Abstract
Adrenocortical carcinoma (ACC) is an aggressive cancer with a 5-year survival rate <35%. Mortality remains high due to lack of targeted therapies. Using bioinformatic analyses, we identified maternal embryonic leucine zipper kinase (MELK) as 4.1-fold overexpressed in ACC compared with normal adrenal samples. High MELK expression in human tumors correlated with shorter survival and with increased expression of genes involved in cell division and growth. We investigated the functional effects of MELK inhibition using newly developed ACC cell lines with variable MELK expression, CU-ACC1 and CU-ACC2, compared with H295R cells. In vitro treatment with the MELK inhibitor, OTSSP167, resulted in a dose-dependent decrease in rates of cell proliferation, colony formation, and cell survival, with relative sensitivity of each ACC cell line based upon the level of MELK overexpression. To confirm a MELK-specific antitumorigenic effect, MELK was inhibited in H295R cells via multiple short hairpin RNAs. MELK silencing resulted in 1.9-fold decrease in proliferation, and 3- to 10-fold decrease in colony formation in soft agar and clonogenicity assays, respectively. In addition, although MELK silencing had no effect on survival in normoxia, exposure to a hypoxia resulted in a sixfold and eightfold increase in apoptosis as assessed by caspase-3 activation and TUNEL, respectively. Together these data suggest that MELK is a modulator of tumor cell growth and survival in a hypoxic microenvironment in adrenal cancer cells and support future investigation of its role as a therapeutic kinase target in patients with ACC.
MELK is overexpressed in human adrenocortical carcinoma cells and drives proliferation, tumorigenesis, and survival in a hypoxic environment. MELK is a therapeutic target for patients with ACC.
Adrenocortical carcinoma (ACC) is an aggressive, orphan malignancy with an estimated 500 new cases in the United States each year (1). ACC affects every age group, from children to older adults, and has a dismal prognosis with the majority of patients having regional or distant metastases at time of diagnosis (1, 2). Surgery is the first-line therapy for ACC; however, many patients present with advanced disease where surgical options are limited (1, 3). Medical therapeutic options are also restricted, including mitotane (o,p′DDT; a derivative of the insecticide dichlorodiphenyl-trichloroethane), the only FDA-approved medication for ACC, and chemotherapy etoposide, doxorubicin, and cisplatin for patients with progressive disease. Both medical treatments have very modest effectiveness (4–6). Overall survival for patients with ACC has remained constant over the last several decades with <35% 5-year survival rates (1).
Recently, two integrated genomic analyses provided insights into the ACC landscape (7, 8). These studies concluded that ACC tumors can be subdivided into two major groups: the C1A group (60%) with putative driver mutations and C1B (40%) without an obvious driver (7, 8). Clinical correlation of a patient’s prognosis with their molecular phenotype suggested that the C1A subtype tumors had a more aggressive clinical course with decreased survival compared with the C1B group (7, 8). However, sparse clinical data available in the analysis limited the identification of specific prognostic biomarkers in the two groups. Approximately 40% of ACC tumors show activation of the β-catenin and canonical WNT pathways and 20% of tumors have a mutation in TP53 (7, 8). To date, however, neither of these pathways have been successfully targeted.
Several other pathways have been studied for possible targeted therapy with little success. The IGF pathway was investigated after in vitro targeting yielded promising antitumor activity (9–14); however, this did not translate to success in clinical trials (15–17). Antiangiogenics or epidermal growth factor receptor–targeted drugs in patients with ACC also have yielded disappointing results (18–22). Frequent driver mutations in TP53, ATM, and CTNNB1, amplification of the CDK4, FGFR1, FGF9, FRS2, and deletions in CDKN2A and CDKN2B suggest molecular abnormalities in genes governing the G1/S phase transition in a subset of patients (23), and represent possible future targets. Given that most ACC arise sporadically without known mutations and respond poorly to existing regimens of chemotherapeutic drugs, identifying vulnerabilities in the DNA damage response pathway, rather than targeting mutated genes, may be particularly important for the future success of ACC treatments.
With the goal of identifying druggable targets in the DNA damage pathway, we analyzed publically available expression data sets and identified maternal embryonic leucine zipper kinase (MELK) as overexpressed in ACC tumor samples. MELK is a member of the AMPK/Snf1 family, a highly conserved serine/threonine kinase that was previously identified as a mediator of radiation resistance in human breast and brain cancer (24–26). In breast cancer cells, MELK acts as an oncogene to increase DNA damage tolerance. Exposure to a MELK inhibitor induced stalled replication forks and also downregulated genes involved in ataxia-telangiectasia mutated signaling, cell cycle control genes involved in chromosomal replication, and genes involved in DNA damage response, ultimately resulting in senescence (27). Loss of MELK in basal breast cancer cells was associated with defective mitosis and subsequent apoptosis (24). Based upon these data, we explored the functional role of MELK in ACC human cell lines.
Using the human ACC cell line, H295R, and our newly developed ACC tumor cell lines, CU-ACC1 and CU-ACC2 (28), we demonstrated that the MELK inhibitor, OTSSP167, suppressed ACC cell proliferation dependent on the levels of MELK overexpression. We identified components of the cell cycle that were coregulated with MELK and demonstrated that MELK has an oncogenic role in ACC by controlling proliferation, colony formation, and anchorage independent colony growth in soft agar. In line with its reported role as a prosurvival factor, silencing of MELK in ACC cells induced apoptosis selectively in a hypoxic microenvironment. Together these findings confirm the protumorigenic role of MELK in ACC and support efforts to further explore the use of MELK inhibitors as therapeutics in patients with ACC.
Material and Methods
Analysis of public genomic data sets
A literature search for publically available gene expression data containing normal adrenal, adenoma, and ACC samples analyzed on the same platform was performed. Two studies that reported gene expression microarray performed on the Affymetrix Human Genome U133A2.0 Plus platform that were deposited in NCBIs Gene Expression Omnibus were identified and CEL files downloaded (GSE10927 and GSE19750). The files contained data on 14 normal adrenals, 22 adrenal adenomas, and 77 ACC samples. The European Network for the Study of Adrenal Tumor (ENSAT) data were also downloaded (N = 45 ACC) [gene expression performed on Affymetrix Human Gene 2.0 ST Array deposited in Gene Expression Omnibus (GSE49278)] and analyzed separately. After batch correction, one-way ANOVA with Fisher least significant difference using the Partek Genomics Suite 6.6 software was used to examine differentially expressed transcripts with a stringent false discovery rate <0.05. An expression change cutoff of more than twofold was applied to generate a set of transcripts with differential expression between normal adrenal and ACC samples from U133A2.0 Plus platform. With a focus to identify new therapeutic targets, a kinase screen was performed with the list of 536 known human kinases on the set of transcripts, which were twofold higher expression in ACC compared with normal tissues.
The raw RNA sequencing (RNA-seq) FASTQ data from The Cancer Genome Atlas (TCGA; phs000178) and Genotype-Tissue Expression project (GTEx; phs000424), both performed on Illumina HiSeq platform, were downloaded from dbGaP. The expression profiles from 79 ACC samples from TCGA and 45 normal adrenal tissues from GTEx were analyzed. Raw FASTQ RNA-seq reads were downloaded and mapped to the human genome sequence using GSNAP. Transcript assembly and expression was calculated by Cufflinks (29) and analyzed for differential gene expression by ANOVA in R (30–33). Clinical correlation between gene expression and time of survival from diagnosis as well as gene coexpression using the TCGA data were performed using the cBio Portal for Cancer Genomics Web site (34, 35).
