Abstract
BACKGROUND:
Among adults undergoing contemporary noncardiac surgery, little is known about the frequency and timing of death and the associations between perioperative complications and mortality. We aimed to establish the frequency and timing of death and its association with perioperative complications.
METHODS:
We conducted a prospective cohort study of patients aged 45 years and older who underwent inpatient noncardiac surgery at 28 centres in 14 countries. We monitored patients for complications until 30 days after surgery and determined the relation between these complications and 30-day mortality using a Cox proportional hazards model.
RESULTS:
We included 40 004 patients. Of those, 715 patients (1.8%) died within 30 days of surgery. Five deaths (0.7%) occurred in the operating room, 500 deaths (69.9%) occurred after surgery during the index admission to hospital and 210 deaths (29.4%) occurred after discharge from the hospital. Eight complications were independently associated with 30-day mortality. The 3 complications with the largest attributable fractions (AF; i.e., potential proportion of deaths attributable to these complications) were major bleeding (6238 patients, 15.6%; adjusted hazard ratio [HR] 2.6, 95% confidence interval [CI] 2.2–3.1; AF 17.0%); myocardial injury after noncardiac surgery [MINS] (5191 patients, 13.0%; adjusted HR 2.2, 95% CI 1.9–2.6; AF 15.9%); and sepsis (1783 patients, 4.5%; adjusted HR 5.6, 95% CI 4.6–6.8; AF 12.0%).
INTERPRETATION:
Among adults undergoing noncardiac surgery, 99.3% of deaths occurred after the procedure and 44.9% of deaths were associated with 3 complications: major bleeding, MINS and sepsis. Given these findings, focusing on the prevention, early identification and management of these 3 complications holds promise for reducing perioperative mortality. Study registration: ClinicalTrials.gov, no. NCT00512109.
Worldwide, 100 million patients aged 45 years and older undergo inpatient noncardiac surgery each year.1,2 Although surgery has the potential to improve and prolong quality and duration of life, it is also associated with complications and mortality.
During the last several decades, advances in perioperative care have included less invasive surgery, improved anesthetic techniques, enhanced intraoperative monitoring and more rapid mobilization after surgery.2 At the same time, the age and the number of comorbidities of patients undergoing surgery have increased substantially.3,4 Hence, in the current context, the frequency and timing of mortality is uncertain, as is the relation between perioperative complications to mortality.
In a large prospective study (The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation [VISION] Study), we systematically followed patients who underwent noncardiac surgery and documented perioperative complications and death. Our a priori objectives included establishing the frequency and timing of death after noncardiac surgery, and the association between perioperative complications and postsurgical death.
Methods
Study design, population and data
We previously reported details of the study design and methods.5,6
VISION was an international, prospective cohort study. Patients were included if they were aged 45 years or older, had undergone noncardiac surgery, had received general or regional anesthesia and remained in hospital for at least 1 night after surgery. Patients were recruited at 28 centres in 14 countries in North and South America, Asia, Europe, Africa and Australia, from August 2007 to November 2013 (Supplemental Table 1, Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190221/-/DC1). If patients underwent surgery and were enrolled in VISION and then had surgery 18 months later, they could not be re-enrolled in VISION. In other words, patients could be enrolled only once. This is a common criterion we use in our observational and randomized controlled trials. Appendix 1 lists the participating centres and investigators, as well as the grants and funding sources (Box 1). Appendix 1A provides the study oversight.
Box 1: Supplementary table of contents for Appendix 1*.
-
Supplemental centres, investigators, and funding sources
Participating centres and investigators: pages 2, 3
Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) funding sources: pages 3–5
-
Supplemental appendices
Appendix 1A. Study oversight: page 6
Appendix 1B. Participant consent: page 7
Appendix 1C. Surgical categories: page 8
Appendix 1D. Study outcomes and their definitions: pages 9, 10
Appendix 1E. Outcome adjudication: page 11
Appendix 1F. Preoperative and surgical variables used in the multivariable analyses to determine the relationships between perioperative complications and 30-day mortality: page 12
-
Supplemental tables
Supplemental Table 1. Recruitment by country and centre: pages 13, 14
Supplemental Table 2. 30-day mortality by region: page 15
Supplemental Table 3. Relationship between region and mortality: page 16
Supplemental Table 4. 30-day mortality by surgical category: pages 17, 18
Supplemental Table 5. Timing and location of death by region: page 19
Supplemental Table 6. Timing and location of death by surgical category: page 20
Supplemental Table 7. Relationship between preoperative patient characteristics and surgical category with 30-day mortality: page 21
Supplemental Table 8. 30-day complications by region: page 22
Supplemental Table 9. Main results and post-hoc analyses evaluating relationship between perioperative complications and 30-day mortality: pages 23, 24
-
Supplemental figures
Supplemental Figure 1. Patient flow chart: page 25
Supplemental Figure 2. Cumulative proportion of events during 30-day follow-up: page 26
Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190221/-/DC1.
