Abstract
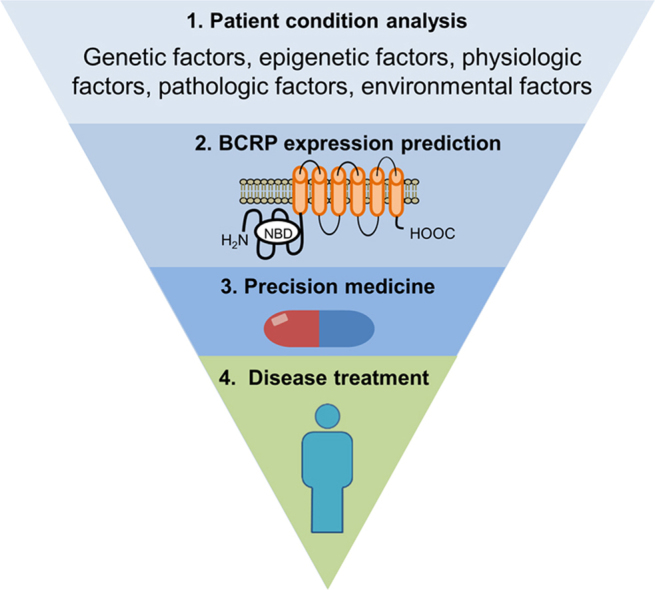
Precision medicine is a rapidly-developing modality of medicine in human healthcare. Based on each patient׳s unique characteristics, more accurate dosages and drug selection can be made to achieve better therapeutic efficacy and less adverse reactions in precision medicine. A patient׳s individual parameters that affect drug transporter action can be used to develop a precision medicine guidance, due to the fact that therapeutic efficacy and adverse reactions of drugs can both be affected by expression and function of drug transporters on the cell membrane surface. The purpose of this review is to summarize unique characteristics of human breast cancer resistant protein (BCRP) and the genetic variability in the BCRP encoded gene ABCG2 in the development of precision medicine. Inter-individual variability of BCRP/ABCG2 can impact choices and outcomes of drug treatment for several diseases, including cancer chemotherapy. Several factors have been implicated in expression and function of BCRP, including genetic, epigenetic, physiologic, pathologic, and environmental factors. Understanding the roles of these factors in controlling expression and function of BCRP is critical for the development of precision medicine based on BCRP-mediated drug transport.
Abbreviations: 3′-UTR, 3′-untranslated region; 5-aza-C, 5-aza-2′-deoxycytidine; ABCG2, ATP-binding cassette subfamily G member 2; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; AUC, area under curve; BCRP, breast cancer resistant protein; miRNA, microRNA; FTC, fumitremorgin C; H3K4me3, histone H3 lysine 4 trimethylation; H3K9me3, histone H3 lysine 9 trimethylation; H3S10P, histone H3 serine 10 phosphorylation; HDAC, histone deacetylase; HIF-1α, hypoxia inducible factor 1 subunit alpha; HIV-1, human immunodeficiency virus type-1; HMG-CoA, β-hydroxy-β-methyl-glutaryl-coenzyme A; MDR, multidrug resistance; MDR1, multidrug resistance 1; NBD, nucleotide binding domain; P-gp, P-glycoprotein; RISC, RNA-induced silencing complex; siRNA, small RNA interference; SNP, Single nucleotide polymorphism; Tat, transactivator protein; TKI, tyrosine kinase inhibitor
Keywords: BCRP, Epigenetics, Gene polymorphisms, Physiologic factors, Precision medicine
Graphical abstract
This review summarizes multiple factors, including genetic, epigenetic, physiologic, pathologic, and environmental factors, which have been reported to affect ABCG2 expression or BCRP function. Understanding how these factors affect BCRP function is critical for the development of precision medicine approaches to achieve optimized therapeutic effects and minimize adverse effects when prescribing specific drugs to patients.
1. Introduction
Precision medicine is a rapidly-developing field in human healthcare that is influencing the practice of medicine. Based on each patient׳s unique characteristics that affect responses to drugs, more accurate doses or drug treatments can be given to the patient to achieve better therapeutic benefits and less adverse reactions using a precision medicine approach.
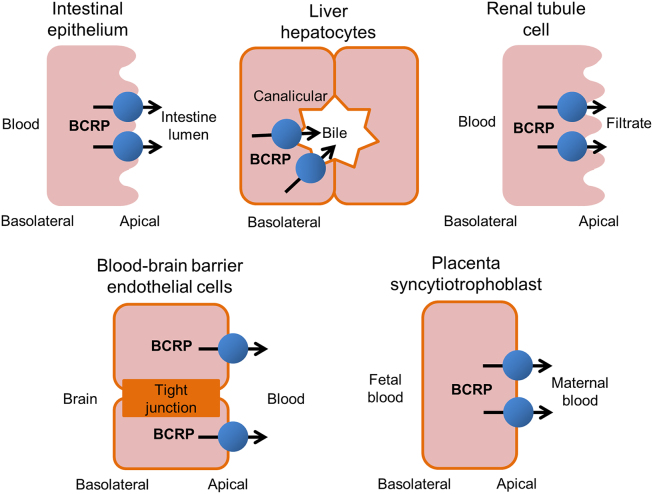
A patient׳s individual characteristics that affect drug transporter action are an important aspect of precision medicine. Drug transporters are a class of proteins located on the membrane surface of cells that allow drugs to enter and exit cells via carrier-mediated mechanisms1. Therapeutic efficacy and adverse reactions of drugs can be affected by the expression and function of drug transporters located in biological membrane barriers of a multitude of target tissues. Human breast cancer resistant protein (BCRP) is a key transporter involved in a patient׳s idiosyncratic functionality with respect to drug transport. BCRP is so called because it was first identified in breast cancer cells that exhibited drug resistance to mitoxantrone, doxorubicin, and daunorubicin2. The human BCRP protein is encoded by the ATP-binding cassette subfamily G member 2 (ABCG2) gene, which is a member of the ATP-binding cassette transporter superfamily. Human BCRP is apically localized in the cell membranes of multiple organs3, including epithelial cells of the small intestine, the canalicular domain of liver hepatocytes, renal tubule cells, brain capillary endothelial cells in blood-brain barrier, and placental syncytiotrophoblasts (Fig. 1)4., 5.. BCRP utilizes the energy generated from ATP hydrolysis to drive the efflux of diverse chemicals across cell membranes6, including both endogenous substrates, such as folic acid7 and uric acid8, and xenobiotics9, as well as environmental toxins, such as pheophorbide A10. Detailed information about BCRP substrates are discussed elsewhere11.
Figure 1.
Tissue distribution of human BCRP. BCRP is highly expressed in several sites in human, including small intestine, liver, kidney, the blood–brain barrier, and placenta. The primary function of BCRP is to pump substrates from the intracellular space to the extracellular space.
BCRP has been extensively studied for its role as an efflux transporter of drugs, leading to drug resistance in target cells and decreased pharmacological effects of substrate drugs. Overexpression of BCRP has been regarded as one of the causes of multi drug resistance (MDR) in diseases, especially cancer. In cell based studies, over expression of BCRP has been found to correlate with MDR in cells derived from several cancers, including breast cancer, ovarian cancer, colon cancer, small cell lung cancer, and myeloma2., 12., 13., 14., 15., 16.. In addition to cellular models, BCRP overexpression has been correlated with MDR and cancer treatment outcomes in clinical settings. BCRP has also been found to be overexpressed in hematopoietic malignancies and solid tumors after chemotherapy treatment. In studies of acute myeloid leukemia (AML), high BCRP levels were reported in 33% of AML blast cells, which also correlated with disease prognosis and overall survival17., 18., 19.. However, no correlation between BCRP expression and overall AML incidence has been found20., 21.. In acute lymphocytic leukemia (ALL), another hematopoietic malignancy, correlations between BCRP expression and drug response differed between studies21., 22.. The roles of BCRP have also been assessed in multiple solid tumors with conflicting conclusions about overall survival or prognosis, including breast carcinoma, non-small cell lung cancer, melanoma, and retinoblastoma23., 24., 25., 26., 27.. The lack of consensus concerning the role of BCRP in these studies might stem from multiple sources, including BCRP detection methods, sample sizes, or patient composition. Despite the fact that the role of BCRP is still not completely understood, inhibition of BCRP has been considered as a pharmacological strategy to improve outcomes of drug treatment28., 29..
Differential expression and altered function of BCRP lead to dramatic inter-individual differences in drug response and disease progression. Several factors have been reported to be responsible for variations of BCRP expression and function. In this review, we summarize some well-documented factors, including genetic, epigenetic, physiologic, pathologic, and environmental factors in the regulation of BCRP expression and alterations of BCRP function. Combinations of these factors form each patient׳s unique characteristics that impact BCRP-mediated drug transport and can then be used to develop precision medicine-based treatments to achieve better therapeutic outcomes.
2. Genetic polymorphisms of the ABCG2 gene
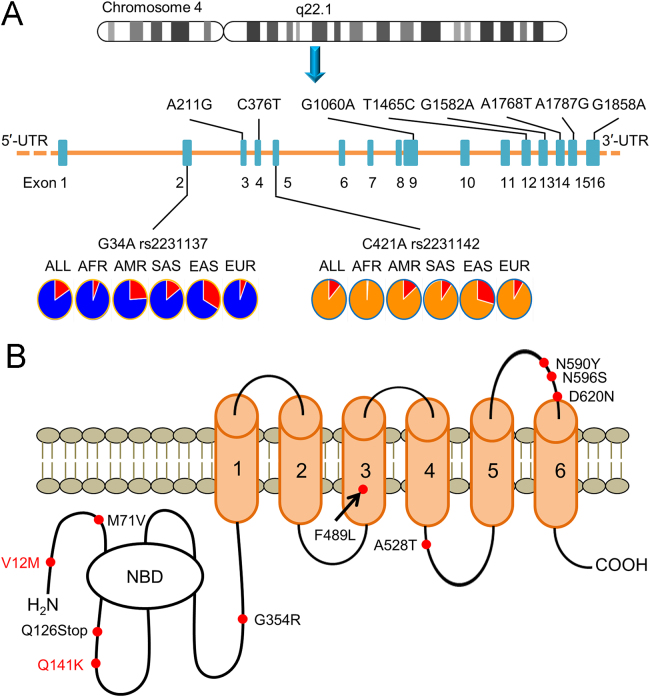
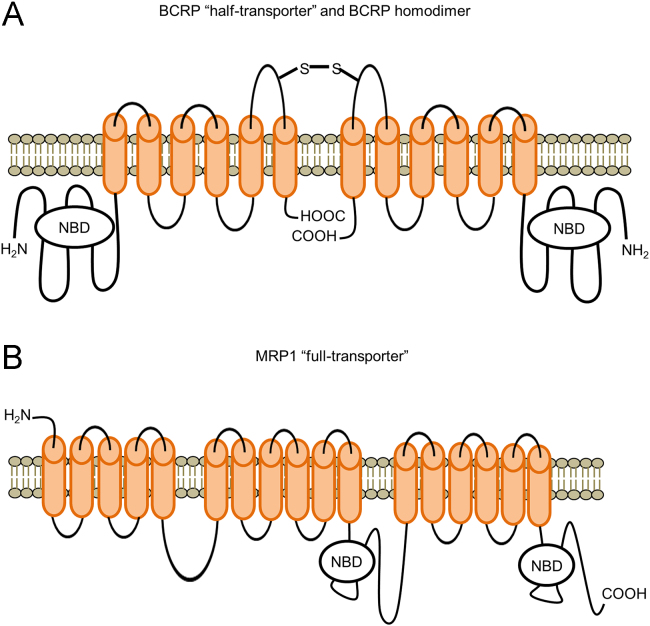
The human ABCG2 gene encodes the BCRP protein and is fully-annotated in the Human Genome build GRCh38/h38 in the UCSC Genome Browser. The ABCG2 gene is located on human chromosome at the locus 4q22.1 and consists of approximately 141 kb of genomic sequence with 16 exons and 15 introns (Fig. 2A). The BCRP protein contains two major domains: a cytoplasmic hydrophilic nucleotide binding domain (NBD), where ATP binds and is hydrolyzed, and a hydrophobic membrane-spanning domain, consisting of six transmembrane domains (Fig. 2B). Unlike other “full transporters”, such as multidrug resistance 1 (MDR1) protein, which has two halves, BCRP is regarded as a “half transporter”. Usually, two BCRP “half transporters” form a homodimer to perform its transporting function as illustrated in Fig. 330., 31..
