ABSTRACT
Background
Following a vegetarian diet is considered to be beneficial for overall health and is associated with a lower risk of chronic disease.
Objective
This study examined whether South Asians in the United States who consume a vegetarian diet have a lower prevalence of cardiometabolic risk factors.
Methods
Data from the Mediators of Atherosclerosis in South Asians Living in America study, which included 892 South Asians (47% women), with an age range of 40–83 y and a mean ± SD age of 55 ± 9.4 y, were used. Participants were classified as vegetarian if they reported no consumption of meat, poultry, or fish in the previous year on a validated and culturally appropriate food-frequency questionnaire. Adjusted linear and logistic regression models were used to examine associations of a vegetarian diet with cardiometabolic risk factors.
Results
Thirty-eight percent of the cohort participants were classified as vegetarian. Vegetarians reported more frequent weekly eating occasions of whole grains (median frequency/wk: 10 compared with 9, P = 0.012) and beans and legumes (median frequency/wk: 8.5 compared with 5.1, P < 0.001), and less frequent weekly eating occasions of sweets and desserts (median frequency/wk: 1.9 compared with 2.3, P < 0.001). Consuming a vegetarian diet was associated with lower body mass index (P = 0.023), fasting glucose (P = 0.015), insulin resistance (P = 0.003), total cholesterol (P = 0.027), and LDL cholesterol (P = 0.004) and lower odds of fatty liver (OR: 0.43; 95% CI: 0.23, 0.78, P = 0.006). The odds of having any coronary artery calcium were lower for vegetarian men (OR: 0.53; 95% CI: 0.32, 0.87, P = 0.013); however, no significant associations were observed among women.
Conclusions
Among US South Asians, a vegetarian diet was associated with fewer cardiometabolic risk factors overall and with less subclinical atherosclerosis among men.
Keywords: South Asians, vegetarian, cardiometabolic risk factors, dietary pattern, subclinical atherosclerosis
Introduction
Dietary patterns and behaviors are important lifestyle factors for overall health and in preventing chronic diseases. A vegetarian diet has been found to be protective for cardiometabolic health (1) and has been shown to be associated with a lower risk of obesity (2), diabetes (3), hypertension (4), lower concentrations of total and LDL cholesterol (5, 6), and a lower risk of subclinical atherosclerosis (6).
South Asians, individuals who originate from countries of the Indian subcontinent including Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka, are one of the fastest growing ethnic groups in the United States (7). Studies have found a higher prevalence of chronic diseases such as type 2 diabetes and cardiovascular disease among South Asians than among non-Hispanic whites (8, 9). A higher proportion of South Asians also follow a vegetarian diet owing to religious beliefs and cultural reasons (10). However, following a vegetarian diet does not always indicate that healthful foods are included as part of the dietary pattern. For example, a recent study comparing vegetarian patterns among a nationally representative sample from the 2003–2004 and 2005–2006 cycles of the NHANES in the United States and Asian Indians living in India found that vegetarians in India consumed more sweets and fried foods than did vegetarians living in the United States (11). In addition, the protective association of a vegetarian diet for cardiometabolic risk was stronger among US vegetarians (3, 11). Associations between a vegetarian diet and cardiometabolic risk factors among South Asians living in the United States have not been extensively studied.
The overall goal of this study was to examine the dietary and nutrient intakes of US South Asians who consume a vegetarian diet, and to examine the cross-sectional associations of a vegetarian diet with cardiometabolic risk factors, independent of overall diet quality.
Methods
Baseline data from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study were used for these analyses. Detailed information on the MASALA study has been provided elsewhere (12). Briefly, this community-based cohort of South Asians from the San Francisco Bay area and the greater Chicago area was recruited between 2010 and 2013 with the use of surname-based recruitment methods. Study data were collected via interviews, and a clinical examination included anthropometric assessments, blood pressure measurement, blood tests, and procedures to ascertain subclinical atherosclerosis (12). The MASALA study included 906 South Asians, and after excluding 14 participants because of missing or unreliable information on dietary intakes a total of 892 participants were included in these analyses. The study protocol was approved by the institutional review boards of University of California, San Francisco and Northwestern University. All participants signed informed consent forms before undergoing study procedures.
Assessment of dietary intakes, estimation of nutrients, and defining “vegetarian”
The validated Study of Health Assessment and Risk in Ethnic groups (SHARE) FFQ, specifically developed for assessing dietary intakes of South Asians in North America, was used as the dietary assessment instrument in the study (13). For these analyses, we considered participants to be vegetarians if they reported on the FFQ that they did not consume any meat, poultry, or fish regardless of egg and dairy product consumption in the previous year.
The weekly eating occasions for 18 foods and food groups, including alcohol, beans and legumes, whole grains, some whole grains, refined grains, fats and oil, fruits, meat, poultry and seafood, egg, dairy, mixed dishes, nuts and oilseeds, snacks, sweets, and desserts, sugar, candy, and jams, sugar-sweetened beverages, starchy vegetables, and vegetables, were derived from the 163-item FFQ. Detailed classification for these has been provided in Supplemental Table 1. Dietary data obtained from the SHARE FFQ were analyzed by the ESHA Food Processor nutrient analysis software version 6.11 (1996), to generate estimates of macro- and micronutrient intakes.
