Abstract
The precise pathogenic mechanisms in the development, persistence and worsening of Hidradenitis Suppurativa (HS) remain ill-defined. This chronic inflammatory dermatosis displays a strong Th1 and Th17 inflammatory signature with elevated levels of TNF-α, IL-1β, IL-17 and IFNγ in lesional and perilesional tissue. HS significantly differs to other chronic inflammatory dermatoses due to the development of hypertrophic scarring and dermal tunnels. The development of scarring and tunnels suggests that fibroblastic stromal cells (including myofibroblasts, fibroblasts, pericytes etc.) may be involved in the development and progression of disease. Heterogeneous populations of fibroblasts have been identified in other inflammatory disorders and malignancy which contribute to inflammation and present novel therapeutic targets for fibrotic disorders. Findings in HS are consistent with these fibroblast subpopulations and may contribute to tunnel formation, aggressive squamous cell carcinoma and the phenotypic presentation of familial HS variants. We describe the existing knowledge regarding these mechanistic pathways and methods to confirm their involvement in the pathogenesis of HS.
Keywords: Hidradenitis Suppurativa, Acne Inversa, Pathogenesis, Fibroblasts, Scarring
1. Introduction:
The precise pathogenic mechanisms in the development, persistence and worsening of Hidradenitis Suppurativa (HS) remain ill-defined1. This chronic inflammatory dermatosis displays a strong Th1 and Th17 inflammatory signature with elevated levels of TNF-α, IL-1β, IL-17 and IFNγ in lesional and perilesional tissue2. HS significantly differs to other chronic inflammatory dermatose3 due to the development of hypertrophic scarring and dermal tunnels4. The development of which suggest that fibroblastic stromal cells (including myofibroblasts, fibroblasts, pericytes etc.) may be involved in the development and progression of disease5. The recent investigations by Sanchez et al5 experimentally demonstrate that biologically active peptides and inflammatory mediators from the extracellular matrix (ECM) engage in inflammatory crosstalk with keratinocytes in HS. This paper begins to lay the foundation for future investigations into the interaction between ECM, fibroblasts and keratinocytes in HS. Such insights may help explain why certain patients are prone to hypertrophic scarring, tunneling and tract formation, and the mechanisms underlying these presentations. This may also lead to the identification of novel therapeutic targets which overlap with pipeline drugs for fibrotic diseases6. A large body of literature exists regarding epidermal-stromal interactions in the setting of cutaneous melanoma7 and other inflammatory disorders such as Rheumatoid Arthritis (RA)8 and Inflammatory Bowel Disease (IBD)9, however, investigations in inflammatory skin disease are limited. Our background knowledge in these conditions may help generate testable hypotheses regarding the mechanisms at play in inflammatory dermatoses including HS.
2. Mesenchymal Stromal Cells as a Heterogeneous Population:
Fibroblast populations in chronic inflammation and malignancy are heterogeneous. Fibroblasts are identified by their spindle shaped cellular morphology in combination with common mesenchymal markers such as vimentin9–12 and develop from multiple sources including resident tissue stromal cells, bone marrow derived stromal cells, Epithelial Mesenchyme Transition (EMT) and Endothelial Mesenchyme Transition (endoMT) from epithelial and endothelial cells, respectively.10,11 The source of these stromal cells can be identified through examination of specific cell markers (such as PDGFRα, PDGFRβ and Podoplanin)9–12, however, no individual marker is known to be predictive of function. Transcriptomics and single cell RNA sequencing (scRNAseq) have recently been able identify and characterize subpopulations of stromal cells9,11 and provide insights into their putative function in malignancy and inflammation. Factors including cellular origin, spatial location, microenvironment and degree of differentiation all contribute to the functional phenotype (i.e. matrix re-modelling, epithelial maintenance) of fibroblasts identified in these disorders9–12. This results in a heterogeneous milieu of fibroblasts which are proposed to be differentially dysregulated in different forms of aberrant wound healing, fibrosis, hypertrophic scarring, malignancy and metastases9–12. Common fibroblast subpopulations have been identified within chronic inflammatory conditions, chronic wound healing, malignancy and metastasis9–12. This indicates common pathways may be at play which are potential therapeutic targets in these various disorders.
