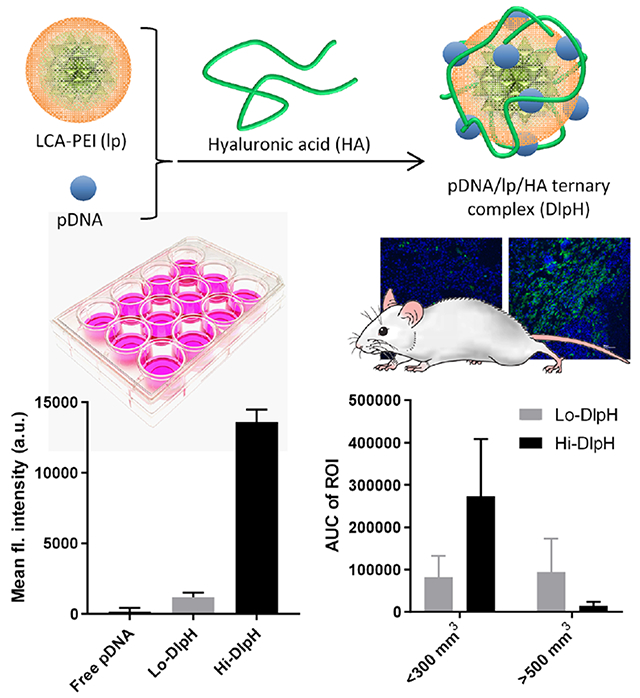
Abstract
Polyethyleneimine (PEI) is widely used for the delivery of nucleic acids, but its clinical application is limited due to high cytotoxicity and instability in biological fluids. To overcome these challenges, linear PEI (2.5 kDa) was modified with lithocholic acid (LCA) to produce a LCA-PEI conjugate (lp), and its complex with plasmid DNA (pDNA) was covered with hyaluronic acid (HA). Ternary complexes of pDNA, lp, and HA (“DlpH”) were prepared in different ratios and tested in cells and tumor-bearing mice for gene transfection efficiency. DlpH with a relatively high lp/pDNA ratio (Hi-DlpH) was more resistant to DNase and heparin treatment and showed more efficient gene transfection than DlpH with a lower lp/pDNA ratio (Lo-DlpH) in vitro. In contrast, Hi- and Lo-DlpH showed distinct transfection efficiency in vivo in a tumor-size dependent manner, where Hi-DlpH showed relatively high gene transfection in tumors of <300 mm3 but performed poorly in tumors of >500 mm3 and Lo-DlpH did the opposite. Tumor-associated macrophages, which increase with tumor growth and preferentially intercept Hi-DlpH, may account for the poor performance of Hi-DlpH in relatively large tumors. Accordingly, suggestions are made for future in vitro screening of new gene formulations to better predict their in vivo performances.
Keywords: Gene delivery, lithocholic acid-polyethyleneimine, tumor-associated macrophages, in vitro screening, in vitro and in vivo discrepancy
Graphical Abstract
1. Introduction
Gene therapy uses nucleic acids as therapeutic agents to restore the production of deficient proteins or to modulate the expression of specific genes governing disease progression. After five decades of efforts, gene therapies are now bringing new therapeutic options for different diseases [1]. In 2017, U.S. Food and Drug Administration (FDA) approved chimeric antigen receptor (CAR)-T cell therapy, cytotoxic T cells transfected with a recombinant anti-CD19 receptor by a viral vector, for the treatment of pediatric patients with B-cell acute lymphoblastic leukemia [2]. The success of gene therapy relies on the development of safe and efficient gene carrier systems. Viral vectors are most widely used as the carrier of therapeutic genes in clinical studies; however, acute immunogenicity, low loading capacity, and difficulty in large-scale production remain their limitations [3, 4]. To overcome this challenge, non-viral vectors based on cationic lipids or polymers have widely been pursued. For example, polyethyleneimine (PEI), a cationic polymer, has been explored as a gene carrier on the basis of high charge density and proton-sponge effects [5].
A weakness of PEI is the cytotoxicity due to the high molecular weight and excessive positive charges. Low molecular weight (MW) PEIs (e.g., 1.8 kDa PEI) show reduced cytotoxicity, but they also lose the transfection efficiency due to inadequate DNA complexation and protection [6]. To compensate for the loss of activity, the polymers may be grafted with hydrophobic moieties, which help form self-assemblies of cationic polymers, providing additional stability to the electrostatic assemblies of nucleic acids and polymers. For example, the conjugation of lipoic acid [7], deoxycholic acid [8, 9], cholesterol [10], or phospholipids [11–13] to PEI have improved the ability of the polymer to protect and deliver genes to cells. Likewise,PEI (2 kDa) grafted with lipids achieved comparable transfection efficiency as that of 25 kDa PEI while maintaining its benefit of low toxicity [8, 14].
On the basis of the earlier studies, we intend to optimize the stability and transfection efficiency of a DNA-PEI complex. We used lithocholic acid (LCA) to stabilize the pDNA-polymer complex, because LCA may also offer anti-cancer effects [15, 16]. Since excessive lipid substitution may interfere with efficient contact between amines of PEI and DNA, rather destabilizing the PEI/DNA complexes [14, 17–19], we modified low MW PEI with a minimal number of LCA and complement the stabilization with additional complexation of hyaluronic acid (HA). HA is an anionic polysaccharide of natural origin and has shown to shield extra cationic charges of PEI to protect a PEI-DNA complex from serum protein binding [20]. HA also enhances gene transfection by loosening the PEI-DNA complex in a timely manner and facilitating the access to the gene transcription machinery [20, 21].
In this study, we produce a low MW linear PEI (PEI, 2.5 kDa) grafted with two LCA molecules per polymer, prepare a ternary complex of pDNA, LCA-PEI (lp) and HA varying the ratio of lp and HA to pDNA, characterize its stability in adverse conditions, and evaluate the gene transfection efficiency in vitro (cell culture) and in vivo (Huh7-tumor bearing nude mice model). Interestingly, we observe an unexpected conflict between the two settings, where high polymer/pDNA ratio yields a relatively high gene delivery efficiency in cell culture, consistent with the existing studies of polymeric gene vectors, but shows a lower gene transfection upon intratumoral injection to large (advanced) tumors. We perform in vitro gene transfection in conditions mimicking different features of advanced tumors to identify tumor-associated macrophages (TAMs) as one of the potential reasons for the in vitro-in vivo discrepancy. On the basis of our observation, we make suggestions for future in vitro evaluation of gene formulations.
