Abstract
Background
Cancer‐related pain is complex and multi‐dimensional but the mainstay of cancer pain management has predominantly used a biomedical approach. There is a need for non‐pharmacological and innovative approaches. Transcutaneous Electric Nerve Stimulation (TENS) may have a role in pain management but the effectiveness of TENS is currently unknown. This is an update of the original review published in Issue 3, 2008.
Objectives
The aim of this systematic review was to determine the effectiveness of TENS for cancer‐related pain in adults.
Search methods
The initial review searched The Cochrane Library, MEDLINE, EMBASE, CINAHL, PsychINFO, AMED and PEDRO databases in April 2008. We performed an updated search of CENTRAL, MEDLINE, EMBASE, CINAHL and PEDRO databases in November 2011.
Selection criteria
We included only randomised controlled trials (RCTS) investigating the use of TENS for the management of cancer‐related pain in adults.
Data collection and analysis
The search strategy identified a further two studies for possible inclusion. One of the review authors screened each abstract using a study eligibility tool. Where eligibility could not be determined, a second author assessed the full paper. One author used a standardised data extraction sheet to collect information on the studies and independently assess the quality of the studies using the validated five‐point Oxford Quality Scale. The small sample sizes and differences in patient study populations of the three included studies (two from the original review and a third included in this update) prevented meta‐analysis. For the original review the search strategy identified 37 possible published studies; we divided these between two pairs of review authors who decided on study selection; all four review authors discussed and agreed final scores.
Main results
Only one additional RCT met the eligibility criteria (24 participants) for this updated review. Although this was a feasibility study, not designed to investigate intervention effect, it suggested that TENS may improve bone pain on movement in a cancer population. The initial review identified two RCTs (64 participants) therefore this review now includes a total of three RCTs (88 participants). These studies were heterogenous with respect to study population, sample size, study design, methodological quality, mode of TENS, treatment duration, method of administration and outcome measures used. In one RCT, there were no significant differences between TENS and placebo in women with chronic pain secondary to breast cancer treatment. In the other RCT, there were no significant differences between acupuncture‐type TENS and sham in palliative care patients; this study was underpowered.
Authors' conclusions
Despite the one additional RCT, the results of this updated systematic review remain inconclusive due to a lack of suitable RCTs. Large multi‐centre RCTs are required to assess the value of TENS in the management of cancer‐related pain in adults.
Keywords: Adult, Humans, Bone Diseases, Bone Diseases/therapy, Neoplasms, Neoplasms/complications, Pain, Pain/etiology, Pain Management, Pain Management/methods, Randomized Controlled Trials as Topic, Transcutaneous Electric Nerve Stimulation, Transcutaneous Electric Nerve Stimulation/methods
Plain language summary
Transcutaneous electrical nerve stimulation (TENS) for cancer‐related pain in adults
Cancer‐related pain is complex and multidimensional but is mostly managed using drug therapy. There is increasing recognition of the need for non‐drug approaches and TENS may have a significant role to play. Only one new study met eligibility criteria for this review update, making at total of three included studies. TENS was given to 15 participants in one study, 41 participants in the other and 24 participants in the most recently included study. The newly included study suggested TENS might improve cancer bone pain on movement, but as a pilot study it was not designed to determine the impact of TENS on pain. The two studies in the previous review did not show that TENS significantly improved cancer pain. One study did not have sufficient participants to determine whether or not TENS had an effect. TENS was well tolerated in all three studies. There were significant differences in participants, treatments, procedures and symptom measurement tools used in the studies. In two of the studies some participants were able to identify when they received active TENS and when they received placebo. Consequently, there is insufficient evidence to judge whether TENS should be used in adults with cancer‐related pain. Further research using well designed clinical trials is needed to improve knowledge in this field.
Background
Description of the condition
There are many reasons why a patient with cancer may experience pain; these include pain associated with the disease, pain associated with the cancer treatments and any associated co‐morbid conditions. The mainstay of cancer pain management has predominantly used the biomedical approach including drug therapy, medical or surgical treatments (Turk 1998). However, it is clear that cancer‐related pain is complex and multidimensional and there is a definite need for a multi‐disciplinary team approach, utilising non‐pharmacological and innovative approaches (Raphael 2010). Physical treatments such as electrical stimulation may have a role for a significant number of patients (Johnson 2008a; Raphael 2010; Simpson 2000).
Description of the intervention
Transcutaneous electrical nerve stimulation (TENS) is a non‐invasive therapeutic intervention which has been widely used for many years to manage a range of acute and chronic pain problems (DeSantana 2008; Johnson 2008b; Johnson 2011; Walsh 1997). TENS is used in a variety of clinical settings and has gained popularity with both patients and healthcare professionals of different disciplines. TENS devices have many advantages in that they are portable, easy to use, have relatively few adverse effects or contra‐indications and allow the user autonomy over their pain control.
How the intervention might work
There are several types of TENS application which are used in clinical practice, but the two most common are high frequency, low intensity (conventional) TENS (HF‐TENS) and low frequency, high intensity (acupuncture‐like) TENS (AL‐TENS) (Jones 2009). Intense low frequency high intensity TENS can be administered for a few minutes at a time to deliver maximum tolerable (painful) TENS paraesthesiae (Jones 2009). More recent developments of TENS have evolved with the aim of improving the efficacy of TENS and these include 'burst' and 'modulated' modes of stimulation. The analgesic action of TENS is mediated by peripheral and central nervious system mechanisms, both spinal and supra spinal (DeSantana 2008). The clinical use of conventional TENS is underpinned by the gate control theory of pain (Melzack 1965), which suggests that there is a 'gating' mechanism in the dorsal horn of the spinal cord which can control nociceptive signals and ultimately influence the pain experience. In summary, the stimulation of large diameter (A‐beta) afferent fibres is thought to 'close the gate' and reduce the perception of pain. Acupuncture‐like TENS stimulates A‐delta and C fibres and is therefore thought to achieve pain control mostly through the descending pain suppression system. In essence, acupuncture‐like TENS is thought to help close the gateway of pain transmission and hence result in a reduction in pain. However, the physiological mechanisms underpinning the action of different TENS modalities may not be this distinct. In mice, high and low frequency TENS activate large diameter afferents, peripheral alpha 2A‐adrenergic receptors appear to contribute to high and low frequency TENS analgesia and blockade of GABA A receptors at a spinal level inhibits the analgesic effect of both modalities (DeSantana 2008).
Why it is important to do this review
There are a number of Cochrane systematic reviews published which address the use of TENS for non‐cancer related pain (Brosseau 2003; Dowswell 2009; Khadilkar 2008; Kroeling 2009; Mulvey 2010; Nnoaham 2008; Proctor 2009; Rutjes 2009; Walsh 2006). In addition, several review articles (Bjordal 2003; Johnson 2001; Reeve 1996) address TENS in benign pain. There is some controversy over the use of TENS in chronic pain, with most review papers citing the need for further research using large multi‐centre RCTs. The single available review in cancer pain addresses non‐drug approaches for symptoms related to cancer and includes the evidence on TENS for pain management (Pan 2000). Although experts in the field suggest that TENS has an important role in the management of cancer‐related pain (Filshie 2000), and a recent case series suggested a potential role in cancer bone pain (Searle 2008), it is clear that there is currently no guidance for clinicians on the use of TENS for oncology and palliative care patients. The clinical benefit of TENS for cancer patients with pain remains controversial.