All centres that enrolled patients were academic hospitals. Research team members identified patients undergoing elective, urgent or emergent surgery during the day and night, and on weekdays and weekends, through daily screening of patient lists in preoperative assessment clinics, daily surgical lists, surgical lists from the previous day, patient lists on surgical wards and in intensive care units, and patients in the preoperative holding areas. In some centres, surgical volume exceeded the capacity of research staff to enroll all eligible patients on consecutive weeks. To facilitate recruitment of a representative sample in these centres, the project office created a recruitment schedule consisting of randomly selected weeks of recruitment or randomly selected surgical services, proportional to the prevalence of the types of surgery at each local centre. Appendix 1B provides the details regarding participant consent.
Research team members interviewed and examined patients and reviewed charts to obtain baseline variables (e.g., comorbidities), type of surgery (Appendix 1C) and type of anesthesia; ensured measurement of troponin T at 6–12 hours after surgery and on days 1, 2 and 3 after surgery; and evaluated patients throughout their hospital stay, reviewed hospital charts and noted outcomes.
All-cause mortality was the primary outcome. The specific complications that we evaluated were major bleeding, myocardial injury after noncardiac surgery (MINS), sepsis, nonsepsis infection, acute kidney injury requiring dialysis, stroke, congestive heart failure, venous thromboembolism and new-onset atrial fibrillation. Appendix 1D provides the study outcomes and their definitions. Study team members phoned patients (or, if the patient was unavailable, next of kin) at 30 days after surgery and documented patient outcomes. We focused on 30-day outcomes because noncardiac surgery is associated with an increased risk of major complications until 30 days after surgery,7 and most perioperative studies focus on 30-day outcomes.8–10
Research team members submitted case report forms and supporting documentation to the data management system (iDataFax, coordinating centre, McMaster University, Hamilton, Ontario). Data monitoring comprised central data consistency checks and on-site monitoring. Outcome adjudicators evaluated the outcomes listed in Appendix 1E; their decisions were used for the analyses.
Statistical analysis
We wrote a statistical analysis plan before undertaking analyses, which is available in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190221/-/DC1). Of the patients who died within 30 days after noncardiac surgery, we determined the proportion who died in the operating room, after surgery during the index admission to hospital and after discharge from the hospital. We determined the risk of death by geographical region and type of surgery.
To determine the relation between perioperative complications and mortality, we used a Cox proportional hazards model in which the dependent variable was mortality up to 30 days after noncardiac surgery. Independent variables included preoperative and surgical variables previously associated with 30-day perioperative mortality5,11 (Appendix 1F) and perioperative complications as time-dependent variables. We included centre in the model as a random effect. Patients who did not complete the 30-day follow-up were censored on the last day their vital status was known. We established our sample population based on the number of events that we would require to include an adequate number of covariates in our risk prediction models. Our sample population of 40 000 patients provided 37 events per variable included in our multivariable analysis,12 which ensured a stable model.
Based on the results of the Cox proportional hazards model, we determined the attributable fraction (AF) for each complication that was independently associated with 30-day mortality.13 This is a measure that represents the proportional reduction in mortality within a population that would occur if the incidence of a complication was reduced to 0, provided that a causal relation existed between that complication and 30-day mortality.
For the Cox proportional hazards models, we report adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). Discrimination was assessed through evaluation of the optimism-corrected C-index. All tests were 2-sided, and we designated p < 0.05 as significant. Analyses were performed using SAS version 9/4 (SAS Institute Inc.) and version R-3.5.1 (R Project).
Ethics approval
Each academic hospital obtained approval from its research ethics board before starting patient enrolment.
Results
Of the 40 037 patients enrolled in VISION, 40 004 were included in these mortality analyses; we were unable to determine survival status at hospital discharge or at 30 days for 31 patients, and 2 patients were missing predictors used in our model (Supplemental Figure 1, Appendix 1). We obtained 30-day follow-up data on 39 651 patients (99.1%).
Table 1 reports patients’ preoperative characteristics, surgical categories and type of anesthesia. Half of the patients were women, and mean age was 63.9 (standard deviation 11.2) yr. The most common comorbidities were hypertension (20 152 patients, 50.5%), active cancer (9832 patients, 24.6%), and diabetes (8332 patients, 20.9%). The most common surgeries were lowrisk surgery (14 383 patients, 36.0%), major general surgery (7950 patients, 19.9%) and major orthopedic surgery (6982 patients, 17.5%). Of all surgeries, 4189 patients (10.5%) underwent urgent or emergent surgery. The most common types of anesthesia were general only (20 760 patients, 51.9%) and neuraxial only (9557 patients, 23.9%). The median length of hospital stay was 4 (interquartile range [IQR] 2–8) days.
