Abstract
Background
Intensive follow-up after surgery for colorectal cancers is common in clinical practice, but evidence of a survival benefit is limited.
Objective
To conduct a systematic review and meta-analysis on the effects of follow-up strategies for nonmetastatic colorectal cancer.
Data sources
We searched Medline, Embase, and CENTRAL databases through May 30, 2018.
Study selection
We included randomized clinical trials evaluating intensive follow-up versus less follow-up in patients with nonmetastatic colorectal cancer.
Interventions
Intensive follow-up
Main outcomes measures
Overall survival.
Results
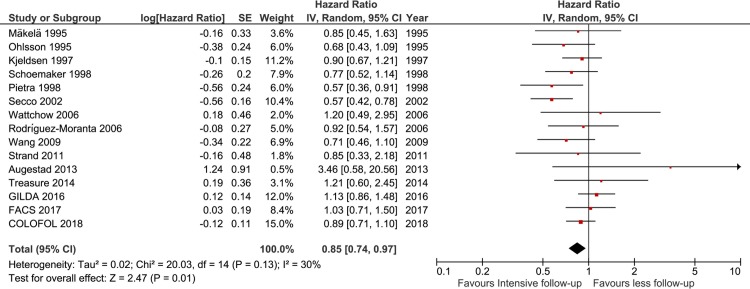
The analyses included 17 trials with a total of 8039 patients. Compared with less follow-up, intensive follow-up significantly improved overall survival in patients with nonmetastatic colorectal cancer after radical surgery (HR 0.85, 95% CI 0.74–0.97, P = 0.01; I2 = 30%; high quality). Subgroup analyses showed that differences between intensive-frequency and intensive-test follow-up (P = 0.04) and between short interval and long interval of follow-up (P = 0.02) in favor of the former one.
Limitations
Clinical heterogeneity of interventions
Conclusions
For patients with nonmetastatic colorectal cancer after curative resection, intensive follow-up strategy was associated with an improvement in overall survival compared with less follow-up strategy. Intensive-frequency follow-up strategy was associated with a greater reduction in mortality compared with intensive-test follow-up strategy.
Introduction
Colorectal cancer is estimated to have affected 140 250 patient in 2018 in the United States. [1] It is the third most common cancer, and the second most common cause of cancer-related deaths. Total tumor resection remains the primary management option in patients with non-metastatic disease. However, the appropriate surveillance strategy after primary surgery is poorly defined. Some have argued for a more intensive follow up for early detection of tumor recurrence or metachronous disease.[2] However, others have argued against the need for intensive follow-up.[3] Additionally, the costs of the different follow-up strategies vary, range from hundreds to several thousands of dollars per patient, thus influencing the implementation of the different follow up strategies.[4]
Current follow up guidelines following complete resection vary. Most agree that these patients should at least undergo 5 year surveillance with computed tomography (CT scan, carcinoembryonic antigen testing (CEA), and lower GI endoscopy.[5–7] Individual trials[8, 9] have generally been underpowered; with the last two systematic reviews[10, 11] failing to show any survival benefit from intensive follow-up. Conclusions are though limited by the small sample sizes, relatively short follow-up, and modest trial quality. More recently, the results of a new randomized trial—the COLOFOL (A Pragmatic Study to Assess the Frequency of Surveillance Tests After Curative Resection in Patients With Stage II and III Colorectal Cancer) trial which was the largest trial on this topic—along with the results of the updated analysis of the FACS (Follow-up After Colorectal Surgery) trial and the GILDA (Gruppo Italiano Lavoro per la Diagnosi Anticipata) trial which were the second and the third largest trials, respectively, changed the landscape of evidence.[8, 12, 13] Thus, we performed an updated, systematic review of randomized clinical trials to determine the effects of intensive follow-up among patients with nonmetastatic colorectal cancer.
Methods
Protocol and guidance
This systematic review was conducted according to the protocol which was registered in PROSPERO database (CRD42018100574). We used established methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions[14] to conduct the meta-analysis and reported the findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA) [15].
Eligibility criteria
Eligible studies met the following PICOS criteria:
Population: nonmetastatic colorectal cancer patients of any age who have been treated with curative surgery.
Intervention: intensive follow-up strategies with control follow-up regimens, which were defined by the individual studies according to the diagnostic tests and frequency of monitoring.
Comparison intervention: less follow-up regimens, which was defined by the individual trials
Primary Outcome: overall survival (measured from the time of randomization in the study). Secondary outcomes were cancer-specific survival, relapse-free survival, salvage surgery, and interval recurrences.
Study design: randomized controlled trial.
Trials including patients with advanced cancer (e.g. Dukes’ stage D), when curative resection is generally not possible, where excluded.