3. The role of Fibroblasts in chronic inflammation and epithelial integrity:
Fibroblast dysregulation has been implicated in impaired wound healing12, inflammatory disorders such as pyoderma gangrenosum14 (associated with HS in the PASH and PAPASH syndromes)15, and in the development of tertiary lymphoid follicles12 in RA and IBD.15 Dysregulation is proposed to involve priming signals in the stromal environment during acute inflammation (mediated by granulocytes) to epigenetically modulate fibroblasts, leading to expression of markers including α-SMA, S100A4, FAP-α16. Such activation is purported to result in epigenetic modifications17 given the persistence of the activated phenotype after isolation of cells and in vitro culture12,15,17. This activation leads to upregulation of ICAM-1, VCAM-1 CXCL13, CCN2, IL-618,19 as well as NLRP3 and Caspase-1 inflammasome activation leading to further IL-1β, IL-18 release, matrix metalloproteinase (MMP) production and release9,10,15, and leucocyte recruitment18,19 . MMPs are required for the activation of pro IL-1β, pro TNF-α, pro TGF-β as well as potentiating the action of IL-820. In normal tissue, the presence of a second maturation and stabilization signal (including activation of the TNFRSF3 Pathway and RORγ pathway)15 ensues, however, blockade of these signals leads to a disorganized collection of mixed inflammatory cells reminiscent of the mixed inflammation in established HS.
Bidirectional stromal-epithelial signaling via the Wnt pathway is integral to maintaining the epidermal stem cell compartment13. Dysregulation of this signaling leads to poor epidermal regeneration and is implicated in impaired wound healing and ulceration in Crohn’s disease9. A specific subpopulation of fibroblasts, found adjacent to the involved epithelium, has been identified which mediates this bidirectional signaling via CD449–12,16, however, communication can occur without direct cell to cell contact21 via microvesicles18 or other not-yet-identified mechanisms. Upregulation of Wnt signaling has been shown to accelerate both fibrosis and re-epithelialization in animal and human models22, with Wnt/B-Catenin signaling being suppressed in cutaneous wounds of diabetic patients22,23, a common comorbidity in HS.
4. Fibroblast Like Stromal Cells in Malignancy and Metastases
In the setting of malignancy, dysregulated fibroblasts are known as cancer-associated fibroblasts (CAFs)10,11,16,24,25. CAFs are associated with increased tumor growth and metastatic potential as well as extra cellular matrix remodeling10,11,16,24,25. CAFs contribute to the pro-inflammatory nature of the tumor microenvironment25 and remodeling of the ECM11,16 . Bidirectional crosstalk with tumor cells has been shown to increase the metastatic potential of melanoma, breast, pancreatic and lung tumours,11,16,24,25. Stromal-derived IL-6 and TGF-β have been documented to suppress cell cycle progression in melanoma27, controlling tumor spread in early stage disease. However, in the setting of malignancy with activated fibroblasts, the altered CAF secretome results in exposure of the tumor cells to growth factors and metabolites which promote tumor propagation28. CAFs have been shown to harbor immunomodulatory mechanisms with upregulation of production of cytokines and chemokines, including PDGF, vascular endothelial growth factor A (VEGFA), prostaglandin E2, IL-6, TNF, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IL-8, hepatocyte growth factor (HGF), and CXCL1228,29. This inflammation results in an autocrine signaling capability in CAFs contributing to ongoing inflammation. Activated CAFs, in a similar fashion to other fibroblasts, produce multiple MMPs including MMP1–3, MMP9, and MMP13–14, as well as tissue inhibitors of metalloproteinases (TIMPs), which together result in ECM remodeling. In malignancy, this remodeling facilitates tumor invasion and metastasis29 . Additionally, activated stromal cells can induce EMT and motility in malignant cells via CD44 signaling16. The cause of this aberrant activation is secondary to the pre-existing inflammatory milieu, including the local microenvironment, EMT and EndoEMT programs, as well as the influx of heterogeneous populations of mesenchymal cells primed for activation from bone marrow. Concurrently, expression of CCN2 and production of miRNAs can activate stromal cells to a CAF phenotype24,25, demonstrating the extensive cross talk in the setting of malignancy.
