Abstract
Purpose of review
Studies of the genetic model organism, Drosophila melanogaster, have unraveled molecular pathways relevant to human physiology and disease. The Malpighian tubule, the Drosophila renal epithelium, is described here, including tools available to study transport; conserved transporters, channels, and the signaling pathways regulating them; and fly models of kidney stone disease.
Recent findings
Tools to measure Malpighian tubule transport continue to advance, including use of a transgenic sensor to quantify intracellular pH and proton fluxes. A recent study generated an RNA sequencing-based atlas of tissue-specific gene expression, with resulting insights into Malpighian tubule gene expression of transporters and channels. Advances have been made in understanding the molecular physiology of the WNK (With No Lysine)-SPAK/OSR1 (Ste20-related proline/alanine rich kinase/oxidative stress response) kinase cascade that regulates epithelial ion transport in flies and mammals. New studies in Drosophila kidney stone models have characterized zinc transporters and used Malpighian tubules to study the efficacy of a plant metabolite in decreasing stone burden.
Summary
Study of the Drosophila Malpighian tubule affords opportunities to better characterize the molecular physiology of epithelial transport mechanisms relevant to mammalian renal physiology.
Keywords: epithelial ion and water transport, signaling, nephrolithiasis, Drosophila genetics, organismal physiology
Introduction
Thomas Hunt Morgan’s pioneering studies in the early 20th century began 110 years of Drosophila melanogaster research, and established the common fruit fly as a powerful genetic model organism (1). Six Nobel prizes have recognized Drosophila studies on fundamental genetic mechanisms, early embryonic development, odorant receptors and the olfactory system, innate immunity, and circadian rhythms, illuminating molecular mechanisms conserved in mammalian physiology. Ongoing work has exploited the fly for study of the nervous system, metabolism, cardiovascular function, cancer, and inflammation (2,3). This review will highlight studies on the Drosophila renal epithelium, the Malpighian tubules, with a focus on epithelial transport.
The Drosophila toolbox
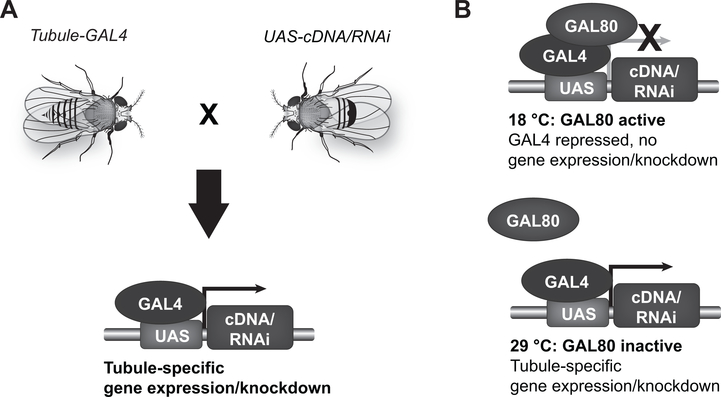
The Drosophila genome, which contains approximately 14,000 genes, was the first animal genome sequenced using a whole-genome shotgun approach (1). Germline mutations exist for many Drosophila genes, and gene disruption and gene-silencing techniques can target additional genes (2). Genes can be expressed or silenced in a temporally and spatially restricted fashion, using the GAL4/UAS system (4,5) (Figure 1).
Figure 1. Temporally and spatially restricted gene expression using the GAL4/UAS system.
A) The yeast transcription factor, GAL4, is transgenically expressed in a tissue or cell type of interest under the control of endogenous genomic enhancers, or engineered cell type-specific promoters. For example, GAL4 lines targeting specific subsets of Malpighian tubule epithelial cells have been generated (6–9). The GAL4-expressing fly is mated to a second fly containing the transgenically expressed GAL4 DNA-binding domain, UAS (Upstream Activating Sequence). In progeny containing both the GAL4 and UAS transgenes, GAL4 binding to UAS results in the transcription of DNA cloned downstream of the UAS. This allows cell-specific expression of a wild-type or mutant Drosophila or mammalian gene. Alternatively, GAL4/UAS-driven transcription of an RNA that is processed into an interfering RNA (RNAi) allows cell-specific gene knockdown. B) Introduction of a temperature-sensitive GAL80 transgene allows temporal control: at 18 °C, GAL80 is active, and represses GAL4; at 28 °C, GAL80 is inactive, allowing GAL4 expression. For example, to achieve adult-specific gene knockdown or expression, flies are reared at 18 °C throughout development (GAL4 off), and then switched to 28 °C in adulthood (GAL4 on).
UAS-RNAi lines targeting nearly every Drosophila gene are available from publicly accessible collections (10,11), or can be generated in individual laboratories using established protocols (12,13), as can transgenic UAS lines to allow expression of wild-type or mutant genes (3,13,14*). Transgenic animals can be made in 2–3 months.
FlyBase (http://flybase.org) is a rich Drosophila resource, including information on genes and the genome, orthologs, references, available tools, and expression data. “Omics” efforts have provided a detailed map of gene expression across developmental timepoints and in different tissues. In particular, FlyAtlas provides information on gene expression in larval and adult Malpighian tubules, as well as many other tissues. The first iteration was based on an Affymetrix microarray platform (15), while FlyAtlas 2 used RNA sequencing technology (16**). Both FlyAtlas (http://flyatlas.gla.ac.uk/flyatlas/index.html) and FlyAtlas2 (http://flyatlas.gla.ac.uk/FlyAtlas2/index.html) have publicly accessible search engines that allow queries for expression patterns of genes of interest. Analysis of these datasets has identified tubule-enriched genes (17,18).
Assays for study of Malpighian tubule function
While Drosophila has podocyte-like cells, called nephrocytes, that also have some of the endocytic functions of mammalian proximal tubule cells (19–21), these are anatomically separate from the four Malpighian tubules, which lie in the abdominal cavity in direct contact with the hemolymph (plasma).
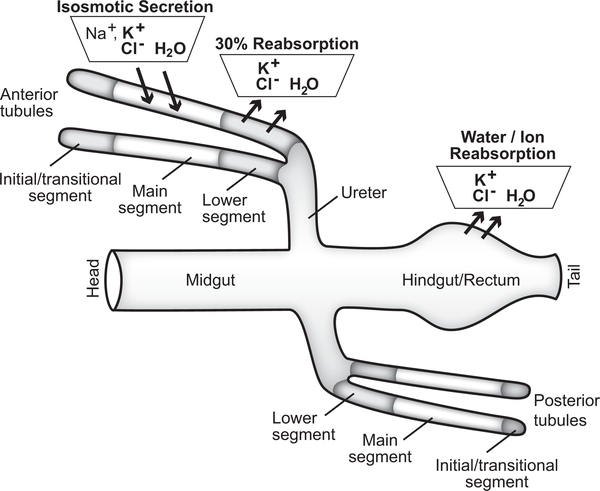
Malpighian tubules are genetically and functionally segmented. GAL4 expression patterns, driven by endogenous genomic enhancers, define a distal initial and transitional segment, a main segment, a proximal lower segment, and an upper and lower ureter (6,17). Urine generation by the blind-ended tubules occurs in the main segment (22), with subsequent modification in the lower segment and hindgut (23–25*) (Figure 2).
