Abstract
Various physiological processes involve appropriate tissue developmental process and homeostasis - the pathogenesis of several diseases connected with deregulatory apoptosis process. Apoptosis plays a crucial role in maintaining a balance between cell death and division, evasion of apoptosis results in the uncontrolled multiplication of cells leading to different diseases such as cancer. Currently, the development of apoptosis targeting anticancer drugs has gained much interest since cell death induced by apoptosis causes minimal inflammation. The understanding of complexities of apoptosis mechanism and how apoptosis is evolved by tumor cells to oppose cell death has focused research into the new strategies designed to induce apoptosis in cancer cells. This review focused on the underlying mechanism of apoptosis and the dysregulation of apoptosis modulators involved in the extrinsic and intrinsic apoptotic pathway, which include death receptors (DRs) proteins, cellular FLICE inhibitory proteins (c-FLIP), anti-apoptotic Bcl-2 proteins, inhibitors of apoptosis proteins (IAPs), tumor suppressor (p53) in cancer cells along with various current clinical approaches aimed to selectively induce apoptosis in cancer cells.
Keywords: Apoptosis, Apoptosis pathways, Cancer, c-FLIP, Caspases, Death receptors, Targeted drugs
Introduction
Cell death is an essential process in the development, tissue homeostasis and integrity of multicellular organisms. The cell proliferation and elimination is necessary to maintain a homeostasis physiological processes in the adult organism.1,2 The unwanted cells removed during the process of metamorphosis, embryogenesis, pathogenesis as well as tissue turnover.3,4 Cell death typically involves two broadly defined mechanisms: programmed cell death and necrosis (Figure 1).5 Cell death which includes a genetically programmed process of cell suicide in response to particular signals is called programmed cell death.6,7 Usually, programmed cell death controlled by a variety of extracellular and intracellular signals which are directed by the environment of the cell and intracellular signals.8 Programmed cell death distinguished from cell necrosis as it has distinct morphological characteristics, maintains tissue homeostasis and regulates the proper number of cells in multicellular organisms by eliminating unwanted cells.3,9 Different endogenous tissue-specific agents and exogenous cell-damaging agents initiate programmed cell death in particular cell type under critical physiological conditions.10 Exogenous activations of programmed cell death include physical agents and infectious agents that act on most types of cells. Physical agents include radiation, physical trauma, and chemotherapeutic drugs while infectious agents include viruses and bacterial toxins.11 Further, internal imbalances such as growth factors withdrawal, ablation of a trophic hormone, treatment with glucocorticoids and loss of matrix attachment can trigger apoptosis.10 Although various research groups have often equated programmed cell death with apoptosis, recent studies have proven that non-apoptotic forms of programmed cell death also exist which lacks involvement of the mechanism of apoptosis. Therefore, programmed cell death and apoptosis should never be considered synonymous.12,13 Kerr et al proposed the term apoptosis used to describe a morphologically distinct pattern of cell death.14,15
Figure 1.
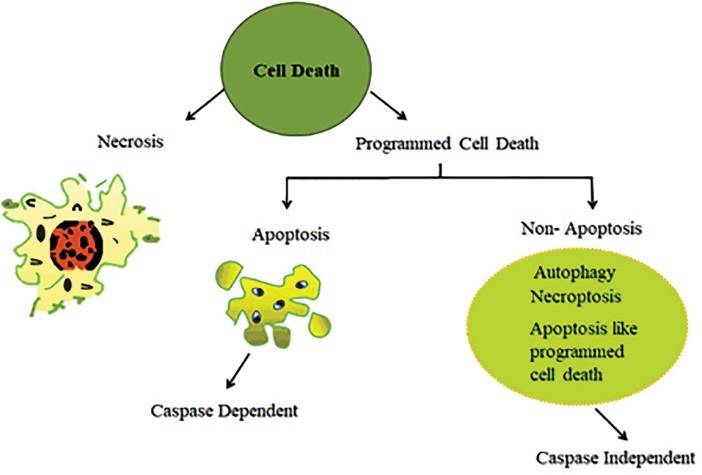
General mode of cancer cell death. Cancer cell death involves two broadly defined mechanisms: programmed cell death and necrosis. Programmed cell death mainly apoptosis and nonapoptosis base cell death, such as autophagy, necroptosis and apoptosis like programmed cell death.
Apoptosis extensively described as a significant mechanism of regulated death that occurs not only as a result of cell damage or external stress but it also takes place during normal development, and morphogenesis.16 Apoptosis tightly regulated by different groups of the executioner and regulatory molecules. Mechanism of action of apoptotic cell death typically characterized by condensation of chromatin material, fragmentation of DNA occurred in the nucleus, cell shrinkage, dynamic membrane blebbing, and loss of adhesion to extracellular matrices. Further, biochemical alterations include; externalization of phosphatidylserine, and the activation of cysteine aspartyl proteases, called caspases which leads to the cell death.14,16-20
Apoptosis typically distinguished from necrosis, which was assumed to represent an opposite way of an unordered cellular explosion in response to severe and irresistible trauma. Interest in non-apoptotic forms of programmed cell death is gradually increasing as more information on this type of cell death is collected.21 Non-apoptotic cell death types include autophagy, necroptosis, and apoptosis-like programmed cell death. Autophagy or autophagic cell death termed as type II cell death. Autophagic cell death is a self-degradative process, and it plays a vital role in the degradation of cellular components inside the dying cell in autophagic vacuoles. Autophagy is also known as vacuolar cell death and is very common in the invertebrate tissue.22,23 Necroptosis is a programmed form of necrotic death, and it initiated by same death signals that induce apoptosis.24 Necroptosis is very common in vivo, in physical traumas, death inflicted by infection and in diverse forms of neurodegeneration. It believed that apoptosis and necroptosis (a regulated and programmed form of cell death) shares several vital processes. Several death receptors (DRs) such as FAS and TNFR that are known to induce apoptosis also induce necroptosis in different cell types.25 However, programmed necrosis has been seen only under a specific condition when apoptosis has been chemically or genetically repressed or blocked.16 Moreover, another form is apoptosis-like programmed cell death describe the type of cell death which involve apoptotic features, but the cell death occurs in a caspase-independent manner.26
Necrosis might happen during several diseases including cancer, neurodegenerative and autoimmune diseases. Necrosis traditionally considered as random, uncontrolled process which is usually initiated by certain stimuli like toxic trauma or physical damage.27,28 Necrosis morphologically characterized by the swelling of cytoplasm and organelles (endoplasmic reticulum and mitochondria), the disruption of the plasma membrane leading to the release of cellular components and cell lysis.16,29 Cell death by necrosis linked to chronic inflammation, caused by necrosis could enhance the proliferation of tumors.30 However, there is another perception that necrosis could also be programmed in nature as well. Programmed necrosis has been seen only under a specific condition in which apoptosis is chemically or genetically repressed or inhibited.31 Various anti-apoptotic proteins of the Bcl-2 family have been shown to inhibit both apoptotic and necrotic. Intracellular ATP depletion can switch an apoptotic response to a necrotic one. Hence, apoptosis and necrosis are not necessarily independent pathways. Instead, they may share some common messengers, activators, and inhibitors.32
Understanding apoptosis pathways for cancer therapeutics
The word cancer was first named by a physician Hippocrates around 460-370 B.C. and originated from a Greek word “karkinos” which means carcinoma.33 Cancer defined as an uncontrolled growth of the cell in an abnormal manner which alters the structure of surrounding tissues.34 Cancer cell genotypes demonstrate seven essential alterations in cell physiology which leads to its progression and metastasis.35 Figure 2 shows the seven “hallmark of cancer” which contribute towards tumor development. The carcinogenesis comprised of complex multiple steps process where single cell converted to the tumor and move to another site via the process of metastasis. Apoptosis is the vital and crucial mechanism which maintains the balance between survival and death in cells to prevent cancer and other related diseases.36
Figure 2.