The aim of this Cochrane review is to determine the effectiveness of TENS in the management of cancer‐related pain and to provide guidance for healthcare professionals and patients on the optimal parameters of TENS for best pain relief.
Objectives
To establish the effectiveness of TENS in the management of cancer‐related pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) (crossover and parallel design); those investigating the use of TENS for the management of cancer‐related pain in adults where the control (placebo) group was clearly defined and was either:
no active stimulation or
no treatment.
We have not included comparisons of TENS with active treatment.
Types of participants
Participants were 18 years of age or older. They had experienced cancer‐related pain, unspecified or persistent cancer treatment‐related pain, or both, for a minimum of three months after any anti‐cancer treatment had been completed. Pain was classified based on commonly used verbal rating scales or pain interference scales.
Types of interventions
We included only studies that evaluated TENS administered using a standard TENS device that delivered monophasic or biphasic pulsed electrical currents in the mA range. We did not include studies that used percutaneous electrical stimulation. We considered conventional TENS as administered using any TENS device which delivered a "strong but comfortable" electrical sensation either: i. in an area of pain where sensation is present; or ii. over nerve bundles proximal to the site of pain.
Our definition of appropriate TENS delivery also included the use of Neuromuscular Electrical Stimulation devices (NMES) and Interferential current devices, providing that a "strong but comfortable" electrical sensation was produced. We considered any parameters of treatment which resulted in this, as was any duration and frequency of treatment. TENS is typically delivered using at least two surface electrodes; however, we also included studies involving single electrical probes (i.e. TENS pens), providing that a "strong but comfortable" electrical sensation was produced. This included the placement of electrodes over an area of pain that co‐incidentally included acupuncture points. Given the above physiological criteria, we excluded studies where TENS was delivered at intensities reported to be "barely perceptible" or "mild".
Types of outcome measures
Primary outcomes
The primary outcome measure was patient reported pain using validated scales (e.g. visual analogue scales (VAS), numerical rating scales).
Secondary outcomes
Secondary outcome measures included any of the following:
patient satisfaction,
function,
range of movement,
quality of life,
mood,
pain coping,
sleep,
analgesic consumption,
hospital attendance and other healthcare interventions e.g. physiotherapy visits, hospice admissions, and
adverse events ‐ major and minor.
Ideally, studies would take outcome measures before, during and after stimulation, but we did not exclude studies which did not do this. We wanted to perform subgroup analyses on outcomes of greater than or equal to 30% reduction in pain from baseline, but this was not possible.
Search methods for identification of studies
The original review searched the following databases on 11 April 2008:The Cochrane Library, MEDLINE (1950 to 2008), EMBASE (1974 to 2008), CINAHL (1982 to 2008), PsychINFO, AMED (1985 to 2008) and PEDRO. We developed detailed search strategies, based on the strategy for MEDLINE but revised appropriately for each database. We also searched various foreign language databases using the terms outlined below. We reviewed reference lists of eligible trials to identify additional studies, and identified relevant RCTs using the following search strategy combined with the Cochrane Sensitive Search Strategy for RCTs (as published in Appendix 5b in the Cochrane Reviewers' Handbook for Systematic Reviews (Alderson 2004)).
We updated the original search strategy and searched the following databases on 29 June 2011, with an update prior to publication on 16 November 2011: CENTRAL, MEDLINE, EMBASE, CINAHL and AMED. We reviewed reference lists of eligible trials to identify additional studies. Our updated MEDLINE search strategy is available in Appendix 1 and all other search strategies in Appendix 2.
Searching other resources
We screened references from retrieved articles for additional publications.
Data collection and analysis
Selection of studies
We used a study eligibility form to screen each abstract. It identified whether the study was randomised, participants were adults with cancer related pain, the study compared TENS with another control group, and reported pain related outcomes. Where study eligibility could not be determined, two review authors assessed the full paper.
Data extraction and management
We used a standardised data extraction sheet to collect information on authors, participants, trial design, characteristics of interventions (TENS settings, application, treatment schedule, concurrent interventions), adverse effects and baseline and end of study outcomes. Two review authors independently assessed the quality of the studies using the validated five‐point Oxford Quality Scale (Jadad 1996) which considers the method of randomisation, blinding and the description of withdrawals or drop‐outs.
Analysis
The small sample sizes and differences in patient study populations of the three included studies prevented meta‐analysis.
Assessment of risk of bias in included studies
We assessed the risk of bias using the five‐point Oxford Quality Scale (Jadad 1996) and The Cochrane Collaboration's risk of bias tool (Figure 1).
1.
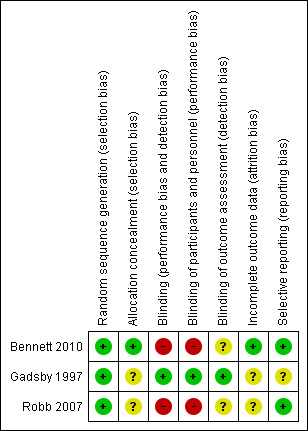
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
When meta‐analysis of continuous data is possible, we will calculate the mean difference, or standardised mean difference if studies use different measurement scales. For dichotomous data we will calculate the risk ratio (RR).
Dealing with missing data
In the event of missing data, we planned to contact the authors of included studies, and to evaluate the amount of data missing and the impact of omission on the overall results. We planned to apply the appropriate method of intention‐to‐treat (ITT) analysis.
Assessment of heterogeneity
We addressed clinical and methodological diversity by extraction and assessment of data regarding participants, intervention, outcomes and study design. We will check statistical heterogeneity when we can include at least two studies in a meta‐analysis. We will quantify inconsistency across studies using the I2 statistic.
Assessment of reporting biases
We did not perform assessment of publication bias.
Data synthesis
We will perform meta‐analysis to describe the overall results when appropriate.
Results
Description of studies
Results of the search
We identified two additional studies by the updated search, only one of which met the eligibility criteria (Bennett 2010). The other study (Sima 2009) investigated the efficacy of electroacupuncture at recognised acupoints. As a percutaneous intervention it was excluded from our review which was concerned exclusively with transcutaneous electrical stimulation. Only two of the 43 potential studies identified by the original search met the eligibility criteria for review (Gadsby 1997; Robb 2007). The most common reasons for exclusion were non‐randomised studies and the published source contained no clinical data (i.e. educational reviews). We have provided a full list of the excluded studies and the reasons for exclusion in the 'Characteristics of excluded studies' table.
Included studies
The two RCTs included in the original review were heterogenous with respect to study population, sample size, study design, methodological quality, mode of TENS, treatment duration, method of administration and outcome measures used (Gadsby 1997; Robb 2007). Participants who had previously used TENS were excluded in both studies. Robb 2007, who is also a member of this review team, compared conventional TENS with Transcutaneous Spinal Electroanalgesia (TSE) and sham TSE in 49 cancer survivors with chronic pain associated with breast cancer treatment. The investigators attempted to mimic clinical practice and used treatment for three weeks duration of each intervention with participants also self‐treating at home as needed. They assessed outcome using measures for pain, anxiety, depression and physical functioning. Gadsby 1997 investigated acupuncture‐like TENS for cancer pain or nausea and vomiting, or both, in 15 terminally ill participants. The investigators administered TENS for 30 minutes daily for five days and assessed the outcome using a quality of life questionnaire and a performance status score (see 'Characteristics of included studies' table for more details).