Assessment of cardiometabolic risk factors
Height, weight, and waist circumference were measured through the use of standard methods. BMI was calculated as kg/m2. Hepatic fat attenuation was assessed by noncontrast computed tomography (CT), and fatty liver was defined as hepatic fat attenuation <40 Hounsfield units (14). Visceral fat area (expressed as cm2) was assessed by a single-slice abdomen CT at the level of L4-L5. Blood pressure was measured in a seated position with an automated blood pressure monitor (V100 Vital Signs Monitor; GE Healthcare). A total of 3 assessments of blood pressure were made and the averages of the second and third readings were used for analyses. Agatston scores for measurement of coronary artery calcium (CAC) were generated with the use of noncontrast gated-cardiac CT scans. CAC was analyzed dichotomously as present or absent (Agatson score >0 compared with 0). High-resolution B-mode ultrasonography was used for measuring common and internal carotid artery intima media thickness (CIMT). Detailed information for all assessments has been provided elsewhere (12).
Laboratory assessments were performed after a 12-h fast. Serum lipids including total cholesterol, TGs, and HDL cholesterol were measured with enzymatic methods (Quest), and LDL cholesterol was calculated via the Friedewald formula (15). Plasma glucose was assessed via the hexokinase method (Ortho Clinical Diagnostics, Johnson & Johnson); insulin was measured by sandwich immunoassay (Roche Elecsys 2010; Roche Diagnostics), and insulin resistance was assessed by the HOMA-IR and calculated as fasting insulin (µIU/mL) multiplied by fasting glucose (mmol/L)/22.5 (16).
Covariates
Selection of covariates was based on univariate analysis, our previous studies with this cohort, and published literature and included age, sex, study site, education, traditional cultural beliefs, smoking status, intentional exercise, alcohol and energy intakes, diet quality, and medication use as assessed by structured interview questions and study questionnaires (12). Education was categorized as having a Bachelor's degree or higher compared with less than a Bachelor's degree. A traditional cultural beliefs scale was developed for this cohort and individuals were categorized as having strong, moderate, or weak traditional South Asian cultural beliefs (17). The Typical Week's Physical Activity Questionnaire was used to assess intentional exercise including walking for exercise, dance, conditional activities, and sports, and total metabolic equivalent minutes per week were calculated and used for analysis (18). Alcohol intake and daily energy intake were derived from the FFQ for analysis and used in the models on a continuous scale as grams of ethanol per day and kilocalories per day, respectively. The Alternative Healthy Eating Index (AHEI) was used as a measure of diet quality, and includes 11 food components with scores ranging from 0 to 10 for each component, based on intake as reported on the SHARE FFQ. The possible range of the total AHEI score is from 0 to 110, with higher AHEI scores indicating a better quality of diet (19). For these analyses, AHEI scores were modeled on a continuous scale.
Statistical analysis
Sociodemographic characteristics were reported as mean ± SD, median (IQR), or percentage by vegetarian status. Student's t test, Mann-Whitney U tests, and chi-square tests were used for univariate analyses comparing sociodemographic characteristics and cardiometabolic risk factors between vegetarian and nonvegetarian participants. The Mann-Whitney U test was also used for comparing the number of eating occasions per week for food groups across vegetarian and nonvegetarian participants. Comparisons by vegetarian status for energy, macro-, and micronutrient intakes were made with the use of linear regression models after adjusting for age, sex, and total energy intake, except for those that were a proportion of energy intake. Multivariable linear regression models were created to determine the associations between vegetarian status and cardiometabolic risk factors including BMI; waist circumference; systolic and diastolic blood pressure; fasting glucose; HOMA-IR; TG; total, HDL, and LDL cholesterol; and common and internal CIMT after adjusting for age, sex, study site, alcohol and energy intake, smoking status, BMI (for outcomes other than BMI and waist circumference), education, traditional cultural beliefs, intentional exercise, and AHEI score. Models for fasting glucose, blood pressure, and total and LDL cholesterol were also adjusted for glucose, blood pressure, and lipid-lowering medication use, respectively. TG, fasting glucose, and HOMA-IR were natural log transformed in the multivariable linear regression models owing to skewed distribution. And lastly, multivariable logistic regression was used to assess the association between vegetarian status and presence of fatty liver and CAC. We checked to see if the association between vegetarian status and the cardiometabolic risk factors varied by sex by adding an interaction term into the model. Because the AHEI score does not include sweets and desserts, we also performed sensitivity analyses, adding this category to the final model (data not presented). Because the Friedewald formula for estimation of LDL cholesterol may be inaccurate for individuals with high TGs, we also performed sensitivity analysis for LDL cholesterol by excluding those with serum TGs >150 mg/dL. All analyses were performed with SAS version 9.4 (SAS Institute, 2013), and a 2-sided P value of <0.05 was considered to be statistically significant.