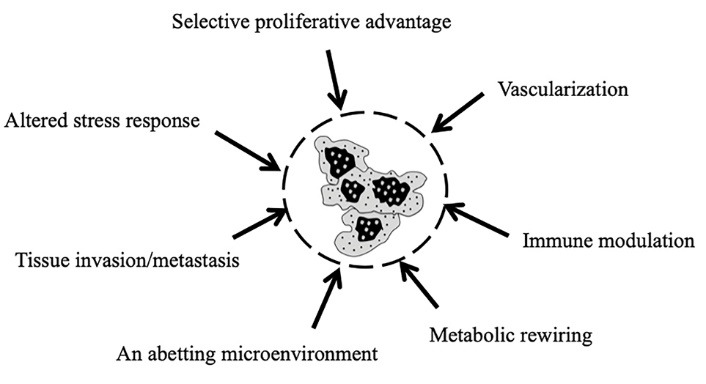
Hallmark of cancer. Various acquire features of cancer cell, include selective proliferative advantage, vascularization, immune modulation, metabolic rewiring, an abetting microenvironment, tissue invasion/metastasis and altered stress response.
Recent advances in cancer research are focused on the development of new drugs that halt the escape behavior of cancer cells via the execution of apoptosis. To this context, novel apoptotic inducers or sensitizers have been used with the combination of current drugs. Defects in the apoptosis-inducing pathways can eventually lead to the multiplication of neoplastic cells. The resistance of apoptosis stimulates aberrant cellular multiplication which eventually leads to tumorigenesis and is a significant hurdle to active cancer treatment.37,38 The induction of apoptosis in cancer cells and limit concurrent death of normal cells is the primary objective of cancer therapy.39 Some proteins have been studied to exert pro- and anti-apoptotic activity in the cell and the proportion of these proteins plays an essential part in the regulation of cell death.40 Similarly, the induction of cancer apoptosis is among the main approaches in cancer gene therapy or immunotherapy. The apoptotic inducer, mediator or executioner gene is routinely incorporated in cancer cells to reverse the deficiency of its endogenous counterpart.41,42 Understanding the cascade of events that trigger apoptosis in response to chemotherapy, and dysfunction of the apoptosis process give insight toward the novel effective therapeutic approach to the development of molecular-targeted specific therapies against cancer.
The mechanism of apoptosis mainly consist of two core pathways involved in inducing apoptosis; extrinsic pathway and intrinsic pathway. Extrinsic pathway refers to DR-mediated pathway, and the intrinsic pathway is a mitochondrial-mediated pathway.15 Both of these apoptotic pathways, extrinsic and intrinsic pathways might be lead to same terminal (execution pathway).15,40
Extrinsic pathway
Apoptotic signaling through the extrinsic pathway engaged when extracellular ligands such as TNF (tumor necrosis factor), Fas-L (Fas ligand), and TRAIL (TNF-related apoptosis-inducing ligand) are attached to the extracellular domain of the DR (transmembrane receptors), i.e., the type 1 TNF receptor (TNFR1), Fas (also called CD95/Apo-1) and TRAIL receptors. The order of events involved in the extrinsic phase of apoptosis well characterized by the FasL/FasR and TNF-α/TNFR1 models.15,43,44 This triggering of DRs by specific death ligands (DLs) results in the formation of a death-inducing signaling complex (DISC).12 This DISC consists of the DD-containing Fas-associated death domain (DD) as an adaptor molecule, procaspase-8, procaspase-10, and the cellular FLICE inhibitory proteins (c-FLIPs). The caspase 8 activate in such a manner that prodomain of caspase 8 remains at the DISC, while active caspase 8 dissociates from the DISC to start the cascade of caspase activation which constitutes the execution phase of apoptosis.45 Experimental evidence shows the excessive role of caspases in apoptosis.46,47 Caspases are essential initiators and executioners of the apoptosis, and their function is very closely related to its structure having different substrate preferences. Some caspases have long pro-domains which involve particular motif like the death effector domain (DED), and caspase recruitment domains (CARD), which allow interacting with other proteins, and connect with signaling pathways. DED includes caspase-8 and caspase-10 while CARD involves caspase-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11 and caspase-12.48 Caspases traditionally classified as initiator and effector or executioner caspases by their position in apoptotic signaling cascades.
Intrinsic pathway
The intrinsic pathway refers to mainly mitochondrial-mediated apoptotic pathway. The intrinsic pathway triggered by various extra and intra-cellular stresses, which include oxidative stress, irradiation, and treatment with cytotoxic drugs.49,50 Figure 3 shows the pathways of apoptosis, the intrinsic pathway is mediated by Bax/Bak insertion into mitochondrial membrane, and subsequently, cytochrome c released from the mitochondrial intermembrane space into the cytosol.51 Bcl-2 and Bcl-xL (Bcl-2 family member) are anti-apoptotic proteins which prevent the release of cytochrome c.52 The cytochrome c combines with Apaf–1 and procaspase-9 to produce apoptosome. Apoptosome is a multi-protein complex which comprised of a seven-spoke ring-shaped complex, which triggers caspase 9 followed by the activation of caspase-3 signaling caspase cascade which leads to the demolition of cells and ends up to apoptosis.43,46,53 Proteins that are generally involved in intrinsic pathway include SMAC/DIABLO (Second mitochondrial activator of caspases/direct IAP binding protein with low PI), Caspase-9 (Cysteinyl aspartic acid-protease-9), Bcl-2 (B-cell lymphoma protein 2), Bcl-w (Bcl-2 like protein), Nox (Phorbol-12-myristate-13-acetate-induced protein 1), Aven (Cell death regulator Aven) and Myc (Oncogene Myc).15 The dysfunctional mitochondrial results in loss of inner mitochondrial membrane potential, hyperproduction of superoxide ions, disturbance in mitochondrial biogenesis, the outflow of matrix calcium glutathione and release of membrane proteins,14,15 hold promising potential for cancer therapeutic strategies via induction of apoptosis in cancer cells which are discussed later in this review.
Figure 3.
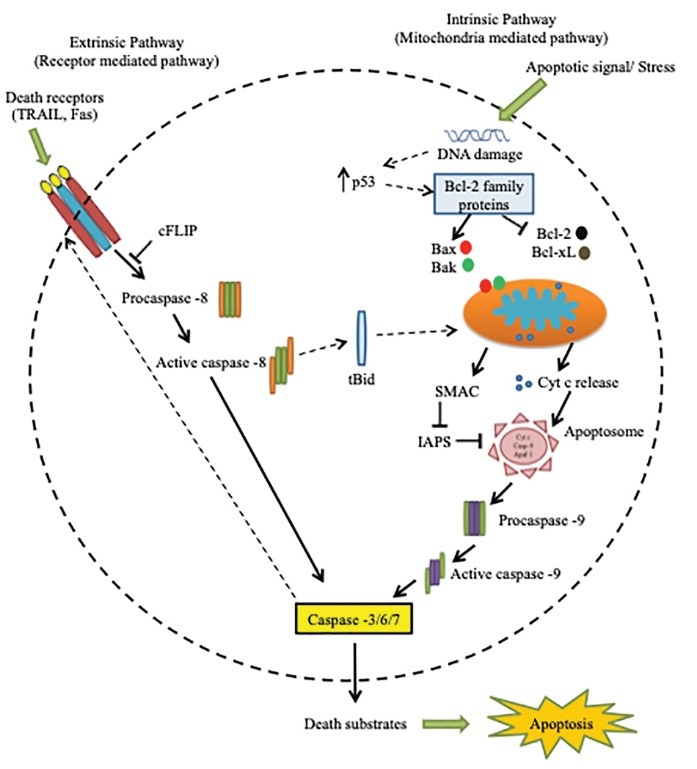
Pathways of Apoptosis. Apoptosis mainly consist of two main pathways and third is executioner pathway of apoptosis. Extrinsic pathway triggered by external stimuli or ligand molecule and particularly involve death receptors (DRs). The intrinsic pathway is mediated by Bax/Bak insertion into mitochondrial membrane, and subsequently, cytochrome c released which combines with Apaf–1 and procaspase-9 to produce apoptosome followed by the activation of caspase 3 cascade of apoptosis. TNF related apoptosis inducing ligand (TRAIL), cellular FLICE inhibitory proteins (cFLIP), Truncated bid (tBid), B-cell lymphoma protein 2 (Bcl-2), Bcl-2 homologue splice variants (Bcl-xL), Cytochrome (Cyt C), Second mitochondrial activator of caspases (SMAC), Inhibitor of apoptosis proteins (IAPs).