Figure 2. Schematic of Drosophila iono- and osmoregulatory epithelia.
The two pairs of Malpighian tubules (anterior and posterior), together with the hindgut, regulate ionic and osmotic homeostasis in the fly. Urine is generated by the transepithelial movement of ions and water across the main segment of the anterior and posterior tubules, resulting in isosmotic secretion of potassium chloride-rich fluid; secreted fluid also contains sodium, and secretion can occur in potassium-free medium (22,25*–28). Urine then flows through the downstream lower segment, where ~30% of the potassium and water secreted by the main segment are reabsorbed (29). The urine then passes through the ureter and enters the hindgut, where further ion and water fluxes occur to match the composition of excreta to the animal’s physiological need (23–25*).
Transepithelial ion and water fluxes can be measured in isolated Malpighian tubules, as first pioneered by Ramsay in stick insects (30), and later adapted for Drosophila tubules (22). Drosophila tubules, which are nearly 3 mm long (25*), are easily dissected under a stereomicroscope, and 20–30 tubules can be studied in a single experiment using the Ramsay assay (31,32). When paired with the use of ion-specific electrodes (31), the Ramsay assay allows measurement of transepithelial fluxes of inorganic and organic ions, including sodium, potassium, and calcium (29); ammonium (33); salicylate (34); and tetraethylammonium (35). When paired with confocal microscopy, the Ramsay assay can measure transport of fluorescent organic anions and cations (36). In vitro tubule perfusion has been performed in Drosophila tubules (37), and allows control over luminal perfusate. Ion secretion and reabsorption have also been measured using self-referencing ion-selective microelectrodes positioned in the unstirred layer (27,35,38).
Transgenic sensors, expressed in the tubule using the GAL4/UAS system, have been used to measure intracellular (9) and mitochondrial calcium (39) in tubule epithelial cells, as well as intracellular chloride (14*,40) and pH (41**). cAMP (adenosine 3’5’-cyclic monophosphate), cGMP (guanosine 3’5’-cyclic monophosphate) and calcium signaling have been manipulated in a cell-specific fashion using optogenetic techniques and by expressing exogenous receptors coupled to these signaling pathways and exposing tubules to their ligands (42,43).
Malpighian tubule ion and water transport
Although the configuration of transporters and channels in the Malpighian tubule differs from the mammalian tubule, in many cases the transporters, and the signaling pathways regulating them, are conserved.
Fluid secretion by the main segment
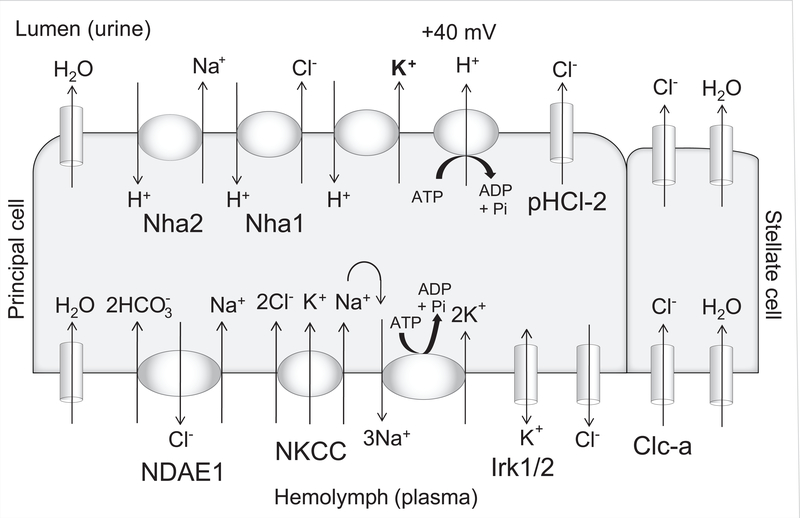
In the fluid-secreting main segment, transepithelial cation flux occurs through principal cells, while chloride flux occurs through the neighboring stellate cells (40,44,45). Fluid secretion is energized by the apical vacuolar proton ATPase (V-ATPase) (22,46–49), a multi-subunit transporter homologous to the mammalian V-ATPase in the collecting duct intercalated cell that is mutated in patients with distal renal tubular acidosis (50,51). The V-ATPase generates a lumen-positive transepithelial potential of ~40 mV (37,44), which is thought to drive proton/cation exchange across the apical membrane (Figure 3).
Figure 3. Cell model of the fluid-secreting Malpighian tubule main segment.
Transporters and channels described in the text are shown in the cation-conducting principal cell and anion-conducting stellate cell. In addition, Nha1 and Nha2 are apically-expressed chloride/proton and sodium/proton exchangers, respectively (52,53); the potassium/proton exchanger is unknown. The apically-expressed pentameric ligand-gated chloride channel, pHCl-2, has a functional role in cAMP-stimulated fluid secretion (54). NKCC and the sodium/potassium-ATPase (Na+/K+-ATPase) are required for normal transepithelial potassium flux (28). The SLC4 family sodium-driven anion exchanger NDAE1 is localized to the basolateral membrane of the principal cell (55,56). Basolateral potassium and chloride conductances have been demonstrated (57); the identity of the chloride channel is unknown, while inwardly-rectifying potassium channels, Irk1 and Irk2, have a demonstrated functional role in transepithelial ion flux and fluid secretion (37). One study has demonstrated a small cell-to-bath driving force for potassium across the basolateral membrane (57), while others have suggested that basolateral potassium channels allow uptake of potassium from the hemolymph into the principal cells (32), as occurs in the Formica (ant) tubule when extracellular potassium is high (58,59). The direction of potassium flow through the basolateral potassium channels may therefore depend on extracellular potassium concentration, which in normal conditions is ~26 mM (60,61). Principal cell knockdown of Irk1 and Irk2 has additive effects with ouabain on decreasing fluid secretion and transepithelial potassium flux, suggesting that these channels are not solely recycling potassium entering through the Na+/K+-ATPase (37).
