Abstract
Do people engaged in joint action form action plans that specify joint outcomes at the group level? EEG was recorded from pairs of participants who performed coordinated actions that could result in different postural configurations. To isolate individual and joint action planning processes, a pre-cue specified in advance the individual actions and/or the joint configuration. Participants had 1200 ms to prepare their actions. Then a Go cue specified all action parameters and participants performed a synchronized action as quickly as possible. Action onsets were shorter when the pre-cue specified the joint configuration, regardless of whether individual action was also specified. EEG analyses showed that specifying joint action parameters in advance reduced ambiguity in a structured joint action plan (reflected in the decrease of the amplitude of the P600) and helped with representing action goals and interpersonal coordination patterns in sensorimotor brain areas (reflected in increased alpha/mu suppression and CNV amplitudes). These results provide clear evidence that joint action is driven not only by action plans that specify individual contributions, but also by action plans that specify joint action outcomes at the group level.
Keywords: Joint action, Social cognition, Action planning, We-representations, EEG
Highlights
-
•
People form individual and group-level representations during joint action planning.
-
•
Information about joint configuration benefits task performance.
-
•
Information about joint configuration reduces ambiguity in joint task representation.
-
•
Evidence for predictive “we-representations” in the sensorimotor system.
-
•
“We-representations” may be formed independently of “I” and “You” representations.
1. Introduction
A wide range of human activities, such as passing a ball, carrying a bed, or performing in a music ensemble, depend on individuals' ability to effectively coordinate their actions (Butterfill, 2011; Clark, 1996; D'Ausilio et al., 2015). It has been postulated that interpersonal coordination can be achieved when co-actors form joint action plans that consist of predictive mental representations of the individual contributions to a joint action (Bolt and Loehr, 2017). Such representations do not only specify abstract outcomes at the cognitive level but also action parameters at the sensorimotor level (Sebanz and Knoblich, 2009). The realization of such joint action plans is made possible through a neural mechanism that matches actions to their perceptual consequences (Hommel et al., 2001; Prinz, 1997), thus allowing individuals to form forward internal models of actions (Wolpert and Flanagan, 2001) in order to simulate and predict the consequences of another person's actions in a similar way as their own actions (Knoblich and Jordan, 2003; Ramnani and Miall, 2004).
An open question is whether joint action plans consist of separate predictive representations for one's own (“I-representations”) and a co-actor's performance (“You-representations”) or whether they include predictive action representations at the group level (Della Gatta et al., 2017; Knoblich et al., 2011; Vesper et al., 2010), also referred to as “We-representations” or the “We-mode” (Gallotti and Frith, 2013). The core idea is that joint action is driven by plans that specify first and foremost what can be achieved by the group and not by what each individual contributes to the joint action. Alternatively, successful joint action could be achieved with action plans that do not explicitly represent the relation between one's own and others' contribution to a joint action.
Empirical evidence for group-level representations has been provided for action mimicry. Tsai et al. (2011) demonstrated that compatibility relations between observed and performed actions at the group level can overrule compatibility relations at the level of individual contributions to a joint action. Later studies have corroborated the role of We-representations for achieving interpersonal synchronization (Novembre et al., 2016; Ramenzoni et al., 2014; Sacheli et al., 2018), and for judging the level of individual and joint control during joint action performance (Dewey et al., 2014; Van der Wel, 2015). A related finding is that joint action partners invest individual effort to reduce joint effort, suggesting that planning at the group level may have priority over individual action planning (Constable et al., 2016; Santamaria and Rosenbaum, 2011).
1.1. Present study
The objective of the present study was to examine whether We-representations are formed during joint action planning and whether We-representations benefit joint action performance. To that end, we developed a pre-cuing task (Rosenbaum, 1980), where a visual pre-cue either specified individual action contributions (i.e. making a hand movement that results in the palm facing inwards or outwards) and/or a joint action configuration, that is, the relation of individual actions required for the joint action (i.e. instructing two co-actors to perform the same or a different hand movement without specifying the movement itself). The participants could use this advance information to prepare a joint action, which they initiated after a visual Go cue that fully specified the joint action to be performed. This means that there was a preparation interval during which participants could form joint action plans before initiating their actions. An important aspect of our design was that in one condition participants were informed in advance of the joint configuration they had to prepare for without the specific individual actions they or their partner needed to perform. Better task performance in this condition would indicate that the formation of We-representations does not require the specification of individual action contributions.
In line with previous work on pre-cueing of individual actions (e.g. Reeve and Proctor, 1984; Rosenbaum and Kornblum, 1982), we hypothesized that participants would be faster to initiate their action when the pre-cue specified parameters of individual action. Importantly, assuming that joint action plans imply representing the relation between co-actors’ actions and that this in turn facilitates implementing joint action plans, participants should be faster to initiate their action when the pre-cue specified aspects of the joint action configuration even when the individual action contributions are not specified.
1.2. EEG investigations of joint action planning
Recording Electroencephalograms (EEG) enabled us to test how joint action plans benefit performance by looking at brain activity in the action planning interval, reflecting the formation of an action plan in response to the pre-cue. We tested whether participants form representations of the joint action plan specifying the joint configuration to be achieved and the two individual actions necessary to achieve this configuration. We expected that specification of the individual action and/or the joint configuration would reduce the ambiguity in the generation of a joint action plan and allow the participants to form more precise action representations at the sensorimotor level.
1.2.1. Disambiguation in action planning
We expected the information that was provided by the pre-cues to reduce ambiguity in this structured representation. This can be investigated through the P600, an Event Related Potential (ERP) that is associated with processing of structured representations in language (Osterhout and Holcomb, 1992), music (Patel et al., 1998), and the action domain (Maffongelli et al., 2015). The P600 over frontal areas, in particular, is believed to reflect processing of ambiguous structures (Friederici et al., 2002; Kaan and Swaab, 2003; Opitz and Kotz, 2012). Consequently, we expected that the P600 would be reduced when the pre-cue reduced ambiguity in a joint action plan by specifying aspects of the joint configuration or the individual actions required to achieve it.
1.2.2. We-representations at the sensorimotor level
Furthermore, we tested whether the information provided by the pre-cues would help participants to form more precise action plans at the sensorimotor level. To this end, we assessed the modulation of the alpha rhythm (∼10Hz) and of the Contingent Negative Variation (CNV) before the onset of the Go stimulus.
The alpha rhythm is the dominant rhythm in the human brain (Bazanova and Vernon, 2013) and suppression of its amplitude reflects activation or disinhibition of the underlying brain areas (Pfurtscheller and Lopes Da Silva, 1999). Alpha suppression over sensorimotor areas (often referred to as the “mu rhythm”) reflects the involvement of the sensorimotor system in the representation of planned, executed, perceived or imagined actions (Behmer and Fournier, 2014; Hari, 2006; Kourtis et al., 2013a, 2013b; Neuper et al., 2005; Pineda, 2005). The suppression of the alpha/mu rhythm is modulated by a person's engagement in social interaction (Oberman et al., 2007; Perry et al., 2011) and it is considered an index of sensorimotor processes that contribute to the representation and the on-line prediction of another person's action goal (Muthukumaraswamy et al., 2004; Southgate et al., 2010).