The additional RCT identified by the updated search performed in June 2011 (Bennett 2010) was a multicentre study assessing the feasibility of a phase III trial of TENS in patients with cancer bone pain receiving palliative care. Unlike other included studies, previous TENS use was not an exclusion criterion. Employing a crossover design, conventional TENS was compared with placebo TENS in 24 patients with heterogenous cancer diagnoses, stable analgesic medication and radiological evidence of bone involvement. A single continuous treatment with active or placebo TENS was applied by a medical researcher for 60 minutes at the bone pain site. Between two and seven days later active or placebo TENS, depending of the previous application, was administered for 60 minutes. Pain intensity and pain relief, at rest and on movement, were measured using Numerical (NRS) and Verbal Rating Scales (VRS); 19 participants completed the cross‐over and provided evaluable data. The Short‐Form McGill Pain Questionnaire (SF‐MPQ) and patient satisfaction questionnaires were also completed.
Risk of bias in included studies
Robb 2007 and Bennett 2010 scored four points and Gadsby 1997 scored three points on the five‐point Oxford Quality Scale (Jadad 1996).
Allocation
All three included studies described the means of randomisation. None discussed allocation concealment.
Blinding
In Gadsby 1997, both participants and the outcome assessor were adequately blinded. Although the researcher completing assessments and participants in Bennett 2010 were technically blind to the intervention, 10 of 19 patients correctly identified the placebo TENS. The assessor was adequately blinded in 15 of 19 participants. In Robb 2007, attempts were made to blind participants but not assessors. In this study it is commented that a minority of participants may have identified the placebo, but this was not formally analysed and numbers were not available.
Incomplete outcome data
In Bennett 2010, five participants did not complete the study. All withdrew from the study after the first treatment: two in the active TENS then placebo arm and three in the other arm. Three participants did not complete due to deteriorating health unrelated to TENS and two withdrew due to increasing pain during TENS application. The data of those who did not complete were excluded from primary analysis. Two participants in the placebo arm did not complete in Gadsby 1997. In both cases it was due to deteriorating health. In Robb 2007, eight participants did not complete. The distribution of these participants was not reported. In two participants withdrawal was due to increasing pain, in two decreasing pain and in one a skin reaction. Three withdrew for other reasons which were not described. There were no statistically significant differences in baseline data between participants lost to follow‐up and those who completed. Median anxiety and depression scores were higher in those who did not complete, but this did reach statistical significance. Neither Gadsby 1997 nor Robb 2007 stated how missing data were addressed in the analysis.
Effects of interventions
Whilst not designed or adequately powered to investigate treatment effect, the findings of Bennett 2010 indicated that cancer bone pain on movement may improve with TENS. On verbal pain relief scores 12 participants (63.2%) experienced good or very good pain relief with active TENS at one hour, compared with five participants (26.3%) on placebo. The difference in the proportion of participants experiencing good or very good pain relief on movement with active compared to placebo TENS was statistically significant: 36.8% (95% CI 7.55 to 66.2%). NRS scores for pain relief and pain intensity on movement did not reach statistical significance. For pain relief on movement, participants had a mean score at one hour of 52.6 for active TENS compared with 38.4 with placebo (difference = 14.2, 95% CI ‐3.34 to 31.76), where higher scores indicated better pain relief. The mean NRS scores for pain intensity on movement at one hour were 2.84 with active TENS and 3.05 with placebo TENS (difference = ‐0.32, CI ‐1.85 to 1.22), where higher scores indicated more intense pain. The study did not indicate that cancer bone pain at rest improved with TENS. Only five of the 22 adverse events were deemed at least possibly related to TENS. Only two withdrawals were related to pain during TENS application. Only one of these was during active TENS application.
Robb 2007 found no significant differences in pain relief scores between TENS or sham TSE. There were also no significant differences in any of the other outcome measures, except one dimension of a patient satisfaction questionnaire where TENS was considered significantly more effective than sham TSE. Twenty‐six of 41 women (63%) who completed the study decided to continue with a device on completion of the trial and of these, the majority (n = 13) decided to continue with TENS, as opposed to sham TSE (n = six). The majority of the women continuing with TENS were still using it to good effect at three months (n = 14) and 12 months (n = 10), with those using sham TSE to good effect at three months and 12 months (n = 4 and n = 2 respectively). Overall, TENS appeared to be well tolerated; women found TENS easy to use and few reported difficulties with electrode placement. Adverse effects were monitored and reported and were minimal in this study.
Gadsby 1997 did not detect any statistically significant differences between AL‐TENS and sham AL‐TENS. However, the study was underpowered, with only five participants randomised into each of the three treatment groups and only 13 participants completing the study.
Discussion
The results of this systematic review examining the effectiveness of TENS for cancer pain in adults remain inconclusive due to a lack of suitable RCTs. Only one RCT, in addition to the two RCTs identified by the initial review, met the inclusion criteria for review, and heterogeneity of these RCTs prevented meta‐analysis. The studies were different with respect to study population, sample size, study design, methodological quality, mode of TENS, treatment duration, method of administration and outcome measures used. Two studies (Bennett 2010; Robb 2007) scored four out of five for the Oxford Quality Score. The former provided little evidence that TENS was superior to a placebo in treating women with chronic pain following breast cancer treatment. Bennett 2010 was not designed to investigate treatment effect and addressed only bone pain. Although pain relief VRS on movement showed greater improvement with active than placebo TENS, no firm conclusion can be drawn from this. The findings of Bennett 2010 did not suggest TENS relieved cancer bone pain at rest. Gadsby 1997 scored three out of five and provided no evidence that TENS was significantly better than placebo in treating pain in palliative care patients. We are unable to comment on important clinical issues such as optimal treatment parameters, as there were insufficient data for analysis.
There have been no previous systematic reviews on TENS in cancer pain and only one review paper has been published (Pan 2000). This paper reviewed the use of complementary and alternative medicine to manage pain and other symptoms associated with end of life. Four studies on TENS were discussed (Avellanosa 1982; Gadsby 1997; Ostrowski 1979; Wen 1977), one of which was included in our review (Gadsby 1997). Pan 2000 concluded that TENS, along with a range of other interventions, may provide pain relief in palliative patients with pain but acknowledged that there is a paucity of data to support this. A major criticism of the majority of studies found in the literature search is that they were mostly case‐series or non‐randomised studies and the bulk of these studies were published in the 1970s and 1980s.
Summary of main results
In summary, there is insufficient evidence to judge whether TENS should be used in adults with cancer‐related pain. Further research is needed to improve knowledge in this field. Bennett 2010 identified pain relief on movement as the most appropriate outcome for future research and calculated the sample size required for further research.
Quality of the evidence
All three RCTs found in the literature search were undersized and lacked sufficient power to detect significant differences. Bennett 2010 was designed as a feasibility study rather than a powered clinical trial. Robb 2007 performed power calculations but failed to recruit a sufficient number of participants, whereas Gadsby 1997 did not perform any power calculations. Although Gadsby 1997 maintained double blind conditions, adequate blinding was not maintained in Robb 2007 and Bennett 2010. As pain is a highly subjective symptom, the inadequacy of blinding introduces considerable risk of bias.