Results
Sociodemographic characteristics by vegetarian status are reported in Table 1. The mean ± SD age of the MASALA cohort was 55 ± 9.4 y with 47% women. Approximately 38% of the participants reported consuming a vegetarian diet, and 58% of vegetarians were women (P < 0.001). Individuals with strong traditional cultural beliefs were more likely to follow a vegetarian diet (P < 0.001). Vegetarians also had slightly lower scores on the AHEI than did nonvegetarians (P < 0.001). In univariate analysis, vegetarians tended to have a lower BMI, lower waist circumference, lower LDL cholesterol, lower HOMA-IR, lower diastolic blood pressure, lower subclinical atherosclerosis as measured by CAC (men only) and CIMT, and a lower proportion of them had fatty liver (P < 0.05 for all) compared with nonvegetarians. The sex-specific distributions of sociodemographic characteristics are shown in Supplemental Tables 2–4. Number of eating occasions per week of foods and food groups showed that vegetarians had less frequent consumption of alcohol, eggs, and sweets and desserts, and higher frequencies of beans and legumes, whole grains, and fats and oil consumption compared with nonvegetarians (P < 0.05 for all) (Table 2).
TABLE 1.
Sociodemographic and health characteristics by vegetarian status in the MASALA cohort1
| Vegetarian | Nonvegetarian | |||
|---|---|---|---|---|
| Total (n = 892) | (n = 335) | (n = 557) | P | |
| Age, y | 55.3 ± 9.4 | 55.6 ± 9.2 | 55.2 ± 9.5 | 0.558 |
| Women, % | 47.1 | 57.6 | 40.8 | <0.001 |
| Current smoker, % | 3.1 | 1.5 | 4.1 | 0.029 |
| Education ≥ Bachelor's degree, % | 87.8 | 88.1 | 87.6 | 0.843 |
| Income ≥ $75K,2 % | 73.6 | 70.8 | 75.2 | 0.158 |
| Traditional cultural beliefs, % | <0.001 | |||
| Strong | 33.4 | 44.7 | 26.4 | |
| Intermediate | 29.9 | 29.0 | 30.4 | |
| Weak | 36.7 | 23.1 | 43.2 | |
| Intentional exercise, MET min/wk | 945 (315–1860) | 945 (420–1760) | 960 (315–1890) | 0.99 |
| Alternative Healthy Eating Index score | 70.1 ± 6.8 | 68.2 ± 5.1 | 71.3 ± 7.4 | <0.001 |
| Alcohol, g/d | 0.2 (0–2.6) | 0 (0–0.3) | 1.0 (0.01–5.3) | <0.001 |
| Energy intake, kcal/d | 1630 (1300–1970) | 1540 (1280–1860) | 1690 (1350–2010) | <0.001 |
| BMI, kg/m2 | 26.0 ± 4.1 | 25.6 ± 4.1 | 26.2 ± 4.1 | 0.027 |
| Waist circumference, cm | 92.7 ± 10.4 | 91.1 ± 10.1 | 93.7 ± 10.5 | <0.001 |
| Serum total cholesterol,2 mg/dL | 188 ± 36.9 | 186 ± 35.5 | 189 ± 37.6 | 0.154 |
| Serum TGs,2 mg/dL | 118 (88–157) | 118 (89–156) | 118 (88–158) | 0.99 |
| Serum LDL cholesterol,2 mg/dL | 111 ± 32.0 | 109 ± 30.2 | 113 ± 32.9 | 0.036 |
| Serum HDL cholesterol,2 mg/dL | 50.1 ± 13.3 | 51.2 ± 13.4 | 49.4 ± 13.3 | 0.055 |
| Statin use, % | 26.6 | 24.2 | 27.7 | 0.255 |
| Fasting plasma glucose,2 mg/dL | 94 (87–106) | 96 (89–105) | 98 (90–109) | 0.054 |
| HOMA-IR2 | 2.5 (1.6–3.9) | 2.2 (1.5–3.3) | 2.6 (1.7–4.3) | <0.001 |
| Diabetes medication use, % | 16.0 | 17.0 | 15.4 | 0.535 |
| Systolic blood pressure, mm Hg | 125 ± 15.8 | 124 ± 15.7 | 125 ± 15.8 | 0.210 |
| Diastolic blood pressure, mm Hg | 73.4 ± 9.8 | 72.0 ± 9.2 | 74.2 ± 10.0 | <0.001 |
| Antihypertensive medication use, % | 30.4 | 28.1 | 31.8 | 0.242 |
| Fatty liver (HU < 40),2 % | 9.1 | 5.1 | 11.5 | 0.001 |
| CAC Agatston score2 | ||||
| Men | 12 (0–139) | 6.5 (0–62) | 18 (0–162) | 0.025 |
| Women | 88 (83–96) | 0 (0–3.7) | 0 (0–0) | 0.167 |
| CAC Agatston score > 0,2 % | ||||
| Men | 58.7 | 52.5 | 61.4 | 0.074 |
| Women | 23.7 | 26.9 | 20.9 | 0.147 |
| Common CIMT,2 mm | 0.84 (0.73–0.96) | 0.83 (0.71–0.95) | 0.84 (0.73–0.99) | 0.047 |
| Internal CIMT,2 mm | 1.11 (0.95–1.34) | 1.09 (0.91–1.23) | 1.13 (0.97–1.39) | 0.006 |
1Values are percentages, means ± SDs, or medians (IQRs). CAC, coronary artery calcium; CIMT, carotid artery intima media thickness; HU, Hounsfield units; MASALA, Mediators of Atherosclerosis in South Asians Living in America; MET, metabolic equivalent.
