Key Points
Question
Meta-analyzing nonrandomized cohort studies, how does clozapine compare with other second-generation antipsychotics (SGAs)?
Findings
In this systematic review and meta-analysis of 63 cohort studies comprising 109 341 participants, although more severely ill patients received clozapine, use of clozapine was associated with better effectiveness outcomes regarding hospitalization, all-cause discontinuation, and overall symptoms but with a higher risk of cardiometabolic-related outcomes vs other SGAs.
Meaning
In a more generalizable sample of patients with schizophrenia than in randomized trials, clozapine was statistically and clinically associated with better effectiveness outcomes compared with other SGAs, but some concerns about safety require careful monitoring and clinical attention.
This systematic review and meta-analysis compares various outcomes of clozapine vs oral nonclozapine second-generation antipsychotics in cohort studies of patients with schizophrenia.
Abstract
Importance
Recent meta-analyses of randomized clinical trials (RCTs) comparing clozapine with nonclozapine second-generation antipsychotics (NC-SGAs) in schizophrenia have challenged clozapine’s superiority in treatment-resistant patients. However, patients in RCTs are not necessarily generalizable to those in clinical practice.
Objective
To conduct a systematic review and meta-analysis to compare various outcomes of clozapine vs oral NC-SGAs in cohort studies.
Data Sources
Systematic literature search in PubMed, PsycINFO, and CINAHL without language restriction from database inception until December 17, 2018.
Study Selection
Nonrandomized cohort studies reporting effectiveness and/or safety outcomes comparing clozapine with NC-SGAs in schizophrenia or schizoaffective disorder.
Data Extraction and Synthesis
Independent investigators assessed studies and extracted data. Using a random-effects model, the study calculated risk ratio (RR) unadjusted for covariates and follow-up duration, number needed to treat/number needed to harm (NNT/NNH) for dichotomous data, and standardized mean difference (SMD) or mean difference (MD) for continuous data.
Main Outcomes and Measures
Coprimary outcomes were hospitalization and all-cause discontinuation. Secondary outcomes included all effectiveness and safety outcomes reported in at least 3 analyzable studies.
Results
Of 8446 hits, 68 articles from 63 individual cohort studies (n = 109 341) (60.3% male; mean [SD] age of 38.8 [6.5] years, illness duration of 11.0 [5.1] years, and study duration of 19.1 [23.3] months) were meta-analyzed. Compared with NC-SGAs, despite greater illness severity (17 studies [n = 38 766]; Hedges g, 0.222; 95% CI, 0.013-0.430; P = .04), clozapine was significantly associated with lower hospitalization risk (19 studies [n = 49 453]; RR, 0.817; 95% CI, 0.725-0.920; P = .001; NNT, 18; 95% CI, 12-40) and all-cause discontinuation (16 studies [n = 56 368]; RR, 0.732; 95% CI, 0.639-0.838; P < .001; NNT, 8; 95% CI, 6-12). Associations were statistically significant for comparisons with quetiapine fumarate and aripiprazole regarding hospitalization and all NC-SGAs, except aripiprazole, for all-cause discontinuation. Clozapine was also significantly associated with better outcomes regarding overall symptoms (SMD, −0.302; 95% CI, −0.572 to −0.032; P = .03) and Clinical Global Impressions scale severity (SMD, −1.182; 95% CI, −2.243 to −0.122; P = .03). Clozapine was significantly associated with increases in body weight (MD, 1.70; 95% CI, 0.31-3.08 kg; P = .02), body mass index (MD, 0.96; 95% CI, 0.24-1.68; P = .009), and type 2 diabetes (RR, 1.777; 95% CI, 1.229-2.570; P = .002; NNH, 27; 95% CI, 13-90).
Conclusions and Relevance
In cohort studies, despite more severely ill patients being treated with clozapine, use of clozapine was associated with better key efficacy outcomes and higher cardiometabolic-related risk outcomes vs NC-SGAs.
Introduction
Although antipsychotics are the main treatment for schizophrenia, approximately 30% patients do not respond to 2 or more antipsychotic trials of adequate dosage and duration.1 These patients are considered to have treatment-resistant schizophrenia (TRS).
Clozapine is the criterion standard medication for TRS,2,3,4,5 whose superior effectiveness is supported by a network meta-analysis of acute treatment associations, although the population was not specifically treatment resistant.6 However, recent meta-analyses of masked randomized clinical trials (RCTs) comparing clozapine with other oral nonclozapine second-generation antipsychotics (NC-SGAs) reported inconsistent results.7,8 One network meta-analysis found superiority of clozapine only in TRS compared with first-generation antipsychotics but not NC-SGAs, either individually (ie, olanzapine, risperidone, or ziprasidone hydrochloride) or pooled.7 These surprising results have been challenged as being related to methodological issues, including lower clozapine dosages than in clinical care, and, especially, the possibility of sampling bias in that patients recruited in RCTs are less representative of real-world patients, being less severely ill and having greater illness insight, and thus more likely to respond to NC-SGAs.9 Studies on long-acting injectable (LAI) antipsychotics yielded similarly conflicting findings10 in that LAI antipsychotics were not superior to oral antipsychotics in a meta-analysis of RCTs,11 whereas meta-analyses of mirror-image studies12 and cohort studies,13 expected to better reflect real-world patients and practice than RCTs, exhibited consistent superiority of LAI antipsychotics.
Therefore, real-world effectiveness of clozapine should also be explored beyond RCTs, as in cohort studies. However, cohort studies also have a potential sampling bias in that more severely ill and treatment-resistant patients are more likely treated with clozapine than with other oral NC-SGAs. Nevertheless, this would be a conservative bias, which would strengthen the results if clozapine was superior to NC-SGAs.
Therefore, to complement the meta-analyses of clozapine RCTs7,8 we conducted a meta-analysis of cohort studies comparing clozapine with oral NC-SGAs. We hypothesized that clozapine would be associated with better effectiveness outcomes compared with NC-SGAs, although clozapine-treated patients would have greater illness severity and/or chronicity.
Methods
We conducted a systematic review and meta-analysis of 68 articles from 63 cohort studies. The meta-analysis was performed in accord with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.14
Literature Search
At least 2 independent authors (T.M., F.M., and M.T.) conducted a systematic literature search in PubMed, PsycINFO, and CINAHL without language restriction from database inception until December 17, 2018, using the following search terms: (clozapin* or clozaril* or leponex* or denzapin* or zaponex*) AND (schizophrenia OR schizoaffective OR schizophreniform). The electronic search was supplemented by a manual review of reference lists from eligible publications and relevant reviews.
Inclusion and Exclusion Criteria
At least 2 authors (T.M., F.M., and M.T.) independently screened selected full-text articles. Any disagreements were resolved by consensus or involvement of the senior author (C.U.C). Eligible study characteristics were (1) nonrandomized, prospective, or retrospective cohort studies; (2) at least 4-week duration (even if study duration differed between groups); (3) effectiveness and/or safety outcomes comparing clozapine with oral NC-SGAs (<30% SGA-LAI antipsychotics); (4) at least 15 patients per study; (5) mean age at least 18 years; and (6) at least 70% of participants diagnosed as having schizophrenia, schizoaffective, or schizophreniform disorder.