The CNV is a slow rising ERP of negative polarity that develops during the delay period between an informative cue and a target/imperative stimulus and peaks at approximately the time of a planned response (Walter et al. 1964). The early CNV is related to attentional and orienting processes towards the informative cue, whereas the late CNV is related to stimulus anticipation and movement planning processes (Leuthold et al., 2004). The late CNV is typically larger over premotor areas and it is enhanced when movement parameters (e.g. force, direction) are specified in advance (Ulrich et al., 2003; Wind-Wall et al., 2003). Furthermore, the late CNV is believed to reflect preparation and organization of a motor program that involves multiple actions performed sequentially or synchronously by an individual or by a pair of individuals (Kourtis et al., 2014; Kourtis et al., 2013a, 2013b; Leuthold and Schröter, 2011; Praamstra et al., 2009).
We hypothesized that specifying the joint configuration as well as the individual actions required to achieve it would enable more precise specification of the ensuing action. This, in turn, should be reflected in the enhancement of alpha/mu suppression and the late CNV amplitude over primary sensorimotor and premotor areas, respectively, before the display of the Go stimulus. Thus, enhanced alpha/mu suppression and late CNV during action planning should not only be observed for pre-cues specifying individual actions but also for pre-cues that solely specify the joint configuration.
2. Methods
2.1. Participants
We based our sample size on previous EEG studies investigating joint action planning (e.g. Kourtis et al., 2014). We recruited twenty-four right-handed volunteers (12 females, mean age = 25.5 yrs (SD = 4.4)). All participants had normal or corrected-to-normal vision and took part in the experiment in pairs. Four pairs of participants consisted of two males, four pairs of two females and four pairs were of mixed gender. All participants provided their informed written consent after full explanation of the study. The study was approved by the ethics committee of the Hungarian Psychological Association (EPKEP).
2.2. Experimental setup and task
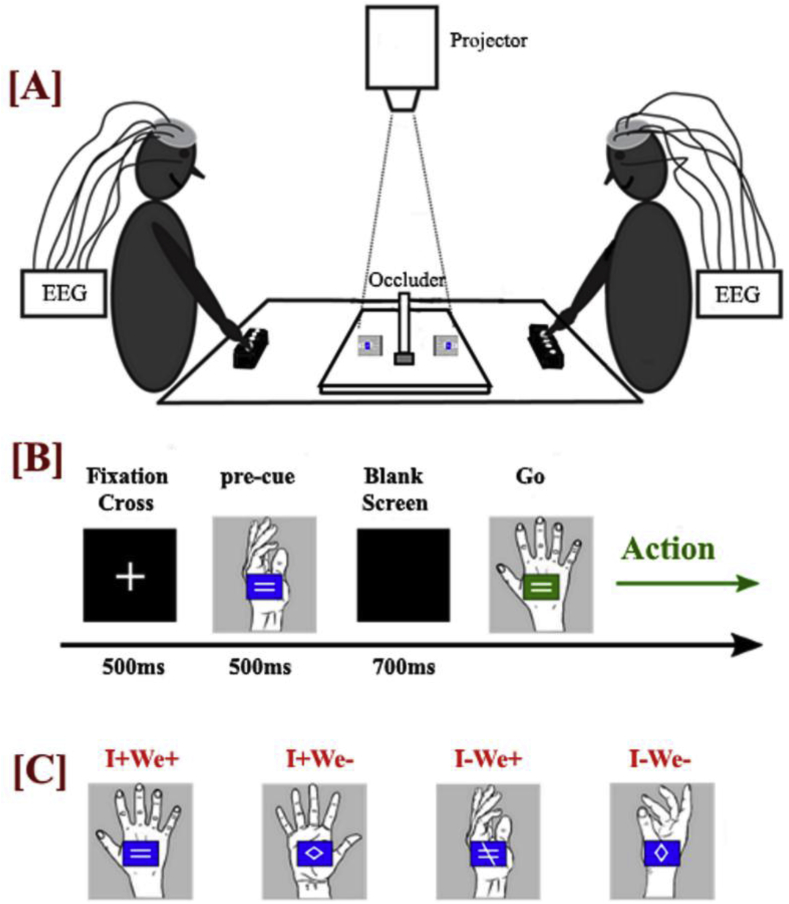
The experiment was run in a quiet, normally illuminated room. EEG was concurrently recorded from two participants who were seated at opposite sides of a rectangular table (Height: 74 cm, Surface: 80 × 80 cm), facing each other (see Fig. 1, Fig. 2). A 50 × 38 x 4 wooden platform was placed centrally on the table. A 40 × 16 × 0.5 cm white wooden occluder was located at the centre of the table. The occluder served to prevent the participants from seeing each other's stimuli. An LCD projector (EPSON, EH-TW490) was used to present the visual stimuli (9 × 9 cm) on the wooden platform at 4 cm distance from either side of the occluder and at a viewing distance of approximately 80 cm. The projector was mounted on the ceiling directly above the middle of the table. Action onsets were recorded by two response boxes (The Black Box Toolkit Ltd, Sheffield, UK), which were placed in front of each participant. All experimental sessions were recorded by a video camera (JVC, CG-XA2) in order to ensure that the participants were correctly performing the designated actions.
Fig. 1.
[A] Schematic drawing of experimental setup. [B] Time-course of a trial. After a fixation cross the pre-cue provided advance information about the joint configuration to be performed, the individual action to be performed, both, or neither. The pre-cue was followed by a blank screen. A Go signal that fully specified the joint configuration and the individual actions indicated to the participants to perform the action with the partner as quickly as possible. [C] Examples of pre-cues for each of the four experimental conditions. From Left to Right: I+We+: Specification of individual action (palm facing outwards) and of joint configuration (partner will perform same action). I+We-: Specification of individual action (palm facing inwards) but not of joint configuration. I-We+: No information regarding individual action, but specification of joint configuration (partner will perform different action). I-We-: No information regarding individual action or joint configuration.
Fig. 2.
Joint actions performed by the participants. In total, there were four different types of joint configurations. In two types of configurations, both participants performed the same individual action (top, both palms facing outwards) and in the other two types of configurations the participants performed different individual actions (bottom, one palm facing outwards, one inwards). Note that the participant on the left performs the same individual action in both instances.
2.3. Procedure and stimuli
Each trial started with a white fixation cross on top of a black background for 500 ms, followed by a pre-cue, presented for 500 ms, providing full, partial or no information regarding the ensuing joint action. The pre-cue was followed by a blank screen for 700 ms, after which a fully-informative Go signal specified all individual and joint action parameters and prompted the participants to act (Fig. 1B).
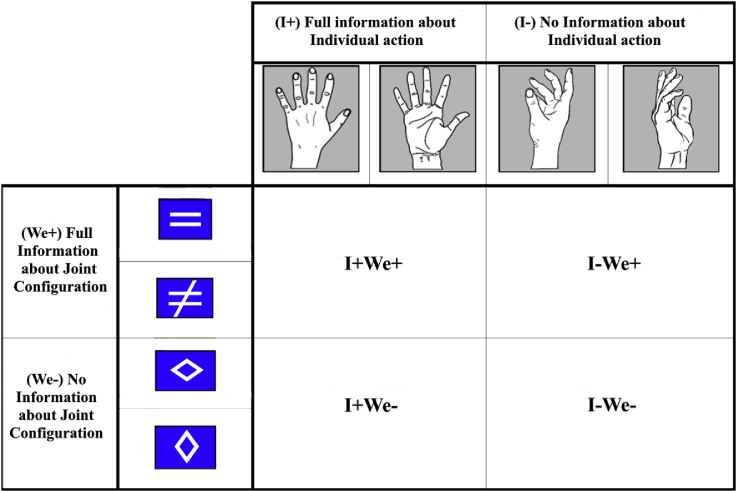
The pre-cue consisted of a drawing of a hand with the palm facing outwards, inwards or sideways. The depicted hand always matched the hand that the participants performed the actions with. The palm orientation provided information regarding the participants’ individual action. For example, a palm facing outwards informed the participant that he/she should plan an arm extension with his/her palm facing outwards (Fig. 2). A palm facing sideways indicated unspecified information about the target hand position. On top of the palm there was a blue box containing one of three white symbols that provided information regarding the joint action configuration that participants were going to produce together: “ = ” for same, “≠” for different, or rhombus for not specified (Figs. 1C and 3).