In the mammalian kidney, SLC12 cation-chloride cotransporters, including the sodium-potassium-2-chloride (NKCC) and sodium-chloride (NCC) cotransporters, play important roles, and are the target of commonly used diuretics (62). Both NKCC2 and NCC are mutated in human salt-losing tubulopathies, as are the inwardly rectifying potassium channels, ROMK (renal outer medullary potassium channel) and Kir4.1 (63). Functional roles in the principal cell have been demonstrated for the Drosophila NKCC (28), encoded by Ncc69 (64,65), and two inwardly rectifying potassium channels, Irk1 and Irk2, expressed in the tubule (37,66–68). While a third putative inwardly rectifying potassium channel, Irk3, is expressed at very high levels in the tubule (17), there was no functional consequence of knocking it down on fluid secretion or potassium flux (37).
The basolateral sodium/potassium-ATPase (Na+/K+-ATPase) (56,69) is required for transepithelial potassium flux, by recycling sodium entering the principal cell through the NKCC (28). Na+/K+-ATPase activity is also required for hormonally-stimulated fluid secretion (70), and provides the driving force for sodium-dependent transport of organic anions like salicylate and para-aminohippuric acid (71,72).
CLC family chloride channels are important for chloride transport in the mammalian kidney, and are mutated in human patients with salt-losing tubulopathy (73). A CLC chloride channel in Drosophila, encoded by Clc-a, is required in stellate cells for transcellular chloride secretion in hormonally stimulated tubules (40). Chloride transport mechanisms in unstimulated tubules are not defined. A paracellular pathway for chloride transport has been demonstrated in Aedes aegypti mosquitos (74,75); whether a similar pathway exists in Drosophila tubules is unknown.
There are 8 aquaporin (AQP) family genes in Drosophila (76). Transcripts for Drip have been localized to the stellate cell of the adult Malpighian tubule, while transcripts for CG17664 and CG4019 have been localized to the principal cell (77), suggesting that transcellular water transport could occur in both cell types. Knockdown of the CG4019 Aedes aegypti homolog, AaAqp5, which is expressed on the apical and basolateral membranes of the larval mosquito principal cell, results in decreased tubule fluid secretion (78).
Calcium, magnesium and phosphate transport
Calcium transport occurs predominantly in the initial/transitional segment, which is larger in anterior tubules compared to posterior tubules (6,38,79–81). Intracellular calcium- and magnesium-rich concretions are found in the distal tubule of Drosophila hydei larvae (82), and active magnesium transport has been demonstrated in Malpighian tubules of larval Aedes campestris mosquitos (83). Mammalian TRPM (transient receptor potential cation channel, subfamily M) channels are important for epithelial magnesium transport (84–87). A Drosophila TRPM channel is predominantly expressed in the initial/transitional segment, and has been implicated in magnesium transport (88), although tubule magnesium fluxes have not been measured in Drosophila.
At least one proven phosphate transporter, MFS13 (major facilitator superfamily 13), is enriched in the Malpighian tubule. Malpighian tubule phosphate fluxes have not been measured, but ablation of tubule epithelial cells in the main segment results in higher hemolymph phosphate concentrations in animals fed a high-phosphate diet (89,90). However, while 42 of 46 human solute carrier (SLC) families are found in insects, the SLC34 family, which includes the mammalian proximal tubule NaPi (sodium phosphate) cotransporters Npt2a and Npt2c, is not found in insects (91).
Other transporters expressed in the Malpighian tubule
Many other transporters, exchangers and channels are also expressed in the Malpighian tubule (17); a few examples will be reviewed here. The SLC5 family of sodium/glucose cotransporters, which includes the mammalian proximal tubule transporters SGLT1 and SGLT2, is comprised of 15 genes in Drosophila (92), 7 of which are enriched in expression in the adult tubule in FlyAtlas 2. Addition of glucose to the bathing medium increases fluid secretion by the tubule (26), but glucose transport has not otherwise been examined.
An important function of the proximal tubule is the secretion of small molecules, including medications and endogenous solutes like uremic toxins (93,94). Similarly, the Malpighian tubule secretes organic anions and cations, including tetraethylammonium, para-aminohippuric acid, ouabain, and human therapeutics like methotrexate, daunorubicin, and salicylate (34–36,70–72,95). Transporter families involved in this process in the mammalian proximal tubule include the ABCC (ATP-binding cassette C, also known as the MRP, or multidrug resistance, transporters), SLC22 (which includes the OAT1 and OAT3 organic anion transporters), and SLCO (also known as OATP, or organic anion transporter P) families (94). These transporter classes are well-represented in the Drosophila genome, and many family members are enriched in expression in the Malpighian tubule in FlyAtlas 2, including nine of fourteen ABCC, eleven of twenty-five SLC22 (76,91,96), and six of eight SLCO/Oatp transporters (70). A functional role for Oatp58Db has been demonstrated in ouabain transport (70), and the roles of dMRP, MET (Methoprene-tolerant, an SLC46 family member), and Oatp58Dc have been explored in organic anion and cation transport (95,97,98).
Signaling pathways regulating ion transport
Signaling pathways regulating epithelial transport are also conserved between flies and mammals. For example, nitric oxide signaling regulates sodium transport in multiple nephron segments in the mammalian kidney (99–102), and also regulates transport in the Malpighian tubule (103–105).
Another conserved regulatory pathway is the WNK (With No Lysine)-SPAK/OSR1 (Ste20-related proline/alanine rich kinase/oxidative stress response) kinase cascade, which regulates ion transport in the thick ascending limb and distal convoluted tubule of the mammalian nephron (106). There are four WNK paralogs in mammals, and WNK1 and WNK4 are mutated in a human syndrome of hypertension and hyperkalemia (107). WNKs phosphorylate two related kinases, SPAK and OSR1, to activate them (108,109). Activated SPAK and OSR1 then phosphorylate SLC12 transporters, including NCC, NKCC1 and NKCC2, to activate them (110–115). Drosophila has a single WNK homolog, which phosphorylates the fly SPAK/OSR1 homolog, encoded by Fray (116–118). Fray phosphorylates fly NKCC (Ncc69), and WNK and Fray regulate Malpighian tubule transepithelial ion transport via NKCC in the principal cell (13). Thus, WNK-SPAK/OSR1 regulation of renal epithelial ion transport is conserved from flies to mammals.
Chloride binds to the active site of WNKs and inhibits their autophosphorylation and activation in vitro (119,120). Acute decreases in intracellular chloride in Malpighian tubule epithelial cells result in WNK activation over 30 to 60 minutes, with stimulation of transepithelial ion flux (13,14*). These findings are relevant to chloride regulation of transport in the mammalian distal convoluted tubule to maintain potassium homeostasis (121,122). A role for the scaffold protein mouse protein 25 (Mo25)/calcium-binding protein 39 to achieve maximum pathway stimulation and ion transport was also demonstrated (14*). Mo25 is expressed in the mammalian distal convoluted tubule and thick ascending limb (123), but its functional role in those nephron segments has so far been unexplored.