Figure 2.
Structure of the ABCG2 gene and BCRP protein. (A) Chromosomal location and composition of the ABCG2 gene. Locations of the 10 SNPs are indicated on the cDNA sequence. Allele frequencies of rs2231137 and rs2231142 in a global population of the 1000 Genome Project samples are indicated. ALL: 2,504 individuals in 26 groups; AFR: Africans from 5 groups; AMR: Native Americans from 6 groups; EUR: Europeans from 5 groups; SAS: South Asians from 5 groups; and EAS: East Asians from 5 groups. (B) “Half transporter” structure of BCRP protein containing six membrane-spanning domains (MSDs) and a nucleotide binding domain (NBD). Relative locations of ten SNPs are indicated on the DNA and amino acid sequences.
Figure 3.
Structure of the BCRP “half-transporter” homodimer and the MDR1 “full-transporter”.
Common genetic polymorphisms exist in the ABCG2 gene in various populations across the world. Single nucleotide polymorphisms (SNPs) are the most common genetic polymorphisms, responsible for more than 90% of all interindividual genetic variations in ABCG232. The detection of SNPs in the human genome has been carried out utilizing recent advanced DNA sequencing technologies in several genomic projects, including protein-coding genetic variations33, the Exome Aggregation Consortium34, and the 1000 Genomes Project35. Over 100 SNPs associated with the ABCG2 gene are retrievable from the ExAC Browser in the Exome Aggregation Consortium database (http://exac.broadinstitute.org/). Table 1 summarizes the ten most common SNPs leading to BCRP amino acid substitutions with minor allele frequencies derived from a dataset of global populations comprising different ethnic groups. Their locations in the ABCG2 gene and in the BCRP amino acid sequence are indicated in Fig. 2A and B, respectively. Among these SNPs, rs2231137 G34A (V12M) in exon 2 and rs2231142 C421A (Q141K) in exon 5 are the two most common ABCG2 variants, each with a minor allele frequency of over 10% in the global population. Other SNPs have minor allele frequencies of less than 1%. The frequencies of the two prevalent ABCG2 variants (Table 236., 37., 38., 39., 40., 41.) differ among various ethnic groups with minor allele frequencies higher (20%–40%) in East Asians (Chinese36., 37., Japanese37., 38., 39., and Korean40) and lower (2%–10%) in Caucasians36., 37., 38., 41. and Africans36., 37., 38.. The minor allele frequencies of these two ABCG2 variants in the 1000 Genomes Project (http://www.internationalgenome.org/) populations show a similar global distribution. The minor allele of rs2231137 A has a frequency of 15.8% in the global populations of the 1000 Genomes Project, but varied significantly in different ethnic groups with 6.3% in Africans, 23.8% in Native Americans, 6.1% in Europeans, 15.4% in South Asians, and 32.6% in East Asians (Fig. 2A, rs2231137 and Table 3). Similarly, the minor allele of rs2231142 A has a frequency of 11.9% in the global populations of the 1000 Genomes Project, but varied with 1.3% in Africans, 14.1% in Native Americans, 9.4% in Europeans, 9.7% in South Asians, and 29.1% in East Asians (Fig. 2A, rs2231142 and Table 3). Higher frequencies in the East Asian populations may indicate higher variability on BCRP-mediated drug transport in these populations.
Table 1.
| SNPs rs number | cDNA and protein variant | Minor allele frequency |
|---|---|---|
| rs2231142 | C421A (Q141K) | 0.1180 |
| rs2231137 | G34A (V12M) | 0.1076 |
| rs34783571 | G1858A (D620N) | 0.0056 |
| rs138606116 | G1060A (G354R) | 0.0026 |
| rs45605536 | G1582A (A528T) | 0.0014 |
| rs192169063 | T1465C (F489L) | 0.0010 |
| rs148475733 | A211G (M71V) | 0.0009 |
| rs147547385 | A1787G (N596S) | 0.0005 |
| rs72552713 | C376T (Q126Stop) | 0.0005 |
| rs34264773 | A1768T (N590Y) | 0.0003 |
Minor allele frequency is derived from the Exome Aggregation Consortium ExAC Browser (http://exac.broadinstitute.org/).
The global population consists of East Asian, Latino, European (Finnish and non-Finish), South Asian, African, and others.
Table 2.
Frequency of C421A and G34A SNP in different ethnic groups.
Table 3.
Frequency of rs2231142 C421A and rs2231137 G34A SNP in the populations of the 1000 Genomes Project.
| Populations of the 1000 Genome Project | rs2231142 | rs2231137 |
|---|---|---|
| C421A frequency | G34A frequency | |
| All | C: 0.881, A: 0.119 | G: 0.842, A: 0.158 |
| AFR: African | C: 0.987, A: 0.013 | G: 0.937, A: 0.063 |
| EUR: European | C: 0.906, A: 0.094 | G: 0.939, A: 0.061 |
| SAS: South Asian | C: 0.903, A: 0.097 | G: 0.846, A: 0.154 |
| AMR: American | C: 0.859, A: 0.141 | G: 0.762, A: 0.238 |
| EAS: East Asian | C: 0.709, A: 0.291 | G: 0.674, A: 0.326 |
African populations: Caribbean in Barbados, African ancestry in Southwest US, Esan in Nigeria, Gambian in the Gambia, Luhya in Kenya, Mende in Sierra Leone, and Yoruba in Nigeria.
European populations: European ancestry in Utah, Finish in Finland, British in England and Scotland, Iberian in Spain, and Toscani in Italy.
South Asian populations: Bengali in Bangladesh, Gujrati Indian in Houston, Indian Telugu in the UK, Punjabi in Pakistan, and Sri Lankan Tamil in the UK.
American populations: Colombian in Colombia, Mexican ancestry in California, Peruvian in Peru, and Puerto Rican in Puerto Rico.
East Asian populations: Chinese Dai, Han Chinese, and Southern Han Chinese in China, Japanese in Japan, and Kinh in Vietnam.
The minor alleles of the two most common ABCG2 variants, rs2231137 G34A (V12M) and rs2231142 C421A (Q141K), may cause a reduced level of BCRP expression. The rs2231137 G34A (V12M) variant was first identified in mitoxantrone-resistant cell lines42. BCRP protein expression levels in placentas are significantly lower in rs2231137 A/A samples compared to rs2231137 C/C39. The rs2231142 C421A (Q141K) variant was also first identified in mitoxantrone-resistant cell lines42. A functional analysis of SNPs of the ABCG2 gene was achieved by transfecting cDNA plasmids containing different variants into LLC-PK1 cells, which showed a significantly lower expression level of BCRP in rs2231142 A construct as compared to rs2231142 C construct43. The rs2231142 A SNP also yielded a reduced amount of cell membrane expression of BCRP in K562 cells as compared to rs2231142 C44. The protein expression level from the rs2231142 A construct was about half that of rs2231142 C construct in Flp-in-293 cells45. The reduced protein expression of rs2231142 A construct is due to the reduced stability of the BCRP protein in the endoplasmic reticulum and increased susceptibility to ubiquitin-mediated proteasomal degradation45. These cell-based mechanistic studies showed the ability to alter BCRP expression by the specific ABCG2 SNPs, which might further cause altered BCRP function in these SNP carriers.
The minor alleles of the two most common ABCG2 variants, rs2231137 G34A (V12M) and rs2231142 C421A (Q141K) may also result in decreased transport of BCRP substrates. Drug resistance profiles of Flp-In-293 cells expressing the two major SNP variants of rs2231137 G34A and rs2231142 C421A showed approximately a 50% reduction in transport of the drug SN-38 in cells expressing the mutated proteins as compared to wild type46. In HEK293 cells transfected with rs2231142 C421A constructs, the A allele construct had an 80% higher intracellular accumulation of the carcinogenic heterocyclic anime 2-amino-1-methyl-6-phenlimidazo[4,5-b]pyridine as compared to the C allele construct47. The reduced transport activity caused by the rs2231142 A allele resulted in increased cytotoxicity when K562 cells were treated with the anticancer drugs imatinib, dasatinib, and nilotinib44. The results generated from these studies could explain the BCRP dysfunction in patients carrying the same SNPs, which might assist in the development of guidance for drug prescription.
Minor alleles of less common ABCG2 variants may also lead to decreased drug transport via BCRP. Several variants, such as F208S and S441N were expressed at markedly low levels in Flp-In-293 cells48. These mutations affect intracellular distribution and ubiquitin-mediated proteasomal degradation of the mutant BCRP protein48. Several variants were also defective in porphyrin transport in insect cells transfected with constructs made by site-directed mutagenesis49. Due to the low variant frequency in human populations, it is difficult to determine if the data from cell models can be extrapolated to humans. However, these studies could potentially be utilized to develop dosing guidelines for patients with rare ABCG2 variants.
2.1. The common ABCG2 variants in prediction and treatment of cancer
The common genetic variants of the ABCG2 gene, rs2231137 G34A and rs2231142 C421A, have been reported to be associated with disease risk, reduced efficacy of drug treatments, and increased adverse reactions in different human diseases. Below we summarize recent findings on how the ABCG2 genetic variants play roles in carcinogenesis, efficacy of chemotherapy treatment, and drug-induced cytotoxicity.
Relationship between carcinogenesis and common ABCG2 variants is controversial in population-based association studies in various types of cancer. Association of ABCG2 rs2231142 C421A with the development of breast cancer was examined in 100 Kurdish patients and 200 healthy controls50. Patients with AA genotype of rs2231142 were at a higher risk of breast cancer progression as compared to patients with rs2231142 CC. Similarly, association of the genetic variations rs2231137 G34A and rs2231142 C421A with breast cancer susceptibility was determined in 1,230 Chinese breast cancer patients51. The study found that rs2231137 GA/AA and rs2231142 AA genotypes as well as rs2231137 A-rs2231142 C and rs2231137 G-rs2231142 A haplotypes were associated with a significantly increased risk for developing breast cancer. Associations of the common ABCG2 variants with cancer susceptibility have also been examined in other types of cancer. A meta-analysis based on 10 studies involving a total of 3593 cases and 5875 controls was performed to evaluate the contribution of the ABCG2 rs2231142 C421A with cancer susceptibility52. The analysis found that the rs2231142 A allele is associated with a lower risk for the development of multiple cancer types, including leukemia and colorectal cancer. Based on these data, the authors conclude that the A allele C421A in BCRP might be a protective factor against cancer development52. In a Danish prospective case-cohort study53, rs2231137 A allele carriers with the high fiber intake exhibited a statistically-significantly decreased risk of colorectal cancer as compared to patients harboring the rs2231137 G allele. However, in a case-controlled study in a Turkish population54, rs2231142 A allele carriers had a significantly higher risk of colorectal cancer, while the rs2231137 A allele was not associated with the risk of colorectal cancer. A study including 170 patients with androgen-independent prostate cancer and 141 healthy control subjects showed no significant difference in the prevalence of prostate cancer in subjects with rs2231142 C421A genetic variants47, but another study reported that patients carrying rs2231142 A allele had a significantly shorter time to prostate cancer recurrence post-prostatectomy than patients carrying the rs2231142 C allele55. From the above results, we can see that the relationship between the common ABCG2 variants and cancer risk is complex and may be different in divergent human populations and variable across cancer types. Further studies are needed to clarify the impacts of BCRP transporter genotypes upon carcinogenesis.
