Abstract
Background
Patients with glioma are at increased risk for tumor-related and treatment-related complications. Few guidelines exist to manage complications through supportive care. Our prior work suggests that a clinical care pathway can improve the care of patients with glioma.
Methods
We designed a quality improvement (QI) project to address the acute care needs of patients with gliomas. We formed a multidisciplinary team and selected 20 best-practice measures from the literature. Using a plan-do-study-act framework, we brainstormed and implemented various improvement strategies starting in October 2013. Statistical process control charts were used to assess progress.
Results
Retrospective data were available for 12 best practice measures. The baseline population consisted of 98 patients with glioma. Record review suggested wide variation in performance, with compliance ranging from 30% to 100%. The team hypothesized that lack of process standardization may contribute to less-than-ideal performance. After implementing improvement strategies, we reviewed the records of 63 consecutive patients with glioma. The proportion of patients meeting criteria for 12 practice measures modestly improved (65% pre-QI; 76% post-QI, P > .1). Unexpectedly, a higher proportion of patients were readmitted within 30 days of hospital discharge (pre-QI: 10%; post-QI: 17%, P > .1). Barriers to pathway development included difficulties with transforming manual measures into electronic data sets.
Conclusions
Creating evidence-based clinical care pathways for addressing the acute care needs of patients with glioma is feasible and important. There are many challenges, however, to developing sustainable systems for measuring and reporting performance outcomes overtime.
Keywords: glioma, outcomes, quality improvement
The majority of primary malignant brain tumors are diffuse infiltrating gliomas.1 Glioblastoma (GBM), the most aggressive form, accounts for the largest number of these cases.1,2 Due to their aggressive nature, gliomas are often associated with poor survival.1 Furthermore, patients with glioma are at increased risk for developing tumor-related and treatment-related complications such as cognitive dysfunction, fatigue, pain, and disability.3,4 These symptoms may affect quality of life to varying degrees during the course of a patient's illness.3 Elderly patients with glioma are more likely to suffer from medical comorbidities, have poorer tolerance of chemotherapeutic agents, and be at increased risk for developing radiation-induced neurotoxicty.5 Finally, brain tumors are an independent risk factor for falls,6 the seventh leading cause of death in those over age 65.7
Few guidelines are available to direct decisions about how to effectively address the supportive care needs of patients with glioma and there are wide variations in patterns of care.8 Cancer-specific clinical care pathways may assist in selecting evidence-based care and lead to improved quality of care.9 We previously developed a clinical care pathway to address the needs of patients with glioma during the perioperative period.10 We found that quality improvement (QI) methods could be used to improve the care provided to patients with glioma.10 Furthermore, we observed a significant improvement in several quality measures including more prompt presentation of patients at tumor board and earlier assessment by social workers.10 These results suggest that it may be beneficial to develop pathways that address other aspects of care of patients with glioma.
Here we report on the second component of this multi-phase project. Using QI methodology, we developed a clinical care pathway to address the needs of patients with glioma during the acute phase of their treatment. The overall aim of our project is to ensure that patients with glioma receive comprehensive, consistent and timely care, with the ultimate goal of improving outcomes.
Materials and Methods
Organizing for Improvement
Approximately 30% of the patients with primary brain tumors treated at our institution carry a diagnosis of glioma. As previously reported, we chartered a multi-phase QI project, beginning with the patients entering into the Neuro-Oncology microsystem and ending with discharge to survivorship or death.10 We defined the microsystem as a group of multidisciplinary, health care professionals who came together to care for a defined population of patients.11
This phase of the project focused on the care of patients from the time of neurosurgery through the first 10 weeks after diagnosis, a period that included the completion of treatment with chemotherapy and/or radiation. The specific aim was to improve the quality of care, to reduce process variation, and to maximize patient safety. We assembled an interdisciplinary team that was comprised of physicians, nursing staff, schedulers, a social worker, and QI experts. The team represented several disciplines including neuro-oncology, neurosurgery, radiation oncology, care management, and Cancer Center leadership. The team met weekly between February and March 2013, monthly April through July 2013, bimonthly August through May 2014, and then quarterly to maintain project gains.
This project was QI work with no research component. Therefore, it was exempt from review by our local institutional review board, the Dartmouth College Committee for the Protection of Human Subjects.
Planning and Implementation
Prior to starting this QI effort, we reviewed the available literature. With the exception of some evidence on rates of perioperative complication12,13 and guidelines that direct chemotherapy, surgery, and radiation,14–16 we found minimal information for which measures led to improved outcomes in patients with glioma during this phase of care. Based on the limited evidence and brainstorming, we proposed 20 objective process and outcome measures that delineated timely and comprehensive care.
