Abstract
Objective
To evaluate pharmacologic treatment options for visual snow and to report prevalence of comorbid diseases.
Methods
Medical charts of patients with a diagnosis of visual snow at the neurology outpatient clinic were reviewed on prescribed medication, and comorbid migraine, tinnitus, and psychiatric conditions including depression and anxiety.
Results
From 2007 to 2018, 58 patients were diagnosed with visual snow. Comorbid migraine was present in 51.7% of patients, lifetime depression in 41.4%, and lifetime anxiety in 44.8%. Lamotrigine was prescribed most frequently (26/58) and resulted in partial remission of symptoms in 5/26 (19.2%). No patients reported complete remission. Adverse events occurred in 13/26 (50.0%) patients. None of the other prescribed drugs (valproate [n = 7], topiramate [n = 4], acetazolamide [n = 2], flunarizine [n = 1]) led to improvement except for topiramate in one patient, who discontinued, however, because of adverse events.
Conclusions
Of medication prescribed (lamotrigine, valproate, acetazolamide, flunarizine), only lamotrigine afforded some improvement in a small minority of patients. Migraine, depression, anxiety, and tinnitus were common comorbid diseases.
Classification of evidence
This study provides Class IV evidence that for some patients with visual snow, lamotrigine resulted in partial remission of symptoms.
Visual snow is characterized by the continuous presence of countless, small, flickering dots in the entire visual field.1 Patients often describe it as seeing “TV static”; that is, the noise of a detuned analogue television. Diagnosis of visual snow is made after exclusion of secondary causes of pan-field visual disturbances, such as lesions in the visual pathway, retinal pathology, and occipital epilepsy. Visual snow syndrome is diagnosed in patients who in addition have ≥2 of the following visual symptoms: palinopsia, enhanced entoptic phenomena, photophobia, or nyctalopia.1 The pathophysiology of visual snow is unknown.2 In the past, visual snow was often believed to be a migraine phenomenon, because many patients also had migraine with aura.1,3 However, in visual snow, classical migraine characteristics such as (scintillating) scotoma or zig-zag lines are absent. Moreover, visual snow may also occur in patients without migraine.
Other diseases such as tinnitus, depression, and anxiety have also been frequently reported in patients with visual snow,1,3 but never studied in an outpatient clinic setting.
Pharmacologic treatment of visual snow is based on expert opinion and single case reports.2,4,5 In one patient, 50 mg lamotrigine BID resulted in complete remission of visual snow.4 More extensive evaluations of the effects of treatment in visual snow are not available.
In the present study, we evaluated treatment responses in 58 patients with visual snow from our outpatient clinic and studied the comorbid diseases, notably migraine, tinnitus, depression, and anxiety disorder.
Methods
We evaluated pharmacologic treatment options and comorbidities in a case series of visual snow patients (Class IV evidence). Patients who were referred to our neurology outpatient clinic with “positive visual disturbances,” between November 2007 and June 2018, were retrospectively included. Diagnosis of visual snow was made according to criteria as proposed earlier.1 If patients had ≥2 additional visual symptoms, they also met visual snow syndrome criteria.1 Medical charts and referral letters were reviewed and data were extracted on demographics, comorbid migraine, medical history, performed diagnostic tests, and prescribed medication. Depression and anxiety were evaluated as part of standard clinical care using a previously published mood disorder questionnaire and algorithm with validated cutoff scores for the Hospital Anxiety and Depression Scale (HADS) and the Center for Epidemiologic Studies Depression Scale (CES-D).6 This is standard for headache patients who visit our clinic, because of the shared comorbidity between migraine and mood disorders.6 Since most visual snow patients were scheduled in headache consulting hours, they also received the questionnaire. Patients filled in the questionnaire before their visit. Lifetime depression was defined as HADS depression score ≥8 or CES-D score ≥16, or (past) depression diagnosed by a physician, or (past) use of antidepressants for depression. Lifetime anxiety was defined as HADS anxiety score ≥8, or (past) therapy for anxiety disorder, or (past) use of antidepressant for anxiety disorder.6 Current depression was defined as HADS depression score ≥8 or CES-D ≥16, and current anxiety was defined as HADS anxiety score ≥8.6
For prescribed medication, dosage, adverse events, and decision to continue or discontinue were documented. Complete remission was defined as absence of visual snow. Partial remission was defined as self-assessed reduction in symptom severity without a strict cutoff in minimum reduction because there is no standardized questionnaire for severity of visual snow.
