Abstract
Background.
People living with HIV (PLWH) are at increased risk for developing several cancers, but less is known about how HIV impacts the rate of progression to advanced cancer or death.
Methods.
We compared stage at presentation and mortality after diagnosis between 14,453 PLWH and 6,368,126 HIV-uninfected patients diagnosed with cancers of the oral cavity, stomach, colorectum, anus, liver, pancreas, lung, female breast, cervix, prostate, bladder, kidney, thyroid, and melanoma using data from the National Cancer Database (2004–2014). Polytomous logistic regression and Cox proportional hazards regression were used to evaluate the association between HIV, cancer stage, and stage-adjusted mortality following diagnosis, respectively. Regression models accounted for the type health facility where cancer treatment was administered and type of individual health insurance.
Results.
HIV-infected cancer patients were more likely to be uninsured (HIV-infected: 5·0% vs. HIV-uninfected: 3·3%; P<0·0001) and were less likely to have private health insurance (25·4% vs. 44·7%; P<0·0001). Compared to non-PLWH, the odds of being diagnosed at advanced stage were significantly elevated in PLWH for melanoma and cancers of the oral cavity, liver, female breast, prostate, and thyroid (OR for stage IV vs. stage I range:1·24–2·06). PLWH diagnosed with Stage I-III disease experienced elevated mortality following diagnosis across 13 of the 14 cancer sites evaluated, with hazard ratios ranging from 1·20 (95%CI 1·14–1·26) for lung cancer to 1·85 (1·68–2·04), 1·85 (1·51–2·27), and 2·93 (2·08–4·13) for female breast, cervix, and thyroid cancers, respectively.
Conclusion.
PLWH were more likely to be diagnosed with advanced-stage cancers and to experience elevated mortality following cancer diagnosis, even after accounting for healthcare-related factors.
Keywords: HIV and cancer, cancer patient mortality, cancer stage, National Cancer Database, health insurance and mortality
Precis:
People living with HIV are more likely to be diagnosed with advanced-stage cancers and to experience elevated mortality following their cancer diagnosis, even after accounting for healthcare-related factors.
INTRODUCTION.
People living with human immunodeficiency virus (PLWH) have an elevated risk of certain cancer types, particularly those caused by viral co-infections.1–9 Rates of many infection-associated cancers among PLWH have declined following the widespread availability of highly active antiretroviral therapy (HAART) to restore patient immunity, although risk remains elevated compared to HIV-uninfected persons.10–13 Unlike infection-associated cancers, some non-infection-related cancers such as prostate, female breast, and colon do not occur at elevated rates in PLWH.14 Nevertheless, these cancers are becoming increasingly prevalent among PLWH because of widespread dissemination of HAART that permits HIV patients to live to older ages when cancers are more common,15, 16 raising the important question of whether HIV-related immunosuppression is associated with tumors that progress to higher stages before being diagnosed or result in poorer outcomes following diagnosis.
Both advanced stage at presentation and elevated mortality following diagnosis can reflect tumor aggressiveness, and recent data suggest that immunosuppressed patients fare worse on both metrics. A prior registry-based study showed that PLWH were more likely to be diagnosed with distant-stage cancers of the lung, female breast, prostate, bladder, and melanoma.17 Stage-adjusted mortality following diagnosis was also elevated in a similar population of HIV-infected cancer patients across a range of sites, including cancers of the colorectum, pancreas, larynx, lung, female breast, prostate, and melanoma.18 It is plausible that advanced-stage disease and elevated mortality following a cancer diagnosis among PLWH are related to the biological effects of HIV-associated immunosuppression.
However, the more advanced-stage cancers and elevated mortality reported among PLWH could also be related to receipt of sub-optimal healthcare, a possibility supported by mounting data that PLWH are less likely to receive cancer treatment.19, 20 Available data have not been able to disentangle these possibilities. To begin addressing this issue of sub-optimal healthcare, we evaluated the association between HIV, cancer stage at presentation, and mortality after a cancer diagnosis after accounting for the type of treating health facility and reported individual health insurance using data from more than six million US cancer patients from the National Cancer Database.
METHODS.
The National Cancer Database (NCDB) is a nationwide, hospital-based registry database jointly sponsored by the American Cancer Society and the American College of Surgeons. Using the NCDB, we ascertained the following primary, malignant cancers occurring in adults (>18 years of age) between 2004–2014: oral cavity/pharynx/larynx (International Classification of Diseases [ICD] for Oncology version 3 topography codes C000-C009, C019-C069, C079-C099, C100-C109, C110-C119, C129-C140, C142, C148, C320-C329), stomach (C160-C169), colorectum (C180-C189, C199, C209), anus (C210, C218), liver (C220), pancreas (C250-C259), lung (C340-C349), female breast (C500-C509), cervix (C530-C539), prostate (C619), bladder (C670-C679), kidney (C649, C659), thyroid (C739), and melanoma (C440-C449 with histology codes 8720–8790). Cancers with the following histology codes were excluded: 9050–9055, 9140, and 9590–9989. Patients with unknown stage, unknown date of diagnosis, or unknown last date of contact were also excluded.
