Abstract
Twik-related K+ (TREK) channels produce background currents that regulate cell excitability. In vivo, TREK-1 is involved in neuronal processes including neuroprotection against ischemia, general anesthesia, pain perception, and mood. Recently, we demonstrated that A-kinase anchoring protein AKAP150 binds to a major regulatory domain of TREK-1, promoting drastic changes in channel regulation by polyunsaturated fatty acids, pH, and stretch, and by G-protein-coupled receptors to neurotransmitters and hormones. Here, we show that the microtubule-associated protein Mtap2 is another constituent of native TREK channels in the brain. Mtap2 binding to TREK-1 and TREK-2 does not affect directly channel properties but enhances channel surface expression and current density. This effect relies on Mtap2 binding to microtubules. Mtap2 and AKAP150 interacting sites in TREK-1 are distinct and both proteins can dock simultaneously. Their effects on TREK-1 surface expression and activation are cumulative. In neurons, the three proteins are simultaneously detected in postsynaptic dense bodies. AKAP150 and Mtap2 put TREK channels at the center of a complex protein network that finely tunes channel trafficking, addressing, and regulation.
Keywords: potassium channels, proteomics, microtubules, trafficking, postsynaptic dense bodies, scaffolding
Introduction
K+ channels allow the passive and selective transport of K+ through the cell membrane. They control K+ homeostasis, cell volume, and excitability. With 77 genes encoding pore-forming subunits, K+ channels form the largest family of ion channels. Among them, 15 are structurally related and form a subfamily of subunits with four membrane-spanning segments (M1 to M4) and two pore (P1 and P2) domains. These K2P channels are active as dimers and produce almost time- and voltage-independent background currents (Lesage and Lazdunski, 2000; Lesage, 2003). These currents drive the membrane potential toward the K+ equilibrium potential and affect input resistance. A subclass of K2P channels including TREK-1, TREK-2, and the more distant Twik-related arachidonic acid-stimulated K+ channel (TRAAK) have unique properties. They display low basal activity when expressed alone (Fink et al., 1996, 1998; Bang et al., 2000; Lesage et al., 2000). They can be strongly stimulated by temperature (Maingret et al., 2000b), mechanical stretch, and cell swelling (Patel et al., 1998), intracellular acidification (Maingret et al., 1999), arachidonic acid, and other polyunsaturated fatty acids (PUFAs) (Fink et al., 1998), lysophospholipids (Maingret et al., 2000a), and PIP2 (phosphatidylinositol-4,5-bisphosphate) (Chemin et al., 2005; Lopes et al., 2005), or by pharmacological agents such as volatile anesthetics (Patel et al., 1999) and riluzole (Duprat et al., 2000). TREK-1 gene inactivation produces mice less sensitive to volatile anesthetics (Heurteaux et al., 2004). These mice have also impaired neuroprotection afforded by PUFAs (Heurteaux et al., 2004) and altered perception of pain (Alloui et al., 2006). They display a depression-resistant phenotype (Heurteaux et al., 2006).
A-kinase anchoring protein 150 (AKAP150) is a constituent of native TREK-1 channels in the brain (Sandoz et al., 2006). AKAP150 interacts with the post-M4 region of TREK-1 that is necessary for activation by PUFAs, phospholipids, stretch and intracellular acidification, and for regulation by neurotransmitters and hormones. Binding of AKAP150 to this regulatory domain switches the low activity outwardly rectifying TREK-1 current into a robust leak conductance. Inhibition of the TREK-1/AKAP150 complex by Gs-coupled receptors is as extensive as for TREK-1 alone, but is faster. Inhibitions of TREK-1/AKAP150 by a PKC activator such as PMA and by Gq-coupled receptors are much reduced compared with TREK-1 alone.
Here, we characterized the interaction of TREK-1 with another of its partners: the microtubule-associated protein Mtap2. Mtap2 promotes an increase of the TREK-1 current without affecting its properties. This current enhancement is attributable to a channel density increase at the plasma membrane and relies on binding of Mtap2 to microtubules. This effect is specific to TREK-1 and TREK-2 and was not observed for other less closely related K2P channels. Mtap2 binds to a site distinct from the AKAP150 interacting site. The three proteins TREK-1, Mtap2, and AKAP150 can interact simultaneously and were codetected in postsynaptic dense bodies of hippocampal neurons.
Materials and Methods
Molecular biology.
Mtap2c was cloned from a mouse brain cDNA library and inserted into pCMV-Myc (Clontech) and pIRES2 HcRed. This vector was derived from pIRES2 enhanced green fluorescent protein (EGFP) (Clontech) by substituting HcRed coding sequence to EGFP. Mtap2c-mut was produced by PCR. Plasmids for expression of AKAP150, TREK-1, and the truncated mutants of TREK-1 have been described previously (Patel et al., 1998; Sandoz et al., 2006).
Cell culture and electrophysiology.