Fig. 3.
Pre-cues: The columns and rows refer to the aspects of the pre-cue that concerned Individual Action and Joint Configuration, respectively.
Thus, the pre-cue specified either both the individual action and the joint configuration (I+We+), only the individual action (I+We-), only the joint configuration (I-We+), or neither (I-We-). In order to have the same number of stimuli per condition, the rhombus was displayed either horizontally or vertically with equal probability and the sideways hand was displayed with the palm facing either to the left or to the right with equal probability. The (always fully informative) Go stimulus was identical to the Full Information pre-cue, with the exception that the colour of the box was green (Fig. 1B).
The experiment consisted of 8 blocks of approximately 5 ½ min each. Each block consisted of 64 trials, which resulted in 128 trials per condition. The main experiment was preceded by two practice blocks of approximately 3 min each. The participants used their right hand in one practice block and in half of the experimental blocks and their left hand in the remaining blocks. The participants were instructed to always keep the index finger of the acting hand on the designated Response Box button, fixate on the cross, pay attention to the pre-cue and to initiate their action only after the Go cue was displayed. The participants were also instructed to fixate on the cross or the stimuli throughout the trials and to only look towards each other during action performance.
2.4. EEG data acquisition
EEG was recorded continuously from both participants using 32 active electrodes (Acticap, BrainProducts GmbH, Germany) per participant, arranged according to an extended version of the 10–20 system at Fz/3/4/7/8, FCz/1/2/5/6, Cz/3/4, CPz/1/2/5/6, Pz/3/4/7/8, Oz/1/2, and T7/8, using carefully positioned nylon caps. All electrodes were referenced to the right mastoid during recording. Vertical and horizontal eye movements were monitored using two pairs of bipolar EOG electrodes: one positioned under the left and right eyes and one lateral to the left and right eyes. Electrode impedance was kept below 20 kΩ. EEG and EOG signals were amplified with a band-pass filter of 0–250 Hz by two BrainAmp DC Amplifiers (Brain Products GmbH, Gilching, Germany) and sampled at 500 Hz.
2.5. Data processing and analysis
The video recordings of each experimental session were visually examined and participants’ performance was evaluated on a trial-by-trial basis. Trials during which a participant did not perform the action designated by the pre-cue or when the action onset was not recorded for mechanical reasons (e.g. when the response button was not pressed hard enough) were removed from the analyses. Action onset was defined as the time interval between the onset of the Go stimulus and the release of the response button. For each participant, all action onsets that were smaller than 100 ms or differed more than two standard deviations from the mean action onset within each condition were removed from further analysis. We also calculated (on a trial-by-trial basis) the action onsets asynchronies within each pair of participants.
Our EEG analyses focused on the 1200 ms time period between the onset of the pre-cue and the Go cue, during which different types of action were planned but not executed. EEG data processing was performed offline using Brain Vision Analyzer 2.1.0 (Brain Products GmbH, Germany). EEG data were first re-referenced to the mean of both mastoid electrodes. Ocular correction was performed using the Gratton–Coles algorithm (Gratton et al., 1983). The data were then filtered using a low cut-off filter of 0.01Hz (24 dB/octave) and a high cut-off filter of 40 Hz (24 dB/octave) to remove the influence of slow drifts and excessive high-frequency noise, respectively. Then, the data were segmented offline into epochs from 400 ms before until 2300 ms after pre-cue onset.
In addition to the trials that were removed for failing to meet behavioural criteria, semi-automatic artefact rejection was performed before averaging in order to remove individual trials containing remaining vertical eye movements or other EEG-related artefacts. An epoch was rejected when the difference between the maximum and minimum value at a single channel exceeded 100 μV. Averages were constructed separately for each condition and each participant. For the ERP analysis, the baseline was defined as the time period from 200 ms before pre-cue onset until pre-cue onset.
Time-frequency analysis was performed using a continuous complex Morlet waveform transformation as implemented in the Brain Vision Analyzer software. Absolute values of wavelets coefficients were calculated in the frequency range of 3–30Hz with 20 frequency steps and Morlet parameter c = 4. The baseline was defined as the interval from 300 ms until 100 ms before pre-cue onset. After averaging, the frequency layer with central frequency 11.4Hz (Gauss borders 8.5Hz and 14.2Hz), was extracted for subsequent analysis.
Statistical analyses were performed by means of repeated measures ANOVAs (Greenhouse–Geisser corrected). Post-hoc comparisons were performed by means of paired t-tests (Bonferroni correction applied). The regions of interests for EEG analyses were defined on the basis of grand average topographies and previous literature. The selection of the time intervals of analyses were based on the aggregate grand average from trials (AGAT) (Brooks et al., 2017). The P600 peaked around 580 ms after pre-cue onset and it was quantified over fronto-central (electrode FCz) and parietal areas (electrode Pz) as the mean amplitude from 540 to 620 ms after pre-cue onset. Visual inspection of the ERPs showed that the absolute P600 amplitude depended on the amplitude of the preceding N500, which peaked around 490 ms after pre-cue onset. Thus, the P600 was measured on a peak-to-peak basis with respect to the N500, which was quantified as the mean amplitude from 470 to 510 ms after pre-cue onset. The Contingent Negative Variation was quantified as the mean amplitude during the last 200 ms before the onset of the Go stimulus over electrode FCz. The suppression of alpha/mu rhythm was lateralized over sensorimotor areas and it was quantified as the mean amplitude during the last 400 ms before the onset of the Go stimulus over the left and the right hemisphere (electrodes (C3, CP1, and CP5) and (C4, CP2 and CP6), respectively.
3. Results
Behavioural and EEG preliminary analyses showed that the hand (left or right) that participants performed their actions with did not interact with the two main factors of interest: cueing of ‘Individual Action’ and ‘Joint Configuration’ (ps >.05). Thus all analyses are reported on pooled data from both hands.
3.1. Behavioural analyses
The overall percentage of the trials that were removed from the analyses was 11.1%. There was no difference in the percentage of trials removed from different conditions (all ps > .47).
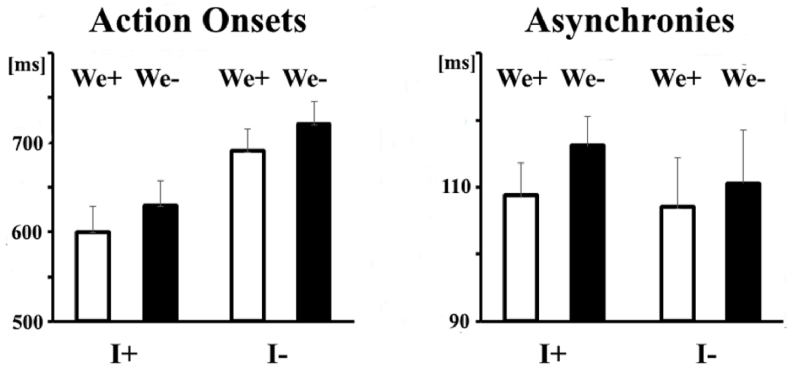
Statistical comparisons were performed by means of repeated measures 2 × 2 ANOVAs (Greenhouse–Geisser corrected) with the factors Individual Action (‘I+’ vs. ‘I-’) and Joint Configuration (‘We+’ vs. ‘We-’). The left panel in Fig. 4 displays the results for action onsets. Participants were faster to initiate their actions when the pre-cue specified the Individual Action (F(1,23) = 47.73, p<.001, np2=.675) or the Joint Configuration (F(1,23) = 26.85, p<.001, np2=.539). The interaction between these factors was not statistically significant (p = .880).