We used commonly accepted QI methods to conduct our project,17,18 including the Define-Measure-Analyze-Improve-Control (DMAIC) model. In the define phase, we described the current system of care and created flowcharts to summarize processes of care. The team members created three process maps, representing neurosurgery, chemotherapy, and radiation therapy and then combined these into one process map. In the measure phase, the team assigned the 20 objective best practice measures to relevant steps in the process (see Supplementary Table S1).19–31
In the analyze phase, we obtained performance data from available electronic medical records (EMR) and from an existing database created through prior QI work. Using a plan-do-study-act framework and tools such as fishbone diagraming,17,18 the team then evaluated how well the current process was performing. The team postulated that there were at least 8 reasons for less-than-ideal process performance and designed 10 improvement interventions to address these concerns (the improvement phase). Once the changes were successfully implemented, the team crafted a more formalized strategy for maintaining the gains that we achieved and identifying additional strategies for improvement (the control phase).
Proposed Improvement Strategies
The team found that there were several reasons for less-than-ideal process performance. For instance, while we assumed that clinicians routinely discuss symptoms of concern and risk factors for venous thrombo-embolic events (VTE) with patients, this was not consistently documented in the medical record. Because clinical documentation plays a key role in facilitating communication between patients and the health care team,32 we were concerned that a lack of written information about the plan of care may result in poorer quality care. Delays in the turn-around time for pathology results to be available in the EMR also disrupted treatment planning in some cases. Furthermore, there was no reliable mechanism for assessing baseline compliance with 8 of the best practice measures and some measures were not even part of the current process. For example, the definition of chemotherapy education varied between team members and there was no agreed upon time when this education should take place.
The team also discovered that there were other nuances in the workflow patterns that may be problematic. For example, the social worker typically met with patients during their scheduled visit with the neuro-oncologist. In the event that this visit went longer than planned, the social worker was unable to complete her psychosocial assessment. Thus, the social worker was unable to assess patients for relevant complications such as psychological distress, depression, and financial concerns. Untreated cancer-related depression may contribute to poorer quality of life and shortened survival.33,34 Furthermore unaddressed financial concerns may result in unnecessary delays in the start of treatment.
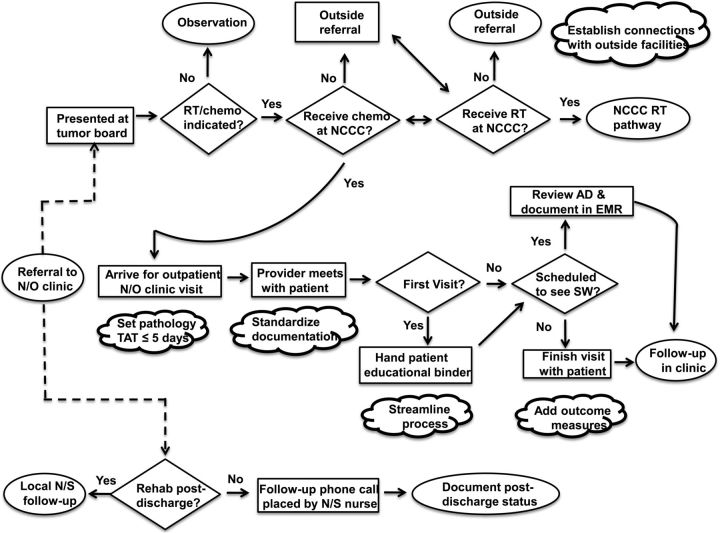
In October 2013, the team piloted several small tests of change to address these concerns. The team created a standardized template to document that patients were counseled about symptoms of concern and informed of the availability of clinical trials. The clinic secretary scheduled independent visits for the social worker to meet with patients and families. Clinicians were encouraged to document clinical outcomes such as treatment complications and interruptions. Pathology was involved in establishing a target goal of providing results within 5 business days. Fig. 1 highlights key process changes.
Fig. 1.
Process flow map for the care of patients with glioma (postintervention phase). AD = advanced directive; Chemo = chemotherapy; EMR = electronic medical record; NCCC = Norris Cotton Cancer Center, Lebanon, NH; N/O = neuro-oncology; N/S = neurosurgery, Rehab = rehabilitation facility; RT = radiation oncology; SW = social worker; TAT = turn-around time. [cloud shaped image] summarize key changes that were made at the respective steps of the process.
The team added these newly identified measures to an existing data dashboard to facilitate ongoing measurement and reporting of process performance. The project leader reviewed the measures on the dashboard each month with the statistician. The team met quarterly to review system performance and to continue to modify the process as necessary.
Outcome Measures
We generated a score for each patient based on maximum standards of care achieved. This score was comprised only of the 12 best-practice measures for which baseline data was available. The numerator represented the number of standards of care met and the denominator represented the number of standards of care that should be achieved by each patient. Depending on symptoms and grade of glioma, certain standards of care did not apply to a subset of patients. For example, patients who were discharged to a rehabilitation facility did not receive a follow-up phone call from nursing staff.
