Abstract
The primary purpose of pulmonary ventilation is to supply oxygen (O2) for sustained aerobic respiration in multicellular organisms. However, a plethora of abiotic insults and airborne pathogens present in the environment are occasionally introduced into the airspaces during inhalation, which could be detrimental to the structural integrity and functioning of the respiratory system. Multiple layers of host defense act in concert to eliminate unwanted constituents from the airspaces. In particular, the mucociliary escalator provides an effective mechanism for the continuous removal of inhaled insults including pathogens. Defects in the functioning of the mucociliary escalator compromise the mucociliary clearance (MCC) of inhaled pathogens, which favors microbial lung infection. Defective MCC is often associated with airway mucoobstruction, increased occurrence of respiratory infections, and progressive decrease in lung function in mucoobstructive lung diseases including cystic fibrosis (CF). In this disease, a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene results in dehydration of the airway surface liquid (ASL) layer. Several mice models of Cftr mutation have been developed; however, none of these models recapitulate human CF-like mucoobstructive lung disease. As an alternative, the Scnn1b transgenic (Scnn1b-Tg+) mouse model overexpressing a transgene encoding sodium channel nonvoltage-gated 1, beta subunit (Scnn1b) in airway club cells is available. The Scnn1b-Tg+ mouse model exhibits airway surface liquid (ASL) dehydration, impaired MCC, increased mucus production, and early spontaneous pulmonary bacterial infections. High morbidity and mortality among mucoobstructive disease patients, high economic and health burden, and lack of scientific understanding of the progression of mucoobstruction warrants in-depth investigation of the cause of mucoobstruction in mucoobstructive disease models. In this review, we will summarize published literature on the Scnn1b-Tg+ mouse and analyze various unanswered questions on the initiation and progression of mucobstruction and bacterial infections.
1. Background
Aerobic processes within a cell consume oxygen (O2) and release carbon dioxide (CO2) during the process of respiration. Pulmonary ventilation is responsible for supplying O2 to and eliminating CO2 from cells undergoing aerobic respiration. In addition to oxygen, aberrant constituents of ambient air such as abiotic insults and airborne pathogens are also inhaled into the airspaces [1]. Upon entering the conducting airways, airborne insults are trapped within the airway surface liquid (ASL) layer, a thin layer of hydrated mucus that lines the airway epithelium. The airway epithelial cells are specialized to constitute a mucociliary clearance (MCC) host defense mechanism that facilitates the removal of trapped insults [1]. Ciliated cells move the layer of mucus containing the airborne insults towards the epiglottis, thus away from airspaces [1].
Defects in the functioning of the mucociliary escalator compromise the MCC of inhaled pathogens and abiotic insults, which favors airspace infection and lung injury, respectively [2]. Impaired MCC is also often associated with airway mucoobstruction in mucoobstructive lung disease patients [2]. The cause-effect relationship between these two responses and their effect on microbial infections are unclear. High morbidity and mortality among mucoobstructive disease patients, high economic and health burden, and lack of scientific understanding of the progression of mucoobstruction warrant in-depth investigation of the pathogenesis of mucoobstruction using mucoobstructive disease models [3–5]. In this review, we will focus our discussion on MCC defect in cystic fibrosis (CF) and its recapitulation in a widely accepted mouse model of CF, i.e., Scnn1b-Tg+ mouse.
2. Physiology of ASL Layer
The ASL layer, a thin layer of fluid that lines the luminal surface of the airway epithelium, is comprised of two distinct layers: the mucus layer and the periciliary layer [1]. The mucus layer is a luminal (superficial) layer of ASL that is exposed to the air and traps the airborne insults [6]. Removal of inhaled pathogens and abiotic insults involves unidirectional movement of the mucus layer towards the epiglottis [1]. Located directly underneath the mucus layer, the aqueous periciliary layer bathes the cilia projecting from the airway epithelium and facilitates ciliary beating [2]. The force generated by the ciliary beating within the periciliary layer fuels the movement of the mucus layer towards the epiglottis [1] (Figures 1(a) and 2(a)).
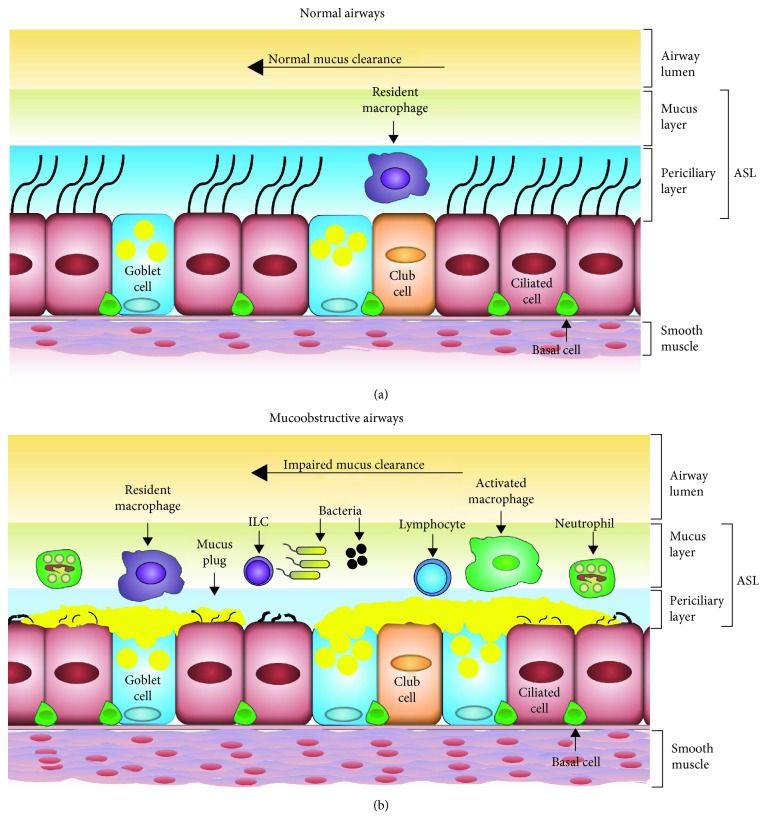
Figure 1.
Diagrammatic comparisons of normal and mucoobstructive airways. (a) In normal airways, the normal functioning of epithelial ion channels maintains a healthy ASL layer. The normal functioning of the mucociliary clearance system efficiently clears aberrant ASL constituents. As a result, the epithelial layer consists of a balanced proportion of various epithelial cell types, including ciliated cells, club cells, and goblet cells. In addition, resident macrophages continue to perform their sentinel roles. (b) In mucoobstructive airways, an ion-channel defect causes ASL dehydration, which leads to mucus hyperconcentration, mucoobstruction, mucous cell metaplasia, bacterial infection, and airway inflammation.
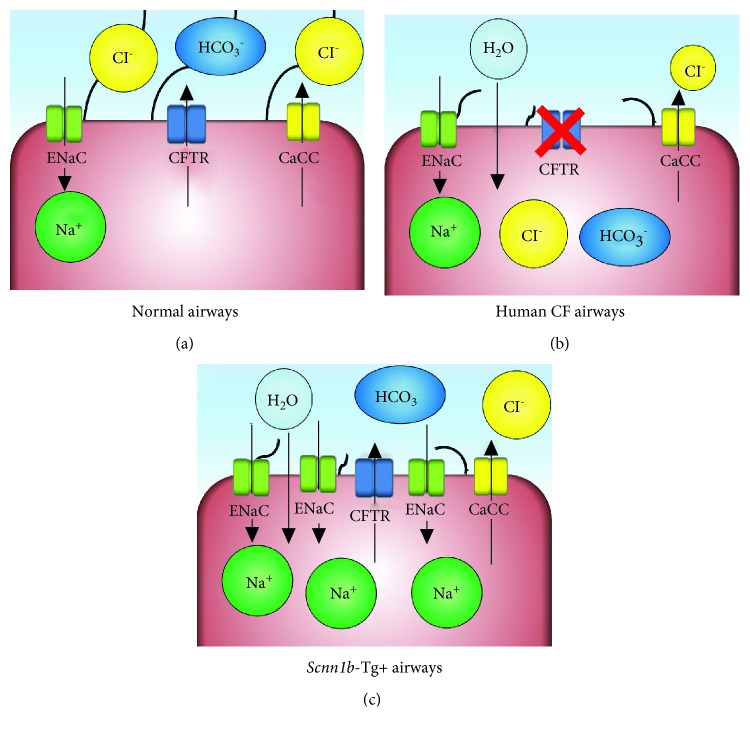
Figure 2.
Ion-channel physiology in airways. (a) The hydration state of normal airways in airway surface liquid (ASL) is regulated by the concerted action of ion channels. Major ion channels responsible for regulating chloride (Cl−)/sodium (Na+) transport are the cystic fibrosis transmembrane conductance regulator (CFTR), calcium-activated chloride channels (CaCCs), and epithelial Na+ channels (ENaC). CFTR and CaCCs are responsible for regulating Cl− transport, while ENaC facilitates epithelial Na+ absorption. CFTR is also responsible for bicarbonate (HCO3−) transport that regulates the local pH of the airways. Balanced ionic transport maintains water contents of ASL in the physiological range. (b) In cystic fibrosis, a dysfunctional CFTR channel results in the net movement of sodium ions into the cytoplasm of airway epithelial cells. The osmotic drive due to sodium hyperabsorption dictates the net movement of ASL water into the cytoplasm of airway epithelial cells. These alterations result in the pathology of CF lung disease. (c) In mice, the chloride ion transport inhibition due to the genetic inactivation of a CFTR channel defect is compensated by relatively more prominent CaCCs. In Scnn1b-Tg+ airways, the overexpression of ENaC results in the hyperabsorption of sodium ion into the cytoplasm of airway epithelial cells, an ionic imbalance defect similar to human CF airways.