Information sources and search strategy
The search strategy was developed and executed with an experienced research librarian (PX) and was independently peer-reviewed by another investigator (YC). Medline, Embase, the Cochrane Central Register of Controlled Trials were searched electronically from inception until June 30, 2018, update on June 5, 2019. A second librarian independently peer-reviewed the search strategy. We consulted ClinicalTrials.gov and European Union Clinical Trials Register for ongoing studies and those completed with reported results. We also searched conference proceedings from American Society for Clinical Oncology, European Society for Therapeutic and Radiation Oncology, International Journal of Radiation Oncology Biology Physics: proceedings of the American Society for Radiation. Database searches were supplemented by screening the reference lists of relevant trials and reviews. No language restrictions were imposed. Details of the search strategy are presented in S1 File.
Study selection
Two independent investigators (Yaqin Zhao and Yu Zhang) screened the titles and abstracts of reports. They screened the full text for potentially relevant studies when both agreed that a citation met the eligibility criteria. Disagreements between the investigators were resolved by consultation with a third investigator (FF). We contacted study authors to obtain missing information and unpublished data when needed to assess the inclusion criteria or when suitable data were not available.
Data collection process
Two independent investigators (Yaqin Zhao and Yu Zhang) extracted data in duplicate using a pre-piloted standardized data-form and created tables for the evidence and outcomes. Articles reporting on the same trial at different follow-up timepoints were considered as a single trial for all analyses. Disagreements between the two investigators were resolved by consultation with a third investigator (FF).
Assessment of risk of bias and quality of evidence
Two independent investigators (Yaqin Zhao and Yu Zhang) performed risk assessment following the approach in the Cochrane Handbook for Systematic Reviews of Interventions[16]. We assessed the following domains for each study: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias. We classified trials at high risk of bias if at least one domain was high risk. To evaluate the quality (certainty) of evidence for each outcome, we used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidance to assess the overall risk of bias, inconsistency, imprecision, indirectness and publication bias and summarized results in an evidence profile. [17]
Data synthesis
We used RevMan 5.3.3 software (a freeware available from The Cochrane Collaboration) to conduct all analyses. All pooling analyses were done using random-effect model regardless of the level of heterogeneity because the included trials varied in clinical and methodological features. Time-to-event outcomes are most appropriately analyzed using hazard ratio (HR). For dichotomous outcomes, we calculated the odds ratio (OR) with 95% CI. We assessed heterogeneity using the Chi2 test (threshold p = 0.10), which was quantified using the I2 test (I2 > 50% being substantial). A P-value <0.05 was set for statistical significance. We planned to use a funnel plot to explore the possibility of publication bias when 10 or more trials were pooled.[18]
We conducted trial sequential analysis (TSA) for primary outcomes to explore whether cumulative data were adequately powered to evaluate outcomes.[19] An optimal information size set to a 2-sided 5% significance, 80% power, relative risk reduction of 20%, and the pooled control-group event rate across the included studies.
We prospectively identified 2 variables for subgroup analyses based on the main controversies in the debate: how frequently and what tests should patients be followed up with. We planned subgroup analyses for primary outcome including the following: 1. intensive follow-up strategy (intensive-frequency follow-up and intensive-test follow-up). Intensive-frequency follow-up was defined as more frequentl intensive follow-up with the same tests; intensive-test follow-up was defined as intensive follow-up with more types of tests at the same frequency. 2. intensive follow-up strategy on frequency of follow-up (short interval≤3 months and long interval >3 months) 3. intensive follow-up strategy on using CT (CT and no CT); 4. intensive follow-up strategy on using CEA (CEA and no CEA).
We perform post-hoc meta-regression based on frequency of follow-up, length of follow-up, mean age, and Dukes’ stage.
We planned sensitivity analyses by performing meta-analyses of results after removing 1 study at a time and removing earlier studies (before 2000).