Table 1:
Patient baseline characteristics
| Characteristic | No. (%) of patients n = 40 004 |
|---|---|
| Age, yr | |
| 45–64 | 22 141 (55.3) |
| 65–74 | 10 160 (25.4) |
| ≥ 75 | 7703 (19.3) |
| Sex, female | 19 877 (49.7) |
| History of | |
| Hypertension | 20 152 (50.5) n = 39 917 |
| Diabetes | 8332 (20.9) n = 39 905 |
| Coronary artery disease | 5159 (12.9) n = 39 876 |
| Peripheral arterial disease | 3203 (8.0) |
| Chronic obstructive pulmonary disease | 3165 (7.9) |
| Coronary revascularization | 2256 (5.7) n = 39 828 |
| Stroke | 1682 (4.2) |
| Congestive heart failure | 1424 (3.6) n = 39 870 |
| High-risk coronary artery disease | 384 (1.0) |
| Cardiac arrest | 235 (0.6) n = 39 868 |
| Coronary revascularization within 6 mo | 138 (0.3) n = 39 827 |
| Active cancer | 9832 (24.6) |
| Atrial fibrillation just before surgery | 1123 (2.8) n = 39 876 |
| Preoperative estimated glomerular filtration rate (mL/min/1.73 m2) | n = 37 290 |
| < 30 | 1515 (4.1) |
| 30–44 | 1774 (4.8) |
| 45–59 | 3707 (9.9) |
| > 60 | 30 294 (81.2) |
| Surgical category* | |
| Major general | 7950 (19.9) |
| Major orthopedic | 6982 (17.5) |
| Major urology and gynecology | 4827 (12.1) |
| Major vascular | 2642 (6.6) |
| Major neurosurgery | 2341 (5.9) |
| Major thoracic | 1165 (2.9) |
| Low-risk surgery only | 14 383 (36.0) |
| Urgent or emergent surgery | 4189 (10.5) |
| Type of anesthesia | n = 39 969 |
| General only | 20 760 (51.9) |
| Neuraxial (spinal or epidural) only | 9557 (23.9) |
| General with nitrous oxide only | 3805 (9.5) |
| General and thoracic epidural only | 1658 (4.1) |
| General and nerve block only | 1252 (3.1) |
| Other | 2937 (7.3) |
No. of patients with 2 major surgery categories = 280; no. of patients with 3 major surgery categories = 3.
During the 30-day follow-up, 715 patients (1.8%, 95% CI 1.7%–1.9%) died. Mortality varied across regions (Supplemental Table 2, Appendix 1), ranging from 96 deaths among 1489 patients (6.4%, 95% CI 5.3%–7.8%) in Africa to 253 deaths among 22 447 patients (1.1%, 95% CI 1.0%–1.3%) in North America, Europe and Australia. Geographical regions were independent predictors of mortality when added to our model (Supplemental Table 3, Appendix 1). Mortality also varied across surgical categories (Supplemental Table 4, Appendix 1), ranging from a mortality of 240 deaths among 7950 patients (3.0%, 95% CI 2.7–3.4) undergoing major general surgery to 24 deaths among 4827 patients (0.5%, 95% CI 0.3–0.7) undergoing major urology and gynecology surgeries; the latter mortality was lower than what were deemed a priori as low-risk surgeries (177 deaths among 14 383 patients [1.2%, 95% CI 1.1–1.4]).
Among the 715 patients who died, 5 deaths (0.7%, 95% CI 0.3%–1.6%) occurred in the operating room, 500 (69.9%, 95% CI 66.5%–73.2%) occurred after surgery during the index admission to hospital and 210 (29.4%, 95% CI 26.1%–32.8%) occurred after discharge from the hospital. The median time to death was 11 (IQR 6–19) days, and the number of deaths was almost evenly distributed over 30-day follow-up (Supplemental Figure 2, Appendix 1).
The timing and location of deaths varied across regions (e.g., Asia, 73 deaths [37.1%] after hospital discharge; Africa, 18 deaths [18.8%] after hospital discharge; Supplemental Table 5, Appendix 1). The timing and location of deaths also varied across surgical categories (Supplemental Table 6, Appendix 1): of the deaths that occurred, death in the operating room was most common in major vascular surgery (2.7%) and death after hospital discharge was least common in major urology or gynecology surgery (12.5%).
