Abstract
The systemic circulation depends upon a highly organized, hierarchal blood vascular network that requires the successful specification of arterial and venous endothelial cells during development. This process is driven by a cascade of signaling events (including Hedgehog, vascular endothelial growth factor (VEGF), Notch, connexin (Cx), transforming growth factor-beta (TGF- β), and COUP transcription factor 2 (COUP-TFII)) to influence endothelial cell cycle status and expression of arterial or venous genes and is further regulated by hemodynamic flow. Failure of endothelial cells to properly undergo arteriovenous specification may contribute to vascular malformation and dysfunction, such as in hereditary hemorrhagic telangiectasia (HHT) and capillary malformation-arteriovenous malformation (CM-AVM) where abnormal vessel structures, such as large shunts lacking clear arteriovenous identity and function, form and compromise peripheral blood flow. This review provides an overview of recent findings in the field of arteriovenous specification and highlights key regulators of this process.
Keywords: artery, vein, endothelial cell specification, vascular development
Introduction
Systemic blood circulation depends upon a highly organized vessel network to efficiently deliver nutrient-rich blood to, and remove waste from, peripheral tissues. The mechanisms that drive development of the blood vasculature are of significant scientific interest with regard to improving our basic understanding of developmental vascular biology and to advancing the fields of personalized and regenerative medicine, where the ability to engineer blood vessels in a laboratory setting would be of significant therapeutic value. In particular, the cell signaling programs that govern the acquisition of specialized endothelial cell identities such as arteries and veins—both crucial for the formation of a functional circulatory system—are the focus of this review.
During early embryonic development, the vasculature forms when primitive endothelial cells coalesce into a primordial microvascular network. Subsequent remodeling of this primitive vasculature organizes the network into the hierarchal architecture typical of mature vessel beds. Specifically, vascular remodeling processes (including endothelial cell proliferation and migration as well as mural cell recruitment and differentiation) drive the formation of anatomically distinct large vascular structures such as arteries and veins (reviewed by dela Paz and D’Amore 1 in 2009). The arterial and venous sides of the systemic circulation are connected to one another at one end by the heart, and at the other end by dense networks of microvessels in the periphery; altogether, this systemic circuit supports circulation of blood throughout the body. Arteries and smaller arterioles are located upstream of the microvasculature and bear a characteristically thick layer of circumferentially organized smooth muscle cells that regulate vessel diameter to influence luminal blood flow and downstream perfusion. In contrast, the smooth muscle layer of veins and venules is thinner and less organized, resulting in a less rigid vessel wall capable of supporting the large blood volume typical of the venous side of the vasculature, and venous valves prevent luminal backflow through these vessels. Microvascular capillaries are a network of thinly walled, small-caliber blood vessels surrounded by a perivascular layer of pericytes that together support exchange of oxygen, fluid, nutrients, and waste into and out of surrounding tissue.
Throughout the vasculature, an inner endothelial layer forms the interface between the vessel lumen and the vessel wall. However, endothelial cells are not a homogeneous cell population: rather, endothelial cells of specialized vascular structures (that is, arteries, veins, and microvessels) express distinct molecular signatures 2 that reflect their individual location and function in the vascular tree. Moreover, acquisition of specialized endothelial cell signatures occurs prior to network formation 1, 3, suggesting that molecular specification of endothelial cells toward arterial or venous identities (termed arteriovenous specification) or other endothelial cell lineages (such as lymphatic or hemogenic) can drive the morphological reorganization of nascent vessel networks. Defects in vascular specification, proliferation, and remodeling can result in arteriovenous malformations (AVMs) that express both arterial and venous markers 4 and may even express markers of lymphatic vasculature 5. Collectively, these data emphasize the importance of proper endothelial cell specification for normal development and function of the vascular system.
Early embryonic vessel development and endothelial specification
During embryogenesis, vascular endothelial cells originate from mesoderm-derived angioblasts (that is, endothelial progenitor cells) that, in mice, first appear at embryonic day 7.0 to 7.5 in the extra-embryonic yolk sac. During the process of vasculogenesis, precursor cells progressively acquire markers of endothelial cell phenotype—vascular endothelial growth factor receptor type 2 (VEGFR2), vascular endothelial cadherin, and so on 6, 7 —and form a primitive vascular plexus in the yolk sac and in the embryo proper 8. Recently, Plein et al. 9 reported that a subset of yolk sac endothelial cells that acquire hemogenic potential to become erythro-myeloid progenitor cells are also capable of re-differentiating into endothelial cells and integrating back into the yolk sac and embryonic vasculature 9, 10. The primitive vasculature that is comprised of these two endothelial cell sources undergoes stepwise remodeling to produce the earliest extra- and intra-embryonic vessels, which collectively form the embryo’s first closed circulatory loop 8. As endothelial cells of the dorsal aorta and the cardinal vein within the embryo progressively acquire expression of arterial and venous markers, respectively, the remaining endothelial cells of primitive plexi undergo sprouting angiogenesis to expand the vascular network, followed by additional remodeling and specification to further reorganize the network into a hierarchal branching architecture.
