HSP101 is dynamically recruited to various distinct cytosolic foci after heat stress, including stress granules, and facilitates protein disaggregation and possibly proteasomal degradation of aggregated proteins
Abstract
Stressful environments often lead to protein unfolding and the formation of cytotoxic aggregates that can compromise cell survival. The molecular chaperone heat shock protein (HSP) 101 is a protein disaggregase that co-operates with the small HSP (sHSP) and HSP70 chaperones to facilitate removal of such aggregates and is essential for surviving severe heat stress. To better define how HSP101 protects plants, we investigated the localization and targets of this chaperone in Arabidopsis (Arabidopsis thaliana). By following HSP101 tagged with GFP, we discovered that its intracellular distribution is highly dynamic and includes a robust, reversible sequestration into cytoplasmic foci that vary in number and size among cell types and are potentially enriched in aggregated proteins. Affinity isolation of HSP101 recovered multiple proteasome subunits, suggesting a functional interaction. Consistent with this, the GFP-tagged 26S proteasome regulatory particle non-ATPase (RPN) 1a transiently colocalized with HSP101 in cytoplasmic foci during recovery. In addition, analysis of aggregated (insoluble) proteins showed they are extensively ubiquitylated during heat stress, especially in plants deficient in HSP101 or class I sHSPs, implying that protein disaggregation is important for optimal proteasomal degradation. Many potential HSP101 clients, identified by mass spectrometry of insoluble proteins, overlapped with known stress granule constituents and sHSP-interacting proteins, confirming a role for HSP101 in stress granule function. Connections between HSP101, stress granules, proteasomes, and ubiquitylation imply that dynamic coordination between protein disaggregation and proteolysis is required to survive proteotoxic stress caused by protein aggregation at high temperatures.
Proteotoxic stress induced by conditions that enhance protein misfolding is detrimental to the survival of all organisms. One main environmental condition that induces such stress is exposure to above-optimal temperatures. Heat stress can result in protein misfolding/unfolding that exposes normally sequestered hydrophobic residues, which can then promote the accumulation of the affected proteins in cytotoxic protein aggregates. To limit the detrimental effects of protein unfolding and aggregation, cells engage several folding/refolding and degradation pathways to restore protein homeostasis (Chen et al., 2011; Mogk et al., 2018). Included are molecular chaperones that limit irreversible protein unfolding or work to restore the correctly folded state, aggregation mechanisms that sequester misfolded proteins, and catabolic pathways that remove damaged proteins when repair fails (Protter and Parker, 2016). For plants, understanding these protective responses has important agronomic value for maintaining yield in the face of unpredictable daily temperature fluctuations and the anticipated impacts of elevated temperatures due to global climate change.
Small heat shock proteins (sHSPs) are the first lines of cellular defense against irreversible protein aggregation. sHSPs assemble into multi-subunit oligomers (12 → 24 subunits, depending on the sHSP) that are in rapid equilibrium with substructural dimers. As temperatures increase, this equilibrium shifts to the dimeric form, which is proposed to be the species that binds heat-sensitive unfolding proteins and helps them regain their near-native conformation (Basha et al., 2012; Haslbeck and Vierling, 2015; Santhanagopalan et al., 2018). Although conserved in all kingdoms of life, sHSP diversity is particularly striking in plants, with at least 11 gene clades found in monocots and eudicots (Waters, 2013). Two major clades of cytosolic sHSPs are found in all land plants, class I (CI; six members in Arabidopsis [Arabidopsis thaliana]) and class II (CII; two members in Arabidopsis; Scharf et al., 2001). Studies with transgenic Arabidopsis, in which expression of either the CI or CII clades was reduced using RNA interference (RNAi) strategies, demonstrated that both clades are required for maximum thermotolerance. While affinity experiments showed that CI sHSPs are more effective than CII sHSPs at capturing substrates during heat stress in vivo, overlap in potential CI and CII clients exists (McLoughlin et al., 2016). Some of these sHSP-associated clients have been identified by their aggregation during heat stress and failure to resolubilize during recovery in plants deficient in either CI or CII sHSPs or the protein disaggregase HSP101 (McLoughlin et al., 2016).
HSP101 belongs to the Hsp100/casein lytic proteinase (Clp) B family of AAA+ chaperones (ATPase associated with diverse cellular activities) present in both prokaryotes and eukaryotes that assemble into homohexamers capable of disaggregating misfolded proteins. Their disaggregation mechanism involves coupling ATP hydrolysis to unfolding of the misfolded state, threading the unfolded polypeptide through a central pore, and then attempting proper refolding to the native state in combination with other molecular chaperones (Parsell et al., 1994; Haslberger et al., 2008; Watanabe et al., 2009). Each monomer contains two nucleotide-binding domains that, when assembled, form two stacked rings connected by a coiled-coil (middle) domain which is unique to Hsp100/ClpB AAA+ proteins (Lee et al., 2003). This middle domain is important for interactions with HSP70, which decorates the surface of protein aggregates and recruits and activates HSP101 (Seyffer et al., 2012; Winkler et al., 2012; Lee et al., 2013).
Bacterial members of the AAA+ protein family, such as ClpA or ClpX (which lack the coiled-coil middle domain of Hsp100/ClpB proteins), associate with proteases like ClpP or ClpQ that can directly break down misfolded proteins if refolding fails (Kirstein et al., 2009). In contrast, the cytosolic version of Hsp100/ClpB in budding yeast (Saccharomyces cerevisiae), Hsp104, favors client protein refolding (Weibezahn et al., 2004) over facilitating degradation and has not been found associated with proteases. In fact, Hsp104 engineered to deliver substrates directly to an associated peptidase was unable to support thermotolerance (Weibezahn et al., 2004; Tessarz et al., 2008), implying that refolding of Hsp104 clients is strongly preferred over degradation during recovery from heat stress. Mammals lack a Hsp100/ClpB-type disaggregation system but harbor a disaggregation machinery comprising HSP110, HSP70, and HSP40 (Nillegoda et al., 2015). They also rely on proteasomes for clearing aggregated proteins, with the shuttle factor Ubiquilin 2 recognizing HSP70-bound clients and delivering them to the proteasome complex for breakdown (Hjerpe et al., 2016).
Plants express multiple nuclear-encoded HSP100/ClpB proteins that are directed to three cellular compartments, the cytosol/nucleus, chloroplasts, and mitochondria. Of these, cytosolic/nuclear HSP101 (ClpB1; At1g74310 in Arabidopsis) is a major isoform essential for tolerance of extreme heat (Hong and Vierling, 2000; Queitsch et al., 2000). While Hsp100/ClpB homologs in nonplant species are reasonably well studied (Mogk et al., 2018), the substrates and interacting (adaptor) proteins for plant HSP100/ClpB proteins remain largely unknown.
Heat stress also induces the formation of cytoplasmic stress granules that coalesce aggregates of heat-labile misfolded proteins as a mechanism to mitigate proteotoxic stress (Cherkasov et al., 2013; Mitrea and Kriwacki, 2016). These membrane-less organelles play important roles in plant development and stress tolerance, as demonstrated by the analysis of mutants eliminating various stress granule constituents that displayed defects in seedling morphology and/or stress acclimation (Cherkasov et al., 2013; Merret et al., 2013; Sorenson and Bailey-Serres, 2014; Perea-Resa et al., 2016). These granules appear as highly dynamic foci with biophysical properties typical of fluid biomolecular condensates that rapidly exchange their contents with the surrounding cellular milieu. They are also highly mobile via actin filament cytosolic streaming, which likely encourages misfolded protein accretion and granule fusion (Hamada et al., 2018). A subset of stress granules accumulates mRNAs and associated RNA-binding proteins that protect translation-stalled mRNAs from degradation (Nover et al., 1989; Protter and Parker, 2016; Gomes and Shorter, 2019). During heat stress recovery, HSP70 and HSP101 are recruited to these granules, which collectively promote granule dissolution and restoration of mRNA translation (Cherkasov et al., 2013; McLoughlin et al., 2016; Merret et al., 2017).
Given the importance of HSP101 to thermotolerance in plants, we addressed several unanswered questions pertaining to its functions during recovery from severe heat stress in Arabidopsis. We document that this chaperone reversibly decorates several morphologically distinct biomolecular condensates during heat stress and recovery, one of which has signatures of stress granules. Mass spectrometry (MS), coimmunoprecipitation, and microscopic studies detected an unanticipated interaction between HSP101 and proteasomes, with HSP101 and this proteolytic complex transiently colocalizing in amorphous foci during heat shock recovery. Differences in ubiquitylated protein profiles from wild-type and hsp101 null seedlings after heat stress indicate that the disaggregase activity of HSP101 is important for clearing insoluble protein fractions modified with ubiquitin. Comparisons of soluble and insoluble proteins enriched in aggregated proteins identified over 600 Arabidopsis proteins whose aggregation is suppressed by HSP101. The subset of proteins most severely affected by heat stress partially overlaps with known stress granule constituents and sHSP-interacting proteins, indicating that HSP101 is important for, but not limited to, stress granule disassembly during heat stress recovery. Although most HSP101 clients appeared destined for refolding, synergy between HSP101 and the proteasome was apparent in clearing a subset of insoluble, ubiquitylated proteins.
RESULTS
HSP101 Forms Dynamic Foci during Acclimation, Heat Stress, and Recovery
HSP101 is typically present at low levels in nonstressed Arabidopsis seedlings but rapidly accumulates upon heat stress (Hong and Vierling, 2001). To study the role(s) of this protein disaggregase during heat stress, we applied a protocol in which seedlings grown at 22°C were treated with a 1.5-h moderate heat shock at 38°C, followed by a 2-h recovery at 22°C (designated as acclimation) to induce the synthesis of HSP101 and other molecular chaperones important for thermotolerance (Hong et al., 2003). The acclimation treatment was followed by a severe heat shock at 45°C for 1 h and a variable-length recovery phase at 22°C, during which HSP101 dynamics and activity were studied (Fig. 1A). The acclimation process protects wild-type seedlings from the severe 45°C treatment, but results in growth arrest of hsp101 null seedlings (hot1-3 allele; Hong and Vierling, 2000). The heat sensitivity of the hsp101 null mutant could be complemented by expressing a HSP101-GFP fusion under control of the native HSP101 promoter (McLoughlin et al., 2016); this HSP101-GFP hsp101 line was used to interrogate HSP101 localization and dynamics. As expected, the fluorescent signal in HSP101-GFP expressing plants was strongly enhanced after heat stress acclimation (Supplemental Fig. S1A).
Figure 1.
HSP101-GFP rapidly accumulates in cytosolic foci during heat stress and recovery. A, Overview of the heat stress regime. Six-day-old HSP101-GFP hsp101 seedlings grown on solid medium at 22°C in long days (16-h light/8-h dark) were heat acclimated at 38°C for 1.5 h and allowed to recover at 22°C for 2 h to induce the synthesis of molecular chaperones, including HSP101 (Acc). Seedlings were then subjected to a more severe heat stress at 45°C (HS) for 1 h followed by a recovery phase (Rec) of variable length at 22°C. B, Intracellular distribution of HSP101-GFP in root cortical cells after heat stress acclimation. Left, Cells were imaged for GFP (green) and propidium iodide (red) by confocal fluorescence microscopy. Right, Overlay of HSP101-GFP images collected at t = 0 (blue) and t = 8 min (yellow) to demonstrate movement of the foci. C and E, Images of root cortical cells (C) and leaf epidermal pavement (E, upper) and guard cells (E, lower) expressing HSP101-GFP at the indicated phases of the heat stress regime. Scale bars = 5 μm (B, C, and E). D, F, and G, Diameter of HSP101 foci measured after heat stress and recovery in root and leaf cells. Each bar represents the measurement of 50 foci from multiple cells plotted in a box and whisker plot. The central line locates the median value, + indicates the average value, the box encompasses the upper and lower quartiles, and the error bars show the maxima and minima of the size distributions.