Cell culture and reagents
H295R ACC cells were a kind gift from Dr. William Rainey (Ann Arbor, University of Michigan). Cells were cultured in DMEM/F12 Medium with HEPES (Gibco, Thermo Fisher Scientific, Waltham, MA) supplemented with 5% NuSerum, 1% Antibiotic Pen/Step, and 1 μg/mL gentamicin (1 μg/mL) at 37°C in humidified 5% CO2. CU-ACC1 and CU-ACC2 ACC cell lines (28) were grown in F medium [3:1 (v/v) F-12 Nutrient Mixture (Ham)–Dulbecco’s modified Eagle’s medium (Invitrogen, Thermo Fisher Scientific, Waltham, MA) containing 5% fetal bovine serum, 0.4 μg/mL hydrocortisone (Sigma-Aldrich, St Louis, MO), 5 μg/mL insulin (Sigma-Aldrich), 8.4 ng/mL cholera toxin (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Invitrogen), and 24 μg/mL adenine (Sigma-Aldrich)] (28). The FOXM1 inhibitor, thiostrepton, was obtained from EDM Millipore (Burlington, MA). The MELK inhibitor, OTSSP167, was purchased from SelleckChem (Houston, TX).
Plasmids and transduction
All short hairpin RNA (shRNA) constructs were from GE Dharmacon (Lafayette, CO) and were components of the TRIPZ-MELK shRNA packaging starter kit. The shScr scrambled control was designed to avoid targeting any known genes. All shRNAs were packaged into lentivirus and cells transduced at a ratio of 1:3 of viral supernatant to media following the manufacturer’s protocol.
Immunoblot analysis
For immunoblot analysis, cells were harvested in 1× radioimmunoprecipitation assay buffer supplemented with protease inhibitor (Sigma-Aldrich). Protein levels were quantified using the bicinchoninic acid protein assay and loaded on an SDS-PAGE gel. Proteins were blotted on nitrocellulose membranes and blocked using 3% bovine serum albumin. Membranes were incubated with primary antibodies overnight, anti-MELK (Abcam, Cambridge, MA) (36), anti-PARP (Cell Signaling, Danvers, MA) (37), anti-Caspase-3 (Cell Signaling) (38), anti-Bub1b (Novus Biologicals, Littleton, CO) (39), and antiβ-tubulin (Abcam) (40) were used at a dilution 1:1000; anti-FoxM-1 (Santa Cruz Biotechnology, Dallas, TX) (41), anti-ERCCL6 (Abcam) (42), and anti-PLK1 (Cell Signaling) (43) were used at a dilution of 1:500. Antibody against GAPDH (Millipore) (44) was used at a dilution of 1:5000. Horseradish peroxidase–conjugated polyclonal anti-mouse IgG or anti-rabbit IgG were used as secondary antibodies. Blots were developed using the ECL kit from Pierce. Densitometry analysis was performed using the National Institutes of Health Image J software. Figures are representative images of at least three biological replicates.
Immunohistochemistry
Immunohistochemistry (IHC) for MELK was performed on 5-μm-thin sections prepared from formalin-fixed paraffin-embedded human ACC tumor samples (N = 18) and normal adrenal tissue (N = 6). Patients with ACC were consented for an institutional review board–approved study at the University of Colorado; normal adrenal tissue was obtained from deidentified archived samples. Sections were deparaffinized and hydrated with standard methods. Antigen retrieval was in a 10-mM sodium citrate (pH 6.0) for 10 minutes at 110°C in a pressure cooker followed by one rinse in 1× Reaction Buffer (Ventana Medical Systems, Tucson, AZ). IHC was performed on a Ventana Benchmark XT autostainer (Ventana Medical Systems) using an Ultraview Ventana detection kit with the MELK antibody (Sigma-Aldrich) (45) 1:100 dilution at 37°C for 32 minutes. Sections were counterstained with hematoxylin and dehydrated and coverslipped. Negative controls to confirm the specificity of the immunostaining included omission of the primary antibody incubation step in the IHC protocol and/or substitution of the primary antibody with nonimmune immunoglobulin of the same isotype at the same concentration as the primary antibodies.
Expression of MELK protein was graded, in a blinded fashion, by a single pathologist (HS) for the intensity of stained tumor cells (1, no staining; 2, slight staining; 3, moderate staining; 4, strong staining). Intensity of staining was graded in comparison with control samples from normal adrenal tissue, see Supplemental Fig. 1.
Quantitative real-time PCR
Quantitative real-time PCR was done using the Applied Biosystems 7500 real-time PCR system. Briefly, total RNA was extracted from H295R, CU-ACC1, and CU-ACC2 cell lines using RNeasy kit (Qiagen, Germantown, MD) and converted to cDNA using the Iscript cDNA synthesis kit (Bio-Rad, Hercules, CA). The Power SYBR Green qPCR Master Mix from Life Technologies (Carlsbad, CA) was used for real-time quantitative analysis. qPCR primers used are provided as Supplemental Fig. 2.
Clonogenicity assay
Three thousand cells were plated per well in a six-well plate and after 11 days cells were fixed in 4% PFA and stained with crystal violet for 1 hour. Images were scanned using Licor Odyssey (Image Studio Lite version 5.2; LI-COR Biosciences, Lincoln, NE). Photographed images were analyzed for number of colonies using a macro built into Image J.
Proliferation assays
Proliferation assays were performed using the Incucyte ZOOM imaging system from Essen Biosciences (Ann Arbor, MI). Cells were labeled with Nuclight from Essen (catalog no. 4626). For live imaging proliferation assays, cells were plated at a concentration of 5000 cells per well in a 96-well plate. Standard scans were conducted following the manufacturer’s protocol under ×4 magnification and images quantified using nuclear counts. Cell counts at days 1 through 10 were measured.
Soft agar assay
Soft agar assays were performed using a 0.5% bottom layer and 0.35% top layer. For the base layer, equal volume of 1% agar and 2× media was mixed, and 1.5 mL was added to each well of a six-well plate. For the top layer, cells were resuspended at a concentration of 10,000 or 20,000 per well in 2× media and mixed with equal volume of 0.7% agar solution. A volume of 1.5 mL was dispensed onto each well. Photographs of colonies were taken 4 weeks postplating for MELK silenced H295R ACC cells at ×2 magnification. Colonies were counted manually and plotted relative to controls. Soft agar assays were performed in MELK silencing experiments only, because small molecule inhibitors do not effectively penetrate through the top agar layer.
Apoptosis assays
Apoptosis assays were performed under hypoxic conditions (1% O2) in the presence of 5% and 0% serum in H295R cells where MELK was silenced. Hypoxic condition with 1% O2 was achieved using Coy Hypoxia Chamber Cell Culture Glove Box (Coy Laboratory Products, Grass Lake, MI) with continuous O2 monitoring set at 1% for these studies. The H295R cells were plated at a confluency of 30% at day 1. On day 3 postplating, media was replaced with 5% or 0% serum and cells were subjected to hypoxic conditions for 24 hours after which they were stained for TUNEL (Promega, Fitchburg, WI; catalog no. G3250) following the manufacturer’s protocol. To assess changes in activated PARP and Caspase 3 protein, H295R cells were incubated for 48 hours in a hypoxic microenvironment and immunoblots performed as above.
Statistical analysis
Data are presented as means ± SEM from three or more separate experiments. P-value calculations were conducted using unpaired Student t test for two-group comparison or ANOVA (with Bonferroni posttest analysis for multiple comparisons). All data were analyzed and presented using GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA).
Results
Gene expression arrays show a differential molecular signature of ACC tumors
Using publically available genomic data performed on Affymetrix Human Genome U133 Plus 2.0 Array platform (46, 47), we analyzed the combined gene expression microarray profiles on 14 normal adrenal, 22 adrenal adenomas, and 77 ACC samples. After batch effect correction, a one-way ANOVA, using Partek Genomic Suite 6.6 on 54,675 gene probes, showed a unique molecular signature of ACC tumors compared with adrenal adenomas and normal adrenal samples (Supplemental Fig. 3A). Using a stringent false discovery rate <0.05 to control for multiple testing, bioinformatic analysis revealed 1156 genes were differentially expressed > 2.0-fold between ACC and normal adrenal samples, with 388 genes upregulated and 768 downregulated in ACC compared with normal. Next, we analyzed the data set for dysregulated genes in the DNA damage pathway, with a focus on altered kinases as potential therapeutic targets. We identified MELK as a candidate kinase involved in the DNA damage response.