5. Mesenchymal Subpopulations Mediate Disparate Functional Pathways in Inflammation, Fibrosis and Fistula Formation in Inflammatory Bowel Disease
Dysregulated fibroblasts are implicated in the development of inflammation, fibrosis and fistula formation in IBD9, several inflammatory characteristics of RA12,15, and enhanced metastatic potential in breast carcinoma11. Specific subpopulations of fibroblasts are associated with the loss of epithelial integrity, ECM remodeling, angiogenesis and development of tertiary lymphoid structures9,11,12,15. PDFGRA+ cells with differentially elevated expression of DCN, LUM, VCAN, Col14A1, FBLN1, FBLN2, SMOC, LOX, LOX1 and CXCL14 display an ECM and EMT related transcriptomic signatures associated with ECM remodeling and stimulation of EMT in epidermal tissues9,10,11,12,15. PDFGRB- fibroblasts with differentially elevated expression of POSTN, BMP2, BMP5, WNT5A, WNT5B, SOX6, SCRG1, SOX9, SOX10, MFAP5 and CCN2 were described as enriched in inflammation9,10,11,12,15, and associated with fibroblasts derived from epidermal tissues via EMT. Podoplanin + fibroblasts were identified as a subgrouping of PDFGRA+ fibroblasts with expression suggestive of an EndoMT origin9,10,11,12,15. This subset has been implicated in the development of tertiary lymphoid follicles in chronic inflammation including IBD and RA9,12,15 .NR2F2 expressing fibroblasts with elevated expression of Notch3, Epas1, and COL18A1 have transcriptomic profiles suggestive of a pro-angiogenic profile with a subset of these cells also expressing high levels of cell cycle related genes9,10,11,12,15. In IBD, an increase proportion of PDGFRA+ cells are implicated in T cell recruitment and barrier dysfunction9. This subpopulation was significantly expanded in IBD patients compared with healthy controls9. PDFGRB- fibroblasts were decreased in IBD and this subpopulation was implicated in epithelial maintenance9.
6. The Inflammatory Signature of Hidradenitis Suppurativa Suggests a Possible Contribution of Fibroblasts
Existing studies in HS demonstrate intriguing evidence of fibroblast-related pathways being activated in lesional tissue. Elevations in IL-1β, IL-6, IL-8, MMP2 and MMP9 as well as extensive matrix remodeling are core manifestations of HS1,2,5. IHC studies have identified co-localization of MMP2 to dermal fibroblasts30 . The presence of isolated keratinocytes in the dermis is a longstanding phenomenon in HS and can be explained by EMT and the motility of keratinocyte derived MSCs which still partially express keratinocyte markers30. The Invasive Proliferative Gelatinous Mass (IPGM), found attached to the epithelium of the sinus tracts of HS, has been characterized as an active inflammatory component of the disease and comprised of Neutrophil Extracellular Traps (NETs)31. Such NETs develop through a process of NETosis which is stimulated by IL-8, IL-6, TNF-α32. NETs have been documented to stimulate EMT and EndoMT in other autoimmune diseases such as lupus nephritis and rheumatoid arthritis33. Microbiota such as porphyromonas sp., which are associated with HS and colonize epithelialized dermal tunnels, are known triggers of NETosis32,34. The extensive tunnel formation in HS may be partially explained by PDFGRA+ fibroblasts stimulating matrix remodeling and tunnel formation, whilst the epithelialization of dermal tunnels may be partially explained by the role of PDFGRB- fibroblasts in stimulating re-epithelialization as a form of aberrant wound healing. Such epithelialized tunnels may then perpetuate Th17-mediated keratinocyte feed forward inflammation, as well as stimulating fibroblasts to produce IL-6 and IL-8. This may partially explain the dramatic reduction in draining fistula counts with IL-23 blockade35.
