Abstract
Obesity is a primary risk factor for osteoarthritis, and previous studies have shown that dietary content may play an important role in osteoarthritis pathogenesis of cartilage and bone in the knee. Several previous studies have shown that the ratio of ω-3 polyunsaturated fatty acids (PUFAs), ω-6 PUFAs, and saturated fatty acids can significantly influence bone structure and osteoarthritis (OA) progression. However, the influence of obesity or dietary fatty acid content on shoulder OA is not well understood. The goal of this study was to investigate the role of dietary fatty acid content on bone and cartilage structure in the mouse shoulder in a model of diet-induced obesity. For 24 weeks, mice were fed control or high-fat diets supplemented with ω-3 PUFAs, ω-6 PUFAs, or saturated fatty acids. The humeral heads were analyzed for bone morphometry and mineral density by microCT. Cartilage structure and joint synovitis were determined by histological grading, and microscale mechanical properties of the cartilage extracellular and pericellular matrices were quantified using atomic force microscopy. Diet-induced obesity significantly altered bone morphology and mineral density in a manner that was dependent on dietary free fatty acid content. In general, high-fat diet groups showed decreased bone quality, with the ω-3 diet being partially protective. Cartilage mechanical properties and OA scores showed no changes with obesity or diet. These findings are consistent with clinical literature showing little if any relationship between obesity and shoulder osteoarthritis (unlike knee osteoarthritis), but suggest that diet-induced obesity may influence other joint tissues.
Keywords: chondrocyte, diabetes, osteoblast, type VI collagen, chondron
INTRODUCTION
Osteoarthritis (OA) is a degenerative joint disease that is characterized by the thinning or loss of the articular cartilage surfaces, as well as altered bone remodeling and synovial inflammation that clinically manifests as joint pain and dysfunction.1 There are currently no disease-modifying pharmacological treatments available for osteoarthritis, and the standard of care involves lifestyle modification (i.e., diet/exercise) and palliative care until ultimately a total joint replacement is required.2 Obesity is one of the primary risk factors for OA, with the historic perspective that increased body weight resulted in more “wear and tear” of the joint; however, it is now clear that metabolic and inflammatory changes associated with obesity are likely to contribute to OA and may significantly interact with mechanical factors to influence the pathogenesis of OA.3,4 For example, the positive association between obesity and OA in non-weight-bearing joints such as the hand indicate that factors other than mechanical overloading may also influence joint health in obesity. It is now hypothesized that the influence of obesity on OA may result primarily from metabolic changes that are reflected through chronic, low-grade systemic inflammation that occurs secondary to increased adiposity and immune cell infiltration into visceral fat depots.3,5–7 However, since normal mechanical loading has been shown to be necessary to maintain cartilage homeostasis,8 the influence of obesity on other joints, such as the shoulder with its particularly complex loading environment, remains unclear, with some studies showing mild or no association between obesity and OA in this joint.9
To study the mechanisms linking obesity and OA more directly, animal models of obesity have been developed using either a high-fat diet10–14 or genetic ablation of leptin signaling (e.g., ob/ob or db/db mice).15 Multiple studies have shown increased severity of spontaneous OA15–17 or injury-induced OA7,13,14 in animals fed a high-fat diet, but a lack of spontaneous OA in obese, leptin-deficient mice fed a standard chow diet.15
There is also increasing evidence that dietary composition itself can contribute to obesity-induced OA. In addition to the caloric content, there is increasing evidence that the composition of the diet, particularly the fatty acid content, may have significant effects on the severity of OA in a joint-specific manner.7,10,18,19 For example, high-fat diets composed of mainly saturated fatty acids (SFAs) or ω-6 polyunsaturated fatty acids (PUFAs) show considerably worse injury-induced knee OA than weight-matched counterparts on a high-fat diet with ω-3 PUFAs or a standard mouse chow diet.10 These studies observed that a high-fat diet supplemented in ω-3 PUFAs protect the knee from injury-induced OA, while high-fat diets rich in saturated fat and ω-6 PUFAs exacerbate cartilage degeneration and synovitis after knee injury. However, no significant effect of increased dietary ω-3 PUFAs has been observed on the development of spontaneous OA in the knee joint.7,10,20
The influence of diet and obesity on joints that do not regularly bear body weight, such as the shoulder, remains unclear. While correlations have been observed between serum adipokine levels and shoulder OA,21 most clinical studies show mild or no association between obesity and OA in this joint.9 However, it is important to note that early changes in OA may be reflected in other joints22 or other tissues such as bone or synovium, depending on the type or cause of OA.23 For example, animal models of OA show an increase in subchondral trabecular separation, and decreases in both bone volume fraction (BV/TV) and mineral content (BMD) within the affected joint,24 and previous studies have shown a decrease in overall bone quality with obesity.25 Furthermore, some of the earliest detectable changes in cartilage are observed in the tissue mechanical properties,26 potentially at the microscale regions immediately surrounding the chondrocytes.27 This surrounding region, known as the pericellular matrix (PCM), differs both in composition and mechanical properties to the bulk extracellular matrix (ECM).28 As this region completely surrounds the chondrocyte, it may reflect some of the earliest cell-mediated changes that occur in the tissue.27 Previous studies have observed decreases in the stiffness of the PCM and the ECM with OA, as well as a decrease in the stiffness gradient between the softer PCM and stiffer ECM.28 which would be expected to alter the mechanical environment of chondrocytes.29
The goal of this study was to determine whether dietary fatty acid content influenced the onset of spontaneous OA and bone structural changes of the shoulder in high-fat diet-induced obese mice. Mice were fed either a low-fat control chow diet, or high-fat diets rich in ω-3 PUFAs, ω-6 PUFAs, or SFAs. We hypothesized that the ω-3 PUFA rich diet would have a protective effect on shoulder cartilage and bone. The bone, cartilage, and synovium of the humeral heads were analyzed for OA-associated changes using micro-computed tomography (MicroCT) to examine bone microstructure, atomic force microscopy (AFM) to examine micro-scale cartilage mechanical properties, and histological grading to assess cartilage degeneration and synovial inflammation.