Fig. 4.
Left: Mean Action Onsets were shorter when the pre-cue specified the Individual Action (I+) and/or the Joint Configuration (We+). Right: Action Onset Asynchronies were smaller only when the pre-cue specified the Joint configuration (We+). Error bars represent standard error of the mean.
The right panel of Fig. 4 displays the action onset asynchronies. These were smaller when the pre-cue specified the Joint Configuration (F(1,11) = 9.92, p = .009, np2=.474) but not when the pre-cue specified the Individual Action (p = 0.601). The interaction between these two factors was not significant (p = .377).
The relatively large number of trials allowed us to perform an additional analysis investigating whether the ‘Joint Configuration’ effect depended on the type of Configuration. We conducted a 2 × 2 ANOVAs separately for the ‘Same’ and ‘Different’ configurations. The analysis showed that action onsets were shorter regardless of whether the specified Joint Configuration was “Same” (F(1,11) = 29.31, p<.001, np2=.560) or “Different” (F(1,11) = 17.71, p<.001, np2=.435). However, the action onset asynchronies were smaller only in the case of ‘Same’ Configuration (F(1,11) = 6.27, p = .029, np2=.363), but not in the case of ‘Different’ Configuration (p = .311).
3.2. EEG analyses
3.2.1. Event-related potentials
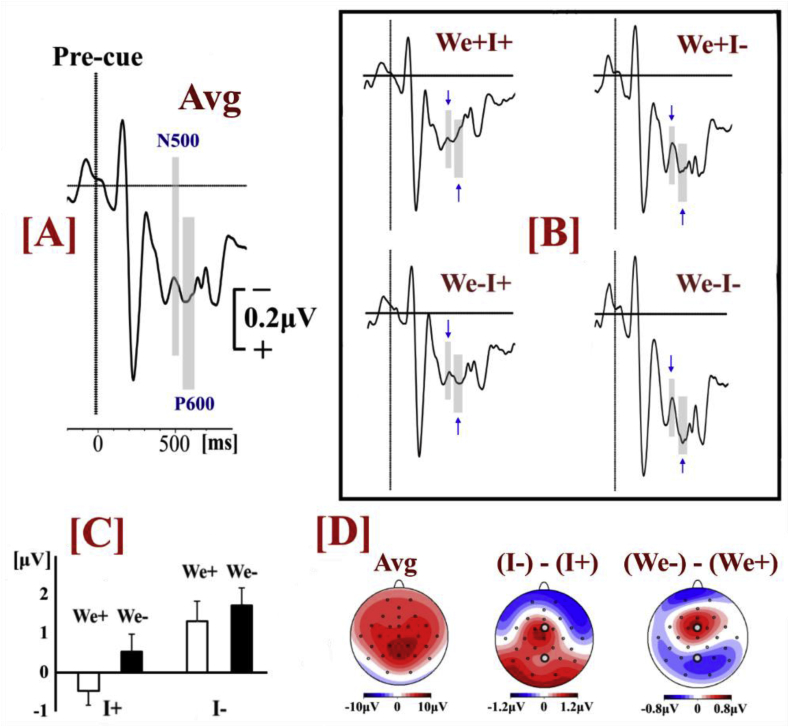
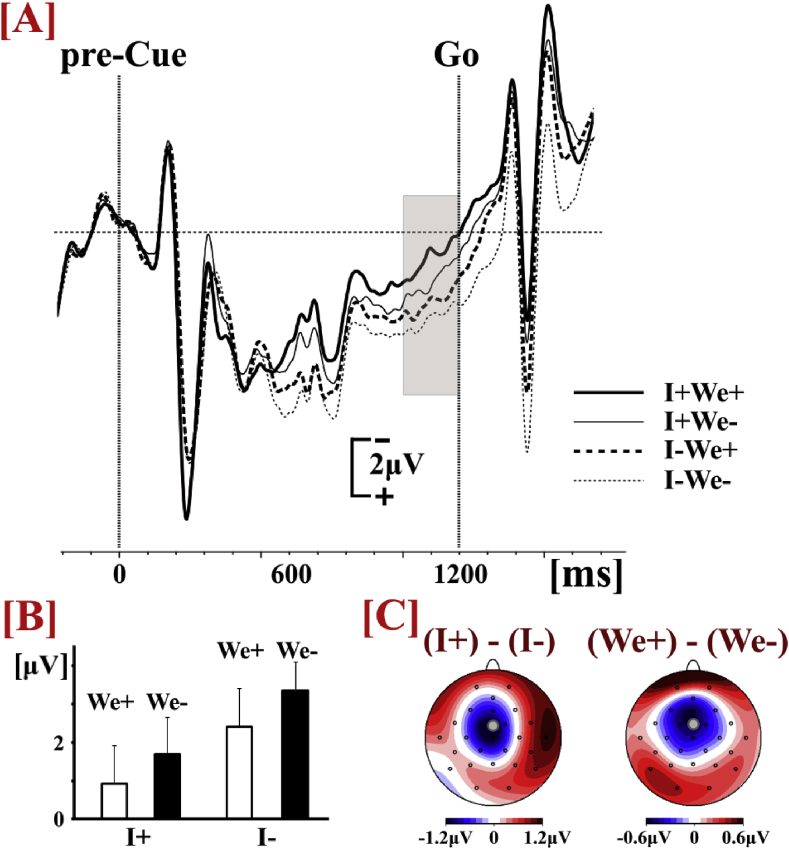
Statistical comparisons on the P600 amplitude (Fig. 5) were performed by means of repeated measures 2 × 2 × 2 ANOVA (Greenhouse–Geisser corrected) with factors Individual Action (‘I+’ vs. ‘I-’), Joint Configuration (‘We+’ vs. ‘We-’) and Location (Fronto-Central (FCz) vs. Parietal (Pz)). The statistical analysis showed that the P600 amplitude was smaller when the pre-cue specified the Individual Action (F(1,23) = 16.12, p = .001, np2=.412). The main effect Joint Configuration was not statistically significant (p = .075); however, there was a significant ROI x Joint Configuration interaction (F(1,23) = 9.95, p = .004, np2=.302). Post-hoc paired t-test (adjusted level of significance a=.025) showed that the P600 amplitude was smaller when the pre-cue specified the Joint Configuration only over fronto-central areas (t(23) = -2.54, p = .018) and not over parietal areas (p = .405). The main effect of ROI and all other interactions were not statistically significant (p>.107).
Fig. 5.
[A] ERP waveforms based on the average data (Avg) of all conditions (electrode FCz). The grey-shaded areas represent the time intervals for the quantification of the peak-to-peak P600 amplitude. [B} ERP waveforms (electrode FCz) in each condition (the arrows point at the N500 and P600 intervals of analysis). [C] Peak-to-peak P600 amplitudes (electrode FCz). [D] Voltage scalp topographies based on average data of all conditions (Avg), the difference between the conditions where the pre-cue did or did not specify the individual action ((I-) – (I+)), and the difference between the conditions where the pre-cue did or did not specify the joint configuration ((We-) – (We+)).