Statistical Analysis
To ascertain whether the maximum standard of care improved over time, we analyzed the results for consecutive patients in an individual values and moving range (XmR) chart. In this statistical process-control (SPC) chart, each data point represents a single observation.35 The SPC chart contains upper and lower control limits, which are set at 3-sigma to minimize the risk of a type I error.35 SPC charts differentiate between 2 types of variation, common and special cause. Processes are considered to be in statistical control when rates over time fall within the upper and lower control limits.35 A statistically significant change or a special cause variation occurs when 1 or more points go beyond the control limits or when there is a process shift in which 8 or more successive values fall on the same side of the overall rate.35,36
SPC charts are useful statistical tools for detecting special cause variation when the control limits are set at 3-sigma. Here the probability of a type 1 error (ie, point falling outside the control limits due to chance) is small (P < .01).37 Similarly, the probability of 8 or more successive values falling on the same side of the overall rate is low.37 Therefore, it is appropriate to infer that a statistically significant change occurred and recalculate the overall rate and limits.
We used a histogram plot to compare the proportion of eligible patients meeting each of the individual 12 measures pre-QI and post-QI work. We relied on the χ2 statistic to evaluate associations between categorical variables. We defined statistically significant as P < .05.
Results
Baseline Performance
The baseline population consisted of 98 patients with newly diagnosed glioma who received surgical care at our institution between June 2011 and September 2013. We found that retrospective data were available for only 12 of the 20 identified best-practice measures; 6 of which could be electronically abstracted from medical records. There was also wide variation in performance, with compliance ranging from 30% to 100%. Table 1 compares the baseline characteristics of the populations included in the pre-QI and post-QI phases.
Table 1.
Baseline characteristics of subjects before and after quality improvement work, June 2011 – September 2013 and October 2013 – March 2015
| Pre-QI, N (%) | Post-QI, N (%) | |
|---|---|---|
| Total | 98 (100) | 63 (100) |
| Sex, Male | 65 (66) | 35 (56) |
| Mean age in years [SD] | 59.6 [15.7] | 59.7 (16.6) |
| Tumor Grade | ||
| Grade I | 3 (3) | 5 (8) |
| Grade II | 9 (9) | 5 (8) |
| Grade III | 10 (10) | 7 (11) |
| Grade IV | 76 (78) | 46 (73) |
| Surgical Management | ||
| Resection | 82 (84) | 48 (76) |
| Biopsy | 16 (16) | 15 (24) |
| Median length of stay, days | 5 | 5 |
QI, Quality Improvement; %, percent, SD, standard deviation.
Impact of Process Improvement
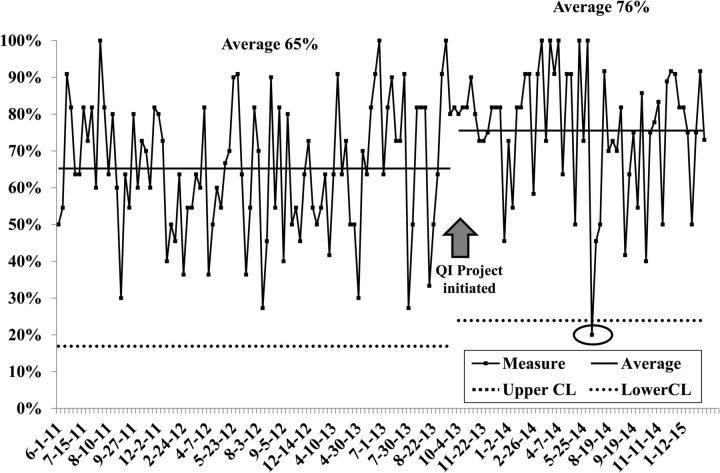
Figure 2 depicts consecutive patients and the proportion of the 12 best-practice measures that were met pre-QI and post-QI work. Before the QI work, the overall mean was 65% and there was wide variation (the lower and upper control limits were 18% and 100%, respectively). After we implemented the improvement interventions starting in October 2013, compliance with the best-practice measures improved with an overall mean of 76%, although the result was not statistically significant (P > .1). There was less variation in the post-QI group, meaning that best practices would be met from 24% (lower control limit) to 100% (every time). The team also identified 1 patient whose best-practice measures fell below the lower control limit of 24% (special-cause result). The team investigated this event and discovered that language barriers likely contributed to a breakdown in the care pathway.
Fig. 2.
Individual value and moving range (XmR) chart of percent standards of care achieved by patients with glioma pre- and post-quality improvement work, June 2011 – September 2013 and October 2013 – March 2015.a,b CL = control limit, % = percent; QI = quality improvement. aCircle delineates a special cause event. bEach data point represents a single observation. Upper and lower control limits are set at 3-sigma to minimize the risk of a type I error.