Results
Study selection and study characteristics
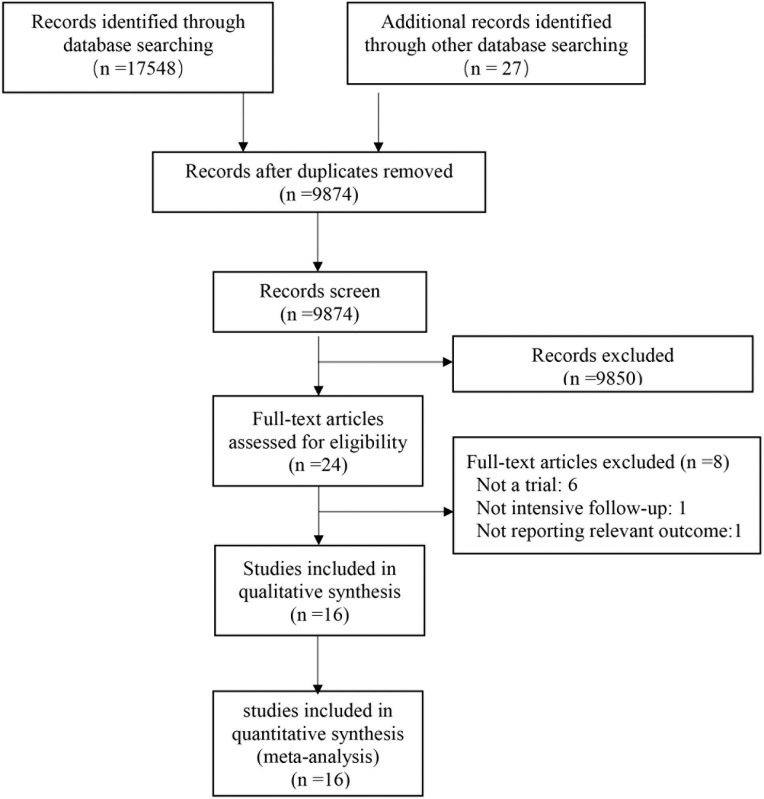
The results of the literature search are shown in Fig 1. Of the 17575 results, 24 pertinent studies were identified and included in full-text review. After review, 8 studies were excluded because they did not meet the inclusion criteria (S1 File).[20–27] A total of 16 trials were selected for the present analysis, including a total of 7908 patients.[8, 12, 13, 28–40] The kappa for systematic searches, selection of studies and data extraction were 1.00, 0.89 and 0.97, respectively.
Fig 1. Flow diagram of the literature search.
Table 1 shows the main characteristics of selected trials. The trials were published between 1995 and 2018. Population sizes ranged from 106 to 2555. Seven trials[12, 30, 31, 33, 34, 38, 39] compared intensive follow-up strategy with less follow-up strategy, whereas 4 trials[13, 32, 35, 36] compared intensive follow-up group with no/minimal any follow-up. The frequency of the follow-up strategy in the included trials varied. Most of the trials carried out follow-up every 3 months for 2 years, then every 6 months for 3 years.
Table 1. The characters of included trials.
| Author | Year | No patients | Country | Dukes’ stage | Intervention group | Control group | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Augestad | 2013 | 110 | Norway | Dukes' A: 24; Dukes' B: 55; Dukes' C: 32 | Surgeon follow-up | GP follow-up | 24 |
| COLOFOL | 2018 | 2555 | Sweden | Unclear | CT (thorax and abdomen) and CEA at 6, 12, 18, 24, and 36 months | CT (thorax and abdomen) and CEA at 12 and 36 months | 60 |
| GILDA | 2016 | 1228 | Italy, Spain, US | Dukes' B: 617; Dukes' C: 611 | 4, 8, 12, 16, 20, 24, 30, 36, 42, 48, and 60 monthly office visits and history and clinical examination, FBC, CEA, and CA 19–9; Colonoscopy and CXR at 12, 24, 36, 48, and 60 months; Liver ultrasound at 4, 8, 12, 16, 24, 36, 48, and 60 months; For rectal participants, pelvic CT at 4, 12, 24, and 48 months | 4, 8, 12, 16, 20, 24, 30, 42, 48, and 60 monthly office visits, including history, examination, and CEA; Colonoscopy at 12 and 48 months; Liver ultrasound at 4 and 16 months; Rectal cancer participants in addition had rectoscopy at 4 months, CXR at 12 months, and liver US at 8 and 16 months. A single pelvic CT was allowed if a radiation oncologist required it as baseline following adjuvant treatment | 96 |
| Kjeldsen | 1997 | 597 | Denmark | Dukes' A 138; Dukes' B: 293; Dukes' C: 166 | At 6, 12, 18, 30, 36, 48, 60, 120, 150 and 180 months, digital rectal examination, colonoscopy, CXR: the same in both groups. | At 60, 120, 180 months, digital rectal examination, colonoscopy, CXR: the same in both groups. | 132 |
| Mäkelä | 1995 | 106 | Finland | Dukes' A: 28; Dukes' B: 48; Dukes' C: 30 | participants who had rectal or sigmoid cancers had flexible sigmoidoscopy with video imaging every 3 months, colonoscopy at 3 months (if it had not been done pre-operation), then annually. They also had ultrasound of the liver and primary site at 6 months, then annually. | participants who had rectal and sigmoid cancers had rigid sigmoidoscopy and barium enema annually | 60 |
| Ohlsson | 1995 | 107 | Finland | Dukes' A: 19; Dukes' B: 47; Dukes' C: 41 | at 3-, 6-, 9-, 12-, 15-, 18-, 21-, 24-, 30-, 36-, 42-, 48-, and 60-month intervals. Performed at each visit were clinical exam, rigid proctosigmoidoscopy, CEA, alkaline phosphatase, gamma-glutaryl transferase, faecal haemoglobin, and CXR. Examination of anastomosis (flexible sigmoidoscopy or colonoscopy, as dictated by the lesion) was performed at 9, 21, and 42 months. Colonoscopy was performed at 3, 15, 30, and 60 months. CT of the pelvis was performed at 3, 6, 12, 18, and 24 months. | no follow-up visits planned. They received written instructions recommending that they leave faecal samples with the district nurse for examination every third month during the first 2 years after surgery then once a year. They were instructed to contact the surgical department if they had any symptoms. | 66–105.6 |