Arteriovenous specification of the endothelial cells that form the dorsal aorta and cardinal vein appears to be molecularly determined prior to the onset of systemic blood flow. Discrete, non-overlapping expression of neuropilin-1 (Nrp1) and neuropilin-2 (Nrp2) is observed in vascular plexi of chick embryos and these endothelial cell populations subsequently segregate into the earliest embryonic arteries and veins, respectively 3. Expression of ephrinB2, enriched in some arterial endothelial cells, and the receptor EphB4, enriched in some venous cells, is also observed in the primitive vasculature prior to the onset of blood flow 11. Despite these findings, the early morphogen or morphogens that first induce formation of the initial vascular plexus and support arteriovenous specification therein remain unclear. Furthermore, it is still uncertain whether these arteriovenous specification pathways are common across all vertebrate species or even whether, within the same organism, all endothelial cells synchronously acquire their arteriovenous identity via the same initiating signal(s) or downstream mechanism(s).
Role of shear stress
The observations that embryonic arterial and venous markers are expressed prior to the onset of blood flow 3, 11, 12 and that blood flow is dispensable for early arteriovenous specification events in the chick embryo 13 suggest that the initiating arteriovenous specification event for angioblasts is not necessarily blood flow. However, blood flow is nonetheless crucial for certain arteriovenous specification events and is necessary for the maintenance of arterial identity.
Supporting this point, loss of systemic blood flow in chick 13, 14 and mice 15, 16 produces defects in arteriovenous specification and induces AVMs. For example, in Ncx1 −/− mouse embryos (which lack a heartbeat), blood flow is not required for initial formation of the dorsal aorta and the cardinal vein but is necessary to induce separation of these vessel structures and to maintain arterial marker expression and suppress venous identity genes in the dorsal aorta 15. Thus, early endothelial cell fate acquisition is dynamic, and hemodynamic signaling is needed to sustain arteriovenous identity in the remodeling vasculature.
Consistent with this model, expression of arterial identity genes is induced in cultured endothelial cells and is greatest when cells are exposed to shear stress magnitudes typical of arterial vessels (~15 dynes/cm 2), relative to higher or lower shear magnitudes 17. Furthermore, maintenance of arterial gene expression in cultured arterial endothelial cells requires pulsatile, not constant, flow 14. These data indicate that endothelial cell specification is tightly calibrated to hemodynamic flow profile and suggest that other endothelial cell types, such as venous and lymphatic, may be similarly promoted by vessel-specific flow profiles. Consistent with the idea that endothelial cell identity is plastic and influenced by hemodynamic flow, several studies show that vessel grafts generally lose markers of their vessels of origin and assume the molecular identity of their grafted location 18. Although the particular mechanosensitive pathways that govern these flow-sensitive specification events remain unclear, activation of mechanosensitive receptors, such as activin receptor-like kinase 1 ( ACVRL1, or Alk1) and Notch1 or Notch4, likely leads to the downstream transactivation of fundamental regulators of endothelial cell specification and vascular remodeling, which is discussed in greater detail below.
Role of cell cycle control
A growing body of evidence suggests that endothelial cell cycle arrest is necessary to enable the acquisition of specialized endothelial cell phenotypes. Cell cycle control critically regulates cell fate decisions during embryonic stem cell differentiation 19, 20, suggesting that a similar process may occur for acquisition of specialized cell phenotypes in other contexts, such as for endothelial cells in the vasculature. In undifferentiated stem cells, cell cycle progression is tightly regulated 21, and cell cycle length governs both pluripotency 20 and cell differentiation 19. In blood vessels, molecular regulation of cell cycle state may similarly be required to achieve a balance between expansion and maturation of vessel networks.
Consistent with this hypothesis, angiogenic endothelial cells are highly proliferative 22, whereas proliferation is substantially suppressed in remodeling vessel networks undergoing arteriovenous specification 17, 23, particularly in developing arterial-associated vascular beds 17. In addition, fluid shear stress at physiologically arterial levels significantly reduces proliferation of endothelial cells in culture 24 and endothelial cells in mature arteries are characteristically quiescent 25. Recent studies show that pharmacological induction of G 1 arrest is sufficient to enable the expression of arterial identity genes in endothelial cells in culture even in the absence of other conventional activators of arterial specification 17. In addition, during coronary vascular development, transition from venous to arterial endothelial cell phenotypes is associated with G 1 growth arrest that is prevented by expression of the venous identity regulator COUP-TFII 26, and endothelial cell G 1 arrest is also required for hemogenic specification 27. Thus, signaling pathways, including those reviewed below, may regulate endothelial specification, at least in part, by modulating cell cycle state to enable subsequent endothelial cell specification events. Whether a specific cell cycle state is necessary to enable venous and lymphatic endothelial cell specification is unclear and this is under investigation.
Regulators of arteriovenous specification
Vascular network morphogenesis and endothelial cell specification require coordinated cell–cell signaling between endothelial cells, mural cells, and adjacent cell types. Specification of endothelial cell identity is regulated by the integrated balance of multiple cell–cell signaling pathways that antagonistically induce arterial or venous identity. In particular, an “arterialization” cascade involving Hedgehog, vascular endothelial growth factor (VEGF), Notch, and connexin (Cx) signaling plays an important role in inducing arterial specification. There is also cross-talk of this pathway with transforming growth factor-beta (TGF-β) signaling and this pathway is inhibited by regulators of venous identity, such as COUP-TFII.
Hedgehog
Binding of the morphogen Sonic Hedgehog (Shh) to its cell surface receptor Patched-1 ( PTCH1) alleviates repression of the central downstream Shh effector, Smoothened (Smo). In turn, Smo induces the expression of numerous gene targets essential for embryonic development 28. In endothelial cells, Shh activates endothelial cell survival and alters cytoskeletal arrangement in culture 29, and studies in zebrafish show that Shh signaling is necessary for arteriovenous specification. Specifically, in zebrafish mutants lacking Shh, endothelial cells of the dorsal aorta fail to acquire expression of the arterial-enriched gene ephrinB2. This is thought to be the result of loss of VEGF expression in Shh-deficient somites, leading to reduced VEGF signaling and reduced downstream Notch 30. However, Shh also regulates endothelial cell identity independent of its stimulation of VEGF/Notch signaling. Specifically, Hedgehog represses venous identity 31 and promotes arterial specification via calcitonin receptor-like receptor (Crlr) signaling 32 as well as by directly upregulating expression of Notch signaling effectors 33.