The common ABCG2 variants are contributors to variations in the efficacy of cancer drug treatments since the primary function of BCRP is to mediate the active efflux of drugs out of cancer cells. In a study with Kurdish breast cancer patients50, rs2231142 AA carriers had a better response rate to the chemotherapeutic agents anthracycline and paclitaxel. In a study of breast cancer with a Chinese patient population51, rs2231142 AA homozygotes had a significantly enhanced therapeutic response in patients treated with neoadjuvant anthracycline-based chemotherapy. Moreover, rs2231137 AA genotype carriers had improved response in patients with cancers positive for the estrogen or progesterone receptors treated with anthracycline-based chemotherapy. A correlation of rs2231137 A and rs2231142 A alleles with altered sorafenib plasma levels was found in a study with 47 French patients with advanced hepatocellular carcinoma, where the heterozygous group showed lower sorafenib plasma concentrations with a tendency toward better clinical response56. Variants of ABCG2 and dysfunction of BCRP were also reported to alter non-BCRP substrate drugs through indirect mechanisms. Prostate cancer patients carrying the rs2231142 A allele showed increased survival rates as compared to patients carrying the rs2231142 C allele after treatment with docetaxel, which is thought not to be a BCRP substrate55. One study showed the involvement of serine/threonine kinase Pim-1 (Pim-1L), which co-localizes and phosphorylates BCRP at the threonine 362 position, as being functionally involved in BCRP mediated docetaxel resistance. This study also showed that transfection of Pim-1L cDNA directly caused docetaxel resistance in LNCaP cells, suggesting that BCRP might be able to alter the transport of a broader range of substrates than previously thought57. In summary, based on genotypes of the common ABCG2 variants, patients with the minor alleles of rs2231137 G34A and rs2231142 C421A may, in general, have a better therapeutic response to anti-cancer drugs. These genetic SNP markers may therefore serve as predictors for efficacious outcomes of chemotherapies.
However, the common ABCG2 variants may also be related to severe drug-induced adverse reactions to chemotherapy. A study with 219 Japanese renal cell carcinoma patients showed a significant association between the rs2231142 C421A genetic variant and severe thrombocytopenia after treatment with sunitinib58. Dose adjustments of sunitinib are needed for patients with the rs2231142 A variant. A prospective clinical study with 83 Japanese non-small-cell lung cancer patients found a statistically-significant association between the rs2231137 A allele and an increased occurrence of skin rash after gefitinib treatment59. The results suggest that rs2231137 G34A might be a useful predictor of grade 2 or worse gefitinib-induced skin toxicity. Knowing the relationship between the common ABCG2 variants and predisposition to drug-induced toxicity may serve to guide a better and more precise prescription of drugs for patients carrying specific ABCG2 variants.
2.2. The role of common ABCG2 variants in prediction and treatment of diseases other than cancer
Considering the fact that BCRP also transports endogenous compounds and non-chemotherapeutic drugs, dysregulation of BCRP expression or function can also impact the progression of a variety of diseases in addition to cancer. BCRP involvement in gout and statin-based hyperlipidemia treatment are two well-studied cases where BCRP function is critical and can be affected by specific ABCG2 variants. In this section, we will summarize and discuss the overall impact of BCRP in disease and the efficacy of certain drugs for gout and hyperlipidemia treatment.
The common ABCG2 variants are associated with the development of gout. Gout is a common inflammatory disease characterized by hyperuricemia and arthritis. Since BCRP plays a critical role in the elimination of uric acid, disrupted BCRP function might lead to reduced urate excretion and higher levels of blood uric acid, which may result in gout development. Recent studies have shown the relationship between the common ABCG2 variants and the etiology of gout. A study with 39,853 subjects consisting of European Americans, African Americans, Mexican Americans, and American Indians found that the rs2231142 A allele was significantly associated with higher serum levels of uric acid and a higher risk of gout60. Similarly, a study with a Chinese Han male population of 352 cases and 350 controls also show a significant association of the rs2231142 A allele with an increased risk of gout61. In addition, a meta-analysis including 9 case-control studies involving 17,942 individuals62 clearly showed an increased gout risk in patients with the rs2231142 A allele as opposed to the rs2231142 C allele. Gender and ethnicity were found to be additional co-factors affecting the association with susceptibility to gout. The rs2231142 C421A variant was also associated with poor response to gout treatment with allopurinol, suggesting this SNP might be a robust genetic marker for the prediction of gout treatment success63. Another study with a Chinese Han population of 143 cases and 310 controls64 identified four additional susceptible ABCG2 SNPs associated with occurrence of gout, including rs2622621, rs3114018, rs17731799, and rs3114020. An ABCG2 haplotype containing these SNPs is associated with a decreased risk of development of gout.
The common ABCG2 variants are associated with efficacy of lowering cholesterol by statin drugs. Statins are a class of drugs widely used in the treatment of hyperlipidemia through their ability to reduce plasma low-density lipoprotein cholesterol by inhibiting β-hydroxy-β-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, a rate-controlling enzyme for cholesterol biosynthesis in liver. The biological target of statins is the hepatocyte and full drug efficacy requires their retention in liver. Rosuvastatin has been shown to be a substrate of BCRP65, indicating that its pharmacokinetics can be affected by common ABCG2 variants. A study with 14 healthy Chinese male individuals showed that rosuvastatin has a higher Cmax and a lower clearance in individuals with rs2231142 CA or AA genotypes as compared to individuals with rs2231142 CC66. Another study also reported that individuals with the ABCG2 rs2231142 A variant had a lower clearance of rosuvastatin67. The ABCG2 rs2231142 C421A variant also showed a similar impact on the pharmacokinetics of atorvastatin and simvastatin in Caucasian and Asian subjects68. Furthermore, a study focusing on adverse drug reactions induced by atorvastatin showed that patients with rs2231142 CA or AA had a higher risk of developing dose-dependent atorvastatin adverse effects as compared to rs2231142 CC patients69. These studies showed the role of BCRP in the transport of several statin drugs, where specific BCRP alleles were able to alter drug therapeutic efficacy and even toxicity. In precision medication, carriers of these ABCG2 variants should receive modified doses of drugs to improve therapeutic efficacy and avoid unnecessary adverse reactions.
In summary, genetic polymorphisms are common in the ABCG2 gene with more than 100 SNPs identified in the human genome. The two most common ABCG2 variants have minor allele frequencies of over 10% in global populations, with higher frequencies in East Asians. The minor alleles of both variants result in amino acid substitutions in the BCRP NBD domain, which have been proven to reduce the expression and function of BCRP and its ability to transport its substrates. Although population-based association studies revealed a potential effect on cancer progression, the minor alleles of these two common ABCG2 variants are associated with a significant better response rate to chemotherapy with various anticancer drugs on a range of cancers. Because of the reduced efflux of substrates out of target cells, minor alleles of these two common ABCG2 variants also have the potential to trigger adverse events during chemotherapy. In addition to cancer, the common ABCG2 variants also have a proven impact on progression of other diseases, such as gout, and therapeutic efficacy of additional drugs, such as statins.
The current literature does not yet give a complete picture of all the genetic polymorphisms of the ABCG2 gene. Current cell-based and population-based studies mainly focused on the two most common ABCG2 variants, rs2231137 G34A (V12M) and rs2231142 C421A (Q141K), while knowledge of other ABCG2 SNPs, particularly for SNPs in the promoter, untranslated regions, and introns, are very limited. Several studies have shown that altered pharmacokinetic behavior of drugs in patients carrying the common ABCG2 variants exist, but there are no dosing guidance or drug labels on how to best use substrate drugs in individuals harboring genetic variants of the ABCG2 gene.
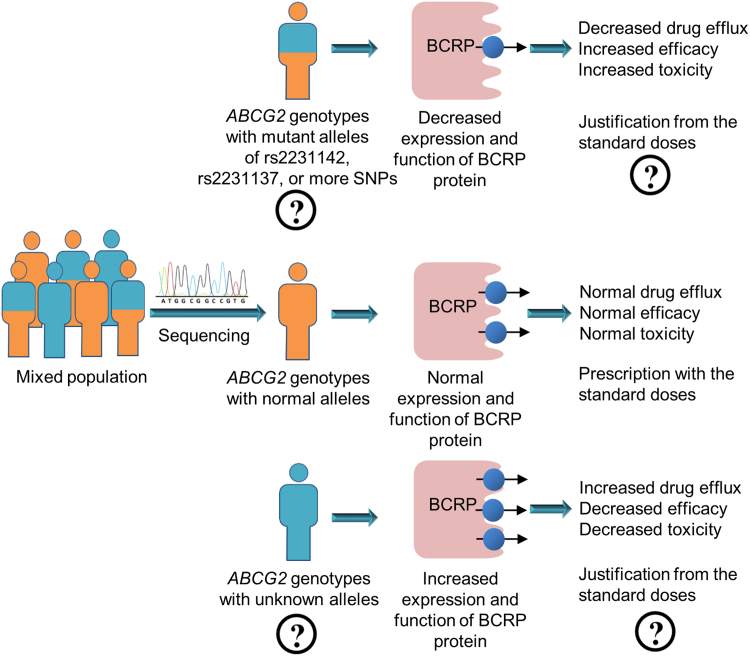
However, sufficient data are now in hand to develop precision medicine approaches based on ABCG2 genetic polymorphisms. As illustrated in Fig. 4, before a patient is prescribed a drug, sequencing of the ABCG2 gene should be performed and this information should be annotated. A genotype associated with decreased expression and function of BCRP protein can be predicted to have decreased drug efflux mediated by BCRP accompanied with increased efficacy, but with risk of increased toxicity. Deviation from standard doses with either an increased dose for better efficacy or a decreased dose for less toxicity could be made to achieve a more precise treatment plan. More accurate associations of ABCG2 genotypes with expression and function of BCRP protein and more precise dose adjustments are the areas that warrant future study in order to develop precision medicine strategies related to ABCG2 genotypes.
Figure 4.
A strategy for the development of a precision medicine approach based on genetic polymorphisms in the ABCG2 gene in a mixed population.
3. Alterations of BCRP expression by epigenetic mechanisms
In addition to the common genetic polymorphisms of the ABCG2 gene, expression and function of BCRP protein can be altered by epigenetic mechanisms. Epigenetic mechanisms refer to the molecular events that can affect gene expression without changes of the DNA sequence per se. DNA methylation, histone modifications, and non-coding RNAs are among the most highly studied epigenetic pathways. Expression of BCRP protein has been shown to be regulated by each of these epigenetic mechanisms.
3.1. Alteration of BCRP expression by DNA methylation
DNA methylation usually occurs at the 5′-carbon position of cytosine residues located in “CpG” islands. DNA methylation at the promoter region of a gene can inhibit transcriptional initiation and leads to repression of transcription.