Hippocampal neurons from neonate mice were isolated and transfected as previously described (Sandoz et al., 2006). Mabin–Darby canine kidney (MDCK) cells were grown in Minimal Essential Medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin in a humidified incubator with 5% CO2 at 37°C. MDCK cells were transfected with Lipofectamine 2000 (Invitrogen). COS-7 cells were cultured and transfected using DEAE-dextran as previously described (Patel et al., 1998). After transfection, 25 mm KCl was added to the culture medium. For electrophysiological recordings, the internal solution contained 150 mm KCl, 3 mm MgCl2, 5 mm EGTA, and 10 mm HEPES at pH 7.2 with KOH, and the external solution contained 150 mm NaCl, 5 mm KCl, 3 mm MgCl2, 10 mm HEPES at pH 7.4 with NaOH.
Defolliculated Xenopus oocytes were injected with cRNA encoding TREK-1 (0.3 ng), AKAP150 (0.3 ng), and Mtap2c (0.3 ng). They were used for electrophysiological studies 2–4 d after injection. In a 0.3 ml perfusion chamber, a single oocyte was impaled with two standard microelectrodes (1–2.5 MΩ resistance) filled with 3 m KCl and maintained under voltage clamp by using a Dagan TEV 200 amplifier, in standard ND96 solution (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 2 mm MgCl2, 5 mm HEPES, pH 7.4 with NaOH). Stimulation of the preparation, data acquisition, and analysis were performed using pClamp software (Molecular Devices).
Cell surface quantification.
To quantify TREK-1 at the cell surface, a hemagglutinin (HA)-tag was introduced in the P2–M4 extracellular loop to generate TREK1-HA. This modification did not affect the functional expression of TREK-1 (data not shown). COS cells were transfected by TREK1-HA in the presence or in the absence of Mtap2c. Twenty-four hours after transfection, cells were incubated at 4°C in the presence of anti-HA antibodies (αHA-ab), (mouse monoclonal antibody HA-7; Sigma-Aldrich). TREK1-HA/αHA-ab complexes were detected with peroxidase-coupled secondary antibodies (sheep polyclonal anti-mouse antibodies; GE Healthcare) and quantified by using a colorimetric assay (Cornet et al., 2002).
Immunocytochemistry.
Transfected cells on coverslips were fixed with 0.1 m phosphate buffer containing 4% paraformaldehyde (15 min at 21–22°C), and then permeabilized with PBS/0.1% Triton X-100 (PBST) and blocked 1 h with 5% horse serum (HS) in PBST. Primary and secondary antibodies were diluted in PBST/5% HS PBST and incubated 1 h at 21–22°C. Three 5 min washes with PBST were performed between each incubation step and at the end of the procedure. Coverslips were mounted in Dako Fluorescent Mounting medium (Dako). The following antibodies were used: rabbit polyclonal TREK-1 antibodies (Fink et al., 1996), mouse monoclonal antibody against Myc epitope (clone 9E10; Roche Diagnostics), mouse monoclonal antibodies against Mtap2 (HM-2; Sigma-Aldrich), rat monoclonal antibody against HA epitope (clone 3F10; Roche Diagnostics), goat polyclonal antibodies against AKAP150 (C-20; Santa Cruz Biotechnology; sc-6445), goat anti-rabbit conjugated to Alexa Fluor 488 (Invitrogen), donkey anti-mouse conjugated to Alexa Fluor 594 (Invitrogen), and donkey anti-rat conjugated to Texas Red (Jackson ImmunoResearch). Microscopic analysis was performed with Axioplan 2 Imaging microscope (Carl Zeiss). The Pearson's correlation was calculated with ImageJ.
Immunoprecipitation and Western blot analysis.
COS-7 cells were homogenized in PBS containing saponin (0.5% w/v), Triton X-100 (0.5% w/v), and protease inhibitors (Roche Diagnostics). Lysate were clarified by centrifugation at 20,000 × g for 30 min. Anti-TREK-1 antibodies were immobilized on protein A-Sepharose 4B Fast Flow (Sigma-Aldrich). The immunoprecipated proteins were separated on 10% SDS polyacrylamide gel and blotted onto nitrocellulose membrane (Hybond-C Extra; GE Healthcare). Western blots were performed using a mouse monoclonal antibody against Myc epitope for Myc-tagged Mtap2c.
Results
Coprecipitation and colocalization of Mtap2 and TREK channels in the brain
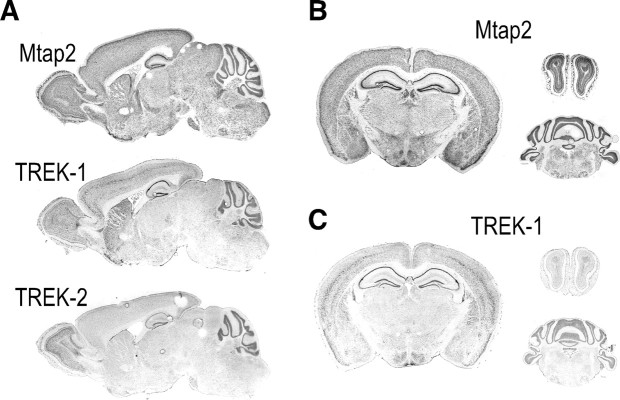
Among the proteins coprecipitated by anti-TREK-1 antibodies from wild-type mice and absent from TREK-1−/− mice (Sandoz et al., 2006) was the microtubule-associated protein Mtap2 (also termed MAP2). Mtap2 binds to microtubules and stabilizes the microtubule network (Felgner et al., 1997). Mtap2 interacts also with protein kinase A (PKA) and links this kinase to microtubules in various brain regions (Harada et al., 2002). In situ hybridization showed that Mtap2 and TREK-1 distributions overlap in adult mouse brain (Lein et al., 2007). Mtap2 is expressed in many areas with the strongest signals found in olfactory bulb, striatum, cortex, hippocampus, and cerebellum (Fig. 1). In these locations, TREK1 is well expressed, particularly in cerebellar granule layer and hippocampus. In the cortex, Mtap2 and TREK-1 are not found in the same layers (Fig. 1A–C). TREK-2, a channel closely related to TREK-1, is not expressed in the striatum and only weakly in the cortex, as previously described in the rat brain (Talley et al., 2001). However, it was detected in olfactory bulb, hippocampus, and cerebellum together with TREK-1 and Mtap2 (Fig. 1A). Because of these overlaps and because TREK channels are known to be associated with the cytoskeleton and to be tightly regulated by PKA (Patel et al., 1998), we studied the functional consequences of the Mtap2/TREK interaction.