Vascular endothelial growth factor
VEGF functions at multiple levels during vasculogenesis and vessel remodeling, including during arteriovenous specification. Although loss of even a single allele of VEGF-A is sufficient to disrupt vessel formation resulting in embryonic lethality in mice 34, VEGF-A knockdown in zebrafish morphants preserves embryonic survival albeit with arteriovenous specification defects 30. Recent studies indicate that whereas early VEGF signaling governs endothelial cell development from angioblasts, mid-somitogenic VEGF signaling primarily influences arterial specification by activating Etv2, a member of the Ets family of transcription factors 35, to regulate downstream Notch signaling effector expression 36, 37.
Recent findings indicate that, in addition to its effects on Etv2 and downstream Notch, VEGF/VEGFR2 activation directly regulates the balance of signaling through either phosphatidylinositol-3-kinase (PI3K) or mitogen-activated protein kinase (MAPK) pathways to determine arterial and venous cell fates. In a small-molecule screen, Hong et al. 38 report that inhibition of PI3K signaling induces ERK1/2 (MAPK signaling) activation—a signaling pathway that regulates endothelial cell proliferation (among other functions) 39—to promote arterial specification. This effect is capable of rescuing arteriovenous defects of the gridlock zebrafish mutant, where the Notch-targeted transcription factor Hey2 is affected, indicating that MAPK signaling influences arterial identity downstream of Notch signaling 38. In contrast, small-molecule inhibitors of MAPK, or constitutive activation of PI3K signaling via induction of protein kinase B (Akt), prevents arterial specification and instead induces venous identity 38, indicating that the antagonistic relationship between MAPK and PI3K signaling pathways strongly influences endothelial cell fate.
Notch
Members of the Notch family of transmembrane receptors, as well as their membrane-bound ligands, are expressed in multiple cell types of the developing and mature vasculature 40. In response to VEGF-activated expression of members of the Sox family of transcription factors (for example, Sox7, Sox17, and Sox18) 41 or stimulation of the Wingless/Integrated (Wnt) signaling pathway 42, primordial endothelial cells are induced to express the transmembrane receptor Notch1 and its ligand Dll4 43. Notch1 and related endothelial-expressed Notch receptors are activated by membrane-bound Notch ligands of adjacent endothelial cells (homocellular signaling) as well as those expressed by other stromal cell types (heterocellular signaling). Indeed, the developing vasculature expresses multiple Notch ligand and receptor types, and some are restricted to specific regions of the expanding and remodeling vascular tree 40. Binding of ligand to the Notch receptor results in proteolytic cleavage of the Notch intracellular domain, which translocates to the nucleus and binds to and activates DNA-binding protein RBPJκ, resulting in transcription of genes that influence endothelial cell cycle status 17 and function, leading to induced expression of arterial identity genes 44.
In animals treated with Notch inhibitors or in transgenic animals lacking either Notch ligands or receptors, sprouting angiogenesis and arteriovenous specification fail to occur normally 17, 22, 45– 47. Instead, vascular endothelial cells hyperproliferate and do not properly remodel into arteriovenous networks 17, 45. In gridlock zebrafish mutants, ephrinB2 expression is lost and formation of the dorsal aorta is compromised but the cardinal vein is enlarged 12.
One possible explanation for the central role for Notch in arteriovenous specification is as a mechanism to couple mechanosensory receptor signaling to downstream endothelial cell specification pathways. Fluid shear stress activates Notch signaling in endothelial cells in a dose-dependent fashion with Notch activation peaking at 17 or slightly above 48 physiologically arterial levels of shear. Ablation of Notch1 signaling compromises classic flow-sensitive endothelial cell responses, including quiescence 17 and cell alignment 48, whereas constitutive activation of Notch4 induces focal vessel enlargement by disrupting normal hemodynamic signaling 49. Although the exact mechanosensory signaling complex or complexes that render Notch signaling flow-sensitive have yet to be identified, ligand-dependent Notch activation is force-dependent 50, 51, which suggests that the Notch receptor itself may participate in an as-yet-undescribed mechanosensory complex.
Other studies suggest that Notch may also enable arteriovenous specification by determining the cell cycle state of remodeling endothelial cells. In response to flow, Notch signaling activation alters the expression of cell cycle regulators 17. In addition, Notch-mediated G 1 arrest is required for acquisition of arterial 17, 45 as well as hemogenic 27 cell fates. In contrast, suppression of Notch signaling by COUP-TFII drives venous specification 52, and transgenic ablation of Notch signaling components enhances lymphatic endothelial cell specification 53. Taken together, these data suggest that, in the developing vasculature, Notch signaling may play a central role in precisely coupling endothelial cell cycle state to hemodynamic flow sensing to achieve proper fate specification. However, it is still unclear whether venous and lymphatic endothelial cell fates are similarly specified in distinct cell cycle states, which requires further intensive investigation. Nonetheless, in support of this hypothesis, dysregulated Notch signaling leads to focal appearance of AVMs at sites of high flow that are associated with failure to acquire (or maintain) specialized endothelial cell identities 49, 54. Whether in animals lacking Notch (or Alk, see below) signaling AVMs are a direct result of disrupted arteriovenous specification or whether EC fail to undergo proper arteriovenous specification as a by-product of enlarged, malformed vessels that result from aberrant responses to shear (such as failure to migrate against the direction of flow 49, 55) remains unclear.