Authors' conclusions
Implications for practice.
The evidence from three RCTs provides insufficient evidence to judge whether TENS should be used to manage cancer‐related and cancer treatment‐related pain.
Implications for research.
Large multi‐centre RCTs are required to assess the value of TENS in the management of cancer‐related pain. Attention should be given to:
power calculations to ensure adequate sample sizes;
selection of participants to ensure homogeneity of pain conditions under study;
consideration of impact on pain at rest and on movement;
optimal stimulation parameters and treatment schedules;
use of valid, reliable outcome measures to assess all dimensions of pain for example NRS of pain intensity and pain relief;
short and long‐term follow‐ups; and
cost analysis in comparison to standard treatment i.e. medications.
What's new
| Date | Event | Description |
|---|---|---|
| 9 April 2015 | Review declared as stable | A search for studies is not likely to identify potentially relevant studies. The authors and editors class this review as 'stable'. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 16 November 2011 | New citation required but conclusions have not changed | One additional RCT (Bennett 2010) containing data from 24 participants was added to this update which previously contained two RCTs. |
| 16 November 2011 | New search has been performed | The search for this review was brought up to date in November 2011. |
Acknowledgements
We would like to acknowledge the work of: Sheila Leadbeater, Senior NHS Librarian in the Leeds General Infirmary in developing our search strategy; Jessica Thomas, Cochrane Pain, Palliative and Supportive Care Managing Editor for her ongoing support; and Phil Wiffen, former Co‐ordinating Editor of the Cochrane Pain, Palliative and Supportive Care Review Group for his feedback on the protocol.
Appendices
Appendix 1. MEDLINE (via OVID) search strategy
1. Electric Stimulation Therapy/
2. (electric and stimulation).mp.
3. electrostimulation.mp.
4. electroanalgesi*.mp.
5. electrotherap*.mp.
6. electromagneti*.mp.
7. interferential.mp.
8. rebox.mp.
9. codetron.mp.
10. likon.mp.
11. TNS or ENS or TENS).mp.
12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13. exp Neoplasms/
14. (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinom* or oncolo*).mp.
15. 13 or 14
16. exp Pain/
17. pain*.mp.
18. 16 or 17
19. 12 and 15 and 18
Appendix 2. Other search strategies
1 CENTRAL search
| Search terms |
| 1. MeSH descriptor Electric Stimulation Therapy explode all trees 2. electric and stimulation 3. electrostumulation 4. electroanalgesi* 5. electrotherap* 6. electromagneti* 7. interferential 8. rebox 9. codetron 10. likon 11. TNS or ENS or TENS 12. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) 13. MeSH descriptor Neoplasms explode all trees 14. cancer* or tumor* or tumour* or malignan* or neoplas* or carcinom* or oncolo* 15. (#13 OR #14) 16. MeSH descriptor Pain explode all trees 17. pain* 18. (#16 OR #17) 19. (#12 AND #15 AND #18) |
2 EMBASE Search
| Search terms |
| 1 exp electrostimulation therapy/ 2 (electric and stimulation).mp. 3 electrostimulation.mp. 4 electroanalgesi*.mp. 5 electrotherap*.mp. 6 electromagneti*.mp. 7 interferential.mp. 8 rebox.mp. 9 codetron.mp. 10 likon.mp. 11 (TNS or ENS or TENS).mp. 12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 exp neoplasm/ 14 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinom* or oncolog*).mp. 15 13 or 14 16 exp pain/ 17 pain*.mp. 18 16 or 17 19 12 and 15 and 18 |
3 AMED Search
| Search terms |
| 1. electric stimulation/ 2. transcutaneous electric nerve stimulation/ 3. (electric and stimulation).mp. 4. electrostimulation.mp. 5. electroanalgesi*.mp. 6. electrotherap*.mp. 7. electromagneti*.mp. 8. interferential.mp. 9. rebox.mp. 10. codetron.mp. 11. likon.mp. 12. (TNS or ENS or TENS).mp. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp neoplasms/ 15. (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinom* or oncolo*).mp. 16. 14 or 15 17. exp pain/ 18. pain*.mp. 19. 17 or 18 20. 13 and 16 and 19 |
4 Search of CINAHL
| Search terms |
| 1.transcutaneous adj electric adj nerve adj stimulation 2. transcutaneous‐electric‐nerve‐stimulation.de. 3. tns 4. electric‐stimulation.de. 5. electric adj stimulation adj therapy 6. percutaneous adj electric adj nerve adj stimulation 7. electric adj stimulation 8. electrostimulation 9. electroanalgesi$ 10. electrothera$ 11. electromagneti$ 12. electrotherapy#.w..de. 13. interferential 14. rebox 15. codetron 16. likon 17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. cancer 19. cancer‐patients.de. 20. cancer adj pain 21. cancer‐pain.de. 22. neoplasm 23. neoplasms#.w..de. 24. tumour 25. carcinoma#.w..de. 26. carcinoma 27. oncolo$ 28. malignan$ 29. tumor 30. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. pain 32. pain#.w..de. 33. pain adj measurement 34. pain‐measurement.de. 35. pain adj scale 36. 31 or 32 or 33 or 34 or 35 37. adult.de. or middle‐age or aged.w..de. or aged‐80‐and‐over 38. clinical adj trial 39. controlled adj clinical adj trial 40. evaluation 41. prospective 42. meta‐analysis 43. randomised adj controlled adj trial 44. validation 45. random adj allocation 46. experimental‐studies#.de. or clinical‐trials#.de. 47. clinical adj research 48. clinical‐research#.de. 49. 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 50. 17 and 30 and 36 51. 17 and 30 and 36 and 49 52. 51 and 37 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennett 2010.