| Pietra | 1998 | 207 | Italy | Dukes' A: 0; Dukes' B: 122; Dukes' C: 85 | At 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, and 60 months, then annually thereafter. At each visit, clinical examination, ultrasound, CEA, and CXR were performed. Annual CT and colonoscopy were performed. | At 6 and 12 months, then annually. At each visit, clinical examination, CEA, and ultrasound were performed. Annual CXR, colonoscopy, and CT were performed. | 60 |
| FACS | 2017 | 1202 | UK | Dukes' A: 254; Dukes' B: 553; Dukes' C: 354 | (1) CEA follow-up: measurement of blood CEA every 3 months for 2 years, then every 6 months for 3 years, with a single chest, abdomen, and pelvis CT scan at 12–18 months if requested at study entry by hospital clinician (n = 300). (2) CT follow-up: CT of the chest, abdomen, and pelvis every 6 months for 2 years, then annually for 3 years (n = 299). (3) CEA and CT follow-up: both blood CEA measurement and CT imaging as above (n = 302). | no scheduled follow-up except a single CT scan of the chest/abdomen/pelvis if requested at study entry by a clinician | 106 |
| Rodriguez-Moranta | 2006 | 259 | Spain | Unclear | Seen with history, examination, and bloods (including CEA) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, and 60 months. US/CT at 6, 12, 18, 24, 30, 36, 42, 48, and 56 months. CXR and colonoscopy at 12, 24, 36, 48, and 56 months | Seen with history, examination, and bloods (including CEA) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, and 60 months | 48 |
| Schoemaker | 1998 | 325 | Australia | Dukes' A: 71; Dukes' B: 153; Dukes' C: 101 | Participants in the experimental arm underwent yearly CXR, CT of the liver, and colonoscopy. | These investigations were only performed in the control group if indicated on clinical grounds or after screening test abnormality, and at 5 years of follow-up, to exclude a reservoir of undetected recurrences. | 60 |
| Secco | 2002 | 227 | Italy | Unclear | They had clinic visits and serum CEA, abdomen/pelvic US scans, and CXR. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR. | Minimal follow-up programme performed by physicians | 61.5 48 |
| Sobhani | 2008 | 130 | French | Unclear | PET performed at 9 and 15 months and conventional follow-up | conventional follow-up | 24 |
| Strand | 2011 | 110 | Sweden | Unclear | surgeon-led follow-up | nurse-led follow-up | 60 |
| Treasure | 2014 | 216 | UK | Dukes' A: 10; Dukes' B: 95; Dukes' C: 74 | Second-look laparotomy | No further action was taken | 300 |
| Wang | 2009 | 326 | China | Dukes' A: 53; Dukes' B: 186; Dukes' C: 93 | Colonoscopy at 3-month intervals for 1 year, at 6-month intervals for the next 2 years, and once a year thereafter | Colonoscopy at six months, 30 months, and 60 months postoperatively | 64–79 |
| Wattchow | 2006 | 203 | Australia | Dukes' A: 47; Dukes' B: 96; Dukes' C: 60 | Follow by surgeons: more ultrasound, colonoscopy and sigmoidoscopy. CEA, CT, Rx, endoscopy: the same in both groups. | Follow-up by general practitioners: more fecal occult blood. CEA, CT, Rx, endoscopy: the same in both groups. | 24 |
Risk of bias and quality of evidence
Risk of bias assessments are reported in S1 File. None trial was judged as low risk of bias, 11 were unclear risk, and 5 were high risk. Table 2 shows GRADE summary of all outcomes.