2Missing data for some characteristics—income: total n = 866, n (vegetarian) = 322, n (nonvegetarian) = 544; total cholesterol and fasting glucose: total n = 888, n (vegetarian) = 333, n (nonvegetarian) = 555; TGs and HDL cholesterol: total n = 889, n (vegetarian) = 334, n (nonvegetarian) = 555; LDL cholesterol: total n = 882, n (vegetarian) = 331, n (nonvegetarian) = 551; HOMA-IR: total n = 828, n (vegetarian) = 314, n (nonvegetarian) = 514; liver fat: total n = 879, n (vegetarian) = 332, n (nonvegetarian) = 547; CAC: total n = 885, n (vegetarian) = 334, n (nonvegetarian) = 551; common CIMT: total n = 891, n (vegetarian) = 334, n (nonvegetarian) = 557; internal CIMT: total n = 890, n (vegetarian) = 334, n (nonvegetarian) = 556.
TABLE 2.
Eating occasions of foods and food groups by vegetarian status in the MASALA cohort1
| Times/wk | |||
|---|---|---|---|
| Vegetarian (n = 335) | Nonvegetarian (n = 557) | P | |
| Alcohol | 0.00 (0–0.23) | 0.60 (0–3.0) | <0.001 |
| Beans and legumes | 8.50 (6.00–10.5) | 5.12 (3.00–8.25) | <0.001 |
| Whole grains | 10.0 (7.00–14.0) | 9.00 (6.00–14.0) | 0.012 |
| Some whole grains | 0.79 (0.29–2.00) | 0.87 (0.29–2.12) | 0.691 |
| Refined grains | 7.3 (3.4–10.0) | 7.0 (3.3–10.2) | 0.813 |
| Fats and oils | 1.00 (0.00–7.00) | 0.500 (0.00–5.00) | 0.018 |
| Fruit | 13.6 (9.00–20.0) | 14.4 (8.89–21.5) | 0.248 |
| Meat, poultry, seafood | 0 | 4.00 (1.46–6.81) | <0.001 |
| Egg | 0 (0.00–0.25) | 1.08 (0.500–3.00) | <0.001 |
| Dairy | 22.3 (15.0–29.3) | 22.0 (15.3–30.1) | 0.773 |
| Mixed dishes | 1.12 (0.50–2.00) | 1.25 (0.56–2.12) | 0.185 |
| Nuts and oilseeds | 6.00 (2.02–7.25) | 6.00 (2.25–7.50) | 0.587 |
| Snacks | 2.25 (1.10–4.60) | 2.12 (1.00–4.37) | 0.664 |
| Sweets and desserts | 1.87 (0.77–3.56) | 2.29 (1.06–4.25) | <0.001 |
| Sugar, candy, and jam | 7.25 (1.00–14.3) | 7.25 (1.12–14.5) | 0.751 |
| SSB | 0.04 (0.00–0.500) | 0.10 (0.00–0.500) | 0.075 |
| Starchy vegetables | 2.50 (1.25–4.00) | 2.37 (1.25–4.00) | 0.576 |
| Vegetables | 26.3 (19.8–36.9) | 27.1 (21.0–38.0) | 0.186 |
1Values are medians (IQRs). MASALA, Mediators of Atherosclerosis in South Asians Living in America; SSB, sugar-sweetened beverage.
Table 3 describes the nutrient intake estimates by vegetarian status. Compared with nonvegetarians, vegetarians had lower intakes of energy, protein, fat, percentage energy from fat SFAs, trans fat, and MUFAs, niacin and vitamin B-12, chromium, selenium, and n–3 and n–6 fatty acids, and higher intakes of carbohydrate, glycemic index and load, fiber, thiamin, vitamin C, folate, calcium, iron, potassium, and zinc (P < 0.05 for all).
TABLE 3.