| Methods | Randomised, placebo‐controlled crossover feasibility study Randomised to active then placebo TENS or placebo then active TENS Sample size: 24 patients randomised, 19 received both applications, 10 in active then placebo arm, 9 placebo then active arm Active or placebo TENS applied to site of bone pain by a medical researcher for continuous 60 minute period After 2 to 7 days placebo or active then applied for 60 minutes Outcome measures recorded at baseline, after 30 minutes and sixty minutes by the patient and research nurse observer both of whom were blinded, telephone follow up after 48 hours. Patient satisfaction and preference questionnaires on completion of study. SF‐MPQ completed before and after active/placebo TENS application |
|
| Participants | Patients with cancer referred to specialist palliative care services in 2 UK cities Inclusion criteria: over 18 years of age, diagnosis of any cancer with bone metastases, radiological evidence of bone metastases, estimated survival of more than 4 weeks, pain intensity of at least 3 out of 10 on NRS at rest and /or movement Exclusion criteria: unable to complete patient related information on entry, no ongoing cancer and TENS contraindicated as per UK Chartered Society of Physiotherapists Guidance for the Clinical Use of Electrophysical Agents, significant change (increase or decrease in opioid dose of 30% or more or addition or removal of co analgesic) to analgesic medication within 48hours of baseline Mean age (years) 72 (median 74 range: 40‐9) Male: 18, female: 6 Primary cancer: prostate (n = 12), breast (n = 5), lung (n = 3), thyroid (n = 1), renal (n = 1). other (n = 1) ECOG performance: 1 (n = 3), 2 (n = 11), 3 (n = 9), 4 (n = 1) Previous and current treatment: radiotherapy (n = 19), bisphosphonates (n = 8), strong opioids (n = 21), paracetamol (n = 15) NSAID (n = 7) strong opioid and paracetamol or NSAID (n = 16) Median 193 days between radiotherapy and randomisation Dropouts: After 1st treatment (n = 5) withdrawal due to deteriorating performance status unrelated to TENS (n = 3), withdrawal due to pain during TENS (n = 2), pain during active TENS (n = 1), pain during placebo (n = 1) |
|
| Interventions | Single channel TENS device and 2 self adhering hypoallergenic gel pads approximately 5 x 5 cm in size placed between 5 and 10 cm apart over area of pain on an area of skin in good condition without signs of altered sensation Parameters: continuous pulse pattern, pulse width: 200 microseconds, pulse frequency 80Hz, intensity increased until TENS sensation strong but comfortable Duration; 2 x 60 min, once placebo, once active, 2‐7 days between treatments |
|
| Outcomes | NRS pain relief and pain intensity, VRS pain relief and pain intensity at baseline 30 minutes and 60 minutes, SF‐MPQ before and after application, after second application: patient satisfaction questionnaire (TENS beneficial, TENS easy to use, most impact at rest or on movement), which application provided most benefit, which outcome scale best represented experience of pain intensity and relief | |
| Notes | Quality: 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized via the Clinical Trials Research Unit (University of Leeds) central randomization system, using stratified permuted block randomization" |
| Allocation concealment (selection bias) | Low risk | Comment: A "central randomization system" was used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: 'After both TENS applications had been completed, 11 out of 19 patients thought that placebo TENS was used in the study and of these 11 patients, 10 correctly identified the placebo. Blinding was judged by the research nurse observer to have been successfully concealed to them in 15 of 19 patients (i.e. the patient did not reveal the sensation that they were experiencing)." "The medical researcher applying the TENS device was not blind to the intervention but did not take part in patient assessments." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "After both TENS applications had been completed, 11 out of 19 patients thought that placebo TENS was used in the study and of these 11 patients, 10 correctly identified the placebo. Blinding was judged by the research nurse observer to have been successfully concealed to them in 15 of 19 patients (i.e. the patient did not reveal the sensation that they were experiencing)." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The medical researcher applying the TENS device was not blind to the intervention but did not take part in patient assessments." "Blinding was judged by the research nurse observer to have been successfully concealed to them in 15 of 19 patients (i.e. the patient did not reveal the sensation that they were experiencing)." Comment: the research nurse observer was the assessor and may have been unblinded in some participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five patients withdrew from the study after first treatment. Three were withdrawn by their clinician due to deteriorating performance status (deemed unrelated to the TENS application) and 2 patients withdrew because of increased pain during TENS application: 1 following active TENS and 1 following placebo TENS." Comment: all those who did complete were excluded from primary analysis. The missing outcome data are balanced in numbers between intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: although the protocol was not available, the study reports on all pre‐specified and relevant outcomes |
Gadsby 1997.
| Methods | Double‐blind RCT Participants were allocated to active AL‐TENS (Gp 1), placebo AL‐TENS (Gp 2) or no treatment (Gp 3) Sample size: total 15 were randomised (Gp 1 n = 5; Gp 2 n = 3; Gp 3 n = 5) Follow‐up: none TENS administered by nurse practitioner (author) Outcome measures at baseline and on day 6. Retrospective analysis of analgesic and anti‐emetic use on day 6. Daily biophysical measurements of body electrical resistance No reporting of adverse effects | |
| Participants | Inclusions: admitted for symptom control; aged 35‐75; pain and/or nausea and vomiting symptoms; Caucasian origin Exclusions: unwilling to provide informed consent; too ill to cope with 30 mins treatment; patients with an on‐demand pacemaker, premenopausal women, patients with vomiting due to intestinal obstruction or raised intracranial pressure or iatrogenic causes, patients previously treated with TENS or AL‐TENS. Gender: 14 females, 1 male. Age range 38‐74 years. All terminal cancer; diagnoses: breast (n = 6), colon (n = 3), pancreas (n = 2), stomach (n = 1), cervical (n = 1) Dropouts: n = 2; both in placebo group, due to a rapid deterioration in their condition | |
| Interventions | AL‐TENS and placebo delivered via 2 gelled carbon electrodes, sealed with tape: one to acupuncture point Pe6 (Neiguan) and one to L14 (Hegu) of dominant hand. Leads attached to V‐TENS stimulator Electrical parameters: pulse rate: 2 Hz, symmetrical biphasic pulsewave in continuous mode; pulse width: 200 ms; amplitude: 2.5 Duration of treatment: 30 minutes; frequency: 5/day | |
| Outcomes | EORTC QOL‐C30 at baseline and on Day 6. Includes dimensions on pain, nausea and vomiting and fatigue, global quality of life and 5 functional scales. Retrospective assessment of analgesic and anti‐emetic use over study period at Day 6 | |
| Notes | Quality: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated trial therapies, via the sealed‐envelope method of colour coded allocation cards to receive active ALTENS, placebo ALTENS or no ALTENS ('no ALTENS' standard control)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: sealed envelopes were used although it is not clear if they were opaque and sequentially numbered. Nor is it clear if enrolling investigators were aware of there content of the envelope prior to assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The high electrical skin resistance of these patients appeared, on questioning the patient for the level of comfort by the trialist, to be blocking the sensory stimulation of the ALTENS from reaching conscious awareness. This blocking of sensory awareness and the communication of such between patient and trialist also helped to reduce the risk of operator bias." "The output leads were colour‐tagged ‐ real and placebo ‐ and the code changed at bi‐weekly intervals during the study in order to help maintain the double blind element. This was undertaken by a second independent observer who kept a record of the codes throughout the trial." Comment: interventions were randomised and colour coded, the investigator applying the interventions did not change or record the changing colour codes. Patients were unable to distinguish between the sensation of active TENS and placebo and therefore were not unblinded and could not unblind the investigator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: neither patients not investigator applying treatment were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the investigator applying the interventions also recorded outcomes. This investigator was blind as to the nature of the intervention. A second investigator kept a record of the colour codes denoting treatment and placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: two patients in the placebo arm were unable to complete the outcome assessment due to rapidly deteriorating health. It was not reported how missing data was handled in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study does not report on all pre‐specified outcomes. It reports pain, nausea and vomiting, fatigue and global quality of life scores but does not report on the functional scales and retrospective drug use over the trial period. The main outcome of interest in this review is pain, which is reported |
Robb 2007.