Standard protocol approvals, registrations, and patient consents
This study was approved by the ethical committee of the Leiden University Medical Centre.
Data availability
Anonymized data can be obtained by request from any qualified investigator for purposes of replicating procedures and results.
Results
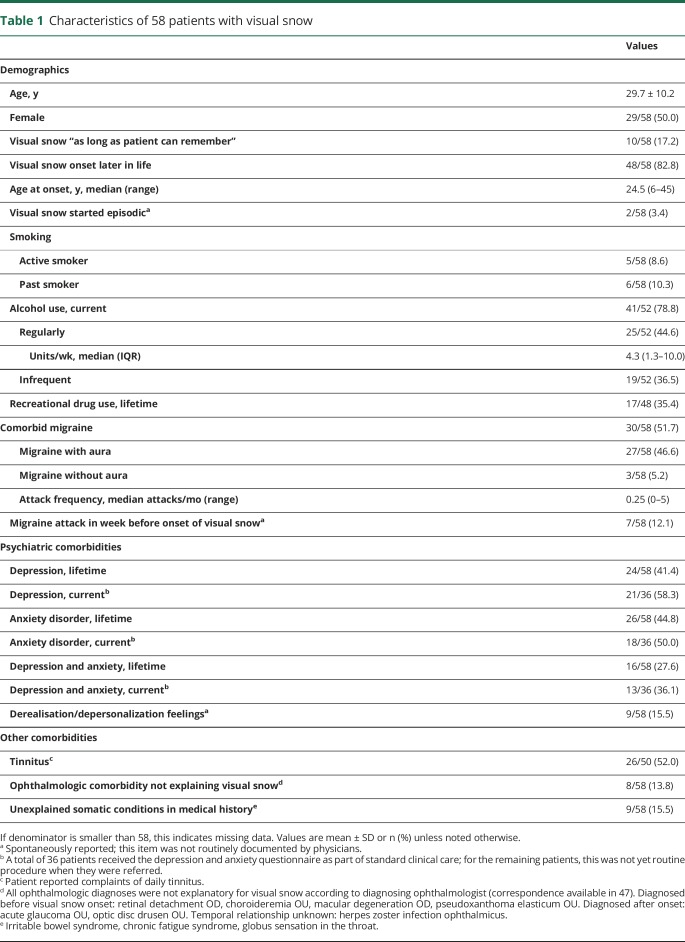
Of 63 referrals, 58 (92.1%) patients (29 female, 29 male) were diagnosed with visual snow and 47 (74.6%) additionally met visual snow syndrome criteria1 (figure). Some patients had self-diagnosed and had explicitly asked their general practitioner for a referral. Clinical characteristics are summarized in table 1. Median age at onset was 24.5 years (range 6–45) for patients who could remember onset of visual snow (48/58 patients). Comorbid migraine was present in 30 patients (51.7%), mainly migraine with aura (27/30). Six patients reported that visual snow started in close temporal association with a migraine with aura attack, in 3 during the attack and in another 3 in the few days thereafter. One patient reported onset of visual snow following a migraine without aura attack. Attack frequency in these 7 patients was low (median 0.5 attacks/month, range 0.1–1.0), making a coincidental temporal association less likely.
Figure. Flowchart of study participants.
