Abstract
Background
Keloids are characterized by an overabundance of collagen deposition due to elevated activity and proliferation of fibroblasts, which lead to hypoxic conditions. Adaptation to these conditions is regulated by the transcription factor hypoxia inducible factor-1α (HIF-1α). Cytoglobin (Cygb), a reactive oxygen species scavenger, is a target gene of HIF-1α. In our previous study, we showed that Cygb expression in keloid tissue was correlated with HIF-1α expression. However, whether HIF-1α regulates Cygb expression and the proliferation of keloid fibroblasts remained unclear. Therefore, this study aimed to determine the role of HIF-1α in Cygb expression and fibroblast proliferation of keloids.
Methods
This was an in vitro study using a primary culture of keloid fibroblasts in which ibuprofen was used to inhibit HIF-1α expression. The expression of HIF-1α and Cygb mRNA were analyzed using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) methods, and their protein levels were analyzed using an enzyme-linked immunosorbent assay (ELISA). Fibroblast proliferation was analyzed using a Trypan blue exclusion assay.
Results
Inhibition of HIF-1α by ibuprofen decreased Cygb mRNA expression but not in all the samples, followed by a decrease in the protein level of Cygb. There was a positive correlation between the HIF-1α protein and Cygb mRNA, probably due to the regulation of Cygb by HIF-1α at the mRNA level, but not the protein level. The proliferation of keloid fibroblasts was significantly decreased and positively correlated with the HIF-1α protein.
Conclusion
HIF-1α regulates Cygb expression and fibroblast proliferation in keloids.
Keywords: HIF-1α, Cygb, Ibuprofen, Proliferation, Fibroblast, Keloid
INTRODUCTION
A keloid is a benign skin tumor that occurs as a result of excessive fibrogenesis as part of the wound healing process.1 Keloid scars show similarities with tumors in terms of overgrowth and rapid proliferation.2 The progression of keloids is characterized by an increase in extracellular matrix deposition as a result of prolonged fibrosis inflammatory processes in keloid tissue.3,4 Fibroblasts stimulate excess collagen synthesis in keloids, and they show the same bioenergetics as cancer cells in terms of ATP formation, which is primarily produced by the glycolysis proccess.5
High fibroblast proliferation in keloids leads to hypoxic conditions.6 Adaptation to these conditions is mediated by hypoxia inducible factor-1 (HIF-1), which consists of two subunits, an α-subunit (HIF-1α) and a β-subunit (HIF-1β). The function of HIF-1 is primarily determined by HIF-1α because HIF-1α expression depends on cellular oxygen levels, whereas HIF-1β is expressed constitutively in cell nuclei.7 Under hypoxic conditions, HIF-1 acts as a transcription factor and aids cell survival, for example, by inducing the expression of genes that contribute to cell proliferation, metabolism, and angiogenesis.8
Hypoxic conditions trigger cells to produce reactive oxygen species, which can be scavenged by cytoglobin (Cygb).9 The latter is involved in fibrogenesis, where it plays an important role in collagen synthesis by providing oxygen for hydroxylation of proline residues during pro-collagen synthesis.10 Hypoxic conditions have been shown to increase Cygb levels. For example, Guo et al. demonstrated the presence of binding sites of HIF-1 on hypoxia response elements (HREs) of the Cygb promoter gene in a cervical adenocarcinoma cell line and in normal human lung cells (BEAS-2B).11
In the previous study, the result showed that Cygb played a role in hypoxia of fibrosis tissue.12 Although Cygb is associated with HIF-1α expression and correlated with fibroblast growth factor and pro-collagen I and III, both of which are important in fibrosis,12 whether Cygb expression and keloid fibroblast proliferation are under the control of HIF-1 remain unclear. Therefore, the aim of this study was to analyze the regulation of Cygb expression and keloid fibroblast proliferation by HIF-1α. With this aim in mind, HIF-1α expression in keloid fibroblasts was inhibited using ibuprofen, a nonsteroidal anti-inflammatory drug that inhibits HIF by suppressing the synthesis and increasing the degradation of the HIF-1α protein.