METHODS
Animal model.
Beginning at 4 weeks of age, male C57BL/6J mice were fed for 24 weeks either a control low-fat (10% kcal fat) or one of three high-fat (60% kcal fat) diets rich in ω-3 PUFAs, ω-6 PUFAs, or SFAs. These mice were originally developed for a previous study investigating how dietary fatty acids affect the development of knee OA, and data on the dietary contents, body weights, knee OA severity, serum cytokine and lipid measurements, and ear wound healing response were reported.7,10 Thus no live animals were used in the present study, but all previous animal use procedures were approved by the Institutional Animal Care and Use Committee.7,10 All specimens were stored at −20˚C following euthanasia. Prior to analysis, specimens were thawed at 4˚C, and humeral heads were isolated.
MicroCT analysis of trabecular and cortical bone.
Humeral heads were fixed in 4% paraformaldehyde for 24 hours at room temperature (n=12-15 per diet group). Specimens were then dehydrated in ethanol and scanned in air with MicroCT (SkyScan 1176, Bruker, Billerica, MA) at 9 μm/pixel resolution, an x-ray voltage of 55 kV, current 455 μA, exposure time 980 ms, 3x frame averaging, and beam filtration with 0.5 mm aluminum filter. Bone mineral density was calibrated using hydroxyapatite phantoms (Bruker). Humeral head trabecular regions were defined as the volume between the subchondral bone plate and the proximal growth plate. Each region was analyzed using the BoneJ extension in ImageJ. Trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th.), and trabecular separation (Tb.Sp.) were calculated. Cortical bone regions were analyzed using the automated CTan software package (Bruker). Cortical bone cross-sectional area and thickness were calculated from 20 slices located at two diaphyseal regions: 0.5 mm from the distal aspect of the humeral head, and at the distal aspect of the deltoid tuberosity (n=6-14).
Atomic Force Microscopy.
Mechanical testing of cartilage microscale properties was performed with an AFM as described previously.28,30 In brief, freshly dissected humeral heads were embedded in optimum cutting temperature (OCT) medium, and 5 μm thick cryosections of humeral head articular cartilage were obtained. Sections were briefly thawed and immunologically stained for collagen VI as a marker of the PCM. Briefly, sections were washed in PBS, blocked in 10% normal goat serum, and incubated with Affinity Purified Rabbit Polyclonal Collagen Type VI Antibody (Fitzgerald Industries, Acton, MA) diluted 1:200 in blocking solution for 1 hour. Sections were rinsed with PBS and stained with AlexaFluor 488 conjugated goat anti-rabbit antibody diluted at 1:200 in blocking solution (Abcam, Cambridge, MA). Sections were maintained in PBS until analysis to preserve hydration. The local mechanical modulus was calculated using an AFM (MFP-3D, Asylum Research, Santa Barbara, CA). Cartilage tissue was probed using a silicon cantilever (k ~ 5.4N/m; Novascan Technologies, Ames, IA) with a 5 μm-diameter borosilicate spherical indenter that was calibrated daily. Regions of interest encompassing a single chondrocyte were identified using fluorescence microscopy and located approximately 1-2 cells away from the outer edges of the sections to avoid edge artifacts while remaining out of deep-zone cartilage and subchondral bone. The AFM analysis region was 10 μm2 and an indentation was performed every 0.5 μm to give a total number of 400 indentations per region. The sample was indented at a rate of 10 μm/s, as previous studies have shown little or no variation in modulus values for indentation speeds between 5 and 25 μm/s.31 The indentation was continued until the trigger force, 300 nN, was reached.
Cantilever deflection and z-piezo movement were recorded for each indentation spot from the Asylum Research software and analyzed using a custom MATLAB script (The MathWorks, Natick, MA). The tissue elastic modulus was defined using a modified Hertz model as previously described.28,31–33 Contact point extrapolation was used to determine the point at which the cantilever contacted the surface. The fluorescence images were overlaid on the stiffness maps to identify each indentation spot as ECM or PCM. Analysis points within the cell body were excluded from further analysis. Data points were also excluded if they exceeded 2.5 times the mean of the surrounding values; in this case, they were replaced with the mean value of the adjacent points. To plot the modulus versus distance from the center of the cell, the fluorescent image of each cell region was thresholded in MATLAB, and average moduli were calculated for each 0.5 μm thick ring defined radially beginning at the edge of the cell. Due to the high density of chondrocytes in murine cartilage, the average modulus was truncated at its maximum value to avoid including regions of PCM from neighboring chondrocytes.
Histologic analysis and grading.