Detailed analysis of root cortical cells from HSP101-GFP hsp101 plants revealed that HSP101 exhibits dynamic localization patterns during heat stress and recovery. During acclimation, HSP101-GFP became concentrated in discrete cytoplasmic foci that were highly mobile (Fig. 1B). By overlaying consecutive images from 0 and 8 min, this cytoplasmic movement was easily documented (Fig. 1B; Supplemental Video S1). The foci were relatively large for intracellular structures, having an average diameter of 0.49 ± 0.12 μm and a maximum diameter of 0.75 μm. Although increased protein aggregation would be expected with the 45°C treatment, the HSP101-GFP signal surprisingly became mostly diffuse after the severe heat stress, implying that HSP101 dissociated from these foci (Fig. 1C). However, after an initial phase of recovery at 22°C, HSP101-GFP again coalesced into small foci that increased in size over time (Fig. 1C; Supplemental Fig. S1B; Supplemental Video S2), resulting in larger mobile foci, with an average diameter of 0.65 ± 0.15 μm and maximum diameter of 0.91 μm (Fig. 1D), after overnight recovery.
To assess whether this dynamic localization of HSP101-GFP occurred in all cell types or was unique to root cortical cells, we studied the response in leaf epidermal pavement and guard cells subjected to the same heat stress regime (Fig. 1E). Although the kinetics for aggregation, dispersal, and reaggregation of HSP101-GFP were nearly indistinguishable to those in roots, several differences were evident. First, the foci in acclimated leaves were ∼3-fold larger than those seen in roots (1.56 ± 0.50 μm average diameter, with a maximum of 2.64 μm; Fig. 1F; Supplemental Video S3). Second, the accretion of HSP101-GFP into small foci during the initial recovery was more pronounced in leaves, although the foci seen after 45 min of recovery were smaller by comparison (1.03 ± 0.29 μm average diameter with a maximum of 1.71 μm; Fig. 1G) and appeared static in consecutive images over a timeframe of 15 min (Supplemental Video S4). Third, the HSP101-GFP signal in guard cells was concentrated at the edges of the cytoplasm adjacent to the neighboring guard cell during severe heat stress (Fig. 1E). Whether this phenomenon reflected a unique positioning of cytoplasm in guard cells or some other novelty of this cell type is unknown. Fourth, the reformation of cytoplasmic foci in guard cells varied even within the same leaf, with some pairs either containing many smaller foci, a diffuse cytoplasmic distribution, or just a few larger foci at the same recovery time point (Supplemental Fig. S1D). Finally, while the foci in root cortical cells persisted even after 16 h of recovery, they dissipated after the same period of recovery in both epidermal pavement cells and the majority of guard cells, which could imply that leaves more quickly disaggregate/refold their protein complement and/or are better protected against protein misfolding/aggregation.
To eliminate the possibility that prolonged confocal imaging induced foci formation, we monitored HSP101-GFP in epidermal pavement cells that were not subjected to severe heat stress; no foci appeared even after 1 h of fluorescence excitation (Supplemental Fig. S1C). In addition, when roots expressing yellow fluorescent protein alone were subjected to the same heat stress regime, they did not accumulate fluorescent foci (Supplemental Fig. S1A). Together, these data show that HSP101 is rapidly recruited to dynamic cytoplasmic foci after heat stress and that the kinetics of this association is dependent on the heat stress regime and cell type.
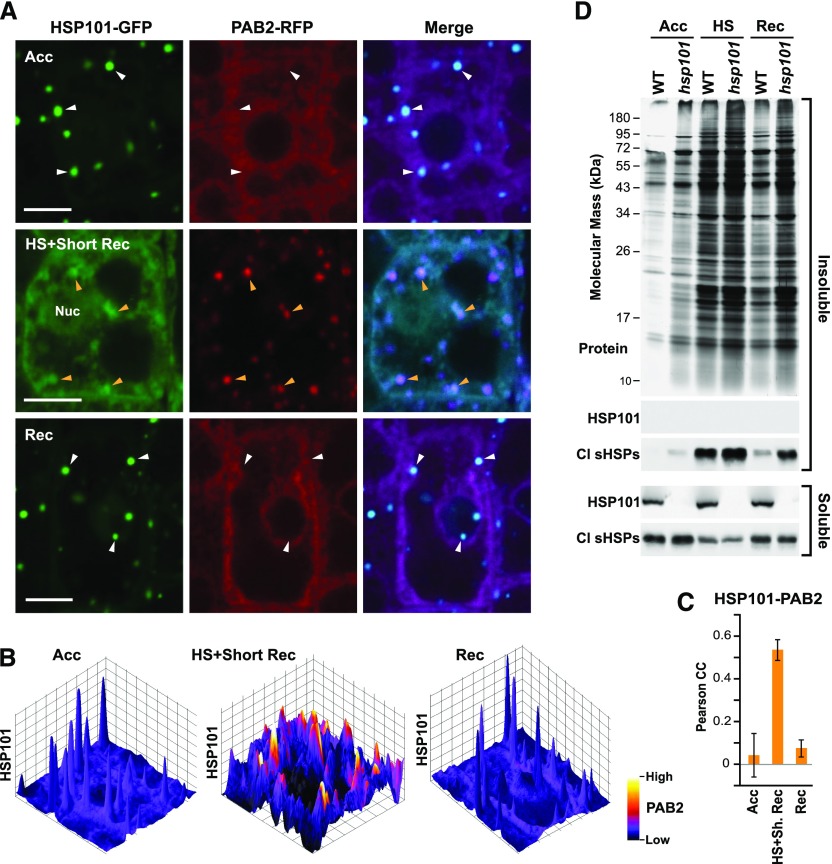
We reported previously that HSP101-GFP colocalizes with sHSPs and several sHSP-interacting proteins in heat stress-induced foci, including translation elongation factor 1B (EF1B; McLoughlin et al., 2016), which is a stress granule constituent in yeast (Cherkasov et al., 2015; Wallace et al., 2015; Jain et al., 2016) and Arabidopsis (Kosmacz et al., 2019). To assess how the HSP101-GFP foci are related to stress granules, we simultaneously localized HSP101-GFP with the stress-granule marker poly(A) binding protein (PAB)-2 fused to red fluorescent protein (RFP; Sorenson and Bailey-Serres, 2014; Merret et al., 2017; Kosmacz et al., 2019). As shown by merged confocal fluorescence images and subsequent rendering and statistical analysis by surface three-dimensional plots (Fig. 2, A–C), both markers could be detected in the same foci in roots after severe heat stress followed by 30 min of recovery. However, unlike HSP101-GFP, PAB2-RFP was not detected in foci during acclimation, nor during the later stages of recovery from severe heat stress, when it instead displayed a diffuse cytosolic distribution (Fig. 2, A–C). Consequently, it appears that HSP101 and PAB2 transiently coexist in the same foci, and that under the conditions examined here, stress granule components appear to be highly dynamic.
Figure 2.
HSP101 is recruited to stress granules during heat stress and is important for disaggregating insoluble proteins during recovery. A, Localization of HSP101-GFP and the stress-granule marker PAB2-RFP in root cortical cells after heat stress acclimation (Acc), and after 30 min (HS+Short Rec) or 5 h (Rec) of recovery following a severe heat stress at 45°C (HS; see Fig. 1A). Colocalization was assessed by merging the fluorescence signals where HSP101-GFP is shown in cyan and PAB2-RFP in magenta. Arrowheads locate positions of HSP101-containing foci; orange arrowheads indicate colocalization with PAB2-RFP and white arrowheads a lack thereof. Nuc, nucleus. Scale bars = 5 μm. B, Evidence for colocalization of HSP101-GFP and PAB2-RFP soon after recovery (Rec) based on analysis of surface three-dimensional plots of the images in A. C, Quantification of colocalization in cytosolic regions by Pearson’s correlation coefficients. Each bar represents the average correlation coefficient (±sd) of five individual cells obtained from multiple roots. D, The effect of HSP101 on protein disaggregation during heat stress recovery. The insoluble (aggregated) proteins enriched by centrifugation from wild-type (WT) and hsp101 seedlings either after acclimation, immediately after a 1-h heat stress at 45°C, or after 3 h of recovery at 22°C. The soluble fractions represent the supernatant, whereas the insoluble fractions represent the repeatedly washed pellets resuspended in an equal volume of buffer. Equal aliquots were subjected to SDS-PAGE and either silver stained for total protein content or immunoblotted with anti-HSP101 or anti-CI sHSP antibodies.
Based on work with yeast showing that Hsp104 and other chaperones interact with protein aggregates (Glover and Lindquist, 1998), along with our studies colocalizing Arabidopsis HSP101, sHSPs, and several sHSP-associated proteins in cytoplasmic foci possibly enriched in aggregated proteins (McLoughlin et al., 2016), we examined the extent of protein aggregation during and after heat stress. The aggregated protein fraction was enriched by centrifugation of total cell extracts and the pellets repeatedly washed and recollected to remove trapped soluble material. As shown in Figure 2D, subjecting seedlings to severe heat stress at 45°C dramatically increased the complexity and abundance of proteins in this aggregated, insoluble fraction, which was further elevated during acclimation, after heat stress, and during recovery in the hsp101 mutant. Consistent with prior studies (Lee et al., 2005; McLoughlin et al., 2016), a substantial portion of the CI sHSP pool also entered the aggregated, insoluble fraction during severe heat stress and was retained during recovery in the hsp101 mutant (Fig. 2D). On the contrary, HSP101 was not bound to this aggregated/insoluble material but instead was retained in the soluble fraction both before and after heat stress (Fig. 2D), suggesting that HSP101 loosely binds to the surface of protein aggregates, similar to that observed for Hsp104 in yeast (O’Driscoll et al., 2015).
HSP101 Interacts with Other Chaperones and the Proteasome during Heat Stress
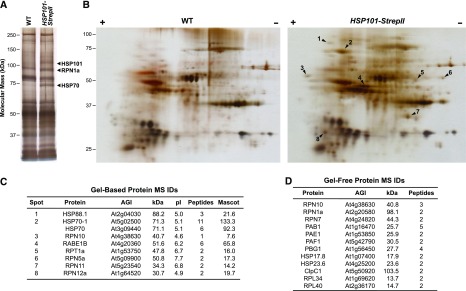
We sought to identify proteins that interact with HSP101 as possible functional partners by using affinity isolation followed by MS. For this purpose, we generated hsp101 plants rescued with a C-terminal Streptavidin II-tagged version of HSP101 expressed under the control of its native promoter. The HSP101-StrepII fusion accumulated at levels similar to that of HSP101 in wild-type plants and fully restored thermotolerance to the hsp101 line, as judged by the maintenance of hypocotyl elongation after heat stress (Supplemental Fig. S2, A and B). Following affinity enrichment from extracts prepared from heat-stressed plants, a number of possible HSP101-StrepII-interacting proteins were detected by one-dimensional SDS-PAGE based on their absence in samples isolated in parallel from wild-type plants also subjected to heat stress (Fig. 3A). Tandem MS analysis of individual bands excised from the gels detected HSP70, which was previously identified as associated with yeast Hsp104, a homolog of Arabidopsis HSP101 (Winkler et al., 2012), thus demonstrating the validity of the approach. Unexpectedly, the proteasome regulatory particle (RP) subunit RPN1a was also identified (Fig. 3A). The interactions between HSP101 and HSP70 or RPN1a were confirmed by immunoblot analyses of the pulldowns with anti-HSP70 and anti-RPN1a antibodies (Supplemental Fig. S2, C and D).