Figure 1.
Localization of TREK-1, TREK-2, and Mtap2 mRNA by in situ hybridization in adult mouse sagittal (A) and coronal sections of brain, olfactory bulb, and cerebellum (B, C). Data were retrieved from the Allen brain database (www.brain-map.org).
Mtap2/TREK-1 channel interaction in transfected cells
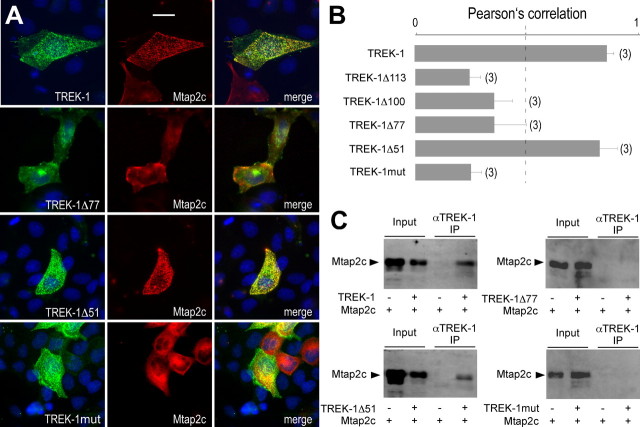
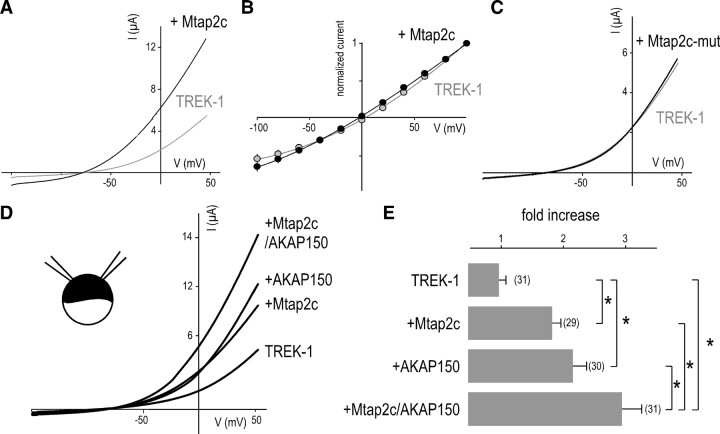
Several splice variants are produced from the gene encoding Mtap2 (Dehmelt and Halpain, 2005). Mtap2c is the shortest isoform. This splice variant shares the same sequence as the longer isoforms and keeps the ability to bind microtubules and PKA (Dehmelt and Halpain, 2005). We first tested the ability of Mtap2c to interact with TREK-1 in transiently transfected cells. Figure 2A shows a strong colabeling of TREK-1 and Mtap2c in transfected COS-7 cells. Expressed alone, Mtap2 has a diffuse cytoplasmic distribution. When expressed with TREK-1, it relocates to membrane ruffles and other actin-rich domains in which TREK-1 is mainly detected when expressed alone (Lauritzen et al., 2005). A similar TREK-1-dependent redistribution of Mtap2 was observed in transfected MDCK cells (see Fig. 4A). The physical interaction between the two proteins was next confirmed by coprecipitating Mtap2 with anti-TREK-1 antibodies from COS-7 lysates (Fig. 2B). In the absence of TREK-1 coexpression, Mtap2c was not precipitated. The functional impact of Mtap2 expression on TREK-1 was studied by whole-cell voltage clamp. As depicted in Figure 2C, Mtap2c expression promotes an increase of the basal TREK-1 current amplitude (2- ± 0.3-fold at 50 mV; n = 19; p < 0.05). Unlike AKAP150, Mtap2c had no effect on the current–voltage (I–V) relationship in symmetrical K+ conditions (data not shown) (for the corresponding I–V curves in Xenopus oocytes, see Fig. 6B) or on the stimulatory effect of arachidonic acid application (data not shown).
Figure 2.