MELK is upregulated in ACC tumors and is associated with worse clinical outcomes
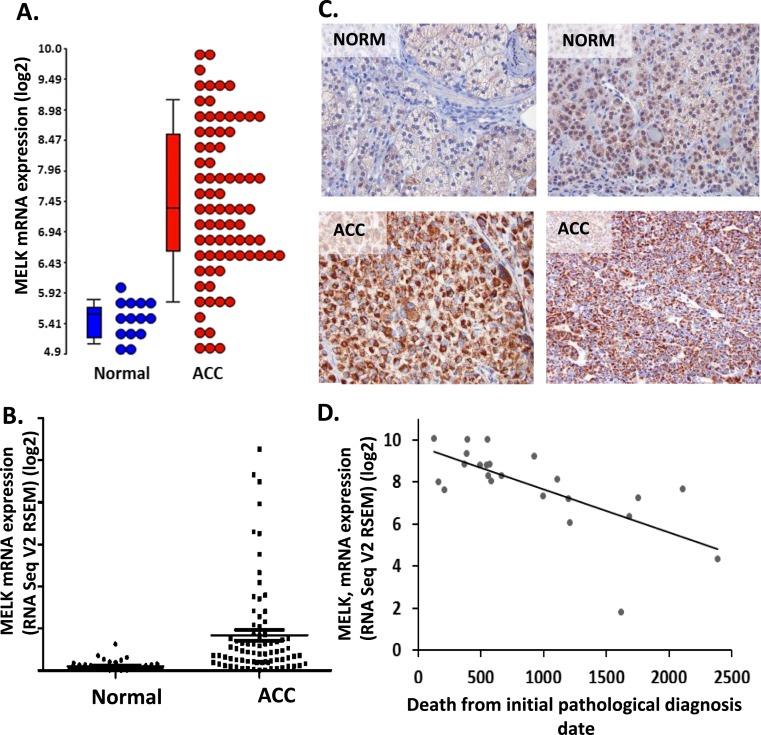
At the transcript level, MELK was upregulated 4.1-fold in ACC samples compared with normal adrenal (P < 0.001) (Fig. 1A) and 4.3-fold upregulated in ACC compared with adrenal adenomas (P < 0.001) (Supplemental Fig. 3B). We assessed the expression of MELK using the RNA-seq data from TCGA ACC data set (which contained no normal samples) compared with normal adrenal expression levels from the GTEx. In these data sets, MELK was upregulated 8.3-fold in ACC samples (P < 0.001) (Fig. 1B). We then confirmed that MELK was dysregulated at the protein level in human ACC samples (N = 18) compared with normal adrenal tissue (N = 6) as shown by IHC (Fig. 1C). Semiquantification of IHC, with representative grading as shown in Supplemental Fig. 1, revealed that 45% of ACC tissue had moderate to high MELK protein expression (grade 3 to 4), whereas 82% of normal adrenal tissue showed mild or no MELK expression (grade 1 to 2). These data correlate with RNA expression from gene microarray and TCGA, which suggest that MELK in overexpressed in 40% to 50% of ACC (Fig. 1A and 1B).
Figure 1.
MELK is upregulated in ACC tissues compared with normal tissues. (A) MELK mRNA expression was upregulated in ACC (red; N = 77) compared with normal adrenal samples (blue; N = 14) as shown on the Log2 transformed dot blot (P < 0.001) (GSE10927 and GSE19750). (B) Similarly, MELK expression levels were higher in ACC tissues (N = 79) (data mined from TCGA) compared with normal tissues (N = 45) [data mined from GTEx project (P < 0.001)]. (C) IHC showing high MELK expression in representative ACC tumor samples (N = 18) compared with normal adrenal samples (N = 6). (D) High MELK expression was associated with poor survival (N = 23) (data derived from TCGA, Pearson correlation = −0.601). NORM, normal.
Using the available clinical information from the TCGA cohort (34, 35), we also examined the correlation between MELK transcript levels and time to death after ACC diagnosis, and determined that MELK expression levels were inversely correlated with survival in patients (Fig. 1D). Similarly, using the ENSAT ACC microarray expression data (7), MELK was 2.2-fold higher in C1A group, reported to have a worse clinical prognosis, as compared with the C1B cohort (Supplemental Fig. 3C).
MELK overexpression is associated with upregulation of BUB1B, NEK2, LMNB1, and PLK1
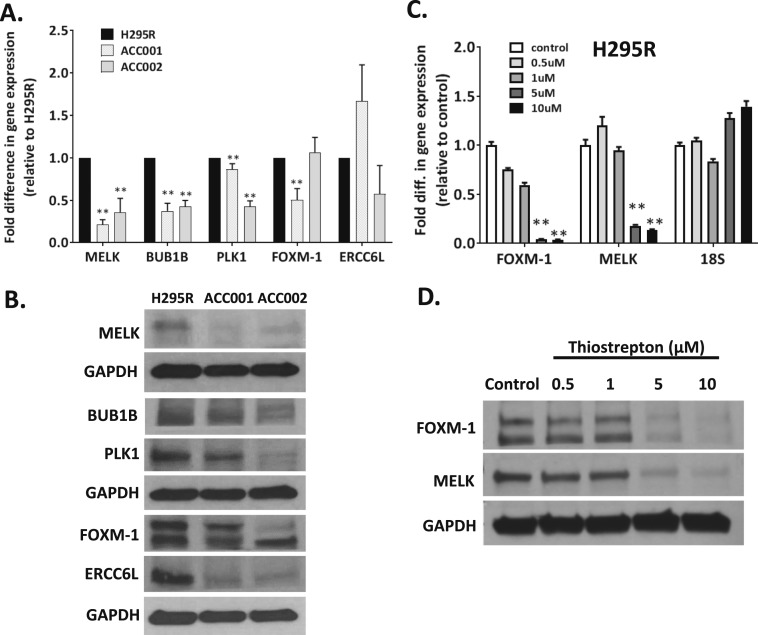
To identify genes that are dysregulated in coordination with MELK, we screened the TCGA RNA-seq data for transcripts with a high MELK coexpression index defined by Spearman score >0.7 (34, 35) and compared the list with microarray data transcripts that showed >2.0-fold upregulation in ACC compared with normal adrenal tissue samples. Ninety-six overlapping transcripts were identified (Supplemental Table 1). Eleven gene transcripts were selected for further analysis based on their fold upregulation and role in cell cycle regulation. To investigate whether MELK expression correlated with upregulation of these 11 genes, we used the ACC cell lines, H295R, CU-ACC1, and CU-ACC2, which have variable MELK expression levels to probe for the expression of the selected candidates at the mRNA and protein level. As shown in Fig. 2A and 2B and Supplemental Fig. 4A and 4B, MELK expression was higher in H295R cells compared with CU-ACC1 or CU-ACC2 cells. Consistent with higher MELK expression levels, several transcripts were consistently upregulated in H295R compared with CU-ACC1 and/or CU-ACC2, including NEK2, BUB1B, PLK1, FOXM1, FANCG, TOP2A, KIF4A, LMNB1, CENPL, and TCF19 (P < 0.01) (Fig. 2A; Supplemental Fig. 4A). BUB1B, PLK1, FOXM1, and ERCC6L were selected to be tested for changes in protein expression by immunoblot because they are known components of the cell cycle. Figure 2B and Supplemental Fig. 4B demonstrate as expected that higher level of MELK was associated with an upregulation of BUB1B, PLK1, FOXM1, and ERCC6L protein levels in H295R compared with CU-ACC1 and CU-ACC2 (P < 0.05). These data suggest that multiple components of the cell cycle are dysregulated in ACC tumors in concert with MELK overexpression.
Figure 2.