2. Materials and methods
2.1. Materials
Linear polyethylenimine base form (PEI base form, MW: 2.5 kDa) and polyethylenimine hydrochloride (PEI salt form, MW 4 kDa equivalent to 2.5 kDa PEI base form) were purchased from Polysciences, Inc. (Warrington, PA). Lithocholic acid (LCA, ≥97%), D-Luciferin potassium salt and EtBr (10 mg/mL) were purchased from Sigma-Aldrich (St. Louis, MO). Luminescence based assay kit was purchased from Promega (Madison, WI). Pierce™ BCA Protein Assay Kit, MassRuler DNA loading Dye (6×), DNase I kit, heparin and HEPES (1 M) were purchased from Thermo Fisher Scientific (Waltham, MA). Lipofectamine™2000 (L2k) and were purchased from Invitrogen (Carlsbad, CA). Sodium hyaluronate (HA, 20 kDa or 1.5 MDa) was purchased from Lifecore Biomedical, LLC (Chaska, MN). Label IT® Plasmid Delivery Control (Fluorescein-conjugated pDNA) was purchased from Mirus Bio LLC (Madison, WI). Dialysis membrane was purchased from Spectrum Labs (Waltham, MA). Enhanced green fluorescent protein (EGFP)-expressing pDNA (pGFP) and firefly luciferase-expressing plasmid DNA (pLuc) were replicated in DH5-α competent Escherichia coli as reported previously [22]. Rat anti-mouse F4/80 antibody was purchased from BioRad (Hercules, CA). Rabbit anti-rat secondary antibody and DyLight 488 conjugated goat anti-rabbit antibody were purchased from Vector Laboratories (Burlingame, CA). ProLong DAPI Gold was purchased from Invitrogen (Carlsbad, CA). Allophycocyanin (APC) conjugated anti-mouse F4/80 antibody was purchased from BioLegend (San Diego, CA).
2.2. Synthesis and characterization of LCA-PEI
8.7 mg of 1,1’-carbonyldiimidazole (CDI) dissolved in 0.7 mL dry chloroform was mixed with 15.8 mg LCA in 2 mL dry chloroform under stirring. After 1 h, the reaction mixture was slowly added into 10 mL of 5 mg/mL PEI base form (2.5 kDa) chloroform solution at 60 °C and reacted for 24 h under stirring. LCA-PEI (lp) was purified by dialysis (MWCO: 1000 Da) against ethanol (containing 0.4 mL of 1N HCl per 200 mL ethanol), followed by DI water and then acidified DI water (5 mL of 1N HCl per 200 mL DI water). The resulting product was dissolved in DMSO and analyzed by a Bruker ARX-300 NMR spectrometer equipped with a 5 mm QNP probe.
2.3. Preparation of DlpH complex
A binary complex of pDNA and lp was first prepared by mixing the two in HEPES buffered saline (HEPES 10 mM, pH 7.4) and incubating at room temperature for 30 min. For preparation of a ternary complex of pDNA (pGFP or pLuc), lp, and HA (DlpH), the Dlp binary complex was added to HA (20 kDa) solution in HEPES buffered saline and incubated at room temperature for 10 min. The typical pDNA/lp/HA ratio was 1/5/0.25 or 1/0.3/0.015 by weight, unless specified otherwise. For comparison, a DNA complex with L2k (pDNA/L2k) was prepared according to the manufacture’s protocol, and a ternary complex of pDNA, PEI salt form and HA (pDNA/PEI/HA) was prepared at a weight ratio of 1/10/0.5, which was found to be optimal for in vitro gene transection, in the same method as DlpH.
2.4. Characterization of DlpH complex
DlpH (equivalent to 0.2 μg of pDNA) was run on a 0.8% agarose gel in 0.5 × TAE buffer and run at 100 V for 40 min. The gel was pre-loaded with ethidium bromide (EtBr), and DNA bands were detected at 302 nm using Azure C300 (Dublin, CA). For stability testing, the gene complexes were challenged with 166 U/mL DNase for 15 min, 8 mg/mL heparin for 2.5 h, or at pH 5.5, 6.5, 7.4 for 24 h and analyzed by agarose gel electrophoresis. The size and zeta potential of DlpH were determined by the Malvern Zetasizer Nano ZS90 (Worcestershire, United Kingdom). The size was measured in HEPES buffer (10 mM, pH 7.4), and the zeta-potential was measured in phosphate buffer (10 mM, pH 7.4). Their morphology was examined by a FEI Tecnai T20 transmission electron microscope (Hillsboro, OR) after negative staining with 1% uranyl acetate.
2.5. Cell culture
SKOV-3 (Human ovarian cancer cell), KB (human oral epidermal carcinoma cells), MCF7 (human breast cancer cells), PC-3 (human prostate adenocarcinoma cells), M109 (mouse lung cancer cells), NIH/3T3 (mouse embryonic fibroblasts), peritoneal MΦ (peritoneal macrophages cell line), LS174T (human hepatocyte carcinoma cells), RAW264.7 (murine macrophages) and B16F10 (mouse melanoma cells) were obtained from ATCC (Manassas, VA). HepG2 (human liver carcinoma cells), Huh7 (human hepatocyte carcinoma cells) were donated by Prof. Wanqing Liu (Wayne State University), and TUBO (murine mammary carcinoma cells) was by Prof. Stephen J. Kron (University of Chicago). SKOV-3, MCF7, PC-3, Huh7, KB, HepG2 and TUBO cells were cultured in RPMI 1640 medium, RAW264.7, PM, NIH/3T3, B16F10 and M109 in DMEM medium, and LS174T cells in MEM medium. The media contained 10% fetal bovine serum (FBS) or 10% fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin.