The DNA methylation status of the ABCG2 promoter contributes to BCRP-mediated chemotherapeutic drug resistance in various cancer cell types. The lung cancer cell line PC-6 showed increased BCRP expression at both mRNA and protein levels after treatment with a DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine (5-aza-C)70. An inverse correlation between methylation status of the CpG sites in the ABCG2 promoter and BCRP expression levels was found in several small cell and non-small cell lung cancer cells70. The study indicated that higher levels of demethylation of the CpG sites in the ABCG2 promoter may serve as a mechanism leading to higher levels of BCRP expression and resistance to SN-38 for anti-cancer therapy in lung cancer cells. A correlation between the DNA methylation status of the ABCG2 promoter and drug resistance was assessed in 32 colorectal cancer cell lines71. Lower levels of BCRP expression were significantly correlated with higher levels of DNA methylation. After treatment with 5-aza-C, enhanced expression of BCRP and decreased sensitivity of the colon cancer cells to the anticancer drugs of 5-fluorouracil, oxaliplatin, and irinotecan were observed71. A high level of DNA methylation at the CpG sites in the ABCG2 promoter and a low level of BCRP expression are also common in T-cell acute lymphoblastic leukemia cells72. Treatment with the chemotherapeutic drugs sulfasalazine and topotecan resulted in demethylation of the CpG sites in the ABCG2 promoter in the T-cell model and a dramatic induction of BCRP mRNA levels as well as consequent acquisition of a BCRP-dependent drug resistant phenotype72. Combinational treatment of malignant glioma cells with a chemotherapeutic drug temozolomide together with melatonin has shown a synergistic toxic effect on the cancer cells. Melatonin-induced DNA methylation at the ABCG2 promoter and a downregulation of the expression and function of BCRP have been suggested to be a potential mechanism for the synergistic toxic effect of melatonin and chemotherapeutic drugs73, therefore, melatonin can be viewed as a promising drug to overcome drug resistance in the treatment of glioblastomas and improve the efficacy of chemotherapy.
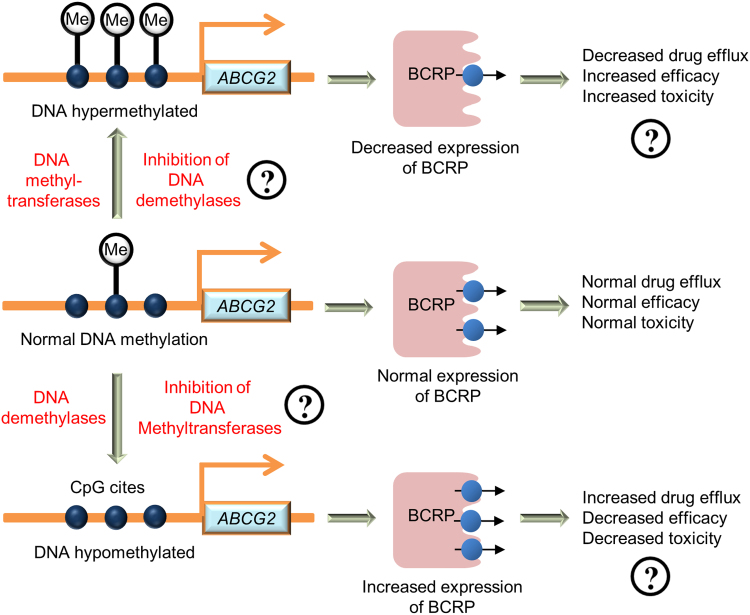
A strategy to develop a precision medication approach based on DNA methylation of the ABCG2 promoter can be developed. As illustrated in Fig. 5, inhibition of DNA demethylases or activation of DNA methyltransferases can serve as a potential strategy for decreasing BCRP expression, whereas inhibition of DNA methyltransferases or activation of DNA demethylases might serve as a potential strategy for increasing BCRP expression and decreasing drug-induced toxicity. Evaluation of existing drugs and new drugs as inhibitors of DNA methyltransferases or demethylases to modulate BCRP-mediated drug transport are areas of future studies for the development of precision medicine strategies.
Figure 5.
A strategy for the development of a precision medicine approach based on DNA methylation in the ABCG2 promoter in a mixed population.
3.2. Alteration of BCRP expression by histone modifications
Histone modification is another well studied epigenetic influence that affects the regulation of gene expression at the transcriptional level. Some amino acid residues (especially lysine and arginine) of the histone tails can be modified by acetylation, methylation, phosphorylation, citrullination, and ubiquitination. Some modifications specifically enhance gene transcription, but others repress.
The histone modification status near the ABCG2 promoter has a great impact on BCRP expression and multidrug-resistant phenotypes. Overexpression of BCRP and subsequent drug resistance are associated with increased acetylated histone H3 at the ABCG2 promoter74. Permissive histone modifications, including histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 serine 10 phosphorylation (H3S10P), were observed to accompany the development of drug resistant phenotypes. The repressive histone H3 lysine 9 trimethylation (H3K9me3) mark has an inhibitory effect on BCRP expression. Histone deacetylase (HDAC) inhibitors have been demonstrated to increase BCRP expression. Transcription of BCRP in acute AML cells was induced by treatment of phenylbutyrate, an HDAC inhibitor75. KG-1a cells treated with phenylbutyrate developed resistance to multiple anticancer drugs, including daunorubicin, mitoxantrone, etoposide, and 5-fluorouracil. The HDAC inhibitors romidepsin, panobinostat, and vorinostat were able to restore normal levels of efflux transport mediated by BCRP in cells bearing the common ABCG2 variant Q141K by enhancing synthesis of BCRP protein and preventing aggresome targeting of the mutated protein76. These results indicate that the involvement of epigenetic regulation in drug resistance transporters and treatment with HDAC inhibitors might increase drug resistance when combined with other anticancer drugs.
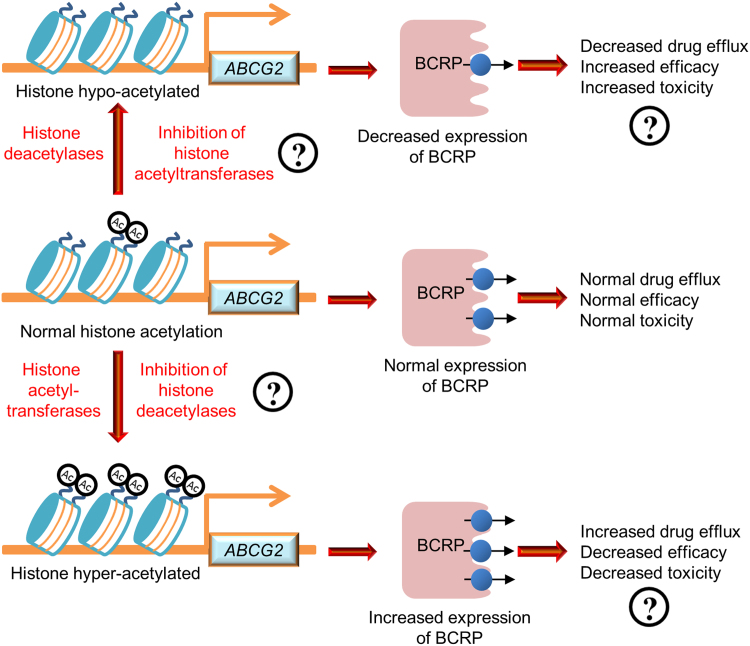
A strategy to develop precision medication approaches based on histone modifications of the ABCG2 gene can be potentially be established. As illustrated in Fig. 6 using histone acetylation as an example for histone modifications, inhibition of histone acetyltransferases or activation of histone deacetylases can serve as a potential strategy for decreasing BCRP expression, thus increasing drug efficacy, whereas inhibition of histone deacetylases or activation of histone acetyltransferases can serve as a potential strategy for increasing BCRP expression, thus enhancing drug disposition and decreasing toxic potential. Evaluation of existing drugs and new drugs as inhibitors of histone acetyltransferases or histone deacetylases for BCRP-mediated drug transport are areas of future study for the development of precision medicine approaches. A similar strategy can be developed for other histone modifications, such as histone methylation and phosphorylation, which have been shown to have a clear implication for the regulation of BCRP expression.
Figure 6.
A strategy for the development of a precision medicine approach based on histone acetylation in the ABCG2 gene in a mixed population.
3.3. Alteration of BCRP expression by microRNAs
MicroRNAs (miRNA) are small non-coding RNAs transcribed from miRNA genes. Mature miRNAs are able to bind with Argonaut protein to form the RNA-induced silencing complex (RISC). An interaction between RISC and target mRNA causes mRNA degradation and/or decreased translational efficiency, which may further affect protein expression and function. The roles of miRNAs in anti-cancer therapy and multidrug resistance have been studied extensively in recent years, where regulation of drug transporters by miRNAs being one of the major mechanisms77.
Expression of BCRP at the post-transcriptional level is regulated in part by miRNAs. Several studies have shown that miRNAs were able to regulate BCRP mRNA or protein expression and alter pharmacokinetic characteristics of BCRP substrate drugs. By targeting the 3′-untranslated region (3′-UTR) of BCRP mRNA, miR-328-3p was able to regulate BCRP expression level and restore the chemosensitivity of MCF7/MX100 cells to Mitoxantrone, a topoisomerase inhibitor used in cancer treatment78., 79.. MiR-487a is another miRNAs that binds to 3′-UTR of BCRP mRNA. By targeting BCRP mRNA, miR-487a also restored the susceptibility of breast cancer cells to mitoxantrone80. Further, inhibition of miR-487a reverses this phenotype80. By comparing the differential expression patterns of miRNAs between the mitoxantrone-resistant breast cancer cell line MCF-7/MX and its parental mitoxantrone-sensitive cell line MCF-7, another miRNA (miR-181a) was found to be the most down-regulated miRNA in MCF-7/MX cells81. Luciferase assays proved that miRNA-181a targets the 3′-UTR of the BCRP mRNA. Overexpression of miR-181a down-regulated BCRP expression and caused mitoxantrone-resistant MCF-7/MX cells to become responsive to mitoxantrone. In addition, miRNAs belonging to the miR-302 family (including miR-302a, miR-302b, miR-302c, and miR-302d) were also able to inhibit BCRP expression by targeting the 3′-UTR of the BCRP mRNA. Consistent with similar targeting by the other miRNAs described here, miRNA-302 also increases the sensitivity of breast cancer cells to mitoxantrone82. Other than directly targeting the 3′-UTR of the BCRP mRNA, a novel mechanism on the regulation of BCRP expression by miRNAs was found for miR-519c83. MiR-519c was able to bind HuR, an mRNA binding protein, and regulate BCRP expression. The indirect regulation of BCRP through miR-519c-HuR regulatory pathway in colorectal cancer tissue was able to cause down-regulation of HuR or up-regulation of miR-519c, which repressed BCRP expression83. These studies indicate that several miRNAs may serve as potential targets for preventing and reversing drug resistance in cancer treatment.
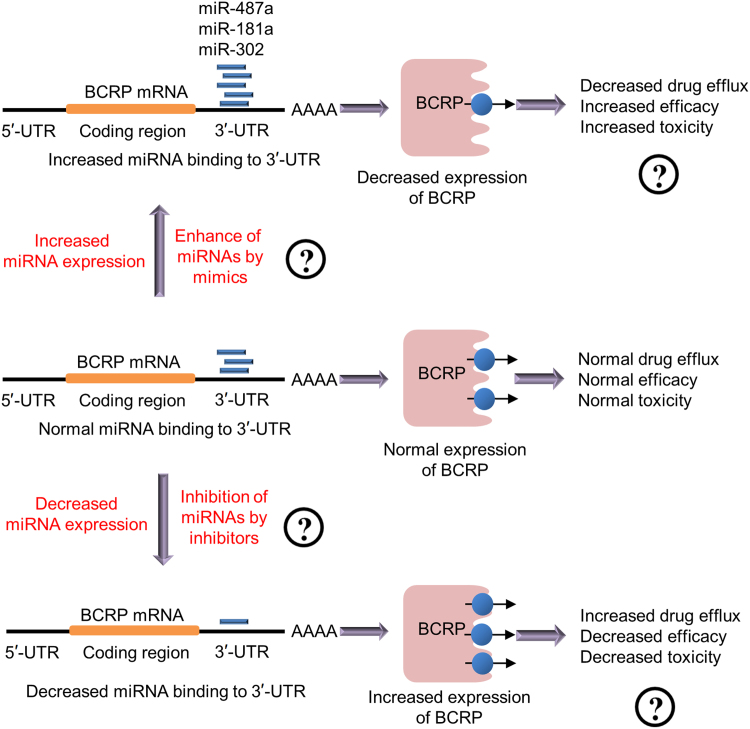
A strategy to develop a precision medication strategy based on miRNA binding to the ABCG2 3′-UTR can be established. As illustrated in Fig. 7, either increased miRNA expression or enhancement of miRNA binding by adding miRNA mimics can serve as a potential strategy for decreasing BCRP expression, whereas decreased miRNA expression or use of small RNA interference (siRNAs) for miRNA inhibition can serve as potential strategies for increasing BCRP expression with associated decreases in toxicity.