MELK expression associates with genes involved in cellular growth and division. (A) Increased MELK in H295R cells, compared with CU-ACC1 and CU-ACC2 cells, is associated with upregulation of BUB1B, PLK1, FOXM1 (**P < 0.01). (B) At the protein level, increased MELK correlated with increased expression of BUB1B, PLK1, FOXM1, and ERCC6L. (C and D) FOXM1 inhibition using thiostrepton was associated with a dose-dependent decrease in (C) FOXM1 and MELK mRNA and (D) FOXM1 and MELK protein expression. Densitometry analysis of the blots are shown in Supplemental Fig. 2B and 2C (**P < 0.01). 18S, 18S ribosomal RNA.
In other systems, FOXM1 has been reported both as MELK regulator and substrate (24, 48, 49). In our analysis, FOXM1 was upregulated at the transcript and protein level with MELK expression in H295R compared with CU-ACC1 cells (P < 0.01). Inhibition of FOXM1 with the FOXMI inhibitor, thiostrepton, in H295R cells demonstrated a dose-dependent decrease in MELK mRNA (P < 0.01) and protein levels (P < 0.01) suggesting that FOXM1 plays a role as an upstream modulator of MELK in ACC tumor cells (Fig. 2C and 2D and Supplemental Fig. 4C). Future studies are needed to dissect whether FOXM1 might also be a substrate of MELK. Together these studies begin to dissect the components of the cell cycle that are coregulated with MELK in human ACC.
The MELK inhibitor (OTSSP167) decreases proliferation and colony formation in ACC cell lines relative to MELK expression level
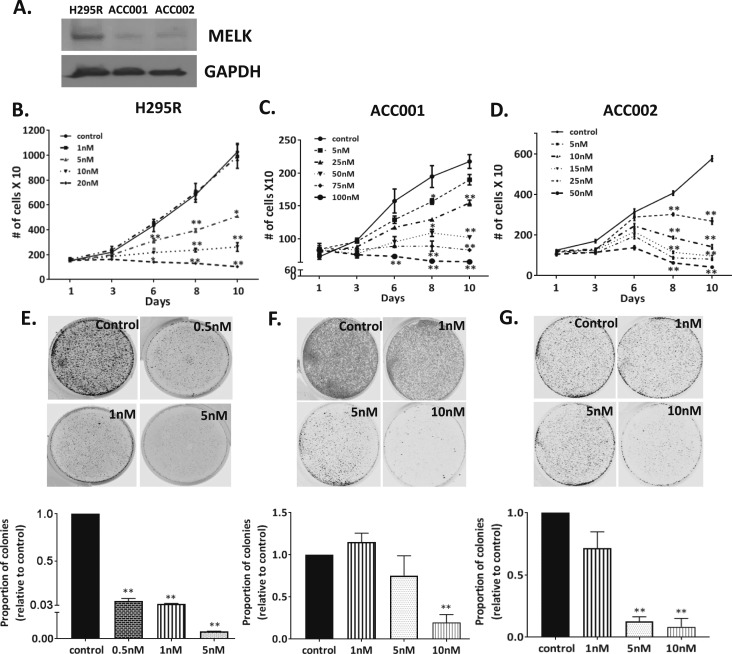
Prior studies have characterized the effects of the MELK inhibitor, OTSSP167, to inhibit the growth of various human cancer cell lines and patient derived xenografts (50). This drug is currently in clinical trials for several solid tumors and leukemia (clinicaltrials.gov; NCT01910545 and NCT02795520). Thus, we tested the effects of OTSSP167 on the rates of proliferation in our multiple human ACC cells. Immunoblotting revealed that H295R cells had higher endogenous levels of MELK protein expression than CU-ACC1 and CU-ACC2 cells (Fig. 3A) and the response to the MELK inhibitor correlated with endogenous levels of MELK (Fig. 3B–3D). H295R cells treated with doses >5 nM OTSSP167 revealed a dose-dependent decrease in rates of proliferation up to 50% at day 8 (P < 0.01). In contrast, the CU-ACC2 and CU-ACC1 cells, with lower endogenous levels of MELK were less sensitive to the MELK inhibitor, and doses >10 and 25 nM, respectively, were required to achieve a >30% to 50% inhibition in proliferation at day 8 (P < 0.05, P < 0.01, respectively). In addition, we examined the efficacy of MELK inhibitor in H295R cells transduced with short hairpin RNA targeting MELK (shMELK). Although scramble control cells showed dose-dependent decrease in proliferation in presence of OTSSP167, the effect of MELK inhibitor on proliferation was abrogated in the cells where MELK was silenced, suggesting MELK inhibitor-specific effect in targeting MELK as a function of proliferation (Supplemental Fig. 5).
Figure 3.
Exposure of ACC cells to the MELK inhibitor, OTSSP167, was associated with dose-dependent decrease in rates of proliferation and colony formation. (A) MELK protein expression in H295R, CU-ACC1, and CU-ACC2 cell lines. (B) MELK inhibition using OTSSP167, the MELK inhibitor, was associated with decreased proliferation in H295R cells at doses >5 nM, (C) in CU-ACC1 cells at doses >25 nM, and (D) in CU-ACC2 cells at doses >5 nM. (E) Exposure to OTSSP167 was associated with decreases in clonogenicity of H295R cells at doses >0.5 nM, (F) in CU-ACC1 cells at doses >10 nM, and (G) in CU-ACC2 cells at doses >5 nM (*P < 0.05; **P < 0.01).
We next examined the effects of OTSSP167 on colony formation in the three ACC cell lines. Similar to effects on rates of proliferation, significant inhibition in colony formation was observed at 0.5 nM of the inhibitor in H295R high MELK expressers (P < 0.01), whereas the concentration needed to inhibit colonies in CU-ACC2 and CU-ACC1 cells with lower endogenous MELK was much higher at 5 and 10 nM, respectively (P < 0.01). Exposure to OTSSP167 was associated with a dose-dependent decrease in clonogenicity (P < 0.01) (Fig. 3E–3G). Taken together, these data suggest the CU-ACC1 and CU-ACC2 cells were less dependent on MELK activities for their growth compared with H295R cells and supported further elucidation of the role of MELK in ACC tumorigenesis.