| Methods | Randomised, placebo‐controlled crossover trial design Participants stratified according to level of average pain prior to randomisation Randomised to 1 of 6 groups for TENS, TSE and placebo TSE Sample size: total 49 were randomised (group 1: 9; group 2: 6; group 3: 9; group 4: 10; group 5: 7; group 6: 8) Treatment duration: 3 weeks of each treatment (12 weeks) with 3 x 1 week breaks in between treatments Follow‐up: 3, 6 and 12 months TENS administered by researcher in clinic and taught to subjects to use at home Self‐report pain and mood questionnaires completed at baseline then weekly thereafter during intervention, then at 3, 6 and 12 months Pain diaries completed daily during intervention Objective measures of shoulder mobility completed by researcher at baseline and at the end of every arm of the trial Self‐report satisfaction questionnaire on completion of the trial | |
| Participants | Inclusions: history of breast cancer and chronic pain for at least 6 months due to cancer treatment Exclusions: under 18 years of age, evidence of recurrent disease, cognitive deficits, pain due to a neurological deficit, absence of skin sensation in the painful area, previous experience of TENS Gender: all female 50% had pain secondary to surgery, 20% has pain secondary to radiotherapy, 30% had a combination Mean age: 58 years (med: 59 range: 38‐60) Mean duration of pain: 51 months (med: 31 range: 6‐182) Majority were Caucasian (87%), married (61%) and in employment (44%) Dropouts: n = 8 (pain increased: n = 2; pain resolved: n = 2; skin reaction: n = 1; other: n = 3). | |
| Interventions | Concurrent treatment: subjects permitted to continue with all current medications but not permitted to start any new treatments during the trial TENS: dual channel stimulator with self‐adhesive pads (Spembly Medical Ltd). Amplitude adjusted to provide a "strong but comfortable" tingling sensation Continuous mode. Pulse width: unknown. Pulse frequency: high (subject adjusted according to comfort). Electrode placement: in area of pain or adjacent dermatome. Two or four electrodes according to size of area. Treatment schedule: as determined by subject, advised on > 1 hour duration; frequency: as determined by pain TSE: single channel stimulator with self‐adhesive pads (Advanced Pain Management Ltd). Pulse frequency: 2000 Hz. Electrode placement: 2 pads para‐vertebrally at C3‐4 level for pain in the neck, arm or hand. Two pads over spinous processes of T1 and T10 for all pain below the neck. Treatment duration: 10‐30 minutes; frequency: as determined by pain Placebo: procedure as for TSE | |
| Outcomes | BPI short form: measured at baseline then weekly thereafter whilst receiving treatment. Post‐treatment measurement at 3, 6 and 12 months HAD: measured as above Range of movement at the ipsilateral shoulder joint (flexion and abduction): measured with a goniometer at baseline and at the end of each intervention Pain diaries documented daily by the subjects: pain relief and analgesic consumption Patient satisfaction questionnaire to evaluate satisfaction with each treatment: recorded on completion of the trial Adverse effects like skin irritation and increased pain were monitored throughout | |
| Notes | Quality: 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized with the help of a trial assistant and a computer‐generated random number chart.". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided regarding allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Overall, few women thought they knew which was the placebo arm." "Although blinding procedures were in place, assessments were not blinded and the assessor knew when a TENS machine was supplied." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Overall, few women thought they knew which was the placebo arm." Comment: the number of women accurately identified placebo was not provided. Nor is it commented if trial staff identified placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Although blinding procedures were in place, assessments were not blinded and the assessor knew when a TENS machine was supplied." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Forty‐nine women commenced the trial and 41 completed the study. The reasons for non completion were pain increased (n = 2), pain resolved (n = 2), skin reaction (n =1), and other (n = 3)." Comment: although it is stated why participants did not complete the distribution across study groups is not provided, the point in the study schedule at which participants left is not stated, nor is it described how these participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐specified and anticipated outcomes were reported on |
AL‐TENS: Acupuncture‐like TENS BPI: Brief Pain Inventory C3‐4: cervical spine level 3‐4 EORTC QOL‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Gp: group HAD: Hospital Anxiety and Depression Scale Hz: hertz mins: minutes ms: microseconds n: number RCT: randomised controlled trial SF‐MPQ: Short‐Form McGIll Pain Questionnaire T1: thoracic spine level 1 T10: thoracic spine level 10 TENS: Transcutaneous electrical nerve stimulation TSE: Transcutaneous spinal electroanalgesia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avellanosa 1982 | Non‐randomised study |
| Bild 1990 | Non‐randomised study |
| Bonakdar 2004 | No clinical data |
| Cata 2004 | Non‐randomised study |
| Cooperman 1975 | Non‐randomised study, not cancer‐related pain |
| Crompton 1992 | Non‐randomised study, not cancer‐related pain |
| De‐Pinto 2006 | No clinical data |
| Dil'Din 1985 | Non‐randomised study |
| Evtiukhin 1998 | Non‐randomised study |
| Hakl 1989 | Non‐randomised study |
| Hamza 1999 | Not cancer‐related pain |
| Hasun 1988 | Non‐randomised study |
| Hidderley 1997 | Cancer patients were not randomised in the main clinical trial |
| Kim 2005 | No clinical data |
| Kleinkort 2005 | Non‐randomised study, not cancer related pain |
| Lamer 1994 | No clinical data |
| Lange 1995 | No clinical data |
| Librach 1988 | No clinical data |
| Long 1991 | No clinical data |
| McCaffery 1992 | No clinical data |
| Miguel 2000 | No clinical data |
| Naveau 1992 | Acute, not chronic treatment‐related pain |
| Oosterwijk 1994 | No clinical data |
| Ostrowski 1979 | Non‐randomised study |
| Pan 2000 | No clinical data |
| Patt 1990 | No clinical data |
| Patt 1992 | No clinical data |
| Picaza 1975 | Non‐randomised study, not cancer related pain |
| Rafter 1986 | Not an RCT |
| Reuss 1985 | Non‐randomised study |
| Robb 2003 | No clinical data |
| Robb 2004 | No clinical data |
| Rutkowski 1980 | Non‐randomised study |
| Sang 2003 | Non‐randomised study |
| Sharp 2003 | No clinical data |
| Sima 2009 | Investigation of percutaneous electrical stimulation |
| Sloan 2004 | No clinical data |
| Tonkin 1998 | No clinical data |
| Urba 1996 | No clinical data |
| Ventafridda 1979 | Non‐randomised study |
| Weinstein 1994 | No clinical data |
| Wen 1977 | Non‐randomised study |
Contributions of authors
Background: KR, MJ
Protocol: SO
Methods: SO, KR
Search strategy: SO, KR
Paper collection: HR
Review of studies (appraisal/quality/data extraction): KS, MB, MJ, SO
Statistical analysis: SO, KR, MB, MJ, KS
Discussion: All
Final production: KR
Contact for Cochrane: KR
Group co‐ordinator: SO, KR
Update 2011: AH, MB
Responsibility for future updates: MB
Sources of support
Internal sources
Barts and the London NHS Trust and Tower Hamlets PCT, UK.