Humeral heads were fixed in 4% paraformaldehyde, decalcified using Calex II (Thermo Fisher Scientific, Waltham, MA) and embedded in paraffin. The samples were sectioned at 8μm and stained using safranin-O, fast green and hematoxylin for OA grading, or hematoxylin and eosin for synovitis grading. The cartilage phenotype was graded using a modified Mankin scoring system by two blinded individuals.7,13 This grading consisted of the following categories: cartilage surface structure (0-11), tidemark duplication (0-3), Safranin-O staining (0-8), chondrocyte clones in uncalcified cartilage (0-2), hypertrophic chondrocytes in calcified cartilage (0-2), and subchondral bone thickness (0-2). The total score possible was 28. The synovial inflammation was graded by two blinded scorers using an established scoring system.13 This consisted of the following categories: enlargement of the synovial lining cell layer (0-3) and density of the cells in the synovial lining (0-3). The thickness of uncalcified and calcified cartilage and the subchondral plate were determined using representative histological images. An average was taken of five thickness measurements for each joint.
Statistical Analysis.
Significance between diet groups for bone and AFM outcomes were determined by one-way ANOVA and Tukey post hoc tests. For discrete histological grading scores, significance was determined using a Kruskal-Wallis test. Data is presented as mean ± standard error of the mean (SEM). Outliers were determined using a ROUT test (Q=1%). All statistics were performed in Prism 7.03 (GraphPad Software, La Jolla, CA).
RESULTS
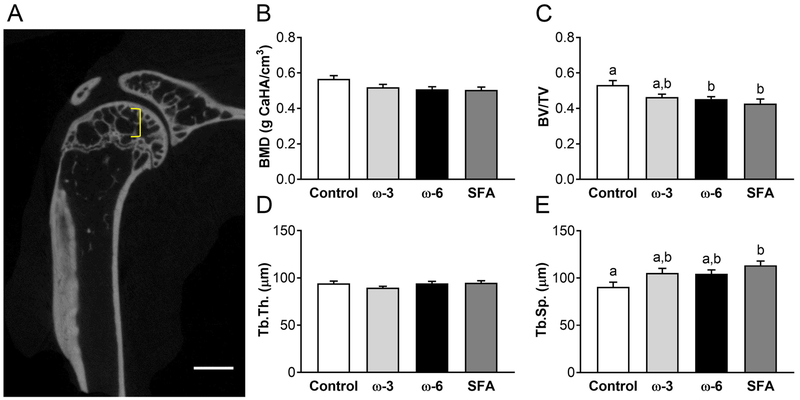
MicroCT analysis of the humeral epiphyseal trabecular region (Figure 1A) showed no effect of dietary fatty acid composition on the BMD (Figure 1B) or the Tb.Th (Figure 1D). Bone volume fraction BV/TV (Figure 1C) and Tb.Sp (Figure 1E) were significantly different between diet groups. BV/TV was significantly decreased in both the ω-6 and saturated fat diet groups, whereas Tb.Sp increased for the saturated fat diet group alone compared to the control diet group.
Figure 1: Trabecular region microCT results.
A) Bracket indicates trabecular region analyzed between the subchondral bone plate and the proximal growth plate. Scale bar: 1mm. B) Trabecular bone mineral density (BMD). C) Bone volume fraction, bone volume/total volume (BV/TV). D) Trabecular thickness (Tb.Th). E) Trabecular separation (Tb.Sp). n=12-15 per diet group. Data presented as mean ± SEM. Groups not sharing the same letter are significantly different from one other, one-way ANOVA and Tukey’s post-hoc test, p<0.05.
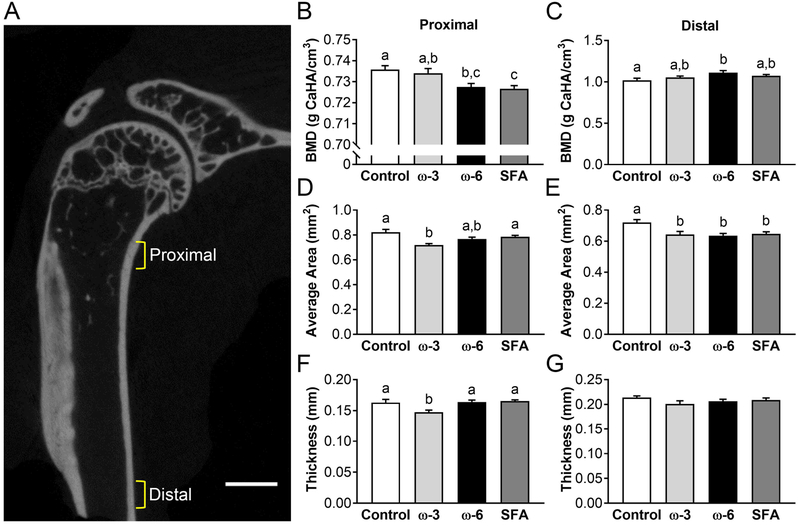
In the cortical bone, two regions were analyzed and are shown as the proximal cortical region and distal cortical region (Figure 2A). More pronounced changes in the bone mineral density can be seen in the distal cortical region, but significant differences due to diet were found in both regions (Figure 2B and 2C). Interestingly, the saturated fat diet shows a lower bone mineral density in the proximal region, but not in the distal region. In the proximal region, only the ω-3 diet group had a decrease in area (Figure 2D); however, all high-fat diets resulted in a significant decrease in the average cross-sectional area in the distal region relative to standard chow controls (Figure 2E). The proximal region cortical thickness in the ω-3 group was also significantly decreased from all other diets (Figure 2F), but the distal region thickness showed no diet-dependence (Figure 2G).