Figure 3.
HSP101 interacts with proteasome subunits and other molecular chaperones following heat stress. Wild-type (WT) and HSP101-StrepII hsp101 were grown on soil for 4 weeks, heat stress acclimated, and subjected to a severe heat stress for 2 h at 45°C. HSP101-StrepII and associated proteins were enriched from total clarified protein extracts by Strep-Tactin affinity chromatography. A, HSP101-StrepII interacts with HSP70 and the proteasome subunit RPN1. Following SDS-PAGE of the affinity-enriched fractions, the indicated bands that were exclusively detected in the HSP101-StrepII eluates were excised and identified via tandem MS. B, HSP101-StrepII interacts with other HSPs and proteasome subunits. Following two-dimensional PAGE of the affinity enriched fractions, spots exclusively detected in the HSP101-StrepII sample were excised (indicated by arrowheads) and identified by tandem MS. C, MS identification of labeled spots in B. D, HSP101-StrepII-interacting proteins identified from 2-week-old seedlings which were grown on agar medium, acclimated, and subjected to a severe heat stress for 1 h at 45°C by tandem MS from affinity-purified samples without PAGE fractionation. Proteins listed were exclusively detected in the HSP101-StrepII samples and were identified by at least two unique peptides (FDR < 5%). The interaction between HSP101 and proteasome subunits was observed in at least five independent experiments.
To expand this analysis, the HSP101-StrepII eluates were separated by two-dimensional PAGE followed by silver staining. Tandem MS analysis of spots specific to the HSP101-StrepII precipitates identified the HSP88.1 chaperone (Hsp90 family) and other RP subunits of the proteasome (RPN5a, RPN10, RPN11, RPN12a, and RPT1a [Yang et al., 2004; Book et al., 2010]) as HSP101 interactors (Fig. 3, B and C). The association of chaperones and proteasome subunits was further confirmed by MS analysis of affinity-purified samples from seedlings without electrophoretic fractionation, which revealed enrichment of several molecular chaperones and additional proteasome subunits, including those for the core protease (CP) subparticle of the proteasome (PAB1, PAE1, PAF1, and PBG1; Fig. 3D). (See Supplemental Datasets S1 and S2 for the full lists of HSP101-interacting proteins identified by MS.) Unexpectedly, some proteins (HSP23.6 and ClpC1) that were enriched in the HSP101-StrepII samples are thought to exclusively localize to organelles; we presume that they represent post-lysis interactions, which likely do not interact with HSP101 in intact cells. Taken together, the data confirm interactions between HSP101 and several chaperones during heat stress and imply that proteasomes are also HSP101 partners.
RPN1a Transiently Localizes with HSP101 after Heat Stress
To further investigate the observed association of HSP101 with proteasomes, we tracked movements of RPN1a-GFP (a RP subunit) and proteasome α-subunit (PAG)1-GFP (a CP subunit) under our heat stress regime for possible colocalization. Prior studies showed that both proteasome reporters faithfully integrate into the holo-proteasome complex and rescue the aberrant phenotypes of the corresponding null mutants (Yao et al., 2012; Marshall et al., 2015). When analyzed by confocal fluorescence microscopy, both RPN1a-GFP and core proteasome α subunit G 1 (PAG1)-GFP displayed a diffuse intracellular pattern under nonstressed conditions, with most of the signal detected in the nucleus, consistent with the concentration of proteasomes in this compartment (Kolodziejek et al., 2011; Marshall et al., 2015). After acclimation, the RPN1a-GFP signal coalesced into large, irregularly shaped condensates (Fig. 4A; Supplemental Fig. S3). Interestingly, the localization pattern of PAG1-GFP only partially overlapped with that of RPN1a-GFP, with PAG1-GFP near exclusively present in the nucleus, suggesting that a sizable pool of proteasomes remains unassembled, or that RP association with the CP is very dynamic in Arabidopsis root cells.
Figure 4.
The proteasome subunit RPN1a accretes in distinct cytosolic foci and colocalizes with HSP101 during recovery from heat stress. Six-day-old seedlings harboring the indicated reporters were subjected to the heat stress regime and imaged by confocal fluorescence microscopy. A, RPN1a-GFP accretes in foci during acclimation (Acc) and heat stress (HS) but is diffuse after 16 h of recovery (Rec). Root cortical cells were imaged for GFP (green) and propidium iodide (red, to locate cell walls) by confocal fluorescence microscopy. Overlays of RPN1a-GFP images collected in acclimated and heat stressed cells at t = 0 (blue) and t = 8 min later (yellow) are shown to demonstrate that the RPN1a-GFP foci are immobile (similar to the images in Fig. 1, B, C, and E). Arrowheads locate the positions of RPN1a-GFP condensates. Nuc, nucleus. B, RPN1a and the stress granule marker PAB2-RFP accumulate in distinct foci during heat stress. Fluorescence from RPN1a-GFP and the stress granule marker PAB2-RFP were visualized in the same cells after a 30-min recovery from severe heat stress. GFP, RFP, and merged signals (RPN1a-GFP in cyan; PAB2-RFP in magenta) are shown. Arrowheads indicate positions of RPN1a-GFP condensates, which do not overlap with PAB2-RFP. C, Poor colocalization of RPN1a-GFP with PAB2-RFP as determined by analysis of surface three-dimensional plots of cells in B subjected to 30 min of recovery. D, Quantification of colocalization in cytosolic regions by Pearson’s correlation coefficients. Each bar represents the average coefficient (±sd) obtained from five individual cells from multiple roots. Colocalization data for HSP101-GFP and PAB2-RFP shown in Figure 2 were included for comparison. E, Diameter measurements of RPN1a and PAB2 condensates/foci that appear after heat stress. Each bar represents the analysis of 50 condensates plotted in a box and whisker plot. The central line locates the median value, + indicates the average value, the box encompasses the upper and lower quartiles, and error bars show the maxima and minima of the size distributions. F, Localization of RPN1a-GFP and HSP101-RFP during acclimation and various times of recovery following a 45°C heat stress for 1 h. Arrowheads locate the positions of RPN1a condensates; orange arrowheads indicate sites of colocalization with HSP101 and white arrowheads a lack thereof. G and H, Colocalization of RPN1a-GFP and HSP101-RFP during the heat stress regime as assessed by surface three-dimensional plots (G) and Pearson’s correlation coefficients (H), as described in C and D. Scale bars = 5 μm (A, B, and F).
Like HSP101, the intracellular distributions of both proteasome reporters were altered following severe heat stress at 45°C. In addition to diffuse background signals, both RPN1a-GFP and PAG1-GFP appeared in discrete cytoplasmic and nuclear foci, in direct contrast to HSP101-GFP, which instead became more dispersed during severe heat stress (Figs. 1C and 4, B–D, Supplemental Figs. S1 and S3). The RPN1a-GFP and PAG1-GFP foci seen during severe heat stress were also morphologically distinct from the foci containing HSP101-GFP that appear during acclimation or recovery. Whereas the HSP101-containing foci were sharply delineated and numerous, the RPN1a-containing foci were fewer, substantially larger, and relatively amorphous. To verify that the proteasome-containing foci were different, we colocalized RPN1a-GFP with the PAB2-RFP stress-granule marker. Unlike the results with HSP101-GFP and PAB2-RFP, little overlap was evident by surface three-dimensional plots and subsequent statistical analysis of the merged fluorescence images (Fig. 4, B–D). The RPN1a-containing foci were also substantially larger than the PAB2-containing foci (Fig. 4E), suggesting that the proteasome condensates are distinct from PAB2-containing stress granules (Fig. 4, B–D).
To test further when and where RPN1a and HSP101 interact, we generated wild-type plants expressing both RPN1a-GFP and an RFP-tagged version of HSP101, and simultaneously imaged both proteins during heat stress recovery. As expected, based on colocalization studies with PAB2, RPN1a-GFP and HSP101-RFP accreted in distinct foci during acclimation, as seen by the surface three-dimensional plots of the fluorescence images (Fig. 4, F–H). Notably, during heat stress recovery, a partial overlap was observed between HSP101 and RPN1a, especially after 3 h of recovery (Fig. 4H). However, by 16 h of recovery the RPN1a-GFP foci disappeared, while the HSP101-RFP signal continued to accumulate in larger foci (Fig. 4, F and G), as can be also seen in Figure 1, C and D, and Supplemental Figure S1A. Altogether, these data suggest that HSP101 localization is strongly dependent on the heat stress regime, and that the protein accretes to distinct cytosolic foci during heat stress recovery.
Possible Functional Interactions between HSP101 and Proteasomes
Given the interaction of HSP101 with proteasome subunits (Fig. 3; Supplemental Fig. S2, C and D) and the transient colocalization of RPN1a-GFP and HSP101-RFP during heat stress recovery (Fig. 4, F–H), we hypothesized that (1) proteasomes could degrade HSP101, (2) HSP101 could impact the abundance or activity of proteasomes, (3) RPN1a could be important for the disaggregase activity of HSP101, or (4) HSP101 could work synergistically with proteasomes to clear protein aggregates. To test the first hypothesis, we monitored HSP101 levels during heat stress recovery in seedlings incubated with the proteasome inhibitor MG132 [(N-benzyloxycarbonyl)-leucinyl-leucinyl-leucinal] or the translational inhibitor cycloheximide. Concurrently, we analyzed seedlings expressing the known, short-lived proteasome substrate His6-HA3-IAA1, which was used as a control (Gilkerson et al., 2015). As shown in Supplemental Figure S4A, the levels of HSP101 were unaffected by the inhibitors during heat stress recovery, while His6-HA3-IAA1 hyper-accumulated in the presence of MG132 and was nearly absent in the presence of cycloheximide. The fact that HSP101 levels remained constant in the presence of MG132 supported the conclusion that HSP101 is not a target of proteasomes.
To test the second hypothesis, that HSP101 impacts proteasome accumulation, we measured the abundance of the RPN1a and PBA1 subunits by immunoblot analysis of total seedling extracts. Levels of both proteins were unaffected by the heat stress regime or the absence of HSP101 (Supplemental Fig. S4B). With the same wild-type and hsp101 samples, we also assayed the activity of proteasomes, using the substrates Suc-LLVY-AMC (N-succinyl-leucyl-leucyl-valyl-tyrosyl-7-amino-4-methylcoumarin) and LFP [(7-methoxycoumarin-4-yl)-acetyl-alanyl-lysyl-valyl-tyrosyl-prolyl-tyrosyl-prolyl-methionyl-glutamyl-(2,4-DNP-(2,3-diaminopropionic acid))-amide], whichmeasure the proteolytic activity of the CP subcomplex and the holo-proteasome, respectively (Smith et al., 2005; Marshall and Vierstra, 2018). The heat stress regime diminished both activities, with severe heat stress causing a 50% decrease, but these activities were the same in hsp101 as in the wild type (Supplemental Fig. S2C). Therefore, HSP101 does not appear to be essential for proteasome activity in response to heat stress.