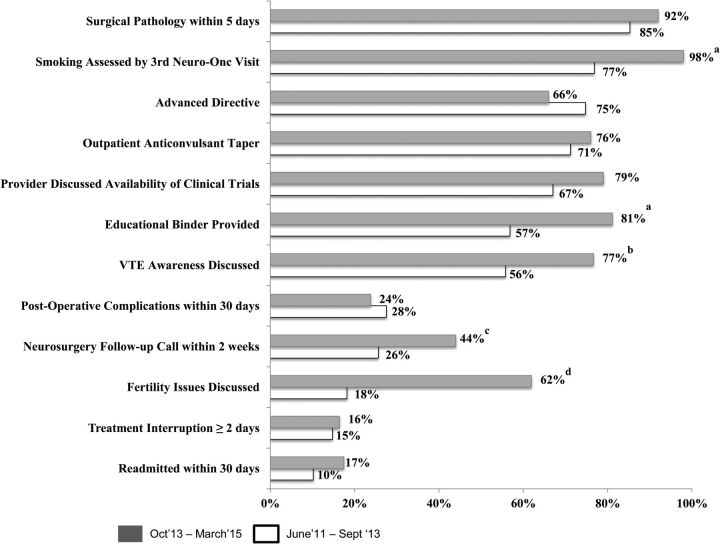
Figure 3 demonstrates the degree to which individual measures changed with these QI efforts. There were significant improvements in measures assessing for (i) educational binder provided to patient (57% vs 81%, P < .01); (ii) discussion of VTE symptoms and risks (56% vs 77%, P = .01); (iii) discussion of fertility risks (18% vs 62%, P = .02); (iv) neurosurgery follow-up call placed within 2 weeks of hospital discharge (26% vs 44%, P = .03); and (v) smoking status assessed by the third neuro-oncology visit (77% vs 98% %, P < .01). Conversely, provision of advanced directives (75% vs 66%, P > .1) and 30-day readmission rates (10% vs 17%, P > .1) worsened, but these results were not significant.
Fig. 3.
Proportion of patients with glioma meeting criteria for individual best-practice measures pre- and post-quality improvement initiative, June 2011 – September 2013 and October 2013 – March 2015. AED = antiepileptic medications; F/u = follow-up; Neuro-Onc = Neuro-Oncology Program; post-op = postoperative; surg path = surgical pathology; VTE = venous-thromboembolic events. aP < .01. bP = .01. cP = .03. dP = .02.
Challenges and Barriers
The team encountered several challenges in conducting this QI work. Roughly 60% of patients received a portion of their treatment (eg, radiation) at outside sites. These facilities were not part of the institutional network and used different EMRs. This made it difficult to track all aspects of patient care. Similarly, there was no standardized system for documenting certain measures such as treatment complications. Instead, this process was highly dependent on patient self-report and provider documentation. Manual data abstraction from the EMR was also quite labor intensive. The team worked with the institution's information technology department to develop more reliable and valid electronic measures. The team also contacted other radiation oncology facilities (involved in the care of our patients) to initiate conversations about improving the system of communication between sites.
We observed that the process for carrying out the post-discharge follow-up phone call was highly operator dependent. If the designated nurse was not available, there was no other staff member assigned to this role. We also observed that the limited amount of social work support available to the clinic impacted at least one of the performance measurements. This prompted a needs assessment for additional social work support for the program.
Unanticipated Opportunities for Improvement
As shown in Table 2, the team found that several patients were readmitted within 30 days of hospital discharge (pre-QI: 10%; post-QI: 17%, P > .1) and/or experienced tumor-related and treatment-related complications (pre-QI: 28%; post-QI: 24%, P > .1). The most frequent potentially avoidable complications included: postoperative urinary tract infections (UTI) (pre-QI: 40%; post-QI: 9%), VTE (pre-QI: 17%; post-QI: 27%), and outpatient falls (pre-QI: 33%; post-QI: 27%). Two falls were associated with significant complications requiring a higher level of care. The team analyzed each of these cases to identify additional opportunities for improvement.
Table 2.
Hospital readmissions and postoperative Complications, pre-QI and post-QI work; June 2011 – September 2013 and October 2013 – March 2015
| Pre-QI, N (%) | Post-QI, N (%) | |
|---|---|---|
| Total | 98 (100) | 63 (100) |
| Patients with ≤30-day Hospital Readmissions | 10 (10) | 11 (17) |
| Patients with any ≤30-day Complications | 27 (28) | 15 (24) |
| Patients with Potentially Avoidable ≤30-day Hospital Readmissionsa | 3/10 (30) | 2/10 (18) |
| DVT/PE | 1 | 0 |
| Wound Infection | 0 | 2 |
| Anticipated Physiologic or Accidental Fallc,d | 2 | 0 |
| Patients with Potentially Avoidable ≤30-day Complicationsb | 24 (89) | 11 (73) |
| Falls | ||
| Anticipated Physiologicc | 9 | 3 |
| Accidentald | 1 | 0 |
| Post-op UTI | 12 | 1 |
| DVT/PE | 5 | 3 |
| Wound Infection | 3 | 3 |
| Seizure | 0 | 1 |
DVT, deep vein thrombosis; N, number; %, percent; PE, pulmonary embolism; Post-op UTI, postoperative urinary tract infection; QI, quality improvement.
