Abstract
Clinical practice presents an opportunity to prevent harm from hazardous environmental exposures however the relevant science is not readily available to healthcare decision-makers. We report the outcome of an interdisciplinary collaboration to help bridge this gap between clinical and environmental health sciences –the Navigation Guide -a systematic and transparent methodology to evaluate the quality of evidence and strength of recommendations about the relationship between the environment and reproductive health in uniform, simple, and transparent summaries that integrates the best practices of evaluation in environmental and clinical health sciences. The Navigation Guide is a critical tool to support evidence-based decision-making in clinical and policy arenas to ensure healthy pregnancies, children, and future generations.
The Need for Timely Action to Prevent Harm
Widespread exposure to environmental chemicals at levels encountered in daily life can adversely impact reproductive and developmental health.(1, 2) Studies have demonstrated that the levels of chemicals to which an average person is exposed can prevent genes from functioning normally and interfere with the hormonal regulation critical to healthy reproduction.(3, 4) For example, environmental chemicals such as polybrominated diphenyl ethers (PBDEs) from flame retardants in furniture and computers,(5) phthalates in commonly used plastics(5) and persistent organochlorine pesticides such as DDT (6, 7) share the ability to alter the endocrine, neurological and/or other biological systems. Virtually everyone in the U.S. incurs ubiquitous exposure to these and many other toxic chemicals found in homes, communities and workplaces.(8, 9)
Exposures to ambient levels of environmental chemicals during critical periods of growth and development, i.e., in utero, and during infancy, childhood and adolescence, are of particular concern because they can have a profound and lasting impact on health.(10–12) Virtually all pregnant women in the U.S. have measured levels of all of the following environmental chemicals in their bodies, and studies have documented that each of these chemicals can be harmful to human reproduction and/or development: lead, mercury, toluene, perchlorate, bisphenol A (BPA), and some phthalates, pesticides, perfluorochemicals (PFCs), polychlorinated biphenyls (PCBs) and PBDEs.(13) Many of these chemicals in pregnant women are at levels associated with adverse health outcomes in human studies.(13) The reproductive and other potential health impacts of daily and simultaneous exposure to environmental chemicals has not been studied, and this shortcoming is recognized by the National Academy of Sciences to be a gap in current scientific methodologies that inform public policy.(1)
Based on their expert assessment of the strength of the existing science, leading scientists and reproductive and other health care professionals recommend timely action to prevent harm.(11, 14–16) The evidence of harm for some chemicals is also strong enough to warrant regulatory action to reduce or prevent exposure, albeit after the chemicals have been allowed to enter the market, environment and people.(17) The inadequacy of this post-market regulatory framework is receiving increased scrutiny by government,(18) non-governmental organizations,(19) industry(20) and professional medical organizations.(21)
Intervening in Clinical Settings to Prevent Harm
While efforts to advance an improved regulatory framework for chemicals in commerce are fundamental to preventing harm, clinical practice offers a complementary point of intervention. Clinical practice presents an opportunity to identify, evaluate and counsel patients about factors that influence their health, and thus to prevent harm from hazardous environmental exposures.
Pediatricians have long been attuned to this opportunity. The American Academy of Pediatrics has had an environmental health committee for over half a century and publishes a clinicians’ handbook for the prevention of childhood diseases linked to environmental exposures.(22) The U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (USEPA) support a network of Pediatric Environmental Health Specialty Units (PEHSUs) across the U.S. to support clinical capacity related to environmental health.(23) The PEHSUs respond to requests for information throughout North America on prevention, diagnosis, management, and treatment of environmentally-related health effects in children.
In light of the importance of preconception and prenatal environmental exposures to the health of the pregnancy, and the child and adult that she or he will become, these pediatric approaches to incorporating environmental health into clinical care are equally relevant to reproductive health. Many individuals hoping to bear children are intensely and justifiably interested in the impact of environmental exposures on their pregnancies and the health of their future children. Health care professionals serving women and men of childbearing age can serve as a science-based source of guidance on how to avoid potentially adverse exposures.(24) More importantly, many people who may eventually have or want to have children are unaware that their home, workplace and/or community environment may influence their fertility and their future children’s health, and do not know steps to take to reduce exposure and potential harm.
Environmental health science provides much evidence about the contribution of the environment to reproductive health, but this information is not readily available to clinicians. One factor that impedes the availability of the science to healthcare decision-makers, including clinicians, patients and policy makers, is the absence of a roadmap for evaluating the evidence in a timely manner. The scientific evidence is voluminous, of variable quality and largely unfamiliar to health professionals caring for women and men of childbearing age. There is no trusted, ready reference or compendium that provides health professionals and women and men of childbearing age with timely, evidence-based advice about exposure to environmental contaminants. While there are many steps and complexities involved in the use of current best evidence in health care settings,(25) the process can be accelerated when knowledge-based information is readily available.(26) Therefore, we undertook an interdisciplinary collaborative process to develop a transparent and systematic methodology to sort the scientific evidence linking environmental exposures to reproductive health outcomes. While the purpose of the methodology is to support development of prevention-oriented guidelines for use in clinical settings, it can also be applied to systematically and transparently review the evidence in broader policy arenas.