The HIV status of each cancer patient at the time of cancer diagnosis was defined using ICD-9 codes, with HIV positivity defined as the presence of codes 04200–04499, 07953, or V08. Cancer stage at presentation was defined as stages I, II, III, or IV using the Collaborative Stage 6th edition (https://cancerstaging.org/cstage/Pages/default.aspx). Factors related to healthcare were ascertained from the NCDB database, including data on the type of health facility where cancer care was administered (community center, comprehensive community center, teaching/research institution, National Cancer Institute [NCI] network cancer center, integrated network, other/unknown, as defined by the Commission on Cancer 21) and the type of reported health insurance (private, Medicaid, Medicare, no insurance, other government insurance). We hypothesized that the type of treating health facility reflects an important aspect of the quality of care received, whereas individual health insurance can influence a patient’s access to care.
Statistical Analysis.
We utilized polytomous logistic regression to estimate the association between HIV status and cancer stage at presentation (stage I as referent group), adjusting for patient sex (male, female), age (18–44, 45–54, 55–64, 65+ years), race (non-Hispanic white, black, Hispanic, other, missing), calendar year of cancer diagnosis (2004–2006, 2007–2010, 2011–2014), and median household income by zip code (<$38000, $38000–47999, $48000–62999, $63000+, missing). Analyses were conducted separately for each cancer site. P-trends were calculated by fitting logistic regression models with stage categorized as an ordinal variable, adjusting for the covariates listed above. To further assess whether any HIV and cancer stage associations were attributable to factors related to sub-optimal care, we adjusted for the type of health facility at which cancer treatment was received and the type of individual health insurance.
We next evaluated whether HIV was associated with overall mortality using Cox proportional hazards regression. Follow-up time for each cancer patient was calculated from the date of cancer diagnosis to the date of death from any cause, with follow-up ending in 2014. Cases diagnosed between 2013 and 2014 were excluded to ensure adequate follow-up time to ascertain mortality. These mortality analyses were limited to Stage I-III disease due to the heterogeneity of disease burden, treatment approaches, and outcomes that occur in patients with metastatic (Stage IV) disease. Analyses were adjusted for age, sex, race, calendar year of cancer diagnosis, median household income, cancer stage, and cancer treatment (categorized as yes/no for receipt of surgery, radiation or chemotherapy). Additional models were adjusted for type of treating health facility and type of individual health insurance. The proportional hazard assumption was tested by examining parallelism in ln(-ln(survival)) versus ln(time) plots. No violations of this assumption were observed for HIV across any cancer site. However, violations were observed for cancer stage and treatment, requiring time-dependent adjustment for these two variables in regression models.
In a supplemental analysis, we ran logistic and Cox models for cancer stage and mortality outcomes, respectively, within patient groups <65 years of age stratified by receipt of specific types of health insurance: (1) private insurance, (2) Medicare, or (3) Medicaid.
RESULTS.
We compared cancer stage at presentation and mortality following cancer diagnosis in over six million US cancer patients from the NCDB, including 14,453 PLWH diagnosed with cancer between 2004 and 2014 (Table 1). HIV-infected cancer patients were younger, more likely to be male and non-white, and had lower median household income than HIV-uninfected cancer patients (P<0·0001). The most common cancer diagnoses in PLWH were lung cancer (29·1% of all cases), colorectal cancer (14·1%), and prostate cancer (10·5%), compared to cancers of the breast (22·8%), prostate (17·8%), and lung (17·7%) among HIV-uninfected patients, reflecting differences in the patient sex distribution by HIV status. We observed significant differences in the proportion of uninsured cancer patients by HIV status (HIV-infected: 5·0% vs. HIV-uninfected: 3·3%; P<0·0001), with PLWH being approximately half as likely to report private health insurance (HIV-infected: 25·4% vs. HIV-uninfected: 44·7%) and approximately 3 times more likely to be on Medicaid (HIV-infected: 17·9% vs. HIV-uninfected: 5·9%). The likelihood of receiving cancer treatment and the type of health facility at which treatment was administered also differed by HIV status (P<0·0001).
Table 1.
Characteristics of patients in the National Cancer Database (2004–2014), according to HIV status
| Total (N=6382579) | HIV-infected (N=14453) | HIV-uninfected (N=6368126) | Chi-square p value | |