2.6. In vitro gene transfection
Cells were seeded in a 24 well plate at a density of 5 × 104 cells/well and cultured at 37 °C with 5% CO2. For evaluation of gene transfection in Huh7 cell and macrophage co-culture system, 2.5 × 104 of Huh7 cells were mixed with 2.5 × 103 (10% of Huh7) or 5 × 103 (20% of Huh7) of RAW264.7 cells and seeded in a 24 well plate. When the cells reached 70% confluence, the culture medium was replaced with 300 μL of transfection medium containing DlpH, pDNA/L2k, or pDNA/PEI/HA equivalent to 0.4 μg pDNA, followed by 24 h incubation at 37 °C. The transfection medium was then replaced with fresh complete medium, and the cells were allowed to grow for another 48 h. For measuring GFP expression, cells were imaged with a Cytation 3 imaging system (Biotek, Winooski, VT). For measuring luciferase expression, cells were rinsed with PBS and lysed with a cell culture lysis buffer, and the luciferase activity of the cell lysate was measured by the Luciferase activity assay kit (Promega, Fitchburg, WI) and a SpectraMax M3 microplate reader (Molecular Devices, Sunnyvale, CA). The total protein amount was measured by Pierce™ BCA Protein Assay. The gene transfection efficiency was expressed as the relative luciferase intensity/mg total protein.
For testing the effects of protein concentrations on gene transfection efficiency, Huh7 cells were seeded in a 24 well plate at a density of 5 × 104 cells/well and cultured to 70% confluence. The culture medium was replaced with 300 μL of transfection medium, which contained Hi-DlpH or Lo-DlpH equivalent to 0.4 μg pDNA as well as 10%, 50% or 80% FBS, and incubated for 24 h at 37 °C. The transfection medium was then replaced with fresh complete medium, and the cells were allowed to grow for another 48 h. The luciferase intensity and total protein amount were measured as above.
2.7. Cellular uptake of *DlpH
Huh7 cells or RAW 264.7 cells were seeded in a 24 well plate at a density of 5 × 104 cell/well and cultured at 37 °C with 5% CO2. After 24 h, the culture medium was replaced with transfection medium containing DlpH equivalent to 0.4 μg of fluorescein-labeled pDNA and incubated for 1, 3 or 6 h. The cells were then rinsed, harvested, and analyzed by the BD Accuri C6 Flow Cytometer (BD Bioscience, Bedford, MA). A total of 10,000 gated events were acquired for each analysis. All experiments were performed in triplicate.
2.8. In vivo gene transfection
5–6 weeks old female athymic nude mouse were purchased from Envigo (Indianapolis, IN) and acclimatized for 7 days prior to the procedure. All animal procedures were approved by Purdue Animal Care and Use Committee, in conformity with the NIH guidelines for the care and use of laboratory animals. A xenograft tumor model was prepared by subcutaneous injection of 107 Huh7 cells in the upper flank of the right hind leg. The length (L) and width (W) of each tumor were measured daily with a digital caliper, and the volume (V) was calculated according to the modified ellipsoid formula: V = (L × W2)/2. When the tumor grown to 500-800 mm3 (defined as late stage tumor) or 100-300 mm3 (defined as early stage tumor), the mouse was treated with a single intratumoral injection of DlpH equivalent to 30 μg pDNA suspended in 150 μL of HEPES-buffered saline (HEPES 10 mM, pH 7.4). Starting from 24 h post-treatment, the animals were imaged with a SPECTRAL AMI imaging system (Spectral Instruments, Tucson, AZ) to visualize the luciferase gene expression. At each imaging, each mouse received 200 μL of 15 mg/mL D-luciferin potassium salt by intraperitoneal injection, followed by continuous monitoring for 20 min. The imaging was repeated every 24 h until no luminescence signal was detected. The luminescence intensity of region of interest (ROI) was quantified by the AMI viewer image software and expressed as radiance (p/s/cm2/sr). The area under the curve (AUC) of ROI was calculated from a plot of ROI vs. time as an estimate of total luminescence signal produced in tumor.
2.9. Histological analysis of tumor-associated macrophages (TAM) population in tumor sections
TAM population in Huh7 tumor tissue was quantified by immunohistochemistry. Briefly, Huh7 tumors were collected and immersed in 30% sucrose solution for cryoprotection. The tissues were snap frozen in the Optimal Cutting Temperature compound by liquid nitrogen and cryosectioned at 5 μm using a Leica CM1860 cryostat and post-fixed in methanol at −20 °C for 10 min. Slides were rinsed twice in PBS and put in a Dako Autostainer Plus for subsequent staining. 2.5% normal horse serum was applied for 20 min. Excess reagent was removed and rat anti-mouse F4/80 antibody applied for 60 min. Slides were rinsed twice with Tris-buffered saline with Tween (TBST), and rabbit anti-rat secondary antibody at a dilution of 1:200 was applied for 30 min. Slides were rinsed twice with TBST, and Dylight 488-conjugated goat anti-rabbit antibody was applied for 30 min. Slides were rinsed in TBST and water, mounted with ProLong DAPI Gold and examined by a Nikon A1R confocal microscope (Nikon America Inc., Melville, NY). Three different sections per tumor tissue were analyzed by Fiji image J software (National Institute of Health, Bethesda, MD) to quantify F4/80+ area and the number of total cell nuclei.The coverage of TAM in each section was expressed as the ratio of F4/80+ area to the number of nuclei.
2.10. Quantitative analysis of TAM population
Huh7 tumors were inoculated in female nude mice as described above and grown to two different sizes (500 and 1200 mm3). The tumors were harvested, minced to small pieces with scissors, and digested by 2 mg/mL collagenase type IV, 0.2 mg/mL DNase I and 0.2 μg/mL hyaluronidase for 2 h at 37 °C. The cell suspension was filtered through 100 μm and 40 μm cell strainers sequentially and centrifuged at 500 ×g for 5 min. Cell pellets were resuspended in 5 mL ACK lysing buffer, incubated for 5 min and washed with PBS for 3 times to remove red blood cells. The single cell suspension was first stained with anti-mouse CD16/32 antibody to block non-specific binding of immunoglobulin to Fc receptors and then stained with APC-conjugated anti-mouse F4/80 antibody. The stained cells were analyzed by the BD Accuri C6 Flow Cytometer (BD Bioscience, Bedford, MA).
2.11. DlpH transport through hyaluronic acid
Sixty microliters of 10 μg/mL HA (1.5 MDa) solution was added into a microchannel of an ibidi μ-Slide VI 0.4 (Gräfelfing, Germany) through the inlet and stabilized for 0.5 h at room temperature. Five microliters of Lo- or Hi-*DlpH was first mixed with 10 μL of 10 mg/mL HA (1.5 MDa) solution and added on top of HA solution through the inlet and stabilized for 10 min. To prevent dehydration of the HA matrix and create a pressure gradient to drive the penetration of DlpH through the matrix, three drops and one drop of lens oil were added to the inlet and outlet, respectively. The penetration of *DlpH was monitored at the inlet and in the middle of the channel by a Nikon A1R confocal microscope (Nikon America Inc., Melville, NY).