Bridging the Gap Between Environmental Health and Clinical Sciences
Timely incorporation of scientific evidence into clinical care to improve health outcomes has long been a goal in the clinical arena, exemplified by the establishment of the Agency for Healthcare Research and Quality (AHRQ).(27) The experience gained in advancing this goal is directly relevant to incorporating environmental health science into clinical practice.
Currently, environmental health scientists utilize many and varied expert opinion-based methodologies to sort the science. Historically, the clinical field largely relied on a system of expert reviews from which to base treatment decisions.(28) However, starting in the 1970s, the role of expert reviews began to be questioned and systematic approaches that harness expertise to a rigorous, transparent and explicit methodology to evaluate a clearly formulated question were advanced. Landmark papers published in the clinical literature, such as Antman et al(27) demonstrated the superiority of systematic reviews for patient outcomes.(28) Antman and colleagues compared expert opinion-based recommendations for treatment of myocardial infarction published in scientific reviews and clinical textbooks to statistical analyses of the combined results of randomized controlled trials. This research documented the lack of timely incorporation of experimental evidence into expert-based recommendations, such that some reviewers did not mention effective therapies and others recommended therapies proven to be ineffective.
In response to these and other similar research findings, by 2002 at least 121 methodologies of variable utility had been developed to evaluate healthcare research to guide clinical decision-making.(29) Subsequently, attempts were made to address the limitations of many of these methods and the related concern that the abundance of methodologies could lead to confusion rather than clarity.(30, 31) An approach emerged, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, based on contemporary principles of evidence-based medicine, and built upon the strengths of existing systems and addressed shortcomings.(32, 33) GRADE systematically rates the quality of evidence and grades the strength of the recommendations to administer -or not administer- an intervention based on the tradeoffs between benefits on the one hand, and risks, burden and -potentially- costs on the other. Grading of recommendations provides healthcare decision-makers with a qualitative estimate of their quality (strong or weak, with weak sometimes called discretionary).
Thus GRADE provided our effort to bridge the gap between clinical and environmental health sciences with a well-developed and transparent organizing framework to evaluate the strength of evidence, integrate expertise and patient values and preferences, and effectively communicate the results. GRADE is also in wide use, having been adopted by over 50 organizations, including the World Health Organization, AHRQ, CDC and Kaiser Permanente.
However, along with these strengths, GRADE, and other evidence-based medicine methodologies, have limitations in terms of direct applicability to environmental health science. These limitations are because: [1] clinical evidence differs in character from evidence streams in environmental science; and [2] clinical decision-making differs in character from decision contexts in environmental health science. Each of these two essential differences is described below.
[1]. Evidence stream differences
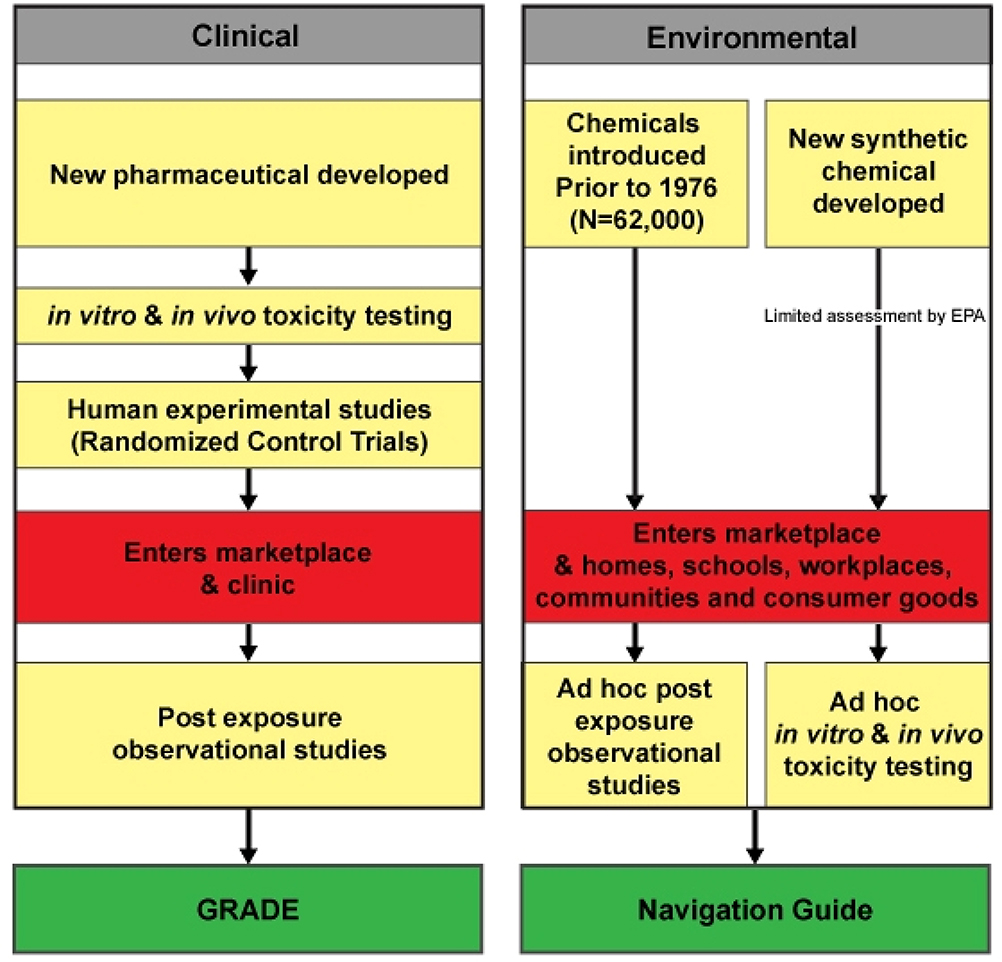
Differences exist between clinical and environmental health science in the types of evidence generally available to decision-makers. The GRADE method considers only human experimental and observational evidence. This is because in vitro and in vivo data have been accounted for by regulatory processes prior to the entry of pharmaceuticals, a primary application of GRADE, into the marketplace (Figure 1). In contrast, clinicians cannot assume, as they do with pharmaceuticals, that adequate in vitro and in vivo testing of environmental contaminants has been undertaken and considered by regulatory agencies before widespread human exposure occurs. The vast majority of chemicals in commerce have entered the marketplace without comprehensive and standardized information on their reproductive or other chronic toxicities (Figure 1).