Statistical comparisons on the CNV amplitude (Fig. 6) were performed by means of repeated measures 2 × 2 ANOVA (Greenhouse–Geisser corrected) with factors Individual Action (‘I+’ vs. ‘I-’) and Joint Configuration (‘We+’ vs. ‘We-’). The statistical analysis showed that the CNV amplitude was larger when the pre-cue specified the Individual Action (F(1,23) = 12.51, p = .002, np2=.352), or the Joint Configuration (F(1,23) = 6.44, p = .018, np2=.219). The interaction between these factors was not statistically significant (p = .800).
Fig. 6.
[A]: ERP waveforms based on the average data of all conditions (electrode FCz). The grey-shaded areas represent the time intervals for the quantification of CNV amplitude. [B] CNV amplitudes. [C] Voltage scalp topographies of the difference between the conditions where the pre-cue did or did not specify the individual action ((I-) – (I+)), and the difference between the conditions where the pre-cue did or did not specify the joint configuration ((We-) – (We+)).
3.2.2. Neuronal oscillations
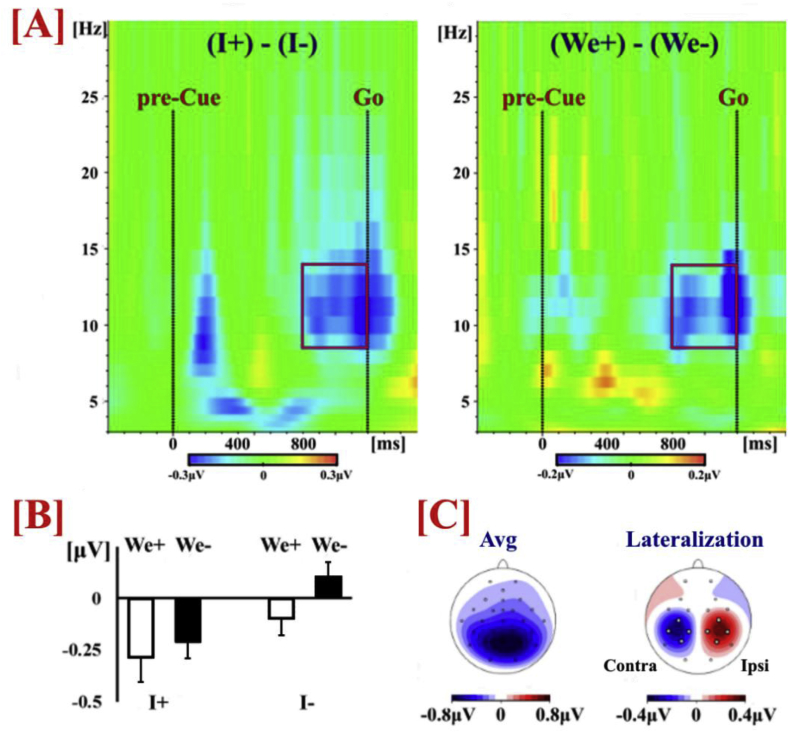
Statistical comparisons on the amplitude of the alpha/mu rhythm (Fig. 7) were performed by means of a repeated measures 2 × 2 × 2 ANOVA (Greenhouse–Geisser corrected) with factors Individual Action (‘I+’ vs. ‘I-’), Joint Configuration (‘We+’ vs. ‘We-’) and Hemisphere (Ipsilateral vs. Contralateral). The statistical analysis showed that alpha suppression was greater not only when the pre-cue specified the Individual Action (F(1,23) = 12.59, p = .002, np2=.354), but also when the pre-cue specified the Joint Configuration (F(1,23) = 5.60, p = .027, np2=.196). In addition, alpha suppression was greater over the contralateral hemisphere to the acting hand (F(1,23) = 6.74, p = .016, np2=.227).
Fig. 7.
[A] Time-frequency plots (average data) of the difference between the conditions where the pre-cue did or did not specify Individual Action (Left) or Joint Configuration (Right). For both factors the difference in alpha suppression (in blue) becomes evident around 400 ms before the onset of the Go cue. [B] Amplitude of alpha/mu rhythm during the last 400 ms before Go onset (average data). [C] Scalp topographies of alpha/mu distribution: Average (Avg) of all conditions and Lateralization of alpha activity before Go onset, created by subtracting the activity of the ipsilateral hemisphere from that of the contralateral hemisphere separately for each hand and then averaging the two differences.
None of the interactions reached statistical significance (ps>.22), with the exception of the three-way Hemisphere x Individual Action x Joint Configuration interaction (F(1,23) = 13.28, p = .001, np2=.366). We explored the source of this interaction by investigating alpha suppression separately for ipsilateral and contralateral hemispheres. A 2 × 2 ANOVA for the ipsilateral hemisphere revealed significant main effects for Individual Action (F(1,23) = 9.39, p = .005, np2=.290) and for Joint Configuration (F(1,23) = 4.87, p = .038, np2=.175), but no significant interaction (p = 936). A 2 × 2 ANOVA for the contralateral hemisphere revealed a significant main effect for Individual Action (F(1,23) = 11.81, p = .002, np2=.339), a numerical but not statistically significant effect for Joint Configuration (p = .062), but also a significant interaction between these two factors (F(1,23) = 7.16, p = .013, np2=.237). Post-hoc paired t-tests (adjusted level of significance, a=.025) revealed that when the pre-cue specified the Individual Action there was no difference (p = .502) if the Joint Configuration was also specified (I+We+ vs. I+We-). When the Individual action was not specified, the specification of the Joint configuration (I-We+ vs. I-We-) induced greater alpha suppression (t(23) = -3.33, p = 0.003). This pattern of results raises the possibility that the ipsilateral hemisphere has a more important role in the representation of interpersonal action parameters. However, this is a tentative interpretation that would require further systematic investigation that is beyond the scope of the present study.
4. Discussion
We investigated whether individuals form action plans at the group-level when they plan to perform a joint action with another person. We showed that specifying whether a joint configuration consisted of the same or of different individual actions had a robust beneficial effect on task performance and modulated the amplitude of several EEG indices of cognitive (P600) and sensorimotor representations (alpha/mu rhythm and late CNV) in action planning. Our findings share similarities with a previous study, which also employed a pre-cueing task to investigate the planning phase of bimanual finger tapping performed by a single individual (Deiber et al., 2005). Despite several methodological differences (e.g. longer planning period, only in-phase tapping movements were analysed), Deiber et al. (2005) showed that specifying in advance the relation between the actions of two different effectors (e.g. in-phase tapping) benefited performance and resulted in enhancement of alpha suppression and CNV amplitude, even if the participants did not know in advance which finger (i.e. index or little finger) they had to tap with. Our findings extend this pattern of results to joint action planning and show that specifying in advance the relation between the actions of effectors that belong to two different individuals affects action planning processes and facilitates joint action performance.