7. Stromal Cell MMP secretion linked to gamma secretase associated pathways:
Gamma secretase associated polymorphisms have been identified in a minority of patients with familial HS36. These polymorphisms involve three of the four subunits of the gamma secretase complex - Nicastrin, Presenilin 1 and the Presenilin enhancer36. The existing hypothesis is that gamma secretase polymorphisms alter Notch signaling which leads to hyperproliferative keratinocytes in the follicular infundibulum leading to follicular occlusion1, however, the possibility of alterations in Notch signaling representing a generalizable inflammatory phenomenon (rather than a mechanistic pathway specific to HS) have not been experimentally tested37. Nicastrin (NCSTN) haploinsufficiency is shown to decrease expression of Interferon related genes in HEK293 cells38, however, does not alter cytokine profiles of stimulated PBMCs39. An only recently appreciated aspect of the gamma secretase complex is its role in fibrosis40–42. Presenilin-1 is the proteolytic component of gamma secretase which is responsible for degradation of CD44 as well as other adhesion molecules such as N-cadherin40 .Cao et al’s Nicastrin knockdown model also revealed downregulated N-Cadherin and upregulation of CXCL1438, which in addition to its immune surveillance properties, is an autocrine factor associated with activated fibroblasts43. Given the role of Nicastrin as the ‘gatekeeper’ of the catalytic activity of the gamma secretase complex41,42, polymorphisms in Nicastrin leading to overactivity of gamma secretase can theoretically lead to profibrotic activity through upregulation of EMT and cellular motility40–42. Presenilin 1 also upregulates TGF-β activity, MMP secretion and the β catenin/Wnt pathway40,44. Upregulation of extracellular proteolysis would also explain the excessive fibrotic extensive matrix remodeling and significant scarring seen in Chinese HS patients with documented Nicastrin mutations37, as opposed to the typical axillary-mammary inflammatory form of the disease1.
8. Stromal Cell Contribution May Explain Aggressive Behaviour of SCC in HS
One of the most serious complications of long-term HS is the development of aggressive, metastatic squamous cell carcinoma45–47. Despite their well differentiated nature, these tumors rapidly metastasize and can be fatal. Suggested contributing factors include human papillomavirus infection48 as well as the propensity for longstanding chronic wounds to develop SCCs49. Despite this well documented association between chronic non-healing wounds, the exact mechanisms remain unclear. Evidence from Epidermolysis Bullosa (Recessive Dystrophic, Generalized Severe subtype)47 implicates a unique dermal microenvironment (including elevated Wnt5A and TSP1 signaling along with a dense population of inflammatory cells and activated fibroblasts) in the development and rapid progression of cutaneous SCC, which are often fatal47. Additionally, gram negative flagellated bacteria may activate TLR5 in RDEB contributing to the pro-tumorogenic environment47. These same upregulated factors are identified in PDFGRB- fibroblasts in Crohn’s disease, and are associated with epithelial maintenance9. This implies a potential common mechanism in the development of highly aggressive RDEB and other diseases including Crohn’s disease and possibly HS.
9. Common mesenchymal pathways may explain the association of Pyoderma Gangrenosum/ IBD/ Arthritis and Neutrophilic Alopecias
Monogenic Autoinflammatory Syndromes manifest in both HS and other inflammatory disorders such as Pyoderma Gangrenosum and Inflammatory Arthropathies including the PASH and PAPASH syndromes50. HS also has a strong epidemiological association with IBD1,51. The coexistence of these conditions implies a possible degree of commonality in their pathogenesis. Dysregulated fibroblasts have been implicated in inflammatory arthropathies and play a significant role in impaired wound healing19,22,23,52. The pro-inflammatory actions of activated fibroblasts are NLRP3 and Caspase-1 inflammasome mediated9,10,15, lending credence to the suggestion that they may be involved in the pathogenesis of PAPA and PASH syndromes, given the association of these syndromes with pathogenic sequence variants in these pathways36. The corollary of this is that other manifestations of these disorders (such as Pyoderma Gangrenosum) may also involve fibroblast dysregulation as a manifestation of aberrant wound healing. The hypothesis would include a role for the increase of PDFGRA+ fibroblasts due to the extensive degree of ECM remodeling and a decrease in PDGFRB- fibroblast which are responsible for epithelial integrity in a similar fashion to colonic ulceration in IBD9. Two other cutaneous conditions bear striking histological, clinical and immunological resemblance to HS, Dissecting Cellulitis of the Scalp and Complex Pilonidal Disease, and the involvement of fibroblasts in these disorders warrants further consideration.