The rate of apoptosis using MELK inhibitor (OTSSP167) is MELK expression dependent
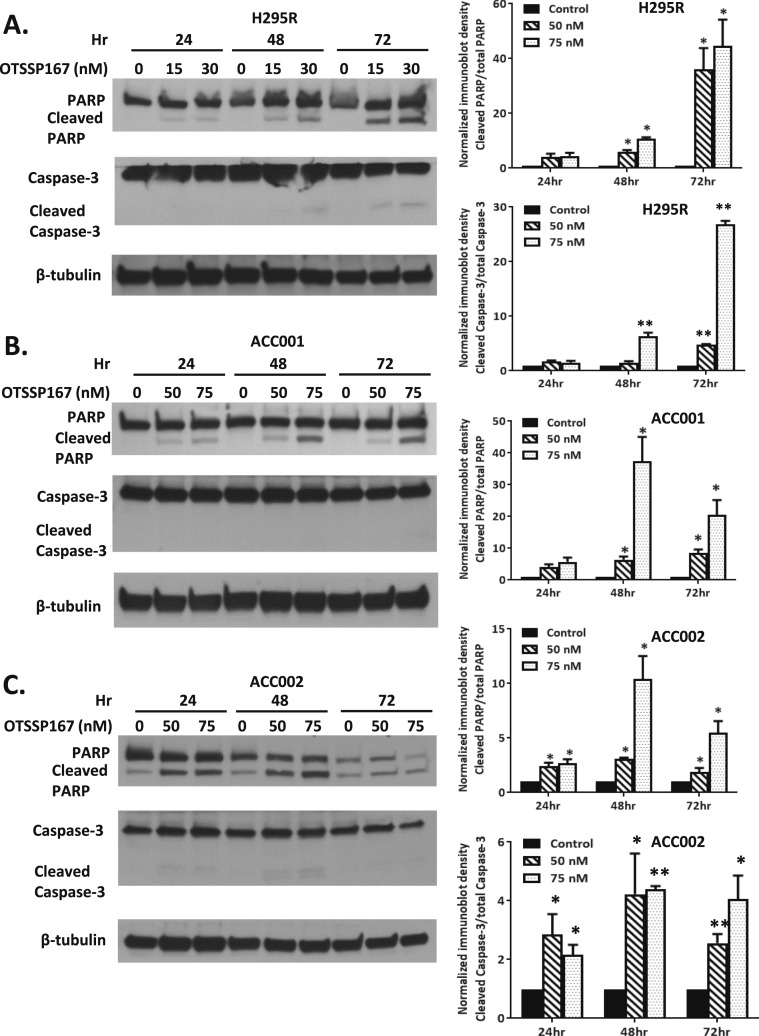
Based upon prior studies suggesting that MELK has a role in cell survival, we also evaluated the effects of the MELK inhibitor (OTSSP167) on rates of cell death. Similar to effects on proliferation rates and colony formation, H295R cells demonstrated higher rates of cell death assessed by PARP and caspase-3 cleavage at lower concentrations of the MELK inhibitor. As shown in Fig. 4A, H295R cells treated with OTSSP167 at >15 nM had increased rates of PARP cleavage at >24 hours and capase-3 cleavage at 72 hours (P < 0.05 and P < 0.01, respectively). In contrast, CU-ACC1 and CU-ACC2 cells required higher doses of the MELK inhibitor for similar effects on rates of apoptosis, with increased PARP cleavage occurring at >50 nM at >48 hours in CU-ACC1 cells (P < 0.05) with no observed caspase-3 cleavage. Effects on cleaved PARP were observed at >50 nM at >24 hours with increases in cleaved caspase-3 seen at >50 nM at >48 hours in CU-ACC2 cells (P < 0.05) (Fig. 4B and 4C). These data showed that the antitumor effects of OTSSP167 on cell survival also correlated in a dose-dependent manner with MELK expression in ACC cell lines.
Figure 4.
The MELK inhibitor, OTSSP167, resulted in a dose-dependent increase in rates of apoptosis in ACC cell lines based upon their endogenous MELK levels. (A) In H295R cells, the MELK inhibitor increased PARP cleavage at >15 nM at >48 h and caspase 3 cleavage at >30 nM at >48 h. (B) In CU-ACC1 cells, cleaved PARP was observed at >50 nM at >48 h and there was no caspase 3 cleavage noted. (C) In CU-ACC2 cells, the MELK inhibitor increased PARP and caspase 3 activation at >50 nM >24 h (*P < 0.05; **P < 0.01).
MELK expression controls proliferation and anchorage independent growth in ACC cell lines
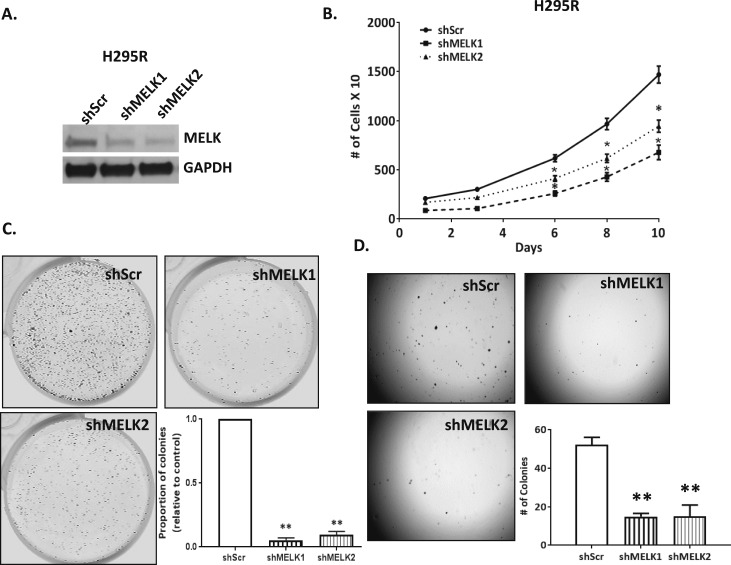
Recent findings suggest that the robust inhibition of proliferation of cancer cell lines mediated by OTSSP167 may also be a result of off target effects on multiple mitotic kinases in addition to MELK (51). To confirm the specific role of MELK in controlling ACC cell responses to the inhibitor on rates of proliferation, we developed in vitro models in which MELK was stably silenced. Because H295R ACC cells had the highest levels of endogenous MELK, MELK expression was silenced using a TRIPZ doxycycline inducible lentiviral shRNA system, with 57% decrease in MELK expression with shMELK1 and 68% decrease in MELK expression with shMELK2 compared with control (Fig. 5A). Using the Incucyte live imaging system, we assessed cell proliferation under normoxic conditions. Stable integration of shMELK1 and 2 decreased rates of cell proliferation on average by 1.9-fold compared with controls at day 10 (P < 0.05) (Fig. 5B). Next, clonogenicity assays were performed to examine the effect of MELK silencing on colony formation. MELK silencing resulted in 10- to 18-fold fewer colonies compared with controls (P < 0.01, for shMELK1 and shMELK2; Fig. 5C). In addition, soft agar assays were performed to assess the tumorigenic potential of loss of MELK. Silencing of MELK resulted in threefold fewer colonies (P < 0.01, for shMELK1 and shMELK2) compared with controls (Fig. 5D).
Figure 5.
MELK silencing decreased rates of proliferation and colony formation in H295R ACC cells. (A) Immunoblot depicting MELK silencing using two shRNA constructs. (B) MELK silencing was associated decreased rates of proliferation, (C) decreased colony formation in clonogenicity assay, and (D) decreased anchorage independent growth colony formation in soft agar (*P < 0.05; **P < 0.01).
Because ACC tumors are often large and necrotic with hypoxic pathway downstream effectors previously reported to play a role (42, 43, 52), we also assessed the effect of MELK silencing in hypoxia (1% O2) and noted decreased rates of cell proliferation on average by 1.8-fold compared with controls at day 10 (P < 0.05) (Supplemental Fig. 6). Together these data support the role of MELK to drive a tumorigenic potential via effects on rates of proliferation in ACC cells.