Daily macro- and micronutrient adjusted mean intakes by vegetarian status in the MASALA cohort1
| Nutrient | Vegetarian (n = 335) | Nonvegetarian (n = 557) | P |
|---|---|---|---|
| Energy, kcal | 1620 ± 27.1 | 1720 ± 20.9 | 0.003 |
| Carbohydrate, g | 256 ± 1.3 | 240 ± 1.0 | <0.001 |
| Carbohydrates, % energy | 58.8 ± 0.3 | 54.8 ± 0.2 | <0.001 |
| Glycemic index | 41.1 ± 0.3 | 39.5 ± 0.3 | <0.001 |
| Glycemic load | 95.2 ± 1.0 | 85.1 ± 0.8 | <0.001 |
| Protein, g | 59.7 ± 0.5 | 63.0 ± 0.4 | <0.001 |
| % Energy from protein | 14.1 ± 0.1 | 15.0 ± 0.1 | <0.001 |
| Fat, g | 52.5 ± 0.5 | 56.2 ± 0.4 | <0.001 |
| Fat, % energy | 27.9 ± 0.3 | 30.0 ± 0.2 | <0.001 |
| SFAs, % energy | 7.4 ± 0.1 | 8.2 ± 0.1 | <0.001 |
| MUFAs, % energy | 11.1 ± 0.1 | 12.1 ± 0.1 | <0.001 |
| PUFAs, % energy | 6.4 ± 0.08 | 6.5 ± 0.06 | 0.570 |
| trans fat, % energy | 0.06 ± 0.004 | 0.07 ± 0.003 | 0.031 |
| Fiber, g | 21.3 ± 0.2 | 20.1 ± 0.2 | <0.001 |
| Vitamin A, IU | 16,300 ± 424 | 15,800 ± 327 | 0.367 |
| Thiamin, mg | 1.5 ± 0.02 | 1.4 ± 0.01 | <0.001 |
| Riboflavin, mg | 1.6 ± 0.02 | 1.6 ± 0.02 | 0.367 |
| Niacin, NE | 1.5 ± 0.08 | 3.0 ± 0.06 | <0.001 |
| Vitamin B-6, mg | 1.9 ± 0.02 | 1.9 ± 0.01 | 0.595 |
| Vitamin B-12, µg | 2.1 ± 0.07 | 2.7 ± 0.05 | <0.001 |
| Vitamin C, mg | 220 ± 3.5 | 210 ± 2.7 | 0.029 |
| Vitamin D, IU | 85.5 ± 4.4 | 84.3 ± 3.4 | 0.828 |
| Vitamin E, IU | 1.8 ± 0.04 | 1.9 ± 0.03 | 0.353 |
| Folate, µg | 440 ± 4.3 | 382 ± 3.3 | <0.001 |
| Calcium, mg | 972 ± 17.8 | 883 ± 13.7 | <0.001 |
| Chromium, µg | 10.6 ± 0.3 | 12.1 ± 0.2 | <0.001 |
| Iron, mg | 15.3 ± 0.1 | 14.4 ± 0.1 | <0.001 |
| Potassium, mg | 3710 ± 32.1 | 3580 ± 24.8 | <0.001 |
| Selenium, mg | 8.3 ± 0.4 | 11.3 ± 0.3 | <0.001 |
| Sodium, mg | 2770 ± 29.7 | 2700 ± 22.9 | 0.071 |
| Zinc, mg | 8.7 ± 0.06 | 8.5 ± 0.05 | 0.018 |
| n–3 fatty acid, g | 0.09 ± 0.01 | 0.16 ± 0.005 | <0.001 |
| n–6 fatty acid, g | 0.34 ± 0.01 | 0.38 ± 0.01 | 0.004 |
1Values are adjusted means ± SEMs. Adjusted for age, sex, and energy intake. IU, International Unit; MASALA, Mediators of Atherosclerosis in South Asians Living in America; NE, niacin equivalent.
The adjusted associations between a vegetarian diet and cardiometabolic risk factors are shown in Table 4 and Figure 1. Consuming a vegetarian diet was associated with lower BMI, visceral fat, waist circumference, fasting glucose, HOMA-IR, total and LDL cholesterol, and lower odds of fatty liver (P < 0.05 for all). The association between vegetarian diet and LDL cholesterol remained significant after excluding those with high TGs. Only for waist circumference were associations attenuated and they became statistically nonsignificant when further adjusted for eating occasions of sweets and desserts (P = 0.07). The association between vegetarian diet and CAC varied by sex (P-interaction = 0.006). The odds of any CAC were lower for vegetarians than for nonvegetarians among men only in both models. A vegetarian diet was not associated with blood pressure and CIMT.
TABLE 4.
Cardiometabolic risk factors by vegetarian status in the MASALA cohort1
| Vegetarian | Nonvegetarian | P | |
|---|---|---|---|
| BMI, kg/m2 | 25.5 ± 0.2 | 26.2 ± 0.2 | 0.023 |
| Waist circumference, cm | 91.8 ± 0.6 | 93.2 ± 0.4 | 0.044 |
| Fasting plasma glucose,2 mg/dL | 99.0 ± 1.3 | 103 ± 1.0 | 0.015 |
| HOMA-IR2 | 3.1 ± 0.4 | 3.8 ± 0.3 | 0.003 |
| Serum total cholesterol, mg/dL | 184 ± 1.9 | 190 ± 1.5 | 0.027 |
| Serum TGs,2 mg/dL | 131 ± 4.0 | 131 ± 3.1 | 0.601 |
| Serum HDL cholesterol, mg/dL | 50.5 ± 0.7 | 49.8 ± 0.5 | 0.399 |
| Serum LDL cholesterol, mg/dL | 108 ± 1.7 | 114 ± 1.3 | 0.004 |
| Visceral fat area, cm2 | 130 ± 2.4 | 137 ± 1.9 | 0.047 |
| Systolic blood pressure, mm Hg | 125 ± 0.8 | 125 ± 0.6 | 0.910 |
| Diastolic blood pressure, mm Hg | 73.0 ± 0.5 | 73.6 ± 0.4 | 0.363 |
| Common CIMT | 0.88 ± 0.01 | 0.87 ± 0.01 | 0.931 |
| Internal CIMT | 1.18 ± 0.02 | 1.23 ± 0.02 | 0.146 |
1Values are adjusted means ± SEMs. Model adjusted for age, sex, study site, alcohol and energy intakes, smoking status, BMI (for outcomes other than BMI and waist circumference), education, traditional cultural beliefs, intentional exercise, and Alternative Healthy Eating Index score; statin use was also adjusted for total cholesterol and LDL cholesterol; diabetes medication use was also adjusted for fasting glucose and HOMA-IR; antihypertensive medication use was also adjusted for systolic and diastolic blood pressure. CIMT, carotid artery intima media thickness; MASALA, Mediators of Atherosclerosis in South Asians Living in America.