To clarify the specific association of clozapine, there were some exclusions. We excluded (1) treatment episode–based studies in which 1 patient could be counted in more than 1 medication group and (2) studies starting the observation period after clozapine had been initiated (except for hospitalization-related outcomes where index discharge was the starting point, which can be after clozapine or NC-SGA initiation).
Outcomes
Coprimary outcomes were hospitalization and all-cause discontinuation. We also compared baseline severity of patients receiving clozapine and NC-SGAs in studies reporting on the coprimary outcomes to examine if patients prescribed clozapine are more severely and/or chronically ill, which would inform the interpretation of nondifferential results. Secondary outcomes included all effectiveness and safety outcomes reported in at least 3 analyzable studies.
Data Extraction
At least 2 authors (T.M., F.M., and M.T.) independently extracted data and assessed study quality with the Newcastle-Ottawa Scale (eTable 1 in the Supplement lists the complete data).15 Non–English language articles were translated by bilingual speakers and double-checked using Google Translate.16 Numerical data reported in figures only were extracted with WebPlotDigitizer.17
For missing data, we e-mailed corresponding authors of studies published after 2005. In the event of nonresponse, they were emailed 3 times.
Data Analysis
For all outcomes, data at the longest study period (and for the most included patients in case of overlapping reports) were analyzed, preferring intent-to-treat data. For dichotomous data, we prioritized the Kaplan-Meier estimates (which better account for dropouts) over crude data. Whenever at least 3 studies provided data for one of the coprimary outcomes, we also (1) investigated the effectiveness at specific time points (6, 12, 24, and 36 months), (2) compared clozapine with specific NC-SGAs, (3) assessed the association of adjusting for follow-up duration (person-years [ie, calculating incidence rate ratios {RaRs}]) and performed post hoc analyses by focusing on studies reporting data as (4) adjusted odds ratios (ORs) and (5) hazard ratios (HRs). After comparing clozapine with specific NC-SGAs across all studies, we conducted sensitivity analyses removing studies with unequal observation periods during which an event could have occurred.
For dichotomous data, we calculated risk ratio (RR). When there was a significant difference in RR, we calculated number needed to treat/number needed to harm (NNT/NNH) from an assumed control group risk,18 which we estimated as the mean occurrence of the respective outcome in the NC-SGA group, as done before.7 For continuous data, we calculated the standardized mean difference (SMD) and the mean difference (MD) for cardiometabolic-related outcomes to also display the data as original units that are clinically more intuitive.
When data for multiple NC-SGAs were reported, we first pooled these NC-SGA groups. We then performed individual NC-SGA comparison analyses.
To analyze baseline illness severity (Hedges g), we used 4 data types, as done before in cohort studies of oral vs LAI antipsychotics in schizophrenia13 (choosing in descending order in case of multiple data). These included (1) psychopathology score (baseline Positive and Negative Syndrome Scale,19 Brief Psychiatric Rating Scale,20 or Clinical Global Impressions scale21); (2) prior number of hospitalizations; (3) prior hospitalization days; and (4) the proportion of hospitalized patients at baseline.
We also conducted subgroup and meta-regression analyses of the coprimary outcomes to explore potential moderators and sources of biases. The subgroup analyses were conducted based on (1) baseline setting (inpatients or outpatients); (2) study design (prospective or retrospective); (3) region; (4) data source (single site, multisite, or database); (5) sample specified as treatment resistant or poorly responding (yes vs no/not reported); (6) pharmaceutical sponsorship; and (7) hospitalization cause definition (all cause or mental health). Meta-regression analyses were conducted exploring the following variables: sex (percentage), sample size, publication year, mean age, illness duration, study duration, follow-up duration, study quality (Newcastle-Ottawa Scale score), and baseline illness severity.
Regarding heterogeneity, I2 and χ2 P values are reported. Publication bias was assessed by Egger regression test22 and the trim-and-fill method by Duval and Tweedie.23
All analyses were conducted using a software program (Comprehensive Meta-Analysis, version 3.3.070; Biostat) based on a random-effects model because considerable between-study heterogeneity was expected.24 In this large, exploratory set of analyses, no statistical adjustments were made for multiple comparisons.
Dealing With Missing Change SD. Data
When calculating SMDs and MDs for psychopathology and safety-related outcomes, some studies reported the number, mean, and SD at baseline and end point but not change SD or convertible data to obtain the SD of the change (ie, P, t, and F value within a group). When analyzing body weight, body mass index (BMI), and triglycerides change, we imputed the pooled change SD from other eligible studies because more than half of studies reported change SD18,25 For other outcomes, we imputed change SD using the correlation coefficient of SDs at baseline and end point18 with 3 types of the correlation coefficients (high is 0.9, medium is 0.5, and low is 0.1) and comparing the SMDs and MDs because the exact correlation coefficients were unknown (eTable 2 in the Supplement lists the complete data). Herein, we show medium correlation coefficient–based SMDs and MDs. Whenever that significance level differed from high or low correlation coefficient–based calculations, we detailed that. All tests were 2 sided with α = .05.
Results
The initial database search produced 12 895 hits. After removing 4449 duplicates, 8446 articles were screened, and 8015 were excluded by title and abstract screening. Of the remaining 431 articles and adding 2 articles through manual search, 68 articles from 63 individual cohort studies (n = 109 341) were meta-analyzed (the Preferred Reporting Items for Systematic Reviews and Meta-analyses [PRISMA] flowchart and study references and characteristics are available in eTable 3 and eFigure 1 in the Supplement). Thirty-three studies had a prospective design (n = 34 434), 30 studies had a retrospective design (n = 74 907), and the number of patients per study ranged from 22 to 30 387 (median, 167). The mean (SD) age was 38.8 (6.5) years, 60.3% (13.7%) were male, illness duration was 11.0 (5.1) years, and study duration was 19.1 (23.3) months (range, 1-132 months; median, 12 months). Fourteen (22.2%) of the 63 studies (n = 7229) included patients characterized as treatment-resistant or suboptimal responders.
Main Analyses of Coprimary Outcomes
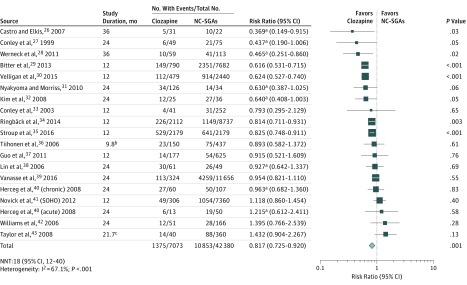
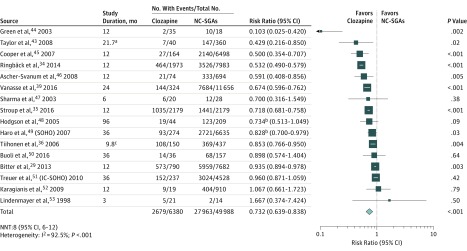
Clozapine was associated with a significantly lower hospitalization risk than NC-SGAs (19 studies26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43 [n = 49 453]; mean [SD] age, 38.8 [5.2] years; mean [SD] study duration, 20.0 (7.9) months; RR, 0.817; 95% CI, 0.725-0.920; P = .001; NNT, 18; 95% CI, 12-40), with significant heterogeneity (I2 = 67.1%; P < .001) (Figure 1). Clozapine was also associated with a significantly lower risk of all-cause discontinuation than NC-SGAs (16 studies29,34,35,36,39,43,44,45,46,47,48,49,50,51,52,53 [n = 56 368]; mean [SD] age, 39.8 [5.0] years; mean [SD] study duration, 23.3 (21.7) months; RR, 0.732; 95% CI, 0.639-0.838; P < .001; NNT, 8; 95% CI, 6-12), with significant heterogeneity (I2 = 92.5%; P < .001) (Figure 2).