The two layers work on the gel-on-brush model in which the large membrane-tethered mucins and mucopolysaccharides of the periciliary layer form a brush-like network of polymers on the epithelial surface [6]. Electron microscopic examination of cultured human bronchial epithelial cells reveals the brush to be a meshwork consisting of large tethered macromolecules, i.e., MUC1, MUC4, MUC16, MUC20, and heparan sulfate, that are attached to the ciliary shaft and epithelial cell surface [6]. These large tethered macromolecules create a semipermeable gradient mesh that becomes tighter near the epithelial surface and is seemingly impenetrable to MUC5B, MUC5AC, and inhaled particles [6]. Button et al. determined that 2 nm dextran particles readily infiltrate the periciliary layer to reach the epithelial surface, while 40 nm particles are excluded from reaching the epithelial surface [6]. Thus, the brush acts as a size-exclusion barrier for infiltrating entities [6].
The periciliary brush also contributes to the regulation of ASL layer hydration by facilitating water distribution between the two layers [6]. Identical charges among the membrane-tethered macromolecules create intermolecular repulsive forces to create an osmotic pressure gradient that stabilizes the periciliary layer by opposing the osmotic pressure gradient created by the overlying mucus layer [6]. In healthy hydrated airways, osmotic pressure created by the brush keeps the mucus layer above the outstretched cilia and facilitates normal MCC [6]. During dehydration of the ASL layer in diseases such as CF, water is first drawn from the mucus layer, increasing the concentration of mucus and osmotic pressure [6]. As the pressure generated from the mucus layer increases, water is drawn from the mucus layer as well as the periciliary layer, resulting in ciliary compression and impaired MCC [6].
Contrary to a previous hypothesis that the periciliary layer is stationary, a study by Matsui et al. reveals that the periciliary layer is moved along with the mucus layer and dextran was cleared at a similar rate by both layers [7, 8]. Simple frictional interaction between the two layers does not account for the similar clearance rates [8]. Matsui et al. propose that a transfer of momentum takes place in order to facilitate the efficient movement of the two layers [8]. Ciliary beating promotes momentum transfer from the mucus layer to the periciliary layer, thus facilitating the concerted movement of both layers [8]. The transport rate and the contribution of both layers towards efficient MCC are determined by the amount and composition of the ASL.
The amount of ASL, expressed as the height of the ASL layer, is a critical factor for the normal functioning of the mucociliary escalator. While the height of the mucus layer varies depending on the airway location (7-70 μm), the optimal height of the periciliary layer in human airways is approximately 7 μm, approximately the height of outstretched cilia [1, 2, 9]. The height of the ASL layer is regulated by a concerted action of various ion channels on the apical surface of the airway epithelium [10]. Major ion channels responsible for regulating chloride (Cl−)/sodium (Na+) transport are the cystic fibrosis transmembrane conductance regulator (CFTR), calcium-activated chloride channels (CaCCs), and epithelial Na+ channels (ENaC) [10]. While epithelial excretion of Cl− is regulated by CFTR and CaCCs, epithelial Na+ absorption is regulated by ENaC [10]. CFTR is also responsible for bicarbonate (HCO3−) transport that regulates the local pH of the airways [11]. The outcome of the concerted action of these ion channels regulates Cl− and Na+ transport across the apical surface of airway epithelial cells, thus regulating the hydration status of the airway epithelium [10].
Another factor determining the efficient functioning of the MCC system is the percent solids in the ASL layer. The constituents of the ASL layer, including secreted mucins, immune cells, ions, antimicrobial peptides, and cytokines, account for approximately 2.5% of the solids in healthy airways [1, 12].
3. ASL Dehydration in CF: A Result of Single Ion-Channel Defect
CF lung disease exemplifies how the defective functioning of a single ion channel, i.e., CFTR, results in serious disturbances in ASL physiology (Figures 1(b) and 2(b)). With the loss of CFTR function in CF epithelial cells, Cl− is retained within the epithelial cells while Na+ absorption by ENaC increases, leading to increased epithelial cytosolic NaCl contents [9, 13]. The increased cytosolic contents of NaCl in epithelial cells create an osmotic drive that promotes net movement of water from the ASL layer into the epithelial cells, thus leading to ASL layer dehydration.
The dehydration of the ASL layer results in the increased concentration of solutes (hyperconcentration) that leads to the compression of the periciliary layer by the overlying mucus layer, resulting in ciliary collapse and impaired MCC [6]. An increase from 2.5% to 6% solids, e.g., in CF airways, in the ASL layer compromises ciliary beat frequency and mucus layer transport [12]. Whether the increase in percent solids in mucoobstructive airways is a direct result of ASL layer dehydration or excessive accumulation or poor clearance of aberrant entities such as mucus plugs, microbes, inflammatory cells, and cellular debris, or a combination of all three outcomes, remains unclear.
4. Animal Models of ASL Dehydration
Although CF affects multiple organs, mucoobstructive lung disease is the major contributor to the morbidity and mortality associated with CF [14]. Various Cftr-knockout animal models including mice, pigs, ferrets, and rats have been generated with the intent of recapitulating mucociliary clearance impairment of human CF airways. In Sections 4.1, 4.2, 4.3, 4.4, and 4.5, the advantages and limitations of various animal models of impaired Cftr functioning and ASL dehydration will be discussed.
4.1. Mice
The availability of strains with genetic alterations of genes related to various inflammatory or pathological outcomes is an unmatched advantage of employing mouse as a disease model. Therefore, to recapitulate human CF-like lung disease, a number of mouse models have been developed over the past two decades (summarized in Table 1).
Table 1.
Mouse models of single ion-channel defect.
| S. No. | Model name | Strain background | Transgene/mutation | Spontaneous onset of lung disease | Airway mucus obstruction | Airway mucous cell metaplasia | Spontaneous airway bacterial infection | Chronic airway inflammation | Mortality | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cftrtm 1UNC | C57BL/6 | Mutation (exon 10) | No | Absent | Present only upon bacterial challenge | Absent | Neutrophilic infiltration at day 30 | Yes | (Snouwaert et al., [15]) |
| 2 | Cftrtm 1HGU | MF1 | Mutation (exon 10) | No | Absent | Present only upon bacterial challenge | Absent | Absent | No | (Dorin et al., [17]) |
| 3 | Cftrtm 1CAM | C57BL/6 | Mutation (exon 10) | No | Absent | Absent | Absent | Absent | Yes | (Ratcliff et al., [19]) |
| 4 | Cftrtm 1BAY | C57BL/6 × 129 | Mutation (exon 3) | No | Absent | Absent | Absent | Absent | Yes | (O'Neal et al., [20]) |
| 5 | Cftrtm 3BAY | 129/Sv | Mutation (exon 2) | No | Absent | Absent | Absent | Absent | Yes | (Hasty et al., [21]) |
| 6 | Cftrtm 2CAM | C57BL/6 | Mutation (ΔF508) | No | Absent | Absent | Absent | Absent | Yes | (Colledge et al., [23]) |
| 7 | Cftrtm 1EUR | FVB | Mutation (ΔF508) | No | Absent | Absent | Absent | Absent | No | (van Doorninck et al., [25]) |
| 8 | Cftrtm 1KTH | C57BL/6 × 129 | Mutation (ΔF508) | No | Absent | Absent | Absent | Absent | Yes | (Zeiher et al., [24]) |
| 9 | Cftrtm 1HSC | 129/SV | Mutation (exon 1) | No | Absent | Absent | Absent | Absent | Yes | (Rozmahel et al., [22]) |
| 10 | CftrG5 51D | CD1/129 | Mutation (G551D) | No | Absent | Absent | Absent | Absent | Yes | (Delaney et al., [26]) |
| 11 | Cftrtm 2HGU | C57BL/6 × 129 | Mutation (G480C) | No | Absent | Absent | Absent | Absent | No | (Dickinson et al., [27]) |
| 12 | Scnn1b-Tg+ | C3H : C57 | Transgene (Scnn1b) | Yes | Yes | Yes | Postnatal | Yes | Yes | (Mall et al., [36]) |
| 13 | Cftrtm 2UTH | C57BL/6 | Mutation (R117H) | No | Absent | Absent | Absent | Absent | No | (van Heeckeren et al., [28]) |
| 14 | Cftrtm 3UTH | C57BL/6 | Mutation (Y122X) | No | Absent | Absent | Absent | Absent | Yes | (van Heeckeren et al., [28]) |
To begin with, in 1992, Snouwaert et al. generated the Cftrtm1UNC mouse via targeted disruption of the Cftr gene (Table 1) [15]. When compared with wild-type (WT) mice, Cftrtm1UNC mice exhibited mortality due to intestinal mucoobstruction; however, contrary to many pathological changes observed in human CF patients, these mice did not exhibit significant pathological changes in the pancreas, male reproductive system, liver, and gallbladder [15]. Although mucoobstruction and bacterial infection were not observed in the airways in Cftrtm1UNC mice, an increase in goblet cells in the proximal airways and impaired MCC were observed [15, 16]. Although the Cftrtm1UNC mouse model exhibited impaired MCC upon bacterial challenge, it did not recapitulate the spontaneously arising airway mucoobstruction and bacterial infection observed in CF patients.