|---|---|---|---|---|
| Demographic Factors | N (%) | N (%) | N (%) | |
| Sex | ||||
| Male | 3146045 (49.3) | 9296 (64.3) | 3136749 (49.3) | p < 0.0001 |
| Female | 3236534 (50.7) | 5157 (35.7) | 3231377 (50.7) | |
| Age at diagnosis (years) | ||||
| 18–44 | 543310 (8.5) | 1452 (10) | 541858 (8.5) | p < 0.0001 |
| 45–54 | 1075558 (16.9) | 3513 (24.3) | 1072045 (16.8) | |
| 55–64 | 1733796 (27.2) | 3597 (24.9) | 1730199 (27.2) | |
| 65+ | 3029915 (47.5) | 5891 (40.8) | 3024024 (47.5) | |
| Race/Ethnicity | ||||
| White, non-Hispanic | 4726015 (74.0) | 7966 (55.1) | 4718049 (74.1) | p < 0.0001 |
| Black | 328450 (5.1) | 1054 (7.3) | 327396 (5.1) | |
| Hispanic | 689689 (10.8) | 4533 (31.4) | 685156 (10.8) | |
| Other | 206283 (3.3) | 177 (1.2) | 206106 (3.3) | |
| Missing | 432142 (6.8) | 723 (5) | 431419 (6.8) | |
| Year of cancer diagnosis | ||||
| 2004–2006 | 1628351 (25.5) | 5141 (35.6) | 1623210 (25.5) | p < 0.0001 |
| 2007–2010 | 2366096 (37.1) | 5912 (40.9) | 2360184 (37.1) | |
| 2011–2014 | 2388132 (37.4) | 3400 (23.5) | 2384732 (37.4) | |
| Median income level a | ||||
| <$38000 | 1120358 (17.6) | 4150 (28.7) | 1116208 (17.5) | p < 0.0001 |
| $38000-$47999 | 1466892 (23) | 3280 (22.7) | 1463612 (23) | |
| $48000-$62999 | 1676914 (26.3) | 3254 (22.5) | 1673660 (26.3) | |
| $63000+ | 2013696 (31.5) | 3444 (23.8) | 2010252 (31.6) | |
| Missing | 104719 (1.6) | 325 (2.2) | 104394 (1.6) | |
| Cancer site | ||||
| Oral Cavity/Pharynx/Larynx | 289643 (4.5) | 939 (6.5) | 288704 (4.5) | p < 0.0001 |
| Stomach | 106060 (1.7) | 290 (2) | 105770 (1.7) | |
| Colorectum | 784001 (12.3) | 2034 (14.1) | 781967 (12.3) | |
| Anus | 30793 (0.5) | 1052 (7.3) | 29741 (0.5) | |
| Liver | 107544 (1.7) | 823 (5.7) | 106721 (1.7) | |
| Pancreas | 211219 (3.3) | 498 (3.4) | 210721 (3.3) | |
| Lung | 1130306 (17.7) | 4201 (29.1) | 1126105 (17.7) | |
| Melanoma | 263127 (4.1) | 214 (1.5) | 262913 (4.1) | |
| Female Breast | 1449954 (22.7) | 1197 (8.3) | 1448757 (22.8) | |
| Cervix | 95222 (1.5) | 330 (2.3) | 94892 (1.5) | |
| Prostate | 1136297 (17.8) | 1513 (10.5) | 1134784 (17.8) | |
| Bladder | 176320 (2.8) | 394 (2.7) | 175926 (2.8) | |
| Kidney | 314810 (4.9) | 708 (4.9) | 314102 (4.9) | |
| Thyroid | 287283 (4.5) | 260 (1.8) | 287023 (4.5) | |
| Individual insurance status | ||||
| Private | 2852824 (44.7) | 3667 (25.4) | 2849157 (44.7) | p < 0.0001 |
| Medicaid | 380678 (6.0) | 2587 (17.9) | 378091 (5.9) | |
| Medicare | 2770671 (43.4) | 7234 (50.0) | 2763437 (43.5) | |
| Uninsured | 211643 (3.3) | 717 (5.0) | 210926 (3.3) | |
| Government | 34223 (0.5) | 72 (0.5) | 34151 (0.5) | |
| Missing | 132540 (2.1) | 176 (1.2) | 132364 (2.1) | |
| Type of treating health facility | ||||
| Community center | 628450 (9.8) | 1410 (9.8) | 627040 (9.8) | p < 0.0001 |
| Comprehensive community center | 2712165 (42.5) | 5435 (37.6) | 2706730 (42.5) | |
| Teaching/research institution | 1466395 (23) | 3833 (26.5) | 1462562 (23) | |
| NCI network cancer center | 731239 (11.5) | 1774 (12.3) | 729465 (11.5) | |
| Integrated Network | 649528 (10.2) | 1463 (10.1) | 648065 (10.2) | |
| Others/Unknown | 194802 (3.1) | 538 (3.7) | 194264 (3.1) | |
|
Receipt of surgery, radiation or chemotherapy |
||||
| Yes | 5758785 (90.2) | 11598 (80.2) | 5747187 (90.3) | p < 0.0001 |
| No | 610101 (9.6) | 2802 (19.4) | 607299 (9.5) | |
| Unknown | 13693 (0.2) | 53 (0.4) | 13640 (0.2) | |
Sums to less than 100% due to missing data
PLWH were more likely (P-trend <0·05) to be diagnosed with advanced-stage cancers of the oral cavity, liver, female breast, prostate, thyroid, and melanoma (Table 2). After adjustment for both the type of treating healthcare facility and individual health insurance, statistically significant trends toward later-stage disease remained, with the association between HIV and Stage IV disease (vs. Stage I) ranging from 1·24 (95%CI 1·02–1·52) for liver cancer to 2·06 (1·70–2·50) for female breast cancer. Of note, HIV was associated with marginally earlier stage at diagnosis for colorectal cancer (Stage IV vs. Stage I: OR=0·89 95%CI 0·78–1·02; P-trend=0·03) and substantially earlier stage anal cancer (OR=0·54; 0·41–0·73; P-trend<0·01). After restricting analyses to patients <65 years of age and stratifying by receipt of either private insurance, Medicare, or Medicaid, results were largely consistent (Supplemental Table 1). Interestingly, the association between HIV and advanced-stage breast cancer was more pronounced among women with private insurance (OR=2·22) or Medicare (OR=2·27), groups assumed to have good access to care, compared to 0·77 in women with Medicaid. The association between HIV and advanced-stage stomach and kidney cancer was not significant overall but was strongly positive among patients with Medicare (stomach: OR=3·46 95%CI 1·67–7·48; kidney: 1·85; 1·09–3·15).
Table 2.