10. IL-17, IL-1, CXCL14, JAK-STAT, Cadherin-11, CDK, miR-203, FRP2/ALX, podoplanin, CCN2 pathways are potential therapeutic targets of Fibroblasts in HS.
There is an urgent need for novel therapies in HS and therefore, identification of novel druggable targets is critical. The presence of distinct fibroblast subpopulations has been identified in lesional and perilesional HS tissue which mirror the expression markers (PDGFRA+, PDGFRA-, NR2F2, Podoplanin) seen in other inflammatory conditions such as Crohn’s disease (Lu et al, unpublished data), although their functions in vivo remain to be established. A number of fibroblast-targeted therapies exist within the rheumatological and immunological therapeutic pipeline53. Along with IL-17, IL-1 and JAK-STAT blockade, (currently in Phase 2 clinical trials)54, other druggable targets include IL-6R, Cadherin 11, cyclin dependent kinases (CDK1, CDK2, CDK4, CDK6), SYK (reducing IL-6 production via the MAPK-PKC pathway), CXCL14, CCN2 as well as Formyl peptide receptor 2 (FRP2/ALX) as a upstream mediator of Stat 1, IL-6 and Podoplanin. One potential complication of note is the disparity between responses to therapy between joint-derived versus skin-derived fibroblasts55 which is reflected in the imperfect efficacy correlation between skin and joint targets in psoriasis and psoriatic arthritis56.
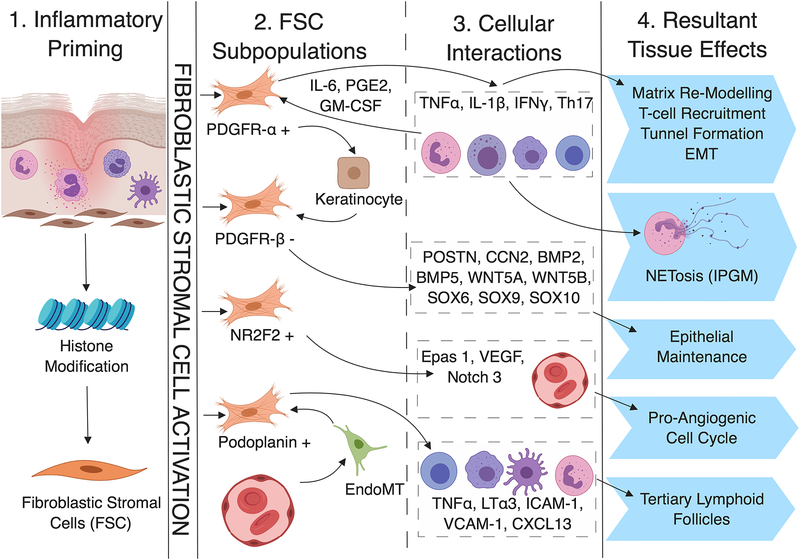
Figure 1:
Proposed Role for Fibroblasts in Tunnel Formation and Inflammation in Hidradenitis Suppurativa. Acute inflammation may act as a priming signal for the activation of fibroblasts through epigenetic mechanisms (1. Inflammatory Priming) leading to the emergence of four discrete subpopulations (2. FSC Subpopulations). Each subpopulation demonstrates specific functional characteristics via actions involving specific inflammatory mediators, transcription factors and cellular pathways (3. Cellular Interactions). Such interactions are associated with (and proposed to be mechanistic causative in) the development of tunnels, epithelialized tracts and fibrosis in HS (4. Resultant Tissue Effects).
