in cerebellar Purkinje cells impairs developmental synapse elimination. Cell Rep. 2014;8(4):1119–29. 10.1016/j.celrep.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 33. Nakayama H, Miyazaki T, Kitamura K, et al. : GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron. 2012;74(2):384–96. 10.1016/j.neuron.2012.02.032 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Ichikawa R, Hashimoto K, Miyazaki T, et al. : Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination. Proc Natl Acad Sci U S A. 2016;113(8):2282–2287. 10.1073/pnas.1511513113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uesaka N, Uchigashima M, Mikuni T, et al. : Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science. 2014;344(6187):1020–3. 10.1126/science.1252514 [DOI] [PubMed] [Google Scholar]
- 36. Uesaka N, Kano M: Presynaptic mechanisms mediating retrograde semaphorin signals for climbing fiber synapse elimination during postnatal cerebellar development. Cerebellum. 2018;17(1):17–22. 10.1007/s12311-017-0888-z [DOI] [PubMed] [Google Scholar]
- 37. Bosman LW, Hartmann J, Barski JJ, et al. : Requirement of TrkB for synapse elimination in developing cerebellar Purkinje cells. Brain Cell Biol. 2006;35(1):87–101. 10.1007/s11068-006-9002-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson EM, Craig ET, Yeh HH: TrkB is necessary for pruning at the climbing fibre-Purkinje cell synapse in the developing murine cerebellum. J Physiol. 2007;582(Pt 2):629–46. 10.1113/jphysiol.2007.133561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choo M, Miyazaki T, Yamazaki M, et al. : Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat Commun. 2017;8(1):195. 10.1038/s41467-017-00260-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker M, Mackenzie IR, Pickering-Brown SM, et al. : Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–9. 10.1038/nature05016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Cruts M, Gijselinck I, van der Zee J, et al. : Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–4. 10.1038/nature05017 [DOI] [PubMed] [Google Scholar]
- 42. Uesaka N, Abe M, Konno K, et al. : Retrograde signaling from progranulin to Sort1 counteracts synapse elimination in the developing cerebellum. Neuron. 2018;97(4):796–805.e5. 10.1016/j.neuron.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 43. Kakegawa W, Mitakidis N, Miura E, et al. : Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron. 2015;85(2):316–29. 10.1016/j.neuron.2014.12.020 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Nakayama H, Abe M, Morimoto C, et al. : Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum. Nat Commun. 2018;9(1):2830. 10.1038/s41467-018-05100-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Miyazaki T, Yamasaki M, Hashimoto K, et al. : Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proc Natl Acad Sci U S A. 2017;114(28):7438–43. 10.1073/pnas.1617330114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamada K, Fukaya M, Shibata T, et al. : Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418(1):106–20. [DOI] [PubMed] [Google Scholar]
- 47. Chaudhry FA, Lehre KP, van Lookeren Campagne M, et al. : Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–20. 10.1016/0896-6273(95)90158-2 [DOI] [PubMed] [Google Scholar]
- 48. Rothstein JD, Martin L, Levey AI, et al. : Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–25. 10.1016/0896-6273(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 49. Watase K, Hashimoto K, Kano M, et al. : Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10(3):976–88. 10.1046/j.1460-9568.1998.00108.x [DOI] [PubMed] [Google Scholar]
- 50. Takayasu Y, Iino M, Shimamoto K, et al. : Glial glutamate transporters maintain one-to-one relationship at the climbing fiber-Purkinje cell synapse by preventing glutamate spillover. J Neurosci. 2006;26(24):6563–72. 10.1523/JNEUROSCI.5342-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iino M, Goto K, Kakegawa W, et al. : Glia-synapse interaction through Ca 2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292(5518):926–9. 10.1126/science.1058827 [DOI] [PubMed] [Google Scholar]
- 52. Guido W: Refinement of the retinogeniculate pathway. J Physiol (Lond). 2008;586(18):4357–62. 10.1113/jphysiol.2008.157115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hooks BM, Chen C: Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52(2):281–91. 10.1016/j.neuron.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 54. Huberman AD: Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17(1):73–80. 10.1016/j.conb.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 55. Chen C, Regehr WG: Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28(3):955–66. 10.1016/S0896-6273(00)00166-5 [DOI] [PubMed] [Google Scholar]
- 56. Ziburkus J, Guido W: Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96(5):2775–84. 10.1152/jn.01321.2004 [DOI] [PubMed] [Google Scholar]