Odds Ratios (ORs) and 95% confidence intervals (CIs) describing the association between patient HIV status and stage of disease at diagnosis
| Cancer Site | Total Cases (N%) | HIV-infected cases (N,%) | HIV-uninfected cases (N,%) | Model 1, not adjusted for healthcare factors a | Model 2, adjusted for healthcare factors b |
|---|---|---|---|---|---|
| Oral Cavity/Pharynx/Larynx | |||||
| Stage I | 65128 (22.5) | 127 (13.5) | 65001 (22.5) | 1 | 1 |
| Stage II | 38100 (13.2) | 108 (11.5) | 37992 (13.2) | 1.32(1.02,1.71) | 1.24(0.96,1.6) |
| Stage III | 50123 (17.3) | 176 (18.7) | 49947 (17.3) | 1.52(1.21,1.91) | 1.38(1.1,1.74) |
| Stage IV | 136292 (47.1) | 528 (56.2) | 135764 (47.0) | 1.54(1.27,1.88) | 1.35(1.1,1.64) |
| P-trend | 0.0001 c | 0.0134 | |||
| Stomach | |||||
| Stage I | 28157 (26.5) | 76 (26.2) | 28081 (26.5) | 1 | 1 |
| Stage II | 15341 (14.5) | 43 (14.8) | 15298 (14.5) | 1.01(0.69,1.47) | 1.03(0.71,1.5) |
| Stage III | 15783 (14.9) | 39 (13.4) | 15744 (14.9) | 0.83(0.56,1.23) | 0.85(0.58,1.26) |
| Stage IV | 46779 (44.1) | 132 (45.5) | 46647 (44.1) | 0.95(0.71,1.26) | 0.94(0.71,1.26) |
| P-trend | 0.7826 | 0.7070 | |||
| Colorectum | |||||
| Stage I | 181014 (23.1) | 465 (22.9) | 180549 (23.1) | 1 | 1 |
| Stage II | 208371 (26.6) | 616 (30.3) | 207755 (26.6) | 1.16(1.03,1.31) | 1.13(1,1.27) |
| Stage III | 227004 (29.0) | 557 (27.4) | 226447 (29.0) | 1(0.88,1.13) | 0.98(0.87,1.11) |
| Stage IV | 167612 (21.4) | 396 (19.5) | 167216 (21.4) | 0.94(0.82,1.08) | 0.89(0.78,1.02) |
| P-trend | 0.1559 | 0.0313 | |||
| Anus | |||||
| Stage I | 6130 (19.9) | 229 (21.8) | 5901 (19.8) | 1 | 1 |
| Stage II | 11996 (39.0) | 385 (36.6) | 11611 (39.0) | 0.86(0.72,1.02) | 0.83(0.7,0.99) |
| Stage III | 10064 (32.7) | 369 (35.1) | 9695 (32.6) | 0.9(0.76,1.08) | 0.86(0.72,1.03) |
| Stage IV | 2603 (8.5) | 69 (6.6) | 2534 (8.5) | 0.57(0.43,0.76) | 0.54(0.41,0.73) |
| P-trend | 0.0100 | 0.0027 | |||
| Liver | |||||
| Stage I | 38333 (35.6) | 242 (29.4) | 38091 (35.7) | 1 | 1 |
| Stage II | 21727 (20.2) | 165 (20.0) | 21562 (20.2) | 1.1(0.9,1.35) | 1.09(0.89,1.33) |
| Stage III | 27884 (25.9) | 246 (29.9) | 27638 (25.9) | 1.27(1.06,1.52) | 1.31(1.09,1.57) |
| Stage IV | 19600 (18.2) | 170 (20.7) | 19430 (18.2) | 1.19(0.97,1.45) | 1.24(1.02,1.52) |
| P-trend | 0.0309 | 0.0084 | |||
| Pancreas | |||||
| Stage I | 17995 (8.5) | 46 (9.2) | 17949 (8.5) | 1 | 1 |
| Stage II | 62661 (29.7) | 142 (28.5) | 62519 (29.7) | 0.84(0.6,1.17) | 0.84(0.6,1.17) |
| Stage III | 22989 (10.9) | 45 (9.0) | 22944 (10.9) | 0.65(0.43,0.99) | 0.65(0.43,0.98) |
| Stage IV | 107574 (50.9) | 265 (53.2) | 107309 (50.9) | 0.83(0.6,1.13) | 0.82(0.6,1.13) |
| P-trend | 0.5813 | 0.5703 | |||
| Lung | |||||
| Stage I | 238623 (21.1) | 790 (18.8) | 237833 (21.1) | 1 | 1 |
| Stage II | 63768 (5.6) | 224 (5.3) | 63544 (5.6) | 0.91(0.79,1.06) | 0.92(0.79,1.07) |
| Stage III | 293173 (25.9) | 1090 (25.9) | 292083 (25.9) | 0.94(0.85,1.03) | 0.93(0.84,1.01) |
| Stage IV | 534742 (47.3) | 2097 (49.9) | 532645 (47.3) | 0.97(0.89,1.05) | 0.97(0.89,1.05) |
| P-trend | 0.8945 | 0.9718 | |||
| Melanoma | |||||
| Stage I | 172448 (65.5) | 110 (51.4) | 172338 (65.5) | 1 | 1 |
| Stage II | 42586 (16.2) | 35 (16.4) | 42551 (16.2) | 1.12(0.77,1.65) | 1.05(0.71,1.54) |
| Stage III | 32356 (12.3) | 38 (17.8) | 32318 (12.3) | 1.52(1.05,2.21) | 1.41(0.97,2.06) |
| Stage IV | 15737 (6.0) | 31 (14.5) | 15706 (6.0) | 2.37(1.58,3.56) | 1.96(1.3,2.97) |
| P-trend | <0.0001 | 0.0022 | |||
| Female breast | |||||
| Stage I | 692293 (47.7) | 453 (37.8) | 691840 (47.8) | 1 | 1 |
| Stage II | 502964 (34.7) | 419 (35) | 502545 (34.7) | 1.2(1.05,1.37) | 1.16(1.02,1.33) |