2.12. Statistical analysis
All statistical analyses were performed with GraphPad Prism 7 (La Jolla, CA). Data were analyzed by t-test or ANOVA followed by the recommended multiple comparisons test. A value of p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Synthesis and characterization of LCA-PEI
LCA was conjugated to PEI via carbonyldiimidazole to form a LCA-PEI conjugate (lp) (Figure 1a). The structures of each component and lp conjugate were examined by 1H NMR.LCA showed distinct chemical shifts at δ 1.0 - 2.3 ppm due to steroid protons, and PEI at δ 2.6 - 3.0 ppm indicating 224 protons of PEI ethylene (Figure S1). The lp conjugate showed shifts of steroid protons of LCA at 0.6 ppm as well as those of PEI ethylene at 2.98 ppm, confirming the conjugation of the two. Of note, the chemical shift of PEI in lp was slightly downfield relative to that of original PEI, likely due to the deprotonation during the purification process, consistent with the literature [23]. According to the integration of 1H NMR peaks, the molar ratio of LCA to PEI was estimated to be 1.7 ± 0.5 (n = 5 independent batches).
Figure 1.
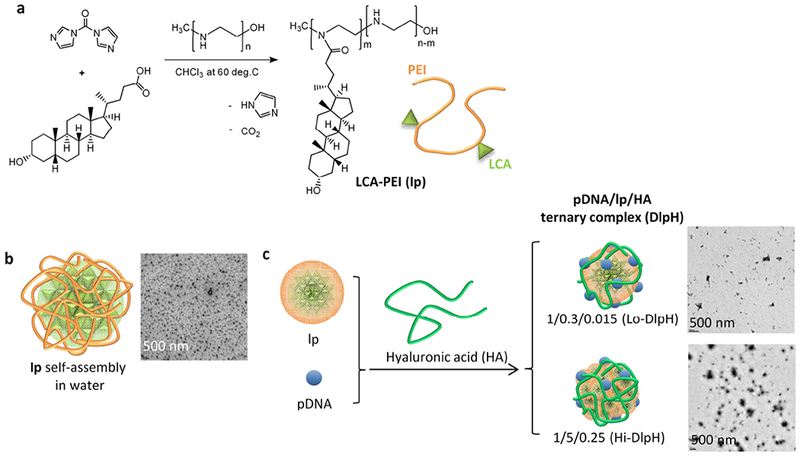
Schematics of (a) LCA-PEI conjugate (lp) synthesis; (b) lp self-assembly in water; and (c) pDNA/lp/HA ternary complexes (DlpH).
3.2. Screening, characterization, and in vitro evaluation of DlpH complex
LCA-PEI (lp) conjugates formed a self-assembly (119.0 ± 23.1 nm based on TEM images: 136 particles analyzed by Image J) in aqueous medium due to the hydrophobic interaction of LCA (Figure 1b). pDNA/lp (Dlp) binary complex was formed by adding pDNA into lp suspension in HEPES buffered saline and incubating for 30 min (Figure 1c). pDNA/lp/HA (DlpH) ternary complex was prepared by mixing Dlp binary complex with HA (Figure 1c). DlpH complexes were prepared with different ratios of lp to pLuc (with a constant lp to HA ratio of 20/1) and tested for luciferase gene expression in Huh-7 cells. Luminescence intensity (quantitative measure of luciferase expression) increased with the lp to pLuc ratio (Figure 2a). Among the tested complexes, DlpH with a pLuc/lp/HA weight ratio of 1/5/0.25 and 1/10/0.5 showed the highest transfection efficiency (luciferase expression per cell protein) (Figure 2c). Since the latter showed significant reduction in protein levels (Figure 2b), indicating a negative effect on cell proliferation, DlpH with a pLuc/lp/HA weight ratio of 1/5/0.25 was chosen as the optimal complex and characterized with respect to physical properties, stability, cellular uptake, and gene transfection activity. Another DlpH with a suboptimal pLuc/lp/HA weight ratio (1/0.3/0.015) was also tested for comparison. DlpH with a pLuc/lp/HA weight ratio of 1/5/0.25 and 1/0.3/0.015 were called Hi-DlpH and Lo-DlpH, respectively.
Figure 2.

Transfection efficiency of DlpH in Huh7 cells with plasmid DNA encoding luciferase (pLuc) as a reporter gene. (a) Luciferase expression represented by luminescence intensity; (b) cell protein levels relative to the untreated control indicating toxicity of DlpH with different ratios of components; and (c) transfection efficiency of DlpH, defined as the luciferase expression divided by the cell protein level. DlpH with a pLuc/lp/HA weight ratio of 1/5/0.25 and 1/0.3/0.015 were called Hi-DlpH and Lo-DlpH, respectively, in the rest of the study. Data are expressed as means and standard deviations of three repeated tests of a representative batch of DlpH.
According to the dynamic light scattering (DLS) measurement, z-averages (in diameter) of Hi-DlpH and Lo-DlpH were 578.7 ± 27.6 nm and 485.0 ± 20.0 nm, respectively (Figure 3a). Zeta potentials of Hi-DlpH and Lo-DlpH were +8.31 ± 0.35 and −37.8 ± 3.4, respectively, reflecting the high content of cationic lp in Hi-DlpH relative to Lo-DlpH. According to TEM images, Hi-DlpH seemed to have formed more condensed complexes than Lo-DlpH (Figure 3b, Figure S2). The particles shown in TEM images appeared to be less than 200 nm, much smaller than the z-averages measured by DLS. The larger particle sizes shown in DLS measurement is likely due to a mild degree of particle aggregation in the buffer (HEPES 10 mM, pH 7.4) in which the particle sizes were measured. When challenged with DNase (representing endogenous nuclease), both types of DlpH protected pDNA from degradation (Figure 3c). The two types of DlpH showed differential sensitivity to heparin (representing negatively charged macromolecules in physiological medium): After 2.5 h treatment with heparin, pDNA was dissociated from Lo-DlpH yielding a strong band, whereas Hi-DlpH showed a relatively weak pDNA band. These results indicate that Hi-DlpH resists adverse conditions better than Lo-DlpH. To evaluate the extent of DlpH uptake by tumor cells, fluorescently-labeled DlpH (called *DlpH) was prepared with fluorescein-conjugated pDNA. Huh7 cells were incubated with *DlpH and analyzed by flow cytometry (Figure 3d). Both Lo-*DlpH and Hi-*DlpH induced a shift in fluorescence intensity compared to the non-treated control cells. The cells incubated with Hi-*DlpH showed significantly higher fluorescence intensity than those with Lo-*DlpH, indicating that Hi-DlpH can more efficiently deliver pDNA into cells than Lo-DlpH (Figure 3d).