aWe defined potentially avoidable hospital readmissions as any events that were clinically related to the patient's initial hospitalization for surgical resection (or biopsy) of glioma and may have been prevented if evidence-based care processes had been followed.38 For example, patients with DVT did not receive postoperative chemoprophylaxis and patients with a history of seizure were not prescribed antiepileptic drugs.
bWe defined potentially avoidable complications as any events that were clinically related to the patient's glioma diagnosis and may have been prevented if evidence-based care processes had been followed. For example, patients who had not received a home safety evaluation and later had an accidental fall in the home.
cAnticipated physiologic falls are due to intrinsic physiological factors such as delirium or confusion. These falls may be preventable.39
Although there was a hospital-wide program to address inpatient falls, there was no standardized protocol for addressing fall risk at the time of discharge. The team collaborated with rehabilitation, care management, and inpatient nursing to begin to develop a protocol for improving the management of outpatient fall risk. These discussions also enabled the team to learn about (and learn from) a similar program that was already underway in the emergency room.
Concurrent with our QI work, the hospital implemented a nursing-led effort to minimize inpatient UTIs. Subsequent to the implementation of this intervention, the team observed that there were no further cases of UTIs among patients with glioma. The team also shared the VTE findings with the section of neurosurgery. These discussions resulted in the formation of a separate interdisciplinary QI initiative to evaluate the role of chemical VTE prophylaxis for patients with glioma. As a result of this work, the neurosurgery service decided to change the protocol for managing VTE risk in hospitalized patients with glioma. Patients without evidence of bleeding on a postoperative MRI will receive chemical prophylaxis with heparin.
Discussion
Developing clinical care pathways to ensure that patients receive comprehensive, consistent, and timely care may reduce their risk for complications and potentially improve their quality of life. Our prior work demonstrated that a clinical care pathway that addressed the needs of patients with glioma during the perioperative period led to significant improvements in several areas of care.10 Similarly, Back et al found that a well-coordinated, multidisciplinary approach resulted in significant improvement in survival for patients with high-grade glioma.41
We used QI methodology to develop a clinical care pathway to address the needs of patients with glioma during the acute phase of their care. We found that the proportion of patients meeting best-practice measures, as defined by the group, improved from 65% pre-QI work to 76% post-QI work, although the results were not statistically significant. There was, however, a change in several best-practice measures including a statistically significant increase in the number of patients who received an educational binder, were assessed for smoking by the third neuro-oncology visit, received a neurosurgery follow-up call within 2 weeks of hospital discharge, and were informed of VTE symptoms and risks and fertility risks. Ongoing engagement of team members in these QI efforts was necessary for maintaining project gains. It was also critical to develop systems of care that incorporated the EMR in order to facilitate project sustainability. Finally, the development of a data dashboard to track measurements over time facilitated root cause analysis of protocol deviations, revealed unexpected areas in need of additional improvement, and enabled us to develop interventions to address these concerns and improve care.
Similar to other studies, we found a high proportion of hospital readmissions and tumor-related and treatment-related complications.42,43 Marcus et al reported a baseline 30-day readmission rate of 13.2% among patients discharged after craniotomy for malignant supratentorial tumors.42 Unlike our results, however, these authors found that these admissions were largely driven by seizures.42 These differences may be attributed to several factors. We had smaller sample sizes and a large proportion of patients in our analysis were diagnosed with GBM. In a separate review of patients with glioma undergoing biopsy or tumor resection, Dickinson et al found that 7.5% experienced an unplanned readmission within 30 days of discharge.43 The authors felt that the majority of these readmissions were preventable.43 In our study, we also found that a large proportion of readmissions were due to potentially avoidable causes.
The high proportion of patients experiencing symptomatic VTE complications after surgery, in spite of the standardized use of external pneumatic devices, was unanticipated. While patients with glioma are known to be at higher risk for developing VTEs,44,45 clinicians are reluctant to use chemical prophylaxis in this population.46,47 Establishing standards of care around VTE prophylaxis in the glioma population is necessary and the benefits of chemoprophylaxis may outweigh the harm.48–50 Relying solely on pneumatic compression devices may not be advisable since compliance with these devices is generally poor, even with patient and staff education.51,52 Furthermore, risk stratification may help to identify those who would most benefit from chemoprophylaxis.45,53 As a result of our work, prophylactic heparin is being incorporated into the routine care of patients with glioma who do not have evidence of bleeding on postoperative MRI. The dashboard will allow us to monitor the incidence of VTE and hemorrhagic complications over time and make modifications accordingly.