| Stage III | 179480 (12.4) | 185 (15.5) | 179295 (12.4) | 1.36(1.14,1.62) | 1.26(1.06,1.5) |
| Stage IV | 75217 (5.2) | 140 (11.7) | 75077 (5.2) | 2.36(1.95,2.85) | 2.06(1.70,2.50) |
| P-trend | <0.0001 | <0.0001 | |||
| Cervix | |||||
| Stage I | 45105 (47.4) | 146 (44.2) | 44959 (47.4) | 1 | 1 |
| Stage II | 14506 (15.2) | 53 (16.1) | 14453 (15.2) | 1.2(0.87,1.65) | 1.05(0.76,1.45) |
| Stage III | 22528 (23.7) | 78 (23.6) | 22450 (23.7) | 1.05(0.79,1.38) | 0.92(0.69,1.22) |
| Stage IV | 13083 (13.7) | 53 (16.1) | 13030 (13.7) | 1.42(1.03,1.96) | 1.22(0.88,1.69) |
| P-trend | 0.0866 | 0.5754 | |||
| Prostate | |||||
| Stage I/II d | 939405 (82.7) | 1190 (78.7) | 938215 (82.7) | 1 | 1 |
| Stage III | 115700 (10.2) | 135 (8.9) | 115565 (10.2) | 0.97(0.82,1.17) | 0.96(0.8,1.15) |
| Stage IV | 81192 (7.1) | 188 (12.4) | 81004 (7.1) | 1.81(1.55,2.12) | 1.57(1.34,1.83) |
| P-trend | <0.0001 | <0.0001 | |||
| Bladder | |||||
| Stage I | 81514 (46.2) | 170 (43.1) | 81344 (46.2) | 1 | 1 |
| Stage II | 46726 (26.5) | 118 (29.9) | 46608 (26.5) | 1.2(0.95,1.52) | 1.19(0.94,1.5) |
| Stage III | 18135 (10.3) | 36 (9.1) | 18099 (10.3) | 0.9(0.63,1.29) | 0.89(0.62,1.28) |
| Stage IV | 29945 (17.0) | 70 (17.8) | 29875 (17.0) | 1.04(0.78,1.38) | 1(0.75,1.33) |
| P-trend | 0.7419 | 0.8901 | |||
| Kidney | |||||
| Stage I | 185725 (59.0) | 412 (58.2) | 185313 (59.0) | 1 | 1 |
| Stage II | 28666 (9.1) | 56 (7.9) | 28610 (9.1) | 0.81(0.61,1.08) | 0.84(0.64,1.11) |
| Stage III | 46784 (14.9) | 105 (14.8) | 46679 (14.9) | 1.05(0.85,1.31) | 1.07(0.86,1.33) |
| Stage IV | 53635 (17.0) | 135 (19.1) | 53500 (17.0) | 1.09(0.89,1.32) | 1.06(0.87,1.3) |
| P-trend | 0.6677 | 0.6752 | |||
| Thyroid | |||||
| Stage I | 207173 (72.1) | 149 (57.3) | 207024 (72.1) | 1 | 1 |
| Stage II | 23217 (8.1) | 23 (8.8) | 23194 (8.1) | 0.94(0.59,1.48) | 0.92(0.58,1.45) |
| Stage III | 35457 (12.3) | 42 (16.2) | 35415 (12.3) | 1.23(0.85,1.78) | 1.15(0.8,1.67) |
| Stage IV | 21436 (7.5) | 46 (17.7) | 21390 (7.5) | 1.95(1.36,2.81) | 1.67(1.16,2.41) |
| P-trend | 0.0007 | 0.0072 |
Model 1 adjustment: age, sex, race, calendar year of cancer diagnosis, median household income (by zip code)
Model 2 adjustment: age, sex, race, calendar year of cancer diagnosis, median household income (by zip code), patient-reported individual health insurance, type of treating cancer facility
Bolded text denotes statistical significant for the P-trend test describing the likelihood of diagnosis with more advanced stages of disease by HIV status
Individual stage groupings with less than 10 patients collapsed into categories to provide more stable effect estimates (e.g., stage I and stage II prostate cancer patients combined into stage I/II disease)
Among patients with Stage I-III disease, HIV was significantly associated with elevated mortality after a cancer diagnosis for 13 of the 14 sites evaluated, even after adjustment for stage at diagnosis, first-course cancer treatment modality, type of treating cancer facility, and type of individual health insurance (Table 3; Supplemental Table 2). The strength of the association between HIV and overall mortality ranged from HRs of 1·20 (95%CI 1·14–1·26) for lung cancer to 1·85 (1·68–2·04), 1·85 (1·51–2·27), and 2·93 (2·08–4·13) for female breast, cervix, and thyroid cancers, respectively. The five cancers with the most pronounced HIV-associated elevations in mortality following diagnosis are illustrated in Figure 1 and include both virus-associated and non-virus-associated tumors. We also confirmed that PLWH experienced significantly elevated mortality even after including patients with metastatic (Stage IV) disease (Supplemental Table 3).