In 1992, Dorin et al. generated the Cftrtm1HGU mouse model also via targeted disruption of exon 10 [17]. Similar to Cftrtm1UNC mice, the Cftrtm1HGU mice exhibited no pathological abnormalities in the pancreas and reproductive system, although one male exhibited increased mucus accumulation in the vas deferens [17]. Unlike the Cftrtm1UNC mouse model, however, the Cftrtm1HGU mouse model exhibited only mild intestinal mucoobstruction and all pups were able to survive past weaning [17]. Although the airway mucoobstructive phenotype associated with CF was not observed, upon challenge with two types of bacteria commonly associated with CF, i.e., Staphylococcus aureus and Burkholderia cepacia, Cftrtm1HGU mice exhibited pathological features of CF lung disease [18]. Cftrtm1HGU mice exhibited difficulty in clearing the bacteria from the airspaces as effectively as WT littermates [18]. The airways of Cftrtm1HGU mice also exhibited a marked increase in the abundance of goblet cells and mucoobstruction in response to bacterial challenge [18]. Although the Cftrtm1HGU mouse model, similar to Cftrtm1UNC, exhibited impaired MCC in response to challenge, it also did not exhibit the spontaneously occurring mucoobstructive phenotype seen in CF airways.
Similar to the previously mentioned models, Ratcliff et al. targeted exon 10 of Cftr to generate the Cftrtm1CAM mouse model [19]. Similar to Cftrtm1UNC, Cftrtm1CAM pups exhibited increased mortality attributed to intestinal mucoobstruction [19]. Similar to human CF patients, Cftrtm1CAM mice exhibited obstruction of the pancreatic ducts, a phenotype not observed in Cftrtm1UNC and Cftrtm1HGU mice [19]. An interesting phenotype observed in the Cftrtm1CAM model that was previously not reported in Cftr−/− mice was the susceptibility to ocular infections and lacrimal gland abnormalities [19]. Although the Cftrtm1CAM mouse exhibited increased mortality, intestinal mucus obstruction, and pancreatic abnormalities, this model still did not exhibit mucus accumulation in the airways as seen in CF [19].
In 1993, O'Neal et al. generated the Cftrtm1BAY mouse model by targeted disruption of exon 3 in the Cftr locus [20]. No pathological abnormalities, i.e., mucus obstruction, were observed in the lungs of Cftrtm1BAY [20]. This mouse model also exhibited increased mortality-associated muco-obstruction of the intestines [20]. In 1995, Hasty et al. targeted exon 2 of the Cftr in order to generate the Cftrtm3BAY mouse model [21]. In accordance to the previous mouse models, Cftrtm3BAY mice exhibited high mortality as a result of severe intestinal mucoobstruction [21]. Cftrtm3BAY mice also did not exhibit an onset of lung disease, liver disease, or obstruction of the pancreatic ducts when examined at birth, one week of age, or three to four weeks of age [21]. A clinical phenotype that is commonly associated with male CF patients is sterility [15]. Contrary to the phenotype observed in male CF patients, Cftrtm3BAY males exhibited no reproductive abnormalities, whereas most females were sterile [21]. In 1996, Rozmahel et al. generated the Cftrtm1HSC mouse model on a 129/SV background by targeted disruption of exon 1 [22]. As seen in previously discussed models, the Cftrtm1HSC mouse model exhibited severe intestinal mucoobstruction that led to high mortality rates [22].
In 1995, two mouse models, i.e., Cftrtm2CAM and Cftrtm1KTH, incorporating a deletion of phenylalanine at position 508 (ΔF508) of the Cftr gene locus, the most common genetic mutation associated with human CF, were generated by two separate groups [23, 24]. Although significant mortality due to intestinal mucoobstruction was observed in both strains, no abnormalities in the pancreas, male reproductive system, or lungs were evident [23, 24]. In 1995, a third mouse, Cftrtm1EUR, with the ΔF508 mutation was generated on an FVB background [25]. As seen in Cftrtm2CAM and Cftrtm1KTH, pathological abnormalities, i.e., mucus retention, were not observed in the lungs, pancreas, liver, or vas deferens in these mice. However, these mice did not exhibit mortality due to intestinal mucus obstruction, but they did exhibit hypertrophy of goblet cells in the intestines [25]. The observed differences in the rate of mortality may be attributed to the strain background.
Also in 1996, Delaney et al. generated a mouse model possessing another mutation associated with human CF, the G551D mutation (CftrG551D) that occurs in approximately 3% of CF patients [26]. The CftrG551D also exhibited increased mortality due to intestinal mucoobstruction [26]. No pathological differences in the lungs, pancreas, and reproductive system were observed in CftrG551D mice [26]. In 2002, Dickinson et al. generated the Cftrtm2HGU targeted integration of the G480C mutation, a mutation associated with human CF [27]. The Cftrtm2HGU exhibited comparable survival to WT littermates, and no intestinal mucoobstruction was observed [27]. Mild goblet cell hypertrophy was observed in the intestines of the Cftrtm2HGU mice [27]. There were no abnormalities reported in the lungs and the reproductive systems [27]. The Cftrtm2UTH model was generated by the integration of the R117H mutation, a mutation characterized by CFTR reaching the apical surface of the epithelium but not properly functioning [28]. Upon challenge with Pseudomonas aeruginosa, the Cftrtm2UTH mouse model exhibited significantly lower neutrophil counts as compared to similar inflammatory responses to a previous Cftr−/− mouse model, i.e., Cftrtm1UNC, but presented no significant differences in inflammatory cytokine levels [28]. The Cftrtm3UTH mouse model was generated by integrating the Y122X mutation [28]. The Cftrtm3UTH mouse model exhibited lower levels of tumor necrosis factor alpha (TNF-α) and - interleukin 1 beta (IL-1β) when compared to the Cftrtm1UNC mouse model in response to Pseudomonas aeruginosa [28]. Taken together, there were no substantial differences between the tested Cftr−/− mouse models in response to Pseudomonas aeruginosa challenge [28]. Although mouse models of Cftr knockdown or various functional mutations recapitulated the intestinal mucoobstruction, none of these models produced the spontaneous onset of airway mucoobstruction and airway bacterial infection exhibited in CF, warranting a need for an animal model that recapitulates human CF.
4.2. Pig
In order to address the limitations observed in CF mouse models, Rogers et al. generated Cftr−/− pigs that exhibited gastrointestinal, pancreatic, and reproductive abnormalities commonly associated with CF [29]. All Cftr−/− piglets exhibited meconium ileus, a phenotype seen in ~15% of human CF patients [29]. The pancreas of Cftr−/− piglets was morphologically smaller when compared with WT littermates and exhibited ductal obstruction [29]. Male Cftr−/− pigs were also infertile, a phenotype commonly associated with human male CF patients [29]. Pertaining to lung disease manifestation, no lung inflammation, mucus obstruction, or infection was observed at 6-12 hours after birth [29, 30]. However, Cftr−/− newborn pigs exhibited difficulty in clearing bacteria upon challenge, i.e., Staphylococcus aureus [30]. Cftr−/− pigs that survived more than two months exhibited delayed onset of lung disease characterized by airway inflammation and mucoobstruction [30]. Although the Cftr−/− pig phenotypically expressed common hallmarks of CF, the mucoobstructive airway phenotypes in this model have been described as variable, ranging from no to severe manifestation [30].
4.3. Ferret
In 2010, Sun et al. generated the Cftr−/− ferret by targeted disruption that exhibited meconium ileus, pancreatic lesions, degenerate or absent vas deferens, dehydration of the ASL layer, severe airway inflammation, and a predisposition to lung infections [31]. Due to the susceptibility to lung infections, antibiotic treatment was necessary for the survival of the Cftr−/− ferret [32]. The Cftr−/− ferret exhibited mortality by the age of six months with antibiotic treatment, with 3 of 11 Cftr−/− ferrets surviving [32]. In order to investigate the progression of lung disease, Sun et al. removed the Cftr−/− ferret from antibiotics at three months of age [32]. Upon cessation of antibiotic treatment, progressive lung disease that resembled human CF, i.e., mucoobstruction and bacterial colonization, was observed in the major and small airways [32]. Thus, antibiotic intervention was needed to enhance survival to induce a more applicable CF lung phenotype in the Cftr−/− ferret.
4.4. Rat
In 2014, Tuggle et al. generated the Cftr−/− rat model by targeted disruption [33]. There was no significant difference in survival between Cftr−/− and WT littermates until weaning but survival was drastically decreased in the Cftr−/− rat by the age of six weeks [33]. Decreased survival was a result of intestinal mucoobstruction and complications [33]. There were no pancreatic abnormalities observed in the Cftr−/− rat [33]. Although the ASL layer in the Cftr−/− rat model was dehydrated, no pathological abnormalities were observed in the lungs at the age of 22 to 42 days [33]. Abnormal lung pathology developed as the Cftr−/− rats aged due to the development of submucosal gland hypertrophy [34]. At six months of age, the small airways of the Cftr−/− rat exhibited increased mucus secretion and accumulation leading to delayed mucus transport [34]. The Cftr−/− rat model did not exhibit airway obstruction or spontaneously arising bacterial infection [34].