The Malpighian tubule also affords opportunities to study mammalian genes in the context of a transporting renal epithelium. For example, knockdown of Drosophila Mo25 was rescued by expression of mouse Mo25α (14*). Knockdown of Drosophila WNK can be rescued by expression of mammalian WNKs, but the three kidney-expressed mammalian WNKs (1, 3 and 4) behave differently (Rodan AR, unpublished data). Because mammalian WNKs compensate for one another (124–126), and also interact (127–129), the ability to express individual or specific combinations of WNKs may allow better definition of the roles of individual WNK paralogs or their combinations.
Malpighian tubule kidney stone models
Kidney stones are increasing in incidence and prevalence, with an associated increase in cost, and are associated with substantial morbidity (130). The Drosophila Malpighian tubule has been developed as a model of stone formation using dietary and genetic approaches (Table 1) (131). These include high oxalate diet, which can contribute to stone formation in humans (132); melamine, which resulted in infant stone disease due to tainted milk powder in China (133,134); and knockdown of Xanthine dehydrogenase (Xdh), which results in stone formation when mutated in humans (xanthinuria type I) (135). Stones can be visualized with light microscopy with polarizing light, microscopic computed tomography or scanning electron microscopy (132–134).
Table 1.
Malpighian tubule kidney stone models.
| Model | Stone type | Chemical Analysis | References |
|---|---|---|---|
| High oxalate feeding | Calcium oxalate | X-ray diffraction | 132 |
| Bathing tubules in Na oxalate (ex vivo) | Calcium oxalate | Not done | 137 |
| Ethylene glycol feeding | Calcium oxalate | Energy-dispersive X-ray spectroscopy | 133 |
| Melamine feeding | Chemically complex (carbon, oxygen, phosphate, chloride, calcium) | Energy-dispersive X-ray spectroscopy | 133, 134 |
| Knockdown of xanthine dehydrogenase or allopurinol feeding | Xanthine, hypoxanthine, and hydroxyapatite | Fourier transform infrared spectroscopy, high performance liquid chromatography-mass spectrometry, micro X-ray absorption near edge spectroscopy | 135 |
The SLC26 anion exchanger family includes nine Drosophila genes (136), six of which show enrichment in the Malpighian tubule in FlyAtlas and FlyAtlas 2, including dPrestin. dPrestin mediates chloride exchange with oxalate, sulfate, thiosulfate and formate (132,136,137). Principal cell knockdown of dPrestin decreased crystal formation in the high-oxalate diet model (132) and the inhibitory effect of sulfate and thiosulfate feeding on stone formation (137). Thus, dPrestin is an important mediator of Malpighian tubule oxalate transport.
A recent study used Drosophila to examine effects of the plant metabolite 3,4,5-tri-O-galloylquinic acid methyl ester (TGAME) on stone formation, and found decreased calcium oxalate formation ex vivo when tubules were incubated in TGAME-containing baths (138**). Thus, this rapidly performed assay may have utility for prioritizing compounds of interest for further study.
Analysis of the concretions in Xdh knockdown flies revealed significant amounts of zinc, which was also found in human samples of Randall’s plaques (precursors for calcium-based kidney stones) and human xanthine kidney stones. Increasing dietary zinc increased Malpighian tubule stone formation, while dietary supplementation with a zinc chelator decreased stones (135). There are two major families of zinc transporters. Transcripts of five of seven SLC30 family genes, and five of ten SLC39 family genes, are enriched in the Malpighian tubule in FlyAtlas 2. Knockdown of three of the tubule-enriched SLC30 family members reduced tubule stone formation (135). CG10006 (Zip10) is an SLC39 zinc transporter highly enriched in the Malpighian tubule. Immunohistochemistry demonstrated apical membrane staining of the Drosophila transporter in the principal cell of the Malpighian tubule, and of the human transporter in the apical membrane of the proximal tubule and cortical collecting duct (139**). Drosophila is thus a useful model to study the role of zinc in stone formation.
Conclusion
The strength of Drosophila melanogaster as a model organism relevant to human physiology has derived from conservation of molecular pathways. Complex interactions between mammalian nephron segments (140), or between the kidney and other organs, may be better modeled in organisms with kidney structures and hormonal signaling more similar to humans (141). On the other hand, as discussed here, transporters and channels important in the mammalian kidney are also present in the Drosophila Malpighian tubule, and some have been functionally characterized. Similarly, signaling pathways relevant to ion transport regulation in the mammalian kidney also regulate transport in the Malpighian tubule. Drosophila thus affords opportunities for ongoing characterization of the molecular physiology of epithelial transport. Unbiased genome-wide forward genetic screening may identify novel pathways interacting with known transporters and signaling pathways, and opportunities also exist for drug screening (3) and characterization (138**).
Key points.
Drosophila melanogaster has a sophisticated genetic and physiological toolkit to characterize transport processes in the fly renal epithelium, the Malpighian tubule.
Many classes of transporters, exchangers and channels important in mammalian kidney function have Drosophila homologs that are expressed in the Malpighian tubule.
Signaling pathways regulating transport are also conserved in Drosophila, such as the WNK-SPAK/OSR1 pathway, and recent studies have characterized the molecular physiology of these pathways.
The Malpighian tubule has also been developed as a model for kidney stone formation, including testing of pharmacological agents for stone treatment, and characterizing the role of zinc in stone formation.
Acknowledgements
The author thanks Michael Romero for discussions on NDAE1 and Diana Lim for assistance with the figures.
Financial support and sponsorship
This work was supported by the Department of Internal Medicine and Molecular Medicine Program, University of Utah, Salt Lake City, UT, the US Department of Veterans Affairs, the National Institutes of Health (R01DK110358) and the American Heart Association (16CSA28530002).
Funding: The author is supported by the National Institutes of Health (R01DK110358) and the American Heart Association (16CSA28530002).
Footnotes
Conflicts of interest
None.