Acknowledgements:
J.W.F. was supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. K.N. was supported by a MSTP grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare
References:
- 1.Vossen ARJV, van der Zee HH and Prens EP. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front. Immunol 2018. 9:2965. doi: 10.3389/fimmu.2018.02965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frew JW, Hawkes JE and Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa [version 1; referees: awaiting peer review]. F1000Research 2018, 7:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czarnowicki T, He H, Leonard A, Kim HJ et al. Blood endotyping distinguishes the profie of vitiligo from that of other inflammatory and autoimmune skin diseases J Allergy CLin Immunol 2019; [DOI] [PubMed] [Google Scholar]
- 4.Scheinfeld N An Atlas of the morphological manifestations of hidradenitis suppurativa Dermatol Online J 2014;20:22373. [PubMed] [Google Scholar]
- 5.Sanchez J, Le Jan S, Muller C Matrix Remodeling and MMP expression/activation is associated with hidradenitis suppurativa skin inflammation. Exp Dermatol 2019; doi: 10.1111/exd.13919 [DOI] [PubMed] [Google Scholar]
- 6.Henrot P, Truchetet ME, Fisher G, Taieb A, Cario M CCN proteins as potential actionable targets in scleroderma Exp Dermatol 2019;28:11–18 [DOI] [PubMed] [Google Scholar]
- 7.Izar B, Joyce CE, Goff S, Cho NL et al. Bidirectional cross talk between patient-derived melanoma and cancer-associated fibroblasts promoted invasion and proliferation Pigment Cell Melanoma Res. 2016;29:656–668 [DOI] [PubMed] [Google Scholar]
- 8.Hirota K, Hashimoto M, Ito Y Matsuura M et al. Autoimmune Th17 cells induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis Immunity 2018;48(6):1220–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinchen J, Chen HH, Parikh K, Antanaiciute A et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease Cell 2018;175:372–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki K, Sugai T, Ishida K, OSakabe M et al. Analysis of cancer-associated fibroblasts and the epithelial mesenchymal transition in cutaneous basal cell carcinoma, squamous cell carcinoma and malignant melanoma Human Pathol 2018;79:1–8 [DOI] [PubMed] [Google Scholar]
- 11.Bartoschek M, Oskolkov N, Bocci M, Lovrot J et al. Spatially and functionally distinct subclasses of breast cancer -associated fibroblasts revelaed by single cell RNA sequencing. Nature Comm 2018;9:5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorraji SE, Hovd AK, Kanapathippillai P, Bakland G Mesenchymal stem cells and T Cells in the formation of Tertiary Lymphoid Structures in Lupus Nephritis. Scientific Reports 2018;8:7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philippeos C, Telerman SB, Oules B, Pisco AO et al. Spatial and Single Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations J Invest Dermatol 2018;138:811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang EA, Steel A, Luxardi G, Mitra A, et al. Classic Ulcerative Pyoderma Gangrenosum Is a T Cell-Mediated Disease Targeting Follicular Adnexal Structures: A Hypothesis Based on Molecular and Clinicopathologic Studies. Frontiers in Immunology, 2018; 8, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barone F, Gardner DH, Nayar S, Steinthal N et al. Stromal Fibroblasts in Tertiary Lymphoid Structures: A Novel Target in Chronic Inflammation Frontiers Immunol 2016;7:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Yang K, Andl T, Wickett RR et al. Perspective of Targeting Cancer-Associated Fibroblasts in Melanoma J Cancer 2015;6:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrengues J, Bertero T, Grasset E Bonon S Epigenetic switch drives the conversion of fibroblasts into pro-invasive cancer-associated fibroblasts Nature Comm 2015;6:10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang P, Bi J, Owen GR, Chen W Keratinocyte Microvesicles Regulate the Expression of Multiple Genes in Dermal Fibroblasts J Invest Dermatol 2015;135:3051–3059 [DOI] [PubMed] [Google Scholar]