To examine the possibility that proteasomes are important for the disaggregase activity of HSP101, we separated by centrifugation total seedling extracts from wild-type, hsp101, and rpn1a-4 seedlings subjected to the heat stress regime into soluble and insoluble fractions as described above (see Fig. 2D), and examined the distribution of HSP101 and RPN1a in each fraction by immunoblot analysis (Supplemental Fig. S5). The rpn1a-4 allele is known to strongly reduce RPN1a protein levels, modestly compromise proteasome function, and confer a hypersensitivity to heat, oxidative stress, and MG132 treatment (Wang et al., 2009), while even stronger alleles have a host of phenotypic defects (Brukhin et al., 2005; Yao et al., 2012). RPN1a partitioned into the soluble fraction along with HSP101 after acclimation and subsequent severe heat stress (Supplemental Fig. S5). In contrast, CI sHSP and the sHSP-interacting proteinstranslation elongation factor (eEF)-1Bα, eEF-1Bβ, and eEF-1Bγ accumulated in the insoluble fraction after severe heat stress and subsequently returned to the soluble fraction during recovery, indicating that the disaggregation activity of HSP101 was not affected by the rpn1a mutation (Supplemental Fig. S5). Interestingly, the levels of sHSP-interacting proteins were reproducibly more abundant in the soluble fraction of rpn1a seedlings as compared to wild type, which could imply that these proteins are substrates of proteasomes (Supplemental Fig. S5).
HSP101 and Proteasomes Work in Concert to Restore Proteostasis
Considering the fourth possibility, as to the potential function(s) of an HSP101/proteasome interaction, we explored whether they might work in concert to clear intracellular aggregates by refolding and/or turnover. We first determined, by immunoblot analysis of the insoluble fractions from wild-type and hsp101 seedlings during heat stress and recovery, whether the levels of ubiquitin-protein conjugates were altered in the absence of HSP101. The insoluble fraction from hsp101 seedlings after acclimation displayed a weak ubiquitin signal that was absent in the wild type, suggesting that even moderate temperature stress results in both increased aggregation/sequestration and ubiquitylation of thermosensitive proteins (Fig. 5A). Strikingly, the levels of insoluble ubiquitylated species dramatically rose after the severe heat stress, with the highest levels seen in the hsp101 mutant, indicating either that ubiquitylated proteins entered the insoluble pool upon modification or that the insoluble proteins that remained aggregated were preferentially modified (Fig. 5, A and B).
Figure 5.
HSP101 and CI sHSPs help clear aggregated ubiquitylated proteins. A, Two-week-old wild-type (WT) and hsp101 agar-plate grown seedlings were harvested after acclimation (Acc), immediately after a 1-h heat stress at 45°C (HS), or after 3 h recovery at 22°C (Rec). Immediately after the 45°C heat stress, the medium was replaced with fresh medium without or with 100 μm MG132. Protein extracts were separated by centrifugation into soluble and insoluble/aggregated fractions and then subjected to immunoblot analysis with anti-ubiquitin antibodies. Coomassie brilliant blue (CBB) staining confirmed near-equal starting material. B and C, Ubiquitin immunoblot signals were densitometrically quantified in the insoluble (B) and soluble (C) fractions after the indicated heat stress regimes and in the presence and absence of MG132. Each value was normalized to the average intensity which was set at 100. Bars represent the average (±sd) of three independent biological replicates. *P < 0.05, as determined by Student’s t test for relevant comparisons. D, Effects of CI and CII sHSPs in clearing the insoluble/aggregated fraction modified by ubiquitylation. Extracts from wild-type seedlings and from seedlings expressing RNAi constructions that downregulate all members of either the CI or CII sHSP families were analyzed by immunoblot analysis with anti-ubiquitin antibodies. Efficacies of the lines in reducing CI and CII sHSP levels are shown by the bottom immunoblot(s) developed with the corresponding antibodies. The gels shown in A and D are representative of at least three biological replicates.
To determine whether there is a contribution of proteasomes to the clearance of heat-induced ubiquitylated aggregates, we subjected wild-type and hsp101 seedlings to heat stress and allowed recovery in the presence of MG132. The insoluble pool of ubiquitin conjugates decreased during the recovery phase in wild-type seedlings but was retained in the hsp101 seedlings (Fig. 5, A and B), implying that HSP101 helps promote their resolubilization and/or turnover. The addition of MG132 had little effect on the profile and abundance of ubiquitylated proteins in the insoluble fraction during this recovery, indicating that most aggregated and ubiquitylated proteins were unavailable for proteasome degradation.
For the soluble fraction, a reduction in ubiquitin conjugates was observed in the heat stress samples, but no obvious differences were seen between wild-type and hsp101 samples during these time points (Fig. 5, A [bottom] and C). However, during recovery, ubiquitin conjugates reappeared in the soluble fraction in the wild-type samples after 3 and 5 h of recovery, indicating that the ubiquitylated aggregated proteins were being disaggregated. This accumulation was more pronounced in the presence of MG132, thus implying that HSP101 and proteasomes work together during heat stress recovery to solubilize and degrade ubiquitylated HSP101 clients after their aggregation.
In parallel, we examined heat-induced changes in protein solubility and ubiquitylation in seedlings with reduced levels of CI or CII sHSPs created by RNAi suppression (McLoughlin et al., 2016). As observed previously, the CI sHSP RNAi line (RNAi-CI) displayed a moderate decrease in CI sHSP protein levels, while a much stronger decrease was seen for the CII sHSP proteins in the RNAi line impacting this group (RNAi-CII; Fig. 5D). When the seedlings were subjected to heat stress and assayed for ubiquitylated proteins in the insoluble fraction, little influence on conjugate levels was seen in either the RNAi-CI or RNAi-CII lines directly after the severe heat stress, as compared to the wild type (Fig. 5D). However, during recovery, the RNAi-CI line retained insoluble, ubiquitylated species to a greater extent than the RNAi-CII line, even though levels of CI sHSP were not impacted as strongly (Fig. 5D). These data imply that CI sHSPs are more influential for dissolving/degrading ubiquitylated proteins after aggregation, which is consistent with the stronger interaction seen between CI sHSPs and their substrates after heat stress (McLoughlin et al., 2016).
HSP101 Affects the Solubility of Numerous Proteins during Heat Stress Recovery
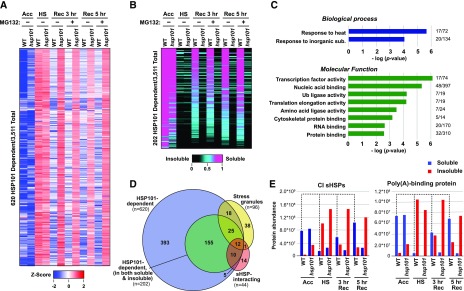
To identify which insoluble (aggregated) proteins were disaggregated by HSP101 and then destined for degradation by the proteasome, we applied tandem MS to the soluble and insoluble fractions prepared from acclimated and 45°C heat-stressed seedlings treated with or without MG132 during recovery. We used the precursor ion intensities of individual peptides from the MS1 scans of trypsinized samples to provide label-free quantification. In total, 3,511 protein groups were abundantly detected using whole seedling extracts, of which ∼2,000 were detected in the insoluble fraction after the acute heat stress (Supplemental Dataset S3). Of this insoluble collection, we focused on 620 proteins that were less abundant in the acclimation and recovery samples versus the acute heat stress samples, as well as being more abundant in hsp101 versus wild type, i.e. possibly representing heat stress-aggregated proteins resolubilized by HSP101. As shown by the heat map in Figure 6A, this collection displayed a profile consistent with proteins that entered the insoluble fraction after acute heat stress and then returned to the soluble fraction upon recovery via a route strongly dependent on HSP101. Notably, the abundance of most of these proteins was unaffected by MG132 during recovery in either wild-type or hsp101 seedlings, implying that these aggregated proteins were not available for proteasomal turnover (Fig. 6A).
Figure 6.
HSP101 disaggregates numerous proteins after heat stress, which are predominantly reconstituted in the soluble fraction during recovery. Two-week-old wild-type (WT) and hsp101 seedlings were subjected to the same treatments as reported in Figure 5, including a 3- and 5-h recovery time point. Total extracts prepared from seedlings harvested after the various heat stress time points were separated into soluble and insoluble/aggregated fractions and subjected to tandem MS using the MS1 precursor ion intensities for label-free quantification. A, A large collection of proteins that aggregate during heat stress are retained in the insoluble fraction in the absence of HSP101. Shown is the abundance of 620 proteins in wild-type and hsp101 seedlings (from a total of 3,511 detected proteins [FDR < 5%]), that were at least 4-fold more abundant in the insoluble fraction after heat stress (HS) as compared to the acclimated (Acc) samples, and showed a subsequent 50% decrease in the insoluble fraction in the wild type, but not in hsp101 samples, during recovery. Shown are their dynamics displayed by a heat map based on Z-scores and ranked by unrooted hierarchical clustering. Blue and red indicate a lower and higher abundance for each protein in the insoluble fraction, respectively. B, HSP101 has a dramatic effect on the solubility of numerous proteins. From the dataset of insoluble proteins identified in A, 202 proteins were selected that were consistently detected in all insoluble and soluble samples from the wild type. Their solubility was calculated by their ratio in the soluble/insoluble fractions after the 45°C heat stress and then ranked by dependency on HSP101, using a heat map to visualize solubility. C, HSP101 client proteins identified in B are enriched in various biological processes and molecular functions. Gene ontology (GO) enrichment was conducted using a singular enrichment analysis. P-values of the most significant and unique GO-terms regarding biological processes and molecular functions were −log transformed and displayed in bar graphs. The numbers at the end of each bar reflect the number of proteins identified compared to the total number of proteins in each GO category. D, Venn diagram showing the overlap between HSP101 clients as determined from the insoluble fraction (as in A), HSP101 clients detected in both the soluble and insoluble fractions (as in B), stress granule constituents, and sHSP-interacting proteins. The number of proteins in each sector is indicated. E, Dynamics of representative HSP101 clients during heat stress and recovery. Shown are the abundance CI sHSPs and poly(A) binding proteins in the soluble and insoluble protein fractions from wild-type and hsp101 seedlings after the indicated stages of the heat stress regime. CI sHSPs and the stress granule marker poly(A) binding protein showed strong partitioning into the insoluble fraction upon heat stress and then returned to the soluble fraction during recovery by a process dependent on HSP101. The dashed lines highlight protein abundance in the soluble fraction of the wild-type samples. The data in this figure are from one representative of three biological replicates.
To more accurately monitor the fate of disaggregated HSP101 clients, we focused on a subset of 202 proteins that were consistently traceable in the soluble fraction during acclimation and in the wild type during recovery (Fig. 6B; Supplemental Dataset S4). When the solubility of each protein was visualized with a heat map reflecting protein abundance, confirmation of their heat lability became evident, as almost all the proteins partitioned into the insoluble/aggregated fraction after the severe heat stress. However, after 3 h of recovery, a substantial percentage of the insoluble fraction (∼30%; shown in magenta) returned mostly, or fully, to the soluble fraction via a process dependent on HSP101, which was even more striking after 5 h of recovery (∼60%; Fig. 6B). Interestingly, a subset remained mostly insoluble during recovery even in the presence of HSP101 and thus might be either disaggregated at a slower rate or permanently misfolded.
GO enrichment analysis of these 202 putative HSP101-clients retrieved several biological processes and molecular functions, including an over-representation of the “response to heat” category that contained many HSPs (Fig. 6C). GO enrichments were also seen for the “transcription factor activity,” “ubiquitin ligase activity,” and “cytoskeletal protein binding” categories. Besides several sHSPs, the list contained numerous sHSP-interacting proteins and predicted orthologs of yeast stress granule constituents, including multiple RNA-binding proteins [including several poly(A)-binding proteins], translation initiation and elongation factors, GTP-binding proteins, profilin2, and several cyclophilins, among others (Supplemental Dataset S5; Cherkasov et al., 2015; Wallace et al., 2015; Jain et al., 2016; McLoughlin et al., 2016; Kosmacz et al., 2019).