4.1. We-representations facilitate joint task performance
Our behavioural results clearly showed that participants took into account the information that specified the joint configuration and utilized it to initiate their actions faster. Action initiation was faster not only when the pre-cue specified the individual action to be performed by the participants, but, importantly, when it merely specified the joint configuration (same or different). This demonstrates that participants did not only form representations of potential individual actions but also represented how their own action would relate to their partner's action. Furthermore, it shows that action representations at the group level (We-representations) do not require the specification of co-actors’ individual action contributions. Rather, they can be formed independently of “I” and “You” action representations. This, in turn, reduced the number of possible joint configurations that the co-acting participants had to prepare for, enabling faster action initiation. To illustrate, when participants received a pre-cue instructing them to execute the “same” action as their partner, this helped them prepare for the remaining two possible configurations (both palms facing inwards or both palms facing outwards) even though they did not know which individual action to plan for. Moreover, action onsets were more synchronized when the pre-cue indicated to participants that they were going to perform the same action, even if they did not know which individual action to perform. Unfortunately, the present setup did not allow us to obtain synchronization measurements during the execution phase, but it is likely that the better alignment of action onsets also implied more synchronous performance. This effect was absent when the participants performed different actions, possibly due to the mechanics of performing the movements required in the present study. Arm extension with the palm facing inwards requires the rotation of the wrist, whereas arm extension with the palm facing outwards does not require wrist rotation. The participants, in our study, were asked to try to synchronize their actions, but they received no instructions as to how to achieve this. Thus, it is conceivable that synchronization of different actions does not necessarily benefit alignment of action onsets if different movement are to be performed.
It may be argued that our findings do not reflect the formation of a joint action plan, but simply the fact that the participants were informed of the other person's expected action. Although our design did not include a condition in which the participants acted in parallel but without the requirement of interpersonal synchronization, we believe that this alternative interpretation cannot account for several aspect of the present results. First, it is unclear why an additional piece of information, seemingly unrelated to a person's planned action, would have a positive effect on the speed of action onsets and interpersonal synchronization. Second, the direct comparison between the I-We+ and the I-We-conditions shows that, although the participants were equally ‘ignorant’ of the other person's action, knowing the relation of their planned (still unspecified) action and the other person's expected (still unspecified) action had a clear beneficial effect for their performance.
The finding that individuals form action plans not only at an individual level, but also at a group level is in line with previous research demonstrating that individuals are capable of forming “We-representations” that may facilitate the performance of a joint task (Della Gatta et al., 2017; Dewey et al., 2014; Ramenzoni et al., 2014; Sacheli et al., 2018; Tsai et al., 2011; Van der Wel, 2015). The present study extends this research by tracking the formation of We-representations and their effects on action preparation during joint action planning.
4.2. Disambiguation in action planning
The EEG analyses provided further insight into the processes of planning a joint task. Confirming our prediction, the P600 amplitude over frontal areas was smaller after pre-cues that reduced the number of possible joint configurations, either by specifying parameters of the individual action or parameters of the joint configuration. The P600 is generally associated with representation of structures (Lelekov-Boissard and Dominey, 2002) and, specifically over frontal areas, the representation of ambiguous structures (Friederici et al., 2002; Kaan and Swaab, 2003; Opitz and Kotz, 2012). A joint action plan may be regarded as a structured mental representation consisting of the co-actors’ potential actions and the ways that they might relate to each other. Accordingly in the present study, the decrease of the P600 amplitude after specification of the joint configuration, regardless of the specification of the individual actions, suggests that the participants were capable of using information that described the task at the group level in order to reduce the number of the potential action outcomes and form a more precise (i.e. less ambiguous) joint action plan.
4.3. We-representations at the sensorimotor level
Also consistent with our predictions, specification of the individual actions as well as of the joint configuration by the pre-cue resulted in enhancement of the alpha/mu rhythm and of the late CNV amplitude. Suppression of the sensorimotor alpha/mu rhythm prior to an action is related to planning processes (Behmer and Fournier, 2014; Kourtis et al., 2013a, 2013b) and it may also reflect processing of interpersonal coordination (Yin et al., 2017). Importantly, the alpha/mu suppression in the present study was lateralized over primary sensorimotor areas contralateral to the acting hand, which strongly suggests that it reflected action preparation and not simply anticipation of the centrally-presented Go cue (Pfurtscheller et al., 1997). According to our hypothesis, this shows that the participants were able to use the information provided by the pre-cue, regarding individual or relational action parameters, in order to reduce the number of the possible joint configuration that they needed to prepare for and form more precise predictive sensorimotor representations of the remaining possible joint action goals/outcomes.
An alternative interpretation is that the lateralization pattern was caused by an amplitude increase of alpha/mu oscillations over the ipsilateral hemisphere to the acting hand, reflecting inhibition of task-irrelevant neuronal populations (Brinkman et al., 2014; Brinkman et al., 2016). Although we did not record any increase in the amplitude of alpha oscillations, it is possible that such effect was masked by the overall suppression of parieto-occipital alpha, which probably indicated activation of visual areas in anticipation of the Go stimulus and the subsequent arm movements (Onoda et al., 2007; Van Dijk et al., 2008). Nevertheless, regardless of whether the lateralization of alpha/mu suppression reflects representation of the remaining possible joint action outcomes or representation of the excluded (by the pre-cue) joint action outcomes, our findings suggest that information about interpersonal action parameters are utilized by primary sensorimotor areas in the service of interpersonal coordination.
In addition to alpha oscillations, information concerning the joint configuration that the two action partners were required to achieve, resulted in the enhancement of the late CNV amplitude. The late CNV in motor tasks is considered and index of optimal, time-locked action preparation (Kononowicz and Penney, 2016; Leuthold et al., 2004; Van Rijn et al., 2011) and it has been shown to be enhanced when two actions are prepared by one or two persons in parallel (Kourtis et al., 2014). The scalp topography of the modulation of the late CNV in the present study is consistent with activation of the Supplementary Motor Area (SMA), which is known to contribute largely to the amplitude of the late CNV (Cui et al., 2000; Leuthold et al., 2004; Nagai et al., 2004) and is central to the planning and coordination of multiple movements (Macuga and Frey, 2012; Obhi et al., 2002). Accordingly, the enhanced late CNV indicates that participants were capable of using advance information about their individual action and more importantly about the joint configuration in order to optimize the planning of a task that required coordination of the two partners’ actions. Crucially, this was the case even in the condition where participants were only informed about the joint configuration that they had to prepare for, but not about the specific individual actions they and their partner were required to perform.
Our findings have broader theoretical implications in the field of sensorimotor control. Research in primates has demonstrated that multiple potential reaching actions can be represented in parallel in distinct neuronal populations within premotor and primary motor areas (Bastian et al., 2003; Cisek and Kalaska, 2005). Comparable results have been also reposted in human participants that showed that the amplitude of several EEG indices of action preparation are modulated by the number of potential actions (Praamstra et al., 2009). This indicates that the processes of action selection and action specification may evolve in parallel and in a continuous manner (Cisek and Kalaska, 2010). The findings of the present study provide an additional dimension, showing that information that concerns the relation of a person's potential actions to a co-actor's potential actions can be processed and represented in advance in the motor system as a means for optimizing interpersonal coordination.
4.4. Implications for social cognition research
The present results also have broader implications for our understanding of social cognition. Much research has been concerned with the question of how theory of mind allows us to overcome the gap between our own and others' minds and contributes to building common ground (e.g., Apperly, 2018; Schaafsma et al., 2015). While many social interactions indeed require the ability to attribute mental states to others and to keep them apart from one's own, it is interesting to note that coordination in joint action may be achieved through joint action plans in the form of We-representations. These provide a fundamental level of sociality that comes from having mental structures that do not merely represent individual actions, but relations between own and others' actions. Planning structures that directly relate the actions of self and other also provide a way to specify the somewhat elusive concept of a “We-mode” (Gallotti and Frith, 2013; Searle, 1990). Interesting questions for future research are whether We-representations can be traced in other forms of joint tasks that do not involve spatiotemporal coordination of actions, and whether their formation hinges on the spatial and/or social proximity between task partners.