Figure 2: Cortical bone microCT results.
A) Brackets indicate two analyzed diaphyseal regions: proximal begins 0.5mm below the end of the humeral head and is 0.5mm thick, distal begins after the deltoid tuberosity and is 0.4mm thick. Scale bar: 1mm. Bone mineral density (BMD) for B) proximal and C) distal. Average cross-sectional area for D) proximal and E) distal. Cortical thickness for F) proximal and G) distal. Data presented as mean ± SEM. Groups not sharing the same letter are significantly different from one other, one-way ANOVA and Tukey’s post-hoc test, p<0.05.
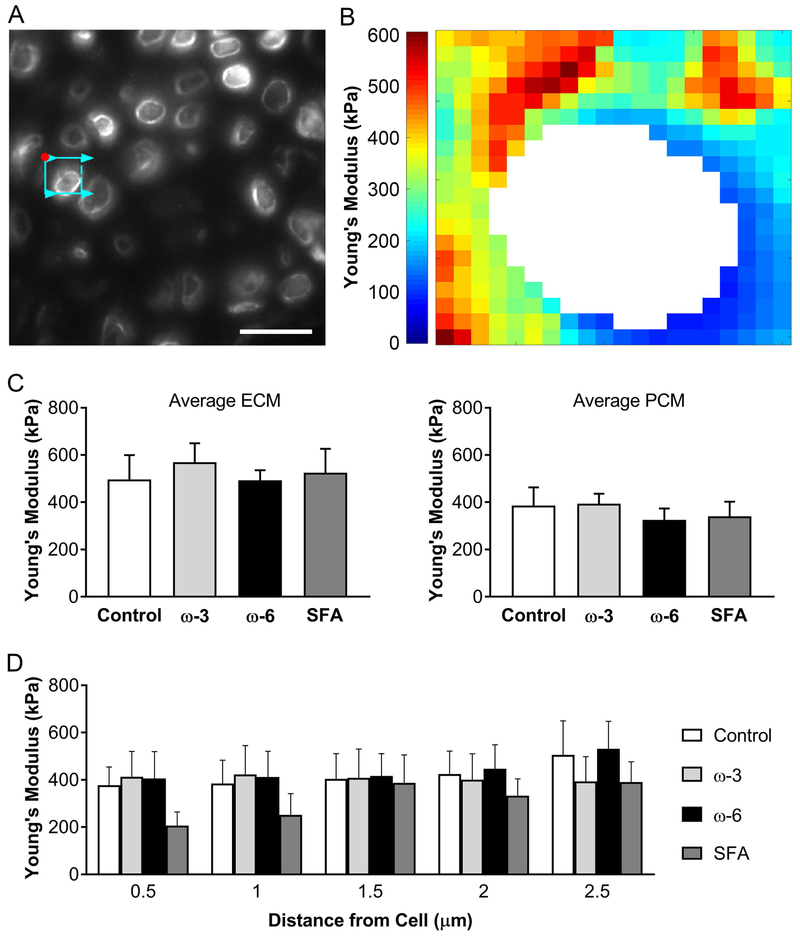
Mechanical testing using immunofluorescence-guided atomic force microscopy (Figure 3A) showed no significant changes in the cartilage modulus values either for the bulk ECM or the PCM surrounding the chondrocyte (Figure 3C). The progression of the modulus from softer PCM to stiffer ECM was not significantly altered by the diet, although the mice fed SFA diet showed a trend toward decreased modulus (Figure 3D). A representative stiffness map (Figure 3B) shows the softer PCM surrounding the cell and the gradual progression to the stiffer ECM.
Figure 3: Atomic force microscopy mechanical mapping results.
A) Fluorescent image of tested area. Red dot indicates start location of AFM probe, arrowed cyan box indicates probe scan area and direction. Scale bar: 20μm. B) Representative stiffness map. Each pixel represents one force measurement for each 0.5μm × 0.5μm area. C) Young’s Modulus averaged for ECM and for PCM. D) Young’s modulus progression out from the cell in concentric rings of 0.5μm. Data presented as mean ± SEM. No statistically significant differences were observed (one-way ANOVA, p<0.05).