MATERIALS AND METHODS
Materials
Material for this studies were as follow: Dulbecco’s modified Eagles medium (DMEM) low-glucose (Gibco, Fisher Scientific, UK), 1% penicillin-streptomycin (Sigma-Aldrich, USA), phosphate buffered saline/PBS (Sigma-Aldrich, USA), 1% amphotericin (Biosera, UK), 10% fetal bovine serum/FBS (Gibco, Thermo Scientific, UK ), 24-well culture plate for primary fibroblast culture (Costar, Corning, USA), Ibuprofen (Sigma-Aldrich, USA) for HIF-1α inhibition; Tripure Isolation reagent (Sigma-Aldrich, USA) for total RNA isolation; Primers of qRT PCR for HIF-1α14 were 5′-TGATGACCAGCAACTTGAGG-3′ (forward) and 5′-TTGATTGAGTGCAGGGTCAG-3′(reverse); while primers for Cygb were 5′-CAGTTCAAGCACATGGAGGA-3′ (forward) and 5′-GTGGGAAGTCACTGGCAAAT-3′ (reverse); 18s rRNA primer as housekeeping gene were 5′-AAACGGCTACCACATCCAAG-3′ (forward) and 5′-CCTCCAATGGATCCTCGTTA-3′ (reverse); cell extraction buffer (Thermo Fisher Scientific, USA) to isolate total protein; bovine serum albumin/BSA (Sigma-Aldrich, USA) and the material for ELISA kit were human HIF-1α ELISA kit (Elabscience, UK) and human Cygb ELISA kit (Elabscience, UK), trypan blue (Sigma-Aldrich, USA) for determination of fibroblast proliferation.
Methods
This was an experimental study conducted on keloid fibroblast primary cultures. This experiment used keloid tissue samples from three patients with keloids. Each sample was cultured to propagate fibroblast keloids. Keloid fibroblast-1 (KF1) was propagated from patient-1; keloid fibroblast 2 (KF2) was propagated from patient-2; and keloid fibroblast 3 (KF3) was propagated from patient-3. Each group was divided into two subgroups: experimental and control subgroups. The experimental subgroups were treated using ibuprofen, and the control groups were untreated (negative control).
This study was conducted in the Molecular Biology Laboratory for Oxidative Stress Studies, Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia. All the subjects involved in this research provided informed consent, and this study was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (No. 472/UN2.F1/ETIK/2016).
1. Keloid fibroblast primary culture
Keloid fibroblast primary culturing was conducted according to Tucci-Vegas,13 with some modifications. The keloid samples were washed six times using 2% penicillin-streptomycin in phosphate buffer saline (PBS). The keloid samples were then incubated in complete medium (DMEM low-glucose medium containing 1% penicillin-streptomycin, 1% amphotericin, and 10% FBS) for 30 min. The dermal layer of the keloid tissue was then separated from the epidermal layer. Using a scalpel, the dermal layer was cut into small fragments of approximately 2 × 2 mm2. Each fragment was placed in a well of a 24-well culture plate that had previously been scraped. The plate was left slightly open under laminar air flow for 30 min. Subsequently, 100 μL of complete medium was added to each well and incubated at 37° C with 95% O2 and 5% CO2. The medium was changed every 2 days, and the cells were observed under an inverted microscope.
2. Inhibition of HIF-1α
To inhibit HIF-1α expression, the fibroblast cells were seeded into each well of a 24-well plate culture at densities of 3.0 × 104 cells in each well in 500 μL of complete medium for 48 h. The medium was then removed, replaced with serum-free medium containing 2 mM ibuprofen, and incubated for 6 h based on previous optimization of the inhibition time (data not shown). Untreated fibroblasts were used as a control. At the end of this incubation period, the fibroblasts were harvested and ready for the total RNA and protein isolation, and for the determination of HIF-α and Cygb mRNA and protein.
3. Total RNA isolation
The total RNA from the fibroblasts was isolated using a Tripure Isolation reagent (Sigma-Aldrich, USA)). RNA concentrations were determined at a wavelength of 260 nm using Varioscan (Flash Multimode Reader, Thermo Scientific, USA). The RNA purity index was assessed by determining the ratio of absorbance at 260 and 280 nm.