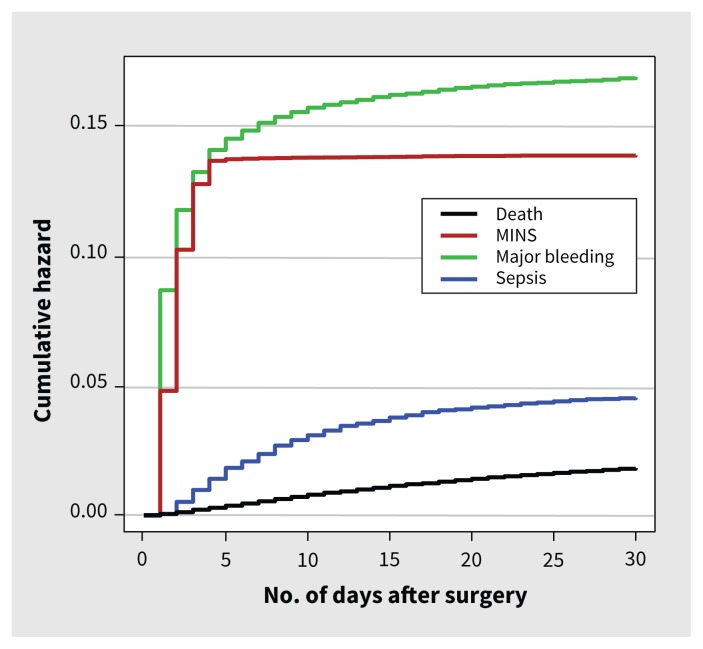
The most common complications were major bleeding (6238 patients, 15.6%), MINS (5191 patients, 13.0%), infection without sepsis (2171 patients, 5.4%) and sepsis (1783 patients, 4.5%) (Table 2). There was variation across surgical categories for major bleeding and MINS. Among the major surgeries, major bleeding was most common in major orthopedic surgery (2164 patients, 31.0%) and least common in thoracic surgery (119 patients, 10.2%). Among the major surgeries, MINS was most common in major vascular surgery (633 patients, 24.0%) and least common in major urologic or gynecologic surgery (503 patients, 10.4%). The median time to major bleeding was on the day (IQR 0–2) of surgery, MINS occurred a median of 1 (IQR 0–1) day after surgery and sepsis occurred a median of 6 (IQR 3–11) days after surgery (Supplemental Figure 2, Appendix 1).
Table 2:
Thirty–day perioperative complications overall and by type of surgery
| Outcome | No. (%, 95% CI) of patients | |||||||
|---|---|---|---|---|---|---|---|---|
| All surgeries n = 40 004 |
Type of major surgery | Low-risk surgery only n = 14 383 |
||||||
| General n = 7950 |
Vascular n = 2642 |
Neurosurgery n = 2341 |
Orthopedic n = 6982 |
Thoracic n = 1165 |
Urology or gynecology n = 4827 |
|||
| Major bleeding | 6238 (15.6, 15.2–16.0) | 1454 (18.3, 17.5–19.2) | 666 (25.2, 23.6–26.9) | 419 (17.9, 16.4–19.5) | 2164 (31.0, 29.9–32.1) | 119 (10.2, 8.6–12.1) | 658 (13.6, 12.7–14.6) | 876 (6.1, 5.7–6.5) |
| MINS | 5191 (13.0, 12.7–13.3) | 980 (12.3, 11.6–13.1) | 633 (24.0, 22.4–25.6) | 301 (12.9, 11.6–14.3) | 1257 (18.0, 17.1–18.9) | 231 (19.8, 17.6–22.2) | 503 (10.4, 9.6–11.3) | 1335 (9.3, 8.8–9.8) |
| Sepsis | 1783 (4.5, 4.3–4.7) | 783 (9.8, 9.2–10.5) | 140 (5.3, 4.5–6.2) | 132 (5.6, 4.8–6.6) | 258 (3.7, 3.3–4.2) | 54 (4.6, 3.6–6.0) | 162 (3.4, 2.9–3.9) | 293 (2.0, 1.8–2.3) |
| Infection without sepsis | 2171 (5.4, 5.2–5.7) | 632 (7.9, 7.4–8.6) | 152 (5.8, 4.9–6.7) | 102 (4.4, 3.6–5.3) | 508 (7.3, 6.7–7.9) | 44 (3.8, 2.8–5.0) | 261 (5.4, 4.8–6.0) | 493 (3.4, 3.1–3.7) |
| Acute kidney injury with dialysis | 118 (0.3, 0.2–0.4) | 49 (0.6, 0.5–0.8) | 25 (0.9, 0.6–1.4) | 4 (0.2, 0.1–0.4) | 14 (0.2, 0.1–0.3) | 3 (0.3, 0.1–0.8) | 7 (0.1, 0.1–0.3) | 17 (0.1, 0.1–0.2) |
| Stroke | 132 (0.3, 0.3–0.4) | 20 (0.3, 0.2–0.4) | 25 (0.9, 0.6–1.4) | 34 (1.5, 1.0–2.0) | 24 (0.3, 0.2–0.5) | 5 (0.4, 0.2–1.0) | 7 (0.1, 0.1–0.3) | 18 (0.1, 0.1–0.2) |
| Venous thromboembolism | 299 (0.7, 0.7–0.8) | 71 (0.9, 0.7–1.1) | 15 (0.6, 0.3–0.9) | 22 (0.9, 0.6–1.4) | 114 (1.6, 1.4–2.0) | 5 (0.4, 0.2–1.0) | 38 (0.8, 0.6–1.1) | 39 (0.3, 0.2–0.4) |
| Congestive heart failure | 372 (0.9, 0.8–1.0) | 113 (1.4, 1.2–1.7) | 46 (1.7, 1.3–2.3) | 5 (0.2, 0.1–0.5) | 120 (1.7, 1.4–2.1) | 12 (1.0, 0.6–1.8) | 30 (0.6, 0.4–0.9) | 53 (0.4, 0.3–0.5) |
| New, clinically important atrial fibrillation | 370 (0.9, 0.8–1.0) | 145 (1.8, 1.6–2.1) | 47 (1.8, 1.3–2.4) | 9 (0.4, 0.2–0.7) | 89 (1.3, 1.0–1.6) | 35 (3.0, 2.2–4.1) | 29 (0.6, 0.4–0.9) | 28 (0.2, 0.1–0.3) |