MELK promotes survival in adrenal carcinogenesis under hypoxic conditions
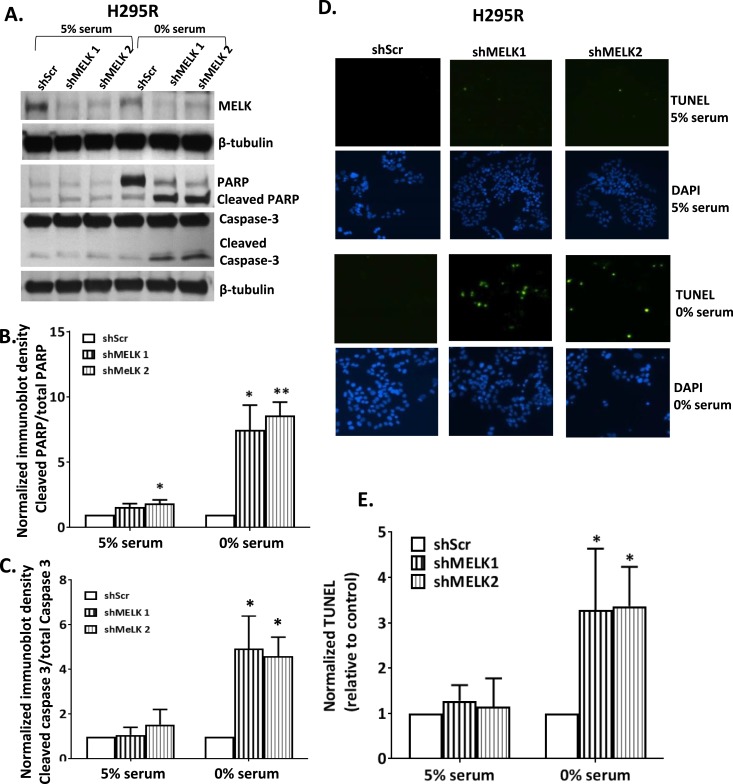
To investigate the specific role of MELK in ACC cell survival, H295R cells with or without silenced MELK were subjected to serum deprivation, initially under normoxic conditions. Unexpectedly, we did not detect differences in rates of tumor cell apoptosis (data not shown). Because ACC tumors are often large and necrotic (53, 54), we hypothesized that these tumors might have developed adaptive mechanisms to allow survival in a hypoxic microenvironment. H295R cells with silenced MELK were exposed to hypoxia (1% O2) in serum depleted and repleted media (Fig. 6A). Incubation in hypoxic conditions (1% O2) in replete serum had no effects as measured by PARP (Fig. 6B) or caspase-3 (Fig. 6C) cleavage. However, serum withdrawal together with a hypoxic microenvironment for 48 hours resulted in increased rates of apoptosis for MELK depleted ACC cells, as assessed by PARP cleavage (7.4- to 8.5-fold, P = 0.03 and P = 0.006 for shMELK1 and shMELK2, respectively) and caspase-3 cleavage (4.6- to 4.9-fold, P = 0.04 and P = 0.02 for shMELK1 and shMELK2, respectively) (Fig. 6A–6C). To examine DNA fragmentation as another measure of cell death, TUNEL assays were performed. As expected, hypoxia alone did not induce TUNEL labeling (Fig. 6D); however, exposure of ACC cells to serum depletion for 24 hours and concomitant hypoxia (1%) led to a threefold increase in apoptosis as assessed by TUNEL (P = 0.04 and P = 0.01 for shMELK1 and shMELK2, respectively) in MELK silenced cells compared with controls (Fig. 6D and 6E).
Figure 6.
MELK silencing promoted apoptosis in ACC cells under hypoxic and growth restricted conditions. (A) Representative immunoblot of shMELK H295R cells compared with scramble controls in 0% serum in hypoxia (1% O2) demonstrating increased caspase-3 and PARP cleavage under serum deprivation with no effects in 5% serum. Both (B) PARP and (C) cleaved caspase activation were increased in growth restricted and hypoxic (1% O2) environment in the presence of silencing of MELK. (D and E) Similarly, shMELK was associated with increased rates of apoptosis by TUNEL [representative pictures in panel (D) and quantified in panel (E)] in the presence of 0% serum and hypoxia (1% O2) for 24 h (*P < 0.05; **P < 0.01).
These experiments support the hypothesis that MELK expression promotes survival in a hypoxic microenvironment under growth-restricted conditions to promote adrenal carcinogenesis. Taken together, these data support the role of MELK to control ACC tumorigenicity via effects on proliferation and survival.
Discussion
ACC is a rare and aggressive malignancy with poor clinical outcomes. Although the progress to discover targeted agents for treatment of patients with ACC has been slow, recent large scale genomic profiling of ACC samples has increased our understanding of molecular pathways and hold promise to identify new druggable targets. In this study, we used multiple publically available genomic databases in conjunction with a literature review to identify a new therapeutic target in ACC. MELK was identified as upregulated in 40% to 50% of ACC tumors in TCGA. Selection of MELK as a potential target in ACC tumors was strengthened by the availability of a MELK inhibitor already in clinical trials in breast cancer (24). Additionally, a homozygous germline knockout of Melk in mice resulted in a normal phenotype, suggesting selective targeting of MELK with specific inhibitors may be useful in the clinic (24).
MELK, a member of the AMPK/Snf1 family, is a highly conserved serine/threonine kinase that was initially cloned in oocytes and identified as a signal transduction factor (55). Although the MELK protein structure has been well conserved across both mammalian and nonmammalian species, its functional roles appear to be species specific. In Xenopus laevis, MELK has a role in cell cycle, proliferation, and mitosis, whereas in mammalian and nonmammalian systems, MELK has been implicated in cell division, stem cell proliferation and organogenesis (56).
MELK overexpression has been reported in several human cancers, including prostate, breast, brain, colorectal, gastric, and acute myeloid leukemia (24, 57–64). We detected overexpression of the MELK transcript in ACC in multiple public platforms, and confirmed this finding at the protein level in our ACC human patient samples by IHC. High MELK expression has been associated with poor prognosis in patients with breast, gastric, and brain cancer (58, 64, 65). Similarly, we found that high MELK expression in the ACC TCGA database correlated with shorter survival from time of ACC diagnosis. This association was also confirmed using the ENSAT ACC data set where MELK was higher in the C1A ACC subgroup who have a more clinically aggressive disease.
To evaluate the interactome of MELK in ACC, we correlated variable endogenous MELK expression in three ACC cell lines with cell cycle transcripts identified as coregulated in TCGA analysis. We identified that increased MELK was associated with increases in expression of NEK2, BUB1B, PLK1, FOXM1, FANCG, TOP2A, KIF4A, LMNB1, CENPL, and TCF19 transcripts. Our findings are consistent with a recent analysis of the transcriptional landscape across multiple human cancer types (66), which demonstrated that these genes are consistently upregulated across different cancers and also are likely to be associated with poor clinical outcome.
Consistent with previous studies that reported that MELK inhibition downregulates FOXM1 levels in leukemia cells (63), FOXM1 expression was increased proportionally to MELK levels in ACC cell lines. Similar to experiments in breast cancer cells (24, 49), treatment of ACC cells with a FOXM1 inhibitor, thiostrepton, decreased MELK levels at both the mRNA and protein levels suggesting that in ACC, FOXM1 is an upstream regulator of MELK; however, the exact regulation and interaction of these proteins needs to be further elucidated. Together, these data begin to identify the components of the MELK pathway in ACC cell lines.
When ACC cells were exposed to the MELK inhibitor, OTSSP167, we confirmed a dose-dependent decrease in rates of proliferation and colony formation and an increase in rates of apoptosis. Selective silencing of MELK was associated with decreased colony formation in soft agar and clonogenicity assays with concomitant decreases in rates of cell proliferation. In contrast to our data and prior literature suggesting that MELK inhibition decreases rates of proliferation of many cancer cells lines (24, 57–64), a recent study (67) using CRISPER/CAS9 mutagenesis reported that MELK knockdown in several cancer cell lines was not associated with decrease in proliferation rates, and cells remained similarly sensitive to OTSSP167 compared with the wild-type controls. In the report, a complete MELK knockdown was achieved. We postulate that activation of redundant pathways in response to complete silencing may explain the discrepant effects compared with our data and the extensive published literature (24, 57–64). Further studies are needed to clarify these differences.
The role of MELK on cell survival has been controversial. Although studies in Caenorhabditis elegans showed that MELK is proapoptotic (68), data in glioblastoma and breast cancer models suggested MELK silencing is associated with increased rates of apoptosis, confirming its prosurvival function (61, 62). In contrast to MELK inhibitor, OTSSP167, where increase in apoptosis was dose dependent in normoxic conditions, in ACC cell lines, variable MELK levels were not associated with changes in survival in a growth factor replete, normoxic environment. These data suggest that the MELK inhibitor likely has additional off target effects, which are MELK independent. However, we showed that MELK does drive the rates of cell survival in a hypoxic microenvironment with growth factor restriction, which is commonly found in the large necrotic human ACC tumors. Interestingly, it has been also reported that other members of AMPK family are activated in hypoxic and serum-depleted environment to play a role in tumor survival (69–72). Together these data suggest that in ACC, MELK levels drive tumorigenesis by effects on both proliferation and alterations in tumor cell survival.