Figure 3.
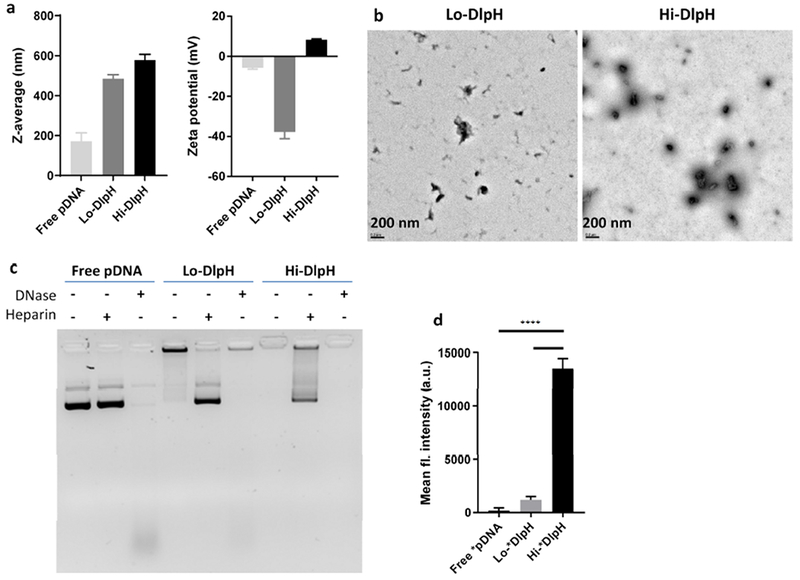
Characteristics of DlpH ternary complex. (a) Z-averages and zeta potentials of free pDNA, Lo-DlpH and Hi-DlpH. (b) TEM images of Lo-DlpH and Hi-DlpH. Scale bar: 200 nm. (c) Agarose gel electrophoresis of free pDNA, Lo-DlpH and Hi-DlpH incubated in DNase or heparin. (d) Cellular uptake of free pDNA, Lo-DlpH and Hi-DlpH by Huh7 cells. Data are expressed as means and standard deviations of three repeated tests of a representative batch of DlpH. ****: p<0.0001 by the Tukey’s multiple comparisons test.
Hi-DlpH, as a preferable complex, was further tested on different cell lines using pLuc and pGFP as reporter genes. The extent of luciferase expression, represented by luminescence signal intensity of the cell lysate, was the highest with SKOV-3 and Huh-7 cells among the tested cell lines, followed by PC3 cells, TUBO cells, MCF-7 cells, B16F10 cells and RAW264.7 cells (Figure S3a). GFP expression showed a similar pattern, with the greatest signal in SKOV-3 and Huh7 cells followed by M109 cells, MCF7 cells, KB cells, NIH3T3, LS174T, HepG2 and peritoneal MΦ cells (Figure S3b). Hi-DlpH was also compared with pDNA/PEI/HA (ternary complex prepared with an HCl salt form of unmodified 2.5 kDa PEI), or pDNA/L2k (complex with Lipofectamine2000), commercial gene transfection agents. The Hi-DlpH tolerated heparin/DNase dual treatment better than a pDNA/PEI/HA ternary polyplex (Figure S4a), demonstrating that LCA conjugation improves the stability of the gene complex. Hi-DlpH showed similar or less cytotoxicity than pDNA/L2k or pDNA/PEI/HA at concentrations equivalent to pDNA 0.4 μg (Figure S4b). In transfecting SKOV-3 and MCF-7 cells, Hi-DlpH was comparable to pDNA/PEI/HA and superior to pDNA/L2k (Figure S4c). With M109 cells, Hi-DlpH was comparable to pDNA/L2k and superior to pDNA/PEI/HA (Figure S4c). In addition, DlpH (pDNA/PEI/HA=1:10:1) showed a longer lasting gene transfection activity than pDNA/L2k (Figure S4d). pDNA/L2k was more efficient than DlpH immediately after the treatment, showing much higher GFP expression when measured at 24 h post-treatment. However, the mean GFP signal intensity decreased over time, indicating that the protein produced early on was diluted through cell proliferation. In contrast, DlpH showed gradually increasing GFP signal intensity, which suggests that it might not release pDNA at once but get passed on to daughter cells to induce persistent gene transfection over generations. Consequently, DlpH showed significantly higher total GFP production (= the cell number multiplied by mean fluorescence intensity per cell) than pDNA/L2k at later time points.
3.3. In vivo gene transfection of DlpH
According to the in vitro cellular uptake (Figure 3d) and gene transfection (Figure 2), we expected that Hi-DlpH would be more efficient than Lo-DlpH in transfecting tumors in vivo. In vivo gene transfection efficiency of Hi- and Lo-DlpH was evaluated in a Huh7 xenograft model with pLuc as a reporter gene. The complexes were injected intratumorally to exclude differences in biodistribution. The evaluation was performed with tumors in two different sizes (<300 mm3 and >500 mm3). In tumors smaller than 300 mm3, Hi-DlpH showed brighter and longer lasting luminescence signal than Lo-DlpH (Figure 4), indicating greater transfection efficiency of Hi-DlpH, consistent with in vitro. In contrast, in >500 mm3 tumors, Hi-DlpH showed weaker luminescence signals than Lo-DlpH.
Figure 4.

Bioluminescence imaging of Huh7 tumor-bearing animals receiving intratumoral injection of Lo-DlpH and Hi-DlpH. The luminescence signal intensity of the region of interest (ROI) was quantified by the AMI viewer image software and expressed as radiance (p/s/cm2/sr). The area under the curve (AUC) of ROI was calculated from the plot of ROI vs. time of each animal. **: p<0.01 by the Sidak’s multiple comparisons test.