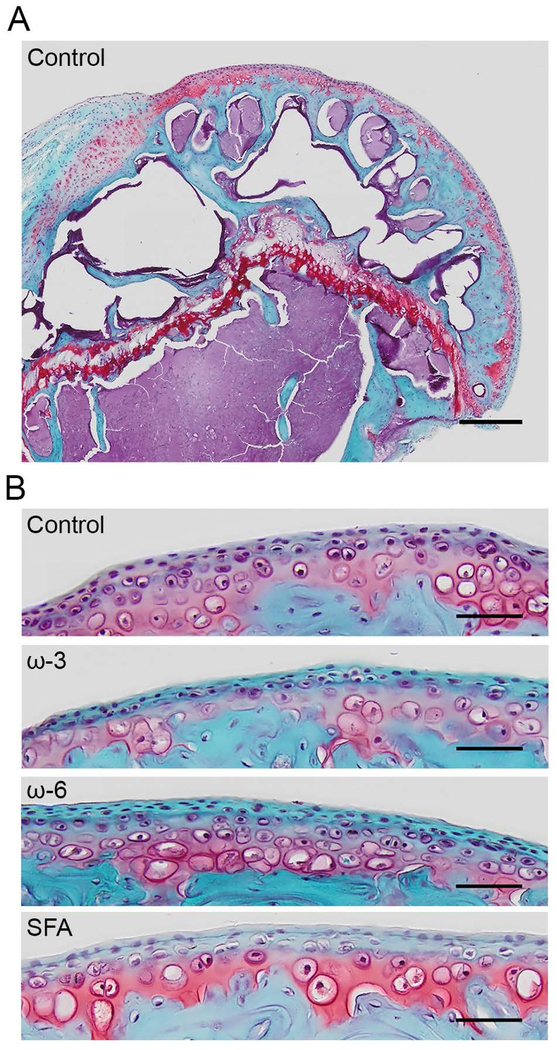
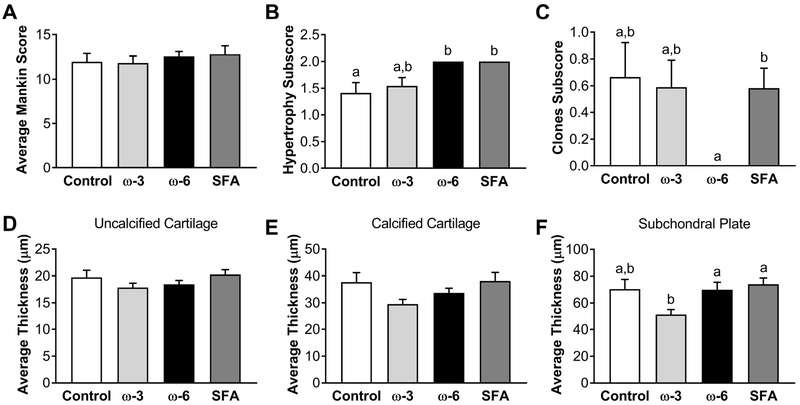
A representative histology image of a humeral head from the control group is shown in Figure 4A, and enlarged images of the cartilage surface from each diet group are shown in Figure 4B. While the overall modified Mankin score showed no differences with diet (Figure 5A), two sub-categories varied with diet. The number of hypertrophic chondrocytes in calcified cartilage (Figure 5B) was significantly increased in the ω-6 and saturated fat groups compared to the ω-3 and control diets. Also, the number of chondrocyte clones in uncalcified cartilage (Figure 5C) was significantly decreased in the ω-6 diet relative to all other diet groups. Corroborating the absent cartilage phenotype, the respective thicknesses of the uncalcified (Figure 5D) and calcified (Figure 5E) cartilage regions was also independent of diet. However, the thickness of the subchondral bone plate measured from the histology images was significantly decreased in the ω-3 diet compared to all other diet groups (Figure 5F).
Figure 4: Representative Safranin-O histology images.
Red: proteoglycans, blue: bone and cartilage. A) Full humeral head from a mouse on the control diet. Scale bar: 0.25mm. B) Representative cartilage surfaces for each diet group. Scale bar: 0.05mm.
Figure 5: Histological results.
A) Average Mankin score. B) Hypertrophy subscore. C) Clones subscore. Average thickness of D) uncalcified cartilage, E) calcified cartilage, and F) subchondral plate. Outliers were eliminated using a ROUT test (Q=1%). Data presented as mean ± SEM. Groups not sharing the same letter are significantly different from one other. Statistical significance for the Mankin scores and subscores was determined using a Kruskal-Wallis test, p<0.05. Groups not sharing the same letter are significantly different from one other (one-way ANOVA and Tukey’s post-hoc test, p<0.05).
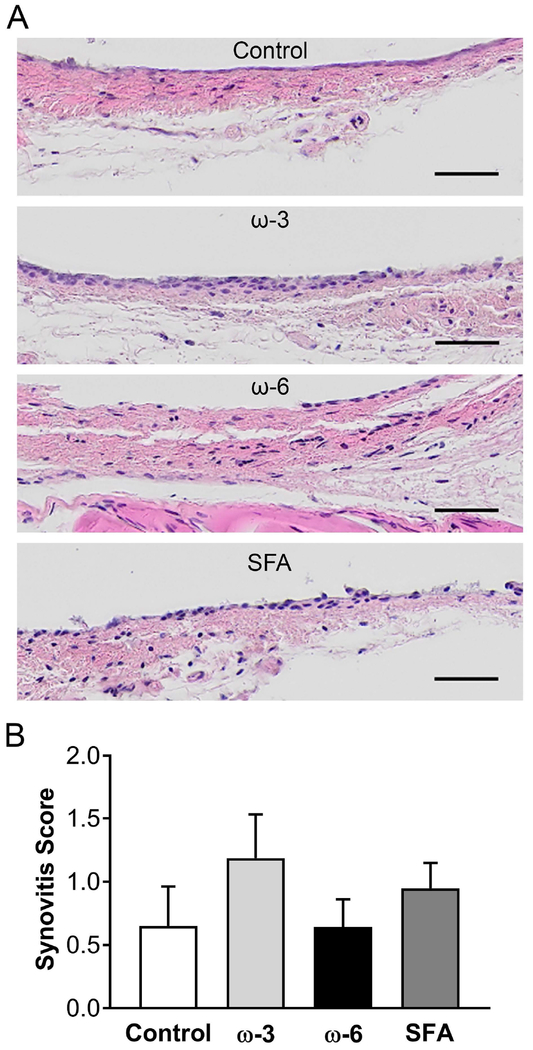
The degree of synovial inflammation was assessed using a synovitis grading scale accounting for both the thickness and cellularity of the synovial lining. Representative histological sections for each diet group are shown in Figure 6A. No statistically significant differences were seen in the overall numerical score for synovial inflammation (Figure 6B).