Figure 1. Forest Plot of Risk Ratio for Hospitalization26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43.
The mean risk for the nonclozapine second-generation antipsychotics (NC-SGAs) (31.9%) was used as the assumed control group risk. White lines within the pooled risk estimate boxes represent that the corresponding 95% CI is narrower than the pooled effect that depends on the size of the sample. NNT indicates number needed to treat; SOHO, Schizophrenia Outpatient Health Outcomes.
aData from the Kaplan-Meier estimates.
bMean, 9.8 (17.0 for clozapine and 7.3 for NC-SGAs).
cMean, 21.7 (23.2 for clozapine and 21.6 for NC-SGAs).
Figure 2. Forest Plot of Risk Ratio for All-Cause Discontinuation29,34,35,36,39,43,44,45,46,47,48,49,50,51,52,53.
The mean risk for the nonclozapine second-generation antipsychotics (NC-SGAs) (51.7%) was used as the assumed control group risk. White lines within the pooled risk estimate boxes represent that the corresponding 95% CI is narrower than the pooled effect that depends on the size of the sample. IC-SOHO indicates Intercontinental Schizophrenia Outpatient Health Outcomes; NNT, number needed to treat; and SOHO, Schizophrenia Outpatient Health Outcomes.
aMean, 21.7 (23.2 for clozapine and 21.6 for NC-SGAs).
bData from the Kaplan-Meier estimates.
cMean, 9.8 (17.0 for clozapine and 7.3 for NC-SGAs).
Publication Bias
There was no indication of publication bias for both coprimary outcomes. Using the trim-and-fill method by Duval and Tweedie to adjust for potentially missing publications, 1 data point was imputed for hospitalization, but the imputation did not change the results (eFigure 2 in the Supplement).
Baseline Illness Severity
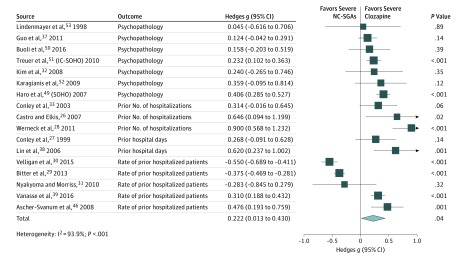
Of 28 studies analyzed for coprimary outcomes, including 7 overlapping studies, 17 provided baseline illness severity and/or chronicity data. Patients receiving clozapine had significantly greater baseline illness severity (17 studies26,27,28,29,30,31,32,33,37,38,39,46,49,50,51,52,53 [n = 38 766]; Hedges g, 0.222; 95% CI, 0.013-0.430; P = .04) (Figure 3).
Figure 3. Forest Plot of Hedges g for Baseline Illness Severity of Studies With Coprimary Outcomes26,27,28,29,30,31,32,33,37,38,39,46,49,50,51,52,53.
IC-SOHO indicates Intercontinental Schizophrenia Outpatient Health Outcomes; NC-SGAs, nonclozapine second-generation antipsychotics; and SOHO, Schizophrenia Outpatient Health Outcomes.
Detailed Analyses of Coprimary Outcomes
Adjusting for follow-up duration by person-year calculation, clozapine was associated with a significantly lower risk than NC-SGAs for hospitalization (11 studies [4526 person-years]; RaR, 0.615; 95% CI, 0.482-0.786; P < .001; NNT, 8; 95% CI, 6-14) and for all-cause discontinuation (5 studies [14 392 person-years]; RaR, 0.542; 95% CI, 0.367-0.802; P = .002; NNT, 5; 95% CI, 3-10). These results are summarized in Table 1 and eFigure 3 in the Supplement.
Table 1. Results of Detailed Analysis of Coprimary Outcomes for Clozapine vs NC-SGAs.
| Outcome | No. of Studies | No. of Patients | Effect Size | Heterogeneity | Effect Size, NNT (95% CI)a | ||
|---|---|---|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | I2, % | P Value | ||||
| Hospitalization (vs Specific NC-SGAs) | |||||||
| Clozapine vs risperidone | 16 | 17 697 | 0.871 (0.745-1.018) | .08 | 61.6 | .001b | NA |
| Clozapine vs olanzapine | 11 | 20 338 | 0.936 (0.765-1.146) | .52 | 76.4 | <.001b | NA |
| Clozapine vs quetiapine fumarate | 7 | 8180 | 0.727 (0.570-0.927) | .01b | 80.2 | <.001b | 14 (9-53) |
| Clozapine vs aripiprazole | 3 | 4529 | 0.698 (0.559-0.872) | .002b | 46.1 | .16 | 20 (14-47) |
| Clozapine vs amisulpride | 3 | 2443 | 0.777 (0.532-1.137) | .19 | 75.3 | .02b | NA |
| Hospitalization (at Specific Period) | |||||||
| At 6 mo | 9 | 1203 | 0.791 (0.462-1.354) | .39 | 59.9 | .01b | NA |
| At 12 mo | 16 | 36 532 | 0.803 (0.699-0.922) | .002b | 63.6 | <.001b | 21 (14-52) |
| At 24 mo | 10 | 13 107 | 0.804 (0.653-0.989) | .04b | 51.0 | .03b | 13 (7-221) |
| All-Cause Discontinuation (vs Specific NC-SGAs) | |||||||
| Clozapine vs risperidone | 14 | 20 836 | 0.702 (0.615-0.800) | <.001b | 87.0 | <.001b | 7 (5-10) |
| Clozapine vs olanzapine | 13 | 27 212 | 0.792 (0.669-0.939) | .007b | 92.9 | <.001b | 10 (7-33) |
| Clozapine vs quetiapine fumarate | 10 | 8642 | 0.654 (0.490-0.874) | .004b | 97.0 | <.001b | 6 (4-15) |
| Clozapine vs aripiprazole | 3 | 4070 | 0.712 (0.338-1.501) | .37 | 99.0 | <.001b | NA |
| Clozapine vs amisulpride | 3 | 2375 | 0.694 (0.495-0.971) | .03b | 85.7 | .001b | 6 (4-57) |
| All-Cause Discontinuation (at Specific Period) | |||||||
| At 6 mo | 6 | 32 230 | 0.779 (0.564-1.076) | .13 | 95.6 | <.001b | NA |
| At 12 mo | 9 | 39 913 | 0.649 (0.530-0.795) | <.001b | 95.1 | <.001b | 6 (5-11) |
| At 24 mo | 5 | 33 862 | 0.707 (0.540-0.926) | .01b | 94.4 | <.001b | 7 (5-28) |
| At 36 mo | 4 | 12 120 | 0.836 (0.688-1.016) | .07 | 65.2 | .04b | NA |
| Adjusted for Person-Years | |||||||
| Hospitalization, person-years | 11 | 4526 | Rate ratio: 0.615 (0.482-0.786) | <.001b | 54.1 | .02b | 8 (6-14) |
| All-cause discontinuation, person-years | 5 | 14 392 | Rate ratio: 0.542 (0.367-0.802) | .002b | 83.7 | <.001b | 5 (3-10) |
| Hazard Ratio | |||||||
| All-cause discontinuation | 5 | NA | Hazard ratio: 0.670 (0.464-0.967)c | .03b | 96.9 | <.001b | NA |
| Adjusted Odds Ratio | |||||||
| Hospitalization | 3 | NA | Adjusted odds ratio: 0.841 (0.620-1.141)d | .27 | 41.5 | .18 | NA |
Abbreviations: NA, not applicable; NC-SGAs, nonclozapine second-generation antipsychotics; NNT, number needed to treat.