To further address the identity of these putative HSP101 clients, we compared this list to 44 known sHSP-interacting proteins (McLoughlin et al., 2016), and 96 previously described stress granule constituents (Fig. 6D; Supplemental Dataset S3; Kosmacz et al., 2019). The HSP101 clients showed a clear overlap with both lists, implying that many are either components of stress granules or proteins that associate following aggregation. To better appreciate the influence of HSP101 on this segregation during severe heat stress, we provided two examples, CI sHSPs and poly(A)-binding proteins within the PAB2 family. Both displayed a dramatic partitioning from the soluble to the insoluble fractions during severe heat stress and a continuing return to the soluble fraction during recovery via a process strongly dependent on HSP101 (Fig. 6E). For example, while most of PAB2-type poly(A)-binding proteins were in the soluble fraction during acclimation (91%), they dramatically sequestered into the insoluble fraction after the severe heat stress (93%) and then eventually repartitioned back to the soluble fraction during 3 and 5 h of recovery (53% and 71%, respectively; Fig. 6E).
HSP101 Clients Are Mostly Destined for Refolding Rather than Proteasomal Degradation
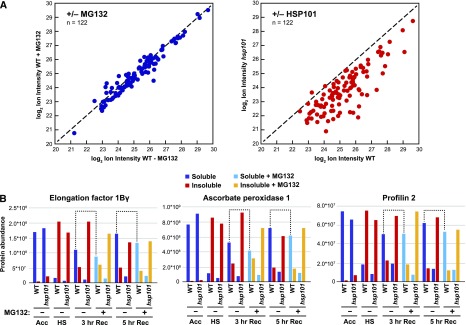
Expecting that some of the proteins that depend on HSP101 for solubility after heat stress eventually become proteasome substrates (see Fig. 5, A and B), we compared the abundance of individual proteins in the soluble fractions after a 5-h recovery with or without MG132 (Fig. 7A). Of the set of 202 proteins described in Figure 6B, we selected those that were detected in each of three independent biological replicates (resulting in a set of 122 proteins) and calculated the average abundance values. When compared by scatter plots, we found that the abundance of most, if not all, was largely identical in wild type ± MG132, implying that proteasomes had little influence on their levels during recovery (Fig. 7A). By contrast, most were substantially more abundant (i.e. resolubilized) when comparing wild type to the hsp101 samples (Fig. 7A). These results suggest that the proteins that aggregated during heat stress were largely reconstituted into the soluble protein pool and likely represent proteins targeted for refolding by the disaggregase activity of HSP101, rather than proteasomal clearance.
Figure 7.
HSP101 clients are predominantly reconstituted in the soluble fraction during recovery. A, HSP101-disaggregated proteins are mainly destined for refolding as opposed to proteasomal turnover. The soluble abundance of 122 proteins that were consistently detected in three independent experiments were selected for analysis of their partitioning into the soluble fraction 5 h after recovery of wild-type (WT) seedlings with or without treatment with MG132 (left), or comparing untreated wild-type and hsp101 seedlings (right). The dashed lines identify the hypothetical point where either the loss of HSP101 or the addition of MG132 has no effect on protein abundance. Each point in the scatter plots represents the average values obtained from three biological replicates. B, Abundance of representative HSP101-clients in the soluble and insoluble fractions prepared from wild-type and hsp101 seedlings after various stages of the heat stress regime in the presence and absence of MG132. Partitioning of known sHSP-interacting proteins and stress granule constituents (EF-1Bγ, ascorbate peroxidase 1, and profilin 2) illustrates the strong impact of HSP101 on protein resolubilization but only a marginal effect by the proteasome inhibitor MG132. Dashed lines highlight the weak effect of MG132 on the amount of each protein in the soluble fraction during recovery. These data are from one representative of three biological replicates.
To better illustrate this point, we analyzed in detail the abundance of three HSP101 clients that were also identified to be present in stress granules and interact with sHSPs during heat stress (Fig. 6D): eEF1Bγ, ascorbate peroxidase, and profilin2 (± MG132 with or without HSP101; Fig. 7B). The addition of MG132 had no discernable effect on the amount of protein that was present in the soluble fraction in the wild type after heat stress recovery. For example, of the percentage of eEF1Bγ that became insoluble during heat stress, 65% and 93% of the polypeptide returned to the soluble fraction after 3 and 5 h of recovery, respectively, with similar percentages evident upon MG132 treatment (56% and 84%, respectively; Fig. 7B). Collectively, these findings implicate HSP101 in the resolubilization of many proteins that aggregate during heat stress in collaboration with stress granules and sHSPs.
DISCUSSION
Proteotoxic stress caused by heat-induced protein aggregation is a major challenge to the survival of all organisms (Queitsch et al., 2000). In their capacities as disaggregases, the Hsp100/ClpB family of chaperones has an essential role in thermotolerance by minimizing protein aggregate formation, disassembling aggregates, and finally enabling the correct refolding of proteins during recovery (Mogk et al., 2018). We examined the intracellular behavior and associations of HSP101 during and after heat stress in Arabidopsis and used an hsp101 mutant to investigate the function and potential clients of this chaperone in cellular proteostasis. Our data demonstrate that HSP101 associates with dynamic, membraneless structures of varying composition as it carries out a central role in clearing heat-induced protein aggregates. HSP101 was also directly linked to proteasome function through affinity interactions with proteasomal subunits and increased ubiquitylation of proteins in an hsp101 null mutant. Proteins dependent on HSP101 for solubility after heat stress were identified by MS and found to represent a diverse subset of molecular functions. Thus, this protein disaggregase participates in complex interactions with multiple cellular factors and heat-sensitive proteins to guard cells from proteotoxicity.
A striking feature of HSP101 was its rapid accumulation in cytoplasmic foci of different sizes, mobility, and content. This behavior presents a fascinating example of how cells spatially and temporally organize functions within the cytosol during stress and recovery without the aid of membranes. Properties of HSP101 cytosolic foci, including whether they were present or not, depended on the severity of the heat stress, stage of recovery, and cell type. Affinity enrichment of HSP101 after severe heat stress identified other chaperones, including HSP70 and sHSPs, as well as sHSP-interacting proteins and proteasomes, as potential partners in HSP101 function and components of the different foci. We previously colocalized HSP101 in foci with CI and CII sHSPs and with sHSP-associated proteins (eEF1Bγ and eEF1Bβ) after an acclimation treatment (McLoughlin et al., 2016). Notably, CI and CII sHSPs also accumulated in separate HSP101-free cytosolic foci, further highlighting how proteins may be functionally segregated in cells. The affinity data presented here imply that the associations of sHSPs with HSP101 continue through severe heat stress and recovery.
We were surprised to see that HSP101, which appeared in cytosolic foci during the acclimation heat treatment, was dispersed in the cytoplasm when the 45°C heat stress was applied. Assuming HSP101 associates with protein aggregates, this behavior could be explained if cells become flooded with unfolded proteins that have not yet coalesced into aggregates during heat stress. HSP101 could adsorb to these species, leading to a diffuse distribution, and then follow the sequestered aggregated proteins to these foci. During recovery, we observed that HSP101-containing foci exhibited changing properties with regard to size and colocalization with other proteins. Localization of PAB2, which is typically associated with RNA-containing stress granules (Sorenson and Bailey-Serres, 2014; Merret et al., 2017), and the proteasomal RP protein RPN1a also exhibited dynamic behavior and were partially colocalized with HSP101 during recovery from heat stress. However, RPN1a and PAB2 were not both present in the same HSP101 foci, indicating that HSP101 activity is distributed in different functional cytosolic compartments during both heat stress and recovery. Another interesting feature is that HSP101-containing foci became less numerous and larger as recovery proceeded, suggesting that a mechanism exists to condense these granules into larger structures. In other organisms, actin filament-based cytosolic streaming has been implicated in granule formation and fusion (Hamada et al., 2018).
From studies on several cell types (root cortical, epidermal pavement, and guard cells), we found that the kinetics of HSP101 foci formation, dispersal, and reformation diverged substantially. We suggest that these variations reflect the different protein composition, metabolic status, and proteostasis demands intrinsic to the cell types. We expect that individual cell types contain a unique composition of heat-sensitive proteins, as well as potentially different levels of other chaperones and proteasomes. These features, along with the metabolic status of the cell, likely contribute to formation, dynamics, and functions of distinct cellular condensates like those containing HSP101.
The observed association of HSP101 with the proteasome was unexpected, and we tested a number of hypotheses about the function of this association. We found no evidence that the association promoted degradation of HSP101, or conversely, directly enhanced the activity of the proteasome. While we cannot exclude that the colocalization is due to mass protein aggregation in response to heat stress, an intriguing possibility is that this association reflects an important stage at which aggregated proteins are triaged to either proceed with degradation or with rescue by refolding. These results further emphasize that there is temporal complexity in the processes occurring during recovery from stress.
An additional connection between proteasomes, HSP101, and aggregated protein removal is provided by our results showing that the insoluble fraction isolated after heat stress accumulates high levels of ubiquitylated proteins via a process that is antagonized by HSP101, CI sHSPs, and possibly CII sHSPs. Most of these HSP101-influenced ubiquitin conjugates were released from the insoluble material during recovery and their accumulation in the soluble fraction was significantly higher in the presence of MG132, implying that at least a portion of this pool is degraded by proteasomes. It is unlikely that their reappearance can be accounted for by new protein synthesis, as our previous studies documented that translation, as assessed by polysome profiling, requires >12 h to significantly recover after heat stress (Zhang et al., 2017).
Our data also underscore how proteins sensitive to misfolding and aggregation upon stress can be readily detected by differential centrifugation, and how this approach can be further employed to determine which factors contribute to restoring proteostasis (Cherkasov et al., 2013; Zhou et al., 2013; Wallace et al., 2015; McLoughlin et al., 2016). This procedure allowed us to demonstrate not only that there was increased accumulation of aggregated, ubiquitylated proteins in the absence of HSP101, but also, when combined with MS, allowed us to identify a collection of Arabidopsis proteins whose solubility is highly susceptible to heat stress and subsequently protected by HSP101. By deep tandem MS analysis of the proteomes of both the soluble and insoluble fractions isolated during acclimation, heat stress, and recovery, we found 620 possible HSP101 clients (from a total set of 3,511 proteins) that matched the expected criteria; i.e. they strongly aggregated during heat stress (i.e. entered the insoluble fraction) and subsequently reentered the soluble fraction during recovery. This reentry could reflect dissolution of stress granule constituents upon recovery or HSP101-mediated refolding of thermo-labile proteins. The list of HSP101 client/binding partners included many chaperones and a number of known or predicted stress granule components (Kosmacz et al., 2019). Also enriched were RNA-binding proteins, including relatives of PAB2 (Merret et al., 2017), proteins affected by heat stress, translation factors, and ubiquitin ligation components. Further characterization of individual HSP101 clients is now needed to determine how these proteins become insoluble, and the cellular mechanism(s) that are required for HSP101 recruitment.