Lastly, Notch is an important regulator of hemogenic endothelial cell development in the yolk sac and embryonic aorta–gonad–mesonephros region 27, 56, and circulating yolk sac-endothelium–derived hematopoietic progenitors have recently been shown to reintegrate into the developing vasculature 9, 10. Thus, it is interesting to speculate that Notch may contribute to arteriovenous network formation, in part, by regulating the relative abundance of these two endothelial cell sources, which may have different propensities for arterial versus venous identity. However, much more work is needed to address these possibilities.
Ephrin/Eph
The ephrin family of transmembrane ligands and their cognate Eph receptors mediate cell–cell signaling between adjacent cells and often involve the repulsion of Eph receptor–expressing cells from ephrin-expressing neighbors. In a landmark study of the developing vasculature, Wang et al. 11 found that ephrinB2 expression is highly enriched in arteries and EphB4 expression is enriched in veins. Expression of both genes is observed prior to the onset of blood flow, suggesting that they participate in a genetic arteriovenous program. Furthermore, EphB4 venous expression depends upon arterial expression of ephrinB2 11, suggesting that during development arterial specification may drive venous specification via ephrin-Eph signaling. Mutations that affect the EphB4 gene or downstream Ras signaling are associated with the autosomal-dominant congenital vascular disease, capillary malformation-arteriovenous malformation (CM-AVM), wherein patients present with numerous cutaneous capillary malformations as well as AVMs 57.
Transforming growth factor-beta
The TGF-β superfamily of soluble ligands and their cognate membrane-bound receptors play a variety of key roles during vessel development. This pathway includes signaling through the TGF-β1-TGFβR2-Alk5 ligand-receptor complex, which predominantly activates Smads2/3 signaling, as well as Bone morphogenetic protein (BMP) 9/10-Alk1-Eng ligand-receptor signaling, which predominantly activates Smads1/5/8 58. Specifically, signaling through TGF-β1-TGFβR2 typically mediates mural cell recruitment and differentiation 59, whereas Alk1/Eng signaling regulates endothelial cell quiescence, limits vessel caliber, and enables arteriovenous specification 54, 55, 60. However, there is also evidence of significant cross-talk between distinct TGF-β signaling pathways 61, 62 as well as between TGF-β superfamily pathways and other cell signaling pathways (for example, Notch) and that it is the balance of Smad signaling activation via these distinct pathways that establishes proper vessel formation 63, 64. Indeed, patients bearing heterozygous mutations affecting either Alk1 or Eng, or downstream Smad4, exhibit the congenital disease hereditary hemorrhagic telangiectasia (HHT), which is characterized by microvascular overgrowth and the focal appearance of large-caliber AVMs that lack clear arterial or venous identity 60, 65.
Several recent studies have focused on the cross-talk between BMP and Notch signaling pathways to modulate endothelial cell behavior during vessel development. BMP-activated sprouting angiogenesis is negatively regulated by Notch upregulation of Smad6, an inhibitor of BMP signaling 66. Meanwhile, nuclear translocation of BMP-activated phospho-Smads not only upregulates BMP target genes but also participates in the Notch/RBPJ-κ gene regulatory complex to regulate Notch-activated transcriptional responses 67. Consequently, inhibited expression of endothelial-expressed BMP regulatory proteins, BMPER and TWSG1, disrupts Notch signaling and expression of arterial identity genes, resulting in increased venous specification in zebrafish embryos 68. Alk1 inhibition also depresses Notch signaling and produces AVMs in mice 54. In separate studies, BMPER is reported to activate ERK1/2 signaling 69 (which promotes arterial specification 38) while the Alk1 co-receptor Endoglin suppresses PI3K/Akt signaling (which promotes venous identity 38) to support endothelial cell migration against the direction of blood flow, a process hypothesized to support arteriogenesis and prevent AVMs 70. Thus, currently available evidence suggests that BMP9/10-Alk1 signaling may regulate arteriovenous specification, at least in part, by modulating endothelial cell responsiveness to VEGF- and Notch-activated signaling. However, other studies suggest that other endothelial cell behaviors, such as responsiveness to shear or migration (or both), are also affected 49, 55.
Connexins
Membrane-expressed connexin (also known as Cx) proteins form multimeric complexes (termed connexons) that dock with connexons of adjacent cells to form intercellular channels (termed gap junction channels) that mediate passage of ions and small signaling molecules to support electrochemical coupling and intercellular communication. At least four connexin proteins (Cx37, Cx40, Cx43, and Cx45) are commonly reported at endothelial cell–cell junctions of the blood vasculature 71, 72, and some studies report endothelial expression of a fifth connexin (Cx32) 73, 74. Furthermore, an additional connexin (Cx47) is expressed in lymphatic endothelial cells and contributes to lymphatic vessel development 75. Of the commonly studied vascular connexins, Cx40 (encoded by the gene Gja5) is well recognized as a potent marker of arterial endothelial cells owing to its high expression in arteries invested with smooth muscle 14, 17. Deletion of this connexin inhibits flow-activated arterial specification in the chick 14 and affects sprouting angiogenesis and mural cell recruitment in the neonatal mouse retina 76. Furthermore, loss of Cx40 potentiates the appearance of AVMs in Alk1-haploinsufficient animals 77, suggesting that it may suppress the formation of these vascular defects in wild-type animals, at least in part, by functioning downstream of BMP9/10-Alk1 signaling. Recently, Su et al. 26 employed a single-cell transcriptomic analysis of developing coronary vessels to identify a Cx40-enriched population of venous-originating “pre-artery” cells that express markers of mature arteries. The majority of these cells were later found to line the coronary arteries and were excluded from coronary veins, suggesting that expression of Cx40 is a critical intermediary step for arterial identity acquisition.