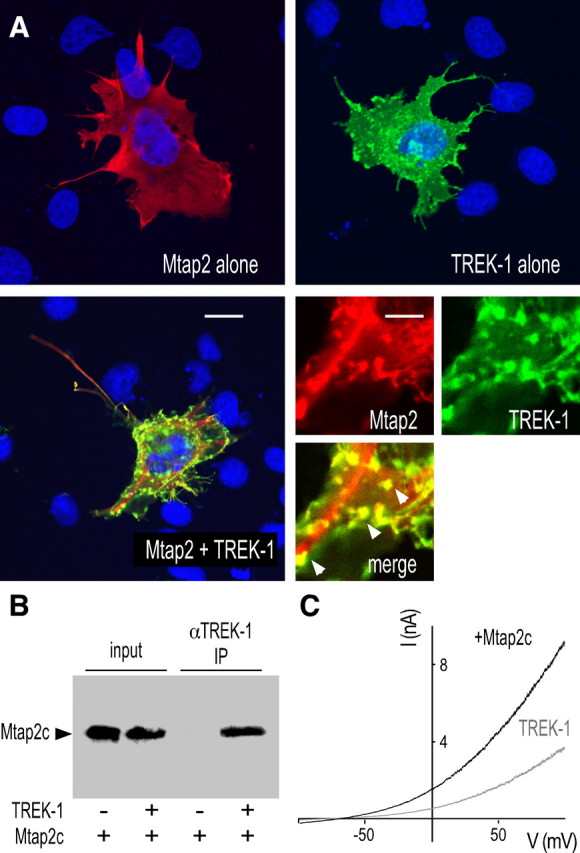
TREK-1/Mtap2 interaction in COS-7 cells. A, Immunodetection in permeabilized cells of myc-tagged Mtap2c (red labeling) and TREK-1 (green labeling) transfected alone (top panels) or together (bottom panels). The cell nuclei are in blue. The bottom left panel shows an entire cell. Scale bar, 15 μm. The three other panels show an enlarged part of this cell. Scale bar, 5 μm. In the merge panels, overlapping green and red labelings are in yellow. The arrowheads indicate actin-rich domains. B, Coprecipitation of myc-tagged Mtap2c by anti-TREK-1 antibodies. IP, Immunoprecipitation. C, Effects of Mtap2c expression on whole-cell currents. Currents were elicited by voltage ramps (from −100 to +100 mV, 1.5 s in duration) from cells expressing TREK-1 in the presence (black) or absence (gray) of Mtap2c.
Figure 4.
Mapping of the Mtap2 binding site in TREK-1. A, Immunolocalization of TREK-1, TREK-1Δ mutants of TREK-1 deleted in its cytoplasmic C-terminal part, or a TREK-1 mutant (TREK-1mut) containing K232P, K347P, R348P, and K349P substitutions (green labelings) and myc-tagged Mtap2c (red labeling) in transfected MDCK cells. Overlapping green and red labelings are in yellow. The cell nuclei are in blue. Scale bar, 10 μm. B, The histograms represent the ratio of the mean Pearson's correlation that is calculated from the colabelings and that is proportional to the extent of colocalization. Error bars indicate SEM. The number of cells tested is shown in parentheses. C, Coprecipitation of myc-tagged Mtap2c by anti-TREK-1 antibodies from transfected cells.
Figure 6.
Mtap2c and AKAP150 act simultaneously on TREK-1. A, Effect of Mtap2c on TREK-1 in Xenopus oocyte. Currents were elicited by voltage ramps (from −150 to +50 mV, 1 s in duration). B, Current–voltage relationships in symmetrical K+ conditions. Steady-state currents were elicited by voltage pulses from a holding potential of 0 mV. C, As in A but for Mtap2c-mut coexpression. D, Effects of Mtap2c and AKAP150 alone or together on TREK-1. Currents were elicited by voltage ramps (from −150 to +50 mV, 1 s in duration). E, The histograms represent the ratio of the mean currents recorded at 0 mV in the four conditions. Error bars indicate SEM. Significant difference, **p < 0.05. The number of cells tested is shown in parentheses.
A Mtap2c mutant unable to bind to microtubules does not stimulate TREK-1
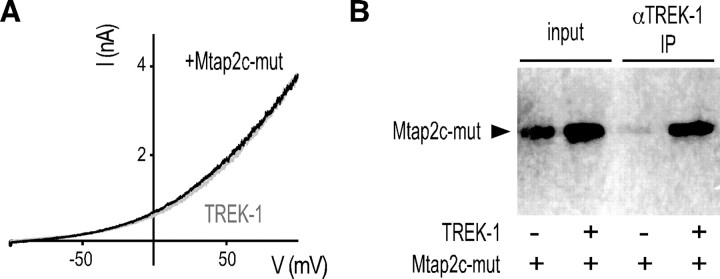
Mtap2 contains three repeated domains involved in the binding to microtubules (Lewis et al., 1988). Phosphorylation of two serine residues S350 and S382 located in these repeats prevents Mtap2c binding to polymerized tubulin. A mutant of Mtap2c in which both serines have been replaced by glutamate residues mimicking a phosphorylated state cannot bind microtubules (Ozer and Halpain, 2000). As shown in Figure 3A, coexpression of this Mtap2 mutant (termed here Mtap2c-mut) failed to significantly increase the TREK-1 current in COS-7 cells (51 ± 9 vs 33 ± 8 nA/nF; n = 18; not significantly different, p > 0.3). However, this mutant coprecipitated (Fig. 3B) and colocalized (data not shown) with TREK-1. These results indicate that the association of Mtap2c with TREK-1 does not require the association of Mtap2 with microtubules, but that the functional effect on the current amplitude relies on this binding. To test the possibility that Mtap2 may affect the microtubule-based vesicular trafficking, and indirectly cell surface expression of TREK-1, we introduced a HA-tag in its P2–M4 extracellular loop to generate TREK1-HA. This modification did not affect the functional expression of TREK-1 and its regulation by Mtap2c (data not shown). TREK-1 channels at the cell surface were quantified by incubating nonpermeabilized cells with anti-HA antibodies, and then by detecting channel/antibody complexes using a peroxidase-coupled secondary antibody and a colorimetric assay. Mtap2c expression promoted an increase of the channel density at the surface. This increase (1.9- ± 0.2-fold; n = 9; p < 0.05) fits quantitatively with the increase of recorded currents in the same expression conditions (2- ± 0.3-fold at 50 mV; n = 18; p < 0.05). Furthermore, Mtap2c-mut did not significantly modify the amount of channels detected at the cell surface (1.1- ± 0.1-fold; n = 9), confirming that the binding of Mtap2c to microtubules is required to stimulate cell surface expression of TREK-1.