References
- 1.Rubin GM, Lewis EB. A brief history of Drosophila’s contributions to genome research. Science. 2000. March 24;287(5461):2216–8. [DOI] [PubMed] [Google Scholar]
- 2.Ugur B, Chen K, Bellen HJ. Drosophila tools and assays for the study of human diseases. Disease Models & Mechanisms. 2016. March 1;9(3):235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011. June;63(2):411–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993. June;118(2):401–15. [DOI] [PubMed] [Google Scholar]
- 5.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. Science Signaling; 2004. February 17;2004(220):pl6–pl6. [DOI] [PubMed] [Google Scholar]
- 6.Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA. 1997. May 13;94(10):5207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terhzaz S, Finlayson AJ, Stirrat L, Yang J, Tricoire H, Woods DJ, et al. Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cellular Signalling. Elsevier Inc; 2010. May 1;22(5):737–48. [DOI] [PubMed] [Google Scholar]
- 8.Terhzaz S, Cabrero P, Robben JH, Radford JC, Hudson BD, Milligan G, et al. Mechanism and Function of Drosophila capa GPCR: A Desiccation Stress-Responsive Receptor with Functional Homology to Human NeuromedinU Receptor. Lee LTO, editor. PLoS ONE. 2012. January 11;7(1):e29897–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosay P, Davies SA, Yu Y, Sözen MA, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci. 1997. August;110 ( Pt 15):1683–92. [DOI] [PubMed] [Google Scholar]
- 10.Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, et al. The Transgenic RNAi Project at Harvard Medical School: Resources and Validation. Genetics. Genetics; 2015. November 12;201(3):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007. July 12;448(7150):151–6. [DOI] [PubMed] [Google Scholar]
- 12.Ni J-Q, Zhou R, Czech B, Liu L-P, Holderbaum L, Yang-Zhou D, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Meth. 2011. April 3;8(5):405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Schellinger JN, Huang C-L, Rodan AR. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem. 2014. September 19;289(38):26131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Wu Y, Jonusaite S, Pleinis JM, Humphreys JM, He H, et al. Intracellular Chloride and Scaffold Protein Mo25 Cooperatively Regulate Transepithelial Ion Transport through WNK Signaling in the Malpighian Tubule. J Am Soc Nephrol. 2018. May;29(5):1449–61.* Measured intracellular chloride in the Malpighian tubule using the transgenic sensor, ClopHensor, and demonstrated roles for chloride and the scaffold protein Mo25 in the regulation of the WNK (With No Lysine)-SPAK/OSR1 (Ste20-related proline/alanine rich kinase/oxidative stress response) signaling in epithelial ion transport.
- 15.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007. June;39(6):715–20. [DOI] [PubMed] [Google Scholar]
- 16.Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 2018. January 4;46(D1):D809–15.** Describes FlyAtlas 2, a searchable resource for gene expression across a broad range of Drosophila tissues, including the Malpighian tubule, based on RNA sequencing technology.
- 17.Wang J, Kean L, Yang J, Allan AK, Davies SA, Herzyk P, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5(9):R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chintapalli VR, Wang J, Herzyk P, Davies SA, Dow JAT. Data-mining the FlyAtlas online resource to identify core functional motifs across transporting epithelia. 2013;14(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. Nature Publishing Group; 2008. October 16;457(7226):322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development. 2009. July;136(14):2335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Zhao Y, Chao Y, Muir K, Han Z. Cubilin and Amnionless Mediate Protein Reabsorption in Drosophila Nephrocytes. Journal of the American Society of Nephrology. 2013. January 31;24(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. Journal of Experimental Biology. 1994. December;197:421–8. [DOI] [PubMed] [Google Scholar]
- 23.Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, et al. Osmoregulation and excretion. Compr Physiol. 2014. April;4(2):405–573. [DOI] [PubMed] [Google Scholar]
- 24.Luan Z, Quigley C, Li H-S. The putative Na+/Cl−-dependent neurotransmitter/osmolyte transporter inebriated in the Drosophila hindgut is essential for the maintenance of systemic water homeostasis. Sci Rep. 2015. January 23;5(1):1218–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yerushalmi GY, Misyura L, MacMillan HA, Donini A. Functional plasticity of the gut and the Malpighian tubules underlies cold acclimation and mitigates cold-induced hyperkalemia in Drosophila melanogaster. J Exp Biol. 2018. March 19;221(Pt 6).* Measured ion fluxes in the Drosophila Malpighian tubule, midgut and hindgut under varying temperature conditions, demonstrating potassium secretion by the Malpighian tubules and, for the first time in Drosophila, potassium reabsorption in the hindgut, which is quantitatively greater in the distal hindgut compared to the proximal hindgut.
- 26.Linton SM, O’Donnell MJ. Contributions of K+:Cl- cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. Journal of Experimental Biology. The Company of Biologists Ltd; 1999. June;202(Pt 11):1561–70. [DOI] [PubMed] [Google Scholar]
- 27.Rheault MR, O’Donnell MJ. Analysis of epithelial K(+) transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. Journal of Experimental Biology. The Company of Biologists Ltd; 2001. July;204(Pt 13):2289–99. [DOI] [PubMed] [Google Scholar]
- 28.Rodan AR, Baum M, Huang C-L. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol, Cell Physiol. 2012. October 15;303(8):C883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell MJ, Maddrell SH. Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. Journal of Experimental Biology. 1995. August;198(Pt 8):1647–53. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay JA. Active Transport of Water by the Malpighian Tubules of the Stick Insect, Dixippus Morosus (Orthoptera, Phasmidae). Journal of Experimental Biology. The Company of Biologists Ltd; 1954. March 1;31(1):104–13. [Google Scholar]
- 31.Schellinger JN, Rodan AR. Use of the Ramsay Assay to Measure Fluid Secretion and Ion Flux Rates in the Drosophila melanogaster Malpighian Tubule. J Vis Exp. 2015. November 25;(105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies SA, Cabrero P, Marley R, Corrales GM, Ghimire S, Dornan AJ, et al. Epithelial Function in the Drosophila Malpighian Tubule: An In Vivo Renal Model. Methods Mol Biol. 2019;1926:203–21. [DOI] [PubMed] [Google Scholar]
- 33.Browne A, O’Donnell MJ. Ammonium secretion by Malpighian tubules of Drosophila melanogaster: application of a novel ammonium-selective microelectrode. Journal of Experimental Biology. 2013. September 25;216(20):3818–27. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell MJ, Rheault MR. Ion-selective microelectrode analysis of salicylate transport by the Malpighian tubules and gut of Drosophila melanogaster. Journal of Experimental Biology. 2005. January;208(Pt 1):93–104. [DOI] [PubMed] [Google Scholar]
- 35.Rheault MR. Organic cation transport by Malpighian tubules of Drosophila melanogaster: application of two novel electrophysiological methods. Journal of Experimental Biology. 2004. May 15;207(12):2173–84. [DOI] [PubMed] [Google Scholar]
- 36.Leader JP, O’Donnell MJ. Transepithelial transport of fluorescent p-glycoprotein and MRP2 substrates by insect Malpighian tubules: confocal microscopic analysis of secreted fluid droplets. Journal of Experimental Biology. 2005. December;208(Pt 23):4363–76. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Baum M, Huang C-L, Rodan AR. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am J Physiol Regul Integr Comp Physiol. 2015. October;309(7):R747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Browne A, O’Donnell MJ. Segment-specific Ca(2+) transport by isolated Malpighian tubules of Drosophila melanogaster: A comparison of larval and adult stages. 2016. April;87:1–11. [DOI] [PubMed] [Google Scholar]
- 39.Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, et al. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. Journal of Biological Chemistry. 2006. July 7;281(27):18849–58. [DOI] [PubMed] [Google Scholar]
- 40.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JAT. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci USA. 2014. September 30;111(39):14301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossano AJ, Romero MF. Optical Quantification of Intracellular pH in Drosophila melanogaster Malpighian Tubule Epithelia with a Fluorescent Genetically-encoded pH Indicator. J Vis Exp. 2017. August 11;(126).** Description of the use of a genetically encoded pH indicator to measure intracellular pH and proton fluxes in Malpighian tubule epithelial cells.