Table 2. Summary of findings and strength of evidence in studies of the effects of intensive follow-up among patients with nonmetastatic colorectal cancer.
| Outcome | No. of patients (Studies) |
Relative effect (95% CI) |
I2 | Absolute effect estimates (per 1000) |
Strength of Evidence (GRADE) |
||
|---|---|---|---|---|---|---|---|
| Less Follow-up | Intensive Follow-up | Difference | |||||
| Overall survival | 7170 (15) |
HR 0.85 (0.74 to 0.97) |
36% | 240 | 206 | -34 (-7 to -59) | High |
| Colorectal survival | 4003 (9) |
HR 0.90 (0.77 to 1.04) |
0% | 112 | 105 | -7 (-9 to 53) | High |
| Relapse-free survival | 5359 (13) |
HR 1.04 (0.94 to 1.16) |
0% | 113 | 120 | 7 (-11 to 27) | High |
| Salvage surgery | 4558 (13) |
OR 2.23 (1.59 to 3.12) |
62% | 62 | 128 | 66 (33 to 109) | Moderate1 |
| Interval recurrences | 5832 (8) |
OR 0.72 (0.44 to 1.19) |
82% | 147 | 110 | -37 (-76 to 23) | Low1,2 |
CI: Confidence interval; HR: Hazard ratio; OR: odds ratio
1 inconsistency
2 imprecisions
Primary outcome: Overall survival
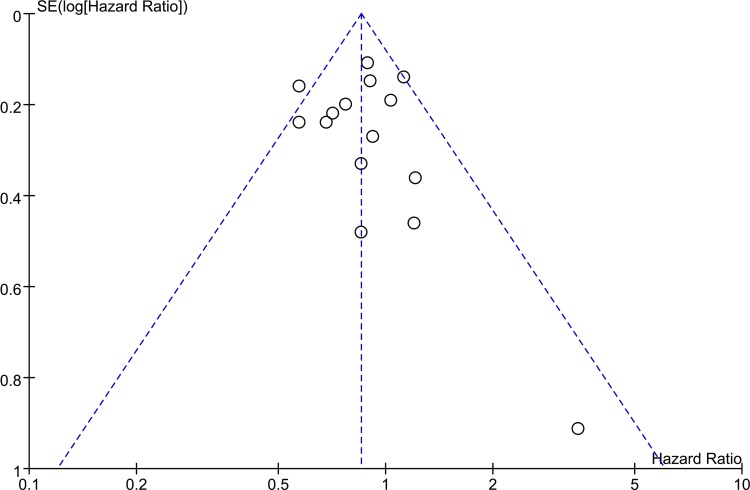
The associations between intensive versus less follow-up and overall survival are shown in Fig 2. Fifteen trials reported outcomes of all-cause mortality. Overall, all-cause mortality was 21.7% (784/3604) in the intensive follow-up group and 24.0% (857/3566) in the less follow-up group (OR 0.82, 95% CI 0.70–0.96; I2 = 29%). Forteen trials reported the data of time-to-event. Pooled HR showed a protective effect of an intensive follow-up strategy on overall survival (HR 0.84, 95% CI 0.74–0.96; I2 = 33%; high quality). Funnel plot analysis did not suggest any asymmetry (Fig 3), and the Egger test did not detect significant publication bias (P = .11). Moreover, TSA confirmed that the required information size was met (S1 File)
Fig 2. Forest plot of overall survival of all trials.
df = degrees of freedom, M-H = Mantel-Haenszel.
Fig 3. Funnel plot analysis of overall survival.
Cancer-specific survival and relapse-free survival
Nine trials presented outcomes of cancer-specific survival. No significant differences were found between intensive and less follow-up in cancer-related survival (HR 0.90, 95% CI 0.77–1.04, I2 = 0%, S1 File). Fourteen trials provided data on relapse-free survival. Also, a pooled HR failed to show a significantly protective effect of intensive follow-up (HR 1.04, 95% CI 0.94–1.16, I2 = 0%).
Salvage surgery and interval recurrences
Among 13 trials, salvage surgery occurred in 11.9% patients in intensive follow-up group and in 6.2% patients in less follow-up group. The odds of salvage surgery in patients receiving intensive follow-up was 2 times as high as that in patients receiving less follow-up (OR 2.23, 95% CI: 1.59–3.12, I2 = 45%, S1 File). Eight trials presented outcomes of interval recurrences. The meta-analysis showed no significant difference in interval recurrences according to the surveillance strategy (OR 0.72, 95% CI 0.44 to 1.19, I2 = 83%, S1 File)
Subgroup analyses, meta-regression, and sensitivity analyses
Overall survival was significantly higher among trials with intensive-frequency follow-up strategy (HR 0.82, 95% CI 0.69–0.97; P for interaction = 0.04; Table 3 and S1 File) and among trials with short interval of follow-up (HR 0.75, 95% CI 0.64–0.87; P for interaction = 0.02; S1 File). Subgroup analyses based on type of tests (CT or CEA) revealed no significant interactions with study settings). Sensitivity analyses (removing 1 study at a time and removing early studies) showed similar results of overall survival (S1 File). Meta-regression showed no interaction based on frequency of follow-up, length of follow-up, mean age, and Dukes’ stage (S1 File).