While nonmurine Cftr−/− models have been somewhat successful in recapitulating the human CF-like spontaneous mucoobstruction and bacterial infection, unlike mice models, it remains challenging to introduce genetic changes into their genomes. The Scnn1b-Tg+ mouse model, although with an intact Cftr gene, exhibits CF-like lung pathology. In Section 4.5, we will review the characteristics of this strain and modulation of Scnn1b-Tg+ lung disease upon various other genetic alterations.
4.5. Scnn1b-Tg+ Mouse Model
None of the Cftr−/− mice models spontaneously recapitulate human CF-like disease, most likely due to the functional compensation by CaCCs [35]. To circumvent this issue, the Scnn1b-Tg+ mouse was generated to accomplish increased Na+ absorption into the airway epithelial cells [36]. The increased Na+ absorption in Scnn1b-Tg+ mice was achieved via overexpressing a transgene encoding sodium channel nonvoltage-gated 1, beta subunit (Scnn1b) in club cells (Figure 2(c)) [36]. Na+ absorption is enhanced in tracheal tissues of adult and neonatal Scnn1b-Tg+ mice; the Cl− secretion remained unaffected (Figure 2(c)) [36].
These mice exhibit various features of mucoobstructive airway diseases. The increased Na+ absorption into the airway epithelium of Scnn1b-Tg+ mice is evident as early as postnatal day (PND) 3 that results in the dehydration of the ASL layer leading to mucoobstruction and impaired MCC [36, 37]. A longitudinal study revealed that high mortality (~50% in the first two weeks of life) is a result of asphyxiation related to airway mucoobstruction [37]. The Scnn1b-Tg+ mice exhibited difficulty in clearing bacteria upon challenge with Haemophilus influenzae and Pseudomonas aeruginosa [36].
The initial microbiological studies on bronchoalveolar lavage fluid (BALF) from Scnn1b-Tg+ adult mice failed to detect spontaneous bacterial infection [36]. Since the initial microbiological studies were conducted only in adult mice and speculating that mucoobstruction creates a microaerophilic environment, Livraghi-Butrico et al. hypothesized that Scnn1b-Tg+ mice would be more susceptible to pulmonary infections by microaerophilic bacteria in neonatal age when the immune system is underdeveloped as compared to adult Scnn1b-Tg+ mice [38]. Under microaerophilic conditions, BALF from Scnn1b-Tg+ neonates showed the presence of polymicrobial bacterial species of oropharyngeal origin [38].
The Scnn1b-Tg+ mouse model also exhibited necrosis of epithelial cells in the airways at newborn (PND 0.5) and neonatal (PND 3.5) stages [37]. Interestingly, epithelial cell hypoxia was observed in the mucoobstructive airways of Scnn1b-Tg+ mice [37]. It is likely that the hypoxic stress to the airway epithelial cells caused by mucoobstruction leads to epithelial necrosis [37]. The blood gas analyses on neonatal (PND 3.5-5.5) Scnn1b-Tg+ mice revealed a significant reduction in the partial pressure of oxygen (PO2) and oxygen saturation, indicative of a systemic hypoxic environment [37]. This is most likely a result of bronchopulmonary dysplasia or emphysematous changes that are evident in Scnn1b-Tg+ mice. The Scnn1b-Tg+ mice manifest airway inflammation accompanied by granulocyte (neutrophil and eosinophil) infiltration and macrophage activation [37].
In the remaining parts of this review, we will discuss various immune cells in the context of muco-obstructive disease evolution in Scnn1b-Tg+ mice.
5. Macrophages
Macrophages are key sentinel cells that express pro- or anti-inflammatory functions based on the external cytokine milieu, broadly classified as M1 and M2 activation, respectively. M1 macrophages are associated with the elimination of pathogens and the secretion of proinflammatory cytokines, e.g., IL-1, IL-6, and IL-23 [39]. M1 macrophages also facilitate the expansion of TH17 lymphocytes that recruit neutrophils through the secretion of IL-17 [39]. Stimulation of M1 macrophages is facilitated by interferon gamma (IFN-γ), lipopolysaccharide (LPS), and other activators of Toll-like receptors (TLRs) [40–42]. Most of the TLRs require an adaptor molecule, myeloid differentiation factor 88 (MyD88), to initiate a downstream intracellular signaling cascade [43]. The MyD88 pathway leads to the activation of nuclear factor-kappa B (NF-κB), a key transcription factor in M1 activation that regulates the expression of a variety of inflammatory genes, e.g., TNF-α, IL1β, and interleukin 6 (IL-6) [44].
M2 macrophages are associated with parasitic infection, tissue remodeling, and promotion of Th2 responses [44]. Stimulation of M2 macrophages is facilitated through IL-4, IL-13, and IL-10 [45–47]. IL-4 and IL-13 facilitate the polarization of M2 macrophages through signal transducer and activator of transcription (STAT) 6, whereas IL-10 acts through STAT3 [48, 49].
Mall et al. initially observed morphological activation of pulmonary macrophages at two weeks, a phenotype that was found to persist into adulthood [37]. To profile molecular signatures of macrophages as they relate to the development of mucoobstructive lung disease, we performed gene expression analyses on purified Scnn1b-Tg+ macrophages at four disease-relevant time-points, i.e., PND 0 (less than 24 hours of age), 3, 10, and 42 [50]. There was evidence of both M1 and M2 macrophages in the BALF of Scnn1b-Tg+ mice at PND 3, with M1 as the more robust polarization state [50]. The predominance of M1 macrophages at PND 3 was found to be consistent with the presence of pulmonary bacterial infection typical of Scnn1b-Tg+ neonates [50]. The macrophage activation status experienced a shift to the M2 state at PND 10, and M2 was found to be more robust at PND 42 [50]. The robust molecular signatures exhibited by pulmonary macrophages during the progression of mucoobstructive lung disease in Scnn1b-Tg+ mice indicated their critical role in disease pathogenesis [50].
To elucidate the role of pulmonary macrophages in neonatal (PND 5-7) Scnn1b-Tg+ mice, we generated Scnn1b-Tg+ mice with macrophage deficiency [51]. In this strain, the expression of apoptosis-inducing diphtheria toxin A (DTA) was targeted to pulmonary macrophages via the myeloid cell-specific Lysozyme M (LysM) promoter [51, 52]. The superimposition of impaired MCC on macrophage depletion (DTA+-Scnn1b-Tg+) resulted in ~51% mortality due to an emaciated phenotype characterized by reduced weight gain, “flaky discoloration,” lethargy, and mortality [51]. Interestingly, macrophage depletion affected various inflammatory characteristics, i.e., alveolar space consolidation, airway inflammation, mucoobstruction, immune cell infiltration, and bacterial infection in Scnn1b-Tg+ [51]. The macrophage-depleted Scnn1b-Tg+ mice exhibited a significantly higher bacterial burden [51]. Although there was a presence of additional bacterial species, Pasteurella pneumotropica remained the predominant microbial inhabitant in the airways of macrophage-depleted mice.
To elucidate the contribution of pulmonary macrophages in mucoobstructive lung disease in adulthood, we compared the lung pathology of surviving macrophage-depleted adult mice [53]. Adult mice with macrophage deficiency exhibited a significantly higher degree of alveolar space consolidation [53]. Interestingly, DTA+-Scnn1b-Tg+ adult mice exhibited a significantly higher degree of mucoobstruction in airways and an increased number of mucus-producing cells compared to DTA−-Scnn1b-Tg+ littermates [53]. Taken together, these mechanistic reports that focused on the numerical depletion of macrophages highlighted the critical roles of these cells in the pathogenesis of lung disease in Scnn1b-Tg+ mice.
Matrix metalloproteinase- (MMP-) 12, a candidate genetic contributor to the development of emphysema, was found to be upregulated in the lungs of Scnn1b-Tg+ mice [54]. MMP12 was also found to be significantly upregulated in BALF macrophages of CF patients [54]. Trojanek et al. determined that MMP12 proteolytic activity was significantly higher on the surface of activated BALF macrophages of Scnn1b-Tg+ mice [54]. The administration of pharmacological inhibitors as well as the genetic deletion of Mmp12 in Scnn1b-Tg+ mice significantly reduced mean linear intercepts and destructive index [54]. Since MMP12 is expressed in non-macrophage cells as well, it remains to be determined whether inactivation or deletion of macrophage-originated Mmp12 accounts for the amelioration of alveolar space pathology in Scnn1b-Tg+ mice. Further investigation employing macrophage-specific deletion of various functionally relevant genes is necessary to determine the effect of functionally compromised macrophages on various pathological features of Scnn1b-Tg+ mice.