4. Total Protein Isolation
Total protein was extracted using Cell Extraction Buffer (Thermo Fisher Scientific, USA). The total protein concentration of the tissues was measured using spectrophotometer at a wavelength of 280 nm. A standard curve was produced using bovine serum albumin solution.
5. Measurement of the Protein Levels of HIF-1α and Cygb
The protein levels of HIF-1α and Cygb were measured using a sandwich-enzyme linked immunosorbent assay according to the manufacturer’s instructions.
6. Measurement of HIF-1α and Cygb mRNA expression
The relative mRNA expression of HIF-1α and Cygb were analyzed using quantitative-reverse transcriptase polymerase chain reaction (qRT-PCR) (Bioneer, Korea). cDNA synthesis and amplification were performed in the same tube using a One Step RT-PCR Kit with SYBR Green Master Mix (Applied Biosystem, USA). The procedure of the reaction was as follows: synthesis of cDNA for 5 min at 42° C, inactivation of reverse transcriptase for 2–5 min at 95° C, 40 PCR cycles at 95° C, with each cycle lasting 10 sec; 30 sec at 54° C for Cygb and 18 sec at 59° C for HIF-1α (through optimization); followed by final step at 72° C for 30 sec. A melting curve analysis was performed for 15 sec at 95° C, 15 sec at 55° C, 15 sec at 95° C, and a 2-min incubation stage. As a reference gene, 18S mRNA was used.
The primers for each gene were as follows:
| - HIF-1α14 | F: 5′-TGATGACCAGCAACTTGAGG-3′, R: 5′-TTGATTGAG TGCAGGGTCAG-3′ |
| - Cygb | F: 5′-CAGTTCAAGCACATGGAGGA-3′ R: 5′-GTGGGAAGTCACTGGCAAAT-3′ |
(The Cygb: NCBI NM_134268.4 primer was designed using Primer3 and check for alignment using BLAST)
| - 18s RNA15 F: | 5′-AAACGGCTACCACATC CAAG-3′ R: 5′-CCTCCAATGGATCCTCGTTA-3′ |
7. Measurement of Fibroblast Proliferation
Fibroblast proliferation was measured using a Trypan blue exclusion assay. Ten microliters of a fibroblast cells suspension were mixed with 10 μL of Trypan blue and then placed in a Luna Slide and read using a Luna Automatic Cell Counter.
8. Statistical Analysis
All data were analyzed and tested for homogeneity and normality using Kolmogorov-Smirnov test and Levene’s test respectively. Data of each sample which treated with ibuprofen were compared to its control. A significant level was determined using an independent t-test, and correlation analysis between ibuprofen treatment with each parameter was conducted using Pearson’s correlation test. Statistical significance was accepted at a level of p < 0.05.
RESULTS
Primary Culture of Fibroblasts from Keloids
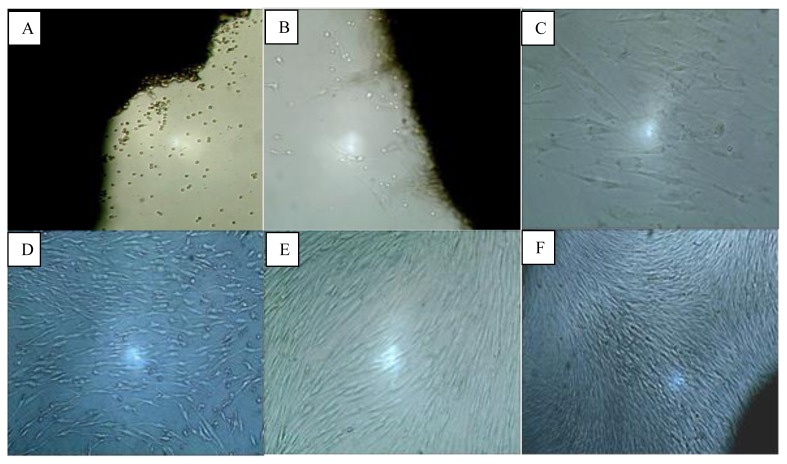
Fibroblasts began to appear on the second day and grew around the explant tissue. On the day-2 the fibroblasts looked like globular cells but later on the day-4 resembled spindle-like cells and adhered to the bottom of the plate. The sizes of these fibroblasts varied from 16–19μm. The fibroblast primary explant culture reached 80% confluency after 13–23 days, depending on the keloid fragment size. Figure 1 shows fibroblast growth from day- 2 until day-16 of the primary culture process.
Figure 1.
Growth of fibroblasts in keloid primary culture on day-1 (A); day-2 (B); day-4 (C); day-8 (D); day-14 (E); and day-16 (F). (Inverted microscope, A, B, D, E, and F: 40× magnification; C, 100× magnification).
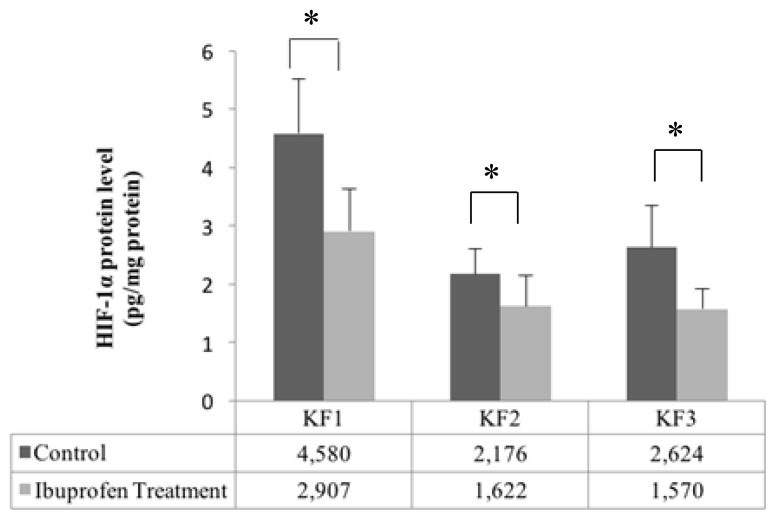
Effect of Ibuprofen on HIF-1α Protein Levels
HIF-1α protein levels in each sample (KF1, KF2, and KF3) significantly decreased after the administration of ibuprofen (t-test, p < 0.05), as shown in Figure 2.
Figure 2.
Effect of ibuprofen treatment on HIF-1α protein levels in a primary culture of keloid fibroblasts (*p < 0.05, independent t-test).
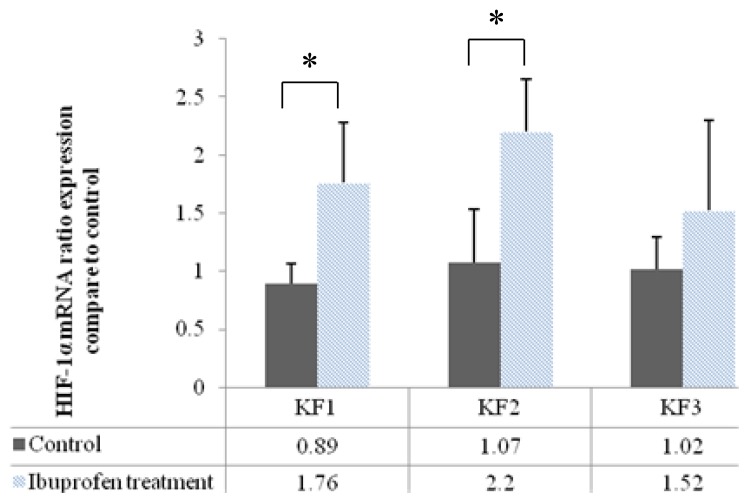
Effects of Inhibition of HIF-1α on HIF-1α mRNA Expression
HIF-1α mRNA expression significantly increased in the KF1 and KF2 samples following the administration of ibuprofen (p < 0.05, independent t-test). Although HIF-1α mRNA expression slightly increased in the KF3 samples, the finding was not statistically significant. Figure 3 shows HIF-1α mRNA expression after inhibition of its protein by ibuprofen.
Figure 3.
Effects of inhibition of the HIF-1α protein by ibuprofen on HIF-1α mRNA expression (*p < 0.05, independent t-test).
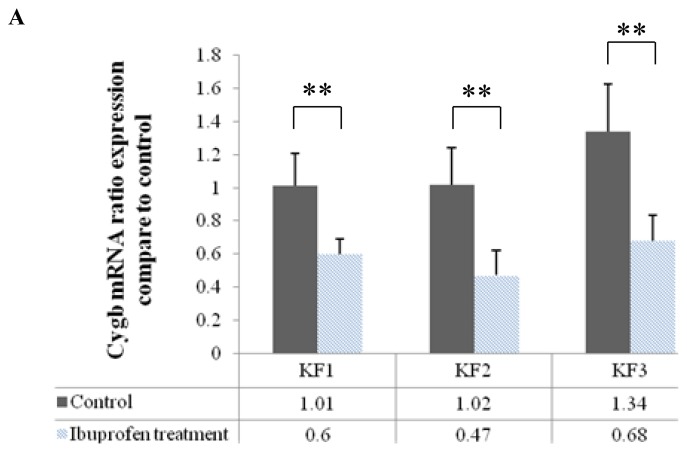
Effects of HIF-1α Inhibition on Cygb mRNA Expression and Protein Level
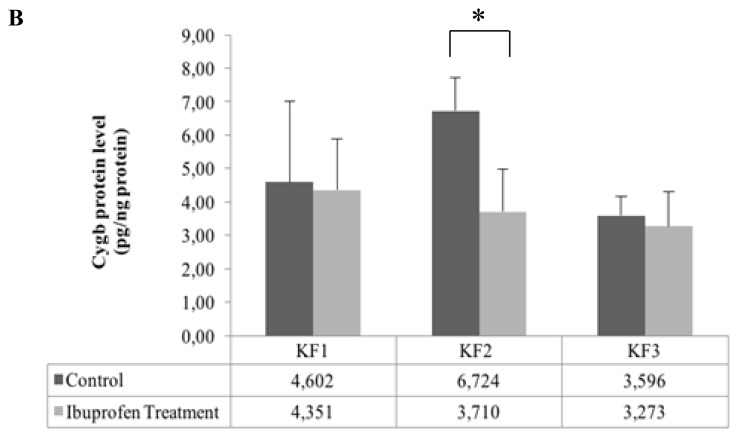
Cygb mRNA expression significantly decreased after inhibition of HIF-1α by ibuprofen in the KF1, KF2, and KF3 samples (p < 0.01, independent t-test) (Fig. 4A). In contrast to the mRNA Cygb level, the Cygb protein level decreased significantly only in the KF2 sample (p < 0.05, independent t-test) after the administration of ibuprofen, but there was no significant difference in the Cygb protein level in the KF1 and KF2 samples as compared with that in the control (Fig. 4B).
Figure 4.
Effects of ibuprofen-induced inhibition of the HIF-1α protein on (A) Cygb mRNA expression (**p < 0.01, independent t-test) and (B) Cygb protein expression (*p < 0.05, independent t-test)
Effects of HIF-1α Inhibition on Fibroblast Keloid Proliferation
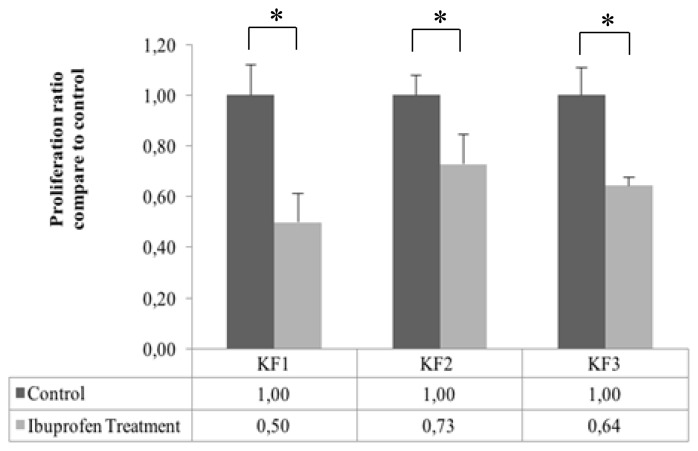
Fibroblast keloid proliferation significantly decreased in the KF1, KF2, and KF3 samples as compared with its control (p < 0.01, independent t-test). The ratios of fibroblast proliferation in the KF1, KF2, and KF3 groups as compared with that in its control were 0:5; 0:73; and 0:64, respectively (Fig. 5).
Figure 5.
Effect of ibuprofen-induced inhibition of the HIF-1α protein on the proliferation of keloid fibroblasts (*p < 0. 05, independent t-test)
Correlation of HIF-1α and Cygb Expression with Fibroblast Proliferation
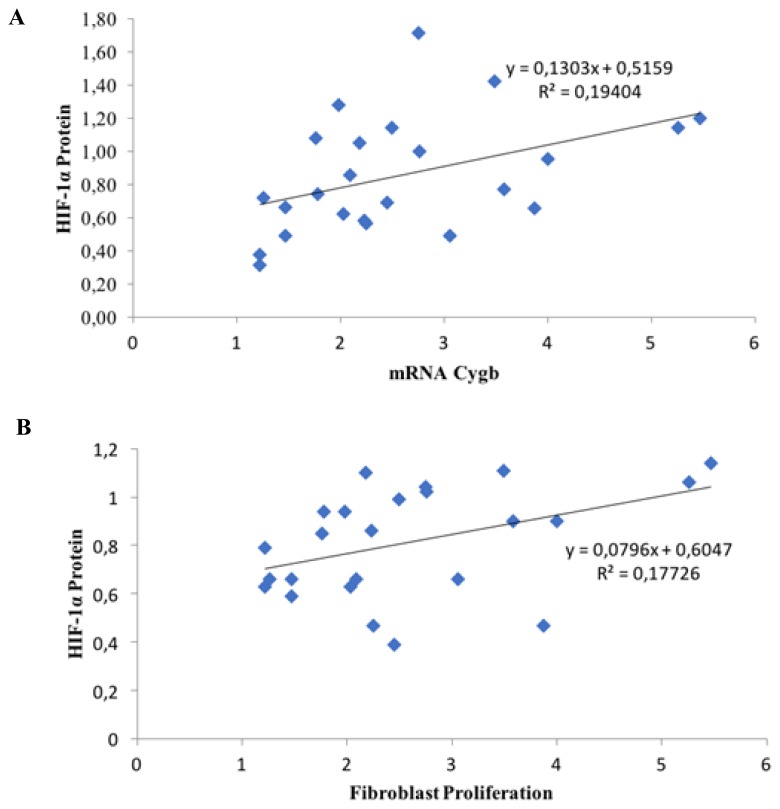
According to Pearson’s correlation test, there was a moderately significant positive correlation between HIF-1α protein and Cygb mRNA expression (Pearson’s test: R = 0.440; p = 0.031; n = 24), as shown in Figure 6A. There was also a moderately significant positive correlation between the HIF-1α protein expression and fibroblast proliferation in keloids (Pearson’s test: R = 0.421; p = 0.040; n = 24), as shown in Figure 6b.
Figure 6.
(A). Correlation between HIF-1α protein and mRNA Cygb (Pearson’s test: R = 0.440; p = 0.031; n = 24); and (B). between HIF-1α protein and fibroblast proliferation (Pearson’s test: R = 0.421; p = 0.040; n = 24).
DISCUSSIONS
Fibroblast cells, the major cellular components of keloids, were successfully grown in this study using the explant method (Fig. 1). The cells began to grow from the edge of the explant. Subsequently, the cells attached to the surface of the well. The morphology of the cells that grew resembled that of fibroblasts (i.e., they were spindle shaped, with a central core).
In our previous study, we showed that the expression of HIF-1α in keloids was higher than that in normal skin.12 Keloid fibroblast proliferation and active collagen synthesis result in an increased oxygen demand. However, the availability of oxygen tends to remain stable. The imbalance between the need for oxygen and oxygen availability causes hypoxic conditions in keloids. Due to the limited availability of oxygen, the prolyl hydroxylase domain (PHD) enzyme cannot hydroxylate the proline residue in HIF-1α. As a result, the HIF-1α protein is not recognized by the von Hippel–Lindau protein for ubiquitination and subsequent degradation by the ubiquitin-proteasome system. Thus, the degradation of HIF-1α by PHD is inhibited which results in HIF-1α stabilization.16
Ibuprofen inhibits HIF-1α through cyclooxygenase-2 (COX-2)-dependent and COX-2-independent pathways. 17,18 Through the COX-2-dependent pathway, it inhibits the synthesis of prostaglandin E2, thereby stimulating HIF expression through the phosphatidyl 3-P kinase (PI3K/Akt) pathway.17 Following hydroxylation by PHD of HIF-1α, the latter is recognized by the von Hippel–Lindau protein and degraded by the ubiquitin proteasome system.18 The results of the present study showed that the HIF-1α protein level decreased after the administration of ibuprofen (Fig. 2). The decrease in the HIF-1α protein level resulted in up-regulation of the synthesis of HIF-1α mRNA (Fig. 3). The latter may represent a positive feedback response to the lack of the HIF-1α protein.
This study showed that inhibition of HIF-1α significantly decreased Cygb mRNA expression (Fig. 4A). In addition, the expression of Cygb mRNA was significantly correlated with the decrease in HIF-1α protein levels (Fig. 6A). These results are in accordance with those of Guo et al.11,19 The results of the present study demonstrate that the HIF-1α protein plays a role in regulating the expression of Cygb.
In our study, the decrease in the mRNA expression of Cygb was not followed by a reduction in the protein level of Cygb in all the samples, with decreased levels found only in the KF2 sample (Fig. 4B). This finding was probably due to HIF-1α regulating the mRNA level of Cygb but not the protein level. The presence of HIF-1α binding sites in the HRE of the Cygb gene indicates that HIF-1α acts as a transcription factor for the Cygb gene.11,19 By decreasing in HIF-1α levels, Cygb gene transcription will be inhibited. As a result, Cygb mRNA will decrease. However, Cygb proteins present in cells may not decrease due to the influence of other factors, such as post-transcription modification in synthesis of Cygb protein.
In the present study, the proliferation of keloid fibroblast cells treated with a HIF-1α inhibitor was significantly decreased (Fig. 5), and the decline was positively correlated with the HIF-1α protein (Fig. 6B). Thus, the HIF-1α protein appears to play an important role in cell proliferation. Previous studies showed that HIF-1α served to induce the expression of various genes, such as nitrite oxide synthase (NOS), insulin-like growth factor-2 (IGF-2), transforming growth factor (TGF), C-MYC, and ID2, that support cell proliferation in hypoxic conditions.8 HIF-1α knockdown reduced cell proliferation has been proved by Xia et al, who conducted experiment using retinoblastoma WERI-Rb-1 cells.20 Therefore, inhibition of the HIF-1α protein influences the expression of genes regulated by HIF, thereby reducing cell proliferation.
Although the role of Cygb remains unclear, it is known to be very important in cell proliferation in hypoxic conditions. As shown in previous research, the Cygb protein plays a role in oxygen binding and supply in cells by ensuring the availability of oxygen for energy-intensive mitotic processes in cell proliferation.10 Keloids are composed of fibrous tissue, which has a high proliferation index as compared with that of normal tissue. Collagen synthesis in keloid tissues is greater than that in normal skin fibroblasts. The high proliferation and activity of fibroblasts in keloids consume a large amount of energy and oxygen. Cygb plays a role in meeting the oxygen demand of fibroblasts and is associated with the activity of fibroblasts, which require oxygen for proline hydroxylation in collagen maturation.12 Thus, constraints on Cygb expression will result in reduced oxygen availability and consequently reduced cell proliferation. This study showed that HIF-1α regulates Cygb expression in keloid fibroblasts and affects the proliferation of keloid fibroblasts. Further research is needed to shed light on the role of Cygb in the proliferation of keloid fibroblasts. Such research should include analyses of the effect of Cygb inhibition on cell proliferation. So far, our experiment lead to understand the mechanism of fibroblast proliferation in keloid tissue and might have clinical implication in treatment and prevent the recurrence of keloid tissue in keloid patient.
ACKNOWLEDGMENTS
We would like to express our gratitude to Directorate of Research and Community Service Universitas Indonesia (Direktorat Riset dan Pengabdian Masyarakat, DRPM UI), which provided the funding to conduct this research through Hibah Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT, grant No 016/SP2H/LT/DRPM/II/2016), and to Dr. Yefta Moenadjat, SpBP(K), Nurse Mila, Rumah Sakit Cipto Mangunkusumo (RSCM), RSIA Budi Kemuliaan, Rumah Sunatan Jati Asih, Rumah Sunatan 123 for providing the samples, and PT Ecosains Hayati for providing the qRT-PCR equipment.
REFERENCES
- 1.Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review) Int J Mol Med. 2009;24:283–293. doi: 10.3892/ijmm_00000231. [DOI] [PubMed] [Google Scholar]
- 2.Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of keloid scar: Article review. J Am Coll Clin Wound Spec. 2015;7:1–7. doi: 10.1016/j.jccw.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidgwick GP, Bayat A. Extracellular matrix molecule implicated in hypertrophic and keloid scarring. JEADV. 2012;26:141–152. doi: 10.1111/j.1468-3083.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 4.Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71:32–38. doi: 10.1016/j.mehy.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Vincent AS, Phan TT, Mukhopadhyay A, Lim HY, Halliwell B, Wong KP. Human skin keloid fibroblasts display bioenergetics of cancer cells. J Invest Dermatol. 2008;128:702–709. doi: 10.1038/sj.jid.5701107. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Chen J, Xu B, Long X, Qin H, Zhao R, et al. Keloid-derived keratinocytes acquire a fibroblast-like appearance and an enhanced invasive capacity in a hypoxic microenvironment in vitro. Int J Mol Med. 2015;35:1246–1256. doi: 10.3892/ijmm.2015.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu C, Wang L, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1 alpha (hif-1alpha) and hif-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/409272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jusman SWA, Iswanti FC, Suyatna FD, Ferdinal F, Wanandi SI, Sadikin M. Cytoglobin expression in oxidative stressed liver during systemic chronic normobaric hypoxia and relation with HIF-1α. Med J Indones. 2014;23:133–138. [Google Scholar]
- 10.Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, Simpson JC, et al. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J Biol Chem. 2004;279:8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Philipsen S, Tan-Un KC. Study of the hypoxia-dependent regulation of human CYGB gene. Biochem Biophys Res Commun. 2007;364:145–150. doi: 10.1016/j.bbrc.2007.09.108. [DOI] [PubMed] [Google Scholar]
- 12.Wulandari E, Jusman SWA, Moenadjat Y, Jusuf AA, Sadikin M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J Med Sci. 2016;62:58–69. [PMC free article] [PubMed] [Google Scholar]
- 13.Tucci-viegas VM, Hochman B, Ferreira LM. Keloid explant culture: a model for keloid fibroblasts isolation and cultivation based on the biological differences of its specific regions. Int Wound Journal. 2010;7:339–348. doi: 10.1111/j.1742-481X.2010.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardiany NS, Sadikin M, Wanandi SI. Ekspresi relatif mRNA Hypoxia Inducible factor-1alfa pada sel glioma penderita. Indonesian Journal of Cancer. 2013;7:1–5. [Google Scholar]
- 15.Hardiany NS, Sadikin M, Siregar NC, Wanandi SI. The suppression of manganese superoxide dismutase decreased the survival of human glioblastoma multiforme T98G. Med J Indones. 2017;25:19–25. [Google Scholar]
- 16.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu XH, Kirschenbaum A, Lu M, Yao S, Dosorets A, James F, et al. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 18.Palayoor ST, Tofilon PJ, Coleman CN. Ibuprofen-mediated reduction of hypoxia-inducible factors hif-1αand hif-2α in prostate cancer cells. Clin Cancer Res. 2003;9:3150–3157. [PubMed] [Google Scholar]
- 19.Guo X, Philipsen S, Tan-un KC. Characterization of human cytoglobin gene promoter region. Biochem Biophys Res Commun. 2006;1759:208–215. doi: 10.1016/j.bbaexp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Xia T, Cheng H, Zhu Y. Knockdown of HIF-1α reduces proliferation, induces apoptosis and attenuates the aggressive phenotype of retinoblastoma WERI-Rb-1 cells under hypoxic conditions. Ann Clin Lab Sci Spring 44. 2014;2:134–44. [PubMed] [Google Scholar]