In summary, MELK is overexpressed in human ACC tumors, and it is correlated with poor clinical prognosis. Multiple human ACC cell lines are responsive in a dose-dependent manner to the currently available MELK inhibitor, in relation to their endogenous levels of MELK. MELK plays an important role in ACC tumorigenesis in vitro, through increases in rates of proliferation and decreases in apoptosis in a hypoxic growth factor restricted microenvironment. Our studies suggest that in ACC tumor cells, MELK is coregulated with cell cycle genes, including BUB1B, LMNB, ERCC6L, and PLK1, and it is downstream of FOXM1. Future studies are needed to further elucidate the mechanisms of MELK actions, as well as to evaluate the effects of MELK inhibitors in our newly established ACC patient-derived xenografts, toward confirming its importance as a therapeutic target for patients with ACC.
Supplementary Material
Acknowledgments
The results in this manuscript contain data generated by the TCGA Research Network: http://cancergenome.nih.gov/ and GTEx Portal https://www.gtexportal.org/home/.
Financial Support: This work was supported by Veterans Affairs Merit Review Award 001 (to M.E.W.), National Institutes of Health Grant K12CA086913-12 (to K.K.-V.), Cancer League of Colorado Award (to K.K.-V. and S.L.), Doris Duke Charitable Foundation University of Colorado–Fund to Retain Clinical Scientists (CU-FRSC) 2015212 (to K.K.-V.), University of Colorado Cancer Center Support Grant P30-CA046934, and the Cancer Center Genomics Core.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- ENSAT
European Network for the Study of Adrenal Tumor
- GTEx
Genotype-Tissue Expression project
- IHC
immunohistochemistry
- MELK
maternal embryonic leucine zipper kinase
- RNA-seq
RNA sequencing
- shRNA
short hairpin RNA
- shMELK
short hairpin RNA targeting maternal embryonic leucine zipper kinase
- TCGA
The Cancer Genome Atlas
References
- 1. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allolio B, Fassnacht M. Clinical review: adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91(6):2027–2037. [DOI] [PubMed] [Google Scholar]
- 3. Grubbs EG, Callender GG, Xing Y, Perrier ND, Evans DB, Phan AT, Lee JE. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17(1):263–270. [DOI] [PubMed] [Google Scholar]
- 4. Berruti A, Grisanti S, Pulzer A, Claps M, Daffara F, Loli P, Mannelli M, Boscaro M, Arvat E, Tiberio G, Hahner S, Zaggia B, Porpiglia F, Volante M, Fassnacht M, Terzolo M. Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab. 2017;102(4):1358–1365. [DOI] [PubMed] [Google Scholar]
- 5. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B; FIRM-ACT Study Group . Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 6. Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. [DOI] [PubMed] [Google Scholar]
- 7. Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libé R, de Reyniès A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607–612. [DOI] [PubMed] [Google Scholar]
- 8. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso MCBV, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Hammer GD, Giordano TJ, Verhaak RGW; Cancer Genome Atlas Research Network . Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29(5):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ilvesmäki V, Kahri AI, Miettinen PJ, Voutilainen R. Insulin-like growth factors (IGFs) and their receptors in adrenal tumors: high IGF-II expression in functional adrenocortical carcinomas. J Clin Endocrinol Metab. 1993;77(3):852–858. [DOI] [PubMed] [Google Scholar]
- 10. Ilvesmäki V, Liu J, Heikkilä P, Kahri AI, Voutilainen R. Expression of insulin-like growth factor binding protein 1-6 genes in adrenocortical tumors and pheochromocytomas. Horm Metab Res. 1998;30(10):619–623. [DOI] [PubMed] [Google Scholar]
- 11. Boulle N, Baudin E, Gicquel C, Logié A, Bertherat J, Penfornis A, Bertagna X, Luton JP, Schlumberger M, Le Bouc Y. Evaluation of plasma insulin-like growth factor binding protein-2 as a marker for adrenocortical tumors. Eur J Endocrinol. 2001;144(1):29–36. [DOI] [PubMed] [Google Scholar]
- 12. Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93(9):3524–3531. [DOI] [PubMed] [Google Scholar]
- 13. Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doghman M, Axelson M, Lalli E. Potent inhibitory effect of the cyclolignan picropodophyllin (PPP) on human adrenocortical carcinoma cells proliferation. Am J Cancer Res. 2011;1(3):356–361. [PMC free article] [PubMed] [Google Scholar]
- 15. Weigel B, Malempati S, Reid JM, Voss SD, Cho SY, Chen HX, Krailo M, Villaluna D, Adamson PC, Blaney SM. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014;61(3):452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. [DOI] [PubMed] [Google Scholar]
- 18. Adam P, Hahner S, Hartmann M, Heinrich B, Quinkler M, Willenberg HS, Saeger W, Sbiera S, Schmull S, Voelker HU, Ströbel P, Allolio B, Fassnacht M. Epidermal growth factor receptor in adrenocortical tumors: analysis of gene sequence, protein expression and correlation with clinical outcome. Mod Pathol. 2010;23(12):1596–1604. [DOI] [PubMed] [Google Scholar]
- 19. Berruti A, Sperone P, Ferrero A, Germano A, Ardito A, Priola AM, De Francia S, Volante M, Daffara F, Generali D, Leboulleux S, Perotti P, Baudin E, Papotti M, Terzolo M. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166(3):451–458. [DOI] [PubMed] [Google Scholar]
- 20. Gross DJ, Munter G, Bitan M, Siegal T, Gabizon A, Weitzen R, Merimsky O, Ackerstein A, Salmon A, Sella A, Slavin S; Israel Glivec in Solid Tumors Study Group . The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13(2):535–540. [DOI] [PubMed] [Google Scholar]
- 21. Quinkler M, Hahner S, Wortmann S, Johanssen S, Adam P, Ritter C, Strasburger C, Allolio B, Fassnacht M. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab. 2008;93(6):2057–2062. [DOI] [PubMed] [Google Scholar]
- 22. Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, Allolio B, Fassnacht M. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162(2):349–356. [DOI] [PubMed] [Google Scholar]
- 23. De Martino MC, Al Ghuzlan A, Aubert S, Assié G, Scoazec JY, Leboulleux S, Do Cao C, Libè R, Nozières C, Lombès M, Pattou F, Borson-Chazot F, Hescot S, Mazoyer C, Young J, Borget I, Colao A, Pivonello R, Soria JC, Bertherat J, Schlumberger M, Lacroix L, Baudin E. Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. J Clin Endocrinol Metab. 2013;98(10):4080–4088. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Lee YM, Baitsch L, Huang A, Xiang Y, Tong H, Lako A, Von T, Choi C, Lim E, Min J, Li L, Stegmeier F, Schlegel R, Eck MJ, Gray NS, Mitchison TJ, Zhao JJ. MELK is an oncogenic kinase essential for mitotic progression in basal-like breast cancer cells. eLife. 2014;3:e01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minata M, Gu C, Joshi K, Nakano-Okuno M, Hong C, Nguyen CH, Kornblum HI, Molla A, Nakano I. Multi-kinase inhibitor C1 triggers mitotic catastrophe of glioma stem cells mainly through MELK kinase inhibition. PLoS One. 2014;9(4):e92546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Speers C, Zhao SG, Kothari V, Santola A, Liu M, Wilder-Romans K, Evans J, Batra N, Bartelink H, Hayes DF, Lawrence TS, Brown PH, Pierce LJ, Feng FY Maternal Embryonic Leucine Zipper Kinase (MELK) as a novel mediator and biomarker of radioresistance in human breast cancer. Clin Cancer Res. 2016;22(23):5864–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beke L, Kig C, Linders JT, Boens S, Boeckx A, van Heerde E, Parade M, De Bondt A, Van den Wyngaert I, Bashir T, Ogata S, Meerpoel L, Van Eynde A, Johnson CN, Beullens M, Brehmer D, Bollen M. MELK-T1, a small-molecule inhibitor of protein kinase MELK, decreases DNA-damage tolerance in proliferating cancer cells. Biosci Rep. 2015;35(6):e00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiseljak-Vassiliades K, Zhang Y, Bagby SM, Kar A, Pozdeyev N, Xu M, Gowan K, Sharma V, Raeburn CD, Albuja-Cruz M, Jones KL, Fishbein L, Schweppe RE, Somerset H, Pitts TM, Leong S, Wierman ME. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer. 2018;25(4):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henderson HH, Timberlake KB, Austin ZA, Badani H, Sanford B, Tremblay K, Baird NL, Jones K, Rovnak J, Frietze S, Gilden D, Cohrs RJ. Occupancy of RNA polymerase II phosphorylated on serine 5 (RNAP S5P) and RNAP S2P on varicella-zoster virus genes 9, 51, and 66 is independent of transcript abundance and polymerase location within the gene. J Virol. 2015;90(3):1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bradford AP, Jones K, Kechris K, Chosich J, Montague M, Warren WC, May MC, Al-Safi Z, Kuokkanen S, Appt SE, Polotsky AJ. Joint MiRNA/mRNA expression profiling reveals changes consistent with development of dysfunctional corpus luteum after weight gain. PLoS One. 2015;10(8):e0135163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maycotte P, Jones KL, Goodall ML, Thorburn J, Thorburn A. Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol Cancer Res. 2015;13(4):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baird NL, Bowlin JL, Cohrs RJ, Gilden D, Jones KL. Comparison of varicella-zoster virus RNA sequences in human neurons and fibroblasts. J Virol. 2014;88(10):5877–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. RRID:AB 10861966.