- 42.Efetova M, Petereit L, Rosiewicz K, Overend G, Haußig F, Hovemann BT, et al. Separate roles of PKA and EPAC in renal function unraveled by the optogenetic control of cAMP levels in vivo. J Cell Sci. 2013. February 1;126(Pt 3):778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerr M, Davies SA, Dow JAT. Cell-Specific Manipulation of Second Messengers. Current Biology. 2004. August;14(16):1468–74. [DOI] [PubMed] [Google Scholar]
- 44.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila Melanogaster. Journal of Experimental Biology. The Company of Biologists Ltd; 1996. May;199(Pt 5):1163–75. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 1998. April;274(4 Pt 2):R1039–49. [DOI] [PubMed] [Google Scholar]
- 46.Du J, Kean L, Allan AK, Southall TD, Davies SA, McInerny CJ, et al. The SzA mutations of the B subunit of the Drosophila vacuolar H+ ATPase identify conserved residues essential for function in fly and yeast. J Cell Sci. 2006. June 15;119(Pt 12):2542–51. [DOI] [PubMed] [Google Scholar]
- 47.Allan AK, Du J, Davies SA, Dow JAT. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics. 2005. July 14;22(2):128–38. [DOI] [PubMed] [Google Scholar]
- 48.Davies SA, Goodwin SF, Kelly DC, Wang Z, Sözen MA, Kaiser K, et al. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem. 1996. November 29;271(48):30677–84. [DOI] [PubMed] [Google Scholar]
- 49.Dow JA, Davies SA, Guo Y, Graham S, Finbow ME, Kaiser K. Molecular genetic analysis of V-ATPase function in Drosophila melanogaster. Journal of Experimental Biology. 1997. January;200(Pt 2):237–45. [DOI] [PubMed] [Google Scholar]
- 50.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999. January;21(1):84–90. [DOI] [PubMed] [Google Scholar]
- 51.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet. 2000. September;26(1):71–5. [DOI] [PubMed] [Google Scholar]
- 52.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci. 2008. August 1;121(Pt 15):2612–9. [DOI] [PubMed] [Google Scholar]
- 53.Chintapalli VR, Kato A, Henderson L, Hirata T, Woods DJ, Overend G, et al. Transport proteins NHA1 and NHA2 are essential for survival, but have distinct transport modalities. Proc Natl Acad Sci USA. 2015. September 15;112(37):11720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feingold D, Starc T, O’Donnell MJ, Nilson L, Dent JA. The orphan pentameric ligand-gated ion channel pHCl-2 is gated by pH and regulates fluid secretion in DrosophilaMalpighian tubules. Journal of Experimental Biology. 2016. August 31;219(17):2629–38. [DOI] [PubMed] [Google Scholar]
- 55.Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. Journal of Biological Chemistry. 2000. August 11;275(32):24552–9. [DOI] [PubMed] [Google Scholar]
- 56.Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na(+)-driven anion exchanger protein NDAE1 from Drosophila melanogaster. AJP: Cell Physiology. 2001. August;281(2):C449–63. [DOI] [PubMed] [Google Scholar]
- 57.Ianowski JP, O’Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl- cotransport and Cl- conductance. Journal of Experimental Biology. 2004. July;207(Pt 15):2599–609. [DOI] [PubMed] [Google Scholar]
- 58.Leyssens A, Van Kerkhove E, Zhang SL. Measurement of intracellular and luminal K+ concentrations in a Malpighian tubule (Formica). Estimate of basal and luminal electrochemical K+ gradients. Journal of insect …. 1993;39(11):945–58. [Google Scholar]
- 59.Leyssens A, Dijkstra S, Van Kerkhove E, Steels P. Mechanisms of K+ uptake across the basal membrane of malpighian tubules of Formica polyctena: the effect of ions and inhibitors. Journal of Experimental Biology. 1994. October;195:123–45. [DOI] [PubMed] [Google Scholar]
- 60.Naikkhwah W, O’Donnell MJ. Salt stress alters fluid and ion transport by Malpighian tubules of Drosophila melanogaster: evidence for phenotypic plasticity. Journal of Experimental Biology. 2011. September 28;214(20):3443–54. [DOI] [PubMed] [Google Scholar]
- 61.MacMillan HA, Hughson BN. A high-throughput method of hemolymph extraction from adult Drosophila without anesthesia. 2014. Apr;63:27–31. [DOI] [PubMed] [Google Scholar]
- 62.Gamba G Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiological Reviews. 2005. April;85(2):423–93. [DOI] [PubMed] [Google Scholar]
- 63.Seyberth HW, Weber S, Kömhoff M. Bartter’s and Gitelman’s syndrome. Current Opinion in Pediatrics. 2017. April;29(2):179–86. [DOI] [PubMed] [Google Scholar]
- 64.Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011. February;59(2):320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Q, Tian E, Turner RJ, Hagen Ten KG. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. AJP: Cell Physiology. 2009. December 16;298(1):C26–C37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLean SJ, Andrews BC, Verheyen EM. Characterization of Dir: a putative potassium inward rectifying channel in Drosophila. Mech Dev. 2002. [DOI] [PubMed] [Google Scholar]
- 67.Döring F, Wischmeyer E, Kühnlein RP, Jäckle H, Karschin A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors Kir channel function. J Biol Chem. 2002. July 12;277(28):25554–61. [DOI] [PubMed] [Google Scholar]
- 68.Evans JM, Allan AK, Davies SA, Dow JAT. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. Journal of Experimental Biology. 2005. October;208(Pt 19):3771–83. [DOI] [PubMed] [Google Scholar]
- 69.Schubiger M, Feng Y, Fambrough DM, Palka J. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron. 1994. February;12(2):373–81. [DOI] [PubMed] [Google Scholar]
- 70.Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, et al. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA. 2004. September 14;101(37):13689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linton SM, O’Donnell MJ. Novel aspects of the transport of organic anions by the malpighian tubules of Drosophila melanogaster. Journal of Experimental Biology. 2000. December;203(Pt 23):3575–84. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-Sanchez E, O’Donnell MJ. Characterization of salicylate uptake across the basolateral membrane of the malpighian tubules of Drosophila melanogaster. J Insect Physiol. 2006. September;52(9):920–8. [DOI] [PubMed] [Google Scholar]
- 73.Teulon J, Planelles G, Sepúlveda FV, Andrini O, Lourdel S, Paulais M. Renal Chloride Channels in Relation to Sodium Chloride Transport. Compr Physiol. 2018. December 13;9(1):301–42. [DOI] [PubMed] [Google Scholar]
- 74.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf). 2011. July;202(3):387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol. 1993. February;132(1):63–76. [DOI] [PubMed] [Google Scholar]