Table 3.
Hazard Ratios (HRs) and 95% confidence intervals (CIs) describing the association between patient HIV status and mortality following cancer diagnosis
| Cancer Site | Total Cases | Deaths (% of cases) | Model 1, not adjusted for healthcare factors a | Model 2, adjusted for healthcare factors b |
|---|---|---|---|---|
| Oral Cavity/Pharynx/Larynx | ||||
| HIV-infected | 353 | 194 ( 55.0% ) | 1.89(1.64,2.18) | 1.66(1.44,1.92) |
| HIV-uninfected | 123760 | 44303 ( 35.8% ) | 1 | 1 |
| Stomach | ||||
| HIV-infected | 141 | 106 ( 75.2% ) | 1.25(1.03,1.52) | 1.20(0.98,1.45) |
| HIV-uninfected | 47263 | 28895 ( 61.1% ) | 1 | 1 |
| Colorectum | ||||
| HIV-infected | 1524 | 903 ( 59.3% ) | 1.65(1.54,1.76) | 1.58(1.48,1.69) |
| HIV-uninfected | 505650 | 181240 ( 35.8% ) | 1 | 1 |
| Anus | ||||
| HIV-infected | 764 | 319 ( 41.8% ) | 1.56(1.38,1.76) | 1.34(1.19,1.52) |
| HIV-uninfected | 21032 | 6601 ( 31.4% ) | 1 | 1 |
| Liver | ||||
| HIV-infected | 515 | 422 ( 81.9% ) | 1.32(1.19,1.46) | 1.29(1.16,1.42) |
| HIV-uninfected | 65652 | 46977 ( 71.6% ) | 1 | 1 |
| Pancreas | ||||
| HIV-infected | 212 | 198 ( 93.4% ) | 1.37(1.19,1.59) | 1.34(1.16,1.54) |
| HIV-uninfected | 81115 | 67982 ( 83.8% ) | 1 | 1 |
| Lung | ||||
| HIV-infected | 1908 | 1547 ( 81.1% ) | 1.24(1.18,1.3) | 1.20(1.14,1.26) |
| HIV-uninfected | 483000 | 340444 ( 70.5% ) | 1 | 1 |
| Melanoma | ||||
| HIV-infected | 145 | 57 ( 39.3% ) | 1.79(1.38,2.32) | 1.65(1.27,2.14) |
| HIV-uninfected | 194402 | 37221 ( 19.1% ) | 1 | 1 |
| Female Breast | ||||
| HIV-infected | 957 | 399 ( 41.7% ) | 1.98(1.80,2.19) | 1.85(1.68,2.04) |
| HIV-uninfected | 1099101 | 173881 ( 15.8% ) | 1 | 1 |
| Cervix | ||||
| HIV-infected | 218 | 95 ( 43.6% ) | 2.06(1.68,2.52) | 1.85(1.51,2.27) |
| HIV-uninfected | 67181 | 17506 ( 26.1% ) | 1 | 1 |
| Prostate | ||||
| HIV-infected | 1170 | 236 ( 20.2% ) | 1.65(1.46,1.88) | 1.56(1.37,1.77) |
| HIV-uninfected | 901852 | 112889 ( 12.5% ) | 1 | 1 |
| Bladder | ||||
| HIV-infected | 302 | 217 ( 71.9% ) | 1.71(1.50,1.96) | 1.66(1.45,1.9) |
| HIV-uninfected | 117311 | 57533 ( 49.0% ) | 1 | 1 |
| Kidney | ||||
| HIV-infected | 514 | 204 ( 39.7% ) | 1.52(1.32,1.75) | 1.41(1.22,1.62) |
| HIV-uninfected | 205898 | 45884 ( 22.3% ) | 1 | 1 |
| Thyroid | ||||
| HIV-infected | 190 | 33 ( 17.4% ) | 3.10(2.20,4.37) | 2.93(2.08,4.13) |
| HIV-uninfected | 206656 | 7841 ( 3.8% ) | 1 | 1 |
Model 1 adjustment: age, sex, race, calendar year of cancer diagnosis, median household income (by zip code), cancer stage and treatment
Model 2 adjustment: Model 1 + type of individual health insurance and treating cancer facility
FIGURE 1.
Hazard ratios (HR) illustrating the association between HIV status and mortality following a cancer diagnosis for the five cancer sites with the strongest effect estimates, according to the type of individual health insurance reported
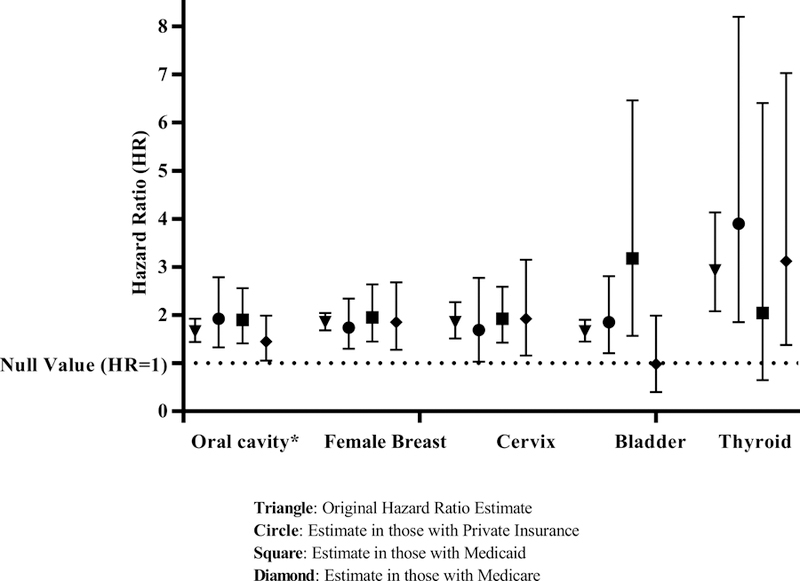
* oral cavity/pharynx/larynx
DISCUSSION.