External sources
No sources of support supplied
Declarations of interest
Karen Robb and Michael I Bennett are lead authors of included studies in this review; the remaining authors are not aware of any conflicts with this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bennett 2010 {published data only}
- Bennett MI, Johnson MI, Brown S, Searle RD, Radford H, Brown JM. Feasibility study of Transcutaneous Electrical Nerve Stimulation (TENS) for cancer bone pain. Journal of Pain 2010;11(4):351‐9. [DOI] [PubMed] [Google Scholar]
Gadsby 1997 {published data only}
- Gadsby JG, Franks A, Jarvis P, Dewhurst F. Acupuncture‐like transcutaneous electrical nerve stimulation within palliative care: a pilot study. Complementary Therapies in Medicine 1997;5:13‐8. [Google Scholar]
Robb 2007 {published data only}
- Robb KA, Newham DJ, Williams JE. Transcutaneous electrical nerve stimulation vs. transcutaneous spinal electroanalgesia for chronic pain associated with breast cancer treatments. Journal of Pain and Symptom Management 2007;33(4):410‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avellanosa 1982 {published data only}
- Avellanosa AM, West CR. Experience with transcutaneous electrical nerve stimulation for relief of intractable pain in cancer patients. Journal of Medicine 1982;13(3):203‐13. [PubMed] [Google Scholar]
Bild 1990 {published data only}
- Bild E, Rosu V. The treatment of cancer. The contribution of transcutaneous electrical nerve stimulation. Revista medico‐chirurgicala a societatii de medici si naturalasti din iasi (iasi) 1990;94(2):407‐10. [PubMed] [Google Scholar]
Bonakdar 2004 {published data only}
- Bonakdar R, Bresler DE, Borwick J. Do CAM therapies work for pain management?. Patient Care for the Nurse Practitioner 2004;September:9. [Google Scholar]
Cata 2004 {published data only}
- Cata JP, Cordella JV, Burton AW, Hassenbusch SJ, Weng H, Dougherty PM. Spinal cord stimulation relieves chemotherapy induced pain: a clinical case report. Journal of Pain and Symptom Management 2004;27(1):72‐8. [DOI] [PubMed] [Google Scholar]
Cooperman 1975 {published data only}
- Cooperman AM, Hall B, Sadar ES, Hardy Jr RW. Use of transcutaneous electrical stimulation in control of post‐operative pain. Surgical Forum 1975;26:77‐8. [PubMed] [Google Scholar]
Crompton 1992 {published data only}
- Crompton AC, Johnson N, Dudek U, Batra N, Tucker A. Is transcutaneous electrical nerve stimulation of any value during cervical laser treatment. British Journal of Obstetrics and Gynaecology 1992;99(6):492‐4. [DOI] [PubMed] [Google Scholar]
De‐Pinto 2006 {published data only}
- De‐Pinto M, Dunbar PJ, Edwards WT. Pain management. Anesthesiology Clinics of North America 2006;24(1):19‐37. [DOI] [PubMed] [Google Scholar]
Dil'Din 1985 {published data only}
- Dil'Din AS, Tikhonova GP, Kozlov SV. Transcutaneous electrostimulation‐method leading to a permeation system of electroanalgesia in oncology patients. Voprosy Onkologii 1985;31:33‐6. [PubMed] [Google Scholar]
Evtiukhin 1998 {published data only}
- Evtiukhin AI, Dunaeyskii IV, Shabut AM, Aleksandrov VA. The use of transcutaneous electrostimulation for pain relief in cancer patients. Voprosy Onkologii 1998;44(2):229‐33. [PubMed] [Google Scholar]
Hakl 1989 {published data only}
- Hakl L, Umlauf R, Haklova O, Zahradka J. Promoting analgesia in cancer sufferers by using TENS through electroacupuncture at D14 and M36 points. Deutsche Zeitschrift Akupunk 1989;32:10‐21. [Google Scholar]
Hamza 1999 {published data only}
- Hamza MA, White PF, Ahmed HE, Ghoname EA. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 1999;91(5):1232‐8. [DOI] [PubMed] [Google Scholar]
Hasun 1988 {published data only}
- Hasun R, Marberger M. Transdermal electric pain control in urologic cancer patients. Schmerz Pain Douleur 1988;9(3):234‐8. [Google Scholar]
Hidderley 1997 {published data only}
- Hidderley M, Weinel E. Clinical practice. Effects of TENS applied to acupuncture points distal to a pain site. International Journal of Palliative Nursing 1997;3(4):185‐8, 190‐1. [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
Kleinkort 2005 {published data only}
- Kleinkort JA. Low‐level laser therapy: new possibilities in pain management and rehab. Orthopaedic Physical Therapy Practice 2005;17(1):48‐51. [PubMed] [Google Scholar]
Lamer 1994 {published data only}
- Lamer TJ. Treatment of cancer‐related pain: when orally administered medications fail. Mayo Clinic Proceedings 1994;69(5):473‐80. [DOI] [PubMed] [Google Scholar]
Lange 1995 {published data only}
- Lange A. Physiotherapy in cancer treatment ‐ therapy for tumour or therapy limited to functional restriction due to malignant disease [Physiotherapie in der Onkologie ‐ Behandlung von tumor oder therapie‐bedingten Funktions ‐ storungen bei malignen Erkrankungen]. Krankengymnastique 1995;47(3):338. [Google Scholar]
Librach 1988 {published data only}
- Librach SL. The use of TENS for the relief of pain in palliative care. Palliative Medicine 1988;2:15‐20. [Google Scholar]
Long 1991 {published data only}
- Long DM. Fifteen years of Transcutaneous Electrical Stimulation for pain control. Stereotactic and Functional Neurosurgery 1991;56(1):2‐19. [DOI] [PubMed] [Google Scholar]
McCaffery 1992 {published data only}
- McCaffery M, Wolff M. Pain relief using cutaneous modalities, positioning and movement. Hospice Journal 1992;8(1/2):121‐53. [DOI] [PubMed] [Google Scholar]
Miguel 2000 {published data only}
- Miguel R. Interventional treatment of cancer pain: the fourth step in the WHO analgesic ladder. Cancer Control 2000;7(2):149‐56. [DOI] [PubMed] [Google Scholar]
Naveau 1992 {published data only}
- Naveau S, Barritault L, Zourabichvili O, Champagne C, Prieur G, Limoge A, et al. Analgesic effect of transcutaneous cranial electrostimulation in patients treated by Nd: YAG laser for cancer of the rectum. A double‐blind randomised trial. Gastroenerologie Clinique et Biologique 1992;16(1):8‐11. [PubMed] [Google Scholar]
Oosterwijk 1994 {published data only}
- Oosterwijk RFA, Meyler WJ, Henley EJ, Scheer SS, Tannenbaum J. Pain control with TENS and team nerve stimulators: a review. Critical Reviews in Physical and Rehabilitation Medicine 1994;6(3):219‐57. [Google Scholar]
Ostrowski 1979 {published data only}
- Ostrowski MJ. Pain control in advanced malignant disease using transcutaneous nerve stimulation. The British Journal of Clinical Practice 1979;33(6):157‐62. [PubMed] [Google Scholar]
Pan 2000 {published data only}
- Pan CX, Morrison RS, Ness J, Fugh‐Berman A, Leipzig RM. Complementary and alternative medicine in the management of pain, dyspnoea and nausea and vomiting near the end of life: a systematic review. Journal of Pain and Symptom Management 2000;20(5):374‐87. [DOI] [PubMed] [Google Scholar]
Patt 1990 {published data only}
- Patt RB. Non‐pharmacologic measures for controlling oncologic pain. The American Journal of Hospice and Palliative Care 1990;7(7):30‐7. [DOI] [PubMed] [Google Scholar]
Patt 1992 {published data only}
- Patt RB. Non‐pharmacologic measures for controlling oncologic pain. The American Journal of Hospice and Palliative Care 1992;9(6):41‐7. [DOI] [PubMed] [Google Scholar]
Picaza 1975 {published data only}
- Picaza JA, Cannon BW, Hunter SE. Pain suppression by peripheral nerve stimulation. Part 1. Observations with transcutaneous stimuli. Surgical Neurology 1975;4(4):105‐14. [PubMed] [Google Scholar]
Rafter 1986 {published and unpublished data}
- Rafter J. TENS and cancer pain. Paper read to the Acupuncture Foundation of Canada on acupuncture and related techniques. November 1986.