Figure 1.
Streams of Evidence for Chemical Toxicity Assessment in Clinical and Environmental Health Sciences
[2]. Decision-context differences
Environmental and clinical sciences also differ in how decisions to expose populations and patients are made. GRADE rates the quality of evidence about exposure to exogenous substances based on how reliably the evidence informs a clinical risk-benefit decision.(34) This is consistent with regulatory and medical ethical requirements that human exposure to pharmaceuticals does not occur in the absence of some potential benefit greater than the known risks. The “gold-standard” for informing clinical risk-benefit decisions about medical interventions is a well-conducted, randomized controlled trial. There is no comprehensive comparable weighing of health benefits and risks in the environmental arena.(35) The benefits of environmental chemicals are mostly not directed towards improving health, and exposures vary and may or may not be significant depending on the toxicity of the agent. Randomized controlled trials on environmental contaminants are virtually precluded from the evidence stream due to ethical considerations.
To bridge this gap between the evidence streams and decision contexts in clinical and environmental health sciences, we undertook an interdisciplinary collaboration to craft an evidence-based medicine methodology to evaluate environmental contaminants and their potential effects on reproductive and developmental health. The result is the Navigation Guide, the product of a yearlong collaboration of 22 clinical and environmental health scientists and/or practitioners, from governmental and non-governmental organizations in the U.S. and Europe.
The Navigation Guide proceeds from GRADE but accounts for the differences in evidence and decision context described above. The method is briefly summarized here and presented in detail in the Appendix. The methodology involves 4 steps (Figure 2):
Specify the study question. The first step is to frame a specific question relevant to healthcare decision-makers about whether human exposure to a chemical or class of chemicals is a “reproductive health risk.”
Select the evidence. This next step involves conducting and documenting a systematic search for published and unpublished evidence. The Navigation Guide does not incorporate most existing lists of “reproductive or developmental toxicants.” This is because such lists have been compiled using a wide range of methodologies to meet varied goals, and the details of the goals and methods are typically not readily apparent. Thus the use of these potentially valuable resources would be inconsistent with our goal of a systematic and transparent methodology.
Rate the evidence. Consistent with GRADE, the Navigation Guide systematically rates the quality of individual studies and the quality of the overall body of evidence based on a priori and transparent criteria. However, due to the nature of the evidence stream in environmental health, the Navigation Guide conducts this process for both human and non-human systems of evidence. As a consequence, the methodology involves an additional step to integrate the quality ratings of each of these two streams of evidence. The end result is one of five possible statements about the overall strength of the evidence: “known”, “probably”, “possibly”, “not classifiable”, or “probably not toxic” to reproductive/developmental health.
Grade the strength of the recommendations. In the final step, the Navigation Guide integrates the strength of the evidence on toxicity with information about exposure, the availability of a less toxic alternative and patient values and preferences. The end result of applying the Navigation Guide is a concise, evidence-based recommendation for prevention, based on all of these considerations, such as “chemical X is known to be toxic to reproductive health. Doing x, y and or z to prevent exposure is strongly recommended. Doing a, b, or c is discretionary.”
Figure 2.
Navigation Guide
Future Directions
A large body of science links exposure to environmental chemicals to adverse reproductive health outcomes across the lifespan of individuals and generations. Thus, there is enormous potential to reduce harm and associated health costs by building collaborative bridges across the divide that separates clinical and environmental health sciences. To this end, the Navigation Guide offers a methodology to transparently and systematically vet the science linking environmental exposure to chemicals to reproductive and/or developmental health. Professional societies, healthcare organizations, government agencies and other potential guideline developers working with toxicologists can use the Navigation Guide to craft consistent and timely recommendations to improve patient, and ultimately population, health outcomes.