5. Conclusion
In conclusion, our study shows that people are capable of forming predictive cognitive as well as sensorimotor representations at the group-level (“We-representations”), which have beneficial effects in the performance of a joint task.
CRediT authorship contribution statement
Dimitrios Kourtis: Formal analysis, Writing - original draft, Conceptualization, Data curation, Methodology, Project administration, Software, Visualization, Writing - original draft, Writing - review & editing. Mateusz Woźniak: Conceptualization, Formal analysis, Methodology, Writing - review & editing, Software. Natalie Sebanz: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing. Günther Knoblich: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing.
Acknowledgments
This research was supported by the European Research Council under the European Union's Seventh Framework Program (FP7/2007–2013)/ERC (European Research Council) grant agreement 609819, SOMICS and by ERC grant agreement 616072, JAXPERTISE (https://erc.europa.eu/).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropsychologia.2019.05.029.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Apperly I. Mindreading and psycholinguistic approaches to perspective taking: Establishing common ground. Top. Cognit. Sci. 2018;10:131–138. doi: 10.1111/tops.12308. [DOI] [PubMed] [Google Scholar]
- Bastian A., Schöner G., Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur. J. Neurosci. 2003;18:2047–2058. doi: 10.1046/j.1460-9568.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Bazanova O.M., Vernon D. Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 2013;44:94–110. doi: 10.1016/j.neubiorev.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Behmer L.P., Jr., Fournier L.R. Working memory modulates neural efficiency over motor components during a novel action planning task: an EEG study. Behav. Brain Res. 2014;260:1–7. doi: 10.1016/j.bbr.2013.11.03. [DOI] [PubMed] [Google Scholar]
- Bolt N.K., Loehr J.D. The predictability of a partner's actions modulates the sense of joint agency. Cognition. 2017;161:60–65. doi: 10.1016/j.cognition.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Brinkman L., Stolk A., Dijkerman H.C., de Lange F.P., Toni I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 2014;34:14783–14792. doi: 10.1523/JNEUROSCI.2039-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman L., Stolk A., Marshall T.R., Esterer S., Sharp P., Dijkerman H.C., de Lange F.P., Toni I. Independent causal contributions of alpha- and beta-band oscillations during movement selection. J. Neurosci. 2016;36:8726–8733. doi: 10.1523/JNEUROSCI.0868-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J., Zoumpoulaki A., Bowman H. Data-driven region-of-interest selection without inflating Type I error rate. Psychophysiology. 2017;54:110–113. doi: 10.1111/psyp.12682. [DOI] [PubMed] [Google Scholar]
- Butterfill S.A. Joint action and development. Philosophical Quaterly. 2011;62:23–47. doi: 10.1111/j.1467-9213.2011.00005.x. [DOI] [Google Scholar]
- Cisek P., Kalaska J.F. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural mechanisms of interacting with a world full of action choices. Annu. Rev. Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Clark H.H. Cambridge University Press; Cambridge: 1996. Using Language. [Google Scholar]
- Constable M.D., Bayliss A.P., Tipper S.P., Spaniol A.P., Pratt J., Welsh T.N. Ownership status influences the degree of joint facilitatory behavior. Psychol. Sci. 2016;27:1371–1378. doi: 10.1177/0956797616661544. [DOI] [PubMed] [Google Scholar]
- Cui R.Q., Egkher A., Huter D., Lang W., Lindinger G., Deecke L. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin. Neurophysiol. 2000;111:1847–1859. doi: 10.1016/s1388-2457(00)00388-6. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A., Novembre G., Fadiga L., Keller P.E. What can music tell us about joint action? Trends Cognit. Sci. 2015;19:111–114. doi: 10.1016/j.tics.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Deiber M.P., Ibañez V., Caldara R., Andrey C., Hauert C.A. Programming effectors and coordination in bimanual in-phase mirror finger movements. Cogn. Brain Res. 2005;23:374–386. doi: 10.1016/j.cogbrainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Della Gatta F., Garbarini F., Rabuffetti M., Viganò L., Butterfill S.A., Sinigaglia C. Drawn together: when motor representations ground joint actions. Cognition. 2017;165:53–60. doi: 10.1016/j.cognition.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Dewey J.A., Pacherie E., Knoblich G. The phenomenology of controlling a moving object with another person. Cognition. 2014;132:383–397. doi: 10.1016/j.cognition.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Hahne A., Saddy D. Distinct neurophysiological patterns reflecting aspects of syntactic complexity and syntactic repair. J. Psycholinguist. Res. 2002;31:45–63. doi: 10.1023/A:1014376204525. [DOI] [PubMed] [Google Scholar]
- Gallotti M., Frith C.D. Social cognition in the we-mode. Trends Cognit. Sci. 2013;17:160–165. doi: 10.1016/j.tics.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Prog. Brain Res. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Hommel B., Müsseler J., Aschersleben G., Prinz W. The theory of event coding (TEC): a framework for perception and action planning. Behav. Brain Sci. 2001;24:849–937. doi: 10.1017/S0140525X01000103. [DOI] [PubMed] [Google Scholar]
- Kaan E., Swaab T.Y. Repair, revision and complexity in syntactic analysis: an electrophysiological differentiation. J. Cogn. Neurosci. 2003;15:98–110. doi: 10.1162/089892903321107855. [DOI] [PubMed] [Google Scholar]
- Knoblich G., Butterfill S., Sebanz N. Psychological research on join action: theory and data. In: Ross B.H., editor. The Physiology of Learning and Motivation. Academic Press; Burlington, VT: 2011. pp. 59–101. [DOI] [Google Scholar]
- Knoblich G., Jordan S. Action coordination in groups and individuals: learning anticipatory control. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29:1006–1016. doi: 10.1037/0278-7393.29.5.1006. [DOI] [PubMed] [Google Scholar]
- Kononowicz T.W., Penney T.B. The contingent negative variation (CNV): timing isn't everything. Current Opinion in Behavioral Sciences. 2016;8:231–237. doi: 10.1016/j.cobeha.2016.02.022. [DOI] [Google Scholar]
- Kourtis D., Knoblich G., Sebanz N. History of interaction and task distribution modulate action simulation. Neuropsychologia. 2013;51:1240–1247. doi: 10.1016/j.neuropsychologia.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Kourtis D., Sebanz N., Knoblich G. Predictive representation of other people's actions in joint action planning: an EEG study. Soc. Neurosci. 2013;8:31–42. doi: 10.1080/17470919.2012.694823. [DOI] [PubMed] [Google Scholar]
- Kourtis D., Knoblich G., Woźniak M., Sebanz N. Attention allocation and task representation during joint action planning. J. Cogn. Neurosci. 2014;26:2275–2286. doi: 10.1162/jocn_a_00634. [DOI] [PubMed] [Google Scholar]
- Lelekov-Boissard T., Dominey P.F. Human brain potentials reveal similar processing of non-linguistic abstract structure and linguistic syntactic structure. Clin. Neurophysiol. 2002;32:72–84. doi: 10.1016/S0987-7053(01)00291-X. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Schröter H. Motor programming of finger sequences of different complexity. Biol. Psychol. 2011;86:57–64. doi: 10.1016/j.biopsycho.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Sommer W., Ulrich R. Preparing for action: inferences from CNV and LRP. J. Psychophysiol. 2004;18:77–88. [Google Scholar]