Analysis of a subset of 202 insoluble proteins that rapidly aggregate after heat stress in vivo but return to solubility in the presence or absence of MG132 revealed that most HSP101 clients are targeted for refolding, with a much less than expected contribution from proteasome-dependent turnover. A similar observation was made in yeast, where most Hsp104 clients are refolded without proteasomal clearance (Wallace et al., 2015). As the refolding efficiency of heat-aggregated proteins likely depends on the severity of the stress (Nollen et al., 1999), it is possible that increasing the strength and duration of the heat stress would increase the importance of proteasomal degradation to stress protection. In addition, autophagy likely contributes through its ability to clear ubiquitylated protein aggregates without dissolution, using ubiquitin-binding receptors such as NBR1 (Zhou et al., 2013). Surprisingly, it has been demonstrated that autophagy mutants are more heat tolerant after a prolonged period of heat stress recovery, possibly due to a lack of autophagic turnover of chaperones such as HSP101 (Sedaghatmehr et al., 2019).
Continued studies on the roles of HSP101 and proteasomes in providing thermotolerance, their localization patterns during heat stress, and the dynamics of the many HSP101-influenced proteins identified here should reveal the importance of HSP101 and proteasomes in restoring protein homeostasis after heat stress. In particular, our MS approach enabled the identification of other chaperones that work with HSP101 (e.g. HSP70, HSP40, and sHSPs), which has been challenged by the high number of paralogs present in plants (Waters, 2013; Sable and Agarwal, 2018). By manipulating these factors, it might be possible to engineer crops that are more tolerant to a variety of environmental stresses. However, given the temporal and spatial regulation observed here, simple strategies involving overexpression of individual components are unlikely to succeed. Rather, we need a better understanding of the overall proteostasis network, and how refolding versus degradation is regulated, to prevent or more rapidly repair damage caused by conditions that disrupt protein homeostasis in plants.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) wild-type, mutant, or transgenic seeds were surface sterilized in 70% (v/v) ethanol for 1 min and in 50% (v/v) bleach for 10 min, washed six times with sterile water, and plated on solid nutrient medium {5 mm KNO3, 2 mm MgSO4, 2 mm Ca(NO3)2, 50 μm Na[Fe(EDTA)], 2.5 mm KH2PO4/K2HPO4 (pH 5.5), 70 μm H3BO3, 14 μm MnCl2, 0.5 μm CuSO4, 1 μm ZnSO4, 0.2 μm Na2MoO4, 10 μm NaCl, and 0.01 μm CoCl2} supplemented with 0.8% (w/v) agar and 0.5% (w/v) Suc. Following stratification at 4°C for 2–3 d, plates were placed in environmental chambers under a long-day (LD) photoperiod (16-h light/8-h dark) with 80 μmol m−2 s−1 of white light and 21°/18°C day/night temperatures. All temperature treatments were conducted in darkness within a calibrated hot air incubator that shielded the plates from incubator air currents to minimize variability. The heat stress regime consisted of growth for 6 d at 22°C, an acclimation phase comprising a 1.5-h sublethal heat stress at 38°C followed by 2 h at 22°C, a severe heat stress for 1 h at 45°C, and finally a recovery phase of variable length (3–16 h) at 22°C (Fig. 1A). These conditions were not lethal for any of the Arabidopsis lines used.
Hypocotyl Elongation Assays
Seeds were plated on solid medium and stratified at 4°C for 3 d before being placed vertically at 22°C in the dark for germination and growth. After 2.5 d, the position of the hypocotyl tip was marked, and seedlings were subjected to the heat stress regime. After an additional 2.5 to 3 d of growth, seedlings were photographed; hypocotyl elongation was determined using ImageJ (https://imagej.nih.gov/ij/index.html; Kim et al., 2017).
Plants Expressing Reporter Constructs
Plants expressing HSP101:HSP101-GFP, RPN1a:RPN1a-GFP, PAG1:PAG1-GFP, 35S:PAB2-RFP, and 35S:YFP were as previously described by McLoughlin et al. (2016), Yao et al. (2012), Marshall et al. (2015), Sorenson and Bailey-Serres (2014), and van Leeuwen et al. (2007), respectively. To generate the HSP101-StrepII transgene, a 734-bp genomic fragment containing the HSP101 promoter and 5′ untranslated region (UTR) was PCR amplified from Arabidopsis genomic DNA and cloned into pBluescript II KS(+) between the KpnI and XhoI restriction sites. The StrepII coding sequence (ATGTGGAGCCACCCGCAGTTCGAAAAA encoding MWSHPQFEK) was appended downstream of the 5′-UTR by around-the-world PCR using the GTGGCTCCACATCTCGAGCGATTAGCTTTTGTA (XhoI site underlined) and CCGCAGTTCGAAAAATGATCCACTAGTTCTAGAGCG (XbaI site underlined) primer pair, which also introduced 5′ XhoI and 3′ XbaI sites relative to the StrepII sequence. The HSP101 coding sequence was PCR amplified from Arabidopsis Col-0 complementary DNA using the CGGCTCGAGATGAATCCAGAGAAATTCAC and GGGCTCGAGATCCTCGATCATTT CCTCATT (XhoI sites underlined) primer pair, digested with XhoI, and inserted into the XhoI site upstream of the StrepII sequence. The KpnI/XbaI fragment containing the promoter, 5′-UTR, and the coding sequences for HSP101 with the StrepII tag was inserted into the pBIN19 binary vector upstream of the transcription terminator and polyadenylation signal from the Cauliflower mosaic virus 35S gene to generate the HSP101:HSP101-StrepII vector.
For the HSP101:HSP101-RFP transgene, a 3,471-bp fragment containing the HSP101 promoter, the 5′-UTR, and the coding sequence was PCR amplified from the HSP101:HSP101-GFP template as previously described (McLoughlin et al., 2016), using the CACCTCTATTTTCAGAAGATCCAAAT and ATCCTCGATCATTTCCTCATTATCG primer pair. This product was inserted into the pENTR/D-TOPO plasmid (Thermo Fisher Scientific) by directional TOPO cloning. The translational fusion of the HSP101 coding sequence with that for RFP was generated by the Gateway LR clonase II reaction (Thermo Fisher Scientific) with pGWB653 binary vector. The HSP101:HSP101-StrepII and HSP101:HSP101-RFP transgenes were transformed into the Agrobacterium tumefaciens strain GV3101, and then into the Arabidopsis hot1-3 (hsp101) mutant (Hong and Vierling, 2001) by the floral-dip method. For affinity purifications involving HSP101-StrepII, transgenic line 1 was used (Supplemental Fig. S1, A and B).
Confocal Fluorescence Microscopy
Arabidopsis seedlings were grown vertically on solid medium under a LD photoperiod for 6 d and exposed to different heat stress regimes. Roots were transferred to 10 μg/mL propidium iodide solution to stain cell walls, before imaging using a Fluoview 1000MPE, IX81 motorized inverted confocal fluorescence microscope (Olympus). Images were captured using a UPLSAPO 60× water lens (NA 1.20) equipped with a Hamamatsu C8484-05G camera. The excitation/emission wavelengths were 473/510, 559/575, and 559/619 nm for GFP, RFP, and propidium iodide, respectively. Sequential line scanning was conducted to reduce bleed-through, line Kalman was used as an averaging factor, and the pictures were processed using FV10-ASW and ImageJ (https://imagej.nih.gov/ij/index.html). Surface three-dimensional plot renderings of the merged fluorescence images and quantification of the foci sizes were determined in Image J. The Pearson’s correlation coefficient was calculated in cytosolic regions of individual cells and used to determine the strength of colocalization.
HSP101-StrepII Affinity Purification
Wild-type (Col-0) and HSP101-StrepII plants were grown on soil for 4 weeks in an environmental chamber, acclimated, and stressed for 2 h at 45°C, or grown on solid medium for 2 weeks, acclimated, and heat stressed for 1 h at 45°C. After the stress, aerial parts of the plants were harvested and homogenized with a mortar and pestle with 3 volumes of extraction buffer (40 mm HEPES [pH 8.0], 20 mm MgCl2, 5% [w/v] glycerol, 2 mm ATP, and 1× Halt protease inhibitor cocktail without EDTA [Thermo Fisher Scientific]). Homogenates were clarified by centrifugation at 10,000 × g for 20 min, resulting in ∼1.5 to 2 mg/mL of protein. HSP101-StrepII and bound proteins were enriched with a 0.2 mL gravity flow columns containing StrepTactin-coated resin (IBA Life Sciences) pre-equilibrated with protein extraction buffer. After incubation with the extract, the columns were washed five times with extraction buffer and eluted with extraction buffer supplemented with 2.5 mm desthiobiotin. The eluate was mixed with 0.25 volumes of 5× SDS-PAGE sample buffer (8% [w/v] SDS, 46% [v/v] glycerol, 20% [v/v] 2-mercaptoethanol, 250 mm Tris-HCl [pH 6.8], and 0.01% [w/v] bromophenol blue), and heated for 2 min at 95°C before separation by SDS-PAGE.
Preparation of Soluble and Insoluble Protein Fractions
Two-week-old seedlings were either untreated or stressed using the stated heat stress regimes described above. In experiments involving MG132 [(N-benzyloxycarbonyl)-leucinyl-leucinyl-leucinal] or cycloheximide, seedlings were transferred to liquid nutrient medium 16 h before the start of the heat stress, and the medium was replaced with either control or inhibitor-containing medium immediately after the 45°C heat treatment. Total extracts were prepared by homogenization at ice temperatures in 1.0 mL per 0.7 g fresh weight of protein isolation buffer (25 mm HEPES [pH 7.5], 200 mm NaCl, 0.5 mm Na2EDTA, 0.1% [v/v] Triton X-100, 5 mm ε-amino-N-caproic acid, 1 mm benzamidine), first using a mortar and pestle and then with a Cole-Parmer PTFE glass tissue grinder. The soluble and insoluble fractions were separated from 1.0 mL of total extract by centrifugation at 16,000 × g for 15 min. The soluble fraction was prepared by adding 0.25 volume of 5× sample buffer and heating for 2 min at 95°C before separation by SDS-PAGE. The insoluble pelleted fraction was washed by repeated resuspension via pipetting and vortexing in protein extraction buffer containing 0.1 g of quartz sand (Sigma-Aldrich) and recollected by centrifugation at 16,000 × g. After five washes, the final wash used 1.0 mL of fresh protein isolation buffer without Triton X-100 followed by centrifugation. This pellet (designated the insoluble fraction) was resuspended in 400 µL hot 2× SDS-PAGE sample buffer and clarified by centrifugation at 1,500 × g for 30 s. Samples for MS analysis were directly processed without solubilization in SDS-PAGE sample buffer.
SDS-PAGE and Immunoblot Analysis
Proteins were separated by SDS-PAGE using 7.5% to 15% (w/v) acrylamide gels. For immunoblot analyses, proteins were electrophoretically transferred onto nitrocellulose membrane (Bio-Rad), and the membranes were blocked with 5% (w/v) fat-free milk and probed with primary antibodies obtained from various collaborators, Abcam, or Agrisera (identified here by their catalog numbers). All antibodies were raised in rabbits, unless stated otherwise, and antibody dilutions were N-ter HSP101 (1:5,000; AS07 253), HSP70 (1:5,000; AS08 371), GAPDH (Ming-Che Shih, University of Iowa; 1:5,000), RPN1a (1:1,000; Yang et al., 2004), human influenza hemagglutinin (1:5,000; Abcam), CI sHSPs (1:3,000; AS07 255), CII sHSPs (1:3,000; AS07 254), ubiquitin (chicken, 1:3,000; Judy Callis, University of California Davis), eEF1Bα (1:3,000; AS10 679), eEF1Bβ (1:3,000; AS10 677), and eEF1Bγ (1:3,000; AS10 676).