The Navigation Guide is not a panacea, but a missing tool in a much larger effort to address the public health impacts of widespread environmental exposure to toxic substances. Like GRADE,(34) the Navigation Guide, does not obviate the need for expert and other judgments, but because it is transparently produced and presented, it will allow others to identify and scrutinize those judgments.
The Navigation Guide uses an evidence based medicine framework. This framework carries many implications for policy and law, for example, by playing a role in brokering the decision-making power of physicians, patients and healthcare system payers about medical treatments.(36) Additionally, the validity of this framework rests the existence of an evidence stream that is directly meaningful to the lives of patients and communities. For example, there is a need to produce evidence capable of informing solutions to the pervasive disparities in healthcare and outcomes associated with race, ethnicity, income, education, geography and other factors.(37) There is also a parallel need to develop the evidence around less-toxic alternatives to current practices, and for solutions to be relevant to the lives and experiences of exposed individuals and communities. Finally, it has been observed that only when likely biases of industry and specialty societies have been either removed or overcome by countervailing interests can the promise of impartial recommendations be achieved.(38)
The evidence stream is rapidly changing in both clinical and environmental health sciences and the Navigation Guide and other evidence-based systems will need constant review to ensure the most current approaches to discerning the evidence are rapidly incorporated and evaluated. It is anticipated that evidence-based medicine will increasingly rely on nonrandomized evidence. The speed and complexity with which new medical interventions and scientific knowledge are being created make it unlikely that the evidence base required for treatment and cost effective health care delivery across subpopulations can be built using only randomized controlled trials.(39) It is also expected that electronic medical records will revolutionize medical research by facilitating instant, comprehensive, longitudinal data that go back years into history and extend indefinitely into the future.(40) Harnessing these changes could greatly accelerate the creation of knowledge about the impact of the environment on human health.
Just as the thalidomide tragedy led to strengthened regulatory oversight of the safety and efficacy of all prescription drugs,(41) recent advances in toxicity testing,(42–45) including substantial investment in USEPA’s Toxcast program of in vitro screening assays,(46, 47) risk assessment,(1, 2, 48, 49) and in efforts to address shortcomings in regulatory policy related to chemicals in commerce,(18–20, 50) are likely to create important change in the amount, type and availability of chemical toxicity data and related health impacts. These anticipated improvements in how environmental chemicals are evaluated and regulated underscore the need for a methodology to ensure timely application of these data to prevention.
In addition to policy improvements in the testing and regulation of chemicals in commerce, public and private incentives to spur safer alternatives and good industrial practices will be required to develop meaningful recommendations for prevention. The Navigation Guide provides a framework to incorporate all of these and related innovations rapidly and transparently as they unfold into guidelines for prevention for patient and population health.
Acknowledgments
The authors acknowledge the other members of the Navigation Guide Work Group: Vincent James Cogliano(International Agency for Research on Cancer), Kate Guyton (Environmental Protection Agency [EPA]), Julia Quint(Retired, California Department of Public Health), Lauren Zeise (CA-EPA), Judith Balk (University of Pittsburgh [UP]), Lisa Bero (UCSF), Jeanne Conry (Kaiser Permanente, American College of Obstetricians and Gynecologists District IX), Daniel M. Fox (Emeritus, Milbank Memorial Fund), David Gee (European Environmental Agency), Rivka Gordon(Association of Reproductive Health Professionals [ARHP]), Sarah Janssen(Natural Resources Defense Council), Beth Jordan (ARHP), Victoria Maizes(University of Arizona), Mark Miller (UCSF), Michele Ondeck (UP), Karen Pierce (San Francisco Department of Public Health), Pablo Rodriguez (Brown Medical School and Women & Infants Hospital of Rhode Island), Heather Sarantis (Collaborative on Health and the Environment), Ted Schettler (Science and Environmental Health Network), and Sandy Worthington (Planned Parenthood Federation of America).
Financial support for development of the Navigation Guide was provided to UCSF Program on Reproductive Health and the Environment by: Clarence Heller Foundation, Passport Foundation, the Heinz Endowments, the Fred Gellert Foundation, Rose Foundation, Kaiser Permanente, New York Community Trust, University of California, San Francisco Institute for Health Policy Studies, Planned Parenthood Federation of America, National Institute for Environmental Health Sciences (ES018135) and US Environmental Protection Agency STAR (RD83467801.
Appendix: Navigation Guide Methodology
The Navigation Guide involves four steps (Figure 2):
Specify the study question
Select evidence
Rate the quality and strength of evidence
Grade the strength of the recommendations
For each step, key assumptions, weightings of quality and types of evidence and values and preferences must be clearly and transparently documented.
Step 1. Specify study question
The Navigation Guide will answer specific questions about whether human exposure to a chemical or class of chemicals is a “reproductive health risk.” “Reproductive health” encompasses all aspects of reproductive and developmental health throughout the life course, including conception, fertility, pregnancy, child and adolescent development, and adult health. “Health risks” can span a range of diseases and conditions, including teratogenic impacts, minor variations in sperm counts and upstream biological perturbations that predict a range of adverse effects. “Health risks” includes assessment of two distinct factors relevant to patient care: 1. the toxicity of the agent; and 2. the nature and extent of patient exposure.