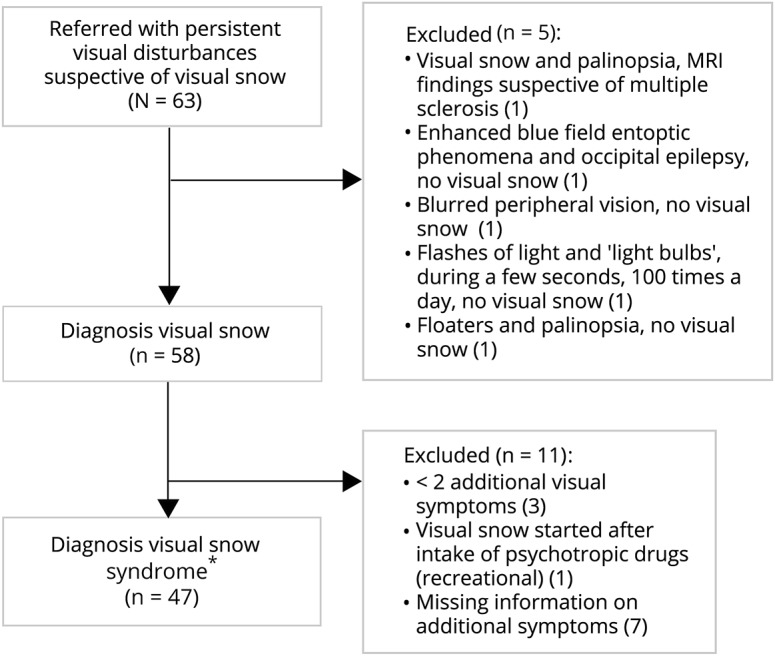
*Visual snow syndrome criteria as proposed by Schankin et al.1
Table 1.
Characteristics of 58 patients with visual snow
Lifetime depression was present in 24/58 (41.4%) patients and anxiety disorder in 26/58 (44.8%) (table 1). Of the 36 patients who filled in the mood disorder questionnaire, current depression was present in 21 (58.3%) and current anxiety in 18 (50.0%). Prevalence of lifetime depression and anxiety was not correlated with the presence of comorbid migraine (both 50.0% in patients without migraine and 33.0% and 40.0%, respectively, in patients with migraine). Daily tinnitus was present in 26/50 (52.0%) patients, absent in 24/50 (48.0%), and unknown in 8 patients.
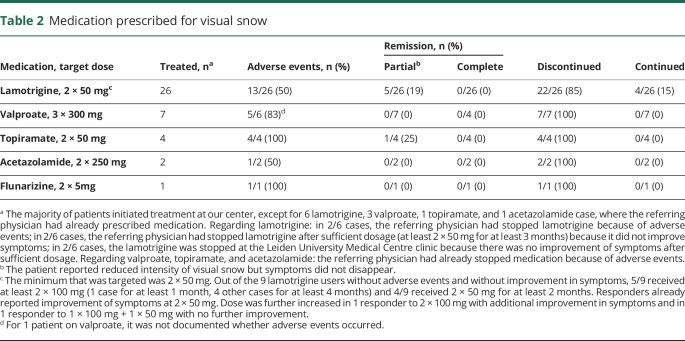
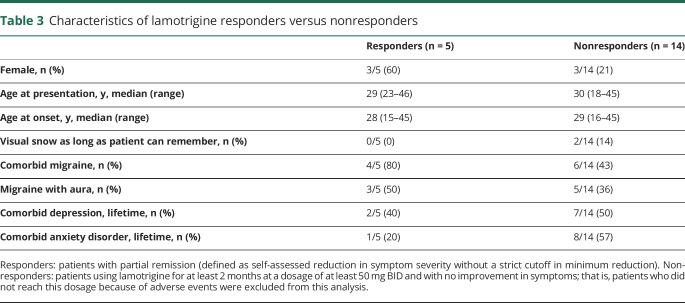
Table 2 summarizes the various treatments and treatment responses. Lamotrigine was prescribed most frequently and resulted in partial remission of symptoms in 5/26 (19.2%) patients; zero patients reported complete remission. Patients with partial remission reported “50% less intensity of visual snow” (n = 1), “substantial reduction in intensity” (n = 1), and “minor improvement in intensity” (n = 3). Adverse events occurred in 13/26 (50.0%) patients. Major adverse events were found in 4/13 patients (n = 3 allergic reactions, n = 1 excessive daytime sleepiness), leading to discontinuation within 1 month. All other adverse events were minor and these patients used lamotrigine for at least 1 month. Characteristics of patients who reported partial remission on lamotrigine (responders) are described in table 3.