| Death | 715 (1.8, 1.7–1.9) | 240 (3.0, 2.7–3.4) | 73 (2.8, 2.2–3.5) | 62 (2.6, 2.1–3.4) | 124 (1.8, 1.5–2.1) | 20 (1.7, 1.1–2.6) | 24 (0.5, 0.3–0.7) | 177 (1.2, 1.1–1.4) |
Note: CI = confidence interval, MINS = myocardial injury after noncardiac surgery.
Eight perioperative complications were independently associated with 30-day mortality (Table 3) after we adjusted for preoperative patient characteristics and surgical categories (Supplemental Table 7, Appendix 1). This model had an optimism-corrected concordance index (C-index) of 0.89. We found that the following complications were independently associated with 30-day mortality: major bleeding (361 deaths; adjusted HR 2.6, 95% CI 2.2–3.1), MINS (314 deaths; adjusted HR 2.2; 95% CI 1.9–2.6), sepsis (215 deaths; adjusted HR 5.6, 95% CI 4.6–6.8), infection without sepsis (55 deaths; adjusted HR 2.3, 95% CI 1.7–3.0), acute kidney injury with new dialysis (49 deaths; adjusted HR 4.2, 95% CI 3.1–5.8), stroke (27 deaths; adjusted HR 3.7, 95% CI 2.5–5.7), venous thromboembolism (15 deaths; adjusted HR 2.2, 95% CI 1.3–3.7) and congestive heart failure (54 deaths; adjusted HR 2.4, 95% CI 1.7–3.2). The highest attributable fractions of risk of mortality were associated with major bleeding (17.0%), MINS (15.9%) and sepsis (12.0%). Of the 715 patients who died, 147 (20.6%, 95% CI 17.8%–23.7%) did not have any of the 8 perioperative complications that were associated with 30-day mortality.
Table 3:
Relation between perioperative complications and 30-day mortality*
| Outcome | No. of patients who died/total no. of patients with the outcome | Percentage (95% CI) of patients who died | Adjusted HR (95% CI) | AF,† % |
|---|---|---|---|---|
| Major bleeding | 361/6238 | 5.8 (5.2–6.4) | 2.6 (2.2–3.1) | 17.0 |
| No major bleeding | 354/33 766 | 1.0 (0.9–1.2) | Ref. | |
|
| ||||
| MINS | 314/5191 | 6.0 (5.4–6.7) | 2.2 (1.9–2.6) | 15.9 |
| No MINS | 401/34 813 | 1.2 (1.0–1.3) | Ref. | |
|
| ||||
| Sepsis | 215/1783 | 12.1 (10.6–13.7) | 5.6 (4.6–6.8) | 12.0 |
| Infection without sepsis | 55/2171 | 2.5 (2.0–3.3) | 2.3 (1.7–3.0) | 2.8 |
| No sepsis or infection | 445/36 050 | 1.2 (1.1–1.4) | Ref. | |
|
| ||||
| Acute kidney injury with dialysis | 49/118 | 41.5 (33.0–50.5) | 4.2 (3.1–5.8) | 1.1 |
| No acute kidney injury with dialysis | 666/39 886 | 1.7 (1.5–1.8) | Ref. | |
|
| ||||
| Stroke | 27/132 | 20.5 (14.5–28.1) | 3.7 (2.5–5.7) | 0.8 |
| No stroke | 688/39 872 | 1.7 (1.6–1.9) | Ref. | |
|
| ||||
| Venous thromboembolism | 15/299 | 5.0 (3.1–8.1) | 2.2 (1.3–3.7) | 0.3 |
| No venous thromboembolism | 700/39 705 | 1.8 (1.6–1.9) | Ref. | |
|
| ||||
| Congestive heart failure | 54/372 | 14.5 (11.3–18.5) | 2.4 (1.7–3.2) | 0.7 |
| No congestive heart failure | 661/39 632 | 1.7 (1.5–1.8) | Ref. | |
|
| ||||
| New, clinically important atrial fibrillation | 44/370 | 11.9 (9.0–15.6) | 1.4 (1.0–2.0) | NA |
| No new, clinically important atrial fibrillation | 671/39 634 | 1.7 (1.6–1.8) | Ref. | |
Note: AF = attributable fraction, CI = confidence interval, HR = hazard ratio, MINS = myocardial injury after noncardiac surgery, NA = not applicable, Ref. = reference.