Figure 7.
A strategy for the development of a precision medicine approach based on miRNA binding in the BCRP mRNA 3′-UTR in a mixed population.
In summary, the epigenetic mechanisms that control BCRP expression are attractive areas for future study. Numerous studies confirmed that epigenetic pathways, including DNA methylation, histone modification, and non-coding RNA action, are able to regulate the transcription or translation of the ABCG2 gene and mRNA, and thus affect function of the BCRP transporter. However, most studies on the epigenetic regulation of BCRP expression and function were performed in cell-based assays. Since those actions of epigenetic modifications are highly specific in different organs, collecting biological specimens and building up an epigenetic database are much more complicated as compared to genotyping approaches. Whether alterations of the epigenetic mechanisms in the target organs can be well represented in surrogate specimens is an area of active research84., 85.. Until biomarkers representing alterations of the epigenetic mechanisms are discovered in clinical specimens, a precision medicine model for dose justification on drugs based on the epigenetic modifications is difficult at best, and more studies are needed in these areas.
4. Impact of physiologic factors upon BCRP expression and function
The expression of BCRP protein can be impacted by seemingly normal physiological factors. Normal human physiological and existential conditions such as gender, age, circadian rhythms, and oxygenation states have impacts on the expression and function of BCRP.
4.1. Impact of gender upon expression of BCRP
Whether or not BCRP expression differs between males and females is a current topic of debate. In one study, the quantification of BCRP protein expression in 65 human liver samples by mass spectrometry revealed no difference between males and females86. Similarly, no differences in BCRP mRNA and protein were observed between males and females in a cohort of 87 human liver samples87. In another study, the quantification of BCRP protein levels in 32 intestinal samples by Western blotting identified a slight higher amount of BCRP protein in females compared to males37. The sample sizes are perhaps not sufficient in some of these studies and only certain organs have been studied. Therefore, the question of whether or not gender is a significant contributor to differential BCRP expression requires further investigation.
4.2. Impact of age upon expression and function of BCRP
It is also unclear whether age should be considered as a physiologic factor that can impact BCRP function, but provocative studies exist. In one study, BCRP expression in 65 human liver samples was not correlated with ages examined (7 and 70 years)86. In a contrary study, BCRP protein levels in 67 human liver samples showed a correlation to age with significantly lower levels in elderly samples (age over 65 years, n=13) as compared to compared to adults of 19–64 years, n=46 and neonates and children (0.02–18 years, n=8)87. Current human studies are not adequate to support any relationship between age and BCRP function. However, other factors such as health condition of tested subject might also lead to inter-individual difference in BCRP function, which covers up the impact of age in BCRP function. Further experiments and larger testing population are still needed to study how BCRP changes with human ages.
Age-related differences in the expression of BCRP have also been assessed in various animal models. BCRP mRNA expression in mouse liver is highest before birth, markedly decreases immediately after birth, stays a consistent level in immature ages, and gradually increases in young adults88. A significantly lower level of BCRP mRNA was found in liver of older female rats (30 and 50 weeks of age) compared to young female rats (10 weeks of age)89. BCRP mRNA expression level in rat kidney was low in fetal samples, increased gradually following birth, and markedly increased on adult90. More detailed studies in both humans and rodents are needed to determine whether or not age should have a significant impact on BCRP expression.
4.3. Impact on expression and function of BCRP by circadian rhythms
Expression of BCRP may be controlled by circadian rhythms. Circadian rhythms are molecular clocks regulating a variety of biological processes across the daily cycle. Circadian clocks have been shown to exert a strong impact on the pharmacokinetics of drug response91, which may result in alterations of therapeutic efficacy or adverse reactions through taking drugs at different times of day. A circadian time-dependent expression pattern of BCRP was found in mouse small intestinal cells, which was controlled by circadian clock-activating transcription factor-4 pathway92. This finding indicates that examinations on whether expression and function of BCRP are regulated by circadian rhythms in various human organs should be investigated to inform future precision medicine approaches to determine the best times of day for drug administration. In addition, since circadian disruption occurs in certain occupations such as shift work and in air travel, these too should be considered for their impact on BCRP function and for pharmacological treatments of shift workers and frequent air travelers93. Future studies focused on circadian alterations of BCRP expression are needed in the development of precision medicine for specific populations with potential circadian disruption and desynchrony.
In summary, implications on alterations of BCRP expression by several physiologic factors, such as gender, age, and circadian, have been studied, but limitations still exist in the current research. The results from the studies examining the relationship of BCRP expression with age and gender are controversial upon different sample pools and no solid conclusions are made. For circadian effects, due to difficulty in collecting human sample and data, the studies were only performed on animals. Overall, the question of whether or not physiologic factors can be used as guidance for precision medicine approaches is unclear.
5. Impact of pathologic factors upon BCRP expression and function
5.1. Impact of diabetes upon expression and function of BCRP
The expression and functionality of BCRP may be altered by chronic disease conditions such as diabetes. Alterations of BCRP expression and function at blood–brain barrier under diabetes and the clinical significances have been discussed94. In a streptozotocin-induced diabetic rat model, a lower level of insulin in diabetic rats is correlated with a lower level of BCRP expression and function in the brain cortex95, and this is accompanied by increases in the distribution of BCRP substrates, such as prazosin and cimetidine, in the brain. Insulin treatment attenuated the impairment of BCRP function in the brain cortex induced by diabetes. In a high fat diet and streptozotocin-induced diabetic rat model, BCRP protein levels were induced in the kidneys, while unchanged in the liver96. These findings in animal models suggest that similar changes might take place in type 2 diabetic patients, and that the pharmacokinetics of drug that are substrates for BCRP may be altered with a potential of increased risk for drug toxicity and decreased therapeutic efficacy. So far, large-scale clinical investigations on a potential correlation between diabetes and altered expression and function of BCRP have not been conducted. However, a positive correlation was observed between levels of BCRP expression in placenta and levels of plasma hemoglobin A1c, a marker of the state of diabetes, in pregnant diabetic women, indicating that placenta BCRP might be affected by poorly managed diabetes97. Even the population-based clinical studies on correlation between diabetes and BCRP expression and function are still limited. The studies above including both animal and human data suggested that diabetic should be considered as a factor in BCRP regulation. Prescription of BCRP substrate drugs to diabetic patients should be adjusted based on patients׳ BCRP function.
5.2. Impact of Alzheimer׳s disease on the expression and function of BCRP
The expression of BCRP may be altered in the brain of Alzheimer׳s patients. A major pathological hallmark of Alzheimer׳s disease is the accumulation of beta amyloid peptide plaques (Aβ peptide) in the brain. BCRP has been referred as a gatekeeper of the blood–brain barrier that acts to prevent blood Aβ peptide from entering the brain, based on an observation of a significantly higher accumulation of Aβ peptide in the brain of Abcg2-null mice as compared to wild type mice98. This study also found a significantly higher level of BCRP expression in the brains of Alzheimer׳s disease patients as compared to age-matched controls. The up-regulation of BCRP expression is considered as a compensatory response in an attempt to reduce the Aβ peptide burden by enhancing efflux of Aβ peptide across the blood brain barrier99. Alternatively, up-regulation of BCRP expression can protect the brains of patients with Alzheimer׳s disease by reducing oxidative stress and inflammatory responses100. Of therapeutic importance, high levels of BCRP expression in the brains of Alzheimer׳s patients may lead to reduced brain drug penetration and efficacy for other neurological disorders.
5.3. Effect of viral infection expression and function of BCRP
The expression and function of BCRP can be altered during viral infection. The transactivator protein (Tat) from human immunodeficiency virus type-1 (HIV-1) has been shown to alter the expression of various multidrug resistant transporters, including BCRP. In a T cell leukemia-derived cell line, overexpression of HIV-1 Tat upregulated BCRP expression, enhanced the BCRP-dependent efflux of doxorubicin, and reduced doxorubicin-induced cytotoxicity101. This strongly suggested that decreased anti-viral drug efficacy may be caused by increase expression of BCRP by Tat.
In summary, BCRP expression and function have been shown to be altered under different disease conditions, where the altered BCRP function might change the efficacy or toxicity of substrate drugs if given to patients. In this case, patients׳ health condition and disease history might be important for prescription, especially BCRP substrate drugs. However, current study on how pathologic factors alter BCRP function is more focus on pre-clinical mechanistic studies with scarce clinical data available. For the development of a precision medicine approach, implications on clinical outcomes mediated by BCRP drug transport by pathologic factors need to be examined in future studies.
6. Impact on BCRP expression and function by environmental factors
6.1. Impact on expression and function of BCRP by diet
Nutritional factors have been shown to impact BCRP expression and function. Oleic acid is present in fat-rich meals. Human epithelial colorectal adenocarcinoma Caco-2 cells treated with oleic acid showed significant intracellular accumulation of the BCRP substrate mitoxantrone102. This accumulation was due to decreased BCRP-mediated efflux caused by oleic acid treatment. Similarly, oleic acid treatment increased intestinal accumulation of mitoxantrone in mice, which was also the result of decreased BCRP-mediated efflux103. Curcumin, an ingredient supplement widely used in food, has been shown to increase the sensitivity of breast cancer cells to several anticancer drugs, including paclitaxel, cisplatin, doxorubicin, and mitomycin C. The combination treatment of curcumin with mitomycin C significantly suppressed tumor growth derived from breast cancer stem cells in mice104. Curcumin treatment renders breast cancer stem cells susceptible to mitomycin C by reducing BCRP expression. These studies show that diet and nutrition can affect BCRP function with obvious implications for drug efficacy.
Flavonoids, common polyphenolic compounds found in foods and herbal products, have been shown to inhibit BCRP function43. Hesperetin, quercetin, daidzein, and stilbene resveratrol were also able to alter BCRP function for the transport of intracellular BCRP substrates105. Considering the possibly of high intake of these natural products, altered BCRP substrate absorption and distribution might happen due to transporter dysfunction.
The fruit extract ellagitannin (containing ellagic acid) can be converted to urolithins by gut microbiotas, which have been shown to possess anti-inflammatory and chemo preventive properties. Urolithins are proven BCRP substrates, which also showed an inhibitory effect on BCRP-mediated efflux of mitoxantrone in MDCKII cells106. The results suggest that physiologically relevant concentrations of the gut microbiota-derived metabolites from fruit extracts could modulate BCRP-mediated transport processes of anticancer drugs and result in increased chemotherapy efficacy.