Step 2. Select evidence
This step involves conducting and documenting a systematic search for published and unpublished evidence including:
Three government lists/sources of information about reproductive and developmental toxicity: U.S. National Toxicology Program, Center for Evaluation of Risks to Human Reproduction (1); U.S. Environmental Protection Agency (USEPA); (2) and California Environmental Protection Agency, Chemicals Known By the State of California to Cause Cancer or Reproductive Toxicity. (3)
Electronic databases
Ad-hoc literature searches
Identifying unpublished studies and other unpublished resources by checking reference lists and personally contacting investigators and other sources of relevant information
A wide range of goals, objectives and approaches are employed to create lists of toxic chemicals and these differences in purpose and methods yield different resultant lists. Because our goal is to ensure consistent and reproducible results based on a single systematic and transparent methodology, the Navigation Guide makes only very limited use of existing sources/lists. We chose the above three sources based on their relevancy to our study question and their use of transparent methodologies including processes for identifying conflicts of interest. (2, 4, 5)
Step 3. Rate quality and strength of evidence
The Navigation Guide rates the quality and strength of evidence of human and non-human evidence streams separately as “sufficient”, “limited” or “inadequate” (Table). Next, the human and non-human evidence ratings are combined to produce one of five possible statements about the overall strength of the evidence of a chemical’s reproductive/developmental toxicity (Figure 2):
Known to be toxic to reproductive/developmental health
Probably toxic to reproductive/developmental health
Possibly toxic to reproductive/developmental health
Not classifiable as toxic to reproductive/developmental health
Probably not toxic to reproductive/developmental health
Table.
Rate the quality and strength of evidence in human and non-human systems1
| Type of evidence | Quality of evidence | Strength of evidence |
|---|---|---|
| Human Systems | ||
| Human observational (Case-control and cohort studies) Human experimental (mechanistic/cell culture, other human systems) 2 |
Causal relationship has been established Chance, bias, and confounding could be ruled out with reasonable confidence |
Sufficient Human |
| Human Observational (Case-control and cohort studies) Human Experimental (mechanistic/cell culture, other human systems) 2 |
Causal interpretation is credible Chance, bias, or confounding could not be ruled out with reasonable confidence |
Limited Human |
| Human Observational (Unsystematic clinical observations, case reports and series) Human experimental (mechanistic/cell culture, other human systems) 2 |
Studies permit no conclusion about a causal association | Inadequate Human |
| Human observational (Case-control and cohort studies) Human experimental (mechanistic/cell culture, other human systems) 2 |
Several adequate studies covering the full range of exposure levels are mutually consistent in not showing a positive association at any observed level of exposure Conclusion is limited to health condition(s) studied |
Human evidence of lack of toxicity |
| Non-Human Systems | ||
| Experimental Animal | Causal relationship has been established through either: - Multiple positive results - Single appropriate study in single species3 |
Sufficient Non-Human |
| Experimental Animal | Data suggest an effect but only in a single study | Limited Non- Human |
| Experimental Animal | Studies permit no conclusion about a toxic effect | Inadequate Non-Human |
| Experimental Animal | Data on an adequate array of endpoints from more than one study with two species that showed no adverse reproductive effects at doses that were minimally toxic in terms of inducing an adverse effect. Information on pharmacokinetics, mechanisms, or known properties of the chemical class may also strengthen the evidence4 Adequate studies in at least two species show that the agent is not toxic * Conclusion is limited to the species, age at exposure, and/or other conditions and levels of exposure studied |
Non-human evidence of lack of toxicity |
The quality and strength of evidence of human and non-human evidence streams are rated separately as “sufficient”, “limited” or “inadequate” and then these 2 ratings are combined to produce one of five possible statements about the overall strength of the evidence of a chemical’s reproductive/developmental toxicity (as shown in Figure 2). The methodology is adapted from the criteria used by the International Agency for Research on Cancer (IARC) to categorize the carcinogenicity of substances (12) except as noted.
IARC’s current method for rating whether a substance is a human carcinogen uses mechanistic or other strong relevant human systems evidence to upgrade a substances classification, i.e., from “not classifiable” to “possibly,” and from “probably” to “known to be a human carcinogen.” What is “strong” evidence in such circumstances is a matter of expert judgment, but it increases as the toxicity classification becomes higher. Upgrading the evidence based perturbations of biological pathways is also consistent with USEPA policy which defines changes in hormonal response perturbations as adverse effects. (18) In recognition of the direction of toxicity testing in the 21st century,(16) the Navigation Guide would incorporate mechanistic and other human systems evidence directly into the evaluation.