2 P values of fasting glucose, HOMA-IR, and TGs were from the models with natural log-transformed fasting glucose, HOMA-IR, and TGs.
FIGURE 1.
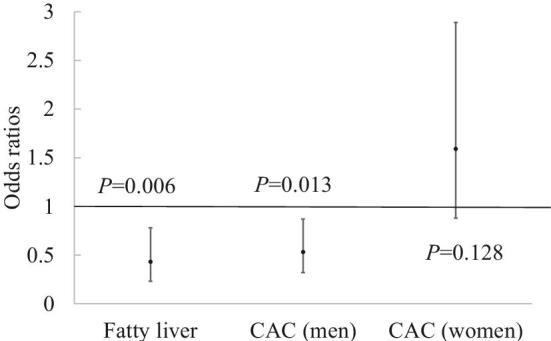
ORs and 95% CIs for health outcomes comparing vegetarians with nonvegetarians in the Mediators of Atherosclerosis in South Asians Living in America cohort. Models were adjusted for age, sex, study site, energy intake, smoking status, BMI, education, traditional cultural beliefs, intentional exercise, alcohol intake, and Alternative Healthy Eating Index score. n = 877 for fatty liver, n = 465 for CAC (men), n = 418 for CAC (women). CAC, coronary artery calcium.
Discussion
We found that 38% of participants in a middle-older aged South Asian cohort in the United States were vegetarian, with a higher prevalence of vegetarianism among women, nonsmokers, and those with strong traditional South Asian cultural beliefs. A vegetarian diet was associated with lower BMI, visceral fat, total and LDL cholesterol, fasting glucose, and insulin resistance, and lower odds of fatty liver and any CAC independent of sociodemographic characteristics and overall diet quality, indicating a protective effect on cardiometabolic health. Following a vegetarian diet was also associated with lower odds of any CAC among South Asian men only.
The prevalence of consuming a vegetarian diet among South Asians in the United States was similar to what has been recently reported from India. Among participants from the India site of the Centre for Cardiometabolic Risk Reduction in South-Asia cohort, 37% followed a vegetarian diet (11). In contrast, in the United States, according to nationally representative data from the NHANES 2003–2004 and 2005–2006 survey cycles, the prevalence of consuming a vegetarian diet among the general US population was only 2.4%, with 1.8% for non-Hispanic whites and 3.7% for other races, much lower than that found in the MASALA study (11).
Dietary acculturation, defined as adopting the dietary habits of the country that people move to, is commonly seen among immigrants (20). Similar to what has been reported for other immigrant populations, after moving to and living in the United States, South Asians have been reported to transition to a Western-style diet that is higher in sugar and fat from the traditional South Asian diet, which includes a higher consumption of legumes and cereals (21). In addition, South Asian vegetarian diets contain a higher intake of fats and appear to be less healthful, whereas vegetarians in the general US population were less likely to eat refined cereals, desserts, and fried foods (11). This could be a result of the nutrition transition that is underway in several low- and middle-income countries, replacing whole grains with refined grains and increasing intake of processed and energy-dense foods (10). In the MASALA study, vegetarians reported more weekly eating occasions of beans and legumes as well as whole grains than did nonvegetarians, which is consistent with a traditional South Asian diet. Nonvegetarians reported more weekly consumption occasions of sweets and desserts, which may partly explain their higher waist circumference. Visceral fat remained significantly higher in nonvegetarians after adjusting for eating occasions of sweets and desserts (while attenuating the differences for waist circumference). This suggests a differential association in the type of fat affected by higher consumption of sweets and desserts (i.e., subcutaneous fat in the waist region). Also, the weekly consumption occasions of fats and oil were higher among vegetarians, which may be attributed to a dietary transition, whereas the weekly consumption occasions of fruits, vegetables, and nut intakes did not differ between the 2 groups. This may partly explain the high prevalence of type 2 diabetes and cardiometabolic risk among South Asians, despite a higher proportion of South Asians being vegetarian. South Asians may follow a vegetarian diet for faith-based, cultural, or familial reasons (22) in contrast to the general US population in which vegetarianism may be a choice made for health and environmental benefits (11). These underlying differences in adoption of a vegetarian diet may explain the food choices made by South Asian vegetarians to some extent.