3.4. Factors affecting in vivo gene transfection in tumors
We speculate that changes in the tumor microenvironment (TME) may be responsible for the dependence of DlpH activity on tumor size. TME consists of tumor cells as well as various immune cells, endothelial cells, stromal cells, and extracellular matrix (ECM) [24, 25]. It also contains soluble proteins such as chemokines, growth factors, interferons, interleukins, and angiogenic factors, synthesized by tumor and stromal cells [26–28]. Furthermore, TME is slightly more acidic (pH 6-7) than normal tissues (pH 7.2-7.4) [29, 30]. These features manifest gradually with the progression of tumors and may account for the differential gene transfection profiles in small (i.e., relatively early stage) and large (i.e., late stage) tumors. To test this, in vitro gene transfection was performed with Huh7 cells in conditions mimicking different features of tumors (acidic pH, presence of extracellular proteins and tumor-associated macrophages).
To test the effect of proteins, cells were transfected with DlpH in media with different protein concentrations. Both Hi- and Lo-DlpH were negatively affected by increasing protein concentrations (Figure S5a); nevertheless, Hi-DlpH maintained consistently higher transfection efficiency than Lo-DlpH. To test the effect of acidic pH, DlpH was incubated in different pH’s (pH 5.5 vs. 7.4) and run on an agarose gel. Hi-DlpH was more stable than Lo-DlpH in all tested pH conditions (Figure S5b). These results indicate that Hi-DlpH is no more sensitive than Lo-DlpH to extracellular proteins and acidic tumoral pH. Therefore, these two conditions may not explain the relatively low performance of Hi-DlpH in advanced tumors.
We then considered the effect of TAMs, which may account for as high as 50% of tumor mass [19, 31]. The TAM population is known to increase with tumor progression [32, 33]. We also observed that macrophage (F4/80) signals in tumor increased with the size by immunostaining of tumor sections (Figure S6) and flow cytometry (Figure S7). We suspected that the increasing number of macrophages might negatively affect tumor transfection of Hi-DlpH. As expected from the phagocytic nature, RAW264.7 macrophages efficiently took up DlpH (Figure 5a, Figure S8). Between Hi-DlpH and Lo-DlpH, the former was more readily taken up, likely due to the positive surface charge of Hi-DlpH [34]. The difference in HA content between Hi-DlpH and Lo-DlpH may also be relevant, given that HA can activate macrophages depending on the molecular weight [35]. However, a similar in vivo gene transfection trend was seen with binary complexes without HA (Figure S9), thus excluding the possibility of HA being responsible for the differential TAM uptake. Both Hi-DlpH and Lo-DlpH showed minimal luciferase expression in macrophages (data not shown). It is well known that degradative enzymes in macrophages destroy the phagocytosed extracellular particles including gene carriers [34]; therefore, the nucleic acid payloads may well have been inactivated upon macrophage uptake. This result suggests that macrophages may intercept DlpH complex and prevent their uptake and translation by tumor cells. Advanced tumors with a large population of TAMs may be more sensitively affected by such interference. To verify this, DlpH transfection was performed in Huh7 tumor cells co-cultured with RAW264.7 macrophages, increasing the fraction up to 20%. The transfection efficiency of Hi-DlpH was significantly reduced in the co-culture system as compared with the single culture (0% macrophages). The transfection efficiency of Hi-DlpH decreased in proportion to the fraction of macrophages in the co-culture, whereas the transfection did not show significant change with Lo-DlpH (Figure 5b). Taken together, these results support that TAMs may, at least partly, be responsible for the poor performance of Hi-DlpH in relatively large tumors.
Figure 5.

(a) Cellular uptake of free *pDNA, Lo-*DlpH and Hi-*DlpH by RAW264.7 cells after incubation for 6 h. Data are expressed as means and standard deviations of three repeated tests of each representative sample. **: p< 0.01, ****: p<0.0001 by the Sidak’s multiple comparisons test. (b) Transfection efficiency of Lo-DlpH and Hi-DlpH in Huh7 cells co-cultured with RAW264.7 macrophages. Data are expressed as means and standard deviations of three repeated tests of a representative batch of each complex. ***: p< 0.001, ****: p<0.0001 vs. 0% RAW264.7 by the Dunnett’s multiple comparisons test.
We note that Hi-DlpH was still more efficient than Lo-DlpH in vitro, given the absolute luminescence signals produced by unit pLuc dose (Figure 5b). This indicates that the competition of TAMs may not fully account for the relatively poor performance of Hi-DlpH in large tumors in vivo. A potential reason may be the differential intratumoral transport of Hi-DlpH and Lo-DlpH due to the difference in surface charge (Hi-DlpH: +8.31 mV vs. Lo-DlpH: −37.80 mV). Efficient intratumoral transport is critical to the nanoparticle-mediated gene delivery [36]. Surface charge plays an important role in intratumoral transport of nanoparticles. Cationic particles are more disadvantaged than anionic particles in vivo, because they show high interactions with cells and extracellular matrix, which present net negatively charges, and thus may not move very far. On the other hand, anionic particles tend to penetrate deeper into tumor tissues than cationic particles due to the lack of distraction [37–39]. Therefore, we speculate that Lo-DlpH, with the negative surface charge, may have traveled further into the tumors from the injection site than Hi-DlpH to achieve widespread gene transfection in tumors. To verify this, we examined the penetration of Hi- and Lo-DlpH through viscous HA (1.5 MDa) solution. HA was chosen as the model matrix as it constitutes one of the main functional components of the tumor extracellular matrix along with collagen [40–42]. HA imparts a strong negative charge and high viscosity, which create an electrostatic and pressure barrier to the transport of cationic particles through tumor extracellular matrix [40, 41]; thus, it is particularly relevant to the DlpH penetration. Fluorescently labeled Hi- and Lo-*DlpH were passed through a microfluidic channel filled with the HA matrix under a pressure gradient, and their movement was observed by the fluorescent microscope. As expected, Hi-*DlpH moved more slowly than Lo-*DlpH in the HA matrix (Figure S10), supporting that the restricted intratumoral transport of Hi-DlpH may account for the relatively poor performance of Hi-DlpH in vivo.