Another significant finding was the high proportion of patients having outpatient falls and UTIs. Patients with glioma are at higher risk for falls and yet, as far we know, there has been no study of interventions to reduce fall risk in the glioma population.6,54,55 Exercise and home safety interventions have been proven to be very effective in minimizing outpatient fall risk in other at-risk populations.56 Similar to other studies, we found that a comprehensive, nursing-led initiative eliminated postoperative, catheter-associated UTIs.57,58 These results suggest that there is a need for institutions to develop standardized practices for addressing tumor-related and treatment-related complications affecting patients with glioma.
Unfortunately, we found that the proportion of patients meeting criteria for advanced directives worsened during our QI initiative. While this process step is overseen by the social worker, only a small portion of social work full-time equivalent is currently allocated to our program. These findings combined with a high number of readmissions and tumor-related and treatment-related complications suggest that social work services should be expanded within the clinic. Some studies have found that the inclusion of social work services in the management of complex medical illness can improve overall care.59–60 For example, Lipani et al found that the use of a preventable admission care team, which was largely led by social work, resulted in a 43% reduction in hospital readmissions and 70% reduction in emergency room visits for patients with complex medical illness.60
There were a number of limitations to our work. We were unable to demonstrate that improvements in our quality measures yielded improved survival, better use of resources, or more satisfied patients and staff. While some studies have found that educating patients about their disease and following up with patients by phone postdischarge may result in better outcomes such as improved patient satisfaction, prior reviews have found that there is limited evidence to support such an association.61,62 Although smoking cessation may not improve the overall survival of patients with glioma, there is modest evidence to suggest that smoking cessation may have other important clinical benefits including a reduction in the risk of VTE.63 During the course of our work, there was also a change in the QI leadership at our institution that may have resulted in some uncertainty among our team and staff members regarding the long-term sustainability of our work. Our small sample size may have limited our ability to demonstrate significant improvement, though we observed positive trends in many of our best-practice measures. Finally, while the best-practice metrics we developed may apply to the care of patients at other institutions, our pathway may not be generalizable. Each institution may need to go through their own QI process to identify a pathway that addresses their unique context.
We hope to address some of the above-named limitations in our future work. Our institution recently implemented a cancer-specific, patient satisfaction survey. These results should provide more insight into the potential effects of QI initiatives on patient satisfaction. We also incorporated questions on patient satisfaction into the postdischarge follow-up phone call. We have shared our results with other neuro-oncology groups with similar QI projects to examine the applicability to other institutions. Finally, we have integrated 8 additional process and outcome measures to the data dashboard with plans to continue to evaluate these with the next phase in the continuum of care.
Conclusions
We believe it is important to develop evidence-based, clinical care pathways to address the needs of patients with glioma during the acute phases of care. However, there are challenges to developing these pathways including lack of consensus or clear evidence about which process measures are associated with improved outcomes. With the arrival of programs such as the Center for Medicare and Medicaid's Hospital-Acquired Condition, which ties reimbursement to quality rather than quantity, there are growing financial pressures on cancer programs to improve the overall quality of care delivered to patients.64
As part of the next steps of our work, we are re-evaluating the protocol for managing VTE risk in our patient population. We are also working with other programs within our institution to develop a protocol for reducing fall risk. The next phase of our project will then focus on developing a pathway to address the chronic care needs of patients afflicted with glioma.
Funding
None declared.
Supplementary Material
Acknowledgments
We would like to acknowledge Kati Fuller, Nancy Lapoint, Mary Robinson, Carissa Thurston, Denise Preston, and Louise Meyer for their assistance in carrying out this QI initiative, as well as Dr. Christopher Dant and Dr. J. Marc Pipas for reviewing our manuscript and providing us with editing suggestions. Finally, we would like to extend our appreciation to the original sponsors to this project including Dr. Mark Israel, Director of Norris Cotton Cancer Center, and Dr. David Roberts, Section Chief of Neurosurgery.
Conflicts of interest statement. The authors have no conflicts of interest to disclose.
References
- 1. Schwartzbaum JA, Fischer JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503. [DOI] [PubMed] [Google Scholar]
- 2. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 3. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical managemnet of brain tumors and the sequelae of treatment. Neuro Oncol. 2015;17(4):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nayak L, Iwamoto F. Primary brain tumors in the elderly. Curr Neurol Neurosci Rep. 2010;10(4):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(7):2128–2133. [DOI] [PubMed] [Google Scholar]
- 7. Rubenstein L. Falls in the elderly. The Merck Manual for Health Care Professionals Available at http://www.merckmanuals.com/professional/geriatrics/falls-in-the-elderly/falls-in-the-elderly Accessed July 4, 2015.