Numbers needed to treat were only calculated when the risk ratio analysis was significant (P < .05). The mean risk for the NC-SGAs was used as the assumed control group risk.
P < .05.
Hazard ratio for hospitalization risk was not calculated due to only 2 studies.
Adjusted odds ratio for all-cause discontinuation risk was not calculated due to only 2 studies.
Performing analyses at specific time points, clozapine was associated with a significantly lower risk of hospitalization at 12 months (RR, 0.803; 95% CI, 0.699-0.922; P = .002; NNT, 21; 95% CI, 14-52) and 24 months (RR, 0.804; 95% CI, 0.653-0.989; P = .04; NNT, 13; 95% CI, 7-221) but not at 6 months (RR, 0.791; 95% CI, 0.462-1.354; P = .39) (Table 1). There were too few analyzable studies at 36 months. Clozapine was associated with a significantly lower risk of all-cause discontinuation at 12 months (RR, 0.649; 95% CI, 0.530-0.795; P < .001; NNT, 6; 95% CI, 5-11) and 24 months (RR, 0.707; 95% CI, 0.540-0.926; P = .01; NNT, 7; 95% CI, 5-28) but only nominally at 6 months (RR, 0.779; 95% CI, 0.564-1.076; P = .13) and 36 months (RR, 0.836; 95% CI, 0.688-1.016; P = .07).
Compared with specific NC-SGAs, clozapine was associated with a significantly lower hospitalization risk than quetiapine fumarate (RR, 0.727; 95% CI, 0.570-0.927; P = .01; NNT, 14; 95% CI, 9-53) and aripiprazole (RR, 0.698; 95% CI, 0.559-0.872; P = .002; NNT, 20; 95% CI, 14-47) but not risperidone (RR, 0.871; 95% CI, 0.745-1.018; P = .08), olanzapine (RR, 0.936; 95% CI, 0.765-1.146; P = .52), or amisulpride (RR, 0.777; 95% CI, 0.532-1.137; P = .19) (Table 1). For all-cause discontinuation, clozapine was associated with a significantly lower risk than risperidone (RR, 0.702; 95% CI, 0.615-0.800; P < .001; NNT, 7; 95% CI, 5-10), olanzapine (RR, 0.792; 95% CI, 0.669-0.939; P = .007; NNT, 10; 95% CI, 7-33), quetiapine (RR, 0.654; 95% CI, 0.490-0.874; P = .004; NNT, 6; 95% CI, 4-15), and amisulpride (RR, 0.694; 95% CI, 0.495-0.971; P = .03; NNT, 6; 95% CI, 4-57) but not aripiprazole (RR, 0.712; 95% CI, 0.338-1.501; P = .37). After removing studies with unequal observation periods, clozapine was associated with a significantly lower risk of hospitalization than all NC-SGAs, including risperidone, except for olanzapine (RR, 0.914; 95% CI, 0.732-1.142; P = .43), and with a lower risk of all-cause discontinuation than all other NC-SGAs, except for aripiprazole (RR, 0.712; 95% CI, 0.338-1.501; P = .37) and amisulpride (RR, 0.792; 95% CI, 0.598-1.049; P = .10) (eTable 4 in the Supplement).
Subgroup and Meta-Regression Analyses
Across 7 subgroup analyses for hospitalization risk (eTable 5-1 in eTable 5 in the Supplement) and 6 subgroup analyses for all-cause discontinuation (eTable 5-2 in eTable 5 in the Supplement), only for all-cause discontinuation 2 variables (treatment setting and study design) differed significantly across subgroups. Clozapine was associated with greater advantages than NC-SGAs in mixed inpatients and outpatients and in retrospective database studies (eTable 5-2 in eTable 5 in the Supplement). In addition, none of the meta-regression analyses for both coprimary outcomes yielded significant moderators (eTable 6 in the Supplement).
Secondary Outcomes
Secondary outcomes were recorded. These results are summarized in Table 2 and eFigure 4 in the Supplement.
Table 2. Results of Secondary Outcomes.
| Outcome | No. of Studies | No. of Patients | Effect Size | Favorsa | Heterogeneity | Effect Size | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SMD (95% CI) | P Value | I2, % | P Value | MD (95% CI) | P Value | I2, % | P Value | ||||
| Effectiveness-Related Outcomes | |||||||||||
| Psychopathology-related outcomes | |||||||||||
| Overall symptoms | 10 | 7767 | −0.302 (−0.572 to −0.032) | .03b | Clozapine | 74.8 | <.001b | NA | NA | NA | NA |
| Positive symptoms | 8 | 8111 | −0.253 (−0.574 to 0.069) | .12 | NS | 76.3 | <.001b | NA | NA | NA | NA |
| Negative symptoms | 9 | 8153 | −0.227 (−0.568 to 0.113) | .19 | NS | 79.5 | <.001b | NA | NA | NA | NA |
| General symptoms | 5 | 357 | −0.050 (−0.351 to 0.251) | .75 | NS | 45.4 | .12 | NA | NA | NA | NA |
| Depressive symptoms | 8 | 8212 | −0.008 (−0.178 to 0.163) | .93 | NS | 30.9 | .18 | NA | NA | NA | NA |
| Cognitive symptoms | 3 | 7818 | −0.124 (−0.235 to −0.014) | .03b | Clozapine | <0.1 | .60 | NA | NA | NA | NA |
| Clinical Global Impressions scale severity | 4 | 170 | −1.182 (−2.243 to −0.122 | .03b | Clozapine | 87.7 | <.001b | NA | NA | NA | NA |
| Hospitalization-related outcomes | |||||||||||
| Length of hospitalization, d | 3 | 2587 | 0.464 (−0.084 to 1.011) | .10 | NS | 64.1 | .06 | NA | NA | NA | NA |
| Frequency of hospitalizationc | 5 | 17 747 | Rate ratio: 1.180 (1.034 to 1.346) | .01b | NC-SGAs | 27.0 | .24 | NA | NA | NA | NA |
| Other outcomes | |||||||||||
| Suicide attempt or self-injurious behavior | 4 | 19 700 | Risk ratio: 0.672 (0.432 to 1.046) | .08 | NS | 13.2 | .33 | NA | NA | NA | NA |
| Study-defined response rate | 6 | 9182 | Risk ratio: 1.066 (0.976 to 1.164) | .16 | NS | 6.2 | .38 | NA | NA | NA | NA |
| Safety and/or Tolerability–Related Outcomes | |||||||||||
| Cardiometabolic-related outcomes | |||||||||||
| Body weight, kg | 9 | 5329 | 0.308 (0.057 to 0.560) | .02b | Clozapine | 81.1 | <.001b | 1.70 (0.31 to 3.08) | .02b | 81.1 | <.001b |
| BMI | 8 | 8606 | 0.317 (0.079 to 0.554) | .009b | Clozapine | 75.5 | <.001b | 0.96 (0.24 to 1.68) | .009b | 77.8 | <.001b |
| Waist circumference, cm | 3 | 453 | 0.272 (−0.042 to 0.586) | .09 | NS | 37.7 | .20 | 1.21 (−0.78 to 3.20) | .23 | 51.1 | .13 |