Our findings, that US-based South Asians following a vegetarian diet have lower BMI and serum lipids, are consistent with the previous literature. In the Adventist Health Study-2 consisting of middle-aged and older non-Hispanic white and black individuals in the United States and Canada, being vegetarian was associated with a 50% lower risk of type 2 diabetes and lower systolic and diastolic blood pressure as well as lower BMI than among nonvegetarians; in addition, the risks of hypertension, high blood total cholesterol, and high blood LDL cholesterol were also lower among vegetarians (4, 5, 23). In a study of South Asians in India comparing urban migrants and their rural siblings, about one-third of participants were vegetarian, and vegetarians were found to have lower concentrations of total and LDL cholesterol, TGs, fasting plasma glucose, and lower levels of systolic and diastolic blood pressure (24). Among the MASALA study participants, those following a vegetarian diet consumed a lower proportion of energy from saturated and trans fats than did nonvegetarians, which may have contributed to the significantly lower LDL cholesterol concentrations in vegetarians than nonvegetarians (25). Although we did observe significant differences for total and LDL cholesterol, there were no differences for plasma TGs across the 2 groups despite a significantly lower BMI for vegetarians and the reasons for these could be several, including a higher intake of carbohydrates by vegetarians, which is known to raise TG concentrations, as well as a lower intake of n–3 fatty acids, which may also increase TG concentrations (26, 27).
In our analyses of MASALA, no significant associations were seen between a vegetarian diet and blood pressure. One reason for this could be the relatively low intake of red and processed meat, which has been associated with higher levels of blood pressure and greater risk of coronary heart disease, among the cohort participants (28). Approximately 25% of the nonvegetarians in the MASALA cohort did not eat any red or processed meat whereas only 13% of participants consumed more than half of their meat intake from red or processed meat. Other reasons why there may have been no difference in blood pressure between vegetarians and nonvegetarians in MASALA include fat, sodium, and potassium intake. Evidence from randomized controlled trials suggests that higher intakes of n–3 fatty acids (≥2 g/d of EPA and DHA) are associated with lower systolic and diastolic blood pressure (29). However, in the MASALA study, although the differences in n–3 fatty acid intakes were statistically significant, intakes in general were relatively low and may have contributed to the nonsignificant association with blood pressure. In contrast to the Indian Migration Study that reported a lower intake of salt among vegetarians, sodium intake did not differ between the 2 groups in MASALA (30). Although potassium intakes were higher among vegetarians, and high potassium intakes have been found to be associated with lower blood pressure (31), our study did not show any protective associations with respect to blood pressure.
Higher consumption of beans and legumes among vegetarians may be the beneficial factor resulting in prevention of cardiometabolic diseases because of the high contents of nutrients such as fiber, folate, and magnesium, and their low glycemic index (32). Epidemiologic investigations have indicated that higher intakes of beans and legumes are associated with a lower incidence of cardiovascular disease (33), and all-cause and cardiovascular disease death (34). In the MASALA cohort, vegetarians reported more frequent eating occasions of beans and legumes, which could explain some of the beneficial associations with health outcomes that were observed.
Our study examined the associations between vegetarian diet and subclinical atherosclerosis, finding a significant association with CAC but not CIMT. Sex was found to be an effect modifier for the association between vegetarian diet and CAC wherein only men who were vegetarian had a lower CAC prevalence but no significant difference was observed for women. There was no association between vegetarianism and CIMT. Our results were consistent with 1 prior study in 88 Brazilian men, which indicated no significant association between vegetarian diet and CIMT after adjusting for covariates (6).
The strengths of our study are that the MASALA cohort is a large community-based sample of South Asians in the United States, and the assessment of diet and derivation of vegetarian status were conducted with the use of a culturally appropriate FFQ, which was previously validated in South Asians in Canada. The MASALA study had several validated measures of cardiometabolic risk factors and subclinical atherosclerosis. In addition, important covariates and confounders were collected and adjusted for in analyses, including information on traditional cultural beliefs because traditional beliefs play an important role in choosing to follow a vegetarian diet. Our study also has limitations. First, no causal relations can be concluded because we lack temporality. Second, the dietary intake was limited only to the past year, which limits our ability to draw conclusions on the long-term effects of diet on cardiometabolic health. And third, the MASALA cohort includes only middle- to older-aged individuals who are mostly highly educated, therefore our results may not be generalizable to younger South Asians or those with lower levels of education.
In conclusion, a vegetarian-style diet was inversely associated with some, but not all, cardiometabolic risk factors among South Asians in the United States. Prospective studies should assess the long-term associations of South Asian vegetarian diet and incident chronic disease in this population.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—SAT: designed the research and had primary responsibility for final content; AMK and NRK: are principal investigators of the MASALA cohort and were responsible for the data collection procedures for the cohort; YJ: analyzed data and wrote the first draft of the paper; LAR: derived the overall diet quality score for the study; and all authors: read and approved the final manuscript.
Notes
The MASALA study was supported by NIH grant R01-HL-093009 (to AMK) and body composition measurements were supported by grant K24HL112827. Data collection at University of California, San Francisco (UCSF) was also supported by NIH/National Center for Research Resources UCSF-Clinical & Translational Science Institute grant UL1 RR024131 (to AMK).