- 8. Chang SM, Parney IF, Huang W et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 9. Phillips C. Clinical pathways in cancer care catching on. NCI Cancer Bulletin. 2012;9: Available at https://wayback.archive-it.org/org-317/20141005065501/http://www.cancer.gov/ncicancerbulletin/090412/page 6 Accessed July 4, 2015. [Google Scholar]
- 10. Riblet N, Schlosser E, Homa K et al. Improving the quality of care for patients diagnosed with glioma during the perioperative period. JOP. 2014;10:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson EC, Batalden PB, Lazar JS et al. Understanding clinical improvement: Foundations of knowledge for change in health care systems, In Nelson EC, Batalden PB, Lazar JS (eds): Practice-Based Learning and Improvement: A Clinical Improvement Action Guide (ed 2). Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 2007, pp. 1–12. [Google Scholar]
- 12. Rahman M, Neal D, Fargen KM et al. Establishing standard performance measures for adult brain tumor patients: a nationwide inpatient sample database study. Neuro Oncol. 2013;15(11):1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang SM, Parney IF, McDermott M et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98(6):1175–1181. [DOI] [PubMed] [Google Scholar]
- 14. Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(Suppl 5):459–466. [DOI] [PubMed] [Google Scholar]
- 15. Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190–v193. [DOI] [PubMed] [Google Scholar]
- 16. Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11(2):152–164. [DOI] [PubMed] [Google Scholar]
- 17. Caldwell C, Butler G, Posten N. Lean-Six Sigma for Healthcare: A Senior Leader Guide to Improving Cost and Throughput .2nd ed. Milwaukee, WI: American Society for Quality, Quality Press; 2009. [Google Scholar]
- 18. Nelson EC. Appendix A, improving care: Clinical improvement worksheets, In Nelson EC, Batalden PB, Lazar JS, eds. Practice-Based Learning and Improvement: A Clinical Improvement Action Guide. 2nd ed. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 2007:135–155. [Google Scholar]
- 19. Rosenblum ML, Mikkelsen T. Developing a brain tumor center. J Neurooncol. 2004;69(1–3):169–180. [DOI] [PubMed] [Google Scholar]
- 20. Easaw JC, Mason WP, Perry J et al. Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol. 2011;18(3):e126–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quality Oncology Practice Initiative (QOPI) and the QOPI Certification Program (QCP). ASCO Institute for Quality. http://www.instituteforquality.org/qopi-qcp, Accessed July 6, 2015.
- 22. Mitty EL, Ramsey G.. Advance directives. In: Capezuti E, Zwicker D, Mezey M, Fulmer T, eds. Evidence Based Geriatric Nursing Protocols for Best Practice. 3rd ed.New York, NY: Springer Publishing Company; 2008:539–563. [Google Scholar]
- 23. Weston J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post-craniotomy seizures. Cochrane Database Syst Rev; 2015;3:CD007286. [DOI] [PubMed] [Google Scholar]
- 24. Center for Medicare & Medicaid Services. Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Updated August 4, 2014. Accessed October 5, 2015.
- 25. Board of Faculty of Clinical Oncology; The Royal College of Radiologists. The timely delivery of radical radiotherapy: standards and guidelines for the management of unscheduled treatment interruptions. 3rd ed https://www.rcr.ac.uk/docs/oncology/pdf/BFCO%2808%296_Interruptions.pdf. Published 2008. Accessed October 5, 2015.
- 26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Supportive Care: Distress Management. Version 1.2015. www.nccn.org Accessed July 5, 2015.
- 27. Lai R, Hershman DL, Doan T, Neugut AI. The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro Oncol. 2010;12(2):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57(2):131–136. [DOI] [PubMed] [Google Scholar]
- 29. Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007;85(3):339–343. [DOI] [PubMed] [Google Scholar]
- 30. Blumenthal DT, Won M, Mehta MP et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jansen M, Yip S, Louis DN. Molecular pathology in adult neuro-oncology: an update on diagnostic, prognostic and predictive markers. Lancet Neurol. 2010;9(7):717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuhn T, Basch P, Barr M, Yackel T; Medical Informatics Committee of the American College of Physicians. Clinical documentation in the 21st Century: executive summary of a policy position paper from the American College of Physicians. Ann Intern Med. 2015;162(4):301–303. [DOI] [PubMed] [Google Scholar]
- 33. Chan CM, Wan Ahmad WA, Md Yusof M, Ho GF, Krupat E. Effects of depression and anxiety on mortality in a mixed cancer group: a longitudinal approach using standardized diagnostic interviews. Psychooncology. 2015;24(6):718–725. [DOI] [PubMed] [Google Scholar]
- 34. Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19(7):734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carey R. Improving Healthcare with Control Charts: Basic and Advanced SPC Methods and Case Studies. Milwaukee, WI: ASQ Quality Press; 2003. [Google Scholar]
- 36. Hart M, Hart R. Statistical Process Control in Health Care. Pacific Grove, CA: Duxbury; 2002. [Google Scholar]
- 37. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. 3M™ Health Information Systems. Potentially Preventable Readmissions Classification System: Methodology Overview. GRP-139. http://multimedia.3m.com/mws/media/531478O/methodology-overview-pprs-05-08.pdf Published March 2008. Accessed August 16, 2015.