Execution pathway
Both extrinsic and intrinsic pathways converge at the same point (execution phase). Execution phase refers to the final pathway of apoptosis.54 Caspase-8, and 9 are initiator caspases while caspase-3, caspase-6 and caspase-7, Caspase-10, CAD (Caspase-activated DNAse) and PARP (Poly (ADP-ribose) polymerase) are classified as effector or executioner caspases.55,56 Initiator caspases activated as a result of autocleavage, which further activates executioner caspases which later proteolyze some substrates leading to apoptosis. They possess long pro-domains that connect large adapter molecules promoting multimerization and result in other caspases activation. However, effector caspases possess short pro-domains which execute apoptosis when activated by initiator caspases. Executioner caspases activate cytoplasmic endonuclease which causes chromatin condensation, the formation of cytoplasmic blebs and apoptotic bodies. Caspases regulate apoptotic cell death via cleavage of numerous target proteins.57,58 The pathway begins with the activation of execution caspases which further activates cytoplasmic endonuclease. Cytoplasmic endonuclease degrades nuclear material, and proteases followed by the degradation of nuclear and cytoskeletal proteins. Among all executioner caspases, caspase-3 is the most important, and any of the initiator caspases can activate it. Endonuclease CAD is activated explicitly by caspase-3, which causes degradation of chromosomal DNA within nuclei and condensation of chromatin. Execution caspases play an essential role in the cytoskeletal reorganization and formation of cytoplasmic blebs and apoptotic bodies.15,46
Therapeutic targets for targeting death receptors: extrinsic pathway
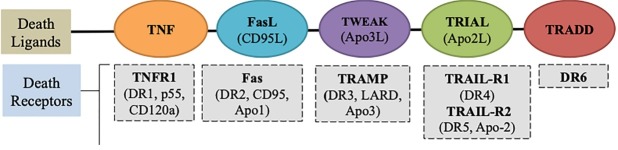
DRs are associates of TNF superfamily and initiate apoptotic signals when similar death ligands bind to the particular cell surface of DRs.59 Death ligands and their respective receptors comprise of TNF-TNFR1, FasL/CD95L-Fas, TWEAK(Apo3L)-TRAMP, TRIAL(Apo2L)-TRAIL-R1 and TRADD-DR6 as shown in Figure 4. The DRs well characterized by cysteine-rich extracellular domains and intracellular cytoplasmic sequence called as DD. This ligand-receptor binding leads to the activation in the cytoplasmic domain, accumulation of receptor and employment of adaptor proteins through the interaction between the adoptors and DD of receptors. Which consequently recruit and activates extrinsic pathway initiator caspases such as caspase -8 and caspase 10.60,61
Figure 4.
Death ligands and their receptors Death ligands and their receptors comprise of Tumor necrosis factor (TNF)- Tumor necrosis factor receptor (TNFR1), Fas ligand (FasL)/CD95L-Fas, TNF-related weak inducer of apoptosis (TWEAK/Apo3L)- TNF receptor–related apoptosis-mediating protein (TRAMP), TNF related apoptosis inducing 13 ligand (TRIAL/Apo2L)- TNF related apoptosis inducing ligand receptor (TRAIL-R1) and Tumor necrosis factor receptor type 1-associated death domain (TRADD)- Death receptor (DR6). TNF-related apoptosis-inducing ligand (TRAIL). This ligand-receptor binding interaction activate the cytoplasmic domain, consequently recruit and activates extrinsic pathway initiator caspases such as caspase -8 and caspase 10.
DRs play a significant role in the extrinsic apoptotic pathway and therefore emerged as a potential cancer therapeutic target. A variety of agents proposed in order to stimulate the apoptotic function of DRs and ligands in the extrinsic pathway, such as DNA damaging chemotherapeutic agents, histone deacetylase (HDAC) inhibitors, proteasome inhibitors, cyclooxygenase-2 inhibitors and a number of antibodies which target the DR. Similarly, DNA damaging chemotherapeutic agents targeting Fas (DR2) expression include cisplatin, mitomycin, methotrexate, mitoxantrone, doxorubicin, and bleomycin. Moreover, etoposide, Ara-C, and camptosar (CPT-11) are used to target TRIAL-R1 (DR4) and TRIAL-R2 (DR5), thereby stimulating their expression.62 A number of HDAC inhibitors like suberoylanilide hydroxamic acid (SAHA), trichostatin A (TSA), LAQ824 (a cinnamic acid hydroxamate), m-carboxycinnamic acid bishydroxamide (CBHA) and MS-275 used in order to stimulate the expression of TRIAL-R1 and TRIAL-R2.63,64 MG132 is an example of proteasome inhibitors which effectively enhance the expression of TRIAL-R2 (DR5) while PS-341 is another proteasome inhibitor which promotes the expression of TRIAL-R1/2 without affecting the expression of pro-apoptotic Bcl-2 family proteins, cFLIP and caspases.65,66 A variety of antibodies have been utilized to target DRs (TRIAL), Apo2L/TRAIL showed as a potential cancer therapeutic agent, induce apoptosis in cancer cells via its two major cell DRs TRAIL-R1 and TRAIL-R2.67,68 They have specifically shown to expressed at higher levels in solid tumors.69 Owing to the ability of TRAIL receptors of inducing cell death specifically in cancer cells, agonistic antibodies against TRAIL receptors have been developed and demonstrated to trigger apoptosis in a number of cancer cells.70 various agonists targeting DRs in clinical trials. Dulanermin targets both TRIAL-R1/2 for colorectal cancer CRC and non-small cell lung cancer NSCLC, Mapatumumab target TRIAL-R1 for advanced solid tumors and NSCLC, PR095780 for Advanced solid tumors, NHL in I and II phase.71-76 Lexatumumab (HGS-ETR2) and Conatumumab (AMG-655) target TRIAL-R2 for Advanced solid tumors in I phase.77-80 Despite all the success of TRAIL targeted cancer therapy, TRAIL resistance is a common hindrance in TRAIL-based therapy that restricts the efficacy of these drugs.81
cFLIP (cellular FLICE-like) inhibitory protein is a crucial anti-apoptotic regulator that suppresses cell death induced by the DRs such as Fas-L, TNF-α and TRAIL.80 cFLIP (27 kDa protein) comprises two DEDs which can inhibit FADD and recruited procaspase-8 interaction by binding to DED of FADD and consequently results in the inactivation of caspase-8. A variety of drugs have been developed to trigger the activation of caspases. Such as apoptin (caspase inducing agent) have been utilized for the activation of caspases-3 and caspase-7. Apoptosis facilitates the execution of apoptosis by causing DNA damage and also aid in the release of cytochrome c from mitochondria.83,84 Moreover, targeting caspase-8 can result in therapeutic effect by utilizing decitabine (5-aza-2´deoxycytidine) which is a cytosine nucleoside analog and endorse demethylation by constraining DNA methyltransferase covalent binding especially in tumors suffering from hypermethylation of caspase-8 promotors, thus restoring the expression of caspase-8.85 Also, gene therapy to induce caspase based apoptosis has adopted by utilizing the genes that encode for inducer, mediator or executioner of apoptosis and also through suppressing the anti-apoptotic gene expression. Selective gene delivery, particular gene expression, and genetic modification by secreting target proteins are some of the strategies adopted to date in apoptosis-based cancer gene therapy.86 The three isoforms of cFLIP in humans include c-FLIPL (long), c-FLIPS (short), and c-FLIPR (splice).60 Generally, higher concentration or enhanced expression of c-FLIPS and c-FLIPL results in the anti-apoptotic function of cFLIP.87 Increased expression of cFLIP observed in different types of cancer leading to enhanced cancer progression.88 Similarly, the reduced expression or down-regulation of cFLIP can inhibit the proliferation of cancer cells and aid in the induction of apoptosis mediated by the DRs and the intrinsic mitochondrial pathway.89 Thus, cFLIP can play an essential role in cancer therapy, specifically if used with TRAIL or conventional chemotherapy.63 Moreover, even though c-FLIPL can perform a dual role in apoptosis (pro- and anti-apoptotic), typically the primary function of cFLIPL have been recognized as an anti-apoptotic regulator of apoptosis in cancer.90
Various approaches that have taken to reduce or suppress the anti-apoptotic function of cFLIP involve the use of siRNA (small interfering RNA), use of many small molecules and agents that down-regulate cFLIP.91 Specifically, siRNA inhibits the expression of c-FLIP and prepare cancer cells to be receptive or sensitize for TRAIL, FASL, and chemotherapeutic agents that induce apoptosis. However, the use of siRNA in vivo involves some restrictions. Besides, the employment of siRNA to inhibit cFLIP depends on the safe delivery of siRNA.92 Many small molecules have been utilized to reduce mRNA and protein intensities of c-FLIPL, however, due to the significantly ordinary homology of cFLIP and caspase-8, use of small molecules for the inhibition of its activity is very challenging.88 Other than utilizing small molecules, different classes of agents have recognized that down-regulate c-FLIP expression by affecting cFLIP transcription, translation and degrading cFLIP.91,93 These agents include conventional chemotherapeutic drugs, DNA damaging agents and HDAC inhibitors. The conventional chemotherapeutic drugs and DNA damaging agents are cisplatin, doxorubicin, camptothecin, 9-nitrocamptothecin, and oxaliplatin. HDAC inhibitors include SAHA and the inhibitors of MEK1/2, PKC, and PI3K.31,86 DR agonists represent an effective therapeutics that mainly target apoptosis. Further, clinical trials of these agents showed the safety of the approach and apoptotic cell death. Forthcoming data from recent trials will also help to demonstrate their clinical activity in different tumor types alone and combinations. Although understanding the mechanism of the TRAIL pathway, studying various factors that might halt response, win over the mechanisms of tumor-cell resistance, and get benefit from these therapies.