3.5. In vitro and in vivo discrepancy: implications for future studies
In developing new gene carriers for tumor therapy, it is a common practice to screen formulations with respect to physicochemical properties and then test promising candidates in cell culture to evaluate the gene transfection efficiency. The cell culture system typically comprises a single type of tumor cells, and the effects of tumor-associated cells are seldom considered. Nevertheless, our study finds that Hi-DlpH, which is superior to Lo-DlpH in stability, cellular uptake, and gene transfection in vitro and in relatively small (early stage) tumors, performs rather poorly in large (advanced) tumors relative to Lo-DlpH, likely due to the increasing population of TAMs that compete with tumor cells non-productively. While our study has focused only on TAMs as a representative non-tumor cell population in TME, there are other supporting cells such as cancer-associated fibroblasts (CAFs), endothelial cells, and immune cells [24, 25, 27] that may similarly affect gene transfection of a carrier in vivo. Our results indicate that typical in vitro cell culture systems based on a single type of cells is limited in predicting in vivo gene transfection efficiency of a carrier. The single cell culture system can even be misleading if the carrier is prone to interactions with non-tumor cells. While the cellular uptake patterns may be individually evaluated using multiple single culture models, they may not help assess how multiple cells compete for the carrier in the microenvironment of target tissues. To circumvent this limitation, it will be desirable to use intratumoral injection or a co-culture model consisting of tumor cells and tumor-associated cells as a routine practice of gene carrier evaluation.
On the other hand, it is worth considering that the tumor microenvironment and tumor-associated cells, given their significance in tumor progression, may be actively exploited to improve therapeutic efficacy of drug products [43]. For example, TAMs, CAFs, and tumor extracellular matrix may be targeted as a way of removing tumor-supportive microenvironment and physicochemical barriers to drug transport [36, 44–48]. Physiological features of tumors such as acidity, hypoxia, and oxidative stress are also explored for improving tumor-specific delivery of nanocarriers [49, 50].
4. Conclusion
Ternary gene complex consisting of pDNA, PEI-LCA conjugate (lp), and HA was developed and optimized with respect to the size, stability, and gene transfection efficiency in vitro and in vivo. Hi-DlpH with a relatively high ratio of lp to pDNA was more stable in adverse conditions and more efficient in cellular uptake and in vitro gene transfection than Lo-DlpH with a lower ratio of lp to pDNA. Hi-DlpH was superior to Lo-DlpH in transfecting Huh7 tumors with a size less than 300 mm3, consistent with the in vitro studies, but performed rather poorly than Lo-DlpH in bigger tumors (>500 mm3). Histological evaluation of tumor sections, macrophage uptake, and transfection in the tumor cell-macrophage co-culture system suggest that increasing TAM populations in the late stage tumors may have competed for Hi-DlpH non-productively, thereby reducing the amount of Hi-DlpH available for tumor cells.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB017791, R01 CA232419, and R01 CA199663) and the scholarship from China Pharmaceutical University. The authors also acknowledge the assistance of the Purdue University Histology Research Laboratory, a core facility of the NIH-funded Indiana Clinical and Translational Science Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supporting Data
Supporting data related to this article can be found on the internet.
Data Availability
The raw data required to reproduce these findings and the processed data required to reproduce these findings are available upon request.
References
- [1].Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M, Gene therapy comes of age, Science 359 (2018). [DOI] [PubMed] [Google Scholar]
- [2].FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma, (2017). Available from URL: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm581216.htm.
- [3].Lostalé-Seijo I, Montenegro J, Synthetic materials at the forefront of gene delivery, Nat. Rev. Chem 2 (2018) 258–277. [Google Scholar]
- [4].Thomas CE, Ehrhardt A, Kay MA, Progress and problems with the use of viral vectors for gene therapy, Nat. Rev. Genet 4 (2003) 346–58. [DOI] [PubMed] [Google Scholar]
- [5].Pandey AP, Sawant KK, Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery, Mater. Sci. Eng. C Mater. Biol. Appl 68 (2016) 904–918. [DOI] [PubMed] [Google Scholar]
- [6].Godbey WT, Wu KK, Mikos AG, Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle, J. Biomed. Mater. Res 45 (1999) 268–75. [DOI] [PubMed] [Google Scholar]
- [7].Zheng M, Zhong Y, Meng F, Peng R, Zhong Z, Lipoic Acid Modified Low Molecular Weight Polyethylenimine Mediates Nontoxic and Highly Potent in Vitro Gene Transfection, Mol. Pharm 8 (2011) 2434–2443. [DOI] [PubMed] [Google Scholar]
- [8].Kim D, Lee D, Jang YL, Chae SY, Choi D, Jeong JH, Kim SH, Facial amphipathic deoxycholic acid-modified polyethyleneimine for efficient MMP-2 siRNA delivery in vascular smooth muscle cells, Eur. J. Pharm. Biopharm 81 (2012) 14–23. [DOI] [PubMed] [Google Scholar]
- [9].Hong J, Ku SH, Lee MS, Jeong JH, Mok H, Choi D, Kim SH, Cardiac RNAi therapy using RAGE siRNA/deoxycholic acid-modified polyethylenimine complexes for myocardial infarction, Biomaterials 35 (2014) 7562–73. [DOI] [PubMed] [Google Scholar]
- [10].Bajaj A, Kondaiah P, Bhattacharya S, Synthesis and gene transfection efficacies of PEI-cholesterol-based lipopolymers, Bioconjug. Chem 19 (2008) 1640–51. [DOI] [PubMed] [Google Scholar]
- [11].Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP, Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery, Biomaterials 33 (2012) 3942–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Navarro G, Essex S, Sawant RR, Biswas S, Nagesha D, Sridhar S, de Ilarduya CT, Torchilin VP, Phospholipid-modified polyethylenimine-based nanopreparations for siRNA-mediated gene silencing: implications for transfection and the role of lipid components, Nanomedicine 10 (2014) 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP, P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells, Nanomedicine (Lond) 7 (2012) 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neamnark A, Suwantong O, K. C RB, Hsu CYM, Supaphol P, Uludağ H, Aliphatic Lipid Substitution on 2 kDa Polyethylenimine Improves Plasmid Delivery and Transgene Expression, Mol. Pharm 6 (2009) 1798–1815. [DOI] [PubMed] [Google Scholar]