Table 2.
Medication prescribed for visual snow
Table 3.
Characteristics of lamotrigine responders versus nonresponders
None of the other drugs (valproate, topiramate, acetazolamide, and flunarizine) resulted in improvement of symptoms, except for topiramate in one patient, who discontinued because of adverse events. Twenty-nine patients did not want to start therapy as no evidence-based therapies could be offered or because of fear of adverse events. It cannot be excluded that symptoms were less severe in these patients.
Discussion
We evaluated treatment response and comorbid diseases in 58 patients with visual snow. Lamotrigine was prescribed most frequently and resulted in partial remission in 5/26 patients. No treatment resulted in complete remission of symptoms. Migraine, depression, anxiety, and tinnitus were highly prevalent comorbid disorders.
Information on treatment of visual snow is limited to case reports. Lamotrigine 50 mg BID was effective in one case (complete remission),4 but not in 2 other cases.5 In our patients, lamotrigine afforded partial remission in 5/26 (19%) patients. A randomized controlled trial is needed to better determine the efficacy of lamotrigine, preferably using a minimum dosage of 100 mg BID. Nonetheless, the present data indicate there will be a large group of patients who will not benefit from lamotrigine. Future research on novel treatment options is necessary, including other approaches such as neuromodulation. The numbers for the other drugs are too small to determine efficacy; however, we consider their adverse events profiles less favorable than lamotrigine.
In line with other studies, migraine was more prevalent than in epidemiologic studies in the general population, in particular migraine with aura. It has therefore been hypothesized that both migraine aura and visual snow share pathophysiologic mechanisms and are a consequence of cortical hyperexcitability.7 Indeed, in some patients, visual snow had started during or shortly after migraine aura, which might support this hypothesis.
Since many patients also had depression and anxiety, we advise to screen patients with visual snow for these conditions. In our study population, ≥50% of patients had active depression or anxiety symptomatology, which is higher than in earlier studies. One study found depression in 11/78 (14.1%) patients and anxiety in 12/78 (15.4%) patients, using the Patient Health Questionnaire 8-item depression scale and 7-item Generalized Anxiety Disorder scale. However, patients were recruited via social media and may differ from those seeking help from a doctor.1 In a neuro-ophthalmology clinic, 6/32 (19%) patients had depression and 14/32 (44%) patients had anxiety disorder, but only medical charts were reviewed for past diagnoses.3
The current study cannot provide an answer on whether there is a causal relationship between these comorbidities. Depression/anxiety may either be cause or consequence of visual snow, or might share underlying (genetic) risk factors, as is the case for the bidirectional relationship between depression and migraine.8,9 We advise to treat depression and anxiety in visual snow patients because these might negatively affect disease burden and coping behavior. For tinnitus, it is known that depression is associated with higher disease burden10 and comorbid depression in migraine patients increases the risk of medication overuse and migraine chronification.11 Furthermore, cognitive therapy is effective in reducing tinnitus severity and impairment.12 Cognitive therapy might therefore be effective in visual snow as well.
The strength of this study is the relatively large number of patients seen at a neurology clinic. There are limitations, however. The retrospective design might have caused selection, recall, and information bias. Additional visual symptoms were not documented for all patients since the criteria for visual snow syndrome were only published in 2014.1,13 Nonetheless, all our patients had the diagnostic symptom of visual snow. Depression/anxiety questionnaire data were only available for 36/58 (62.1%) patients. Evaluation of treatment was based on medical charts rather than a standardized protocol. However, this did not change the observation that the vast majority of patients stopped therapy. Patients were referred to a headache center, which could have led to selection of cases with comorbid migraine. However, patients were not referred for their migraines but for persistent visual complaints.