IARC’s criteria for sufficient evidence of carcinogenicity in animals requires multiple positive results (species, studies, sexes). The Navigation Guide integrates USEPA’s minimum criteria for animal data for a reproductive or developmental hazard, i.e., data demonstrating an adverse reproductive effect in a single appropriate, well-executed study in a single test species. (9) The Navigation Guide would also incorporate USEPA’s ”sufficient evidence category” which includes data that “collectively provide enough information to judge whether or not a reproductive hazard exists within the context of effect as well as dose, duration, timing, and route of exposure. This category may include both human and experimental animal evidence.” (9) The USEPA statement for developmental hazards is slightly different but includes the same relevant information regarding dose, duration, timing, etc.(15)
Based on minimum data requirements according to USEPA Guidelines for Reproductive Toxicity. (9)
The Navigation Guide’s methodology to rate the quality and strength of the evidence is based on integrating:
Features of the methodology established by the International Agency for Research on Cancer (IARC) to evaluate the evidence on whether substances cause cancer, specifically IARC’s systematic and transparent methods to: 1. rate the quality and strength of human and non-human streams of evidence, including applying standard criteria to individual studies to account for design strengths and limitations and potential sources of bias; 2. integrate the ratings of human and non-human evidence streams into an overall strength of evidence rating; and 3. produce a clear and concise statement about a substance’s toxicity based on integration of all evidence streams. (6)
USEPA criteria for reproductive (2) and developmental toxicity (7) in all instances where use of USEPA criteria would enhance the overall strength of the evidence.
Mechanistic or other strong relevant human systems data as primary sources of evidence of toxicity. These data are incorporated to align the Navigation Guide with the direction of toxicity testing which will increasingly rely on in vitro systems of evidence (e.g., stem cells).
The Navigation Guide makes no blanket assumption about threshold levels of exposure below which effects do not occur. Increasing evidence indicates the use of the threshold assumption for reproductive and developmental toxicants can give a false assurance of safety when incremental doses are added to existing background exposures, when they are part of complex exposures to multiple chemicals that act on a common adverse outcome, or that occur in people who are at a susceptible stage of development or who have pre-existing conditions that might enhance toxicity. (8, 9) (10)
The influence of financially conflicted sources of funding is well recognized in the clinical world (11), and widely used examples of systems that utilize best practices for weighing and communicating the strength of the scientific evidence (12) prohibit sponsorship by any commercial source or sources i.e., Cochrane reviews (13) or recommend that the quality of potentially conflicted evidence be downgraded when evaluated, i.e., GRADE. (14) The Navigation Guide similarly allows the quality of any individual study to be downgraded for a conflict of interest in the sponsorship or funding, or, if the findings are published in a journal that does not have a funding conflict of interest disclosure policy.
The Navigation Guide also provides for the overall strength of human and non-human evidence to be upgraded when the magnitude of effect is very large and/or serious (i.e., teratogenicity) or if all plausible biases would decrease the magnitude of an apparent effect. In this manner, the Navigation Guide provides for upgrading the weight of evidence for serious and rare health endpoints when they serve as crucial and reliable early warning systems.
Step 4. Grade strength of recommendations
The Navigation Guide initially grades the strength of the recommendation on two criteria (Figure 2):
Strength of evidence about toxicity – i.e., the overall rating from step 3.
Exposure – a qualitative measure (i.e., high, medium, low) that incorporates an estimate of the amount of exposure (concentration and duration) and the timing of exposure (i.e., when in the course of development does the exposure occur).
The details of how the strength of evidence of toxicity and exposure are incorporated into the matrix are shown in Figure 2. This produces an initial grade of either “strong” or “discretionary.” A “strong” grade denotes “we recommend,” whereas a “discretionary” grade denotes “we suggest.”
Availability of a less toxic alternative
The initial grading of the strength of the recommendation is then evaluated according to the availability of a less toxic alternative. For example, a substance that is rated “probably toxic” and “high exposure” would receive an initial recommendation of “strong” (Figure 2). However this recommendation might be downgraded in the absence of a less toxic alternative, i.e., avoiding exposure to pesticides in food would be “discretionary” if most individuals cannot adopt a diet of organic food due to high cost or lack of availability. Conversely, a recommendation for a substance rated “not classifiable as toxic” and “medium exposure” might be upgraded from “discretionary” to “strong” if less toxic alternatives are widely available, i.e., avoiding exposure to cleaning products which lack toxicity data would warrant a “strong” recommendation when water, soap, or other benign and effective ingredients are widely available. Notably, the frequent dearth of information about the toxicity of alternatives may reduce the influence of this factor on decision-making.
Patient values and preferences
The Navigation Guide distinguishes the contribution of quality of evidence from the values and preferences that patients, communities, policy-makers and other potential end-users bring to decision-making. It is widely accepted that patient values and preferences are a critical component of decision making in the clinical context but there is no consensus on how to involve patients in clinical practice guideline development. The Navigation Guide shares the need to identify and document effective mechanisms for gathering information on patient values and preferences in order that the recommendations reflect the concerns and priorities of exposed patients and populations. For example, cost, resource allocation and utility are not specifically incorporated into grading the recommendation, but may be considered in the context of values and preferences.