Therapeutic targets for targeting anti-apoptotic protein of Bcl-2 family: intrinsic pathway
Bcl-2 family proteins that comprise of pro- and anti-apoptotic proteins are known to play an essential role in the regulation of intrinsic pathway of apoptosis.94 The categorization of Bcl-2 family proteins based on the existence of shared blocks of sequence homology, named as Bcl-2 homology (BH). The equilibrium between pro- and anti-apoptotic Bcl-2 family proteins is an essential element for the initiation of mitochondrial outer membrane permeabilization (MOMP).95 Bcl-2, Bcl-XL, and Mcl-1 are anti-apoptotic proteins and their role is to prevent the release of cytochrome c and maintain mitochondrial integrity while Bax, Bak, Bad, and Bok are pro-apoptotic proteins of Bcl-2 family which allow the release of cytochrome c from the mitochondrial intermembrane space into the cytosol to promote the induction of apoptosis eventually aid in cancer therapeutics.51,52 Up-regulation of pro-apoptotic Bcl-2 proteins and down-regulation of anti-apoptotic Bcl-2 effectively linked to the mechanism of cell death. For example, the ratio between pr-apoptotic (Bax) and anti-apoptotic (Bcl-2) proteins is generally used to determine the fate of the cell.96 Inactivation of pro-apoptotic proteins with multidomain (Bax and Bak) is a crucial feature of carcinogenesis.97 Similarly, elevated levels of anti-apoptotic proteins multidomain (BCL-2, BCL-xL, BCL-w, Bfl-1, and Mcl-1) encourage the deregulation of apoptosis in cancer cells and also aid cancer cells to become resistant to immune-surveillance.98 However, single proapoptotic domain BH3, i.e., BID, BIM, BAD, PUMA (p53 upregulated controller of apoptosis) and NOXA play a primary role in regulating and triggering apoptosis act as sensitizer and serve as an excellent therapeutic target. The overexpression of anti-apoptotic Bcl-2 family members or underexpression of pro-apoptotic Bcl-2 family members usually associated with chemoresistance. Further, BCL-2 over-expression has found in acute myeloid leukemia, chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma (NHL), myeloma, melanoma and hepatocellular, lung, breast, prostate carcinomas.99-101
Potential therapeutic agents with improved efficacy have been developed to target the down-regulation of anti-apoptotic and up-regulating pro-apoptotic Bcl-2 protein.102 Effective strategies have been adopted to inhibit the anti-apoptotic effects of Bcl-2 family, which include: using antisense oligonucleotides, development of small drug molecules and inhibit the gene transcription.96 An example of novel Bcl-2 antisense is Oblimersen Sodium (G3139, Genasense), 18-base antisense phosphorothioate oligonucleotide used in I and II phase of clinical trials in advance solid cancer lymphoma.103,104 It has also tested in combination with other anticancer agents, such as Oblimersen with rituximab used for NHL in II phase, Oblimersen with dacarbazine for myeloma in III phase, Oblimersen with docetaxel for castration-resistant prostate cancer (CRPC) and breast cancer in II and I phase, non-small-cell lung carcinoma (NSCLC) or small-cell lung carcinoma (SCLC) in III phase and HRPCa (EORTC) in II phase.105-109
HDACs are attractive therapeutic targets in cancer and inflammatory diseases.110 A significant controllers of gene expression work enzymatically in removing the acetyl group from histones proteins.111-113 Genetic knock-down has been shown the role of HDACs induce apoptosis and cell cycle arrest in different tumor types, such as colon, lung, breast carcinomas and acute promyelocytic leukemia, highlighting its activity as a critical indicator of survival in cancer cells.114 Further, over-expression of HDACs has been linked to various critical events of tumorigenesis, includes epigenetic repression of CDKN1A (encoding the cyclin-dependent kinase inhibitor p21) tumor suppressor gene and essential genes, like breast cancer 1, early onset BRCA1 and ataxia telangiectasia and Rad 3 related (ATR).94,95 The Sodium butyrate is small molecule Inhibitors (HDAC inhibitor) involved in gene expression alteration in regulation of proapoptotic proteins. Another molecule Flavopiridol (cyclin-dependent kinase inhibitor) and are used to down-regulate the Bcl-2 and Bcl-xl, Mcl-1 expression respectively. Also, Fenretinide, which is a synthetic cytotoxic retinoid, acts by down-regulating the activity of Bcl-2 and Mcl-1 without altering the expression of pro-apoptotic protein Bax.40 Many HDACi have entered phase I to III clinical trials such as, CHR-3996 used for a Refractory solid tumor in phase I.115 Another inhibitory agent Panobinostat (LBH589) used for Relapsed or refractory NHL and advanced solid tumors and Panobinostat (LBH589) along with melphalan for Relapsed or multiple refractory myelomas in I and II phase.116-118
Natural and synthetic small-molecules BH3-mimicking agents have successfully antagonized antiapoptotic Bcl-2 protein family members, such as Obatoclax, Gossypol, ATB-263 and ATB-199.119-122 All BH3 proteins composed of the single domain called α-helical BH3 domain has been demonstrated to play a crucial role in cancer therapy.123-125 The BH-3 mimetics have developed which precisely bind to the hydrophobic groove (which facilitate the binding between pro- and anti-apoptotic proteins), and by this means they oppose the function of anti-apoptotic Bcl-2 family proteins.95 Bcl-2 antisense causes the down-regulation of Bcl-2 proteins by affecting the corresponding mRNA. Potential BH-3 mimetic drugs such as, Obatoclax Mesylate (GX15- 070MS) for SCLC and myelofibrosis and Gossypol/ AT-101 for Metastatic breast cancer and CRPC in I & II phase which inhibits Bcl-2, Bcl-xL, and Mcl- 1 expression. ATB-263 and ATB-199 inhibit/block Bcl-2 for Advanced hematological cancers and CLL 73,74 and MIM1 which inhibits Mcl-1 in clinical trials.126
Therapeutic agents for targeting the Tumor suppressor protein: p53
Tumor suppressor gene p53 is the primary entities involved in carcinogenesis plays an essential part in cancer concerning both cell cycle arrest, and apoptosis.127 Tumor suppressor genes are responsible for controlling DNA repair and cell division. dysfunctional tumor suppressor genes could result in uncontrolled multiplication of cells leading to cancer. Many aspects like chemicals, ionizing radiation, and viruses can cause alterations in proto-oncogenes and tumor suppressor genes.127-129 The expression of p53 is deficient in normal cells under non-stressed conditions. However, p53 can be activated by any stress stimuli; DNA damage or in the response of oncogene activation. Extra and intracellular stress signals change latent p53 to an active form and encourage p53 to accumulate in a cell nucleus. The stability of activated p53 regulated through various post-translational chemical modifications like phosphorylation, acetylation, and methylation.130,131 The fundamental role of p53 is its capability to induce apoptosis by transcription-dependent and transcription-independent manner. p53 performs its function by transcription activation of pro-apoptotic Bcl-2 family proteins and transcription suppression of anti-apoptotic Bcl-2 family proteins. Moreover, it can directly interact with Bax that successively stimulates the release of cyt C via MOMP and aid in the induction of apoptosis.132