Figure 6: Synovitis grading results.
A) Representative hematoxylin and eosin histology images for each diet group. Pink: tissue, dark purple: cell nuclei. Scale bar: 50 μm. B) Overall synovitis score. Data presented as mean ± SEM. No statistically significant differences were observed (Kruskal-Wallis test, p<0.05).
DISCUSSION
This study showed that the fatty acid composition of high fat diets, in the absence of joint injury, caused significant changes in the bone quality of the humerus. Particularly, the ω-3 fatty acid rich diet protected against humeral bone loss compared to the ω-6 and saturated fat rich high fat diets. In contrast, few changes were observed in the properties of the articular cartilage with dietary fatty acid composition, even on the microscale. The changes that were observed, e.g. increased number of hypertrophic chondrocytes, may be associated with bone remodeling.
Overall, the modulus of the ECM, PCM, and the modulus gradient between the PCM and ECM were not affected by the different diets. Furthermore, only minor histologic changes in the cartilage and synovium were observed in the shoulder joint with any of the high-fat diets. These findings are consistent with clinical studies showing little or no increased risk of shoulder OA with obesity, although diet-dependent changes in bone structure and mineral density were apparent in our model. The effects of obesity on bone quality are complex and appear to vary with a number of factors, including age, sex, and site. Obesity has been linked to a site-specific increase in fracture risk, including an increased risk in the proximal humerus.34 This risk has been partially attributed to a decrease in mineral content, but the relationship between obesity and bone mineral density is incompletely understood. Some clinical studies indicate a positive relationship between body mass index (BMI) and bone mineral density (BMD),35 but others show the opposite or no change.36 Similarly, animal models of obesity show varying bone responses to obesity, with some studies showing an increase in bone formation and others a decrease.37,38 Our results are consistent with a previous report showing that mice fed a 45% SFA-rich high-fat diet exhibited a decrease in cancellous but not cortical bone mass in tibia.39 Furthermore, this study also reported a decrease in trabecular bone volume fraction as well as trabecular separation, as we also observed. However, Cao et al. show no significant effects of high-fat feeding on tibial cortical thickness or total area.39 Differences in the diet composition, age or strain of the mice, length of the study, and particularly the site (tibia versus humerus) could account for these discrepancies.40 It has been hypothesized that obesity initially leads to overall bone formation due to the increase in body weight, but eventually the systemic inflammation results in a decrease in bone formation.41 It is also proposed that the process of bone remodeling may occur at different rates in different bones, stressing the need for further investigation into the response of different bone sites to obesity.42
The type of fatty acid present in the diet plays an essential role in regulating bone changes in high-fat feeding. The ω-3 PUFA have been associated with positive cardiovascular health outcomes, but a study found a decrease in cortical area and maximal load with high levels of ω-3 PUFA supplementation in developing rabbits.43 This decrease in cortical area agrees with our results in the upper cortical region. The decrease in bone volume for the ω-3 PUFA diet may be related to the upregulation of adiponectin in these mice, as reported previously.10 Adiponectin, acting through FoxO1, has been shown in some situations to decrease osteoblast proliferation and promote apoptosis.44 Under what conditions ω-3 PUFAs result in catabolic or anabolic affects is still an active area of research, with many studies reporting protective effects of ω-3 PUFA supplementation on bone.36 Although awareness of ω-3 PUFA benefits is increasing, the average American diet has proportionally more ω-6 PUFA and saturated fats.45 The relationship between ω-6 PUFA and bone is under investigation, but prostaglandin E2, a downstream metabolite of a prevalent ω-6 PUFA, is linked to an increase in inflammation and may inhibit bone formation at high doses of ω-6 PUFA intake.37,42 Intermediate doses of ω-6 PUFA show conflicting results, with clinical studies finding a positive correlation between ω-6 PUFA consumption and overall BMD in a study of post-menopausal women.46 Most, but not all, studies suggest that saturated fats lead to detrimental bone changes.37,38 An in vitro study showed that saturated fat led to an increase in osteoclast survival and an in vivo study showed a decrease in bone volume fraction and an increase in trabecular separation after a high-fat diet.47,48 These in vivo results correspond with our findings in this study.