- 76.Limmer S, Weiler A, Volkenhoff A, Babatz F, Klämbt C. The Drosophila blood-brain barrier: development and function of a glial endothelium. Front Neurosci. 2nd ed. 2014;8(353):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaufmann N, Mathai JC, Hill WG, Dow JAT, Zeidel ML, Brodsky JL. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. AJP: Cell Physiology. 2005. August;289(2):C397–407. [DOI] [PubMed] [Google Scholar]
- 78.Misyura L, Yerushalmi GY, Donini A. A mosquito entomoglyceroporin, Aedes aegypti AQP5, participates in water transport across the Malpighian tubules of larvae. J Exp Biol. 2017. October 1;220(Pt 19):3536–44. [DOI] [PubMed] [Google Scholar]
- 79.Dube KA, McDonald DG, O’Donnell MJ. Calcium homeostasis in larval and adult Drosophila melanogaster. Arch Insect Biochem Physiol. 2000. May;44(1):27–39. [DOI] [PubMed] [Google Scholar]
- 80.Dube K, McDonald D, O’Donnell M. Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophila melanogaster: roles of sequestration and secretion. 2000. November 1;46(11):1449–60. [DOI] [PubMed] [Google Scholar]
- 81.Chintapalli VR, Terhzaz S, Wang J, Bratty Al M, Watson DG, Herzyk P, et al. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS ONE. 2012;7(4):e32577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wessing A, Zierold K, Hevert F. Two types of concretions in Drosophila Malpighian tubules as revealed by X-ray microanalysis: a study on urine formation. 1992;38(7):543–54. [Google Scholar]
- 83.Phillips JE, Maddrell SH. Active transport of magnesium by the malpighian tubules of the larvae of the mosquito, Aedes campestris. Journal of Experimental Biology. 1974. December;61(3):761–71. [DOI] [PubMed] [Google Scholar]
- 84.Voets T, Nilius B, Hoefs S, van der Kemp AWCM, Droogmans G, Bindels RJM, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. Journal of Biological Chemistry. 2004. January 2;279(1):19–25. [DOI] [PubMed] [Google Scholar]
- 85.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002. May 28;31(2):166–70. [DOI] [PubMed] [Google Scholar]
- 86.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002. May 28;31(2):171–4. [DOI] [PubMed] [Google Scholar]
- 87.Chubanov V, Ferioli S, Wisnowsky A, Simmons DG, Leitzinger C, Einer C, et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife. 2016. December 19;5:863–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofmann T, Chubanov V, Chen X, Dietz AS, Gudermann T, Montell C. Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLoS ONE. 2010. May 6;5(5):e10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergwitz C, Wee MJ, Sinha S, Huang J, DeRobertis C, Mensah LB, et al. Genetic determinants of phosphate response in Drosophila. PLoS ONE. 2013;8(3):e56753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergwitz C, Rasmussen MD, DeRobertis C, Wee MJ, Sinha S, Chen HH, et al. Roles of major facilitator superfamily transporters in phosphate response in Drosophila. PLoS ONE. 2012;7(2):e31730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Höglund PJ, Nordström KJV, Schiöth HB, Fredriksson R. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Molecular Biology and Evolution. 2011. April;28(4):1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dus M, Ai M, Suh GSB. Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nat Neurosci. 2013. May;16(5):526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang K, Kestenbaum B. Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin J Am Soc Nephrol. 2018. August 7;13(8):1291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015. January;14(1):29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chahine S, O’Donnell MJ. Physiological and molecular characterization of methotrexate transport by Malpighian tubules of adult Drosophila melanogaster. J Insect Physiol. 2009. October;55(10):927–35. [DOI] [PubMed] [Google Scholar]
- 96.Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics. 2004. June 17;18(1):12–24. [DOI] [PubMed] [Google Scholar]
- 97.Chahine S, Campos A, O’Donnell MJ. Genetic knockdown of a single organic anion transporter alters the expression of functionally related genes in Malpighian tubules of Drosophila melanogaster. J Exp Biol. 2012. August 1;215(Pt 15):2601–10. [DOI] [PubMed] [Google Scholar]
- 98.Chahine S, Seabrooke S, O’Donnell MJ. Effects of genetic knock-down of organic anion transporter genes on secretion of fluorescent organic ions by Malpighian tubules of Drosophila melanogaster. Arch Insect Biochem Physiol. 2012. December;81(4):228–40. [DOI] [PubMed] [Google Scholar]
- 99.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide–induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension. 1999. [DOI] [PubMed] [Google Scholar]
- 100.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol. 1999. January;276(1 Pt 2):F159–63. [DOI] [PubMed] [Google Scholar]
- 101.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, et al. Renal Collecting Duct NOS1 Maintains Fluid–Electrolyte Homeostasis and Blood Pressure. Hypertension. 2013. July;62(1):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao Y, Stuart D, Takahishi T, Kohan DE. Nephron-Specific Disruption of Nitric Oxide Synthase 3 Causes Hypertension and Impaired Salt Excretion. J Am Heart Assoc. 2018. July 11;7(14):e009236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, et al. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. AJP: Regulatory, Integrative and Comparative Physiology. 2002. May 1;282(5):R1297–307. [DOI] [PubMed] [Google Scholar]
- 104.Dow JA, Maddrell SH, Davies SA, Skaer NJ, Kaiser K. A novel role for the nitric oxide-cGMP signaling pathway: the control of epithelial function in Drosophila. Am J Physiol. 1994. May;266(5 Pt 2):R1716–9. [DOI] [PubMed] [Google Scholar]
- 105.Davies SA, Stewart EJ, Huesmann GR, Skaer NJ, Maddrell SH, Tublitz NJ, et al. Neuropeptide stimulation of the nitric oxide signaling pathway in Drosophila melanogaster Malpighian tubules. Am J Physiol. 1997. August;273(2 Pt 2):R823–7. [DOI] [PubMed] [Google Scholar]
- 106.Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016. February 10;78:367–89. [DOI] [PubMed] [Google Scholar]
- 107.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001. August 10;293(5532):1107–12. [DOI] [PubMed] [Google Scholar]
- 108.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S-I, Uchida S, Natsume T, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. Journal of Biological Chemistry. American Society for Biochemistry and Molecular Biology; 2005. December 30;280(52):42685–93. [DOI] [PubMed] [Google Scholar]
- 109.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005. October 1;391(Pt 1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anselmo AN, Earnest S, Chen W, Juang Y-C, Kim SC, Zhao Y, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006. July 18;103(29):10883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dowd BFX, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem. 2003. July 25;278(30):27347–53. [DOI] [PubMed] [Google Scholar]