- 19.Georganas C, Liu H, Perlman H, Hoffman A et al. Regulation of IL-6 and IL-8 Expression in Rheumatoid Arthritis Synovial Fibroblasts: the Dominant Role for NF-kB but not C/EBPBor c-Jun J Immunol 2000;165:7199–7206 [DOI] [PubMed] [Google Scholar]
- 20.Nissinen L, Kahari VM Matrix Metalloproteinases in inflammation Biochima et Biophysica Acta (BBA)- General Subjects 2014;1840:2571–2580 [DOI] [PubMed] [Google Scholar]
- 21.Hutchenreuther J, Vincent KM, Carter DE, Postovit LM, Leask A CCN2 Expression by tumor Stroma is required for Melanoma Metastasis J Invest Dermatol 2015;135:2805–2813 [DOI] [PubMed] [Google Scholar]
- 22.McBride JD, Jankins AJ, Liu X, Zhang B Elevated circulation levels of an antiangiogenic SERPIN in patients with diabetic microvascular complications impair wound healing though suppression of Wnt signaling. J Invest Dermatol 2014;134:1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Nie X, Shi X, Zhao J Regulatory Mechanisms of the Wnt/B-Catenin Pathway in Diabetic Cutaneous Ulcers Front Pharmacol 2018;9:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchenreuther J, Vincent K, NOrley C, Racanelli M Activcation of cancer-associated fibroblasts is required for tumor neovasculairization in a murine model of melanoma Matrix Biol 2018;74:52–61 [DOI] [PubMed] [Google Scholar]
- 25.Hutchenreuther J, Vincent KM, Carter DE, POstovit LM, Leask A CCN2 Expression by Tumor Stroma is Required for Melanoma Metastasis J Invest Dermatol 2015;135: 2805–2813 [DOI] [PubMed] [Google Scholar]
- 26.Balachander GM, Talukdar PM, Debnath M, Rangarajan A, Chatterjee K Inflammatory Role of Cancer-Associated Fibroblasts in Invasive Breast Tumors Revealed Using a Fibrous Polymer Scaffold ACS Appl Mater Interfaces 2018;10:33814–33826 [DOI] [PubMed] [Google Scholar]
- 27.Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent Proc Natl Acad Sci USA 2014;111:17188–17193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkasalias T, Moyano-Galceran L, Arsenian-Henrikson M, Lehti K Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int J Mol Sci 2018;19:1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 30.Frew JW, Hawkes JE and Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis F1000Research 2018, 7:1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidacki M, Cong Z, Flamm A, Helm K, Danby F and Nelson A (2019), ‘Invasive proliferative gelatinous mass’ of hidradenitis suppurativa contains distinct inflammatory components. Br J Dermatol. doi: 10.1111/bjd.17541 [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Biermann MH, Brauner JM, Liu Y et al. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation Front Immunol 20165;7:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KH, Kronbicheler A, Park DDY, Park YM et al. Neutrophil Extracellular Traps (NETs) in autoimmune diseases: a comprehensive review Autoimmunity Rev 2017;16:1160–1173 [DOI] [PubMed] [Google Scholar]
- 34.Ring HC, Sigsgaard V, Thorsen J, Fuursted K et al. The microbiome of tunnels in hidradenitis suppurativa patients J Eur Acad Dermatol Venereol 2019;doi: 10.1111/jdv.15597 [DOI] [PubMed] [Google Scholar]
- 35.Kovacs M and Podda M (2019), Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol, 33: e140–e141. doi: 10.1111/jdv.15368 [DOI] [PubMed] [Google Scholar]
- 36.Frew J, Vekic D, Woods J and Cains G (2017), A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol, 177: 987–998. [DOI] [PubMed] [Google Scholar]
- 37.Frew JW We Need to Talk About Notch: Notch dysregulation as an epiphenomenon in inflammatory skin disease. Br J Dermatol. 2019. February;180(2):431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao L, Morales-Heil DJ, Roberson EDO Nicastrin haploinsufficiency alters expression of type-1 interferon stimulated genes in two immortalized human cell lines Clin Exp Dermatol. 2019. January 17. doi: 10.1111/ced.13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, He Y, Hui Y, Xiao X et al. NCSTN mutations in hidradenitis suppurativa/acne inversa do not influence cytokine production by peripheral blood mononuclear cells Br J Dermatol 2017;176:270–280 [DOI] [PubMed] [Google Scholar]