Author disclosures: YJ, AMK, NRK, LAR, and SAT, no conflicts of interest.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- AHEI
Alternative Healthy Eating Index
- CAC
coronary artery calcium
- CIMT
carotid artery intima media thickness
- CT
computed tomography
- MASALA
Mediators of Atherosclerosis in South Asians Living in America
- SHARE
Study of Health Assessment and Risk in Ethnic Groups.
References
- 1. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 2017;57(17):3640–9. [DOI] [PubMed] [Google Scholar]
- 2. Rosell M, Appleby P, Spencer E, Key T. Weight gain over 5 years in 21 966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes 2006;30(9):1389–96. [DOI] [PubMed] [Google Scholar]
- 3. Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients 2017;9(6):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2). Public Health Nutr 2012;15(10):1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fraser G, Katuli S, Anousheh R, Knutsen S, Herring P, Fan J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr 2015;18(3):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Acosta-Navarro J, Antoniazzi L, Oki AM, Bonfim MC, Hong V, Acosta-Cardenas P, Strunz C, Brunoro E, Miname MH, Salgado Filho W. Reduced subclinical carotid vascular disease and arterial stiffness in vegetarian men: the CARVOS study. Int J Cardiol 2017;230:562–6. [DOI] [PubMed] [Google Scholar]
- 7. Hoeffel EM, Rastogi S, Kim MO, Hasan S. The Asian population: 2010. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2012. https://www.census.gov/content/dam/Census/library/publications/2012/dec/c2010br-11.pdf (accessed September 16, 2018). [Google Scholar]
- 8. Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, Dave SS, Qureshi F, Kandula NR. Understanding the high prevalence of diabetes in US south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 2014;37(6):1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000;356(9226):279–84. [DOI] [PubMed] [Google Scholar]
- 10. Singh PN, Arthur KN, Orlich MJ, James W, Purty A, Job JS, Rajaram S, Sabaté J. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am J Clin Nutr 2014;100(suppl_1):359S–64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaacks LM, Kapoor D, Singh K, Narayan KV, Ali MK, Kadir MM, Mohan V, Tandon N, Prabhakaran D. Vegetarianism and cardiometabolic disease risk factors: differences between South Asian and US adults. Nutrition 2016;32(9):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanaya AM, Kandula N, Herrington D, Budoff MJ, Hulley S, Vittinghoff E, Liu K. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol 2013;36(12):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelemen LE, Anand SS, Vuksan V, Yi Q, Teo KK, Devanesen S, Yusuf S. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc 2003;103(9):1178–84. [DOI] [PubMed] [Google Scholar]
- 14. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. Am J Roentgenol 2007;188(5):1307–12. [DOI] [PubMed] [Google Scholar]
- 15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–19. [DOI] [PubMed] [Google Scholar]
- 17. Mukherjea A, Underwood KC, Stewart AL, Ivey SL, Kanaya AM. Asian Indian views on diet and health in the United States: importance of understanding cultural and social factors to address disparities. Fam Community Health 2013;36(4):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 19. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satia-Abouta J, Patterson RE, Neuhouser ML, Elder J. Dietary acculturation: applications to nutrition research and dietetics. J Am Diet Assoc 2002;102(8):1105–18. [DOI] [PubMed] [Google Scholar]
- 21. Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr Rev 2008;66(4):203–15. [DOI] [PubMed] [Google Scholar]
- 22. Caplan P. Crossing the veg/non-veg divide: commensality and sociality among the middle classes in Madras/Chennai. S Asia: J S Asian Stud 2008;31(1):118–42. [Google Scholar]
- 23. Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009;32(5):791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shridhar K, Dhillon PK, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS, Ebrahim S, Indian Migration Study Group The association between a vegetarian diet and cardiovascular disease (CVD) risk factors in India: the Indian Migration Study. PLoS One 2014;9(10):e110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- 26. Smith HA, Gonzalez JT, Thompson D, Betts JA. Dietary carbohydrates, components of energy balance, and associated health outcomes. Nutr Rev 2017;75(10):783–97. [DOI] [PubMed] [Google Scholar]
- 27. Roche HM, Gibney MJ. Effect of long-chain n−3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am J Clin Nutr 2000;71(1):232s–7s. [DOI] [PubMed] [Google Scholar]
- 28. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus. Circulation 2010;121(21):2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens 2014;27(7):885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shridhar K, Dhillon PK, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS, Ebrahim S. Nutritional profile of Indian vegetarian diets – the Indian Migration Study (IMS). Nutr J 2014;13(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food 2013;16(3):185–98. [DOI] [PubMed] [Google Scholar]
- 33. Nouri F, Sarrafzadegan N, Mohammadifard N, Sadeghi M, Mansourian M. Intake of legumes and the risk of cardiovascular disease: frailty modeling of a prospective cohort study in the Iranian middle-aged and older population. Eur J Clin Nutr 2016;70(2):217. [DOI] [PubMed] [Google Scholar]
- 34. Nöthlings U, Schulze MB, Weikert C, Boeing H, van der Schouw YT, Bamia C, Benetou V, Lagiou P, Krogh V, Beulens JW. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr 2008;138(4):775–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