Endothelial-expressed Cx37 is also almost exclusively expressed in large arteries of the adult vasculature 78 as well as in developing arteries and arterioles of remodeling vessels 17. However, unlike Cx40, Cx37 is additionally expressed in remodeling capillaries and in arteriolar vessels that have yet to be invested with mural cells 17, suggesting that it may play an earlier role than Cx40 in arteriovenous specification during development. Deletion of Cx37 disrupts developmental and injury-induced vessel growth and remodeling 17, 79, and transgenic ablation of both connexins in combination results in embryonic lethality due to failure of the vasculature to form 80, suggesting that Cx37 and Cx40 play essential and possibly distinct roles during vessel development. For example, many connexins regulate cell proliferation, and Cx37 is a particularly potent inhibitor of cell cycle progression 81. Cx37 directly modulates endothelial cell cycle status downstream of flow-activated Notch signaling by upregulating p27, causing late G 1 arrest to enable expression of arterial genes Cx40 and ephrinB2 17. It is possible that Cx37 plays a similar cell cycle arrest role to enable specification towards other endothelial cell fates. In support of this possibility, transgenic ablation of one or both copies of Cx37 is associated with endothelial cell hyperproliferation and defects in not only arterial development 17 but also venous 82 and lymphatic 83, 84 development.
MicroRNAs
A growing body of evidence indicates that microRNA (miRNA) species likely play an important, if currently underappreciated, role in endothelial cell specification. Although several studies show that miRNAs are necessary for endothelial cell differentiation from angioblasts, less is known about their involvement in specifying endothelial cell phenotypes. Endothelial and mural cells express numerous miRNA species, and miRNA processing machinery, including Drosha and Dicer, appears to be crucial for vessel development 85, 86. Mutants lacking expression of Dicer in Etv2-positive mesodermal progenitor cells exhibit defects in vessel remodeling and patterning due, at least in part, to loss of miR-130a expression 87. Meanwhile, miR-27b is required for venous formation in zebrafish 88, and miR-181 destabilizes expression of Prox1, a key regulator of lymphatic endothelial cell specification and maintenance 89. In addition, endothelial-specific mutation of Drosha in mice produces leaky, dilated microvessels and aberrant arteriovenous connections (but lack clear AVMs), and missense point mutations in the Drosha gene are more prevalent among patients with HHT compared with healthy populations, suggesting that miRNA processing defects may contribute to the pathogenesis of this disease or modulate its severity or do both 86. Taken together, these studies suggest that miRNAs likely play a broad role in endothelial cell specification and vascular remodeling. However, more work is needed to identify critical miRNA regulators of these processes and to fully elucidate their molecular roles.
Conclusions
Endothelial cell specification toward arterial and venous fates is critical for the formation and remodeling of the blood circulatory system during development and post-natally. Failure of the vasculature to properly undergo arteriovenous specification may contribute to the malformation or dysfunction of blood vessels, such as occurs in patients with HHT, who exhibit aberrant vessel structures that compromise quality of life and that can even be fatal.
During normal development, acquisition of arterial identity is driven by a molecular program (see proposed model, Figure 1) that includes Hedgehog, VEGF, Notch, and connexin signaling and downstream PI3K and MAPK signaling. This pathway is modulated by TGF-β signaling and miRNAs and is antagonized by COUP-TFII, which promotes venous formation. Although early endothelial specification events may occur via a “hardwired” genetic program prior to the onset of blood flow, hemodynamic flow is precisely calibrated to arteriovenous identity and vessel identity is sustained by blood flow forces. Recent studies suggest that flow-sensitive regulation of Notch signaling 48 may play a central role in modulating endothelial cell cycle state to enable the specification of different endothelial cell phenotypes in distinct cell cycle states 17; however, this requires further investigation.
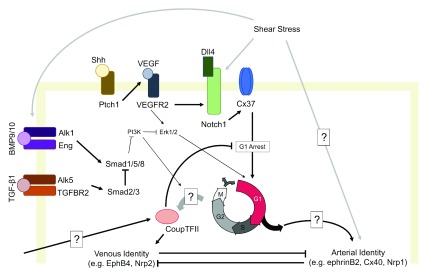
Figure 1. A proposed model of the regulation of arteriovenous specification in primitive endothelial cells, highlighting key players and some of the evidence of cell signaling cross-talk.
A proposed model of the regulation of arteriovenous specification in primitive endothelial cells, highlighting key players and some of the evidence of cell signaling cross-talk.
An improved understanding of the molecular mechanisms that regulate the initial acquisition of endothelial cell identity, as well as the signals that sustain specialized vessel structures and functions, is therefore an important frontier for new and ongoing research in the field of vascular biology. Additional insights into the molecular regulation of arteriovenous specification will profoundly influence our understanding of the physiology of vessel maintenance and the pathophysiology of numerous diseases involving disorganized vessel growth and remodeling.