6.2. Impact on expression and function of BCRP by hypoxic conditions
BCRP expression is altered by hypoxic environments in organs. The alteration of BCRP expression by hypoxic conditions can occur either in tissues exposed to low oxygen environments107 or in persons at a high attitude108. BCRP function in the apical membranes of the placenta syncytiotrophoblasts of pregnant women has a direct impact on protection of the fetus from exposure to various endogenous and exogenous substrates. An adequate supply of oxygen is critical for fetal development during pregnancy. A hypoxic state may have an impact on fetal development through modulation of BCRP expression in the placenta. Using human BeWo choriocarcinoma cells, a study revealed decreased expression of BCRP mRNA and protein under hypoxic conditions (3% oxygen), which is accompanied by increased hypoxia inducible factor 1 subunit alpha (HIF-1α) signaling108. Hypoxia-induced functional impairment of BCRP was also demonstrated by accumulation of the BCRP substrate Hoechst 33342. The same study also found that women who gave birth at a high altitude (3100 m above the sea level) exhibited signs of chronic placental hypoxia in comparison with women at a moderate altitude (1600 m above the sea level)108. The women exposed to high altitude hypoxic conditions had enhanced expression of the HIF-1α protein and reduced BCRP protein in microvillus membranes. This study indicates that the regulation of placental BCRP expression by hypoxia may be an important factor for the protection of the fetus from exposure to chemicals during early development and in hypoxia-related pregnancy disorders. In addition, the fetus under hypoxic conditions may be more sensitive to drug-induced toxicity caused by BCRP-mediated efflux drugs.
BCRP expression in cancer can also be regulated by oxygen levels. Exposure of human para-carcinoma tissues and the pancreatic cancer cell line Capan-2 to hypoxic conditions induced BCRP expression109. The induction was due to increased HIF-1α binding of the ABCG2 promoter and consequent increased transcription. This hypoxia-induced chemo-resistance via BCRP overexpression is indicative of a therapeutic potential for cancer treatment through an inhibition on the HIF-1α signaling. Hypoxic conditions or doxorubicin exposure individually or in combination could also result in induced BCRP expression in a colon cancer cell line, causing drug resistance110. All these results indicate that oxygen levels play an important role in regulating BCRP expression with tissue-dependent impact. Controlling oxygen levels or related signaling pathways could be a potential target for the improvement of cancer treatments by modulating BCRP-mediated drug efflux transport. However, the effect of hypoxia on BCRP function has been studied only in cell culture models, and clinical data of BCRP expression in pregnant women has not been assessed.
6.3. Impact on expression and function of BCRP by drugs
The expression or function of BCRP can be altered by drug treatment, potentially triggering drug–drug interactions. Modulation of BCRP function with drugs influences both cancer and non-cancer drug treatment outcomes.
With the potential role of BCRP in MDR, inhibition of BCRP function has been regarded as an attractive strategy to re-sensitize BCRP-overexpressing cancer cells to chemotherapy drugs. To date, several types of BCRP inhibitors have been discovered and reported. Fumitremorgin C (FTC), the first identified BCRP inhibitor, can reverse the resistance of several BCRP substrate anticancer drugs in vitro111., 112.. Ko143, a tetracyclic analogue of FTC, also showed specific and potent inhibition of BCRP both in vitro and in vivo, possibly through an ATPase inhibitory mechanism113. There are also several other reported FTC-type BCRP inhibitors, including diketopiperazines and tryprostatin A114., 115.. PZ-39 is another identified BCRP inhibitor with the ability to cause BCRP inhibition by both binding to BCRP as well as acceleration of transporter degradation116., 117., 118.. Several MDR inhibitors targeting other transporters were later found to inhibit BCRP function. Elacridar (GF120918), a P-glycoprotein (P-gp) inhibitor, was reported to resensitize cell to the BCRP substrate drug topotecan by increasing its oral bioavailability in a pharmacokinetic study119. Other than selective BCRP inhibitors, tyrosine kinase inhibitors (TKIs) are a group of drugs that display effective inhibition of MDR-associated ABC transporters. By targeting the ATP-binding pocket, TKIs block the hydrolysis of ATP and the energy-dependent transport function of ABC transporters. Several TKIs have shown BCRP inhibition and resensitization of cells to BCRP substrate drugs, including imatinib, dasatinib, ponatinib, lapatinib, sunitinib, and olmutinib120., 121., 122., 123., 124., 125., 126.. Detailed information about the TKI mechanism is reviewed elsewhere127., 128..
BCRP also has been reported to play an important role in the absorption and retention of statin drugs. Fostamatinib is a proven BCRP inhibitor. A cell-based study using polarized Caco-2 cells demonstrated a strong drug–drug interaction on accumulation of rosuvastatin influenced by inhibition of BCRP function by fostamatinib129. The study concludes that inhibition of intestinal BCRP function by fostamatinib can result in an up to 2-fold accumulation of rosuvastatin, which suggests that a reduced dose of rosuvastatin could be indicated in clinical practice. Co-administration of rosuvastatin with eltrombopag, an orally thrombopoietin receptor agonist, in MDCKII cells caused a strong cellular accumulation of rosuvastatin. The interaction of eltrombopag with rosuvastatin can also be explained by inhibition of BCRP-mediated efflux of rosuvastatin by eltrombopag130. In support of this, concomitant administration of eltrombopag with rosuvastatin in 42 healthy adult volunteers proved an increased plasma rosuvastatin area under curve (AUC) in the overall study population131.
These studies indicate that BCRP might be responsible for drug-drug interactions in clinical treatments. Drugs, which can alter BCRP function, should be paid with extra attention when given together with a BCRP substrate drug. However, there is only limited knowledge about which chemical or drugs can alter BCRP function and to avoid BCRP-related drug–drug interaction, further studies are still needed.
7. Conclusions
Interindividual differences in BCRP expression can alter pharmacokinetic properties of substrate drugs and cause variations in drug response and disease risk. In this review, we summarized several factors that might contribute to individual variability of BCRP function for drug transport.
Utilizing advanced sequencing technologies, BCRP genetic polymorphisms and single nucleotide polymorphisms have been identified and their clinical significance has been studied extensively. Several of the most common ABCG2 SNPs were summarized here and their roles in cancer prediction or treatment have been discussed using data from multiple clinical experiments. Epigenetic regulations, including DNA methylation, histone modifications, and non-coding RNAs are also shown to be involved in the regulation of BCRP expression. Other factors, including dietary factors or environmental toxicants might also impact BCRP function and influence BCRP substrate drug treatment. Physiologic conditions of patients, including age, gender, and health conditions, may have implications in the regulation of BCRP expression, but further studies are needed to determine their impacts on the development of precision medicine. Especially, considering the expression of BCRP in placenta, pregnant women should be carefully prescribed and the function of BCRP should be taken into consideration.
In summary, expression and function of BCRP protein in various human tissues are controlled by multiple individual factors, leading to significant variability of BCRP function for drug transport. Understanding these factors and their impacts on BCRP function is critical for the development of precision medicine approaches for better therapeutic efficacy and less adverse toxicity.
Acknowledgment
This study was supported in the part by National Institutes of Health National Institute of General Medical Sciences, USA (R01GM118367 to Xiao-bo Zhong, USA).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Petzinger E., Geyer J. Drug transporters in pharmacokinetics. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:465–475. doi: 10.1007/s00210-006-0042-9. [DOI] [PubMed] [Google Scholar]
- 2.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao Q., Unadkat J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—an update. AAPS J. 2015;17:65–82. doi: 10.1208/s12248-014-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle L., Ross D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 5.Cole S.P., Bhardwaj G., Gerlach J.H., Mackie J.E., Grant C.E., Almquist K.C. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J.T. Use of arrays to investigate the contribution of ATP-binding cassette transporters to drug resistance in cancer chemotherapy and prediction of chemosensitivity. Cell Res. 2007;17:311–323. doi: 10.1038/cr.2007.15. [DOI] [PubMed] [Google Scholar]
- 7.Ifergan I., Shafran A., Jansen G., Hooijberg J.H., Scheffer G.L., Assaraf Y.G. Folate deprivation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression. A role for BCRP in cellular folate homeostasis. J Biol Chem. 2004;279:25527–25534. doi: 10.1074/jbc.M401725200. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama A., Matsuo H., Takada T., Ichida K., Nakamura T., Ikebuchi Y. ABCG2 is a high-capacity urate transporter and its genetic impairment increases serum uric acid levels in humans. Nucleosides Nucleotides Nucleic Acids. 2011;30:1091–1097. doi: 10.1080/15257770.2011.633953. [DOI] [PubMed] [Google Scholar]
- 9.Lemos C., Jansen G., Peters G.J. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br J Cancer. 2008;98:857–862. doi: 10.1038/sj.bjc.6604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robey R.W., Steadman K., Polgar O., Morisaki K., Blayney M., Mistry P. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 11.Mo W., Zhang J.T. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3:1–27. [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 13.Maliepaard M., van Gastelen M.A., de Jong L.A., Pluim D., van Waardenburg R.C., Ruevekamp-Helmers M.C. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 14.Perego P., De Cesare M., De Isabella P., Carenini N., Beggiolin G., Pezzoni G. A novel 7-modified camptothecin analog overcomes breast cancer resistance protein-associated resistance in a mitoxantrone-selected colon carcinoma cell line. Cancer Res. 2001;61:6034–6037. [PubMed] [Google Scholar]
- 15.Kawabata S., Oka M., Shiozawa K., Tsukamoto K., Nakatomi K., Soda H. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280:1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 16.Turner J.G., Gump J.L., Zhang C., Cook J.M., Marchion D., Hazlehurst L. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108:3881–3889. doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross D.D., Karp J.E., Chen T.T., Doyle L.A. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96:365–368. [PubMed] [Google Scholar]
- 18.Benderra Z., Faussat A.M., Sayada L., Perrot J.Y., Tang R., Chaoui D. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–7772. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 19.Benderra Z., Faussat A.M., Sayada L., Perrot J.Y., Chaoui D., Marie J.P. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 20.Sargent J.M., Williamson C.J., Maliepaard M., Elgie A.W., Scheper R.J., Taylor C.G. Breast cancer resistance protein expression and resistance to daunorubicin in blast cells from patients with acute myeloid leukaemia. Br J Haematol. 2001;115:257–262. doi: 10.1046/j.1365-2141.2001.03122.x. [DOI] [PubMed] [Google Scholar]
- 21.Sauerbrey A., Sell W., Steinbach D., Voigt A., Zintl F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br J Haematol. 2002;118:147–150. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- 22.Plasschaert S.L., van der Kolk D.M., de Bont E.S., Kamps W.A., Morisaki K., Bates S.E. The role of breast cancer resistance protein in acute lymphoblastic leukemia. Clin Cancer Res. 2003;9:5171–5177. [PubMed] [Google Scholar]