Different small molecules MDM2 inhibitors that have been developed to trigger wild-type p53 activity, such as Nultlin-3, MI-219, and RITA. The role of Nultlin-3 and MI-219 is to prevent the interaction of MDM2 and p53, activating p53 signaling and suppressing the tumor growth.133-135 However, the pharmacological action of Nutlin-3 is via both the transcription-dependent and - independent p53 apoptotic pathways.32,136,137 Nutlin-3 has been shown to induce mitotic arrest rather than apoptosis mainly.138 MDM2 can also trigger, in response to low genotoxic damage, the downregulation of p53 apoptotic activator HIPK2.139 Interestingly, Nutlin-3 along with zinc ion inhibit the MDM2 ligase activity favoring HIPK2 stabilization results in an induction of p53 apoptotic activity.140 Similarly, co-treatment of Nutlin-3 and (ABT-737) Bcl-2 inhibitor has been shown to enhance the sensitivity to apoptosis of cancer cells greatly.141 Further, multi-target anticancer approach, inhibition of both MDM2 and Bcl-2 could be a positive tool in cancer treatment.142 Another reported small molecule MDM2 inhibitor is CP-31398, which increases the transcriptional activity of p53 in cells.143 Furthermore, three different types of p53 vaccines such as peptide-based vaccines, dendritic-cell based vaccines, and recombinant virus-based vaccines are undergoing clinical trials to assist in the induction of antitumor immune responses.144-146 Another research reveals a class of small molecules that reactivates the wild-type function of mutant p53 in so doing permit p53 to induce apoptotic cell death. PRIMA-1 and its analog APR-246 are examples of this class of small molecules and are undergoing preclinical and clinical trials (phase I) to functions as reactivating mutant p53.147
Therapeutic agents for targeting Inhibitory apoptosis Proteins: IAPs
The IAPs is family of protein which functions as endogenous inhibitors of apoptosis. Elevated expression levels of IAPs were significantly resulting in improved cell survival, increased tumor growth and consequent metastasis. IAPs targeting strategy has become increasingly attractive to sensitize cancer cells towards various therapeutics such as chemotherapies, antibody based-therapies. Besides apoptosis, IAPs observed to play a part in necroptosis, immune regulation, chromosomal and cytoplasmic division.148 IAPs can inhibit both intrinsic and extrinsic pathway of apoptosis. The execution of DR-mediated extrinsic pathway and mitochondrial triggered a family of structurally diverse IAPs modulates intrinsic pathway; X-linked (XIAP), cellular (cIAP1, cIAP2), neuronal (NIAP), testis-specific (Ts-IAP), Bir-ubiquitin conjugating enzyme (BRUCE), Survivin and Livin. Structurally, IAPs are approximately 70 amino acids long and contain zinc finger BIR (Baculovirus IAP Repeat) domains that are responsible for deregulation properties of IAPs where they prevent the conversion of zymogenic (inactive) pro-caspases to active caspases.149,150 Over-expression of IAPs linked to increased chemo-resistance in several types of cancer.151,152 As a controlled expression of IAPs could encourage apoptotic cell death, different strategies have been adopted to inhibit IAPs, and these include: anti-sense facilitated interference of XIAP and survivin oligonucleotides and siRNA expression and inhibition of IAPs by SMAC mimetic compounds.153-155 XIAP inhibits both the extrinsic and intrinsic apoptotic pathways via direct inhibition of enzymes caspases and may be limited by its initiation of cell protective effects via NF-kB signaling and cIAP1/2 through proteasomal degradation or ubiquitination.156 Knockout strategies in cancer cells were highlighting their role in resistance to various anti-cancer therapies. For example, increased apoptosis suppressed tumorigenicity and re-sensitized was reported ovarian cancer cells to cisplatin therapy and in nude mice through shRNA mediated knockdown of XIAP.157 The successful results in acute myeloid leukemia patients undergoing therapy using antisense oligonucleotide AEG35156 that target XIAP in phase II trials.158,159 However, despite this initial success and confirmed on-target knockdown,157 a later trial failed to report a similarly improved outcome in patients with advanced pancreatic cancer.160 while gene silencing is an attractive prospect, its potential clinical relevance is limited by lower knockdown efficiency in patient samples, compared to those demonstrated in cell culture.158 and by the transient nature of XIAP repression.160 Still, strategies for RNAi remain important tools to dissect the mechanistic and functional role of IAPs in cancer.
SMAC mimetics release into the cytosol as a result of MOMP binds to the BIR domain cellular IAPs (cIAP1 and cIAP2) and XIAP, restoring the function of effector caspases by blocking inhibitory role of IAPs.161 To date, some inhibitors of IAP proteins have been developed these include: SH122, SH130, SM164, AZD5582, JP1201, AEG35156, LY2181308 and YM155.151 SMAC mimetic SH130 and SH122 target human prostate cancer cell line by inhibiting IAPs.162,163 AZD5582 and JP1201 are SMAC mimetics which target CLL and pancreatic cancer cell line respectively to enhance apoptosis by TRAIL.164,165 YM155 suppresses survivin expression and induce apoptosis in human cancer cell lines.166,167 AEG35156 and LY2181308 are antisense oligonucleotides and small siRNA molecules which targets survivin expression and also down-regulate XIAP.168,169 Various past researches demonstrated that SMAC mimetics, along with anticancer drugs and TRAIL remarkably enhance apoptotic cell death in several cancer cell types in vitro, such as T98G glioblastoma cells, HeLa cells, and lung adenocarcinomas.170-172 Further, the SMAC mimetics role as sensitizer enhance the sensitivity of various agents, such as paclitaxel, etoposide, and doxorubicin in MCF-7 breast cancer cells.173 Various apoptotic pathways targeted strategies as shown in Figure 5, such as DRs, antiapoptotic Bcl-2 family, IAPs, caspases and p53 need intense focus in development of effective drug therapy. Studies suggested that IAPs may be useful as single agents in cancer also usefulness antagonists in combination with alternative cancer drugs. As further research progress, the better improvements in understanding of therapeutic design which may enhance DR, Bcl-2, IAP and p53 mediated cell death in single as well as combined treatment.
Figure 5.
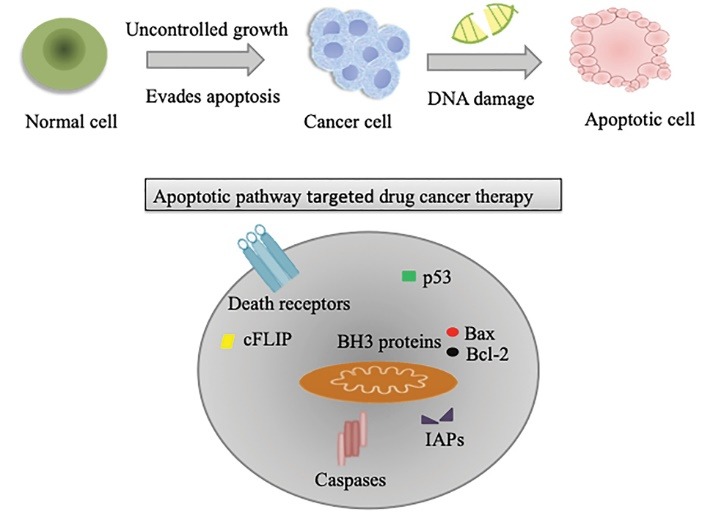
Apoptotic pathway targeted drug therapy Apoptotic pathways targeted strategies such as death receptors, antiapoptotic Bcl-2 family, IAPs, caspases and p53 showed positive results in previous studies need to be investigate more to get effective therapeutics.