Abbreviations
Akt, protein kinase B; Alk, activin receptor-like kinase; AVM, arteriovenous malformation; BMP, bone morphogenetic protein; COUP-TFII, COUP transcription factor 2; Cx, connexin; HHT, hereditary hemorrhagic telangiectasia; MAPK, mitogen-activated protein kinase; miRNA, microRNA; PI3K, phosphatidylinositol-3-kinase; Shh, Sonic Hedgehog; Smo, Smoothened; TGF-β, transforming growth factor-beta; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor type 2
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Helen Arthur, Institute of Genetic Medicine, Centre for Life, Newcastle University, Newcastle, UK
Ondine Cleaver, Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, TX, USA
Funding Statement
This work was supported by the National Institutes of Health (HL128064, HL096360, and EB017103) and CT Innovations (15-RMB-YALE-04 and 15-RMB-YALE-07).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. dela Paz NG, D'Amore PA: Arterial versus venous endothelial cells. Cell Tissue Res. 2009;335(1):5–16. 10.1007/s00441-008-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aranguren XL, Agirre X, Beerens M, et al. : Unraveling a novel transcription factor code determining the human arterial-specific endothelial cell signature. Blood. 2013;122(24):3982–92. 10.1182/blood-2013-02-483255 [DOI] [PubMed] [Google Scholar]
- 3. Herzog Y, Guttmann-Raviv N, Neufeld G: Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev Dyn. 2005;232(4):1047–55. 10.1002/dvdy.20257 [DOI] [PubMed] [Google Scholar]
- 4. Hirashima M, Suda T: Differentiation of arterial and venous endothelial cells and vascular morphogenesis. Endothelium. 2006;13(2):137–45. 10.1080/10623320600698078 [DOI] [PubMed] [Google Scholar]
- 5. Shoemaker LD, Fuentes LF, Santiago SM, et al. : Human brain arteriovenous malformations express lymphatic-associated genes. Ann Clin Transl Neurol. 2014;1(12):982–95. 10.1002/acn3.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kässmeyer S, Plendl J, Custodis P, et al. : New insights in vascular development: vasculogenesis and endothelial progenitor cells. Anat Histol Embryol. 2009;38(1):1–11. 10.1111/j.1439-0264.2008.00894.x [DOI] [PubMed] [Google Scholar]
- 7. Rohban R, Prietl B, Pieber TR: Crosstalk between Stem and Progenitor Cellular Mediators with Special Emphasis on Vasculogenesis. Transfus Med Hemother. 2017;44(3):174–82. 10.1159/000477677 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Chong DC, Koo Y, Xu K, et al. : Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn. 2011;240(9):2153–65. 10.1002/dvdy.22706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plein A, Fantin A, Denti L, et al. : Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature. 2018;562(7726):223–8. 10.1038/s41586-018-0552-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Iruela-Arispe ML: A dual origin for blood vessels. Nature. 2018;562(7726):195–7. 10.1038/d41586-018-06199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang HU, Chen ZF, Anderson DJ: Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. 10.1016/s0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- 12. Zhong TP, Childs S, Leu JP, et al. : Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414(6860):216–20. 10.1038/35102599 [DOI] [PubMed] [Google Scholar]
- 13. Le Noble F, Moyon D, Pardanaud L, et al. : Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131(2):361–75. 10.1242/dev.00929 [DOI] [PubMed] [Google Scholar]
- 14. Buschmann I, Pries A, Styp-Rekowska B, et al. : Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137(13):2187–96. 10.1242/dev.045351 [DOI] [PubMed] [Google Scholar]
- 15. Hwa JJ, Beckouche N, Huang L, et al. : Abnormal arterial-venous fusions and fate specification in mouse embryos lacking blood flow. Sci Rep. 2017;7(1):11965. 10.1038/s41598-017-12353-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Lucitti JL, Jones EA, Huang C, et al. : Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–26. 10.1242/dev.02883 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Fang JS, Coon BG, Gillis N, et al. : Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun. 2017;8(1):2149 10.1038/s41467-017-01742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf K, Hu H, Isaji T, et al. : Molecular identity of arteries, veins, and lymphatics. J Vasc Surg. 2019;69(1):253–62. 10.1016/j.jvs.2018.06.195 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Pauklin S, Vallier L: The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155(1):135–47. 10.1016/j.cell.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vallier L: Cell Cycle Rules Pluripotency. Cell Stem Cell. 2015;17(2):131–2. 10.1016/j.stem.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 21. Pietras EM, Warr MR, Passegué E: Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195(5):709–20. 10.1083/jcb.201102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benedito R, Roca C, Sörensen I, et al. : The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–35. 10.1016/j.cell.2009.03.025 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Pitulescu ME, Schmidt I, Giaimo BD, et al. : Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol. 2017;19(8):915–27. 10.1038/ncb3555 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Levesque MJ, Nerem RM, Sprague EA: Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11(9):702–7. 10.1016/0142-9612(90)90031-K [DOI] [PubMed] [Google Scholar]
- 25. Langille BL, Reidy MA, Kline RL: Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986;6(2):146–54. 10.1161/01.ATV.6.2.146 [DOI] [PubMed] [Google Scholar]
- 26. Su T, Stanley G, Sinha R, et al. : Single-cell analysis of early progenitor cells that build coronary arteries. Nature. 2018;559(7714):356–62. 10.1038/s41586-018-0288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Marcelo KL, Sills TM, Coskun S, et al. : Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Dev Cell. 2013;27(5):504–15. 10.1016/j.devcel.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salybekov AA, Salybekova AK, Pola R, et al. : Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. Int J Mol Sci. 2018;19(10): pii: E3040. 10.3390/ijms19103040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Chinchilla P, Xiao L, Kazanietz MG, et al. : Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9(3):570–9. 10.4161/cc.9.3.10591 [DOI] [PubMed] [Google Scholar]