- 40.Kryczka J and Boncela J Proteases Revisited: Roles and Therapeutic Implications in Fibrosis Mediatos INflamm 2017:2570154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Strooper B, Nicastrin: gatekeeper of the gamma-secretase complex Cell 2005;122(3):318–320 [DOI] [PubMed] [Google Scholar]
- 42.Shah S, Lee SF, Tabuchi K, Hao YH Nicastrin functions as a gamma-secretase substrate receptor Cell 2005;122(3):435–447 [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Chatterjee M, Schmid H, Beck S, & Gawaz M (2016). CXCL14 as an emerging immune and inflammatory modulator. Journal of inflammation (London, England), 13, 1. doi: 10.1186/s12950-015-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patil PU, D’Ambrosio J, Inge LJ, Mason RW, and Rajasekaran AK, “Carcinoma cells induce lumen filling and EMT in epithelial cells through soluble E-cadherin-mediated activation of EGFR,” Journal of Cell Science, vol. 128, no. 23, pp. 4366–4379, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juviler PG, Patel AP, Qi Y. Infiltrative squamous cell carcinoma in hidradenitis suppurativa: A case report for early surgical intervention. Int J Surg Case Rep. 2019;55:50–53. doi: 10.1016/j.ijscr.2019.01.006. Epub 2019 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jourabchi N, Fischer AH, Cimino-Mathews A, Waters KM, Okoye GA. Squamous cell carcinoma complicating a chronic lesion of hidradenitis suppurativa: a case report and review of the literature. Int Wound J. 2017. April;14(2):435–438. doi: 10.1111/iwj.12671. Epub 2016 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M, Murrell DF. Update on the pathogenesis of squamous cell carcinoma development in recessive dystrophic epidermolysis bullosa. Eur J Dermatol. 2015. April;25 Suppl 1:30–2. doi: 10.1684/ejd.2015.2552. [DOI] [PubMed] [Google Scholar]
- 48.Scheinfeld N A case of a patient with Stage III Familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J 2014;20:3. [PubMed] [Google Scholar]
- 49.Reich-Schupke S, Doerler M, Wollina U, Dissemond J, et al. Plattenepithelkarzinome in chronischen venösen Ulcera crurum. Daten aus dem deutschen Marjolin-Register und Übersichtsdarstellung. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2015; 13: 1006–1014. [DOI] [PubMed] [Google Scholar]
- 50.Gasparic J, Theut Riis P, Jemec GB Recognizing syndromic hidradenitis suppurativa: a review of the literature J Eur Acad Dermatol Venereol 2017;31:1809–1816 [DOI] [PubMed] [Google Scholar]
- 51.Vilarrasa Rull E, Gonzalez Lama Y Clinical features of hidradenitis suppurativa and Crohns disease: what do these two entities have in common? Actas Dermosifiliogr 2016;107:S21–26 [DOI] [PubMed] [Google Scholar]
- 52.Caley MP, Martins VL, & O’Toole EA (2015). Metalloproteinases and Wound Healing. Advances in wound care, 4(4), 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dakin SG, Coles M, Sherlock JP, Powrie F et al. Pathogenic stromal cells as therapeutic targets in joint inflammation Nat Rev Rheum 2018;14:714–726 [DOI] [PubMed] [Google Scholar]
- 54.Van Straalen KR, Schneider-Burns S, Prens EP Current and Future treatment of hidradenitis suppurativa Br J Dermatol 2018;doi: 10.1111/bjd.16768 [DOI] [PubMed] [Google Scholar]
- 55.Noack M, Miossec P P067 Il-17 resulting from cell interactions during chronic inflammation: comparison between joint-derived- and skin-derived-mesenchymal cells Annals of the Rheumatic Diseases 2018;77:A41–A42. [Google Scholar]
- 56.Hawkes Jason E., Yan Bernice Y., Chan Tom C., Krueger James G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis J Immunol 2018, 201:1605–1613; [DOI] [PMC free article] [PubMed] [Google Scholar]