It is well recognized that bone formation and quality may, at least, partially dependent on the activity levels (i.e., potential loading on the skeleton)49. Despite the fact that we did not observe significant alterations in spontaneous locomotor activity in the mice fed high-fat or control chow diets in our previous published work,10 it remains unclear whether spontaneous locomotion is an optima model to investigate weight loading on shoulder skeleton. To examine how activity level affects shoulder bone quality, future studies may wish to use behavioral tests that are more focused on upper limb activity such as vertical climbing.50
The effects of dietary FAs and obesity on cartilage and OA have been reported previously, although almost exclusively in the knee joint. Using a surgically induced OA model, we recently demonstrated that mice fed a high-fat diet rich in ω-3 fatty acids (FAs) exhibited decreased knee OA severity compared to the mice fed a high-fat diet rich in either saturated FAs or ω-6 FAs. Importantly, using multivariable linear regression, as well as comparisons of weight-matched mice, we also observed that systemic inflammation (i.e., pro-inflammatory serum adipokine levels and dietary fatty acid composition), rather than body weight, was the major contributor in injury-induced knee OA in obesity. In the present study, which examined the shoulders of these same mice, we observed that the mice fed a high-fat did not exhibit cartilage degeneration or synovial inflammation in shoulder. Taken together, the results from our studies also suggest that injury may be a critical factor in inducing joint OA in obese mice. Indeed, other studies also observed little or no difference in Mankin OA scores between a chow diet and a high-fat diet in un-injured joints.10,13 Additionally, our results also suggest that shoulder may be less susceptible to develop spontaneous OA in obesity as compared to other joints such as knee and temporomandibular joint (TMJ). This implication is supported by the results of other work showing an increase in spontaneous OA with a high-fat (lard) diet, with OA severity being positively correlated to the gain in body fat in knee joint, as well as a positive trend toward proteoglycan loss in TMJ.15,16 Nevertheless, our current study indicates that obesity is associated with decreased shoulder bone quality but not overt cartilage degeneration or synovial inflammation even without injury as a pre-dispositional factor. Two of the subscores from the modified Mankin scoring system showed significant differences between diet groups: the number of hypertrophic chondrocytes in the calcified cartilage and the number of chondrocyte clones in the uncalcified region. In the absence of proteoglycan loss or cartilage structural changes, the physiologic relevance of these changes remains to be determined. Another histological measurement, the average thickness of the subchondral plate, was significantly reduced in the ω-3 diet group compared to the ω-6 diet and saturated fat diet, but no difference in the thickness of the cartilage was seen between diet groups.
These minimal effects of diet and obesity on cartilage structure were supported by the results that our high-fat diets did not alter PCM and ECM mechanical properties. We previously demonstrated that alterations in both ECM and PCM modulus, as well as a change in the gradient of mechanical properties from the PCM to ECM, reflect the matrix degradation in OA, particularly near individual chondrocytes.28 While these findings suggest that the shoulder is not readily susceptible to obesity-induced OA, the lack of macro- or microscale cartilage alterations could be due to other factors. For example, such changes may occur on a longer time scale than 24 weeks on a high fat diet, and the time scale of disease progression, as well as the degree of damage, may be joint-specific.
In summary, we observed a number of diet-specific changes in bone due to obesity, while the cartilage structure and mechanical properties remained relatively unchanged. Studies have shown joint-specific differences in response to obesity-related OA, with correlations being seen in knee and hand OA but with little or no correlation for hip and shoulder OA.21 Despite relatively sparse literature on shoulder OA, our findings are consistent with a lack of clinical evidence for obesity as a risk factor for shoulder OA, but indicate that underlying bone changes occur in this model. This study lays groundwork for a better understanding of the pathogenesis of shoulder OA as well as the link between obesity and changes in musculoskeletal tissues in the shoulder. This work provides further rationale for examining the role of obesity and dietary intervention in the health of the musculoskeletal system, including other joints such as the shoulder, in addition to the knee and hip.
ACKNOWLEDGMENTS
The authors thank Sara Oswald for providing technical writing support for the manuscript, and Dr. Kelsey Collins for discussions and assistance with the joint grading. This study was supported in part by NIH grants AR50245, AR48852, AG15768, AR48182, AG46927, AR073752, OD10707, AR060719, the Washington University Musculoskeletal Research Center (NIH P30 AR057235), the Arthritis Foundation, and the Nancy Taylor Foundation for Chronic Diseases.