Figure 3.
A mutant of Mtap2c unable to bind to microtubules associates with TREK-1, but which does not promote a current increase. A, Effect of Mtap2c-mut expression on TREK-1 currents. Currents were elicited by voltage ramps (from −100 to +100 mV, 1.5 s in duration) from COS-7 cells expressing TREK-1 in the presence (black) or the absence (gray) of Mtap2c-mut. B, Coprecipitation of myc-tagged Mtap2c-mut by anti-TREK-1 antibodies from transfected COS-7 cells.
Mapping of the Mtap2c binding site in TREK-1
In MDCK cells, TREK-1 and Mtap2c were colocalized (Fig. 4A). TREK-1 was detected in actin-rich membrane protrusions present at the cell periphery giving a punctuated labeling (Lauritzen et al., 2005). MDCK cells were chosen for K2P channel immunocytochemistry because they are better than COS-7 cells for this type of experiment (Decressac et al., 2004). The labeling overlap was quantified by calculating the Pearson's correlation coefficient (Pc). The closest to 1 this coefficient is, the strongest is the colocalization of the considered labeled proteins. Below 0.5, there is no significant codistribution. For TREK-1 and Mtap2c coexpression, Pc reached 0.86 ± 0.04. The deletion of the cytosolic C-ter domain of TREK-1 (TREK-1Δ113) resulted in a loss of colocalization (Pc = 0.20 ± 0.07), indicating that the Mtap2 binding site is present in this region. To map this site, we engineered a series of mutants in which the C-ter region of TREK-1 was progressively restored and tested the capacity of these mutants to colocalize with Mtap2c (Fig. 4B). No quantitative overlaps were observed with TREK-1Δ100 (Pc = 0.35 ± 0.1) and TREK-1Δ77 (Pc = 0.35 ± 0.18). A good colocalization was recovered for TREK-1Δ51 (Pc = 0.81 ± 0.1) (Fig. 4A). The Pearson's correlation coefficient was not significantly different from the coefficient calculated for the complete channel. As expected, Mtap2 was coprecipitated by anti-TREK-1 antibodies when coexpressed with TREK-1 and TREK-1Δ51 but not when coexpressed with TREK-1Δ77 (Fig. 4C). These results indicate the Mtap2c binding site is situated between residues 335 and 360 in TREK-1. This region includes an 8 aa segment (residues 342–349) containing four positively charged residues conserved in TREK-1 and TREK-2 (K342, K347, R348, K349). The equivalent segment in TRAAK has no charges but contains several proline residues (see supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In TREK-1, we substituted these basic residues by prolines to get TREK-1mut. TREK-1mut (K342P/K347P/R348P/K349P) was neither able to colocalize with Mtap2 (Fig. 4A,B) nor to coprecipitate Mtap2 (Fig. 4C). Its coexpression with Mtap2 in Xenopus oocytes did not provoke a statistically significant current increase (0.4 ± 0.04 μA, n = 24, vs 0.32 ± 0.03 μA, n = 24 at 0 mV; p > 0.16). These results confirm the role of this region for Mtap2 binding to TREK-1. They also demonstrate that, in the absence of physical interaction with the channel, Mtap2 has no effect on the current levels.