- 39. Morse JM. Preventing Patient Falls. Thousand Oaks, CA: Sage; 1997. [Google Scholar]
- 40. Connell BR. Role of the environment in falls prevention. Clin Geriatr Med. 1996;12(4):859–880. [PubMed] [Google Scholar]
- 41. Back MF, Ang EL, Ng WH et al. Improvements in quality of care resulting from a formal multidisciplinary tumour clinic in the management of high-grade glioma. Ann Acad Med Singapore. 2007;36(5):347–351. [PubMed] [Google Scholar]
- 42. Marcus LP, McCutcheon BA, Noorbakhsh A et al. Incidence and predictors of 30-day readmission for patients discharged home after craniotomy for malignant supratentorial tumors in California (1995–2010). J Neurosurg. 2014;120(5):1201–1211. [DOI] [PubMed] [Google Scholar]
- 43. Dickinson H, Carico C, Nuno M et al. Unplanned readmissions and survival following brain tumor surgery. J Neurosurg. 2015;122(1):61–68. [DOI] [PubMed] [Google Scholar]
- 44. Smith TR, Nanney AD 3rd, Lall RR et al. Development of venous thromboembolism (VTE) in patients undergoing surgery for brain tumors: results from a single center over a 10 year period. J Clin Neurosci. 2015;22(3):519–525. [DOI] [PubMed] [Google Scholar]
- 45. Chaichana K, Pendleton C, Jackson C et al. Deep vein thrombosis and pulmonary embolism in adult patients undergoing craniotomy for brain tumors. Neurol Res. 2013;35(2):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carman TL, Kanner AA, Barnett GH, Deitcher SR. Prevention of thromboembolism after neurosurgery for brain and spinal tumors. South Med J. 2003;96(1):17–22. [DOI] [PubMed] [Google Scholar]
- 47. Swann KW, Black PM. Deep vein thrombosis and pulmonary emboli in neurosurgical patients: a review. J Neurosurg. 1984;61(6):1055–1062. [DOI] [PubMed] [Google Scholar]
- 48. Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122(6):1933–1937. [DOI] [PubMed] [Google Scholar]
- 49. Constantini S, Kanner A, Friedman A et al. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg. 2001;94(6):918–921. [DOI] [PubMed] [Google Scholar]
- 50. Knovich MA, Lesser GJ. The management of thromboembolic disease in patients with central nervous system malignancies. Curr Treat Options Oncol. 2004;5(6):511–517. [DOI] [PubMed] [Google Scholar]
- 51. Stewart D, Zalamea N, Waxman K, Schuster R, Bozuk M. A prospective study of nurse and patient education on compliance with sequential compression devices. Am Surg. 2006;72(10):921–923. [PubMed] [Google Scholar]
- 52. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influences of patient and hospital factors on compliance. BMC Urol. 2013;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kimmell K, Jahromi B. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J Neurosurg. 2015;122(5):1004–1011. [DOI] [PubMed] [Google Scholar]
- 54. Wildes TM, Dud P, Fowler SA et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Capone LJ, Albert NM, Bena JF, Tang AS. Predictors of a fall event in hospitalized patients with cancer. Onc Nurs Forum. 2012;39(5):E407–E415. [DOI] [PubMed] [Google Scholar]
- 56. Gillespie LD, Robertson MC, Gillespie WJ et al. Interventions for preventing falls in older people living in the community: review. Cochrane Database Sys Rev. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoon B, McIntosh S, Rodriquez L, Holley A, Faselis C, Liappis A. Changing behavior among nurses to track indwelling urinary catheters in hospitalized patients. Interdiscip Perspect Infect Dis. 2013;2013:405041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bernard MS, Hunter KF, Moore KN. A review of strategies to decrease the duration of indwelling urethral catheters and potentially reduce the incidence of catheter-associated urinary tract infections. Urol Nurs. 2012;32(1):29–37. [PubMed] [Google Scholar]
- 59. Cherry DL, Vickrey BG, Schwankovsky L, Heck E, Plauche M, Yep R. Interventions to improve quality of care: the Kaiser Permanente-alzheimer association dementia care project. Am J Manag Care. 2004;10(8):553–560. [PubMed] [Google Scholar]
- 60. Lipani M, Bernstein S, Stulman J et al. PACT: preventable admission core team: a unique approach to reducing 30 day readmissions. Circulation. 2011;124:21 (supplement):A17449. [Google Scholar]
- 61. Coulter A. Patient engagement--what works? J Ambul Care Manage. 2012;35(2):80–89. [DOI] [PubMed] [Google Scholar]
- 62. Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006;4:CD004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheng Y-J, Liu Z-H, Yao F-J. Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLOS Med. 2013;10(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Understanding the Hospital-Acquired Condition Required Program. Lake Superior Quality Innovation Network . http://www.stratishealth.org/documents/HAC_fact_sheet.pdf. Accessed March 16, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.