Conclusion
Targeting apoptosis is a new standard in cancer drug development: a significant regulatory mechanism inside cells, a cellular process that is tightly regulated by various membrane-bound and freely available cytoskeleton molecules. Apoptosis is a fundamental regulatory mechanism of normal cells, any dysregulation in apoptosis could trigger the uncontrol multiplication of cells. This regulatory process characterized by a series of specific morphological changes along with biochemical features which involve extrinsic and intrinsic pathways via a different protein that plays a crucial role overall. Therefore, a detailed mechanistic understanding of the apoptotic signaling pathways required for the development of effective cancer therapeutics. The up-regulation of apoptotic pathways via activation of pro-apoptotic pathway proteins (initiator and effector caspases, box, bak, bad and bok along with inhibition of cFLIP and sensitize DRs to trigger apoptotic pathway). The understanding of apoptotic pathways needs intense effort for the development of new approaches to drug discovery and therapy. However, some apoptotic pathways proteins which induce apoptosis selected as a target for drugs are in clinical trials. Positive results of antibodies along with recombinant TRAIL specifically target the DRs in clinical trials against a range of solid tumors. However, much understanding of evading apoptosis in cancer cells is needed to get the positive results in maturing clinical data. The novel agents along with combined apoptotic inhibitors strategy show significant synergistic effects and being in the current study.
Ethical Issue
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV. et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L. et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14(7):1237–43. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 3. Hakem R, Harrington L. Cell death. 4th ed. New York: The McGraw-Hill Companies Inc; 2005.
- 4.Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25(1):82–8. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Programmed cell death (apoptosis) - Molecular biology of the cell. 4th ed. New York: Garland Science; 2002.
- 7.Gorski S, Marra M. Programmed cell death takes flight: genetic and genomic approaches to gene discovery in Drosophila. Physiol Genomics. 2002;9(2):59–69. doi: 10.1152/physiolgenomics.00114.2001. [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc AC. Natural cellular inhibitors of caspases. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):215–29. doi: 10.1016/s0278-5846(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8(2):119–30. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 10.Neuman MG, Katz GG, Malkiewicz IM, Mathurin P, Tsukamoto H, Adachi M. et al. Alcoholic liver injury and apoptosis--synopsis of the symposium held at ESBRA 2001: 8th Congress of the European Society for Biomedical Research on Alcoholism, Paris, September 16, 2001. Alcohol. 2002;28(2):117–28. doi: 10.1016/S0741-8329(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 11.Duckett CS, Li F, Wang Y, Tomaselli KJ, Thompson CB, Armstrong RC. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18(1):608–15. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venderova K, Park DS. Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–59. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Proskuryakov SY, Gabai VL, Konoplyannikov AG. Necrosis is an active and controlled form of programmed cell death. Biochemistry (Mosc) 2002;67(4):387–408. doi: 10.1023/a:1015289521275. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19(4):555–66. doi: 10.1007/s10495-014-0967-2. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry G, Islamiah M, Ismail N, Mohamad H, Sung YY, Muhammad TST. Induction of apoptosis by aaptos sp, fractions in human breast cancer cell line, mcf-7. Int J Res Pharm Sci. 2018;9(2):328–37. doi: 10.26452/ijrps.v9i2.1469. [DOI] [Google Scholar]
- 20.Hudayah T, Chaudhry G, Taib M, Ismail N, Mohammad TST. Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7. Int J Res Pharm Sci. 2017;8(4):667–75. [Google Scholar]
- 21.Szende B, Keri G, Szegedi Z, Benedeczky I, Csikos A, Orfi L. et al. Tyrphostin induces non-apoptotic programmed cell death in colon tumor cells. Cell Biol Int. 1995;19(11):903–11. doi: 10.1006/cbir.1995.1028. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 23.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najafov A, Chen H, Yuan J. Necroptosis and Cancer. Trends Cancer. 2017;3(4):294–301. doi: 10.1016/j.trecan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135(7):1161–3. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kaczanowski S, Sajid M, Reece SE. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit Vectors. 2011;4:44. doi: 10.1186/1756-3305-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artal-Sanz M, Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579(15):3287–96. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B. et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proskuryakov SY, Gabai VL, Konoplyannikov AG. Necrosis is an active and controlled form of programmed cell death. Biochemistry (Mosc) 2002;67(4):387–408. doi: 10.1023/a:1015289521275. [DOI] [PubMed] [Google Scholar]
- 30.Lotze MT, Demarco RA. Dying dangerously: Necrotic cell death and chronic inflammation promote tumor growth. Discov Med. 2004;4(24):448–56. [PubMed] [Google Scholar]
- 31.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119(1):3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- 33.Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2009;1(2):1–4. doi: 10.4172/1948-5956.100000e2. [DOI] [PubMed] [Google Scholar]
- 34.Nithya M, Ambikapathy V, Panneerselvam A, Thajuddin N. Anti-tumour activity of different extracts of Ganoderma lucidum (curt: fr) p karst. World J Pharm Res. 2014;3(4):2204–14. [Google Scholar]
- 35.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–36. [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb) 2011;3(4):279–96. doi: 10.1039/c0ib00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onyeagucha B, Subbarayalu P, Abdelfattah N, Rajamanickam S, Timilsina S, Guzman R. et al. Novel post-transcriptional and post-translational regulation of pro-apoptotic protein BOK and anti-apoptotic protein Mcl-1 determine the fate of breast cancer cells to survive or die. Oncotarget. 2017;8(49):85984–96. doi: 10.18632/oncotarget.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26(2):263–70. doi: 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- 40.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16(6):2129–44. doi: 10.7314/APJCP.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 41.Sayers TJ. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol Immunother. 2011;60(8):1173–80. doi: 10.1007/s00262-011-1008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiuti A, Bachoud-Levi AC, Blesch A, Brenner MK, Cattaneo F, Chiocca EA. et al. Progress and prospects: gene therapy clinical trials (part 2) Gene Ther. 2007;14(22):1555–63. doi: 10.1038/sj.gt.3303033. [DOI] [PubMed] [Google Scholar]
- 43.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–63. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 44.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH. et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16(10):2794–804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Los M, Van de Craen M, Penning LC, Schenk H, Westendorp M, Baeuerle PA. et al. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995;375(6526):81–3. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 47.Stergiou L, Hengartner MO. Death and more: DNA damage response pathways in the nematode C elegans. Cell Death Differ. 2004;11(1):21–8. doi: 10.1038/sj.cdd.4401340. [DOI] [PubMed] [Google Scholar]
- 48.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M. et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46(8):497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 49.Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76(1):169–75. doi: 10.1189/jlb.0903435. [DOI] [PubMed] [Google Scholar]
- 50.Hashemi M, Karami-Tehrani F, Ghavami S. Cytotoxicity effect of Cladribine on the MCF-7 human breast cancer cell line. Iran Biomed J. 2004;8(1):7–12. [Google Scholar]
- 51.Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103(8):1551–60. doi: 10.1002/cncr.20947. [DOI] [PubMed] [Google Scholar]
- 52.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 53.Yuan S, Akey CW. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21(4):501–15. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sankari SL, Masthan KM, Babu NA, Bhattacharjee T, Elumalai M. Apoptosis in cancer--an update. Asian Pac J Cancer Prev. 2012;13(10):4873–8. doi: 10.7314/apjcp.2012.13.10.4873. [DOI] [PubMed] [Google Scholar]
- 55.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 56.Hu Q, Wu D, Chen W, Yan Z, Shi Y. Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J Biol Chem. 2013;288(21):15142–7. doi: 10.1074/jbc.M112.441568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1(1):19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 58. Cairrao F, Domingos PM. Apoptosis: molecular mechanisms. In: Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons Ltd; 2010.