- 30. Lawson ND, Vogel AM, Weinstein BM: sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3(1):127–36. 10.1016/S1534-5807(02)00198-3 [DOI] [PubMed] [Google Scholar]
- 31. Williams C, Kim SH, Ni TT, et al. : Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev Biol. 2010;341(1):196–204. 10.1016/j.ydbio.2010.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Wilkinson RN, Koudijs MJ, Patient RK, et al. : Hedgehog signaling via a calcitonin receptor-like receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood. 2012;120(2):477–88. 10.1182/blood-2011-10-383729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coultas L, Nieuwenhuis E, Anderson GA, et al. : Hedgehog regulates distinct vascular patterning events through VEGF-dependent and -independent mechanisms. Blood. 2010;116(4):653–60. 10.1182/blood-2009-12-256644 [DOI] [PubMed] [Google Scholar]
- 34. Carmeliet P, Ferreira V, Breier G, et al. : Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–9. 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- 35. Koyano-Nakagawa N, Garry DJ: Etv2 as an essential regulator of mesodermal lineage development. Cardiovasc Res. 2017;113(11):1294–306. 10.1093/cvr/cvx133 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Wythe JD, Dang LT, Devine WP, et al. : ETS factors regulate Vegf-dependent arterial specification. Dev Cell. 2013;26(1):45–58. 10.1016/j.devcel.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casie Chetty S, Rost MS, Enriquez JR, et al. : Vegf signaling promotes vascular endothelial differentiation by modulating etv2 expression. Dev Biol. 2017;424(2):147–61. 10.1016/j.ydbio.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Hong CC, Peterson QP, Hong JY, et al. : Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16(13):1366–72. 10.1016/j.cub.2006.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Zachary I: Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280(6):C1375–86. 10.1152/ajpcell.2001.280.6.C1375 [DOI] [PubMed] [Google Scholar]
- 40. Hofmann JJ, Luisa Iruela-Arispe M: Notch expression patterns in the retina: An eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns. 2007;7(4):461–70. 10.1016/j.modgep.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiang IK, Fritzsche M, Pichol-Thievend C, et al. : SoxF factors induce Notch1 expression via direct transcriptional regulation during early arterial development. Development. 2017;144(14):2629–39. 10.1242/dev.146241 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Corada M, Nyqvist D, Orsenigo F, et al. : The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18(6):938–49. 10.1016/j.devcel.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Liu ZJ, Shirakawa T, Li Y, et al. : Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23(1):14–25. 10.1128/mcb.23.1.14-25.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iso T, Maeno T, Oike Y, et al. : Dll4-selective Notch signaling induces ephrinB2 gene expression in endothelial cells. Biochem Biophys Res Commun. 2006;341(3):708–14. 10.1016/j.bbrc.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 45. Lawson ND, Scheer N, Pham VN, et al. : Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–83. [DOI] [PubMed] [Google Scholar]
- 46. Krebs LT, Xue Y, Norton CR, et al. : Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14(11):1343–52. [PMC free article] [PubMed] [Google Scholar]
- 47. Duarte A, Hirashima M, Benedito R, et al. : Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18(20):2474–8. 10.1101/gad.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Mack JJ, Mosqueiro TS, Archer BJ, et al. : NOTCH1 is a mechanosensor in adult arteries. Nat Commun. 2017;8(1):1620. 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Murphy PA, Kim TN, Huang L, et al. : Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc Natl Acad Sci U S A. 2014;111(50):18007–12. 10.1073/pnas.1415316111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meloty-Kapella L, Shergill B, Kuon J, et al. : Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22(6):1299–312. 10.1016/j.devcel.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Langridge PD, Struhl G: Epsin-Dependent Ligand Endocytosis Activates Notch by Force. Cell. 2017;171(6):1383–1396.e12. 10.1016/j.cell.2017.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. You LR, Lin FJ, Lee CT, et al. : Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435(7038):98–104. 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Murtomaki A, Uh MK, Choi YK, et al. : Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140(11):2365–76. 10.1242/dev.083865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larrivée B, Prahst C, Gordon E, et al. : ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22(3):489–500. 10.1016/j.devcel.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rochon ER, Menon PG, Roman BL: Alk1 controls arterial endothelial cell migration in lumenized vessels. Development. 2016;143(14):2593–602. 10.1242/dev.135392 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Gritz E, Hirschi KK: Specification and function of hemogenic endothelium during embryogenesis. Cell Mol Life Sci. 2016;73(8):1547–67. 10.1007/s00018-016-2134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amyere M, Revencu N, Helaers R, et al. : Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Circulation. 2017;136(11):1037–1048. 10.1161/CIRCULATIONAHA.116.026886 [DOI] [PubMed] [Google Scholar]
- 58. Kamato D, Burch ML, Piva TJ, et al. : Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25(10):2017–24. 10.1016/j.cellsig.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 59. Hirschi KK, Rohovsky SA, D'Amore PA: PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141(3):805–14. 10.1083/jcb.141.3.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sorensen LK, Brooke BS, Li DY, et al. : Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261(1):235–50. 10.1016/S0012-1606(03)00158-1 [DOI] [PubMed] [Google Scholar]