- [15].Goldberg AA, Beach A, Davies GF, Harkness TA, Leblanc A, Titorenko VI, Lithocholic bile acid selectively kills neuroblastoma cells, while sparing normal neuronal cells, Oncotarget 2 (2011) 761–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chae SY, Jin CH, Shin JH, Son S, Kim TH, Lee S, Youn YS, Byun Y, Lee MS, Lee KC, Biochemical, pharmaceutical and therapeutic properties of long-acting lithocholic acid derivatized exendin-4 analogs, J. Control. Release 142 (2010) 206–13. [DOI] [PubMed] [Google Scholar]
- [17].Teo PY, Yang C, Hedrick JL, Engler AC, Coady DJ, Ghaem-Maghami S, George AJT, Yang YY, Hydrophobic modification of low molecular weight polyethylenimine for improved gene transfection, Biomaterials 34 (2013) 7971–7979. [DOI] [PubMed] [Google Scholar]
- [18].Bagai S, Sun C, Tang T, Lipid-Modified Polyethylenimine-Mediated DNA Attraction Evaluated by Molecular Dynamics Simulations, J. Phys. Chem. B 118 (2014) 7070–7076. [DOI] [PubMed] [Google Scholar]
- [19].Sun C, Tang T, Uludag H, A molecular dynamics simulation study on the effect of lipid substitution on polyethylenimine mediated siRNA complexation, Biomaterials 34 (2013) 2822–2833. [DOI] [PubMed] [Google Scholar]
- [20].Xu P, Quick GK, Yeo Y, Gene delivery through the use of a hyaluronate-associated intracellularly degradable crosslinked polyethyleneimine, Biomaterials 30 (2009) 5834–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ito T, Iida-Tanaka N, Niidome T, Kawano T, Kubo K, Yoshikawa K, Sato T, Yang Z, Koyama Y, Hyaluronic acid and its derivative as a multi-functional gene expression enhancer: protection from non-specific interactions, adhesion to targeted cells, and transcriptional activation, J. Control. Release 112 (2006) 382–8. [DOI] [PubMed] [Google Scholar]
- [22].Feng M, Ibrahim BM, Wilson EM, Doh K-O, Bergman BK, Park C, Yeo Y, Stabilization of a hyaluronate-associated gene delivery system using calcium ions, Biomater. Sci 2 (2014) 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holycross DR, Chai M, Comprehensive NMR Studies of the Structures and Properties of PEI Polymers, Macromolecules 46 (2013) 6891–6897. [Google Scholar]
- [24].Whiteside TL, The tumor microenvironment and its role in promoting tumor growth, Oncogene 27 (2008) 5904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L, Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy, Ann. Oncol 27 (2016) 1482–92. [DOI] [PubMed] [Google Scholar]
- [26].Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA, Chronic Inflammation and Cytokines in the Tumor Microenvironment, J. Immunol. Res 2014 (2014) 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Balkwill FR, Capasso M, Hagemann T, The tumor microenvironment at a glance, J. Cell Sci 125 (2012) 5591–5596. [DOI] [PubMed] [Google Scholar]
- [28].Huang W, Luo S, Burgess R, Yi YH, Huang GF, Huang RP, New Insights into the Tumor Microenvironment Utilizing Protein Array Technology, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cardone RA, Casavola V, Reshkin SJ, The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis, Nat. Rev. Cancer 5 (2005) 786–795. [DOI] [PubMed] [Google Scholar]
- [30].Gerweck LE, Seetharaman K, Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer, Cancer Res 56 (1996) 1194–1198. [PubMed] [Google Scholar]
- [31].Vinogradov S, Warren G, Wei X, Macrophages associated with tumors as potential targets and therapeutic intermediates, Nanomedicine (Lond) 9 (2014) 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Redente EF, Orlicky DJ, Bouchard RJ, Malkinson AM, Tumor Signaling to the Bone Marrow Changes the Phenotype of Monocytes and Pulmonary Macrophages during Urethane-Induced Primary Lung Tumorigenesis in A/J Mice, Am. J. Pathol 170 (2007) 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW, A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth, PloS One 4 (2009) e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H, Nanoparticle Uptake: The Phagocyte Problem, Nano today 10 (2015) 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA, High and low molecular weight hyaluronic acid differentially influence macrophage activation, ACS Biomater. Sci. Eng 1 (2015) 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Guan X, Lin L, Chen J, Hu Y, Sun P, Tian H, Maruyama A, Chen X, Efficient PD-L1 gene silence promoted by hyaluronidase for cancer immunotherapy, J. Control. Release 293 (2019) 104–112. [DOI] [PubMed] [Google Scholar]
- [37].Kim B, Han G, Toley BJ, Kim C.-k., Rotello VM, Forbes NS, Tuning payload delivery in tumour cylindroids using gold nanoparticles, Nat. Nanotechnol 5 (2010) 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang K, Boerhan R, Liu C, Jiang G, Nanoparticles Penetrate into the Multicellular Spheroid-on-Chip: Effect of Surface Charge, Protein Corona, and Exterior Flow, Mol. Pharm 14 (2017) 4618–4627. [DOI] [PubMed] [Google Scholar]
- [39].Holback H, Yeo Y, Intratumoral drug delivery with nanoparticulate carriers, Pharm. Res 28 (2011) 1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Walker C, Mojares E, Del Río Hernández A, Role of Extracellular Matrix in Development and Cancer Progression, Int. J. Mol. Sci 19 (2018) 3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stylianopoulos T, Poh M-Z, Insin N, Bawendi MG, Fukumura D, Munn LL, Jain RK, Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions, Biophys J 99 (2010) 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM, Therapeutic Targeting of Hyaluronan in the Tumor Stroma, Cancers 4 (2012) 873–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR, Targeting Tumor Microenvironment for Cancer Therapy, Int. J. Mol. Sci 20 (2019) 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pang L, Pei Y, Uzunalli G, Hyun H, Lyle LT, Yeo Y, Surface Modification of Polymeric Nanoparticles with M2pep Peptide for Drug Delivery to Tumor-Associated Macrophages, Pharm. Res 36 (2019) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mpekris F, Papageorgis P, Polydorou C, Voutouri C, Kalli M, Pirentis AP, Stylianopoulos T, Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy, J. Control. Release 261 (2017) 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, Lieber A, Raines EW, Pun SH, Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival, Proc. Natl. Acad. Sci. U S A. 110 (2013) 15919–15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miao L, Wang Y, Lin CM, Xiong Y, Chen N, Zhang L, Kim WY, Huang L, Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin, J. Control. Release 217 (2015) 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Park J, Park J, Castanares MA, Collins DS, Yeo Y, Magnetophoretic Delivery of a Tumor-Priming Agent for Chemotherapy of Metastatic Murine Breast Cancer, Mol. Pharm 16 (2019) 1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mo R, Sun Q, Xue J, Li N, Li W, Zhang C, Ping Q, Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery, Adv. Mater 24 (2012) 3659–65. [DOI] [PubMed] [Google Scholar]
- [50].Fernandes C, Suares D, Yergeri MC, Tumor Microenvironment Targeted Nanotherapy, Front Pharmacol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