6. Neutrophils
Neutrophils are cells of the innate immune system that are typically the first cells to be recruited during inflammation and serve to eliminate invading pathogens [55]. Neutrophils employ a variety of mechanisms for bacterial killing, e.g., phagocytosis, degranulation, or release of neutrophil extracellular traps (NETs) [56]. In the process of phagocytosis, neutrophils engulf pathogens that are subsequently encapsulated in phagosomes [55]. Encapsulated pathogens are killed by the use of NADPH oxygenase-dependent mechanisms (reactive oxygen species) or antibacterial proteins contained within the neutrophilic granules [55]. These neutrophilic granules can also be released extracellularly through the process of degranulation in order to act upon extracellular pathogens [55]. Highly activated neutrophils produce NETs that can immobilize the pathogens for subsequent phagocytosis or directly kill the entrapped pathogens [56]. NETs are also composed of antimicrobial proteins and enzymes, e.g., lactoferrin, cathepsin, and neutrophil elastase (NE), responsible for the elimination of invading pathogens [56]. Interestingly, NE has been linked to both beneficial and detrimental roles in the pathogenesis of CF [57].
The Scnn1b-Tg+ mice exhibited neutrophilic airspace infiltration accompanied by increased expression of neutrophil chemoattractants, i.e., keratinocyte chemoattractant (KC), lipopolysaccharide-induced CXC chemokine (LIX), macrophage inflammatory protein 2 (MIP-2), and granulocyte-colony-stimulating factor (G-CSF), beginning in the neonatal stages and persisting into adulthood [37, 38]. NE has been implicated in the induction of emphysema [58], mucous cell metaplasia (MCM), and mucus hypersecretion [59, 60]. The ablation of Ne in Scnn1b-Tg+ mice resulted in a significant decrease in lung volume, mean linear intercepts, and destructive index as compared to Scnn1b-Tg+ littermates [57]. The Ne−/−-Scnn1b-Tg+ mice also had reduced MCM and expression levels of genes associated with goblet cells and mucus secretion, i.e., Gob5, Muc5ac, and Muc5b, involved in this response [57]. These results suggested that compromised neutrophil function via NE deletion ameliorates lung pathology in Scnn1b-Tg+ mice [57].
Myeloid differentiation primary response 88 (MyD88) is a cytosolic adaptor molecule that is required for the downstream signaling upon TLR ligation. The ablation of the Myd88 gene in Scnn1b-Tg+ mice resulted in significantly increased mortality when compared to Myd88+/--Scnn1b-Tg+ littermates [38]. Myd88−/−-Scnn1b-Tg+ mice also exhibited significantly increased bacterial burden by a greater diversity of bacterial species [61]. As compared to Myd88+/--Scnn1b-Tg+ mice, the Myd88−/−-Scnn1b-Tg+ mice exhibited a significant reduction in neutrophils and BALF levels of neutrophil chemokines, i.e., KC, LIX, MIP-2, and G-CSF [38]. These data suggested that the ablation of TLR signaling in Scnn1b-Tg+ mice leads to the reduced production of neutrophil chemoattractants and poor neutrophil recruitment; thus, there is poor bacterial clearance.
7. Eosinophils
Eosinophils are granulated cells of the innate immune system that respond to helminths and allergies [62]. The eosinophilic granules have been found to contain IL-4, IL-6, IL-10, and TNF-α [63]. As compared to WT mice, the Scnn1b-Tg+ mice exhibited significantly increased eosinophilia that, unlike neutrophilia that persisted into adulthood, peaked during the juvenile (2-3 weeks) stages and subsided during the adult stages [37]. The eosinophil chemoattractant, Eotaxin 1, was found to be overexpressed in the Scnn1b-Tg+ mouse as compared to WT littermates [37]. The ablation of interleukin- (IL-) 4 receptor alpha (Il4rα), the receptor for IL-4 and IL-13, significantly reduced eosinophilic infiltration in 10-day old Scnn1b-Tg+ mice, suggesting the involvement of IL4Rα ligands in eosinophilic recruitment [64, 65]. However, the exact role of eosinophils in Scnn1b-Tg+ lung disease is not yet clear.
8. Natural Killer Cells
Natural killer (NK) cells are cells of the innate immune system that are responsible for eliminating tumor cells and virally infected cells [66, 67]. Johannson et al. found that NK cells determine “self” from “nonself” through the recognition of major histocompatibility complex class I (MHC-I) [68]. NK cells not only possess the ability to kill target cells but also possess the ability to produce IFN-γ and TNF-α [69]. Through the production of IFN-γ, NK cells have also been shown to be involved in the differentiation of Th1 lymphocytes [70]. While the levels of IFN-γ and TNF-α are found to be elevated in the BALF from Scnn1b-Tg+ mice, whether NK cells are involved in the pathogenesis of Scnn1b-Tg+ mice remains unexplored.
9. T-Lymphocytes
The cells of the adaptive immune system, i.e., T- and B-lymphocytes, possess antigen-specific surface receptors that undergo recombination in order to mature [71]. The recombination is facilitated through recombinase activating gene- (RAG-) 1 and RAG-2 [72]. T-lymphocytes are cells of the adaptive immune system and can functionally be divided into subsets, e.g., Th1, Th2, Th17, and T regulatory lymphocytes (Tregs) [73].
Th1 lymphocytes are responsible for controlling intracellular pathogens and are associated with the production of TNF-α and interferon-gamma (IFN-γ) [73]. Th2 lymphocytes are associated with the production with IL-4, IL-5, and IL-13 [73, 74]. As discussed previously, IL-4 and IL-13 have been linked to MCM and increased mucus production [75, 76]. Th17 lymphocytes secrete IL-17, a key proinflammatory cytokine associated with neutrophil recruitment [77]. Th17 lymphocytes are present during the early stages of CF, and a significant correlation exists between IL-17 and the total number of neutrophils [78]. Pseudomonas aeruginosa infection in BALF from CF patients is associated with significantly higher Th17-associated cytokines (IL-17, IL-6, IL-1β, and IL-8) [79]. Tregs are associated with the suppression of exacerbated Th2/Th17 inflammation [80]. CF patients with chronic Pseudomonas aeruginosa infection exhibited lower Treg counts when compared to CF patients without Pseudomonas aeruginosa infection [81].
Lymphocyte counts tend to be higher in BALF from Scnn1b-Tg+ mice as compared to their WT littermates, but a significant increase is evident only in adult Scnn1b-Tg+ mice [37]. While IFN-γ remains comparable between Scnn1b-Tg+ and their WT littermates, BALF levels of TNF-α, a Th1-associated cytokine, are elevated in the BALF of Scnn1b-Tg+ neonates [37]. Mall et al. found significantly higher levels of IL-13 starting at one week of age and waning after three weeks of age [37]. During this time, the Scnn1b-Tg+ mouse model exhibited significantly increased MCM and mucoobstruction [37]. A detailed analysis of the lungs for the presence of various subtypes of Th cells is warranted to completely understand the Th-associated responses in the Scnn1b-Tg+ mice.
10. B-Lymphocytes
B-lymphocytes (B-cells) secrete antigen-specific immunoglobulins (Ig) that constitute antigen-specific humoral immunity. In addition to bone marrow, spleen, and lymph nodes, B-cells localize in the tertiary lymphoid structures such as bronchus-associated lymphoid tissue (BALT) that surrounds bronchi in the lungs. BALTs are frequently found in CF patients and Scnn1b-Tg+ adult mice [38, 53, 82].
CF patients exhibit significantly higher levels of Pseudomonas-specific IgG antibodies [83]. Secretory IgA levels are also significantly upregulated in the nasal secretions of CF patients infected with Pseudomonas aeruginosa [84]. Livraghi-Butrico et al. found that Myd88−/−-Scnn1b-Tg+ mice exhibited significantly more lymphoid aggregates at eight weeks of age than Myd88+/--Scnn1b-Tg+ littermates [38]. In our recent report, a significant increase in the presence of BALTs in the lung parenchyma of macrophage-deficient Scnn1b-Tg+ adults was observed [53]. The presence of these lymphoid aggregates was associated with higher levels of immunoglobulin (Ig) subtypes, i.e., IgA, IgM, IgG1, IgG2b, and IgG3, in BALF from macrophage-deficient Scnn1b-Tg+ adults [53]. While the antigen specificity of these immunoglobulins remains to be investigated, their increased levels in mice with BALTs likely reflect local adaptive response to bacterial infections.
11. Innate Lymphoid Cells (ILCs)
Innate lymphoid cells (ILCs) are innate immune cells that secrete Th effector cytokines but lack antigen-specific receptors that require recombination [85]. In simpler description, the ILCs (ILC1, 2, and 3) are the amnestic equivalent of Th subtypes (Th1, Th2, and Th17) [85]. ILCs delineate separately from T lymphocytes based on the expression of the transcription factor inhibitor of DNA binding 2 (Id2) [86]. ILCs also require the common cytokine receptor γ-chain (also known as Il2rg) [87]. ILCs have been classified based on their expression of transcription factors and cytokines [87].
ILC1s differentiate independently from NK cells from Id2 expressing common helper ILC precursors (ChILPs) [86]. Moro et al. identified ILC2s in mouse mesentery that produce high levels of Th2-associated cytokines, i.e., IL-5 and IL-13, in response to IL-33 [88]. Subsequent studies revealed that ILC2s could also produce the Th2-associated cytokine IL-4 and rely on GATA-3 for differentiation and maintenance [89, 90]. Takatori et al. identified ILC3s that produce Th17-associated cytokines, i.e., IL-17 and IL-22, in response to IL-1β as well as IL-23 and produce IL-17 and IL-22 [91]. ILC3s rely on the expression of rare orphan receptor- (ROR-) γt for differentiation [87]. The role of ILCs in the pathogenesis and progression of lung disease in the Scnn1b-Tg+ mouse model remains unclear and warrants extensive investigation.