Blots prepared for fluorescent detection were incubated with IRDye 700CW donkey anti-rabbit antibodies (1:20,000) and analyzed using a Li-Cor Odyssey CLx imager. Ubiquitin conjugates were detected using three antibodies: ubiquitin (chicken, primary), rabbit anti-chicken-horseradish peroxidase (secondary; 1:5,000; Abcam), and IRDye 700CW donkey anti-rabbit antibodies (tertiary). For chemiluminescent assays, blots were incubated withenhanced chemiluminescence (ECL) donkey anti-rabbit IgGs (1:10,000; GE Healthcare), and visualized using the Pierce ECL immunoblot substrate in combination with a Syngene G:box Imaging System. As an alternative for detecting ubiquitin conjugates, proteins were transferred onto Immobilon-P membrane (Millipore) and autoclaved for 20 min on a liquid cycle. After cooling, the membrane was blocked with 3% (w/v) bovine serum albumin in Tris-buffered saline, incubated with primary rabbit anti-ubiquitin antibodies (1:1000; Marshall et al., 2015) and subsequently with goat anti-rabbit secondary antibodies conjugated to alkaline phosphatase (1:5,000). Proteins were visualized by combining 100 mg/mL nitro-blue tetrazolium chloride and 50 mg/mL 5-bromo-4-chloro-3-indolylphosphate (Research Products International) in alkaline phosphatase development buffer (100 mm diethanolamine, 100 mm NaCl, 5 mm MgCl2, pH 9.5) at room temperature for 5 to 30 min, and protein quantification was conducted using ImageJ. For the two-dimensional gels, separation in the isoelectric focusing dimension was performed using 3–10 nonlinear strips (11 cm; GE Healthcare) for 2 h at 150 V, 2 h at 300 V, 5 h at 500 V, and 7 h at 3,500 V. The second dimension was electrophoresed in 12% SDS-PAGE gels at 200 V. Gels were stained with silver as described (Rabilloud et al., 1988).
Tandem MS
For MS identification of HSP101-StrepII-interacting proteins following electrophoresis, gel spots or bands identified by silver staining were excised, destained as previously described (Shevchenko et al., 1996), and cleared of salt and SDS with a 2-D Clean-up Kit (GE Healthcare). Gel pieces were reduced, alkylated, trypsin digested, and analyzed as previously described (McLoughlin et al., 2016).
For identification of HSP101-interacting proteins in bulk, samples were separated briefly by SDS-PAGE (∼2 cm) using a 4% to 20% gradient gel and stained for protein using the Pierce silver stain designed for MS (Thermo Fisher Scientific). The region surrounding HSP101 was excised to reduce interference; the remainder of the gel was destained, cut into 1 × 1-mm pieces, and incubated in 1.0 mL of water. After 30 min, the water was replaced with 100 μL of 250 mm ammonium bicarbonate and the gel slices were reduced for 30 min at 50°C by addition of 20 μL of 45 mm dithiothreitol (DTT), alkylated at room temperature by addition of 20 μL of 100 mm iodoacetamide, and washed twice in 25 mm ammonium bicarbonate and 50% (v/v) acetonitrile at room temperature for 1 h. The gel slices were dehydrated with 100% (v/v) acetonitrile, vacuum dried, rehydrated, and digested overnight at 37°C in 50 mm ammonium bicarbonate containing 0.3 µg of sequencing-grade modified porcine trypsin and 0.01% ProteaseMAX (Promega). The supernatants were collected, and gel slices were further dehydrated for 30 min using 100 μL of 4:1 acetonitrile and 1% formic acid); the two peptide extractions were combined and vacuum dried.
Nano-scale liquid chromatography separation of tryptic peptides was performed on a Nano Acquity UPLC system (Waters). Protein digests were loaded onto a 2 cm × 100 µm C18 Magic 5 µL particle trap column and separated on a 75 µm × 25 cm C18 Magic 3 µm particle analytical column using a 1-h linear 5% to 35% acetonitrile gradient in 0.1% formic acid at a flow rate of 250 nL/min. MS analysis of eluted tryptic peptides was performed online using a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific) possessing a Nanospray Flex ion source (Thermo Fisher Scientific) operated in positive electro-spray ionization mode. Data-dependent acquisition of full MS scans within a mass range of 300 to 1,750 mass to charge ratio (m/z) at a resolution of 70,000 was performed, combined with high-energy collision-induced dissociation fragmentation of the top 10 most intense peaks at a resolution of 17,500 with an isolation width of 1.6 D. Peptides were assigned by Mascot (version 1.4.1.14) against the Arabidopsis proteome database (TAIR10_pep_20101214; www.arabidopsis.org), using up to two missed trypsin cleavages, parent mass tolerances of 10 ppm, and fragment mass tolerances of 0.05 D. Carbamidomethylation of cysteines was specified as a static modification, while Gln→pyro-Glu modifications, oxidation of Met, and N-terminal acetylation were specified as variable modifications. Peptide identifications were accepted if they could be established at >95% probability by the Peptide Prophet algorithm within Scaffold (version 4.4.8, Proteome Software Inc.; Keller et al., 2002). Protein identifications were accepted if they could be established at >95% probability and included at least two different matching peptides. Proteins containing related peptides that could not be differentiated based on tandem MS analysis alone were grouped to satisfy the principles of parsimony.
For MS analysis of the soluble and insoluble protein fractions, 150 µL of each sample was precipitated twice in 4:1:3 (v/v) methanol/chloroform/water and collected by centrifugation. The second pellet was vacuum dried, resuspended in 100 µL 8 m urea, reduced for 1 h at 22°C with 10 mm DTT, and alkylated with 20 mm iodoacetamide for 1 h. The reaction was quenched with 20 mm DTT and digested overnight at 37°C with 0.5 µg of sequencing-grade modified porcine trypsin (Promega). Peptides were acidified with 10% trifluoroacetic acid and desalted using a 100 µL Bond Elut OMIX C18 pipette tip according to the manufacturer’s instructions (Agilent Technologies).
Nano-scale liquid chromatography separation of the tryptic peptides was performed using a Dionex Ultimate 3000 Rapid Separation system equipped with a 75 µm × 25 cm Acclaim PepMap RSLC 2 µm particle C18 column (Thermo Fisher Scientific) using a 2-h linear 4% to 36% acetonitrile gradient in 0.1% formic acid and a flow rate of 250 nL/min. Eluted peptides were analyzed online by a Q-Exactive Plus mass spectrometer in the positive electrospray ionization mode. Data-dependent acquisition of full MS scans (mass range of 380–1,500 m/z) at a resolution of 70,000 was collected, with the automatic gain control target set to 3 x 106, and the maximum fill time set to 200 msec. High-energy collision-induced dissociation fragmentation of the 15 strongest peaks was performed with an intensity threshold of 4 x 104 counts and an isolation window of 3.0 m/z, and excluded precursors that had unassigned, +1, +7, +8, or > +8 charge states. MS1 scans were conducted at a resolution of 17,500, with an automatic gain control target of 2 × 105 and a maximum fill time of 100 ms. Dynamic exclusion was performed with a repeat count of 2 and an exclusion duration of 30 s, while the minimum MS ion count for triggering the MS2 run was set to 4 × 103 counts. Each sample was analyzed in quadruplicate; the first two runs were performed without exclusion, while the third and fourth runs were performed with an exclusion list containing the 5,000 most abundant peptides that were detected in the first two runs to increase sample coverage (McLoughlin et al., 2018). Raw files with and without exclusion lists were merged, resulting in two technical replicates per sample. A digest of cytochrome c (Thermo Fisher Scientific) was analyzed every 18th run to monitor sensitivity and retention time drift.
The resulting tandem MS datasets were queried by Proteome Discoverer (version 2.0.0.802; Thermo Fisher Scientific) against the Arabidopsis protein database (TAIR10_PEP_20101214_UPDATED; http:/www.arabidopsis.org) supplemented with a list of common protein contaminants. Peptides were assigned by SEQUEST HT (Eng et al., 1994), allowing a maximum of two missed tryptic cleavages, a minimum peptide length of 6, a precursor mass tolerance of 10 ppm, and fragment mass tolerances of 0.02 D. Carbamidomethylation of Cys and oxidation of Met were specified as static and dynamic modifications, respectively. A false discovery rate (FDR) of 0.01 (high confidence) and 0.05 (medium confidence) validated peptide spectral matches. Label-free quantification based on MS1 precursor ion intensities was performed in Proteome Discoverer with a minimum Quan value threshold set to 0.0001 for unique peptides. The “3 Top N” peptides were used for area calculation (Silva et al., 2006).
Proteasome Activity Assay
To assay proteasome activity, 2-week-old wild-type or hsp101 seedlings grown on solid medium were frozen in liquid nitrogen after various stages within the heat stress regime and ground to a fine powder at liquid nitrogen temperatures. Samples were extracted using 1.0 volume of lysis buffer (50 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 1 mm Na2EDTA, 10% [v/v] glycerol), filtered through two layers of Miracloth (Calbiochem), and clarified at 30,000 × g for 20 min at 4°C. Equal amounts of protein (10 μg), as determined by the Bradford assay (Bio Rad), were used to measure proteasome activity in the presence or absence of 100 μm MG132. Each sample was incubated for 20 min at 37°C in 1 mL of assay buffer (50 mm Tris-HCl, pH 7.0, and 2 mm MgCl2, with 1 mm ATP and 2 mm 2-mercaptoethanol added immediately before use) containing 100 μm of the fluorogenic substrates N-succinyl-leucyl-leucyl-valyl-tyrosyl-7-amino-4-methylcoumarin (Sigma-Aldrich) or (7-methoxycoumarin-4-yl)-acetyl-alanyl-lysyl-valyl-tyrosyl-prolyl-tyrosyl-prolyl-methionyl-glutamyl-(2,4-DNP-(2,3-diaminopropionic acid))-amide [MCA-AKVYPYPME-Dpa(Dnp)-amide, also known as LFP; GenScript]. Reactions were quenched by the addition of 1 mL of 80 mm sodium acetate (pH 4.3), and the resulting fluorescence was monitored with an excitation wavelength of 365 nm and an emission wavelength of 460 nm. Linearity of activity was confirmed using various concentrations of protein extract.
Accession Numbers
The raw tandem MS sequence, .msf and .xml files for the whole proteome data sets are available in the ProteomeXchange database under accession number PXD011483 within the PRIDE repository (http://www.proteomexchange.org/). The lists of HSP101 interacting proteins and proteins susceptible to aggregation and resolubilization after heat stress can be found in Supplemental Datasets S1 to S4. Sequence data can be found in the GenBank/EMBL data libraries under the following accession numbers: HSP101 (At1G74310), PAB2 (At4G34110), RPN1a (At2G20580), HSP17.4 (At3G46230), HSP17,6II (At5G121020), eEF1Bγ1 (At1G57720), ascorbate peroxidase 1 (At1G07890), and profilin 2 (At4G29350).
Supplemental Data
The following supplementary materials are available in the online version of this article.
Supplemental Figure S1. HSP101-GFP is rapidly recruited to cytoplasmic foci during recovery.
Supplemental Figure S2. HSP101-StrepII functionally replaces HSP101 and interacts with proteasomes.
Supplemental Figure S3. HSP101 and the proteasome subunits RPN1a and PAG1 have variable and dynamic cellular localization patterns during heat stress and recovery.
Supplemental Figure S4. HSP101 and proteasome activities are not influenced by each other during heat stress.
Supplemental Figure S5. The rpn1 mutant does not impact HSP101 mediated protein disaggregation.