- 112.Gagnon KBE, England R, Delpire E. Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. AJP: Cell Physiology. 2006. January;290(1):C134–42. [DOI] [PubMed] [Google Scholar]
- 113.Richardson C, Rafiqi FH, Karlsson HKR, Moleleki N, Vandewalle A, Campbell DG, et al. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. The Company of Biologists Ltd; 2008. March 1;121(Pt 5):675–84. [DOI] [PubMed] [Google Scholar]
- 114.Richardson C, Sakamoto K, de Los Heros P, Deak M, Campbell DG, Prescott AR, et al. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci. 2011. March 1;124(Pt 5):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HKR, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006. July 1;397(1):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000. December;28(3):793–806. [DOI] [PubMed] [Google Scholar]
- 117.Sato A, Shibuya H. WNK signaling is involved in neural development via Lhx8/Awh expression. Verheyen EM, editor. PLoS ONE [Internet]. Public Library of Science; 2013;8(1):e55301 Available from: http://dx.plos.org/10.1371/journal.pone.0055301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serysheva E, Berhane H, Grumolato L, Demir K, Balmer S, Bodak M, et al. Wnk kinases are positive regulators of canonical Wnt/β-catenin signalling. EMBO Rep [Internet]. 2013. August;14(8):718–25. Available from: http://embor.embopress.org/cgi/doi/10.1038/embor.2013.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014. May 6;7(324):ra41–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang C-L, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney International. 2016. January;89(1):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodan AR. Intracellular chloride: a regulator of transepithelial transport in the distal nephron. Curr Opin Nephrol Hypertens. 2019. March 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodan AR. WNK-SPAK/OSR1 signaling: lessons learned from an insect renal epithelium. Am J Physiol Renal Physiol. 2018. October 1;315(4):F903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grimm PR, Taneja TK, Liu J, Coleman R, Chen Y-Y, Delpire E, et al. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem. 2012. November 2;287(45):37673–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oi K, Sohara E, Rai T, Misawa M, Chiga M, Alessi DR, et al. A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC co-transporters in vivo. Biology Open. 2012. February 15;1(2):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mederle K, Mutig K, Paliege A, Carota I, Bachmann S, Castrop H, et al. Loss of WNK3 is compensated for by the WNK1/SPAK axis in the kidney of the mouse. Am J Physiol Renal Physiol. 2013. May 1;304(9):F1198–209. [DOI] [PubMed] [Google Scholar]
- 126.Roy A, Goodman JH, Begum G, Donnelly BF, Pittman G, Weinman EJ, et al. Generation of WNK1 knockout cell lines by CRISPR/Cas-mediated genome editing. Am J Physiol Renal Physiol. 2015. February 15;308(4):F366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thastrup JO, Rafiqi FH, Vitari AC, Pozo Guisado E, Deak M, Mehellou Y, et al. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012. January 1;441(1):325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chavez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, et al. WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension. Lippincott Williams & Wilkins; 2014. November;64(5):1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Argaiz ER, Chavez-Canales M, Ostrosky-Frid M, Rodríguez-Gama A, Vazquez N, González-Rodríguez X, et al. Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC. Am J Physiol Renal Physiol. 2018. May 30;306(3):F1507–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Investig Clin Urol. 2017. September;58(5):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller J, Chi T, Kapahi P, Kahn AJ, Kim MS, Hirata T, et al. Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol. 2013. November;190(5):1648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hirata T, Cabrero P, Berkholz DS, Bondeson DP, Ritman EL, Thompson JR, et al. In vivo Drosophilia genetic model for calcium oxalate nephrolithiasis. AJP: Renal Physiology. 2012. December 1;303(11):F1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Y-H, Liu H-P, Chen H-Y, Tsai F-J, Chang C-H, Lee Y-J, et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney International. 2011. August;80(4):369–77. [DOI] [PubMed] [Google Scholar]
- 134.Chen W-C, Lin W-Y, Chen H-Y, Chang C-H, Tsai F-J, Man K-M, et al. Melamine-induced urolithiasis in a Drosophila model. J Agric Food Chem. 2012. March 14;60(10):2753–7. [DOI] [PubMed] [Google Scholar]
- 135.Chi T, Kim MS, Lang S, Bose N, Kahn A, Flechner L, et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE. 2015;10(5):e0124150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirata T, Czapar A, Brin L, Haritonova A, Bondeson DP, Linser P, et al. Ion and solute transport by Prestin in Drosophila and Anopheles. 2012. April;58(4):563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Landry GM, Hirata T, Anderson JB, Cabrero P, Gallo CJR, Dow JAT, et al. Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2016. January 15;310(2):F152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abd El-Salam M, Bastos JK, Han JJ, Previdi D, Coelho EB, Donate PM, et al. The Synthesized Plant Metabolite 3,4,5-Tri-O-Galloylquinic Acid Methyl Ester Inhibits Calcium Oxalate Crystal Growth in a Drosophila Model, Downregulates Renal Cell Surface Annexin A1 Expression, and Decreases Crystal Adhesion to Cells. J Med Chem. 2018. February 22;61(4):1609–21.** Demonstrates the inhibition of stone formation by the plant metabolite 3,4,5-tri-O-galloylquinic acid methyl ester in the Drosophila Malpighian tubule.
- 139.Landry GM, Furrow E, Holmes HL, Hirata T, Kato A, Williams P, et al. Cloning, function, and localization of human, canine, and Drosophila ZIP10 (SLC39A10), a Zn2+ transporter. Am J Physiol Renal Physiol. 2019. February 1;316(2):F263–73.** Examines transport characteristics and kidney expression patterns of SLC39A10 zinc transporters from fly, dog and human.
- 140.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, et al. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest. 2015. May;125(5):2136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dow JAT, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol. 2010. December;299(6):F1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]