- 59.Kumar R, Herbert PE, Warrens AN. An introduction to death receptors in apoptosis. Int J Surg. 2005;3(4):268–77. doi: 10.1016/j.ijsu.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Tsuchiya Y, Nakabayashi O, Nakano H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int J Mol Sci. 2015;16(12):30321–41. doi: 10.3390/ijms161226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther. 2008;7(2):163–73. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- 63.Shankar S, Davis R, Singh KP, Kurzrock R, Ross DD, Srivastava RK. Suberoylanilide hydroxamic acid (Zolinza/vorinostat) sensitizes TRAIL-resistant breast cancer cells orthotopically implanted in BALB/c nude mice. Mol Cancer Ther. 2009;8(6):1596–605. doi: 10.1158/1535-7163.mct-08-1004. [DOI] [PubMed] [Google Scholar]
- 64.Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med. 2005;16(6):1125–38. doi: 10.3892/ijmm.16.6.1125. [DOI] [PubMed] [Google Scholar]
- 65.Guo N, Peng Z. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol. 2013;9(1):6–11. doi: 10.1111/j.1743-7563.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Yue P, Chen S, Hu L, Lonial S, Khuri FR. et al. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 2007;67(10):4981–8. doi: 10.1158/0008-5472.can-06-4274. [DOI] [PubMed] [Google Scholar]
- 67.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10(1):66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 68.Khan KH, Blanco-Codesido M, Molife LR. Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev Oncol Hematol. 2014;90(3):200–19. doi: 10.1016/j.critrevonc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA. et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–62. doi: 10.1172/jci6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T. et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7(8):954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 71.Yee L, Burris HA, Kozloff M, Wainberg Z, Pao M, Skettino S. et al. Phase Ib study of recombinant human Apo2L/TRAIL plus irinotecan and cetuximab or FOLFIRI in metastatic colorectal cancer (mCRC) patients (pts): preliminary results. J Clin Oncol. 2009;27(15 Suppl):4129. doi: 10.1200/jco.2009.27.15s.4129. [DOI] [Google Scholar]
- 72.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E. et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(33):4442–51. doi: 10.1200/jco.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 73.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A. et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14(11):3450–5. doi: 10.1158/1078-0432.ccr-07-1416. [DOI] [PubMed] [Google Scholar]
- 74.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W. et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61(1):82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 75.Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C. et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer. 2010;102(3):506–12. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camidge DR. Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use in the treatment of solid tumors. Expert Opin Biol Ther. 2008;8(8):1167–76. doi: 10.1517/14712598.8.8.1167. [DOI] [PubMed] [Google Scholar]
- 77.Pacey S, Plummer R, Attard G, Bale C, Calvert A, Blagden S. et al. Phase I and pharmacokinetic study of HGS-ETR2, a human monoclonal antibody to TRAIL R2, in patients with advanced solid malignancies. J Clin Oncol. 2005;23(16):3055. [Google Scholar]
- 78.LoRusso P, Hong D, Heath E, Kurzrock R, Wang D, Hsu M. et al. First-in-human study of AMG 655, a pro-apoptotic TRAIL receptor-2 agonist, in adult patients with advanced solid tumors. J Clin Oncol. 2007;25(18):3534. [Google Scholar]
- 79.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L. et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16(23):5883–91. doi: 10.1158/1078-0432.ccr-10-0631. [DOI] [PubMed] [Google Scholar]
- 80.Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2013;32(11):1341–50. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R. et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21(2):376–81. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34(3):176–84. [PMC free article] [PubMed] [Google Scholar]
- 83.Burek M, Maddika S, Burek CJ, Daniel PT, Schulze-Osthoff K, Los M. Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene. 2006;25(15):2213–22. doi: 10.1038/sj.onc.1209258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rohn JL, Noteborn MH. The viral death effector Apoptin reveals tumor-specific processes. Apoptosis. 2004;9(3):315–22. doi: 10.1023/B:APPT.0000025808.48885.9c. [DOI] [PubMed] [Google Scholar]
- 85.Hensley P, Mishra M, Kyprianou N. Targeting caspases in cancer therapeutics. Biol Chem. 2013;394(7):831–43. doi: 10.1515/hsz-2013-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia LT, Chen SY, Yang AG. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat Rev. 2012;38(7):868–76. doi: 10.1016/j.ctrv.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19(1):36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Safa AR. Roles of c-FLIP in apoptosis, necroptosis, and autophagy. J Carcinog Mutagen. 2013;Suppl 6 doi: 10.4172/2157-2518.s6-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285(15):11498–507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42(2):210–3. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 91.Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel) 2011;3(2):1639–71. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang JK. FLIP as an anti-cancer therapeutic target. Yonsei Med J. 2008;49(1):19–27. doi: 10.3349/ymj.2008.49.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 95.Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23(12):620–33. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naseri MH, Mahdavi M, Davoodi J, Tackallou SH, Goudarzvand M, Neishabouri SH. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015;15:55. doi: 10.1186/s12935-015-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ierano C, Chakraborty AR, Nicolae A, Bahr JC, Zhan Z, Pittaluga S. et al. Loss of the proteins Bak and Bax prevents apoptosis mediated by histone deacetylase inhibitors. Cell Cycle. 2013;12(17):2829–38. doi: 10.4161/cc.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S. et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17(1):61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97(4):1754–9. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wissink EH, Verbrugge I, Vink SR, Schader MB, Schaefer U, Walczak H. et al. TRAIL enhances efficacy of radiotherapy in a p53 mutant, Bcl-2 overexpressing lymphoid malignancy. Radiother Oncol. 2006;80(2):214–22. doi: 10.1016/j.radonc.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 101.Belka C, Schmid B, Marini P, Durand E, Rudner J, Faltin H. et al. Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene. 2001;20(17):2190–6. doi: 10.1038/sj.onc.1204318. [DOI] [PubMed] [Google Scholar]
- 102.Karami H, Baradaran B, Esfahani A, Asghari Estiar M, Naghavi-Behzad M, Sakhinia M. et al. siRNA-mediated silencing of survivin inhibits proliferation and enhances etoposide chemosensitivity in acute myeloid leukemia cells. Asian Pac J Cancer Prev. 2013;14(12):7719–24. doi: 10.7314/APJCP.2013.14.12.7719. [DOI] [PubMed] [Google Scholar]
- 103.Khan KH, Blanco-Codesido M, Molife LR. Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev Oncol Hematol. 2014;90(3):200–19. doi: 10.1016/j.critrevonc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Brinkmann K, Kashkar H. Targeting the mitochondrial apoptotic pathway: a preferred approach in hematologic malignancies? Cell Death Dis. 2014;5:e1098. doi: 10.1038/cddis.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F. et al. Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. Br J Haematol. 2008;143(3):355–60. doi: 10.1111/j.1365-2141.2008.07353.x. [DOI] [PubMed] [Google Scholar]
- 106.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U. et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–45. doi: 10.1200/jco.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 107.Tolcher AW, Chi K, Kuhn J, Gleave M, Patnaik A, Takimoto C. et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11(10):3854–61. doi: 10.1158/1078-0432.ccr-04-2145. [DOI] [PubMed] [Google Scholar]
- 108.Marshall J, Chen H, Yang D, Figueira M, Bouker KB, Ling Y. et al. A phase I trial of a Bcl-2 antisense (G3139) and weekly docetaxel in patients with advanced breast cancer and other solid tumors. Ann Oncol. 2004;15(8):1274–83. doi: 10.1093/annonc/mdh317. [DOI] [PubMed] [Google Scholar]
- 109.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15(4):1126–32. doi: 10.1158/1078-0432.ccr-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26(37):5420–32. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 111.Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase inhibitors and genomic instability. Cancer Lett. 2009;274(2):169–76. doi: 10.1016/j.canlet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Hrabeta J, Stiborova M, Adam V, Kizek R, Eckschlager T. Histone deacetylase inhibitors in cancer therapy A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(2):161–9. doi: 10.5507/bp.2013.085. [DOI] [PubMed] [Google Scholar]
- 113.Becher I, Dittmann A, Savitski MM, Hopf C, Drewes G, Bantscheff M. Chemoproteomics reveals time-dependent binding of histone deacetylase inhibitors to endogenous repressor complexes. ACS Chem Biol. 2014;9(8):1736–46. doi: 10.1021/cb500235n. [DOI] [PubMed] [Google Scholar]
- 114.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–9. doi: 10.1172/jci69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banerji U, van Doorn L, Papadatos-Pastos D, Kristeleit R, Debnam P, Tall M. et al. A phase I pharmacokinetic and pharmacodynamic study of CHR-3996, an oral class I selective histone deacetylase inhibitor in refractory solid tumors. Clin Cancer Res. 2012;18(9):2687–94. doi: 10.1158/1078-0432.ccr-11-3165. [DOI] [PubMed] [Google Scholar]
- 116.Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM. et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30(18):2197–203. doi: 10.1200/jco.2011.38.1350. [DOI] [PubMed] [Google Scholar]
- 117.Fukutomi A, Hatake K, Matsui K, Sakajiri S, Hirashima T, Tanii H. et al. A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30(3):1096–106. doi: 10.1007/s10637-011-9666-9. [DOI] [PubMed] [Google Scholar]
- 118.Berenson JR, Hilger JD, Yellin O, Boccia RV, Matous J, Dressler K. et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann Hematol. 2014;93(1):89–98. doi: 10.1007/s00277-013-1910-2. [DOI] [PubMed] [Google Scholar]
- 119.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N. et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74(3):481–5. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15(9):3172–6. doi: 10.1158/1078-0432.ccr-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP. et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–59. doi: 10.1016/s1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–96. doi: 10.1200/jco.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vaux DL. Cell Death and Cancer. In: Wu H, eds. Cell Death. New York, NY: Springer; 2014. p. 121-34.