Given the predominance of Th-mediated responses in Scnn1b-Tg+ mice of different ages, it is critical to characterize ILC as well as Th populations in the Scnn1b-Tg+ mice. These studies when followed by ILCs and Th subtype depletion studies will dissect cell-specific roles in mucoobstructive lung disease in Scnn1b-Tg+ mice.
12. Spontaneous Bacterial Infection in CF
CF is characterized by early bacterial colonization by microbes originating from the oral cavity and progressively shifts to a pathogen-dominated environment [92]. Muhlebach et al. conducted a longitudinal study to characterize the microbiome in young CF patients [92]. The lower airways of CF infants were determined to be relatively sterile, but microbes commonly associated with the oral cavity, e.g., Streptococcus and Prevotella, were predominant in the airways by the age of two years [92]. Of note, the origin of spontaneous bacterial colonization in Scnn1b-Tg+ neonates was also determined to be oropharyngeal [38]. At four years of age, the microbiome analyses from CF patients revealed the presence of pathogenic bacterial species, e.g., Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa [92]. The presence of a pathogenic species in CF patients was associated with significantly increased inflammation and structural damage in the lungs [92]. Coburn et al. found that CF patients over the age of 25 exhibited a prevalence of Pseudomonas aeruginosa that was associated with declining lung function [93]. The progressive decline in lung function associated with Pseudomonas aeruginosa infection leads to respiratory failure and death in CF patients [94].
13. Does Infection Lead to the Airway Inflammation?
Whether inflammatory responses in mucoobstructive airways originate from infectious agents remained unclear until recently. To determine if the bacterial infection is essential for airway inflammation in Scnn1b-Tg+ mice, Livraghi-Butrico et al. rederived Scnn1b-Tg+ mice in a germ-free environment [38]. While, as expected, the germ-free Scnn1b-Tg+ mice did not exhibit airway bacterial colonization, other phenotypes including airway inflammation, macrophage activation, MCM, and airway mucoobstruction were still present [38]. Indeed, the macrophage activation was found to be more exaggerated in germ-free Scnn1b-Tg+ mice as compared to specific pathogen-free Scnn1b-Tg+ mice [50]. These results suggested that the inflammatory responses observed in germ-free Scnn1b-Tg+ mice were not dependent on the presence of microbes or pathogen-associated molecular patterns (PAMPs). Along the same lines, antibiotic treatment of spontaneously-infected Cftr−/− ferrets failed to mitigate airway inflammation [95]. Therefore, it is likely that the ASL dehydration-induced stress to the airway cells, i.e., epithelium and immune cells, induces the release of proinflammatory damage-associated molecular patterns (DAMPs) that, in turn, mediates inflammatory responses in the airways.
Various DAMPs have been implicated in the pathogenesis of mucoinflammatory outcomes including airway inflammation, mucin hypersecretion, and MCM. IL-1α, a potent inducer of neutrophilic recruitment, is present at significantly higher levels in BALF from 5-day-old Scnn1b-Tg+ pups [96, 97]. The genetic deletion of Il1r1, a gene encoding the receptor for IL-1α and IL-1β, abolishes airway neutrophilia and significantly reduces mortality, mucoobstruction, and emphysema in Scnn1b-Tg+ pups [97]. Another DAMP, high-mobility group box 1 (HMGB1), is elevated in the sputum from CF patients [98]. Interestingly, HMGB1 levels are also elevated in the BALF from Scnn1b-Tg+ mice [98]. Since HMGB1 acts as a ligand for TLR2 and TLR4, its effect is expected to produce responses similar to PAMPs (LPS and lipoteichoic acid) [99, 100].
IL-33, a potent stimulator of Th2-associated responses, acts as a potent DAMP upon release by airway epithelial cells into the airspaces [101]. IL-33 binds to the ST2 receptor that is present on mast cells, macrophages, Th2 cells, and type 2 innate lymphoid cells (ILC2s) [102]. Administration of IL-33 induces the production of cytokines by Th2 lymphocytes in vivo [103]. IL-33 has also been linked to the activation of ILC2s that also release Th2-associated cytokines [104]. IL-33 levels are elevated in the juvenile Scnn1b-Tg+ mice [105]. Secondhand-smoke exposure to Scnn1b-Tg+ mice results in diminished IL-33 expression and BALF levels, which is strongly associated with diminished MCM and reduced expression of MCM-associated genes [105]. Further investigation on the mice with a genetic deletion of IL-33 on an Scnn1b-Tg+ background will confirm the role of IL-33 in the manifestation of mucoobstructive responses.
14. Does Mucous Cell Metaplasia (MCM) Lead to Mucoobstruction?
MCM refers to an epithelial remodeling response that increases the number of mucous cells in the airway epithelium and upregulates the expression of genes involved in mucin expression and secretion. Scnn1b-Tg+ mice exhibit a significantly higher number of mucous cells in proximal and distal airways as compared to their WT littermates [37]. Interestingly, the neonatal (PND 3.5) Scnn1b-Tg+ pups exhibit mucoobstruction in the trachea but in the absence of MCM, suggesting that mucus accumulation, rather than mucus overproduction, contributes to mucus plugging at this early age [37]. However, in 2−3-week-old Scnn1b-Tg+ mice, mucoobstruction along with MCM is found to be most prominent in the large and distal conducting airways, a feature that persisted into the adult Scnn1b-Tg+ mice [37, 105].
MCM is commonly associated with Th2-associated cytokines, i.e., IL-4 and IL-13 [75, 76]. As discussed before, the ablation of Il4rα, a common receptor for IL-4 and IL-13, significantly decreases neonatal mortality, MCM, and eosinophilic inflammation in the 10-day-old Scnn1b-Tg+ mice [65]. Interestingly, the ablation of Il4rα does not alter the severity of mucus plugging [65]. It appears that the normal production rate of mucus in the ASL-dehydrated state is capable of producing mucoobstruction; however, further experiments are required to ascertain this possibility.
15. Conclusions
Due to the high morbidity and mortality associated with CF-like mucoobstructive lung disease, an in-depth investigation of the immunological responses initiated as a result of ASL dehydration and mucoobstruction is warranted. Although several mice models incorporating different Cftr mutations are available, none of the mouse models effectively recapitulate CF-like mucoobstructive lung disease. Although not modulating the functioning of CFTR channels, the Scnn1b-Tg+ mouse model effectively demonstrates how a single ion-channel defect results in an imbalance in ion transport, which ultimately leads to ASL dehydration and associated lung disease.
The earliest manifestation of lung disease, i.e., ASL layer dehydration, mucoobstruction, immune cell infiltration, and spontaneous bacterial infections, exhibited in the Scnn1b-Tg+ mouse model provides a most representative model for the investigation of the pathogenesis and progression of human CF-like lung disease. The Scnn1b-Tg+ mouse also presents an outstanding tool to investigate the impact of various environmental insults, e.g., cigarette smoke, nanoparticles, and fungal spores, on the development and progression of mucoobstructive lung disease [105–107].
A complete understanding of the evolution of various pathological manifestations in this strain is still unclear. The availability of numerous genetic strains on a congenic C57BL/6 background presents an opportunity to investigate the development of complex mucoobstructive lung disease which otherwise is challenging to pursue. A list of studies employing various genetic alterations has been summarized in Table 2. Selective introduction of additional genetic alterations into the Scnn1b-Tg+ strain have already begun to dissect the pathway-specific roles of various genes in the pathogenesis of mucoobstructive lung disease.
Table 2.
Various genetic modifications in the Scnn1b-Tg+ mouse model.