- 23.Kanzaki A., Toi M., Nakayama K., Bando H., Mutoh M., Uchida T. Expression of multidrug resistance-related transporters in human breast carcinoma. Jpn J Cancer Res. 2001;92:452–458. doi: 10.1111/j.1349-7006.2001.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger H., Foekens J.A., Look M.P., Meijer-van Gelder M.E., Klijn J.G., Wiemer E.A. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–836. [PubMed] [Google Scholar]
- 25.Diestra J.E., Scheffer G.L., Catala I., Maliepaard M., Schellens J.H., Scheper R.J. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 26.Yoh K., Ishii G., Yokose T., Minegishi Y., Tsuta K., Goto K. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 27.Deichmann M., Thome M., Egner U., Hartschuh W., Kurzen H. The chemoresistance gene ABCG2 (MXR/BCRP1/ABCP1) is not expressed in melanomas but in single neuroendocrine carcinomas of the skin. J Cutan Pathol. 2005;32:467–473. doi: 10.1111/j.0303-6987.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y.L., Patel A., Kumar P., Chen Z.S. Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 2012;31:51–57. doi: 10.5732/cjc.011.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasanabady M.H., Kalalinia F. ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J Biosci. 2016;41:313–324. doi: 10.1007/s12038-016-9601-5. [DOI] [PubMed] [Google Scholar]
- 30.Ni Z., Mark M.E., Cai X., Mao Q. Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int J Biochem Mol Biol. 2010;1:1–11. [PMC free article] [PubMed] [Google Scholar]
- 31.Haider A.J., Briggs D., Self T.J., Chilvers H.L., Holliday N.D., Kerr I.D. Dimerization of ABCG2 analysed by bimolecular fluorescence complementation. PLoS One. 2011;6:e25818. doi: 10.1371/journal.pone.0025818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao S.L., Chong S.S., Lee C.G. The role of single nucleotide polymorphisms (SNPs) in understanding complex disorders and pharmacogenomics. Ann Acad Med Singap. 2000;29:376–382. [PubMed] [Google Scholar]
- 33.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh R., Thomson K.L., Ware J.S., Funke B.H., Woodley J., McGuire K.J. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19:192–203. doi: 10.1038/gim.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pybus M., Dall׳Olio G.M., Luisi P., Uzkudun M., Carreno-Torres A., Pavlidis P. 1000 Genomes Selection Browser 1.0: a genome browser dedicated to signatures of natural selection in modern humans. Nucleic Acids Res. 2014;42:D903–D909. doi: 10.1093/nar/gkt1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong F.A., Marsh S., Mathijssen R.H., King C., Verweij J., Sparreboom A. ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10:5889–5894. doi: 10.1158/1078-0432.CCR-04-0144. [DOI] [PubMed] [Google Scholar]
- 37.Zamber C.P., Lamba J.K., Yasuda K., Farnum J., Thummel K., Schuetz J.D. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Sakiyama M., Matsuo H., Takada Y., Nakamura T., Nakayama A., Takada T. Ethnic differences in ATP-binding cassette transporter, sub-family G, member 2 (ABCG2/BCRP): genotype combinations and estimated functions. Drug Metab Pharmacokinet. 2014;29:490–492. doi: 10.2133/dmpk.DMPK-14-SC-041. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi D., Ieiri I., Hirota T., Takane H., Maegawa S., Kigawa J. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33:94–101. doi: 10.1124/dmd.104.001628. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.S., Jeong H.E., Yi J.M., Jung H.J., Jang J.E., Kim E.Y. Identification and functional assessment of BCRP polymorphisms in a Korean population. Drug Metab Dispos. 2007;35:623–632. doi: 10.1124/dmd.106.012302. [DOI] [PubMed] [Google Scholar]
- 41.Backstrom G., Taipalensuu J., Melhus H., Brandstrom H., Svensson A.C., Artursson P. Genetic variation in the ATP-binding cassette transporter gene ABCG2 (BCRP) in a Swedish population. Eur J Pharm Sci. 2003;18:359–364. doi: 10.1016/s0928-0987(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 42.Honjo Y., Morisaki K., Huff L.M., Robey R.W., Hung J., Dean M. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1) Cancer Biol Ther. 2002;1:696–702. doi: 10.4161/cbt.322. [DOI] [PubMed] [Google Scholar]
- 43.Kondo C., Suzuki H., Itoda M., Ozawa S., Sawada J., Kobayashi D. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21:1895–1903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 44.Skoglund K., Boiso Moreno S., Jonsson J.I., Vikingsson S., Carlsson B., Green H. Single-nucleotide polymorphisms of ABCG2 increase the efficacy of tyrosine kinase inhibitors in the K562 chronic myeloid leukemia cell line. Pharmacogenet Genom. 2014;24:52–61. doi: 10.1097/FPC.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa T., Wakabayashi K., Tamura A., Nakagawa H., Morishima Y., Osawa Y. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res. 2009;26:469–479. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura A., Wakabayashi K., Onishi Y., Takeda M., Ikegami Y., Sawada S. Re-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2. Cancer Sci. 2007;98:231–239. doi: 10.1111/j.1349-7006.2006.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner E.R., Ahlers C.M., Shukla S., Sissung T.M., Ockers S.B., Price D.K. Association of the ABCG2 C421A polymorphism with prostate cancer risk and survival. BJU Int. 2008;102:1694–1699. doi: 10.1111/j.1464-410X.2008.07913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa H., Tamura A., Wakabayashi K., Hoshijima K., Komada M., Yoshida T. Ubiquitin-mediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter. ABCG2. Biochem J. 2008;411:623–631. doi: 10.1042/BJ20071229. [DOI] [PubMed] [Google Scholar]
- 49.Tamura A., Watanabe M., Saito H., Nakagawa H., Kamachi T., Okura I. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol. 2006;70:287–296. doi: 10.1124/mol.106.023556. [DOI] [PubMed] [Google Scholar]
- 50.Ghafouri H., Ghaderi B., Amini S., Nikkhoo B., Abdi M., Hoseini A. Association of ABCB1 and ABCG2 single nucleotide polymorphisms with clinical findings and response to chemotherapy treatments in Kurdish patients with breast cancer. Tumour Biol. 2016;37:7901–7906. doi: 10.1007/s13277-015-4679-1. [DOI] [PubMed] [Google Scholar]
- 51.Wu H., Liu Y., Kang H., Xiao Q., Yao W., Zhao H. Genetic variations in ABCG2 gene predict breast carcinoma susceptibility and clinical outcomes after treatment with anthracycline-based chemotherapy. Biomed Res Int. 2015;2015:279109. doi: 10.1155/2015/279109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen P., Zhao L., Zou P., Xu H., Lu A., Zhao P. The contribution of the ABCG2 C421A polymorphism to cancer susceptibility: a meta-analysis of the current literature. BMC Cancer. 2012;12:383. doi: 10.1186/1471-2407-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kopp T.I., Andersen V., Tjonneland A., Vogel U. Polymorphisms in ATP-binding cassette transporter genes and interaction with diet and life style factors in relation to colorectal cancer in a Danish prospective case-cohort study. Scand J Gastroenterol. 2015;50:1469–1481. doi: 10.3109/00365521.2015.1056224. [DOI] [PubMed] [Google Scholar]
- 54.Sari F.M., Yanar H.T., Ozhan G. Investigation of the functional single-nucleotide polymorphisms in the BCRP transporter and susceptibility to colorectal cancer. Biomed Rep. 2015;3:105–109. doi: 10.3892/br.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobek K.M., Cummings J.L., Bacich D.J., O׳Keefe D.S. Contrasting roles of the ABCG2 Q141K variant in prostate cancer. Exp Cell Res. 2017;354:40–47. doi: 10.1016/j.yexcr.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tandia M., Mhiri A., Paule B., Saffroy R., Cailliez V., Noe G. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): monocentric study. Cancer Chemother Pharmacol. 2017;79:759–766. doi: 10.1007/s00280-017-3268-y. [DOI] [PubMed] [Google Scholar]
- 57.Xie Y., Xu K., Linn D.E., Yang X., Guo Z., Shimelis H. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283:3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- 58.Low S.K., Fukunaga K., Takahashi A., Matsuda K., Hongo F., Nakanishi H. Association study of a functional variant on ABCG2 gene with sunitinib-induced severe adverse drug reaction. PLoS One. 2016;11:e0148177. doi: 10.1371/journal.pone.0148177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamura M., Kondo M., Horio M., Ando M., Saito H., Yamamoto M. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J Med Sci. 2012;74:133–140. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Spencer K.L., Voruganti V.S., Jorgensen N.W., Fornage M., Best L.G. Association of functional polymorphism rs2231142 (Q141K) in the ABCG2 gene with serum uric acid and gout in 4 US populations: the PAGE study. Am J Epidemiol. 2013;177:923–932. doi: 10.1093/aje/kws330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou D., Liu Y., Zhang X., Gu X., Wang H., Luo X. Functional polymorphisms of the ABCG2 gene are associated with gout disease in the Chinese Han male population. Int J Mol Sci. 2014;15:9149–9159. doi: 10.3390/ijms15059149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong Z., Guo S., Yang Y., Wu J., Guan M., Zou H. Association between ABCG2 Q141K polymorphism and gout risk affected by ethnicity and gender: a systematic review and meta-analysis. Int J Rheum Dis. 2015;18:382–391. doi: 10.1111/1756-185X.12519. [DOI] [PubMed] [Google Scholar]
- 63.Roberts R.L., Wallace M.C., Phipps-Green A.J., Topless R., Drake J.M., Tan P. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenomics J. 2017;17:201–203. doi: 10.1038/tpj.2015.101. [DOI] [PubMed] [Google Scholar]
- 64.Jiri M., Zhang L., Lan B., He N., Feng T., Liu K. Genetic variation in the ABCG2 gene is associated with gout risk in the Chinese Han population. Clin Rheumatol. 2016;35:159–163. doi: 10.1007/s10067-015-3105-9. [DOI] [PubMed] [Google Scholar]
- 65.Huang L., Wang Y., Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738–742. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W., Yu B.N., He Y.J., Fan L., Li Q., Liu Z.Q. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta. 2006;373:99–103. doi: 10.1016/j.cca.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Wan Z., Wang G., Li T., Xu B., Pei Q., Peng Y. Marked alteration of rosuvastatin pharmacokinetics in healthy Chinese with ABCG2 34G>A and 421C>A homozygote or compound heterozygote. J Pharmacol Exp Ther. 2015;354:310–315. doi: 10.1124/jpet.115.225045. [DOI] [PubMed] [Google Scholar]
- 68.Birmingham B.K., Bujac S.R., Elsby R., Azumaya C.T., Wei C., Chen Y. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol. 2015;71:341–355. doi: 10.1007/s00228-014-1801-z. [DOI] [PubMed] [Google Scholar]
- 69.Mirošević Skvrce N., Macolić Šarinić V., Šimić I., Ganoci L., Muačević Katanec D., Božina N. ABCG2 gene polymorphisms as risk factors for atorvastatin adverse reactions: a case-control study. Pharmacogenomics. 2015;16:803–815. doi: 10.2217/pgs.15.47. [DOI] [PubMed] [Google Scholar]
- 70.Nakano H., Nakamura Y., Soda H., Kamikatahira M., Uchida K., Takasu M. Methylation status of breast cancer resistance protein detected by methylation-specific polymerase chain reaction analysis is correlated inversely with its expression in drug-resistant lung cancer cells. Cancer. 2008;112:1122–1130. doi: 10.1002/cncr.23285. [DOI] [PubMed] [Google Scholar]
- 71.Moon H.H., Kim S.H., Ku J.L. Correlation between the promoter methylation status of ATP-binding cassette sub-family G member 2 and drug sensitivity in colorectal cancer cell lines. Oncol Rep. 2016;35:298–306. doi: 10.3892/or.2015.4342. [DOI] [PubMed] [Google Scholar]