- 61. Mancini ML, Terzic A, Conley BA, et al. : Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev Dyn. 2009;238(10):2479–93. 10.1002/dvdy.22066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oh SP, Seki T, Goss KA, et al. : Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97(6):2626–31. 10.1073/pnas.97.6.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goumans MJ, Lebrin F, Valdimarsdottir G: Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13(7):301–7. 10.1016/S1050-1738(03)00142-7 [DOI] [PubMed] [Google Scholar]
- 64. Lebrin F, Deckers M, Bertolino P, et al. : TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65(3):599–608. 10.1016/j.cardiores.2004.10.036 [DOI] [PubMed] [Google Scholar]
- 65. Urness LD, Sorensen LK, Li DY: Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–31. 10.1038/81634 [DOI] [PubMed] [Google Scholar]
- 66. Mouillesseaux KP, Wiley DS, Saunders LM, et al. : Notch regulates BMP responsiveness and lateral branching in vessel networks via SMAD6. Nat Commun. 2016;7:13247. 10.1038/ncomms13247 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Osmanagic-Myers S, Rezniczek GA: Arteriovenous specification: BMPER and TWSG1 determine endothelial cell fate via activation of synergistic BMP and Notch signaling. FEBS J. 2018;285(8):1399–402. 10.1111/febs.14439 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Esser JS, Steiner RE, Deckler M, et al. : Extracellular bone morphogenetic protein modulator BMPER and twisted gastrulation homolog 1 preserve arterial-venous specification in zebrafish blood vessel development and regulate Notch signaling in endothelial cells. FEBS J. 2018;285(8):1419–36. 10.1111/febs.14414 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Heinke J, Wehofsits L, Zhou Q, et al. : BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103(8):804–12. 10.1161/CIRCRESAHA.108.178434 [DOI] [PubMed] [Google Scholar]
- 70. Jin Y, Muhl L, Burmakin M, et al. : Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat Cell Biol. 2017;19(6):639–52. 10.1038/ncb3534 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Figueroa XF, Duling BR: Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11(2):251–66. 10.1089/ars.2008.2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dejana E: Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–70. 10.1038/nrm1357 [DOI] [PubMed] [Google Scholar]
- 73. Okamoto T, Akiyama M, Takeda M, et al. : Connexin32 protects against vascular inflammation by modulating inflammatory cytokine expression by endothelial cells. Exp Cell Res. 2011;317(3):348–55. 10.1016/j.yexcr.2010.10.018 [DOI] [PubMed] [Google Scholar]
- 74. Okamoto T, Akita N, Kawamoto E, et al. : Endothelial connexin32 enhances angiogenesis by positively regulating tube formation and cell migration. Exp Cell Res. 2014;321(2):133–41. 10.1016/j.yexcr.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 75. Meens MJ, Kutkut I, Rochemont V, et al. : Cx47 fine-tunes the handling of serum lipids but is dispensable for lymphatic vascular function. PLoS One. 2017;12(7):e0181476. 10.1371/journal.pone.0181476 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Haefliger JA, Allagnat F, Hamard L, et al. : Targeting Cx40 (Connexin40) Expression or Function Reduces Angiogenesis in the Developing Mouse Retina. Arterioscler Thromb Vasc Biol. 2017;37(11):2136–46. 10.1161/ATVBAHA.117.310072 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Gkatzis K, Thalgott J, Dos-Santos-Luis D, et al. : Interaction Between ALK1 Signaling and Connexin40 in the Development of Arteriovenous Malformations. Arterioscler Thromb Vasc Biol. 2016;36(4):707–17. 10.1161/ATVBAHA.115.306719 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Haefliger JA, Polikar R, Schnyder G, et al. : Connexin37 in normal and pathological development of mouse heart and great arteries. Dev Dyn. 2000;218(2):331–44. [DOI] [PubMed] [Google Scholar]
- 79. Fang JS, Angelov SN, Simon AM, et al. : Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol. 2011;301(5):H1872–81. 10.1152/ajpheart.00683.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Simon AM, McWhorter AR: Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251(2):206–20. 10.1006/dbio.2002.0826 [DOI] [PubMed] [Google Scholar]
- 81. Burt JM, Nelson TK, Simon AM, et al. : Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol. 2008;295(5):C1103–12. 10.1152/ajpcell.299.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Munger SJ, Kanady JD, Simon AM: Absence of venous valves in mice lacking Connexin37. Dev Biol. 2013;373(2):338–48. 10.1016/j.ydbio.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kanady JD, Dellinger MT, Munger SJ, et al. : Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol. 2011;354(2):253–66. 10.1016/j.ydbio.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Kanady JD, Munger SJ, Witte MH, et al. : Combining Foxc2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling. Dev Biol. 2015;405(1):33–46. 10.1016/j.ydbio.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu D, Krueger J, Le Noble F: The role of blood flow and microRNAs in blood vessel development. Int J Dev Biol. 2011;55(4–5):419–29. 10.1387/ijdb.103220dl [DOI] [PubMed] [Google Scholar]
- 86. Hata A, Lagna G: Deregulation of Drosha in the pathogenesis of hereditary hemorrhagic telangiectasia. Curr Opin Hematol. 2019;26(3):161–9. 10.1097/MOH.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Singh BN, Tahara N, Kawakami Y, et al. : Etv2-miR-130a-Jarid2 cascade regulates vascular patterning during embryogenesis. PLoS One. 2017;12(12):e0189010. 10.1371/journal.pone.0189010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Biyashev D, Veliceasa D, Topczewski J, et al. : miR-27b controls venous specification and tip cell fate. Blood. 2012;119(11):2679–87. 10.1182/blood-2011-07-370635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kazenwadel J, Michael MZ, Harvey NL: Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116(13):2395–401. 10.1182/blood-2009-12-256297 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation