Abstract
Immune memory is a central feature of the mammalian adaptive immune system. The more primitive innate immune system of insects has also been shown to comprise memory, or immune priming. A recent study has shed new light on how Plasmodium primes the mosquito immune system for greater resistance to a subsequent infection with the same pathogen.
Keywords: immune priming, memory, mosquito, Plasmodium
The complex lifestyle of blood sucking mosquitoes comprises both aquatic and terrestrial stages that involve interactions with the microbe-rich breeding water, plant nectars and vertebrate hosts. The mosquito’s innate immune system has evolved to cope with this diverse and continuous microbial exposure through a variety of defense mechanisms. Plasmodium infection of the mosquito’s gut epithelium triggers a potent immune response that involves a variety of effector molecules and mechanisms mediating parasite destruction. The Toll, immune deficiency (IMD), c-Jun N-terminal kinase (JNK) and Janus kinase–signal transducer and activator of transcription (JAK-STAT) immune signaling pathways play key roles in regulating the defense against Plasmodium infection in the mosquito, with some degree of specificity for different parasite species [1]. Plasmodium infection of Anopheles gambiae has been shown to enhance the immune response to a subsequent malaria parasite infection when the mosquito’s gut microbiota is present, indicating the existence of a memory mechanism [2]. As in mammals and fruit flies, this memory mechanism is mediated by blood cells, or hemocytes as they are called in insects [3]. Plasmodium infection was shown to induce the secretion of the soluble hemocyte differentiation factor (HDF) into the mosquito’s open circulatory system, the hemolymph. HDF would prime the immune system of naive mosquitoes, when injected, rendering them more immune competent to deal with Plasmodium infection, through an increase in granulocytes (Figure 1). Silencing of the anti-Plasmodium immune signaling pathways Toll, IMD, JNK and JAK-STAT did not affect HDF levels in the hemolymph, while all but the IMD pathway were necessary for hemocyte differentiation to occur in response to HDF [4]. This is consistent with a model where HDF is a soluble factor acting upstream of these immune pathways. Various bioassays in combination with extraction and fractionation procedures showed that HDF was a likely heat-stable protein-lipid complex. Bioassay-guided fractionation and mass spectrometry analyses showed that the protein portion of HDF represented a mosquito homologue of vertebrate lipocalins, which was named Evokin. The Evokin gene was induced by Plasmodium infection, and its RNAi – mediated depletion abolished the ability of hemolymph from immune challenged mosquitoes to induce hemocyte differentiation when injected into naive mosquitoes [3]. Further mass spectrometry and biochemical studies revealed the lipid component as lipoxin, and that this lipid was produced from arachidonic acid precursors in an immune challenge–dependent manner. Injection of mosquitoes with synthetic lipoxin induced hemocyte differentiation and boosted anti-Plasmodium immunity. Gene silencing studies of Evokin in conjunction with the administration of synthetic lipoxin finally showed its implication in the lipoxin–mediated immune priming, thereby indicating it as the lipoxin carrier [3]. The authors speculated that immune stimulation induces prolonged expression of lipoxin biosynthesis, as it does for Evokin, likely through epigenetic regulation. While some of the anti-Plasmodium immune signaling pathways are required for hemocyte differentiation upon HDF stimulation, the specific pathways that regulate HDF production, and hence immune priming, are unknown [3, 4]. It was hypothesized that a signal from the midgut epithelium is inducing a long-lasting increase of Evokin in the mosquito body wall [3]. Since the vertebrate lipoxins have anti-inflammatory action and promote phagocytosis of bacteria among other processes, and the mosquito lipoxin is mediating immune priming, the authors suggested that lipoxins may play a similar role in the vertebrate immune system, preparing the host for subsequent infections [3]. The significance of Plasmodium-induced immune priming for a subsequent malaria parasite infection is questionable from an evolutionary perspective, given the low prevalence of Plasmodium exposure of mosquitoes in the field, and the unclear impact of this exposure on the mosquito’s overall reproductive fitness at field conditions [5, 6, 7]. There is little evidence, if any, suggesting that Plasmodium would exert selective pressure on the mosquito’s immune system [8, 9]. Hence, the Evokin/Lipoxin complex–mediated immune priming mechanism is likely to have evolved to combat infections with more prevalent and virulent mosquito pathogens, such as bacteria and fungi. This is consistent with the observation that many of the immune genes and signaling pathways that mediate anti-bacterial and anti-fungal defenses are also implicated in anti-Plasmodium immunity [1, 10]. One can envision that the Evokin/Lipoxin complex-mediated immune priming prepares a mosquito to better cope with the microbial exposure of a particular ecological niche. In this way, the Evokin/Lipoxin complex–mediated immune priming may also represent an integral feature of the microbiome’s influence on malaria transmission; exposure of a mosquito to particular microbes in its environment may prime its immune system in a manner that would influence its ability to transmit the malaria parasite. Conversely, malaria parasite infection may also prime the immune system for greater resistance to bacterial and fungal infection, thereby increasing mosquito survival and hence malaria transmission. Further studies are needed to elucidate the role of the Evokin/Lipoxin complex–mediated immune priming on the mosquito’s competence to deal with a diverse microbial exposure, including the malaria parasite, in the field, and how this knowledge could be harnessed to render mosquitoes resistant to the parasite for malaria control.
Figure 1. A model of the mechanism of parasite-induced immune priming.
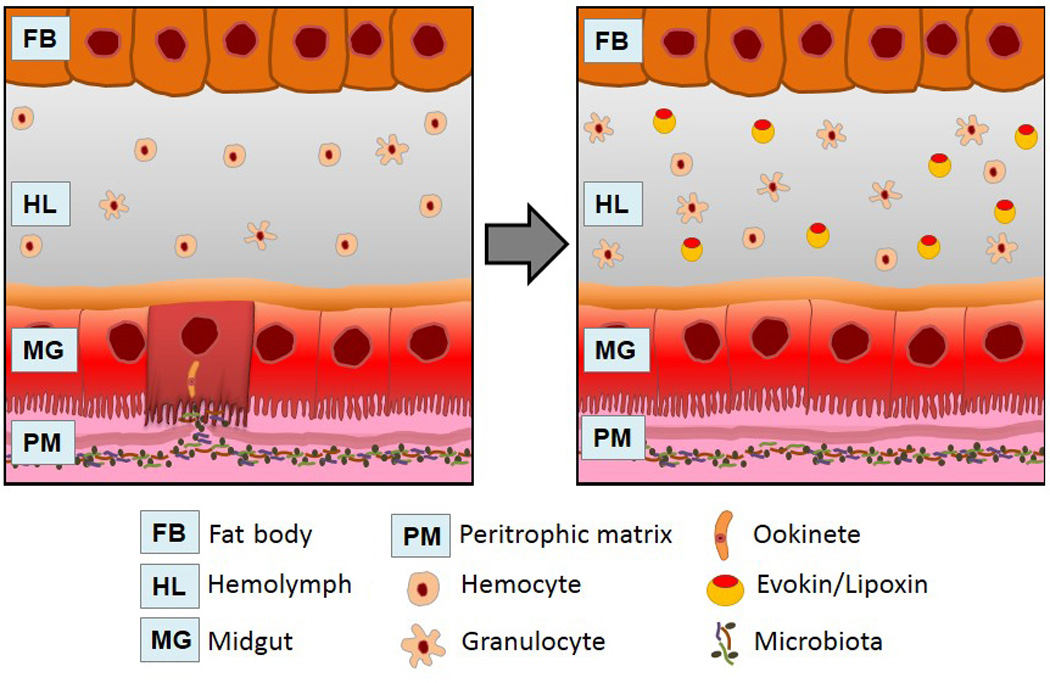
Plasmodium ookinete midgut invasion leads to bacterial contact with the injured invaded cell. This triggers immune priming through the production of the Evokin/Lipoxin complex that stimulates an increase of granulocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clayton AM, et al. The Anopheles innate immune system in the defense against malaria infection. J Innate Immunity. 2014;6(2):169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues J, et al. Haemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez JL, et al. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat Comm. 2015;6:7403. doi: 10.1038/ncomms8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez JL, et al. The role of haemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate Immun. 2014;6:119–128. doi: 10.1159/000353765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vézilier J, et al. Plasmodium infection brings forward mosquito oviposition. Biol Lett. 2015;11(3):pii: 20140840. doi: 10.1098/rsbl.2014.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vézilier J, et al. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc Biol Sci. 2012;279(1744):4033–4041. doi: 10.1098/rspb.2012.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangare I, et al. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infect Genet Evol. 2014;25:57–65. doi: 10.1016/j.meegid.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Rottschaefer SM, et al. Exceptional diversity, maintenance of polymorphism, and recent directional selection on the APL1 malaria resistance genes of Anopheles gambiae. PLoS Biol. 2011;9(3):e1000600. doi: 10.1371/journal.pbio.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obbard DJ, et al. Population genetics of Plasmodium resistance genes in Anopheles gambiae: no evidence for strong selection. Mol Ecol. 2007;16(16):3497–3510. doi: 10.1111/j.1365-294X.2007.03395.x. [DOI] [PubMed] [Google Scholar]
- 10.Dong Y, et al. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathogens. 2009;5(5):e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]