REFERENCES
- 1.Loeser RF. 2012. The effects of aging on the development of osteoarthritis. HSS J 8:18–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers TJ, Blumenthal JA, Guilak F, et al. 2012. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: A randomized controlled study. Pain 153:1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courties A, Gualillo O, Berenbaum F, et al. 2015. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage 23:1955–1965. [DOI] [PubMed] [Google Scholar]
- 4.June RK, Liu-Bryan R, Long F, et al. 2016. Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis. J Orthop Res 34:2048–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada D, Maynard R, Schott E, et al. 2016. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheum 68:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Hunter D, Xu J, et al. 2015. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage 23:22–30. [DOI] [PubMed] [Google Scholar]
- 7.Wu CL, Kimmerling KA, Little D, et al. 2017. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci Rep 7:44315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Adams J, Leddy HA, McNulty AL, et al. 2014. The mechanobiology of articular cartilage: Bearing the burden of osteoarthritis. Curr Rheumatol Rep 16:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HJ, Morey V, Kang JY, et al. 2015. Prevalence and risk factors of spine, shoulder, hand, hip, and knee osteoarthritis in community-dwelling koreans older than age 65 years. Clin Orthop 473:3307–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C-l, Jain D, Mcneill JN, et al. 2015. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunner AM, Henn CM, Drewniak EI, et al. 2012. High dietary fat and the development of osteoarthritis in a rabbit model. Osteoarthritis Cartilage 20:584–592. [DOI] [PubMed] [Google Scholar]
- 12.Collins KH, Paul HA, Reimer RA, et al. 2015. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthritis Cartilage 23:1989–1998. [DOI] [PubMed] [Google Scholar]
- 13.Louer CR, Furman BD, Huebner JL, et al. 2012. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum 64:3220–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooney RA, Sampson ER, Lerea J, et al. 2011. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res Ther 13:R198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin TM, Huebner JL, Kraus VB, et al. 2009. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum 60:2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin TM, Huebner JL, Kraus VB, et al. 2012. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum 64:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin TM, Guilak F. 2008. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology 45:387–398. [PMC free article] [PubMed] [Google Scholar]
- 18.Collins KH, Hart DA, Seerattan RA, et al. 2018. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res 7:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas S, Browne H, Mobasheri A, et al. 2018. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology (Oxford) 57:iv61–iv74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai A, Hutchison E, Hudson J, et al. 2014. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthritis Cartilage 22:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi R, Takahashi M, Rizek R, et al. 2012. Obesity-related adipokines and shoulder osteoarthritis. J Rheumatol 39:2046–2048. [DOI] [PubMed] [Google Scholar]
- 22.Laslett LL, Otahal P, Hensor EMA, et al. 2016. Knee pain predicts subsequent shoulder pain and the association is mediated by leg weakness: Longitudinal observational data from the osteoarthritis initiative. J Rheumatol 43:2049–2055. [DOI] [PubMed] [Google Scholar]
- 23.Baker-LePain JC, Lane NE. 2012. Role of bone architecture and anatomy in osteoarthritis. Bone 51:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Yin J, Gao J, et al. 2013. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res Ther 15:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapses SA, Sukumar D. 2012. Bone metabolism in obesity and weight loss. Annu Rev Nutr 32:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadjab I, Sim S, Karhula SS, et al. 2018. Electromechanical properties of human osteoarthritic and asymptomatic articular cartilage are sensitive and early detectors of degeneration. Osteoarthritis Cartilage 26:405–413. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F, Nims RJ, Dicks A, et al. 2018. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol 71-72:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilusz RE, Zauscher S, Guilak F. 2013. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage 21:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E, Guilak F, Haider MA. 2008. The dynamic mechanical environment of the chondrocyte: A biphasic finite element model of cell-matrix interactions under cyclic compressive loading. J Biomech Eng 130:061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilusz RE, DeFrate LE, Guilak F. 2012. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Interface 9:2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelenski NA, Leddy HA, Sanchez-Adams J, et al. 2015. Type vi collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage. Arthritis Rheum 67:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darling EM, Zauscher S, Guilak F. 2006. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage 14:571–579. [DOI] [PubMed] [Google Scholar]
- 33.Darling EM, Wilusz RE, Bolognesi MP, et al. 2010. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J 98:2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh JS, Vilaca T. 2017. Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int 100:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukumar D, Schlussel Y, Riedt CS, et al. 2011. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int 22:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapses SA, Pop LC, Wang Y. 2017. Obesity is a concern for bone health with aging. Nutr Res 39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau BY, Fajardo VA, McMeekin L, et al. 2010. Influence of high-fat diet from differential dietary sources on bone mineral density, bone strength, and bone fatty acid composition in rats. Appl Physiol Nutr Metab 35:598–606. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Dellatore P, Douard V, et al. 2016. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res 36:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao JJ, Gregoire BR, Gao H. 2009. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 44:1097–1104. [DOI] [PubMed] [Google Scholar]
- 40.Devlin MJ, Robbins A, Cosman MN, et al. 2018. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female c57bl/6j vs. Fvb/nj mice. Bone Rep 8:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecka-Czernik B, Stechschulte LA, Czernik PJ, et al. 2015. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol 410:35–41. [DOI] [PubMed] [Google Scholar]
- 42.Corwin RL. 2003. Effects of dietary fats on bone health in advanced age. Prostaglandins Leukot Essent Fatty Acids 68:379–386. [DOI] [PubMed] [Google Scholar]
- 43.Judex S, Wohl GR, Wolff RB, et al. 2000. Dietary fish oil supplementation adversely affects cortical bone morphology and biomechanics in growing rabbits. Calcif Tissue Int 66:443–448. [DOI] [PubMed] [Google Scholar]
- 44.Kajimura D, Lee HW, Riley KJ, et al. 2013. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through foxo1. Cell Metab 17:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enos RT, Velázquez KT, McClellan JL, et al. 2014. Reducing the dietary omega-6:Omega-3 utilizing α-linolenic acid; not a sufficient therapy for attenuating high-fat-diet-induced obesity development nor related detrimental metabolic and adipose tissue inflammatory outcomes. PLoS One 9:e94897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Järvinen R, Tuppurainen M, Erkkilä AT, et al. 2012. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur J Clin Nutr 66:496–503. [DOI] [PubMed] [Google Scholar]
- 47.Oh S-R, Sul O-J, Kim Y-Y, et al. 2010. Saturated fatty acids enhance osteoclast survival. J Lipid Res 51:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao JJ, Sun L, Gao H. 2010. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci 1192:292–297. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh YF, Turner CH. 2001. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res 16:918–924. [DOI] [PubMed] [Google Scholar]
- 50.Green DJ, Richmond BG, Miran SL. 2012. Mouse shoulder morphology responds to locomotor activity and the kinematic differences of climbing and running. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 318:621–638. [DOI] [PubMed] [Google Scholar]