| Systolic blood pressure, mm Hg | 3 | 437 | 0.247 (0.034 to 0.459) | .02b | Clozapine | <0.1 | .37 | 2.22 (0.15 to 4.28) | .04b | 9.3 | .33 |
| Diastolic blood pressure, mm Hg | 3 | 437 | 0.336 (0.111 to 0.561) | .003b | Clozapine | 9.0 | .33 | 1.92 (0.03 to 3.81) | .047b | 53.8 | .12 |
| Total cholesterol, mg/dL | 4 | 433 | 0.297 (−0.186 to 0.781) | .23 | NS | 75.3 | .007b | 11.06 (−7.66 to 29.78) | .25 | 72.8 | .01b |
| Triglycerides, mg/dL | 7 | 725 | 0.162 (−0.041 to 0.365) | .12 | NS | 23.9 | .25 | 11.66 (2.93 to 20.38) | .009b | <0.1 | .59 |
| Glucose, mg/dL | 7 | 880 | 0.486 (0.230 to 0.743) | <.001b | Clozapine | 55.6 | .04b | 8.09 (4.56 to 11.62) | <.001b | 9.6 | .18 |
| Insulin, μIU/mL | 3 | 394 | 0.391 (0.115 to 0.667) | .005b | Clozapine | <0.1 | .41 | 3.50 (−1.04 to 8.04) | .13 | 36.2 | .21 |
| HOMA-IR | 4 | 422 | 0.382 (0.119 to 0.645) | .004b | Clozapine | <0.1 | .49 | 0.99 (0.01 to 1.96) | .047b | 15.6 | .31 |
| Metabolic syndrome risk | 3 | 378 | Risk ratio: 1.630 (0.937 to 2.834) | .08 | NS | 23.7 | .27 | NA | NA | NA | NA |
| Weight gain risk | 5 | 4793 | Risk ratio: 1.075 (0.751 to 1.537) | .69 | NS | 77.3 | .001b | NA | NA | NA | NA |
| Type 2 diabetes risk | 5 | 5539 | Risk ratio: 1.777 (1.229 to 2.570) | .002b | Clozapine | <0.1 | .87 | NNH: 27 (13 to 90)d | NA | NA | NA |
| Other safety-related outcomes | |||||||||||
| Death | 5 | 45 272 | Risk ratio: 0.920 (0.708 to 1.194) | .53 | NS | 26.8 | .24 | NA | NA | NA | NA |
| EPS risk or anticholinergic use | 7 | 9734 | Risk ratio: 0.640 (0.412 to 0.996) | .048b | NC-SGAs | 62.1 | .02b | NNH: −16 (−1359 to −10)d | NA | NA | NA |
| EPS score | 3 | 306 | 0.068 (−0.179 to 0.314) | .59 | NS | <0.1 | .72 | NA | NA | NA | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EPS, extrapyramidal symptoms; HOMA-IR, homeostatic model assessment of insulin resistance; MD, mean difference; NA, not applicable; NC-SGAs, nonclozapine second-generation antipsychotics; NNH, number needed to harm; NS, not significant; SGA, second-generation antipsychotic; SMD, standardized mean difference.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; triglycerides level to millimoles per liter, multiply by 0.0113; glucose level to millimoles per liter, multiply by 0.0555; and insulin level to picomoles per liter, multiply by 6.945.
For effectiveness-related outcomes, “favors” denotes “superiority” (ie, better effectiveness); for safety and/or tolerability–related outcomes “favors” denotes “inferiority” (ie, higher burden of adverse effects).
P < .05.
Number of hospitalizations per person-years.
Number needed to treat and number needed to harm were only calculated when the risk ratio analysis was significant (P < .05). The mean risk for the NC-SGAs was used as the assumed control group risk.
Effectiveness-Related Outcomes
In 11 effectiveness-related analyses, clozapine was associated with a significant reduction in overall symptoms (SMD, −0.302; 95% CI, −0.572 to −0.032; P = .03), cognitive symptoms (SMD, −0.124; 95% CI, −0.235 to −0.014; P = .03), and Clinical Global Impressions scale severity (SMD, −1.182; 95% CI, −2.243 to −0.122; P = .03) but not positive symptoms (SMD, −0.253; 95% CI, −0.574 to 0.069; P = .12), negative symptoms (SMD, −0.227; 95% CI, −0.568 to 0.113; P = .19), general symptoms (SMD, −0.050; 95% CI, −0.351 to 0.251; P = .75), and depressive symptoms (SMD, −0.008; 95% CI, −0.178 to 0.163; P = .93) (Table 2). Only for cognitive symptoms, there was a difference in statistical significance depending on the pre-post correlation coefficients (no significance when using low correlation coefficients) (eTable 2-1 in eTable 2 in the Supplement). Clozapine was associated with a significantly increased number of hospitalizations (RaR, 1.180; 95% CI, 1.034-1.346; P = .01), but only numerical differences favoring clozapine but missing statistical significance were found regarding hospitalization days (SMD, 0.464; 95% CI, −0.084 to 1.011; P = .10) and suicide attempt or self-injurious behavior (RR, 0.672; 95% CI, 0.432-1.046; P = .08) (Table 2).
Safety-Related Outcomes
Among 16 safety-related analyses, clozapine was significantly associated with an increase in body weight (SMD, 0.308; 95% CI, 0.057-0.560; P = .02; MD, 1.70; 95% CI, 0.31-3.08 kg; P = .02), BMI (SMD, 0.317; 95% CI, 0.079-0.554; P = .009; MD, 0.96; 95% CI, 0.24-1.68; P = .009), systolic blood pressure (SMD, 0.247; 95% CI, 0.034-0.459; P = .02; MD, 2.22; 95% CI, 0.15-4.28 mm Hg; P = .04), diastolic blood pressure (SMD, 0.336; 95% CI, 0.111-0.561; P = .003; MD, 1.92; 95% CI, 0.03-3.81 mm Hg; P = .047), triglycerides (SMD, 0.162; 95% CI, −0.041 to 0.365; P = .12; MD, 11.66; 95% CI, 2.93-20.38 mg/dL; P = .009), glucose (SMD, 0.486; 95% CI, 0.230-0.743; P < .001; MD, 8.09; 95% CI, 4.56-11.62 mg/dL; P < .001), insulin (SMD, 0.391; 95% CI, 0.115-0.667; P = .005; MD, 3.50; 95% CI, −1.04 to 8.04 μIU/mL; P = .13), homeostatic model assessment of insulin resistance (SMD, 0.382; 95% CI, 0.119-0.645; P = .004; MD, 0.99; 95% CI, 0.01-1.96; P = .047), and type 2 diabetes risk (RR, 1.777; 95% CI, 1.229-2.570; P = .002; NNH, 27; 95% CI, 13-90) (Table 2) (to convert triglycerides level to millimoles per liter, multiply by 0.0113; glucose level to millimoles per liter, multiply by 0.0555; and insulin level to picomoles per liter, multiply by 6.945). Clozapine was associated with significantly lower extrapyramidal symptoms (EPS) risk or anticholinergic use (RR, 0.640; 95% CI, 0.412-0.996; P = .048; NNH, −16; 95% CI, −1359 to −10). There were no significant differences for waist circumference, total cholesterol, metabolic syndrome risk, study-defined weight gain risk, and EPS score between clozapine and NC-SGAs.