Table 3. Subgroup analysis of the effect of intensive follow-up on overall survival.
| Subgroup title | Trial | I2 | HR (95% CI) | P |
|---|---|---|---|---|
| Intensive follow-up strategy | ||||
| Intensive frequency | 4 | 17% | 0.82 [0.69, 0.97] | 0.04 |
| Intensive test | 4 | 0% | 1.07 [0.88, 1.31] | |
| Frequency of follow-up | ||||
| Short interval(≤3 months) | 9 | 11% | 0.75 [0.64, 0.87] | 0.02 |
| Long interval (>3 months) | 4 | 0% | 0.96 [0.84, 1.11] | |
| Using CEA | ||||
| CEA | 5 | 0% | 0.97 [0.84, 1.13] | 0.66 |
| No CEA | 1 | - | 0.90 [0.67, 1.21] | |
| Using CT | ||||
| CT | 7 | 0 | 0.93 [0.82, 1.06] | 0.57 |
| No CT | 2 | 53% | 1.31 [0.40, 4.23] | |
Discussion
This meta-analysis demonstrates that, intensive follow-up significantly improved overall survival in patients with nonmetastatic colorectal cancer after radical surgery. Moreover, this meta-analysis suggested that intensive follow-up resulted in a significant increase in the odds of salvage surgery. Finally, this meta-analysis showed no evidence of effects of intensive follow-up on benefits of relapse-free survival or cancer-specific survival.
Compared with other studies
Earlier reviews[41–43] on this topic suggested that intensive follow-up was associated with improved survival. Yet, contrary to our study, the last two reviews[10, 11] which were carried out in 2016 failed to replicate the benefits on survival. A Cochrane review, including a total of 15 trials randomizing 5403 patients after surgery for colorectal cancer, failed to identify credible effect on survival from intensive follow-up (HR 0.90; 95% CI 0.78–1.02). In parallel, an additional systematic review by Mokhles et al., which included 16 trials and 7081 patients, also failed to find a favorable effect on survival (HR 0.98; 95% CI 0.87–1.11).
Differences between our study and the last studies[10, 11] might be explained by our study including a recently published trail and two updated trial, which were the top 3 trials on this topic and accounted for 63.0% (4985/7908) of the total number of patients. Our meta-analysis has made it possible to provide improved precision concerning the effects of intensive follow-up and met minimum information size in TSA. Further, we quantified two new findings of subgroup analyses that frequently intensive follow-up has a reasonable survival profile in nonmetastatic colorectal cancer.
Strengths and limitations
Strengths of this meta-analysis include a utilizing a published protocol in PROSPERO, a comprehensive search, duplicable assessment of eligibility, risk of bias, and data abstraction, the use of a more conservative random effects model, no restriction on language or time of publication for included trials, and the use of an outcome measure which incorporates the time-to-event nature of the data. Strengths of this study also include rigorous assessment of the quality of evidence (and found the quality for primary outcome high), of the TSA (and met the minimum information size), of the credibility of subgroup analyses (and identified crucial differences in frequency of intensive follow-up).
This study has limitations. First, the results of this meta-analysis were weakened by significant heterogeneity of definition of intervention across included trials, with a moderate degree of detected heterogeneity for the primary outcome (I2 = 30%), justifying the use of random-effects models. To explore the source of heterogeneity, we conducted several subgroup analyses. In subgroup analyses based on surveillance strategy (intensive-frequency follow-up and intensive-test follow-up), heterogeneity could be resolved, and significant subgroup difference was found.
Second, the protocol of including trials was developed several decades ago; since then, treatments and diagnostic techniques for colorectal cancer have evolved. For example, some trials were initiated in an era before the widespread use of the CEA blood test as a monitoring test for recurrence of colorectal cancer. However, specificity and sensitivity in detecting disease recurrence depends largely on the definition of abnormal CEA levels. However, the cutoff CEA varies in era and location.
Third, this review did not report adverse effects. Those were rare events, and thus this review was underpowered to evaluate the safety. Observational studies may be more appropriate than trials to evaluate the safety, as these often include more individuals and follow-up may be longer.
Applicability
In practice, there was considerable variance in strategy for surveillance after surgery for colorectal cancer. [44, 45] The follow-up strategies vary in major societies. [5–7] There are also considerable differences in the costs of in the different health systems and reimbursed ways of the services, ranging from hundreds to several thousands of dollars per patient.[4, 46] The results of this study suggest that intensive follow-up for patients with colorectal cancer after curative surgery improves overall survival. Meta-analysis of subgroups investigating frequently intensive follow-up suggests a favorable effect on all-cause mortality. According to these findings, follow-up should be considered at every 3 months. Moreover, intensive follow-up strategies have potentially important resource and financial implications for health services. Application of economic analysis is beyond the scope of this study; however, the present study should serve as a basis for health economic modeling in future studies.