Mtap2 interaction and its functional effect is restricted to TREK-1 and TREK-2
TREK-2, TRAAK, and TASK-1 (TWIK-related acid-sensitive K+ channel) channels were expressed with Mtap2c in MDCK cells for immunocytochemistry and in COS-7 cells for electrophysiology. A strong colocalization of Mtap2c and TREK-2 was observed (Fig. 5A), which is characterized by a Pearson's correlation coefficient of 0.84 ± 0.11 (Fig. 5B), a value close to Pc calculated for TREK-1. The Mtap2 binding sites of TREK-1 and TREK-2 share 72% of sequence homology (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In contrast, there is no significant conservation of this sequence in TRAAK and TASK1 subunits. This sequence divergence was correlated with an absence of quantitative colocalization (Pc = 0.36 ± 0.27 for TRAAK and 0.2 ± 0.18 for TASK-1) (Fig. 5A,B). Furthermore, Mtap2c was not coprecipitated with TRAAK or TASK-1 from transfected cells (data not shown). In terms of functional effects, Mtap2c induced a twofold increase of the TREK-2 current (84 ± 15 vs 43 ± 6 nA/nF at 50 mV; n = 21; p < 0.05) (Fig. 5C), whereas Mtap2c was unable to modify TRAAK and TASK-1 currents (data not shown). Together, these results demonstrate that Mtap2 interacts physically and functionally with the closely related channels TREK-1 and TREK-2 but not with the more distant K2P channels TRAAK and TASK-1. They also show that the Mtap2 effect is specific and does not affect the vesicular trafficking of TRAAK and TASK-1 channels at the cell surface.
Figure 5.
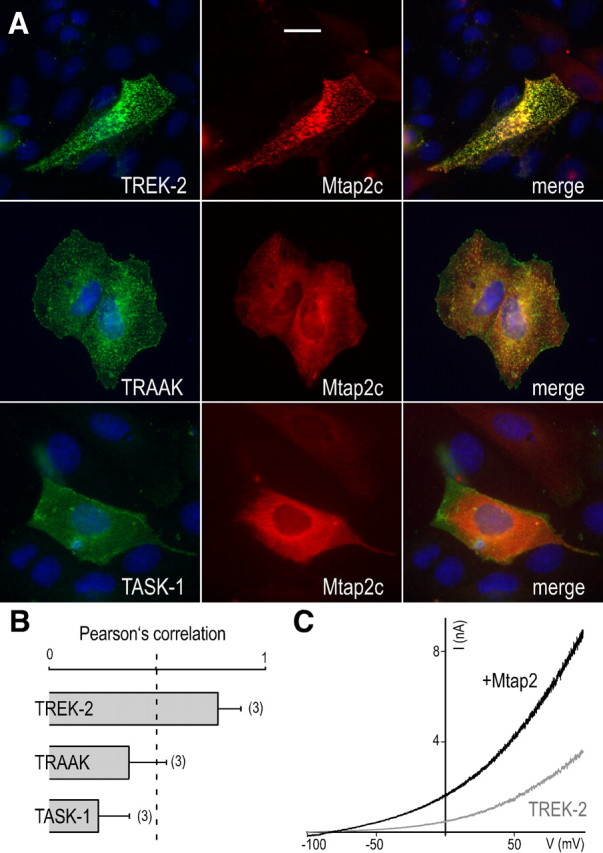
Mtap2c interacts with TREK-1 and TREK-2, but not with the distantly related K2P channels TRAAK and TASK-1. A, Immunolocalization of myc-tagged Mtap2c and TREK-2, TRAAK, and TASK-1 in permeabilized MDCK cells. Channel subunits are in green and Mtap2c in red. Overlapping green and red labelings are in yellow. The cell nuclei are in blue. Scale bar, 5 μm. B, The histograms represent the ratio of the mean Pearson's correlation calculated from the colabelings. Error bars indicate SEM. The number of cells tested is shown in parenthesis. C, Effect of Mtap2c expression on TREK-1 currents. Currents were elicited by voltage ramps (from −100 to +100 mV, 1.5 s in duration) from COS-7 cells expressing TREK-1 in the presence (black) or the absence (gray) of Mtap2c, as displayed.
Mtap2c and AKAP150 simultaneously interact with TREK-1
We previously showed that AKAP150 interacts with TREK-1 and converts it into an open leak channel. Binding occurs in a short domain located between residues 298 and 311. Mtap2c acts on channel density at the cell surface by interacting between residues 335 and 360. The distinct locations of the two binding sites suggested that the two associated proteins could be able to display simultaneously effects on TREK-1 activity. This hypothesis was tested in Xenopus oocyte in which heterologous coexpression of more than two proteins can be easily obtained. First, we confirmed the effects observed in COS-7 cell. Mtap2c induced a similar twofold increase of TREK-1 (3 ± 0.2 vs 1.5 ± 0.2 μA; n = 48) (Fig. 6A,E). Unlike AKAP150, Mtap2c did not modify the I–V relationship in symmetrical K+ conditions (I−80 mV/I+80 mV = 0.61 ± 0.1 vs 0.69 ± 0.1 for TREK-1 alone; n = 25) (Fig. 6B). As observed in COS-7 cells, the stimulatory effect of Mtap2c depends on its binding to microtubules because Mtap2c-mut was unable to modify TREK-1 current density (2.2 ± 0.2 vs 1.7 ± 0.2 μA at 0 mV; n = 28; not significantly different) (Fig. 6C). In Xenopus oocytes, AKAP150 expression induced an increase of the TREK-1 current as previously reported (2.2- ± 0.2-fold at 0 mV) (Fig. 6D,E). When TREK-1 was expressed with both AKAP150 and Mtap2, the current increase was significantly larger than that induced by only one of the partner proteins (2.93- ± 0.31-fold) (Fig. 6D,E). This result suggests that Mtap2 and AKAP150 have independent and additive effects on TREK-1, Mtap2 promoting more channels at the cell surface and AKAP150 stimulating activity of these channels.