- 124.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL. et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44(4):517–31. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA. et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186(3):355–62. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohen NA, Stewart ML, Gavathiotis E, Tepper JL, Bruekner SR, Koss B. et al. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem Biol. 2012;19(9):1175–86. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rice H, Bryant S, Handley C, Hall M. Oncogenes and tumor suppressor genes: An essential building block of cancer. Chemist. 2014;87(2):15–8. [Google Scholar]
- 128.Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358(5):502–11. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 129.Rosai J, Ackerman LV. The pathology of tumors, part III: grading, staging & classification. CA Cancer J Clin. 1979;29(2):66–77. doi: 10.3322/canjclin.29.2.66. [DOI] [PubMed] [Google Scholar]
- 130.Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011;3(1):994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017;429(11):1595–606. doi: 10.1016/j.jmb.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chi SW. Structural insights into the transcription-independent apoptotic pathway of p53. BMB Rep. 2014;47(3):167–72. doi: 10.5483/BMBRep.2014.47.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 134.Azmi AS, Philip PA, Beck FW, Wang Z, Banerjee S, Wang S. et al. MI-219-zinc combination: a new paradigm in MDM2 inhibitor-based therapy. Oncogene. 2011;30(1):117–26. doi: 10.1038/onc.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M. et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–8. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 136.Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108(3):993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787(5):414–20. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rinaldo C, Prodosmo A, Siepi F, Moncada A, Sacchi A, Selivanova G. et al. HIPK2 regulation by MDM2 determines tumor cell response to the p53-reactivating drugs nutlin-3 and RITA. Cancer Res. 2009;69(15):6241–8. doi: 10.1158/0008-5472.can-09-0337. [DOI] [PubMed] [Google Scholar]
- 139.Rinaldo C, Prodosmo A, Mancini F, Iacovelli S, Sacchi A, Moretti F. et al. MDM2-regulated degradation of HIPK2 prevents p53Ser46 phosphorylation and DNA damage-induced apoptosis. Mol Cell. 2007;25(5):739–50. doi: 10.1016/j.molcel.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 140.Nardinocchi L, Puca R, Givol D, D’Orazi G. Counteracting MDM2-induced HIPK2 downregulation restores HIPK2/p53 apoptotic signaling in cancer cells. FEBS Lett. 2010;584(19):4253–8. doi: 10.1016/j.febslet.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 141.Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5(23):2778–86. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 142.Wade M, Rodewald LW, Espinosa JM, Wahl GM. BH3 activation blocks Hdmx suppression of apoptosis and cooperates with Nutlin to induce cell death. Cell Cycle. 2008;7(13):1973–82. doi: 10.4161/cc.7.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang W, Takimoto R, Rastinejad F, El-Deiry WS. Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding. Mol Cell Biol. 2003;23(6):2171–81. doi: 10.1128/mcb.23.6.2171-2181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Speetjens FM, Kuppen PJ, Welters MJ, Essahsah F, Voet van den Brink AM , Lantrua MG. et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res. 2009;15(3):1086–95. doi: 10.1158/1078-0432.ccr-08-2227. [DOI] [PubMed] [Google Scholar]
- 145.Svane IM, Pedersen AE, Johnsen HE, Nielsen D, Kamby C, Gaarsdal E. et al. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a phase I study. Cancer Immunol Immunother. 2004;53(7):633–41. doi: 10.1007/s00262-003-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N. et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–87. doi: 10.1158/1078-0432.ccr-05-2013. [DOI] [PubMed] [Google Scholar]
- 147.Liu J, Zhang C, Feng Z. Tumor suppressor p53 and its gain-of-function mutants in cancer. Acta Biochim Biophys Sin (Shanghai) 2014;46(3):170–9. doi: 10.1093/abbs/gmt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Finlay D, Teriete P, Vamos M, Cosford NDP, Vuori K. Inducing death in tumor cells: roles of the inhibitor of apoptosis proteins. F1000Res. 2017;6:587. doi: 10.12688/f1000research.10625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104(5):781–90. doi: 10.1016/S0092-8674(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 150.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388(6639):300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 151.Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016;7:e2058. doi: 10.1038/cddis.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115(10):2673–8. doi: 10.1172/jci26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fulda S. Molecular pathways: targeting inhibitor of apoptosis proteins in cancer--from molecular mechanism to therapeutic application. Clin Cancer Res. 2014;20(2):289–95. doi: 10.1158/1078-0432.ccr-13-0227. [DOI] [PubMed] [Google Scholar]
- 154.Dai DJ, Lu CD, Lai RY, Guo JM, Meng H, Chen WS. et al. Survivin antisense compound inhibits proliferation and promotes apoptosis in liver cancer cells. World J Gastroenterol. 2005;11(2):193–9. doi: 10.3748/wjg.v11.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Karami H, Baradaran B, Esfehani A, Sakhinia M, Sakhinia E. Down-regulation of Mcl-1 by small interference RNA induces apoptosis and sensitizes HL-60 leukemia cells to etoposide. Asian Pac J Cancer Prev. 2014;15(2):629–35. doi: 10.7314/APJCP.2014.15.2.629. [DOI] [PubMed] [Google Scholar]
- 156.Lau R, Pratt MA. The opposing roles of cellular inhibitor of apoptosis proteins in cancer. ISRN Oncol. 2012;2012:928120. doi: 10.5402/2012/928120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma JJ, Chen BL, Xin XY. XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):222–6. doi: 10.1016/j.ejogrb.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 158.Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS. et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27(28):4741–6. doi: 10.1200/jco.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carter BZ, Mak DH, Morris SJ, Borthakur G, Estey E, Byrd AL. et al. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38- cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis. 2011;16(1):67–74. doi: 10.1007/s10495-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mahadevan D, Chalasani P, Rensvold D, Kurtin S, Pretzinger C, Jolivet J. et al. Phase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. Am J Clin Oncol. 2013;36(3):239–43. doi: 10.1097/COC.0b013e3182467a13. [DOI] [PubMed] [Google Scholar]
- 161.Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21(9):1350–64. doi: 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dai Y, Liu M, Tang W, Li Y, Lian J, Lawrence TS. et al. A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai Y, Liu M, Tang W, DeSano J, Burstein E, Davis M. et al. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res. 2008;14(23):7701–10. doi: 10.1158/1078-0432.ccr-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhuang J, Laing N, Oates M, Lin K, Johnson G, Pettitt AR. Selective IAP inhibition results in sensitization of unstimulated but not CD40-stimulated chronic lymphocytic leukaemia cells to TRAIL-induced apoptosis. Pharmacol Res Perspect. 2014;2(6):e00081. doi: 10.1002/prp2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P. et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–61. doi: 10.1158/0008-5472.can-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y. et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15(11):3872–80. doi: 10.1158/1078-0432.ccr-08-1946. [DOI] [PubMed] [Google Scholar]
- 167.Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E. et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(10):2601–6. doi: 10.1093/annonc/mdt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.LaCasse EC. Pulling the plug on a cancer cell by eliminating XIAP with AEG35156. Cancer Lett. 2013;332(2):215–24. doi: 10.1016/j.canlet.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 169.Rodel F, Frey B, Leitmann W, Capalbo G, Weiss C, Rodel C. Survivin antisense oligonucleotides effectively radiosensitize colorectal cancer cells in both tissue culture and murine xenograft models. Int J Radiat Oncol Biol Phys. 2008;71(1):247–55. doi: 10.1016/j.ijrobp.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 170.Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K. et al. Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-kappaB-dependent manner. Oncogene. 2013;32(8):988–97. doi: 10.1038/onc.2012.108. [DOI] [PubMed] [Google Scholar]
- 171.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305(5689):1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 172.Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T. et al. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 2003;63(4):831–7. [PubMed] [Google Scholar]
- 173.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277(46):44236–43. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]