Discussion
In this systematic review and meta-analysis, we examined various outcomes related to the effectiveness and safety of clozapine vs oral NC-SGAs from 63 nonrandomized cohort studies and 109 341 patients. We found that clozapine was associated with a significant reduction in hospitalization risk by 18% (NNT, 18) and all-cause discontinuation by 27% (NNT, 8), despite greater illness severity and/or chronicity in patients treated with clozapine. The results were not altered by study quality or publication bias. In pairwise comparisons and studies with equal observation time, clozapine was associated with lower hospitalization risk than all NC-SGAs with such data, except for olanzapine, while clozapine was associated with reduction in all-cause discontinuation compared with all NC-SGAs, except for aripiprazole and amisulpride. We were unable to identify significant moderators and mediators of the advantage of clozapine regarding hospitalization risk, indicating that the advantage of clozapine applies across broad patient, setting, and study design features. The same was true for clozapine’s advantage regarding all-cause discontinuation, except that clozapine was associated with a significantly larger effect size in retrospective database studies where all eligible patients are included, no consent occurs, and observation biases are absent.
In addition, clozapine was also associated with a significantly greater reduction than NC-SGAs regarding Clinical Global Impressions scale severity (effect size, −1.182) and overall symptoms (effect size, −0.302) and cognitive symptoms (effect size, −0.124) but not positive, negative, or depressive symptoms. While reducing EPS or anticholinergic use risk by 36% (NNH, −16), clozapine was associated with greater risk of cardiometabolic-related outcomes, including increased body weight, BMI, triglycerides (MD only), glucose, insulin (SMD only), insulin resistance, and type 2 diabetes risk (78%; NNH, 27).
The observed effectiveness-related advantages of clozapine in the meta-analyzed cohort studies are in contrast to meta-analytic results of masked RCTs,7,8 which provided little or inconsistent evidence for the superiority of clozapine vs SGAs. Because participants in RCTs do not necessarily represent real-world patients or settings, the results from RCTs cannot readily be generalized to clinical practice.10 Therefore, a meta-analysis of cohort studies can provide complementary information about clozapine’s association with influence, effectiveness, and safety and tolerability.13 Cohort studies of longitudinal and registry databases also have the advantage of longer-term follow-up than RCTs.
Hospitalization was not evaluated in recent meta-analyses of masked RCTs.7,8 One meta-analysis of 3 RCTs (n = 369) and 19 observational studies (n = 44 349) investigated the association of clozapine with hospitalization, showing also that clozapine was associated with a significant reduction in the proportion of individuals hospitalized compared with NC-SGAs (13 studies [n = 29 559]; RR, 0.75; 95% CI, 0.67-0.83; P < .001).54 Although in our much larger meta-analysis clozapine was not associated with a significantly lower risk than NC-SGAs for hospitalization at 6 months, this result may reflect a type II error because there were fewer events at 6 months than at later periods, and 6 months may be too short to reliably evaluate hospitalization risk. Unexpectedly, clozapine was associated with a higher frequency of hospitalizations than NC-SGAs. However, because clozapine-treated patients had greater illness severity, they probably had been hospitalized more frequently before clozapine initiation. Only an average of 40% of clozapine-treated patients with TRS are considered responders55; therefore, it is likely that a subgroup of the most severely ill patients treated with clozapine contributed to the higher number of hospitalizations, despite lower overall hospitalization risk in the entire group of clozapine-treated patients. Because adherence was neither reported nor assured, it is possible that some of the severely ill patients receiving clozapine were not sufficiently adherent.
Clozapine was also associated with a lower risk of all-cause discontinuation, reflecting effectiveness. Reduced all-cause discontinuation in cohort studies is inconsistent with the lack of difference in meta-analyses of masked RCTs.7,8 Considering that clozapine has been associated with serious adverse effects and requires blood monitoring,56 continuation reflects treatment acceptability, although a “surveillance association” may influence outcomes. Clozapine is considered a last-resort treatment for TRS.1 Therefore, clozapine might have been continued, despite minimal effectiveness. In analyses at specific periods, there were no statistically significant differences in all-cause discontinuation between clozapine and NC-SGAs at 6 and 36 months, but these analyses were likely underpowered; early adverse effect–related discontinuations with clozapine may also have had a role.
Numerical advantages for associations favoring clozapine regarding study-defined response and suicide attempt or self-injurious behavior missed statistical significance. Only 3 to 6 studies contributed such data, likely yielding insufficient power.
Regarding tolerability, clozapine was associated with a lower risk of EPS, consistent with short-term trials in acutely ill patients,6 but was associated with significantly greater cardiometabolic risk than NC-SGAs in 9 of the 13 specific meta-analyzable adverse effects. This finding is consistent with clozapine being one of the most cardiometabolically problematic antipsychotics.57,58,59,60,61 Therefore, a careful risk-benefit evaluation is needed before initiating clozapine, and cardiometabolic parameters (body weight, fasting glucose and lipid levels, and glycated hemoglobin levels) should be monitored regularly.
Despite greater cardiometabolic adverse effects than with almost all other antipsychotics, mortality rates with clozapine have not increased but even decreased,62,63 including cardiometabolic-related mortality,64 likely due to effectiveness-related indirect benefits on healthy lifestyle and monitoring and management of physical health risk factors and comorbidities. However, in contrast to a recent meta-analysis reporting a significant association with a reduction in all-cause mortality with clozapine vs other antipsychotics,65 we did not observe this advantage. Reasons for this difference could include that in the other meta-analysis65 first-generation antipsychotics and NC-SGAs were pooled, and the significant finding only emerged in studies where patients used clozapine continuously (RR, 0.56; 95% CI, 0.36-0.85; P = .007) and not in studies where patients could have stopped clozapine (“ever use”) (RR, 0.74; 95% CI, 0.38-1.45; P = .376), while we did not make this distinction due to too few studies comparing clozapine specifically with NC-SGAs. Moreover, the follow-up duration in our meta-analysis was shorter (median, 1 year vs 5.4 years65), and we did not adjust the results by person-years.