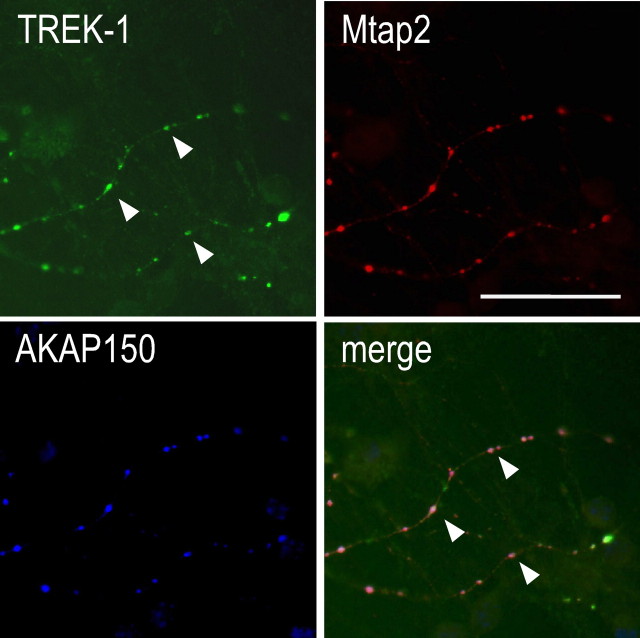
The next important question was to know whether complexes including TREK-1, AKAP150, and Mtap2 are also formed in neuronal cells. Previously, we showed that TREK-1 and AKAP150 are particularly present in postsynaptic dense bodies of hippocampal neurons (Sandoz et al., 2006). Figure 7 shows that endogenous Mtap2 can be detected together with TREK-1 and AKAP150 in the same postsynaptic dense bodies.
Figure 7.
Labeling of endogenous Mtap2 and AKAP150 and transfected TREK-1 in cultured hippocampal neurons. In the merge panel, the TREK-1 labeling is in green, Mtap2c in blue, and AKAP150 in red. The overlapping green, blue, and red labelings are in white. The arrowheads show postsynaptic dense bodies in which the three proteins can be detected. Scale bar, 20 μm.
Discussion
We previously showed that AKAP150 binds TREK-1, affecting its functional properties and its regulation by receptors to neurotransmitters and hormones (Sandoz et al., 2006). The present work demonstrates that Mtap2 is another protein that interacts physically and functionally with TREK-1. Like AKAP150, Mtap2 promotes an increase of the TREK-1 current amplitude (Fig. 2). However, unlike AKAP150, Mtap2c does not directly stimulate channel activity but acts on its expression level at the cell membrane. This effect relies on the capacity of Mtap2 to bind to microtubules (Fig. 2). AKAP150 and Mtap2 associate with TREK-1 at distinct sites of its cytosolic C-ter. Their effects on TREK-1 surface addressing and channel activity are cumulative (Fig. 6).
How does Mtap2 affect TREK-1 density at the cell membrane of transfected cells? Microtubules, a polymer of α- and β-tubulins, are dynamic structures of the cell cytoskeleton serving as rails along which cargoes can be transported. The microtubule-based transport of vesicles facilitates several membrane trafficking steps including endocytosis and exocytosis, contributing to the maintenance of membrane proteins at the cell surface. One class of microtubule-binding proteins comprises motor protein kinesins and dyneins. These proteins mediate intracellular transport of membranous organelles, vesicles, and protein complexes along microtubules in many different cell types (for review, see Goldstein and Yang, 2000). A second class of microtubule-binding proteins is formed by the MAPs (microtubule-associated proteins), to which Mtap2 belongs. These proteins are not directly necessary for microtubule assembly and microtubule-based transport but modulate the dynamic assembly of microtubules (Gamblin et al., 1996; Al-Bassam et al., 2002). By acting on microtubule stability, Mtap2 may indirectly affect the trafficking of TREK-1 in COS-7 cells and Xenopus oocytes. However, this effect is specific to TREK-1 and TREK-2, and was not seen with other K2P channels that do not bind Mtap2 (Fig. 5). This demonstrates that a simultaneous docking of Mtap2 to microtubules and TREK-1 is crucial for controlling the microtubule-based transport of the latter. Mtap2 interacts with the kinase PKA (Obar et al., 1989), whose activity is known to control motor protein activity (Kashina et al., 2004). Recruitment of PKA by Mtap2 to close proximity of vesicles containing TREKs, and modulation of adjacent motor proteins by PKA, may provide a molecular basis for the observed effect on TREK trafficking. Whether this increase in surface expression is attributable to stimulation of the forward transport or to inhibition of endocytosis remains to be determined.