Reuss 1985 {published data only}
- Reuss R, Meyer SC. The use of TENS in the management of cancer pain. Physical Therapy and Clinical Management 1985;5(5):26‐8. [Google Scholar]
Robb 2003 {published data only}
- Robb K. Chronic pain management in breast cancer patients. Chartered Society of Physiotherapy Annual Conference Proceedings. 2003:125.
Robb 2004 {published data only}
- Robb KA. Managing chronic pain in breast cancer survivors. PPA News 2004;17:26‐7. [Google Scholar]
Rutkowski 1980 {published data only}
- Rutkowski B, Kaczor T, Niedzialkowska T, Otto J. Electroanalalgesia in the treatment of pain in oncologic patients. Polski Tygodnik Lekarski 1980;35(48):1865‐6. [PubMed] [Google Scholar]
Sang 2003 {published data only}
- Sang CN, Max MB, Gracely RH. Stability and reliability of detection thresholds for human A‐beta and A‐delta sensory afferents determined by cutaneous electrical stimulation. Journal of Pain and Symptom Management 2003;25(1):64‐73. [DOI] [PubMed] [Google Scholar]
Sharp 2003 {published data only}
- Sharp SM. Placebo controls in the evaluation of medical devices. Research Practitioner 2003;4(4):134‐43. [Google Scholar]
Sima 2009 {published data only}
- Sima L, Yin C. Efficacy of electroacupuncture for bone metastatic cancer patients with neuropathic pain: a randomized controlled trial. Journal of Clinical Oncology 2009;27(15s):9534. [Google Scholar]
Sloan 2004 {published data only}
- Sloan PA. The evolving role of interventional pain management in oncology. Journal of Supportive Oncology 2004;2(6):491‐503. [PubMed] [Google Scholar]
Tonkin 1998 {published data only}
- Tonkin J. Breast cancer: easing the pain. Physiotherapy Frontline 1998;4(5):18. [Google Scholar]
Urba 1996 {published data only}
- Urba SG. Non‐pharmacologic pain management in terminal care. Clinics in Geriatric Medicine 1996;12(2):301‐11. [PubMed] [Google Scholar]
Ventafridda 1979 {published data only}
- Ventafridda V. TENS in cancer pain. Advances in Pain Research and Therapies 1979;2:509‐15. [Google Scholar]
Weinstein 1994 {published data only}
- Weinstein SM. Cancer pain. Physical Medicine and Rehabilitation 1994;8(2):279‐96. [Google Scholar]
Wen 1977 {published data only}
- Wen HL. Cancer pain treated with acupuncture and electrical stimulation. Modern Medicine of Asia 1977;13:12‐6. [Google Scholar]
Additional references
Bjordal 2003
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous Electrical Nerve Stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for post‐op pain. European Journal of Pain 2003;7:181‐8. [DOI] [PubMed] [Google Scholar]
Brosseau 2003
- Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, et al. TENS for the treatment of rheumatoid arthritis in the hand. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004377] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeSantana 2008
- DeSantana J, Walsh DM, Vance C, Rakel B, Sluka KA. Effectiveness of Transcutaneous Electrical Nerve Stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10(6):492‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowswell 2009
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filshie 2000
- Filshie J, Thompson JW. Acupuncture and TENS. Cancer pain management: a comprehensive approach. Oxford: Oxford University Press, 2000. [Google Scholar]
Jadad 1996
- Jadad JA, Moore AR, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Johnson 2001
- Johnson MI. Transcutaneous Electrical Nerve Stimulation (TENS) and TENS‐like devices: do they provide pain relief?. Pain Reviews 2001;8(3‐4):121‐58. [Google Scholar]
Johnson 2008a
- Johnson M. Transcutaneous Electrical Nerve Stimulation. In: Watson T editor(s). Electrotherapy: Evidence based practice. 12th Edition. Edinburgh: Churchill Livingstone, 2008. [Google Scholar]
Johnson 2008b
- Johnson M, Oxberry S, Robb K. Stimulation‐induced analgesia. In: Sykes N, Bennett M, Yuan CS editor(s). Cancer Pain. Vol. 2nd, London: Hodder Arnold, 2008. [Google Scholar]
Johnson 2011
- Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Review of Neurotherapeutics 2011;11(5):735‐53. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones I, Johnson MI. Transcutaneous electrical nerve stimulation. Continuing Education in Anaesthesia, Critical Care & Pain 2009;9(4):130‐5. [Google Scholar]
Khadilkar 2008
- Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low‐back pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003008.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroeling 2009
- Kroeling P, Gross A, Goldsmith CH, Burnie SJ, Haines T, Graham N, et al. Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004251.pub4] [DOI] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971‐99. [DOI] [PubMed] [Google Scholar]
Mulvey 2010
- Mulvey MR, Bagnall A‐M, Johnson MI, Marchant PR. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007264.pub2] [DOI] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003222.pub2] [DOI] [PubMed] [Google Scholar]
Proctor 2009
- Proctor M, Farquhar C, Stones W, He L, Zhu X, Brown J. Transcutaneous electrical nerve stimulation for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raphael 2010
- Raphael J, Hester J, Ahmedzai S, Barrie J, Farqhuar‐Smith P, Williams J, et al. Cancer pain: part 2: physical, interventional and complimentary therapies; management in the community; acute, treatment‐related and complex cancer pain: a perspective from the British Pain Society endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain Medicine 2010;11:872–96. [DOI] [PubMed] [Google Scholar]
Reeve 1996
- Reeve J, Menon D, Corabian P. Transcutaneous Electrical Nerve Stimulation: a technology assessment. International Journal of Technology Assessment in Healthcare 1996;12:299‐324. [DOI] [PubMed] [Google Scholar]
Rutjes 2009
- Rutjes AWS, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Searle 2008
- Searle RD, Bennett MI, Johnson MI, Callin S, Radford H. Letter to editor: transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. Palliative Medicine 2008;22:878‐9. [DOI] [PubMed] [Google Scholar]
Simpson 2000
- Simpson K. Philosophy of cancer pain management. Cancer Pain Management: A Practical Approach. Vol. 1, Oxford: Oxford University Press, 2000. [Google Scholar]
Turk 1998
- Turk DC. Adaptation to metastatic cancer pain, regional/local cancer pain and non cancer pain: role of psychological and behavioural factors. Pain 1998;74:247‐56. [DOI] [PubMed] [Google Scholar]
Walsh 1997
- Walsh DM. TENS: clinical applications and related theory. 1st Edition. Edinburgh: Churchill Livingstone, 1997. [Google Scholar]
Walsh 2006
- Walsh DM, Howe TE, Johnson MI, Moran F, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006142.pub2] [DOI] [PubMed] [Google Scholar]