Migraine, depression, anxiety, and tinnitus are prevalent in visual snow. Different treatments were prescribed for visual snow but all failed except for some improvement in a small minority of patients using lamotrigine.
Glossary
- CES-D
Center for Epidemiologic Studies Depression Scale
- HADS
Hospital Anxiety and Depression Scale
Footnotes
Author contributions
Robin Marc van Dongen: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis. Lindy Waaijer: data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, database design. Gerrit Onderwater: drafting/revising the manuscript, data acquisition, study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data. Michel Ferrari: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision, obtaining funding. Gisela Terwindt: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision.
Study funding
This study was not industry-sponsored. This study was supported by the Netherlands Organization for Scientific Research (VICI grant 918.56.601 and Spinoza 2009 to M.D.F.; Clinical Fellowship 90700217 and VIDI grant 917.11.31 to G.M.T.) and European Community (EUROHEADPAIN no. 602633 to M.D.F.).
Disclosure
R. van Dongen, L. Waaijer, and G. Onderwater report no disclosures relevant to the manuscript. M. Ferrari reports grants and consultancy or industry support from Medtronic, Novartis, Lilly, and TEVA and independent support from NWO, ZonMW, NIH, European Community, and the Dutch Heart Foundation. G. Terwindt reports consultancy/industry support from Novartis and Lilly and independent support from ZonMW, European Community, NIH, Dutch Brain Foundation, Dutch Heart Foundation, and the CAA foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Schankin CJ, Maniyar FH, Digre KB, et al. “Visual snow”: a disorder distinct from persistent migraine aura. Brain 2014;137:1419–1428. [DOI] [PubMed] [Google Scholar]
- 2.Puledda F, Schankin C, Digre K, Goadsby PJ. Visual snow syndrome: what we know so far. Curr Opin Neurol 2018;31:52–58. [DOI] [PubMed] [Google Scholar]
- 3.Lauschke JL, Plant GT, Fraser CL. Visual snow: a thalamocortical dysrhythmia of the visual pathway? J Clin Neurosci 2016;28:123–127. [DOI] [PubMed] [Google Scholar]
- 4.Unal-Cevik I, Yildiz FG. Visual snow in migraine with aura: further characterization by brain imaging, electrophysiology, and treatment: case report. Headache 2015;55:1436–1441. [DOI] [PubMed] [Google Scholar]
- 5.Wang YF, Fuh JL, Chen WT, Wang SJ. The visual aura rating scale as an outcome predictor for persistent visual aura without infarction. Cephalalgia 2008;28:1298–1304. [DOI] [PubMed] [Google Scholar]
- 6.Louter MA, Wardenaar KJ, Veen G, et al. Allodynia is associated with a higher prevalence of depression in migraine patients. Cephalalgia 2014;34:1187–1192. [DOI] [PubMed] [Google Scholar]
- 7.Chen WT, Fuh JL, Lu SR, Wang SJ. Persistent migrainous visual phenomena might be responsive to lamotrigine. Headache 2001;41:823–825. [DOI] [PubMed] [Google Scholar]
- 8.Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology 2003;60:1308–1312. [DOI] [PubMed] [Google Scholar]
- 9.The Brainstorm Consortium. Analysis of shared heritability in common disorders of the brain. Science 2018;360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trevis KJ, McLachlan NM, Wilson SJ. Psychological mediators of chronic tinnitus: the critical role of depression. J Affect Disord 2016;204:234–240. [DOI] [PubMed] [Google Scholar]
- 11.Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain 2013;136:3489–3496. [DOI] [PubMed] [Google Scholar]
- 12.Cima RF, Maes IH, Joore MA, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 2012;379:1951–1959. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;1:1–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be obtained by request from any qualified investigator for purposes of replicating procedures and results.