- 37. RRID:AB 2160739.
- 38. RRID:AB 331439.
- 39. RRID:AB 10001219.
- 40. RRID:AB 2210370.
- 41. RRID:AB 631521.
- 42. RRID:AB 941613.
- 43. RRID:AB 2252687.
- 44. RRID:AB 2107445.
- 45. RRID:AB 1853798.
- 46. Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15(2):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Demeure MJ, Coan KE, Grant CS, Komorowski RA, Stephan E, Sinari S, Mount D, Bussey KJ. PTTG1 overexpression in adrenocortical cancer is associated with poor survival and represents a potential therapeutic target. Surgery. 2013;154(6):1405–1416, discussion 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joshi K, Banasavadi-Siddegowda Y, Mo X, Kim SH, Mao P, Kig C, Nardini D, Sobol RW, Chow LM, Kornblum HI, Waclaw R, Beullens M, Nakano I. MELK-dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells. Stem Cells. 2013;31(6):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kato T, Inoue H, Imoto S, Tamada Y, Miyamoto T, Matsuo Y, Nakamura Y, Park JH. Oncogenic roles of TOPK and MELK, and effective growth suppression by small molecular inhibitors in kidney cancer cells. Oncotarget. 2016;7(14):17652–17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chung S, Suzuki H, Miyamoto T, Takamatsu N, Tatsuguchi A, Ueda K, Kijima K, Nakamura Y, Matsuo Y. Development of an orally-administrative MELK-targeting inhibitor that suppresses the growth of various types of human cancer. Oncotarget. 2012;3(12):1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simon M, Mesmar F, Helguero L, Williams C. Genome-wide effects of MELK-inhibitor in triple-negative breast cancer cells indicate context-dependent response with p53 as a key determinant. PLoS One. 2017;12(2):e0172832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fenske W, Völker HU, Adam P, Hahner S, Johanssen S, Wortmann S, Schmidt M, Morcos M, Müller-Hermelink HK, Allolio B, Fassnacht M. Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer. 2009;16(3):919–928. [DOI] [PubMed] [Google Scholar]
- 53. Bharwani N, Rockall AG, Sahdev A, Gueorguiev M, Drake W, Grossman AB, Reznek RH. Adrenocortical carcinoma: the range of appearances on CT and MRI. AJR Am J Roentgenol. 2011;196(6):W706-14. [DOI] [PubMed] [Google Scholar]
- 54. Sasano H, Suzuki T, Moriya T. Recent advances in histopathology and immunohistochemistry of adrenocortical carcinoma. Endocr Pathol. 2006;17(4):345–354. [DOI] [PubMed] [Google Scholar]
- 55. Heyer BS, Warsowe J, Solter D, Knowles BB, Ackerman SL. New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol Reprod Dev. 1997;47(2):148–156. [DOI] [PubMed] [Google Scholar]
- 56. Ganguly R, Mohyeldin A, Thiel J, Kornblum HI, Beullens M, Nakano I. MELK-a conserved kinase: functions, signaling, cancer, and controversy. Clin Transl Med. 2015;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuner R, Fälth M, Pressinotti NC, Brase JC, Puig SB, Metzger J, Gade S, Schäfer G, Bartsch G, Steiner E, Klocker H, Sültmann H. The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J Mol Med (Berl). 2013;91(2):237–248. [DOI] [PubMed] [Google Scholar]
- 58. Pickard MR, Green AR, Ellis IO, Caldas C, Hedge VL, Mourtada-Maarabouni M, Williams GT. Dysregulated expression of Fau and MELK is associated with poor prognosis in breast cancer. Breast Cancer Res. 2009;11(4):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakano I, Masterman-Smith M, Saigusa K, Paucar AA, Horvath S, Shoemaker L, Watanabe M, Negro A, Bajpai R, Howes A, Lelievre V, Waschek JA, Lazareff JA, Freije WA, Liau LM, Gilbertson RJ, Cloughesy TF, Geschwind DH, Nelson SF, Mischel PS, Terskikh AV, Kornblum HI. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res. 2008;86(1):48–60. [DOI] [PubMed] [Google Scholar]
- 60. Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, Yi S, Bai W, Frantz G, Zhang Z, Koeppen H, de Sauvage FJ, Davis DP. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 2005;65(21):9751–9761. [DOI] [PubMed] [Google Scholar]
- 61. Du T, Qu Y, Li J, Li H, Su L, Zhou Q, Yan M, Li C, Zhu Z, Liu B. Maternal embryonic leucine zipper kinase enhances gastric cancer progression via the FAK/Paxillin pathway. Mol Cancer. 2014;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin ML, Park JH, Nishidate T, Nakamura Y, Katagiri T. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res. 2007;9(1):R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alachkar H, Mutonga MB, Metzeler KH, Fulton N, Malnassy G, Herold T, Spiekermann K, Bohlander SK, Hiddemann W, Matsuo Y, Stock W, Nakamura Y. Preclinical efficacy of maternal embryonic leucine-zipper kinase (MELK) inhibition in acute myeloid leukemia. Oncotarget. 2014;5(23):12371–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li S, Li Z, Guo T, Xing XF, Cheng X, Du H, Wen XZ, Ji JF. Maternal embryonic leucine zipper kinase serves as a poor prognosis marker and therapeutic target in gastric cancer. Oncotarget. 2016;7(5):6266–6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marie SK, Okamoto OK, Uno M, Hasegawa AP, Oba-Shinjo SM, Cohen T, Camargo AA, Kosoy A, Carlotti CG Jr, Toledo S, Moreira-Filho CA, Zago MA, Simpson AJ, Caballero OL. Maternal embryonic leucine zipper kinase transcript abundance correlates with malignancy grade in human astrocytomas. Int J Cancer. 2008;122(4):807–815. [DOI] [PubMed] [Google Scholar]
- 66. Li M, Sun Q, Wang X. Transcriptional landscape of human cancers. Oncotarget. 2017;8(21):34534–34551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin A, Giuliano CJ, Sayles NM, Sheltzer JM. CRISPR/Cas9 mutagenesis invalidates a putative cancer dependency targeted in on-going clinical trials. eLife. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Denning DP, Hatch V, Horvitz HR. Programmed elimination of cells by caspase-independent cell extrusion in C. elegans. Nature. 2012;488(7410):226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278(33):31000–31006. [DOI] [PubMed] [Google Scholar]
- 70. Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278(34):31629–31639. [DOI] [PubMed] [Google Scholar]
- 71. Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21(39):6082–6090. [DOI] [PubMed] [Google Scholar]
- 72. Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Lavin MF, Esumi H. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J Biol Chem. 2003;278(1):48–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.