Conclusion
Among patients with colorectal cancer after curative surgery, intensive follow-up strategy was associated with an improvement in overall survival. This benefit was observed in intensive-frequency follow-up strategy but not intensive-test follow-up strategy.
Supporting information
(DOCX)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work is supported by the projects of National Natural Science Foundation of China (contract/grant number: 81100925, 81472361). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. Epub 2018/01/10. 10.3322/caac.21442 . [DOI] [PubMed] [Google Scholar]
- 2.Colibaseanu DT, Mathis KL, Abdelsattar ZM, Larson DW, Haddock MG, Dozois EJ. Is curative resection and long-term survival possible for locally re-recurrent colorectal cancer in the pelvis? Dis Colon Rectum. 2013;56(1):14–9. Epub 2012/12/12. 10.1097/DCR.0b013e3182741929 . [DOI] [PubMed] [Google Scholar]
- 3.Waghorn A, Thompson J, McKee M. Routine surgical follow up: do surgeons agree? BMJ (Clinical research ed). 1995;311(7016):1344–5. Epub 1995/11/18. 10.1136/bmj.311.7016.1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgo KS, Vernava AM, Longo WE, McKirgan LW, Johnson FE. Cost of patient follow-up after potentially curative colorectal cancer treatment. Jama. 1995;273(23):1837–41. Epub 1995/06/21. . [PubMed] [Google Scholar]
- 5.Glimelius B, Tiret E, Cervantes A, Arnold D, Group EGW. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi81–8. Epub 2013/10/23. 10.1093/annonc/mdt240 . [DOI] [PubMed] [Google Scholar]
- 6.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64–72. Epub 2013/10/23. 10.1093/annonc/mdt354 . [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(35):4465–70. Epub 2013/11/14. 10.1200/JCO.2013.50.7442 . [DOI] [PubMed] [Google Scholar]
- 8.Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27(2):274–80. Epub 2015/11/19. 10.1093/annonc/mdv541 . [DOI] [PubMed] [Google Scholar]
- 9.Sanoff HK. Best Evidence Supports Annual Surveillance for Resected Colorectal Cancer. Jama. 2018;319(20):2083–5. Epub 2018/05/26. 10.1001/jama.2018.5817 . [DOI] [PubMed] [Google Scholar]
- 10.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. The Cochrane database of systematic reviews. 2016;11:CD002200 Epub 2016/11/25. 10.1002/14651858.CD002200.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, et al. Meta-analysis of colorectal cancer follow-up after potentially curative resection. The British journal of surgery. 2016;103(10):1259–68. Epub 2016/08/05. 10.1002/bjs.10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wille-Jorgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, et al. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. Jama. 2018;319(20):2095–103. Epub 2018/05/26. 10.1001/jama.2018.5623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, et al. A randomised controlled trial to assess the cost-effectiveness of intensive versus no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health technology assessment (Winchester, England). 2017;21(32):1–86. Epub 2017/06/24. 10.3310/hta21320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.JPT Higgins SGE. the Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, Oxford. 2011;Version 5.1.0.
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W65–94. Epub 2009/07/23. 10.7326/0003-4819-151-4-200908180-00136 . [DOI] [PubMed] [Google Scholar]
- 16.Shinichi A. Cochrane Handbook for Systematic Reviews of Interventions. Online Kensaku. 2014;35(3):154–5. . [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed). 2008;336(7650):924–6. Epub 2008/04/26. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. Epub 1997/10/06. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–9. Epub 2008/04/16. 10.1016/j.jclinepi.2007.10.007 . [DOI] [PubMed] [Google Scholar]
- 20.Detry R. Follow-up after curative surgery for colorectal cancer. Acta Gastroenterol Belg. 2001;64(3):268–71. Epub 2001/10/30. . [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Mayer RJ. Follow-up strategies after curative resection of colorectal cancer. Semin Oncol. 2003;30(3):349–60. Epub 2003/07/19. . [DOI] [PubMed] [Google Scholar]
- 22.Papagrigoriadis S. Follow-up of patients with colorectal cancer: the evidence is in favour but we are still in need of a protocol. Int J Surg. 2007;5(2):120–8. Epub 2007/04/24. 10.1016/j.ijsu.2006.04.004 . [DOI] [PubMed] [Google Scholar]