- 57. Hooks BM, Chen C: Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28(18):4807–17. 10.1523/JNEUROSCI.4667-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong YK, Park S, Litvina EY, et al. : Refinement of the retinogeniculate synapse by bouton clustering. Neuron. 2014;84(2):332–9. 10.1016/j.neuron.2014.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Tschetter WW, Govindaiah G, Etherington IM, et al. : Refinement of spatial receptive fields in the developing mouse lateral geniculate nucleus is coordinated with excitatory and inhibitory remodeling. J Neurosci. 2018;38(19):4531–42. 10.1523/JNEUROSCI.2857-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Huh GS, Boulanger LM, Du H, et al. : Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290(5499):2155–9. 10.1126/science.290.5499.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee H, Brott BK, Kirkby LA, et al. : Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509(7499):195–200. 10.1038/nature13154 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Bjartmar L, Huberman AD, Ullian EM, et al. : Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26(23):6269–81. 10.1523/JNEUROSCI.4212-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stevens B, Allen NJ, Vazquez LE, et al. : The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Nimmerjahn A: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Paolicelli RC, Bolasco G, Pagani F, et al. : Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Schafer DP, Lehrman EK, Stevens B: The “quad-partite”synapse: Microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. 10.1002/glia.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tremblay MÈ, Lowery RL, Majewska AK: Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Wake H, Moorhouse AJ, Jinno S, et al. : Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–80. 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schafer DP, Lehrman EK, Kautzman AG, et al. : Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Lehrman EK, Wilton DK, Litvina EY, et al. : CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100(1):120–134.e6. 10.1016/j.neuron.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Okazawa H, Motegi S, Ohyama N, et al. : Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174(4):2004–11. 10.4049/jimmunol.174.4.2004 [DOI] [PubMed] [Google Scholar]
- 72. Oldenborg PA, Zheleznyak A, Fang YF, et al. : Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–4. 10.1126/science.288.5473.2051 [DOI] [PubMed] [Google Scholar]
- 73. Chung WS, Clarke LE, Wang GX, et al. : Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Hochreiter-Hufford A, Ravichandran KS: Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. 10.1101/cshperspect.a008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tung TT, Nagaosa K, Fujita Y, et al. : Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper. J Biochem. 2013;153(5):483–91. 10.1093/jb/mvt014 [DOI] [PubMed] [Google Scholar]
- 76. Bialas AR, Stevens B: TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16(12):1773–82. 10.1038/nn.3560 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; F1000 Recommendation
- 77. Vainchtein ID, Chin G, Cho FS, et al. : Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359(6381):1269–73. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Amir RE, Van den Veyver IB, Wan M, et al. : Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–8. 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- 79. Lewis JD, Meehan RR, Henzel WJ, et al. : Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69(6):905–14. 10.1016/0092-8674(92)90610-o [DOI] [PubMed] [Google Scholar]
- 80. Noutel J, Hong YK, Leu B, et al. : Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70(1):35–42. 10.1016/j.neuron.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Louros SR, Hooks BM, Litvina L, et al. : A role for stargazin in experience-dependent plasticity. Cell Rep. 2014;7(5):1614–25. 10.1016/j.celrep.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Thompson AD, Picard N, Min L, et al. : Cortical feedback regulates feedforward retinogeniculate refinement. Neuron. 2016;91(5):1021–33. 10.1016/j.neuron.2016.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Godwin DW, van Horn SC, Erişir A, et al. : Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci. 1996;16(24):8181–92. 10.1523/JNEUROSCI.16-24-08181.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vidnyánszky Z, Görcs TJ, Négyessy L, et al. : Immunocytochemical visualization of the mGluR1a metabotropic glutamate receptor at synapses of corticothalamic terminals originating from area 17 of the rat. Eur J Neurosci. 1996;8(6):1061–71. 10.1111/j.1460-9568.1996.tb01273.x [DOI] [PubMed] [Google Scholar]
- 85. Narushima M, Uchigashima M, Yagasaki Y, et al. : The metabotropic glutamate receptor subtype 1 mediates experience-dependent maintenance of mature synaptic connectivity in the visual thalamus. Neuron. 2016;91(5):1097–109. 10.1016/j.neuron.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 86. Cheadle L, Tzeng CP, Kalish BT, et al. : Visual experience-dependent expression of Fn14 is required for retinogeniculate refinement. Neuron. 2018;99(3):525–539.e10. 10.1016/j.neuron.2018.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Kakizawa S, Yamasaki M, Watanabe M, et al. : Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20(13):4954–61. 10.1523/JNEUROSCI.20-13-04954.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rabacchi S, Bailly Y, Delhaye-Bouchaud N, et al. : Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256(5065):1823–5. 10.1126/science.1352066 [DOI] [PubMed] [Google Scholar]
- 89. Huberman AD, Feller MB, Chapman B: Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. 10.1146/annurev.neuro.31.060407.125533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Penn AA, Riquelme PA, Feller MB, et al. : Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279(5359):2108–12. 10.1126/science.279.5359.2108 [DOI] [PubMed] [Google Scholar]