Cox proportional hazards model in which the dependent variable was 30-day mortality and the independent variables included preoperative and surgical variables previously associated with 30-day perioperative mortality and perioperative complications as time-dependent variables.
The AF is a measure that represents the proportional reduction in mortality within a population that would occur if the incidence of a complication was reduced to 0, provided that a causal relation existed between that complication and 30-day mortality. We used frequency of a complication and the association between the complication and mortality to calculate the AF.
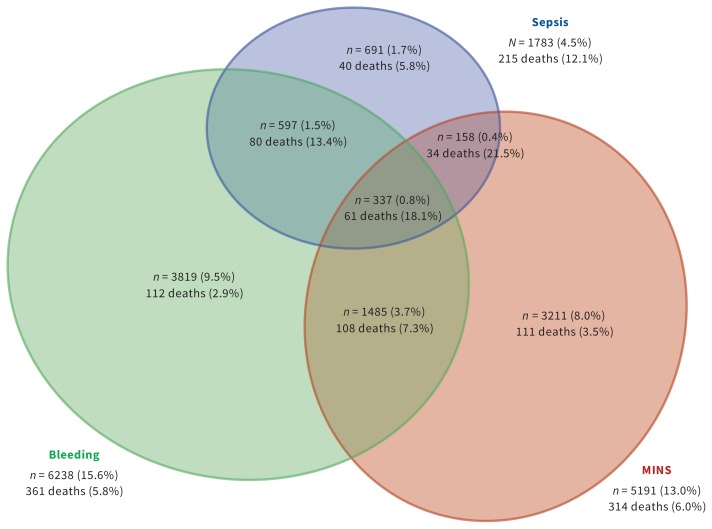
Figure 1 shows the Kaplan–Meier estimates of mortality, MINS, major bleeding and sepsis. These complications varied across regions (Supplemental Table 8, Appendix 1). The Venn diagrams show the outcomes of patients who did and did not have major bleeding, MINS and sepsis, and patients who had combinations of these events (Figure 2).
Figure 1:
Kaplan–Meier curves for death, MINS, major bleeding and sepsis. Note: MINS = myocardial injury after noncardiac surgery.
Figure 2:
Outcomes among patients who had major bleeding, MINS and sepsis, and patients who had combinations of these events. Notes: MINS = myocardial injury after noncardiac surgery; 29 706 (74.3%) of patients had no bleeding, sepsis or MINS, of whom 169 died (0.6%).
Post hoc analyses that included preoperative estimated glomerular filtration rate and hemoglobin showed similar relations between perioperative complications and mortality (Supplemental Table 9, Appendix 1).
Interpretation
In our study, involving 40 004 patients who underwent inpatient noncardiac surgery, 715 died (1.8%) within 30 days after surgery. Death in the operating room was uncommon (i.e., 5 deaths); however, death after discharge from hospital was common, accounting for 29.4% of all deaths. The 3 perioperative complications that were independently associated with mortality and had the highest attributable fractions were major bleeding, MINS and sepsis. The median time to major bleeding was the day of surgery, median time to MINS was 1 day after surgery and median time to sepsis was 6 days after surgery.