Characteristics of studies in our meta-analysis of cohort studies differ in 4 ways from the 3 most recent meta-analyses.7,8,54 These include (1) lack of randomization in all studies, increasing generalizability of included patients but introducing a channeling bias of more severely ill patients being treated with clozapine (creating a conservative bias that strengthens the outcomes); (2) longer duration of our trials vs the meta-analyses that included RCTs only (median, 12 months vs 11 weeks7 and 12 weeks8); (3) inclusion of more recently published evidence (up to 2018 vs 2012,7 2009,8 and 201654); and (4) inclusion of more study participants comparing clozapine specifically with NC-SGAs (eg, 7767 vs 1319 individuals7 and 1429 individuals8 for overall symptom reduction and 49 453 vs 29 559 individuals54 for hospitalization in the sole meta-analysis that included a subsample of cohort studies and that analyzed only hospitalization-related outcomes).
Limitations
This meta-analysis has several limitations. Although risk estimates adjusting for time (HRs) and other relevant covariates would have been ideal, too few such data were available for meta-analysis. For example, only 3 studies provided adjusted ORs (eFigure 3 in the Supplement). Nevertheless, because clozapine is more likely prescribed to patients with more severe treatment resistance, the lack of adjusting for baseline illness severity creates a conservative bias against clozapine. Therefore, clozapine’s association with many better effectiveness outcomes is a sign of the robustness of the association, and effect sizes are an underestimate if anything. Furthermore, despite significant heterogeneity across studies and results, few if any significant moderators or mediators emerged in sensitivity, subgroup, and meta-regression analyses of both coprimary outcomes. Not only clozapine’s influence and effectiveness but also the frequency of clinical contacts and monitoring could alter the lower risk of hospitalization and all-cause discontinuation, but we lacked data to control for frequency of clinical contacts.
Conclusions
This comprehensive meta-analysis of cohort studies, reflecting clinical practice more than RCTs, found clozapine to be associated with better effectiveness outcomes than NC-SGAs, despite more severely ill patients being treated with clozapine, but with significantly greater risk of cardiometabolic adverse outcomes, both of which require consideration when making treatment choices. Because cohort studies have inherent biases, large pragmatic RCTs with broad inclusion and minimal exclusion criteria that better reflect real-world practice are needed.
eTable 1. Results of Secondary Outcomes Using Different Types of Pre/Post Coefficients
eTable 2. Study and Patient Characteristics With Analyzed Outcomes
eTable 3. Sensitivity Analyses of Co-Primary Outcomes Removing Studies With Unequal Observation Periods for Comparing Clozapine With Specific Non-Clozapine SGAs
eTable 4. Subgroup Analyses for Co-Primary Outcomes
eTable 5. Results of Meta-Regression Analyses for Co-Primary Outcomes
eTable 6. Newcastle-Ottawa Quality Assessment Scale of Studies With Co-Primary Outcomes
eFigure 1. PRISMA Diagram of the Systematic Literature Search
eFigure 2. Funnel Plots for Co-Primary Outcomes
eFigure 3. Forest Plots of Detailed Analyses of Co-Primary Outcomes
eFigure 4. Forest Plots of Analyses of Secondary Outcomes
References
- 1.Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216-229. doi: 10.1176/appi.ajp.2016.16050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence Psychosis and schizophrenia in adults: prevention and management. Clinical Guideline CG178. https://www.nice.org.uk/guidance/cg178. Published February 2014. Updated March 2014. Accessed August 12, 2017.
- 3.Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller HJ; WFSBP Task Force on Treatment Guidelines for Schizophrenia . World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: acute treatment of schizophrenia. World J Biol Psychiatry. 2005;6(3):132-191. doi: 10.1080/15622970510030090 [DOI] [PubMed] [Google Scholar]
- 4.Lehman AF, Lieberman JA, Dixon LB, et al. American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 2004;161(2)(suppl):1-56. [PubMed] [Google Scholar]
- 5.Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410-472. doi: 10.1177/0004867416641195 [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 7.Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73(3):199-210. doi: 10.1001/jamapsychiatry.2015.2955 [DOI] [PubMed] [Google Scholar]
- 8.Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi: 10.1192/bjp.bp.115.177261 [DOI] [PubMed] [Google Scholar]
- 9.Kane JM, Correll CU. The role of clozapine in treatment-resistant schizophrenia. JAMA Psychiatry. 2016;73(3):187-188. doi: 10.1001/jamapsychiatry.2015.2966 [DOI] [PubMed] [Google Scholar]
- 10.Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66(8)(suppl):S37-S41. doi: 10.1016/j.jclinepi.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213. doi: 10.1093/schbul/sbs150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. doi: 10.4088/JCP.13r08440 [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619. doi: 10.1093/schbul/sbx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 12, 2017.
- 16.Google Translate https://translate.google.com. Accessed May 22, 2017.
- 17.WebPlotDigitizer https://automeris.io/WebPlotDigitizer/. Accessed May 22, 2017.
- 18.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed August 12, 2017.
- 19.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 20.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812. doi: 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- 21.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Government Printing Office; 1976. Department of Health, Education, and Welfare Publication ABM 76-338. [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7-10. doi: 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Castro AP, Elkis H. Rehospitalization rates of patients with schizophrenia discharged on haloperidol, risperidone or clozapine. Braz J Psychiatry. 2007;29(3):207-212. doi: 10.1590/S1516-44462007000300004 [DOI] [PubMed] [Google Scholar]
- 27.Conley RR, Love RC, Kelly DL, Bartko JJ. Rehospitalization rates of patients recently discharged on a regimen of risperidone or clozapine. Am J Psychiatry. 1999;156(6):863-868. doi: 10.1176/ajp.156.6.863 [DOI] [PubMed] [Google Scholar]
- 28.Werneck AP, Hallak JC, Nakano E, Elkis H. Time to rehospitalization in patients with schizophrenia discharged on first generation antipsychotics, non-clozapine second generation antipsychotics, or clozapine. Psychiatry Res. 2011;188(3):315-319. doi: 10.1016/j.psychres.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 29.Bitter I, Katona L, Zámbori J, et al. Comparative effectiveness of depot and oral second generation antipsychotic drugs in schizophrenia: a nationwide study in Hungary. Eur Neuropsychopharmacol. 2013;23(11):1383-1390. doi: 10.1016/j.euroneuro.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Velligan DI, Carroll C, Lage MJ, Fairman K. Outcomes of Medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133. doi: 10.1176/appi.ps.201300085 [DOI] [PubMed] [Google Scholar]
- 31.Nyakyoma K, Morriss R. Effectiveness of clozapine use in delaying hospitalization in routine clinical practice: a 2 year observational study. Psychopharmacol Bull. 2010;43(2):67-81. [PubMed] [Google Scholar]