Our data on more than six million US cancer patients is consistent with PLWH experiencing a more aggressive disease course for cancer – we report significant associations between HIV and both advanced stage at presentation and elevated mortality following diagnosis. Specifically, PLWH were significantly more likely to be diagnosed with advanced-stage cancers of the oral cavity, liver, female breast, prostate, thyroid, and melanoma. Furthermore, HIV was associated with elevated mortality after a cancer diagnosis for 13 of the 14 cancer sites evaluated, including an almost doubling and tripling of mortality for female breast and thyroid cancers, respectively. The persistence of these associations after adjustment for factors related to receipt of healthcare provides support for a biological link between HIV-related immunosuppression and cancer progression.
Only one prior study broadly examined cancer stage in PLWH; in that study, HIV was associated with more advanced stage in patients with cancers of the lung, breast, prostate, bladder, and melanoma.17 However, that study did not have information on type of care received by HIV status. To mitigate this fact, the authors compared results with those observed among solid organ transplant recipients, an immunosuppressed patient group that, unlike PLWH, has increased utilization of health care. Consistent results for more advanced-stage diagnoses of bladder cancer and melanoma suggested a possible biological role for immunosuppression in the progression of these cancers. Our current study was unique in that availability of information on health insurance in NCDB enabled us to more directly address potential non-biological explanations for later-stage cancer diagnosis in PLWH. We confirmed prior associations between HIV and later-stage breast cancer, prostate cancer, and melanoma.17 In addition, we report associations between HIV and advanced-stage oral cavity, liver and thyroid cancers. Since these associations were independent of the type of health insurance a patient reported, it appears that access to healthcare is not the sole explanation for the stage shifts.
Our results do highlight one notable exception – PLWH were substantially more likely to be diagnosed with less advanced anal cancers than their HIV-uninfected counterparts. This potentially represents an example of increased healthcare being directed to the HIV population, a distinct possibility given the ongoing targeting of anal cancer screening procedures to HIV-infected men who have sex with men.22 Importantly, even though these immunosuppressed patients were more likely to be diagnosed with less-advanced disease, their stage-adjusted mortality was higher than HIV-uninfected patients. Mortality following an anal cancer diagnosis was ~40% higher in PLWH compared to HIV-uninfected patients diagnosed with the same disease stage in our study.
The association of HIV with elevated mortality following a cancer diagnosis was present for Stage I-III tumors regardless of their initiating etiology. Elevations in cancer patient mortality ranged from 20% (lung cancer) to ~3-fold (thyroid cancer) in PLWH, with the largest HIV-related differences observed for cancers with a generally good prognosis (e.g., breast and thyroid cancer).23 These data are consistent with prior work showing higher rates of cancer patient mortality in the setting of HIV.18, 24–26 However, this study not only accounted for clinically-important prognostic factors such as cancer stage and treatment, but also demonstrated higher mortality in HIV-infected cancer patients after accounting for the type of treating health facility (quality of cancer care) and individual health insurance (access to care). Further, the NCDB includes only cancer patients who at least initiated cancer care, as opposed to our prior study that included diagnoses of cancer among all PLWH, regardless of healthcare utilization.18
Ultimately, cancer patients with the same stage at presentation, same initial treatment modality, reporting similar health insurance, and receiving cancer care at the same type of healthcare facility still died more often if they were HIV-infected at the time of cancer diagnosis. A biological explanation for this persistent effect of immunosuppression on cancer patient outcomes is plausible when considered alongside data suggesting that immunosuppressed solid organ transplant recipients also experience elevated mortality after a cancer diagnosis,27 recent advances in cancer survival associated with immune-boosting therapies,28–30 and data suggesting that additional cancer-specific outcomes (i.e., relapse after successful first-course treatment) are poorer in the presence of HIV.31 However, it must be acknowledged that this evaluation focused on overall mortality as the clinically-relevant outcome rather than death due specifically to cancer. It is possible that PLWH in this study died more often due in part to a higher risk of additional causes of death, including AIDS and other HIV-associated comorbidities.
Our study is notable not only for the size and national scope of the NCDB but also because prior studies have not been able to examine the possibility that variation in receipt of healthcare could offer a non-biological explanation for more advanced-stage cancer and elevated mortality following cancer diagnosis among PLWH. Instead of proving a non-biological explanation for the link between HIV and the chosen metrics of tumor aggressiveness – advanced stage and elevated mortality – our data suggest that biology could underlie this association.