| Genotype | Description | Macrophage infiltration | Neutrophil infiltration | Eosinophil infiltration | Lymphocyte infiltration | Mucous cell metaplasia | Airway mucus obstruction | Distal airspace enlargement | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Tnfα−/−-Scnn1b-Tg+ | Global deletion of Tnfα | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | (Livraghi et al., [65]) |
| Tnfr1−/−-Scnn1b-Tg+ | Global deletion of Tnfr1 | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | No significant difference | (Livraghi et al., [65]) |
| Il4rα−/−-Scnn1b-Tg+ | Global deletion of Il4rα | No significant difference | No significant difference | Significantly reduced at PND 10 and 5 weeks of age | No significant difference | Significantly reduced at PND 10 | No significant difference | No significant difference | (Livraghi et al., [65]) |
| Myd88−/−Scnn1b-Tg+ | Global deletion of Myd88 | Significantly higher at PND 10; no significant difference at other observed time-points | Significantly reduced | No significant difference | Lymphoid hyperplasia significantly increased at 8 weeks of age | Significantly reduced at PND 5-7, but not at other time-points | Significantly reduced at PND 5-7, but not at any other observed time-point | Not reported | (Livraghi-Butrico et al., [38]) |
| Ne−/−-Scnn1b-Tg+ | Global deletion of neutrophil elastase | No significant difference | Significantly reduced | No significant difference | No significant difference | Significantly reduced | No significant difference | Significantly reduced | (Gehrig et al., [57]) |
| Il1r−/−-Scnn1b-Tg+ | Global deletion of Il1r | No significant difference | Significantly reduced | No significant difference | No significant difference | Not reported | Significantly reduced | Significantly reduced | (Fritzsching et al., [97]) |
| DTA+-Scnn1b-Tg+ | Partial deficiency of macrophages | No significant difference in total number of macrophage infiltration, significantly reduced in total percentage | Significantly increased | No significant difference | Increased occurrence of lymphoid aggregates in adult mice; significant infiltration in nonemaciated phenotype | Significantly reduced in emaciated phenotype | Significantly reduced in emaciated phenotype | No significant difference | (Saini et al., [51, 53]) |
| Muc5b−/−-Scnn1b-Tg+ | Global deletion of Muc45b | No significant difference | No significant difference | Not reported | Increased lymphoid aggregates, but no significant difference in BALF lymphocytes | Not reported | Significantly reduced | No significant difference | (Livraghi-Butrico et al., [108]) |
| Muc5ac−/−-Scnn1b-Tg+ | Global deletion of Muc5ac | No significant difference | No significant difference | Not reported | No significant difference in incidence of lymphoid aggregates | Not reported | Significantly reduced | Not reported | (Livraghi-Butrico et al., [108]) |
| Spdef−/−-Scnn1b-Tg+ | Global deletion of Spdef | No significant difference | Significantly increased in neonates | No significant difference | No significant difference | Not reported | No significant difference | Not reported | (Chen et al., [109]) |
Acknowledgments
The research was supported by a Flight Attendant Medical Research Institute grant (YS), the National Institute of Health (NIH) grant R01 ES 030125, and a start-up package (YS) from the School of Veterinary Medicine, Louisiana State University.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
BWL, SP, and YS wrote the review.
References
- 1.Knowles M. R., Boucher R. C. Mucus clearance as a primary innate defense mechanism for mammalian airways. The Journal of Clinical Investigation. 2002;109(5):571–577. doi: 10.1172/JCI0215217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy J. V., Dickey B. F. Airway mucus function and dysfunction. The New England Journal of Medicine. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z., Duerr J., Johannesson B., et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. Journal of Cystic Fibrosis. 2011;10:S172–S182. doi: 10.1016/S1569-1993(11)60021-0. [DOI] [PubMed] [Google Scholar]
- 4.Krauth C., Jalilvand N., Welte T., Busse R. Cystic fibrosis: cost of illness and considerations for the economic evaluation of potential therapies. PharmacoEconomics. 2003;21(14):1001–1024. doi: 10.2165/00019053-200321140-00002. [DOI] [PubMed] [Google Scholar]
- 5.Schibler A., Bolt I., Gallati S., Schoni M. H., Kraemer R. High morbidity and mortality in cystic fibrosis patients compound heterozygous for 3905insT and ∆F508. The European Respiratory Journal. 2001;17(6):1181–1186. doi: 10.1183/09031936.01.00034601. [DOI] [PubMed] [Google Scholar]
- 6.Button B., Cai L. H., Ehre C., et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satir P., Sleigh M. A. The physiology of cilia and mucociliary interactions. Annual Review of Physiology. 1990;52(1):137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H., Randell S. H., Peretti S. W., Davis C. W., Boucher R. C. Coordinated clearance of periciliary liquid and mucus from airway surfaces. The Journal of Clinical Investigation. 1998;102(6):1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proceedings of the American Thoracic Society. 2004;1(1):42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 10.Randell S. H., Boucher R. C., University of North Carolina Virtual Lung Group Effective mucus clearance is essential for respiratory health. American Journal of Respiratory Cell and Molecular Biology. 2006;35(1):20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatric Pulmonology. 2015;50:2S4–S30. doi: 10.1002/ppul.23247. [DOI] [PubMed] [Google Scholar]
- 12.Boucher R. C. Evidence for airway surface dehydration as the initiating event in CF airway disease. Journal of Internal Medicine. 2007;261(1):5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 13.Knowles M., Stutts M., Spock A., Fischer N., Gatzy J., Boucher R. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 14.Rowe S. M., Miller S., Sorscher E. J. Cystic fibrosis. The New England Journal of Medicine. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 15.Snouwaert J. N., Brigman K. K., Latour A. M., et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 16.Cowley E. A., Wang C. G., Gosselin D., Radzioch D., Eidelman D. H. Mucociliary clearance in cystic fibrosis knockout mice infected with Pseudomonas aeruginosa. The European Respiratory Journal. 1997;10(10):2312–2318. doi: 10.1183/09031936.97.10102312. [DOI] [PubMed] [Google Scholar]
- 17.Dorin J. R., Dickinson P., Alton E. W., et al. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992;359(6392):211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- 18.Davidson D. J., Dorin J. R., McLachlan G., et al. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nature Genetics. 1995;9(4):351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliff R., Evans M. J., Cuthbert A. W., et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genetics. 1993;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- 20.O’Neal W. K., Hasty P., McCray P. B., Jr., et al. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Human Molecular Genetics. 1993;2(10):1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- 21.Hasty P., O’Neal W. K., Liu K. Q., et al. Severe phenotype in mice with termination mutation in exon 2 of cystic fibrosis gene. Somatic Cell and Molecular Genetics. 1995;21(3):177–187. doi: 10.1007/BF02254769. [DOI] [PubMed] [Google Scholar]
- 22.Rozmahel R., Wilschanski M., Matin A., et al. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nature Genetics. 1996;12(3):280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 23.Colledge W. H., Abella B. S., Southern K. W., et al. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nature Genetics. 1995;10(4):445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 24.Zeiher B. G., Eichwald E., Zabner J., et al. A mouse model for the delta F508 allele of cystic fibrosis. The Journal of Clinical Investigation. 1995;96(4):2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doorninck J. H., French P. J., Verbeek E., et al. A mouse model for the cystic fibrosis delta F508 mutation. The EMBO Journal. 1995;14(18):4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney S. J., Alton E. W., Smith S. N., et al. Cystic fibrosis mice carrying the missense mutation G551D replicate human genotype-phenotype correlations. The EMBO Journal. 1996;15(5):955–963. doi: 10.1002/j.1460-2075.1996.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson P., Smith S. N., Webb S., et al. The severe G480C cystic fibrosis mutation, when replicated in the mouse, demonstrates mistrafficking, normal survival and organ-specific bioelectrics. Human Molecular Genetics. 2002;11(3):243–251. doi: 10.1093/hmg/11.3.243. [DOI] [PubMed] [Google Scholar]
- 28.van Heeckeren A. M., Schluchter M. D., Drumm M. L., Davis P. B. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;287(5):L944–L952. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 29.Rogers C. S., Stoltz D. A., Meyerholz D. K., et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoltz D. A., Meyerholz D. K., Pezzulo A. A., et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Science Translational Medicine. 2010;2(29, article 29ra31) doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X., Sui H., Fisher J. T., et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. The Journal of Clinical Investigation. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X., Olivier A. K., Liang B., et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator–knockout ferrets. American Journal of Respiratory Cell and Molecular Biology. 2014;50(3):502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuggle K. L., Birket S. E., Cui X., et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;9(3, article e91253) doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birket S. E., Davis J. M., Fernandez C. M., et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight. 2018;3(1) doi: 10.1172/jci.insight.97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grubb B. R., Paradiso A. M., Boucher R. C. Anomalies in ion transport in CF mouse tracheal epithelium. American Journal of Physiology-Cell Physiology. 1994;267(1):C293–C300. doi: 10.1152/ajpcell.1994.267.1.C293. [DOI] [PubMed] [Google Scholar]
- 36.Mall M., Grubb B. R., Harkema J. R., O’Neal W. K., Boucher R. C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nature Medicine. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 37.Mall M. A., Harkema J. R., Trojanek J. B., et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+channel-overexpressing mice. American Journal of Respiratory and Critical Care Medicine. 2008;177(7):730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livraghi-Butrico A., Kelly E. J., Klem E. R., et al. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunology. 2012;5(4):397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosser D. M., Edwards J. P. Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. The Journal of Experimental Medicine. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Means T. K., Jones B. W., Schromm A. B., et al. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. Journal of Immunology. 2001;166(6):4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 42.Jones B. W., Means T. K., Heldwein K. A., et al. Different Toll-like receptor agonists induce distinct macrophage responses. Journal of Leukocyte Biology. 2001;69:1036–1044. [PubMed] [Google Scholar]
- 43.Medzhitov R., Preston-Hurlburt P., Kopp E., et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Molecular Cell. 1998;2(2):253–258. doi: 10.1016/S1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 44.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Frontiers in Immunology. 2014;5:p. 614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. The Journal of Experimental Medicine. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doherty T. M., Kastelein R., Menon S., Andrade S., Coffman R. L. Modulation of murine macrophage function by IL-13. The Journal of Immunology. 1993;151(12):7151–7160. [PubMed] [Google Scholar]