- 72.Bram E.E., Stark M., Raz S., Assaraf Y.G. Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance. Neoplasia. 2009;11:1359–1370. doi: 10.1593/neo.91314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martín V., Sanchez-Sanchez A.M., Herrera F., Gomez-Manzano C., Fueyo J., Alvarez-Vega M.A. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer. 2013;108:2005–2012. doi: 10.1038/bjc.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.To K.K., Polgar O., Huff L.M., Morisaki K., Bates S.E. Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug-resistant cells. Mol Cancer Res. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauswald S., Duque-Afonso J., Wagner M.M., Schertl F.M., Lubbert M., Peschel C. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15:3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 76.Basseville A., Tamaki A., Ierano C., Trostel S., Ward Y., Robey R.W. Histone deacetylase inhibitors influence chemotherapy transport by modulating expression and trafficking of a common polymorphic variant of the ABCG2 efflux transporter. Cancer Res. 2012;72:3642–3651. doi: 10.1158/0008-5472.CAN-11-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An X., Sarmiento C., Tan T., Zhu H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm Sin B. 2017;7:38–51. doi: 10.1016/j.apsb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan Y.Z., Morris M.E., Yu A.M. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Xin, Tian Ye, Tu Mei-Juan, Ho Pui Yan, Batra Neelu, Yu Ai-Ming. Bioengineered miR-27b-3p and miR-328-3p modulate drug metabolism and disposition via the regulation of target ADME gene expression. Acta Pharm Sin B. 2019;9:639–647. doi: 10.1016/j.apsb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma M.T., He M., Wang Y., Jiao X.Y., Zhao L., Bai X.F. MiR-487a resensitizes mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by targeting breast cancer resistance protein (BCRP/ABCG2) Cancer Lett. 2013;339:107–115. doi: 10.1016/j.canlet.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Jiao X., Zhao L., Ma M., Bai X., He M., Yan Y. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2) Breast Cancer Res Treat. 2013;139:717–730. doi: 10.1007/s10549-013-2607-x. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Zhao L., Xiao Q., Jiang L., He M., Bai X. miR-302a/b/c/d cooperatively inhibit BCRP expression to increase drug sensitivity in breast cancer cells. Gynecol Oncol. 2016;141:592–601. doi: 10.1016/j.ygyno.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 83.To K.K., Leung W.W., Ng S.S. Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp Cell Res. 2015;338:222–231. doi: 10.1016/j.yexcr.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Istas G., Declerck K., Pudenz M., Szic K.S.V., Lendinez-Tortajada V., Leon-Latre M. Identification of differentially methylated BRCA1 and CRISP2 DNA regions as blood surrogate markers for cardiovascular disease. Sci Rep. 2017;7:5120. doi: 10.1038/s41598-017-03434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowe R., Gemma C., Beyan H., Hawa M.I., Bazeos A., Leslie R.D. Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics. 2013;8:445–454. doi: 10.4161/epi.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prasad B., Lai Y., Lin Y., Unadkat J.D. Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci. 2013;102:787–793. doi: 10.1002/jps.23436. [DOI] [PubMed] [Google Scholar]
- 87.Riches Z., Abanda N., Collier A.C. BCRP protein levels do not differ regionally in adult human livers, but decline in the elderly. Chem Biol Interact. 2015;242:203–210. doi: 10.1016/j.cbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui J.Y., Gunewardena S.S., Yoo B., Liu J., Renaud H.J., Lu H. RNA-Seq reveals different mRNA abundance of transporters and their alternative transcript isoforms during liver development. Toxicol Sci. 2012;127:592–608. doi: 10.1093/toxsci/kfs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawase A., Ito A., Yamada A., Iwaki M. Age-related changes in mRNA levels of hepatic transporters, cytochrome P450 and UDP-glucuronosyltransferase in female rats. Eur J Drug Metab Pharmacokinet. 2015;40:239–244. doi: 10.1007/s13318-014-0208-7. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y.J., Wang Y., Lu Y.F., Xu S.F., Wu Q., Liu J. Age-associated differences in transporter gene expression in kidneys of male rats. Mol Med Rep. 2017;15:474–482. doi: 10.3892/mmr.2016.5970. [DOI] [PubMed] [Google Scholar]
- 91.Musiek E.S., Fitzgerald G.A. Molecular clocks in pharmacology. Handb Exp Pharmacol. 2013:243–260. doi: 10.1007/978-3-642-25950-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamdan A.M., Koyanagi S., Wada E., Kusunose N., Murakami Y., Matsunaga N. Intestinal expression of mouse Abcg2/breast cancer resistance protein (BCRP) gene is under control of circadian clock-activating transcription factor-4 pathway. J Biol Chem. 2012;287:17224–17231. doi: 10.1074/jbc.M111.333377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voigt R.M., Forsyth C.B., Keshavarzian A. Circadian disruption: potential implications in inflammatory and metabolic diseases associated with alcohol. Alcohol Res. 2013;35:87–96. [PMC free article] [PubMed] [Google Scholar]
- 94.Liu L., Liu X.D. Alterations in function and expression of ABC transporters at blood–brain barrier under diabetes and the clinical significances. Front Pharmacol. 2014;5:273. doi: 10.3389/fphar.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y.C., Liu H.Y., Yang H.W., Wen T., Shang Y., Liu X.D. Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem Pharmacol. 2007;74:1766–1772. doi: 10.1016/j.bcp.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 96.Nowicki M.T., Aleksunes L.M., Sawant S.P., Dnyanmote A.V., Mehendale H.M., Manautou J.E. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008;2:11–17. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- 97.Anger G.J., Cressman A.M., Piquette-Miller M. Expression of ABC efflux transporters in placenta from women with insulin-managed diabetes. PLoS One. 2012;7:e35027. doi: 10.1371/journal.pone.0035027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiong H., Callaghan D., Jones A., Bai J., Rasquinha I., Smith C. ABCG2 is upregulated in Alzheimer׳s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood–brain barrier for Aβ1–40 peptides. J Neurosci. 2009;29:5463–5475. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abuznait A.H., Kaddoumi A. Role of ABC transporters in the pathogenesis of Alzheimer׳s disease. ACS Chem Neurosci. 2012;3:820–831. doi: 10.1021/cn300077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen S., Callaghan D., Juzwik C., Xiong H., Huang P., Zhang W. ABCG2 reduces ROS-mediated toxicity and inflammation: a potential role in Alzheimer׳s disease. J Neurochem. 2010;114:1590–1604. doi: 10.1111/j.1471-4159.2010.06887.x. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y., Zhang K., Yin X., Nie Q., Ma Y. HIV-1 Tat protein enhances expression and function of breast cancer resistance protein. AIDS Res Hum Retroviruses. 2016;32:1–3. doi: 10.1089/aid.2015.0117. [DOI] [PubMed] [Google Scholar]
- 102.Aspenstrom-Fagerlund B., Tallkvist J., Ilback N.G., Glynn A.W. Oleic acid decreases BCRP mediated efflux of mitoxantrone in Caco-2 cell monolayers. Food Chem Toxicol. 2012;50:3635–3645. doi: 10.1016/j.fct.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 103.Aspenstrom-Fagerlund B., Tallkvist J., Ilback N.G., Glynn A.W. Oleic acid increases intestinal absorption of the BCRP/ABCG2 substrate, mitoxantrone, in mice. Toxicol Lett. 2015;237:133–139. doi: 10.1016/j.toxlet.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Q., Ye M., Lu Y., Zhang H., Chen Q., Huang S. Curcumin improves the tumoricidal effect of mitomycin c by suppressing ABCG2 expression in stem cell-like breast cancer cells. PLoS One. 2015;10:e0136694. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cooray H.C., Janvilisri T., van Veen H.W., Hladky S.B., Barrand M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317:269–275. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 106.Gonzalez-Sarrias A., Miguel V., Merino G., Lucas R., Morales J.C., Tomas-Barberan F. The gut microbiota ellagic acid-derived metabolite urolithin A and its sulfate conjugate are substrates for the drug efflux transporter breast cancer resistance protein (ABCG2/BCRP) J Agric Food Chem. 2013;61:4352–4359. doi: 10.1021/jf4007505. [DOI] [PubMed] [Google Scholar]
- 107.Krishnamurthy P., Schuetz J.D. The ABC transporter ABCG2/BCRP: role in hypoxia mediated survival. Biometals. 2005;18:349–358. doi: 10.1007/s10534-005-3709-7. [DOI] [PubMed] [Google Scholar]
- 108.Francois L.N., Gorczyca L., Du J., Bircsak K.M., Yen E., Wen X. Down-regulation of the placental BCRP/ABCG2 transporter in response to hypoxia signaling. Placenta. 2017;51:57–63. doi: 10.1016/j.placenta.2017.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He X., Wang J., Wei W., Shi M., Xin B., Zhang T. Hypoxia regulates ABCG2 activity through the activation of ERK1/2/HIF-1alpha and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol Ther. 2016;17:188–198. doi: 10.1080/15384047.2016.1139228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinzon-Daza M.L., Cuellar-Saenz Y., Nualart F., Ondo-Mendez A., Del Riesgo L., Castillo-Rivera F. Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions. J Cell Biochem. 2017;118:1868–1878. doi: 10.1002/jcb.25890. [DOI] [PubMed] [Google Scholar]
- 111.Rabindran S.K., He H., Singh M., Brown E., Collins K.I., Annable T. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 112.Rabindran S.K., Ross D.D., Doyle L.A., Yang W., Greenberger L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 113.Allen J.D., van Loevezijn A., Lakhai J.M., van der Valk M., van Tellingen O., Reid G. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 114.van Loevezijn A., Allen J.D., Schinkel A.H., Koomen G.J. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg Med Chem Lett. 2001;11:29–32. doi: 10.1016/s0960-894x(00)00588-6. [DOI] [PubMed] [Google Scholar]
- 115.Woehlecke H., Osada H., Herrmann A., Lage H. Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A. Int J Cancer. 2003;107:721–728. doi: 10.1002/ijc.11444. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed-Belkacem A., Pozza A., Muñoz-Martínez F., Bates S.E., Castanys S., Gamarro F. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65:4852–4860. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- 117.Peng H., Dong Z., Qi J., Yang Y., Liu Y., Li Z. A novel two mode-acting inhibitor of ABCG2-mediated multidrug transport and resistance in cancer chemotherapy. PLoS One. 2009;4:e5676. doi: 10.1371/journal.pone.0005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng H., Qi J., Dong Z., Zhang J.T. Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells. PLoS One. 2010;5:e15276. doi: 10.1371/journal.pone.0015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kruijtzer C.M., Beijnen J.H., Rosing H., ten Bokkel Huinink W.W., Schot M., Jewell R.C. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 120.Hirota S., Isozaki K., Moriyama Y., Hashimoto K., Nishida T., Ishiguro S. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 121.Tiwari A.K., Sodani K., Wang S.R., Kuang Y.H., Ashby C.R., Jr, Chen X. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–161. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 122.Giannoudis A., Davies A., Lucas C.M., Harris R.J., Pirmohamed M., Clark R.E. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008;112:3348–3354. doi: 10.1182/blood-2007-10-116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sen R., Natarajan K., Bhullar J., Shukla S., Fang H.B., Cai L. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol Cancer Ther. 2012;11:2033–2044. doi: 10.1158/1535-7163.MCT-12-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dai C.L., Liang Y.J., Wang Y.S., Tiwari A.K., Yan Y.Y., Wang F. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 125.Shukla S., Robey R.W., Bates S.E., Ambudkar S.V. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–365. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Z., Guo X., To K.K.W., Chen Z., Fang X., Luo M. Olmutinib (HM61713) reversed multidrug resistance by inhibiting the activity of ATP-binding cassette subfamily G member 2 in vitro and in vivo. Acta Pharm Sin B. 2018;8:563–574. doi: 10.1016/j.apsb.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu P., Nielsen T.E., Clausen M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 128.Hartmann J.T., Haap M., Kopp H.G., Lipp H.P. Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 129.Elsby R., Martin P., Surry D., Sharma P., Fenner K. Solitary inhibition of the breast cancer resistance protein efflux transporter results in a clinically significant drug–drug interaction with rosuvastatin by causing up to a 2-fold increase in statin exposure. Drug Metab Dispos. 2016;44:398–408. doi: 10.1124/dmd.115.066795. [DOI] [PubMed] [Google Scholar]
- 130.Takeuchi K., Sugiura T., Matsubara K., Sato R., Shimizu T., Masuo Y. Interaction of novel platelet-increasing agent eltrombopag with rosuvastatin via breast cancer resistance protein in humans. Drug Metab Dispos. 2014;42:726–734. doi: 10.1124/dmd.113.054767. [DOI] [PubMed] [Google Scholar]
- 131.Allred A.J., Bowen C.J., Park J.W., Peng B., Williams D.D., Wire M.B. Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol. 2011;72:321–329. doi: 10.1111/j.1365-2125.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]