The African Surgical Outcomes Study, a large prospective cohort study that included patients undergoing noncardiac surgery in 25 countries in Africa, reported a lower risk of perioperative mortality (2.1%) compared with our study (i.e., 6.5% risk of mortality in Africa) that may be explained by the younger population and shorter duration of follow-up in the African study.14 A prospective cohort study involving patients undergoing noncardiac surgery in 28 countries in Europe reported a higher risk of perioperative mortality (4.0%)15 than our study (1.2% risk of mortality in Europe). This study recruited patients over 7 days in each hospital, and it is possible that recruitment did not reflect a representative sample within the participating centres. Moreover, this study reported that 25% of patients underwent urgent or emergent surgery, whereas 10% of patients in our study underwent urgent or emergent surgery. We found that urgent or emergent surgery was independently associated with a higher risk of mortality (Supplemental Table 7, Appendix 1).
A 2011 large retrospective study evaluated surgeries with death rates greater than 2.0%16 and, similar to our study, reported that 23.2% of 30-day deaths occurred after hospital discharge. Similar to our results, a 2018 study involving adults undergoing noncardiac surgery in Switzerland reported, using a model predicting 30-day mortality, adjusted HRs associated with MINS of 2.3 (95% CI 1.2–4.4) and with sepsis of 4.5 (95% CI 2.2–9.2).17 A large randomized controlled trial that investigated the effects of perioperative β-blockers in patients undergoing noncardiac surgery reported that significant perioperative bleeding was independently associated with 30-day mortality (adjusted HR 1.7, 95% CI 1.1–2.4),8 which is similar to our finding.
In our study of patients aged 45 years and older who underwent noncardiac surgery, 1.8% of patients died within 30 days of surgery. Assuming that worldwide, 100 million adults aged 45 years and older undergo noncardiac surgery annually,2 then about 1.8 million adults die within 30 days of noncardiac surgery each year. This indicates that perioperative mortality is a substantial global health problem.
We found that death in the operating room was uncommon (i.e., 5 patients) and accounted for 0.7% of deaths. In contrast, postoperative mortality was substantial (i.e., 710 deaths), accounting for 99.3% of deaths. Moreover, 29.4% of all deaths occurred after patients were discharged from the hospital. These data suggest the need for improved monitoring and management of patients after surgery and into the home setting.
Anesthetic-related mortality has decreased 100-fold over the last 100 years.18 Improvements in intraoperative mortality have been attributed largely to increased monitoring during surgery, through the use of electronic monitors (e.g., frequent blood pressure, continuous pulse oximetry and electrocardiography) and the development of a culture of vigilance in anesthesia, as shown by the development of protocols and standards for intraoperative monitoring and care.19,20
In the postoperative setting, most patients receive care from their surgeon, who is often busy performing surgery. After discharge from the hospital, these patients receive care weeks later in their physician’s office. After surgery, when patients are usually receiving analgesics that can mask symptoms (e.g., chest pain) of some complications,5 they typically have their vital signs checked every 4–8 hours on a surgical floor.21 After hospital discharge, most patients receive only monitoring at their 3- to 4-week follow-up.
Studies that obtained continuous pulse oximetry and blood pressure readings from patients on surgical floors have shown that many patients have prolonged hypoxia and hypotension that is not identified by health care providers.22,23 Given that some studies have shown that hypoxia and hypotension are precursors to postoperative complications,24,25 remote automated monitoring technology with a health care provider who is available to respond to early signs of an impending complication may improve outcomes after surgery, similar to how anesthesiologists and enhanced monitoring improved intraoperative outcomes. These interventions require evaluation in prospective studies.
Our study has several strengths: a large sample population of patients from 28 centres in 14 countries, study team members systematically followed all patients and 99.1% of the patients completed the 30-day follow-up. Our mortality model showed excellent discrimination.
Limitations
Our study has some limitations. We did not adjudicate some of our outcomes (i.e., major bleeding, sepsis, infection without sepsis, congestive heart failure and acute kidney injury with dialysis). It is possible that this led to an overestimation of some of these events; however, based on these outcome definitions and our data checks, it is likely that these outcomes were accurately reported. The data from Africa were based on a single centre, whereas all other continental data included 4 centres or more and at least 4 times the number of participants. Clinicians should view our finding of higher risk-adjusted mortality in Africa as hypothesis generating.