- 1.NTP. Center for the Evaluation of Risks to Human Reproduction. Research Triangle Park: National Toxicology Program; 2010. [cited 2010 April 13]; Available from: http://cerhr.niehs.nih.gov/chemicals/index.html. [Google Scholar]
- 2.USEPA. Guidelines for Reproductive Toxicity Risk Assessment. Washington, DC: 1996. [Google Scholar]
- 3.OEHHA. Safe Drinking Water and Toxic Enforcement Act of 1986. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity (Proposition 65 List). [cited 2010 April 14]; Available from: http://www.oehha.org/prop65/prop65_list/files/P65single040210.pdf.
- 4.NTP. National Toxicology Program Non-Cancer Evaluation Criteria. Research Triangle Park: National Toxicology Program; 2009. [Google Scholar]
- 5.OEHHA. Mechanisms for Listing and Delisting Chemicals Under Proposition 65. California Environmental Protection Agency Office of Environmental Health Hazard Assessment; 2007. [cited 2010 April 13]; May 15, 2007:[Descriptions of the listing and delisting mechanisms]. Available from: http://www.oehha.ca.gov/prop65/policy_procedure/listde051007.html. [Google Scholar]
- 6.IARC. Preamble to the IARC Monographs (amended January 2006). Lyon: World Health Organization; 2006. [Google Scholar]
- 7.USEPA. Guidelines for Developmental Toxicity Risk Assessment. Washington, DC: Risk Assessment Forum; 1991. [Google Scholar]
- 8.National Academy of Science; Science and Decisions: Advancing Risk Assessment. Washington, DC: National Research Council Committee on Improving Risk Analysis Approaches Used by the U.S. EPA; 2008. [Google Scholar]
- 9.National Research Council. Phthalates and Cumulative Risk Assessment: The Task Ahead Committee on the Health Risks of Phthalates, editor. Washington, D.C.: National Academies Press; 2008. [PubMed] [Google Scholar]
- 10.Woodruff TJ, Zeise L, Axelrad DA, Guyton KZ, Janssen S, Miller M, et al. Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ Health Perspect. 2008. November;116(11):1568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennie D Integrity in scientific publishing. Health Serv Res. June;45(3):885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ). 2002. March(47):1–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. Chichester, UK:2006. [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008. April 26;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the US Environmental Protection Agency or any other government agency.
References
- 1.National Research Council (U.S.). Committee on Improving Risk Analysis Approaches Used by the U.S. EPA., National Research Council (U.S.). Board on Environmental Studies and Toxicology., National Research Council (U.S.). Division on Earth and Life Studies. Science and decisions: advancing risk assessment. Washington, D.C.: National Academies Press; 2009. [Google Scholar]
- 2.National Research Council (U.S.). Committee on the Health Risks of Phthalates., National Academies Press (U.S.). Phthalates and cumulative risk assessment : the task ahead. Washington, D.C.: National Academies Press; 2008. [Google Scholar]
- 3.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. [Review]. 2003. June;111(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palanza P, Morellini F, Parmigiani S, vom Saal FS. Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neurosci Biobehav Rev. [Review]. 1999. November;23(7):1011–27. [DOI] [PubMed] [Google Scholar]
- 5.Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. [Review]. 2009. July 27;364(1526):2079–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevrier J, Eskenazi B, Holland N, Bradman A, Barr DB. Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Am J Epidemiol. 2008. August 1;168(3):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maervoet J, Vermeir G, Covaci A, Van Larebeke N, Koppen G, Schoeters G, et al. Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ Health Perspect. 2007. December;115(12):1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Disease Control. Third national report on human exposure to environmental chemicals. Atlanta: Centers for Disease Control;2005. [Google Scholar]
- 9.Center for Disease Control. Fourth national report on human exposure to environmental chemicals. Atlanta: Centers for Disease Control;2009. [Google Scholar]
- 10.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. [Review]. 2008. October;90(4):911–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandjean P, Bellinger D, Bergman A, Cordier S, Davey Smith G, Eskenazi B, et al. The Faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102(2):73–5. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff TJ. Environmental impacts on reproductive health and fertility. Cambridge; New York: Cambridge University Press; 2010. [Google Scholar]
- 13.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect. 2011. Epub Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the summit on environmental challenges to reproductive health and fertility: executive summary. Fertil Steril. 2008. February;89(2 Suppl):e1–e20. [DOI] [PubMed] [Google Scholar]
- 15.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. [Review]. 2009. June;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuben SH, Panel PsC. Reducing Environmental Cancer Risk: What We Can Do Now. Bethesda: National Cancer Institute;2010. [Google Scholar]
- 17.Cranor CF. Legally poisoned: how the law puts us at risk from toxicants. Cambridge, Mass: Harvard University Press; 2011. [Google Scholar]
- 18.U.S. Environmental Protection Agency. Essential Principles for Reform of Chemicals Management Legislation. 2010. [cited 2011 March 9]; Available from: http://www.epa.gov/oppt/existingchemicals/pubs/principles.html.
- 19.Chemicals Safer, Healthy Families Coalition. The Health Case for Reforming the Toxic Substances Control Act. 2011. [cited 2011 March 21]; Available from: http://www.saferchemicals.org/about/want.html.
- 20.American Chemistry Council. 10 Principles for Modernizing TSCA. 2011. [cited 2011 March 21]; Available from: http://www.americanchemistry.com/s_acc/sec_article_acc.asp?CID=2178&DID=9939.