- 32.Kim JH, Kim D, Marder SR. Time to rehospitalization of clozapine versus risperidone in the naturalistic treatment of comorbid alcohol use disorder and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):984-988. doi: 10.1016/j.pnpbp.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 33.Conley RR, Kelly DL, Love RC, McMahon RP. Rehospitalization risk with second-generation and depot antipsychotics. Ann Clin Psychiatry. 2003;15(1):23-31. doi: 10.3109/10401230309085667 [DOI] [PubMed] [Google Scholar]
- 34.Ringbäck Weitoft G, Berglund M, Lindström EA, Nilsson M, Salmi P, Rosén M. Mortality, attempted suicide, re-hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden: a register-based study. Pharmacoepidemiol Drug Saf. 2014;23(3):290-298. doi: 10.1002/pds.3567 [DOI] [PubMed] [Google Scholar]
- 35.Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi: 10.1176/appi.ajp.2015.15030332 [DOI] [PubMed] [Google Scholar]
- 36.Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224. doi: 10.1136/bmj.38881.382755.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo X, Fang M, Zhai J, et al. Effectiveness of maintenance treatments with atypical and typical antipsychotics in stable schizophrenia with early stage: 1-year naturalistic study. Psychopharmacology (Berl). 2011;216(4):475-484. doi: 10.1007/s00213-011-2242-3 [DOI] [PubMed] [Google Scholar]
- 38.Lin CH, Lin SC, Chen MC, Wang SY. Comparison of time to rehospitalization among schizophrenic patients discharged on typical antipsychotics, clozapine or risperidone. J Chin Med Assoc. 2006;69(6):264-269. doi: 10.1016/S1726-4901(09)70254-0 [DOI] [PubMed] [Google Scholar]
- 39.Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi: 10.1111/acps.12621 [DOI] [PubMed] [Google Scholar]
- 40.Herceg M, Jukić V, Vidović D, et al. Two-year rehospitalization rates of patients with newly diagnosed or chronic schizophrenia on atypical or typical antipsychotic drugs: retrospective cohort study. Croat Med J. 2008;49(2):215-223. doi: 10.3325/cmj.2008.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novick D, Ascher-Svanum H, Haro JM, Bertsch J, Takahashi M. Schizophrenia Outpatient Health Outcomes study: twelve-month findings. Pragmat Obs Res. 2012;3:27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams R, Kopala L, Malla A, Smith G, Love L, Balshaw R. Medication decisions and clinical outcomes in the Canadian National Outcomes Measurement Study in Schizophrenia. Acta Psychiatr Scand Suppl. 2006;(430):12-21. doi: 10.1111/j.1600-0447.2006.00757.x [DOI] [PubMed] [Google Scholar]
- 43.Taylor M, Shajahan P, Lawrie SM. Comparing the use and discontinuation of antipsychotics in clinical practice: an observational study. J Clin Psychiatry. 2008;69(2):240-245. doi: 10.4088/JCP.v69n0210 [DOI] [PubMed] [Google Scholar]
- 44.Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophr Res. 2003;60(1):81-85. doi: 10.1016/S0920-9964(02)00231-1 [DOI] [PubMed] [Google Scholar]
- 45.Cooper D, Moisan J, Grégoire JP. Adherence to atypical antipsychotic treatment among newly treated patients: a population-based study in schizophrenia. J Clin Psychiatry. 2007;68(6):818-825. doi: 10.4088/JCP.v68n0601 [DOI] [PubMed] [Google Scholar]
- 46.Ascher-Svanum H, Zhu B, Faries DE, Lacro JP, Dolder CR, Peng X. Adherence and persistence to typical and atypical antipsychotics in the naturalistic treatment of patients with schizophrenia. Patient Prefer Adherence. 2008;2:67-77. doi: 10.2147/PPA.S2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi: 10.1007/s00213-003-1506-y [DOI] [PubMed] [Google Scholar]
- 48.Hodgson R, Belgamwar R, Al-tawarah Y, MacKenzie G. The use of atypical antipsychotics in the treatment of schizophrenia in North Staffordshire. Hum Psychopharmacol. 2005;20(2):141-147. doi: 10.1002/hup.669 [DOI] [PubMed] [Google Scholar]
- 49.Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D; SOHO Study Group . Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17(4):235-244. doi: 10.1016/j.euroneuro.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 50.Buoli M, Kahn RS, Serati M, Altamura AC, Cahn W. Haloperidol versus second-generation antipsychotics in the long-term treatment of schizophrenia. Hum Psychopharmacol. 2016;31(4):325-331. doi: 10.1002/hup.2542 [DOI] [PubMed] [Google Scholar]
- 51.Treuer T, Martenyi F, Saylan M, Dyachkova Y. Factors associated with achieving minimally symptomatic status by patients with schizophrenia: results from the 3-year intercontinental Schizophrenia Outpatients Health Outcomes study [published correction appears in Int J Clin Pract. 2010;64(10):1459]. Int J Clin Pract. 2010;64(6):697-706. doi: 10.1111/j.1742-1241.2009.02331.x [DOI] [PubMed] [Google Scholar]
- 52.Karagianis J, Williams R, Davis L, et al. Antipsychotic switching: results from a one-year prospective, observational study of patients with schizophrenia. Curr Med Res Opin. 2009;25(9):2121-2132. doi: 10.1185/03007990903102966 [DOI] [PubMed] [Google Scholar]
- 53.Lindenmayer JP, Iskander A, Park M, et al. Clinical and neurocognitive effects of clozapine and risperidone in treatment-refractory schizophrenic patients: a prospective study. J Clin Psychiatry. 1998;59(10):521-527. doi: 10.4088/JCP.v59n1005 [DOI] [PubMed] [Google Scholar]
- 54.Land R, Siskind D, McArdle P, Kisely S, Winckel K, Hollingworth SA. The impact of clozapine on hospital use: a systematic review and meta-analysis. Acta Psychiatr Scand. 2017;135(4):296-309. doi: 10.1111/acps.12700 [DOI] [PubMed] [Google Scholar]
- 55.Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777. doi: 10.1177/0706743717718167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613. doi: 10.4088/JCP.12r08064 [DOI] [PubMed] [Google Scholar]
- 57.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. doi: 10.1038/nrendo.2011.156 [DOI] [PubMed] [Google Scholar]
- 58.Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97-107. doi: 10.1016/j.molmed.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119-136. doi: 10.1002/wps.20204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339-347. doi: 10.1002/wps.20252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166-174. doi: 10.1002/wps.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-Year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627. doi: 10.1016/S0140-6736(09)60742-X [DOI] [PubMed] [Google Scholar]
- 63.Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2017;S0920-9964(17)30762-4. [DOI] [PubMed] [Google Scholar]
- 64.Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663. doi: 10.1093/schbul/sbu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU, de Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull. 2018. doi: 10.1093/schbul/sby015.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Results of Secondary Outcomes Using Different Types of Pre/Post Coefficients
eTable 2. Study and Patient Characteristics With Analyzed Outcomes
eTable 3. Sensitivity Analyses of Co-Primary Outcomes Removing Studies With Unequal Observation Periods for Comparing Clozapine With Specific Non-Clozapine SGAs
eTable 4. Subgroup Analyses for Co-Primary Outcomes
eTable 5. Results of Meta-Regression Analyses for Co-Primary Outcomes
eTable 6. Newcastle-Ottawa Quality Assessment Scale of Studies With Co-Primary Outcomes
eFigure 1. PRISMA Diagram of the Systematic Literature Search
eFigure 2. Funnel Plots for Co-Primary Outcomes
eFigure 3. Forest Plots of Detailed Analyses of Co-Primary Outcomes
eFigure 4. Forest Plots of Analyses of Secondary Outcomes