Conclusion
Given that 99.3% of deaths among adults undergoing noncardiac surgery occur after the procedure, efforts to improve postsurgical care — in the hospital and home setting — has the potential to reduce mortality. We identified 8 perioperative complications that were independently associated with 30-day mortality. Three of these complications (i.e., major bleeding, MINS and sepsis) could explain 44.9% of the deaths. These complications are promising targets for research on prevention, early identification and management to decrease perioperative mortality. The median time to these events provides insight about when monitoring for each complication is likely to have the greatest effect. Focusing on the prevention, early identification and management of major bleeding, MINS and sepsis holds promise for decreasing perioperative deaths.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.190882
Footnotes
Competing interests: Clara Chow received support from the National Health and Medical Research Council of Australia and The Heart Foundation (Australia) for a career development fellowship. Robert Sapsford received nonfinancial support in the form of a research nurse funded by the National Institutes of Health Research, and lecture fees from Eli Lilly, MSD and Novo Nordisk. Denis Xavier received grants from Cadila Pharmaceuticals, Boehringer Ingelheim, Astra Zeneca India, Sanofi Aventis, Pfizer, Bristol–Myers Squibb, Medical Research Council (United Kingdom) and Wellcome Trust outside the submitted work. Emmanuelle Duceppe received a grant as a coapplicant on an investigator-initiated study and lecture fees from Roche Diagnostics. Philip J. Devereaux is a member of a research group with a policy of not accepting honorariums or other payments from industry for their own personal financial gain. They do accept honorariums or payments from industry to support research endeavours and costs to participate in meetings. Based on study questions Dr. Devereaux has originated and grants he has written, he has received grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Coviden, Octapharma, Philips Healthcare, Roche Diagnostics, Siemens and Stryker. Dr Devereaux has participated in advisory board meetings for GlaxoSmithKline and Boehringer Ingelheim. He also attended an expert panel meeting with AstraZeneca and Boehringer Ingelheim. Roche Diagnostics provided Troponin T assays and financial support for the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Philip J. Devereaux designed the work. P.J. Devereaux, Jessica Spence, Matthew Chan, C.Y. Wang, Alben Sigamani, Denis Xavier, Rupert Pearse, Pablo Alonso-Coello, Ignacio Garutti, Sadeesh Srinathan, Emmanuelle Duceppe, Michael Walsh, Flavia Kessler Borges, German Malaga, Valsa Abraham, Atiya Faruqui, Otavio Berwanger, Bruce Biccard, Juan Carlos Villar, Daniel Sessler, Andrea Kurz, Clara Chow, Carisi Polanczyk, Wojciech Szczeklik, Gareth Ackland, Amit Garg, Michael Jacka, Robert Sapsford, Colin Williams, Olga Lucía Cortes, Pierre Coriat, Ameen Patel, Maria Tiboni, Emilie Belley-Cote, Stephen Yang, Michael McGillion, Holger Schunemann and Shirley Pettit contributed to the concept of the work and acquired the data. Yannick LeManach and Diane Heels-Ansdell analyzed the data. P.J. Devereaux and Jessica Spence drafted the manuscript. All of the authors interpreted the data, revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing: Our data are not available for use by other researchers at this time.
Funding: See Appendix 1.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–44. [DOI] [PubMed] [Google Scholar]
- 2.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015;373:2258–69. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery —1995 to 2009. CMAJ 2012;184:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smilowitz NR, Gupta N, Guo Y, et al. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart 2018;104:1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564–78. [DOI] [PubMed] [Google Scholar]
- 6.Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642–51. [DOI] [PubMed] [Google Scholar]
- 7.Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction: a general population, propensity score–matched cohort study. Arthritis Rheumatol 2015;67:2771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371:1839–47. [DOI] [PubMed] [Google Scholar]
- 9.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1494–503. [DOI] [PubMed] [Google Scholar]
- 10.Myles PS, Leslie K, Chan MT, et al. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet 2014;384:1446–54. [DOI] [PubMed] [Google Scholar]
- 11.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–304. [DOI] [PubMed] [Google Scholar]
- 12.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–10. [DOI] [PubMed] [Google Scholar]
- 13.Rückinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol 2009;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biccard BM, Madiba TE, Kluyts HL, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. Lancet 2018;391:1589–98. [DOI] [PubMed] [Google Scholar]
- 15.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7-day cohort study. Lancet 2012;380:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu P, Chang DC, Osen HB, et al. NSQIP reveals significant incidence of death following discharge. J Surg Res 2011;170:e217–24. [DOI] [PubMed] [Google Scholar]
- 17.Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018;137:1221–32. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Warner M, Lang BH, et al. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology 2009;110:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichhorn JH. Practical current issues in perioperative patient safety. Can J Anaesth 2013;60:111–8. [DOI] [PubMed] [Google Scholar]
- 20.John Doyle D, Dahaba AA, LeManach Y. Advances in anesthesia technology are improving patient care, but many challenges remain. BMC Anesthesiol 2018;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc 2008;10:111–5. [PubMed] [Google Scholar]
- 22.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015;121:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turan A, Chang C, Cohen B, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology 2019;130:550–9. [DOI] [PubMed] [Google Scholar]
- 24.Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1504–13. [DOI] [PubMed] [Google Scholar]
- 25.Gill NP, Wright B, Reilly CS. Relationship between hypoxaemic and cardiac ischaemic events in the perioperative period. Br J Anaesth 1992;68:471–3. [DOI] [PubMed] [Google Scholar]