- Maffongelli L., Bartoli E., Sammler D., Kölsch S., Campus C., Olivier E., Fadiga L., D'Ausillo A. Distinct brain signatures of content and structure violation during action observation. Neuropsychologia. 2015;75:30–39. doi: 10.1016/j.neuropsychologia.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Macuga K.L., Frey S.H. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 2012;59:2798–2807. doi: 10.1016/j.neuroimage.2011.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Johnson B.W., NcNair N.A. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Critchley H.D., Featherstone E., Fenwick P.B., Trimble M.R., Dolan R.J. Brain activity relating to the contingent negative variation: an fMRI investigation. Neuroimage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Neuper C., Scherer R., Reiner M., Pfurtscheller G. Imagery of motor actions: differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Cogn. Brain Res. 2005;25:668–677. doi: 10.1016/j.cogbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Novembre G., Sammler D., Keller P. Neural alpha oscillations index the balance between self-other integration and segregation in real-time joint action. Neuropsychologia. 2016;89:414–425. doi: 10.1016/j.neuropsychologia.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Pineda J.A., Ramachandran V.S. The human mirror system: a link between action observation and social skills. Soc. Cognit. Affect Neurosci. 2007;2:62–66. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obhi S.S., Haggard P., Taylor J., Pascual-Leone A. rTMS to the supplementary motor area disrupts bimanual coordination. Mot. Control. 2002;6:319–332. doi: 10.1123/mcj.8.2.111. [DOI] [PubMed] [Google Scholar]
- Onoda K., Okamoto Y., Shishida K., Hashizume A., Ueda K., Yamashita H., Yamawaki S. Anticipation of affective images and event-related desynchronization (ERD) of alpha activity: an MEG study. Brain Res. 2007;1151:134–141. doi: 10.1016/j.brainres.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Opitz B., Kotz S.A. Ventral premotor cortex lesions disrupt learning of sequential grammatical structures. Cortex. 2012;48:664–673. doi: 10.1016/j.cortex.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Osterhout L., Holcomb P.J. Event-Related brain potentials elicited syntactic anomaly. J. Mem. Lang. 1992;31:785–806. doi: 10.1016/0749-596X(92)90039-Z. [DOI] [Google Scholar]
- Patel A.D., Gibson E., Ratner J., Besson M., Holcomb P.J. Processing syntactic relations in language and music: an event-related potential study. J. Cogn. Neurosci. 1998;10 doi: 10.1162/089892998563121. 1988. [DOI] [PubMed] [Google Scholar]
- Perry A., Stein L., Bentin S. Motor and attentional mechanisms involved in social interaction. Evidence from mu and alpha EEG suppression. Neuroimage. 2011;58:895–904. doi: 10.1016/j.neuroimage.2011.06.060. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes Da Silva F.H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C., Flotzinger D., Pregenzer M. EEG-based discrimination between imagination of right and left hand movement. Electroencephalogr. Clin. Neurophysiol. 1997;103:642–651. doi: 10.1016/s0013-4694(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. The functional significance of mu rhythm: translating “seeing” and “hearing” into “doing”. Brain Research Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Praamstra P., Kourtis D., Nazarpour K. Simultaneous preparation of multiple potential movements: opposing effects of spatial proximity mediated by premotor and parietal cortex. J. Neurophysiol. 2009;102:2084–2095. doi: 10.1152/jn.00413.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. Eur. J. Cogn. Psychol. 1997;9:129–154. doi: 10.1080/713752551. [DOI] [Google Scholar]
- Ramenzoni V.C., Sebanz N., Knoblich G. Scaling up perception-action links: evidence from synchronization with individual and joint action. J. Exp. Psychol. Hum. Percept. Perform. 2014;40:1551–1565. doi: 10.1037/a0036925. [DOI] [PubMed] [Google Scholar]
- Ramnani N., Miall R.C. A system in the human brain for predicting the action of others. Nat. Neurosci. 2004;7:85–90. doi: 10.1038/nn1168. [DOI] [PubMed] [Google Scholar]
- Reeve T.G., Proctor R.W. On the advance preparation of discrete finger responses. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:541–553. doi: 10.1037/0096-1523.10.4.541. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A. Human movement initiation: specification of arm, direction and extend. J. Exp. Psychol. Gen. 1980;109:444–474. doi: 10.1037/0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A., Kornblum S. A priming method for investigating the selection of motor responses. Acta Psychol. 1982;51:223–243. doi: 10.1016/0001-6918(82)90036-1. [DOI] [Google Scholar]
- Sacheli L.M., Arcangeli E., Paulesu E. Evidence for a dyadic motor plan in joint action. Sci. Rep. 2018;8:5027. doi: 10.1038/s41598-018-23275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria J.P., Rosenbaum D.A. Etiquette and effort: holding doors for others. Psychol. Sci. 2011;22:584–588. doi: 10.1177/0956797611406444. [DOI] [PubMed] [Google Scholar]
- Schaafsma S.M., Pfaff D.W., Spunt R.P., Adolphs R. Deconstructing and reconstructing theory of mind. Trends Cognit. Sci. 2015;19:65–72. doi: 10.1016/j.tics.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle J.R. Collective intentions and actions. In: Cohen P.R., editor. Intentions in Communication. MIT Press; 1990. pp. 401–415. [Google Scholar]
- Sebanz N., Knoblich G. Prediction in joint action: what, when, and where. Topics in Cognitive Science. 2009;1:353–367. doi: 10.1111/j.1756-8765.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- Southgate V., Johnson M.H., El Karoui I., Csibra G. Motor system activation reveals infants' on-line prediction of others' goals. Psychol. Sci. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Tsai J.C.C., Sebanz N., Knoblich G. The GROOP effect: groups mimic group actions. Cognition. 2011;118:135–140. doi: 10.1016/j.cognition.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Ulrich R., Leuthold H., Sommer W. Motor programming of response force and movement direction. Psychophysiology. 2003;35:721–728. [PubMed] [Google Scholar]
- Van der Wel R.P.R.D. Me and We: metacognition and performance evaluation of joint actions. Cognition. 2015;140:49–59. doi: 10.1016/j.cognition.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Van Dijk H., Schoffelen J.M., Oostenveld R., Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn H., Kononowicz T.W., Meck W.H., Ng K.K., Peney T.B. Contingent negative variation and its relation to time estimation: a theoretical evaluation. Front. Integr. Neurosci. 2011;5:91. doi: 10.3389/fnint.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper C., Butterfill S., Knoblich G., Sebanz N. A minimal architecture for joint action. Neural Network. 2010;23:998–1003. doi: 10.1016/j.neunet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Walter W.G., Cooper R., Aldridge V.J., McCallum W.C., Winter A.L. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wind-Wall N., Sangals J., Sommer W., Leuthold H. Are fingers special? Evidence about movement preparation from event-related potentials. Psychophysiology. 2003;40:7–17. doi: 10.1111/1469-8986.00002. [DOI] [PubMed] [Google Scholar]
- Wolpert D.M., Flanagan J.R. Motor prediction. Curr. Biol. 2001;11:R729–R732. doi: 10.1016/S0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Yin J., Ding X., Xu H., Zhang F., Shen M. Social coordination information in dynamic chase modulates EEG mu rhythm. Sci. Rep. 2017;7:4782. doi: 10.1038/s41598-017-04129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.