Certain limitations of this study should be noted. First, we cannot rule out the possibility of HIV-related differences in access to healthcare that we were not able to account for in our models. For example, our classification of insurance into large groupings (e.g., private insurance) may have failed to adequately adjust for variation according to insurance provider or coverage levels. Simple adjustment for receipt of health insurance also likely did not capture variation in the utilization of health services (i.e., having and using insurance are not equivalent). Additional misclassification may have also existed for our exposure - hospital discharge diagnoses likely under-ascertain HIV status. As noted above, our evaluation of overall mortality following a cancer diagnosis was not able to capture potentially important HIV-related differences in cancer-specific mortality. Finally, we were not able to address the association between clinical measures of immunosuppression (e.g., CD4 count) or HIV treatment (e.g., anti-retroviral therapy) and outcomes as NCDB does not collect this information. Future studies that include clinical HIV data should evaluate these more refined metrics of patient immune status.
In summary, in our study of over six million US cancer patients, those infected with HIV were more likely to be diagnosed with advanced-stage cancers of the oral cavity, liver, female breast, prostate, thyroid, and melanoma. PLWH diagnosed with Stage I-III cancer also experienced elevated mortality following diagnosis across a range of tumor etiologies. These associations persisted in PLWH even after accounting for HIV-related differences in the type of treating cancer facility and patient-reported individual health insurance. Although the possibility remains that our findings could be partly explained by differences in healthcare utilization, differences in applied cancer treatment, or mortality from non-cancer comorbidities, our results support a possible biological association between HIV and tumor behavior.
Supplementary Material
ACKNOWLEDGEMENTS.
The data used in the study are derived from a limited data set of the National Cancer Data Base (NCDB). The authors acknowledge the efforts of the American College of Surgeons, the Commission on Cancer, and the American Cancer Society in the creation of the National Cancer Data Base. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the authors.
Funding. This research was supported by the Intramural Research Programs of the National Cancer Institute (National Institutes of Health) and the American Cancer Society. Dr. Suneja is supported by grants K08CA228631 and P30AI064518 from the US National Institutes of Health.
Footnotes
Disclosures. Xuesong Han and Ahmedin Jemal are employed by the American Cancer Society, which receives grants from private and corporate foundations, including foundations associated with companies in the health sector for research outside the submitted work. The authors are not funded by or key personnel for any of these grants and their salary is solely funded through American Cancer Society funds. The other authors declared no conflicts of interest.
Previous presentation. This work was presented as a poster at the 16th International Conference on Malignancies in HIV/AIDS in Bethesda, MD in October 2017.
References
- 1.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001;285: 1736–1745. [DOI] [PubMed] [Google Scholar]
- 2.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr 2009;52: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009;101: 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis 2009;49: 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011;20: 2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370: 59–67. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017. [DOI] [PMC free article] [PubMed]
- 8.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008;123: 187–194. [DOI] [PubMed] [Google Scholar]
- 9.Bertisch B, Franceschi S, Lise M, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol 2013;178: 877–884. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids 2006;20: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 11.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst 2002;94: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1999;21 Suppl 1: S34–41. [PubMed] [Google Scholar]
- 13.Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. Aids 2014;28: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill AE, Engels EA, Schymura MJ, Mahale P, Shiels MS. Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst 2018. [DOI] [PMC free article] [PubMed]
- 15.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer Burden in the HIV-Infected Population in the United States. J Natl Cancer Inst 2011;103: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med 2018;168: 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiels MS, Copeland G, Goodman MT, et al. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer 2015;121: 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coghill A, Shiels MS, Suneja G, Engels EA. Elevated Cancer-specific Mortality among HIV-infected Persons in the US. Journal of Clinical Oncology 2015;In Press. [DOI] [PMC free article] [PubMed]
- 19.Suneja G, Boyer M, Yehia BR, et al. Cancer Treatment in Patients With HIV Infection and Non-AIDS-Defining Cancers: A Survey of US Oncologists. J Oncol Pract 2015;11: e380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suneja G, Shiels MS, Angulo R, et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol 2014;32: 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Commission on Cancer. Available from URL: https://www.facs.org/quality-programs/cancer/coc/apply/categories).
- 22.Palefsky JM. Screening to prevent anal cancer: Current thinking and future directions. Cancer Cytopathol 2015;123: 509–510. [DOI] [PubMed] [Google Scholar]
- 23.Zucchetto A, Virdone S, Taborelli M, et al. Non-AIDS-Defining Cancer Mortality: Emerging Patterns in the Late HAART Era. J Acquir Immune Defic Syndr 2016;73: 190–196. [DOI] [PubMed] [Google Scholar]
- 24.Chao C, Xu L, Abrams D, et al. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. Aids 2010;24: 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus JL, Chao C, Leyden WA, et al. Survival Among HIV-Infected and HIV-Uninfected Individuals with Common Non-AIDS-Defining Cancers. Cancer Epidemiol Biomarkers Prev 2015. [DOI] [PMC free article] [PubMed]
- 26.Sigel K, Crothers K, Dubrow R, et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer 2013;109: 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Arcy M Survival after cancer diagnosis among solid organ transplant recipients. Cancer 2018;In Press. [DOI] [PMC free article] [PubMed]
- 28.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372: 320–330. [DOI] [PubMed] [Google Scholar]
- 30.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015. [DOI] [PMC free article] [PubMed]
- 31.Ferreira MP, Coghill AE, Chaves CB, et al. Outcomes of cervical cancer among HIV-infected and HIV-uninfected women treated at the Brazilian National Institute of Cancer. Aids 2017;31: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