Supplemental Dataset S1. HSP101-StrepII interacting proteins (Gel-based, mature plants).
Supplemental Dataset S2. HSP101-StrepII interacting proteins (Gel-free, seedlings).
Supplemental Dataset S3.Protein solubility raw dataset.
Supplemental Dataset S4. HSP101 clientele solubility.
Supplemental Dataset S5. HSP101 client orthologs in yeast.
Supplemental Video S1. Mobility of HSP101 foci in root cortex cells during acclimation.
Supplemental Video S2. HSP101 foci formation in root cortex cells after heat stress.
Supplemental Video S3.Mobility of HSP101 foci in leaf epidermal pavement cells during acclimation.
Supplemental Video S4.Foci formation in leaf epidermal pavement cells after heat stress.
Acknowledgments
We thank Dr. Dingzhong Tang for supplying the RPN1a-GFP line, Dr. Judy Callis for the His6-HA3-IAA1 line and anti-ubiquitin antibodies, Dr. Julia Bailey-Serres for the PAB2-RFP line, and Dr. David C. Gemperline for technical assistance.
Footnotes
This work was supported by grants from the National Institutes of Health/National Institute of General Medical Science (GM-124452) and the National Science Foundation Plant Genome Research Program (IOS-1339325) to R.D.V., and by grants from the U.S. Department of Energy (DE-SC0006646) and the Massachusetts Life Sciences Center (New Faculty Research Award) to E.V.
Articles can be viewed without a subscription.
References
- Basha E, O’Neill H, Vierling E (2012) Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions. Trends Biochem Sci 37: 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book AJ, Gladman NP, Lee SS, Scalf M, Smith LM, Vierstra RD (2010) Affinity purification of the Arabidopsis 26S proteasome reveals a diverse array of plant proteolytic complexes. J Biol Chem 285: 25554–25569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brukhin V, Gheyselinck J, Gagliardini V, Genschik P, Grossniklaus U (2005) The RPN1 subunit of the 26S proteasome in Arabidopsis is essential for embryogenesis. Plant Cell 17: 2723–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Retzlaff M, Roos T, Frydman J (2011) Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol 3: a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, Bukau B (2013) Coordination of translational control and protein homeostasis during severe heat stress. Curr Biol 23: 2452–2462 [DOI] [PubMed] [Google Scholar]
- Cherkasov V, Grousl T, Theer P, Vainshtein Y, Glässer C, Mongis C, Kramer G, Stoecklin G, Knop M, Mogk A, et al. (2015) Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett 589: 3654–3664 [DOI] [PubMed] [Google Scholar]
- Eng JK, McCormack AL, Yates JR (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989 [DOI] [PubMed] [Google Scholar]
- Gilkerson J, Kelley DR, Tam R, Estelle M, Callis J (2015) Lysine residues are not required for proteasome-mediated proteolysis of the auxin/indole acidic acid protein IAA1. Plant Physiol 168: 708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82 [DOI] [PubMed] [Google Scholar]
- Gomes E, Shorter J (2019) The molecular language of membraneless organelles. J Biol Chem 294: 7115–7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Yako M, Minegishi M, Sato M, Kamei Y, Yanagawa Y, Toyooka K, Watanabe Y, Hara-Nishimura I (2018) Stress granule formation is induced by a threshold temperature rather than a temperature difference in Arabidopsis. J Cell Sci 131: jcs216051. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E (2015) A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J Mol Biol 427: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslberger T, Zdanowicz A, Brand I, Kirstein J, Turgay K, Mogk A, Bukau B (2008) Protein disaggregation by the AAA+ chaperone ClpB involves partial threading of looped polypeptide segments. Nat Struct Mol Biol 15: 641–650 [DOI] [PubMed] [Google Scholar]
- Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M, et al. (2016) UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166: 935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2001) Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J 27: 25–35 [DOI] [PubMed] [Google Scholar]
- Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R (2016) ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392 [DOI] [PubMed] [Google Scholar]
- Kim M, McLoughlin F, Basha E, Vierling E (2017) Assessing plant tolerance to acute heat stress. BioProtoc 7: e2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J, Molière N, Dougan DA, Turgay K (2009) Adapting the machine: Adaptor proteins for Hsp100/Clp and AAA+ proteases. Nat Rev Microbiol 7: 589–599 [DOI] [PubMed] [Google Scholar]
- Kolodziejek I, Misas-Villamil JC, Kaschani F, Clerc J, Gu C, Krahn D, Niessen S, Verdoes M, Willems LI, Overkleeft HS, et al. (2011) Proteasome activity imaging and profiling characterizes bacterial effector syringolin A. Plant Physiol 155: 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmacz M, Gorka M, Schmidt S, Luzarowski M, Moreno JC, Szlachetko J, Leniak E, Sokolowska EM, Sofroni K, Schnittger A, et al. (2019) Protein and metabolite composition of Arabidopsis stress granules. New Phytol 222: 1420–1433 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim JH, Biter AB, Sielaff B, Lee S, Tsai FT (2013) Heat shock protein (Hsp) 70 is an activator of the Hsp104 motor. Proc Natl Acad Sci USA 110: 8513–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W, Yoshida M, Tsai FT (2003) The structure of ClpB: A molecular chaperone that rescues proteins from an aggregated state. Cell 115: 229–240 [DOI] [PubMed] [Google Scholar]
- Lee U, Wie C, Escobar M, Williams B, Hong SW, Vierling E (2005) Genetic analysis reveals domain interactions of Arabidopsis Hsp100/ClpB and cooperation with the small heat shock protein chaperone system. Plant Cell 17: 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Vierstra RD (2018) Proteasome storage granules protect proteasomes from autophagic degradation upon carbon starvation. eLife 7: e34532. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marshall RS, Li F, Gemperline DC, Book AJ, Vierstra RD (2015) Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in Arabidopsis. Mol Cell 58: 1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F, Basha E, Fowler ME, Kim M, Bordowitz J, Katiyar-Agarwal S, Vierling E (2016) Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol 172: 1221–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F, Augustine RC, Marshall RS, Li F, Kirkpatrick LD, Otegui MS, Vierstra RD (2018) Maize multi-omics reveal roles for autophagic recycling in proteome remodelling and lipid turnover. Nat Plants 4: 1056–1070 [DOI] [PubMed] [Google Scholar]
- Merret R, Descombin J, Juan YT, Favory JJ, Carpentier MC, Chaparro C, Charng YY, Deragon JM, Bousquet-Antonelli C (2013) XRN4 and LARP1 are required for a heat-triggered mRNA decay pathway involved in plant acclimation and survival during thermal stress. Cell Rep 5: 1279–1293 [DOI] [PubMed] [Google Scholar]
- Merret R, Carpentier MC, Favory JJ, Picart C, Descombin J, Bousquet-Antonelli C, Tillard P, Lejay L, Deragon JM, Charng YY (2017) Heat shock protein HSP101 affects the release of ribosomal protein mRNAs for recovery after heat shock. Plant Physiol 174: 1216–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrea DM, Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Bukau B, Kampinga HH (2018) Cellular handling of protein aggregates by disaggregation machines. Mol Cell 69: 214–226 [DOI] [PubMed] [Google Scholar]
- Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, et al. (2015) Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524: 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH (1999) In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol 19: 2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Neumann D (1989) Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9: 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll J, Clare D, Saibil H (2015) Prion aggregate structure in yeast cells is determined by the Hsp104-Hsp110 disaggregase machinery. J Cell Biol 211: 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S (1994) Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478 [DOI] [PubMed] [Google Scholar]
- Perea-Resa C, Carrasco-López C, Catalá R, Turečková V, Novak O, Zhang W, Sieburth L, Jiménez-Gómez JM, Salinas J (2016) The LSM1-7 complex differentially regulates Arabidopsis tolerance to abiotic stress conditions by promoting selective mRNA decapping. Plant Cell 28: 505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26: 668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabilloud T, Carpentier G, Tarroux P (1988) Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis 9: 288–291 [DOI] [PubMed] [Google Scholar]
- Sable A, Agarwal SK (2018) Plant heat shock protein families: Essential machinery for development and defense. J. Biol. Sci. Med. 4: 51–64 [Google Scholar]
- Santhanagopalan I, Degiacomi MT, Shepherd DA, Hochberg GKA, Benesch JLP, Vierling E (2018) It takes a dimer to tango: Oligomeric small heat shock proteins dissociate to capture substrate. J Biol Chem 293: 19511–19521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Siddique M, Vierling E (2001) The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins). Cell Stress Chaperones 6: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghatmehr M, Thirumalaikumar VP, Kamranfar I, Marmagne A, Masclaux-Daubresse C, Balazadeh S (2019) A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ 42: 1054–1064 [DOI] [PubMed] [Google Scholar]
- Seyffer F, Kummer E, Oguchi Y, Winkler J, Kumar M, Zahn R, Sourjik V, Bukau B, Mogk A (2012) Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nat Struct Mol Biol 19: 1347–1355 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mortensen P, Shevchenko A, Boucherie H, Mann M (1996) Linking genome and proteome by mass spectrometry: Large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA 93: 14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ (2006) Absolute quantification of proteins by LCMSE: A virtue of parallel MS acquisition. Mol Cell Proteomics 5: 144–156 [DOI] [PubMed] [Google Scholar]
- Smith DM, Kafri G, Cheng Y, Ng D, Walz T, Goldberg AL (2005) ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol Cell 20: 687–698 [DOI] [PubMed] [Google Scholar]
- Sorenson R, Bailey-Serres J (2014) Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 111: 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarz P, Mogk A, Bukau B (2008) Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol Microbiol 68: 87–97 [DOI] [PubMed] [Google Scholar]
- van Leeuwen W, Vermeer JE, Gadella TW Jr., Munnik T (2007) Visualization of phosphatidylinositol 4,5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J 52: 1014–1026 [DOI] [PubMed] [Google Scholar]
- Wallace EW, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, et al. (2015) Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162: 1286–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kurepa J, Smalle JA (2009) The Arabidopsis 26S proteasome subunit RPN1a is required for optimal plant growth and stress responses. Plant Cell Physiol 50: 1721–1725 [DOI] [PubMed] [Google Scholar]
- Watanabe YH, Nakazaki Y, Suno R, Yoshida M (2009) Stability of the two wings of the coiled-coil domain of ClpB chaperone is critical for its disaggregation activity. Biochem J 421: 71–77 [DOI] [PubMed] [Google Scholar]
- Waters ER. (2013) The evolution, function, structure, and expression of the plant sHSPs. J Exp Bot 64: 391–403 [DOI] [PubMed] [Google Scholar]
- Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FT, et al. (2004) Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119: 653–665 [DOI] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A (2012) Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J Cell Biol 198: 387–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Fu H, Walker J, Papa CM, Smalle J, Ju YM, Vierstra RD (2004) Purification of the Arabidopsis 26S proteasome: Biochemical and molecular analyses revealed the presence of multiple isoforms. J Biol Chem 279: 6401–6413 [DOI] [PubMed] [Google Scholar]
- Yao C, Wu Y, Nie H, Tang D (2012) RPN1a, a 26S proteasome subunit, is required for innate immunity in Arabidopsis. Plant J 71: 1015–1028 [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu X, Gaikwad K, Kou X, Wang F, Tian X, Xin M, Ni Z, Sun Q, Peng H, et al. (2017) Mutations in eIF5B confer thermosensitive and pleiotropic phenotypes via translation defects in Arabidopsis thaliana. Plant Cell 29: 1952–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Cheng Y, Chi YJ, Fan B, Yu JQ, Chen Z (2013) NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet 9: e1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]