- 23.Renehan AG, O'Dwyer ST , Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ (Clinical research ed). 2004;328(7431):81 Epub 2004/01/13. 10.1136/bmj.328.7431.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder RA, Hu C, Cuddy A, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. Jama. 2018;319(20):2104–15. 10.1001/jama.2018.5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verberne CJ, Zhan Z, van den Heuvel E, Grossmann I, Doornbos PM, Havenga K, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: Results of the randomized "CEAwatch" trial. Eur J Surg Oncol. 2015;41(9):1188–96. Epub 2015/07/18. 10.1016/j.ejso.2015.06.008 . [DOI] [PubMed] [Google Scholar]
- 26.Wichmann MW, Muller C, Hornung HM, Lau-Werner U, Schildberg FW, Colorectal Cancer Study G. Results of long-term follow-up after curative resection of Dukes A colorectal cancer. World J Surg. 2002;26(6):732–6. Epub 2002/06/08. 10.1007/s00268-002-6221-z . [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Chan SW, You L, Wen Y, Peng L, Liu W, et al. The effectiveness of a self-efficacy-enhancing intervention for Chinese patients with colorectal cancer: a randomized controlled trial with 6-month follow up. Int J Nurs Stud. 2014;51(8):1083–92. Epub 2014/01/11. 10.1016/j.ijnurstu.2013.12.005 . [DOI] [PubMed] [Google Scholar]
- 28.Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold T, et al. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ open. 2013;3(4). 10.1136/bmjopen-2012-002391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.E. S, I. N, L. B, K. S. Nurse or surgeon follow‐up after rectal cancer: a randomized trial. Colorectal Disease. 2011;13(9):999–1003. 10.1111/j.1463-1318.2010.02317.x [DOI] [PubMed] [Google Scholar]
- 30.Kjeldsen BJ, Kronborg O, Fenger C, Jorgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. The British journal of surgery. 1997;84(5):666–9. Epub 1997/05/01. . [PubMed] [Google Scholar]
- 31.Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: Results of a prospective randomized trial. Archives of Surgery. 1995;130(10):1062–7. 10.1001/archsurg.1995.01430100040009 [DOI] [PubMed] [Google Scholar]
- 32.Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum. 1995;38(6):619–26. Epub 1995/06/01. 10.1007/bf02054122 . [DOI] [PubMed] [Google Scholar]
- 33.Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum. 1998;41(9):1127–33. Epub 1998/09/28. 10.1007/bf02239434 . [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Moranta F, Saló J, Arcusa À, Boadas J, Piñol V, Bessa X, et al. Postoperative Surveillance in Patients With Colorectal Cancer Who Have Undergone Curative Resection: A Prospective, Multicenter, Randomized, Controlled Trial. Journal of Clinical Oncology. 2006;24(3):386–93. 10.1200/JCO.2005.02.0826 [DOI] [PubMed] [Google Scholar]
- 35.Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114(1):7–14. Epub 1998/01/15. 10.1016/s0016-5085(98)70626-2 . [DOI] [PubMed] [Google Scholar]
- 36.Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol. 2002;28(4):418–23. Epub 2002/07/09. . [DOI] [PubMed] [Google Scholar]
- 37.Sobhani I, Tiret E, Lebtahi R, Aparicio T, Itti E, Montravers F, et al. Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 2008;98(5):875–80. Epub 2008/02/28. 10.1038/sj.bjc.6604263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ open. 2014;4(5). 10.1136/bmjopen-2013-004385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc. 2009;69(3 Pt 2):609–15. Epub 2009/01/13. 10.1016/j.gie.2008.05.017 . [DOI] [PubMed] [Google Scholar]
- 40.Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer. 2006;94(8):1116–21. Epub 2006/04/20. 10.1038/sj.bjc.6603052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ (Clinical research ed). 2002;324(7341):813 Epub 2002/04/06. 10.1136/bmj.324.7341.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. The Cochrane database of systematic reviews. 2002;(1):CD002200 Epub 2002/03/01. 10.1002/14651858.CD002200 . [DOI] [PubMed] [Google Scholar]
- 43.Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, Lopez-Calvino B, Seoane-Pillado T, Pertega-Diaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26(4):644–56. Epub 2014/11/21. 10.1093/annonc/mdu543 . [DOI] [PubMed] [Google Scholar]
- 44.Soreide K, Traeland JH, Stokkeland PJ, Glomsaker T, Soreide JA, Korner H. Adherence to national guidelines for surveillance after curative resection of nonmetastatic colon and rectum cancer: a survey among Norwegian gastrointestinal surgeons. Colorectal Dis. 2012;14(3):320–4. Epub 2011/06/22. 10.1111/j.1463-1318.2011.02631.x . [DOI] [PubMed] [Google Scholar]
- 45.Virgo KS, Wade TP, Longo WE, Coplin MA, Vernava AM, Johnson FE. Surveillance after curative colon cancer resection: practice patterns of surgical subspecialists. Ann Surg Oncol. 1995;2(6):472–82. Epub 1995/11/01. . [DOI] [PubMed] [Google Scholar]
- 46.Richert-Boe KE. Heterogeneity of cancer surveillance practices among medical oncologists in Washington and Oregon. Cancer. 1995;75(10):2605–12. Epub 1995/05/15. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.