- 21.American Medical Association. Proceedings of the American Medical Association House of Delegates: 157th Annual Meeting Chicago: American Medical Association 2008. [Google Scholar]
- 22.Etzel RA, Balk SJ, American Academy of Pediatrics Committee on Environmental Health. Pediatric environmental health. 2nd ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003. [Google Scholar]
- 23.Trasande L, Newman N, Long L, Howe G, Kerwin BJ, Martin RJ, et al. Translating Knowledge About Environmental Health to Practitioners: Are We Doing Enough? Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77(1):114–23. [DOI] [PubMed] [Google Scholar]
- 24.Solomon G, Janssen S. Communicating with patients and the public about environmental exposures and reproductive risk In: Woodruff TJ, editor. Environmental impacts on reproductive health and fertility. Cambridge New York: Cambridge University Press; 2010. p. 214–26. [Google Scholar]
- 25.Liang L The gap between evidence and practice. Health Aff (Millwood). 2007. [DOI] [PubMed] [Google Scholar]
- 26.Titler MG. The Evidence for Evidence-based practice implementation In: Hughes R, editor. Patient safety and quality : an evidence-based handbook for nurses. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Dept. of Health and Human Services; 2008. [PubMed] [Google Scholar]
- 27.Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. Jama. 1992. July 8;268(2):240–8. [PubMed] [Google Scholar]
- 28.Rennie D, Chalmers I. Assessing authority. Jama. 2009. May 6;301(17):1819–21. [DOI] [PubMed] [Google Scholar]
- 29.West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ). 2002. March(47):1–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Swiglo BA, Murad MH, Schunemann HJ, Kunz R, Vigersky RA, Guyatt GH, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008. March;93(3):666–73. [DOI] [PubMed] [Google Scholar]
- 31.Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004. Jan-Feb;17(1):59–67. [DOI] [PubMed] [Google Scholar]
- 32.GRADE Working Group. Frequently Asked Questions. 2011. [cited 2011 March 21]; Available from: http://www.gradeworkinggroup.org/FAQ/index.htm.
- 33.Montori VM, Guyatt GH. Progress in evidence-based medicine. Jama. 2008. October 15;300(15):1814–6. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008. April 26;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffensperger C, Tickner JA. Protecting public health& the environment: implementing the precautionary principle. Washington, D.C.: Island Press; 1999. [Google Scholar]
- 36.Rodwin MA. The politics of evidence-based medicine. J Health Polit Policy Law. 2001. April;26(2):439–46. [DOI] [PubMed] [Google Scholar]
- 37.Casale CR, Clancy CM. Commentary: Not About Us Without Us. Academic Medicine. 2009;84(10):1333–5. [DOI] [PubMed] [Google Scholar]
- 38.Shaneyfelt TM, Centor RM. Reassessment of clinical practice guidelines: go gently into that good night. Jama. 2009. February 25;301(8):868–9. [DOI] [PubMed] [Google Scholar]
- 39.Peterson E, editor. Research methods to speed the development of better evidence-the registries example Institue of Medicine Annual Meeting: Evidence-based medicine and the changing nature of health care; 2007; Washington, D.C.: The National Academies Press. [Google Scholar]
- 40.Halvorson GC, editor. Electronic medical records and the prospect of real time evidence development Institue of Medicine Annual Meeting: Evidence-based medicine and the changing nature of health care; 2007; Washington, D.C.: The National Academies Press. [Google Scholar]
- 41.U.S. Food and Drug Administration. This Week In FDA History - July 15, 1962. 2009. [cited 2011 March 21]; Available from: http://www.fda.gov/AboutFDA/WhatWeDo/History/ThisWeek/ucm117836.htm.
- 42.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26(4):491–510. [DOI] [PubMed] [Google Scholar]
- 43.Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007. January;95(1):5–12. [DOI] [PubMed] [Google Scholar]
- 44.Lein P, Locke P, Goldberg A. Meeting report: alternatives for developmental neurotoxicity testing. Environ Health Perspect. 2007. May;115(5):764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokstad E Putting chemicals on a path to better risk assessment. Science. 2009. August 7;325(5941):694–5. [DOI] [PubMed] [Google Scholar]
- 46.Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, et al. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010. April;118(4):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin MT, Mendez E, Corum DG, Judson RS, Kavlock RJ, Rotroff DM, et al. Profiling the reproductive toxicity of chemicals from multigeneration studies in the toxicity reference database. Toxicol Sci. 2009. July;110(1):181–90. [DOI] [PubMed] [Google Scholar]
- 48.Weiss B, Cory-Slechta D, Gilbert SG, Mergler D, Miller E, Miller C, et al. The new tapestry of risk assessment. Neurotoxicology. [Review]. 2008. September;29(5):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callahan MA, Sexton K. If cumulative risk assessment is the answer, what is the question? Environ Health Perspect. 2007;115(5):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson MP, Chia DA, Ehlers BC. Green chemistry in California: a framework for leadership in chemicals policy and innovation. New Solut. 2006;16(4):365–72. [DOI] [PubMed] [Google Scholar]