- 47.Lang R., Patel D., Morris J. J., Rutschman R. L., Murray P. J. Shaping gene expression in activated and resting primary macrophages by IL-10. Journal of Immunology. 2002;169(5):2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 48.Terabe M., Matsui S., Noben-Trauth N., et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunology. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 49.O’Farrell A. M., Liu Y., Moore K. W., Mui A. L. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. The EMBO Journal. 1998;17(4):1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saini Y., Dang H., Livraghi-Butrico A., et al. Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics. 2014;15(1):p. 726. doi: 10.1186/1471-2164-15-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saini Y., Wilkinson K. J., Terrell K. A., et al. Neonatal pulmonary macrophage depletion coupled to defective mucus clearance increases susceptibility to pneumonia and alters pulmonary immune responses. American Journal of Respiratory Cell and Molecular Biology. 2016;54(2):210–221. doi: 10.1165/rcmb.2014-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreisel D., Nava R. G., Li W., et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini Y., Lewis B. W., Yu D., et al. Effect of LysM+ macrophage depletion on lung pathology in mice with chronic bronchitis. Physiological Reports. 2018;6(8, article e13677) doi: 10.14814/phy2.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trojanek J. B., Cobos-Correa A., Diemer S., et al. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysema. American Journal of Respiratory Cell and Molecular Biology. 2014;51(5):709–720. doi: 10.1165/rcmb.2013-0407OC. [DOI] [PubMed] [Google Scholar]
- 55.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews. Immunology. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 56.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 57.Gehrig S., Duerr J., Weitnauer M., et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. American Journal of Respiratory and Critical Care Medicine. 2014;189(9):1082–1092. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro S. D., Goldstein N. M., Houghton A. M., Kobayashi D. K., Kelley D., Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. The American Journal of Pathology. 2003;163(6):2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voynow J. A., Fischer B. M., Malarkey D. E., et al. Neutrophil elastase induces mucus cell metaplasia in mouse lung. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;287(6):L1293–L1302. doi: 10.1152/ajplung.00140.2004. [DOI] [PubMed] [Google Scholar]
- 60.Voynow J. A., Young L. R., Wang Y., Horger T., Rose M. C., Fischer B. M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1999;276(5):L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 61.Livraghi-Butrico A., Grubb B. R., Kelly E. J., et al. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiological Genomics. 2012;44(8):470–484. doi: 10.1152/physiolgenomics.00185.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weller P. F., Spencer L. A. Functions of tissue-resident eosinophils. Nature Reviews. Immunology. 2017;17(12):746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu V. T., Berek C. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. European Journal of Immunology. 2012;42(1):130–137. doi: 10.1002/eji.201141953. [DOI] [PubMed] [Google Scholar]
- 64.Nelms K., Keegan A. D., Zamorano J., Ryan J. J., Paul W. E. The IL-4 receptor: signaling mechanisms and biologic functions. Annual Review of Immunology. 1999;17(1):701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 65.Livraghi A., Grubb B. R., Hudson E. J., et al. Airway and lung pathology due to mucosal surface dehydration in (beta)-epithelial Na+ channel-overexpressing mice: role of TNF-(alpha) and IL-4R(alpha) signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. Journal of Immunology. 2009;182(7):4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiessling R., Klein E., Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. European Journal of Immunology. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 67.Welsh R. M., Brubaker J. O., Vargas-Cortes M., O’Donnell C. L. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. Journal of Experimental Medicine. 1991;173(5):1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansson M. H., Bieberich C., Jay G., Karre K., Hoglund P. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. The Journal of Experimental Medicine. 1997;186(3):353–364. doi: 10.1084/jem.186.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfe S. A., Tracey D. E., Henney C. S. Induction of “natural killer” cells by BCG. Nature. 1976;262(5569):584–586. doi: 10.1038/262584a0. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Fontecha A., Thomsen L. L., Brett S., et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature Immunology. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 71.Alt F. W., Yancopoulos G. D., Blackwell T. K., et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. The EMBO Journal. 1984;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 73.Constant S. L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annual Review of Immunology. 1997;15(1):297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 74.McKenzie A. N., Culpepper J. A., de Waal Malefyt R., et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dabbagh K., Takeyama K., Lee H. M., Ueki I. F., Lausier J. A., Nadel J. A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. The Journal of Immunology. 1999;162(10):6233–6237. [PubMed] [Google Scholar]
- 76.Zhu Z., Homer R. J., Wang Z., et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. The Journal of Clinical Investigation. 1999;103(6):779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aujla S. J., Dubin P. J., Kolls J. K. Th17 cells and mucosal host defense. Seminars in Immunology. 2007;19(6):377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan H. L., Regamey N., Brown S., Bush A., Lloyd C. M., Davies J. C. The Th17 pathway in cystic fibrosis lung disease. American Journal of Respiratory and Critical Care Medicine. 2011;184(2):252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiringer K., Treis A., Fucik P., et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. American Journal of Respiratory and Critical Care Medicine. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 80.Josefowicz S. Z., Lu L. F., Rudensky A. Y. Regulatory T cells: mechanisms of differentiation and function. Annual Review of Immunology. 2012;30(1):531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hector A., Schafer H., Poschel S., et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic Pseudomonas infection. American Journal of Respiratory and Critical Care Medicine. 2015;191(8):914–923. doi: 10.1164/rccm.201407-1381OC. [DOI] [PubMed] [Google Scholar]
- 82.Lammertyn E. J., Vandermeulen E., Bellon H., et al. End-stage cystic fibrosis lung disease is characterised by a diverse inflammatory pattern: an immunohistochemical analysis. Respiratory Research. 2017;18(1):p. 10. doi: 10.1186/s12931-016-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pressler T., Frederiksen B., Skov M., Garred P., Koch C., Hoiby N. Early rise of anti-pseudomonas antibodies and a mucoid phenotype of Pseudomonas aeruginosa are risk factors for development of chronic lung infection—a case control study. Journal of Cystic Fibrosis. 2006;5(1):9–15. doi: 10.1016/j.jcf.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Aanaes K., Johansen H. K., Poulsen S. S., Pressler T., Buchwald C., Hoiby N. Secretory IgA as a diagnostic tool for Pseudomonas aeruginosa respiratory colonization. Journal of Cystic Fibrosis. 2013;12(1):81–87. doi: 10.1016/j.jcf.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Eberl G., Colonna M., Di Santo J. P., McKenzie A. N. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237, article aaa6566) doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klose C. S. N., Flach M., Mohle L., et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157(2):340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 87.Spits H., Artis D., Colonna M., et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nature Reviews Immunology. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 88.Moro K., Yamada T., Tanabe M., et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 89.Mjosberg J., Bernink J., Golebski K., et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37(4):649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 90.Hoyler T., Klose C. S., Souabni A., et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takatori H., Kanno Y., Watford W. T., et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. The Journal of Experimental Medicine. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muhlebach M. S., Zorn B. T., Esther C. R., et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathogens. 2018;14(1, article e1006798) doi: 10.1371/journal.ppat.1006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coburn B., Wang P. W., Diaz Caballero J., et al. Lung microbiota across age and disease stage in cystic fibrosis. Scientific Reports. 2015;5(1, article 10241) doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Medicine. 2011;9(1):p. 32. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen B. H., Evans T. I. A., Moll S. R., et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. American Journal of Respiratory and Critical Care Medicine. 2018;197(10):1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rider P., Carmi Y., Guttman O., et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. The Journal of Immunology. 2011;187(9):4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 97.Fritzsching B., Zhou-Suckow Z., Trojanek J. B., et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. American Journal of Respiratory and Critical Care Medicine. 2015;191(8):902–913. doi: 10.1164/rccm.201409-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowe S. M., Jackson P. L., Liu G., et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. American Journal of Respiratory and Critical Care Medicine. 2008;178(8):822–831. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park J. S., Svetkauskaite D., He Q., et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of Biological Chemistry. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 100.Park J. S., Gamboni-Robertson F., He Q., et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology. Cell Physiology. 2006;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 101.Cayrol C., Girard J. P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current Opinion in Immunology. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Chen S., Chen B., Wen Z., Huang Z., Ye L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget. 2017;8(20):32407–32418. doi: 10.18632/oncotarget.16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmitz J., Owyang A., Oldham E., et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 104.Bartemes K. R., Iijima K., Kobayashi T., Kephart G. M., McKenzie A. N., Kita H. IL-33-responsive lineage—CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. Journal of Immunology. 2012;188(3):1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewis B. W., Sultana R., Sharma R., et al. Early postnatal secondhand smoke exposure disrupts bacterial clearance and abolishes immune responses in muco-obstructive lung disease. Journal of Immunology. 2017;199(3):1170–1183. doi: 10.4049/jimmunol.1700144. [DOI] [PubMed] [Google Scholar]
- 106.Geiser M., Quaile O., Wenk A., et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Particle and Fibre Toxicology. 2013;10(1):p. 19. doi: 10.1186/1743-8977-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geiser M., Wigge C., Conrad M. L., et al. Nanoparticle uptake by airway phagocytes after fungal spore challenge in murine allergic asthma and chronic bronchitis. BMC Pulmonary Medicine. 2014;14(1):p. 116. doi: 10.1186/1471-2466-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livraghi-Butrico A., Grubb B. R., Wilkinson K. J., et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunology. 2017;10(2):395–407. doi: 10.1038/mi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen G., Volmer A. S., Wilkinson K. J., et al. Role of Spdef in the regulation of Muc5b expression in the airways of naive and mucoobstructed mice. American Journal of Respiratory Cell and Molecular Biology. 2018;59(3):383–396. doi: 10.1165/rcmb.2017-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]