What is the role of the TREK-1/Mtap2 interaction in neurons? In neurons, kinesin and dynein motor proteins transport a wide variety of cargoes required at synapses, and in axons and dendrites themselves. Regionalization of microtubule-associated proteins is believed to contribute to selective transport and neuronal protein sorting (Burack et al., 2000). Mtap2 is mainly found in dendrites, dendritic spines, and postsynaptic densities (Cáceres et al., 1983; Langnaese et al., 1996). One of its functions may be to regulate microtubule-based transport and expression of TREK-1 in these postsynaptic locations. Mtap2 has a recognized role of scaffolding for the localization of signal transduction apparatus in dendrites, particularly near spines. It binds the actin skeleton and is found in close association with key dendritic proteins, including PKA, tyrosine kinases Src, Grb2, and Fyn (Dehmelt and Halpain, 2005), voltage-dependent calcium channel Cav1.2 (Davare et al., 1999), and NMDA receptors (Husi et al., 2000; Buddle et al., 2003). Mtap2 places TREK-1 at the center of dynamic postsynaptic protein complexes that comprises cytoskeleton elements, ion channels, neurotransmitters receptors, and regulatory proteins. In addition, a recent study has provided evidence for a presynaptic location and a presynaptic role of TREK-1. It has shown that gene inactivation of TREK-1 significantly reduces the potency of halothane to inhibit 4-aminopyridine-evoked releases of both glutamate and GABA from cerebrocortical nerve terminals (Westphalen et al., 2007). It is also known that TREK-1 accumulates above and below a ligature in rat sciatic nerve (Bearzatto et al., 2000). The process is rapid and bidirectional, hence compatible with microtubule-based transport of channel-containing vesicles. At the early stage of neuronal development, Mtap2 is present in axon and in the axonal growth cone (Cáceres et al., 1986). In mature neurons, Mtap2 is mostly excluded from axons (Cáceres et al., 1986), but to our knowledge, its presence in the axon terminal has not been extensively studied. If present in nerve terminals, Mtap2 may not only help to transport TREK-1 along axons but also to stabilize TREK-1 at the presynaptic level.
Mtap2 has also been associated with the formation of neurites at early developmental stages and with the dendritic scaffold on maturation (Harada et al., 2002). During neurite initiation, microtubules align to form a tight bundle, and actin filaments reorganize to produce a growth cone. The molecular mechanisms underlying this cytoskeleton reorganization are poorly known. Mtap2 binds both microtubules and F-actin and has been proposed as a candidate to play a key role in these rearrangements. Inactivation of Mtap2 promotes a reduction of dendrite length in vitro and in vivo (Sharma et al., 1994; Harada et al., 2002). However, TREK-1 expression has been reported to induce the formation of actin-rich protrusions, and its gene invalidation significantly alters the growth cone morphology of cultured embryonic striatal neurons (Lauritzen et al., 2005). TREK-1 and Mtap2 interaction may be involved in some of the coordinated cytoskeletal rearrangements controlling the transition from an undifferentiated state to neurite-bearing morphology.
Phosphorylation of Mtap2 affects its ability to bind and stabilize microtubule (Ozer and Halpain, 2000), but also to modulate TREK-1 expression level (Fig. 3). Neurotrophins such as BDNF prevent destabilization of the microtubule network and favor the microtubule-based transport of NMDA receptors to dendritic membrane (Yuen et al., 2005). It is also known that expression levels and distributions of Mtap2 can be affected by oxygen–glucose deprivation in adult hippocampal slices (Buddle et al., 2003). Therefore, regulation of NMDA receptors and TREK-1 background channels via Mtap2 phosphorylation states or expression levels potentially provides important mechanisms for regulating synaptic plasticity and associated learning and memory.
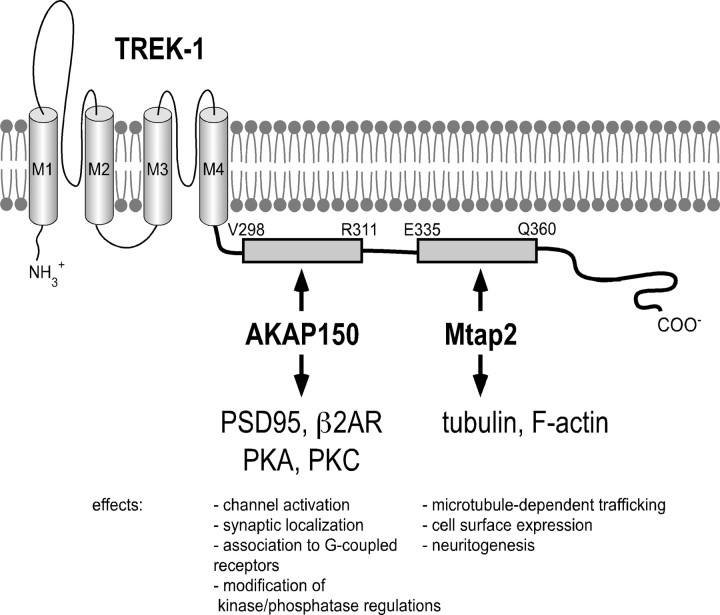
In conclusion, analysis of the native TREK channels has revealed that these channels use AKAP150 and Mtap2, two multifunctional proteins, as partners for their trafficking, addressing, and activity regulation in the brain. These proteins are scaffolding proteins that put TREKs at the center of complex protein networks (Fig. 8). Depending on brain localization and the nature of the expressed proteins, a large number of dynamic signaling complexes can be formed around TREK-1 and they are expected to play important roles in normal and pathological brain functions.
Figure 8.
Mtap2 and AKAP150 place TREK-1 at the center of protein networks that regulate its trafficking to the cell surface, its addressing to postsynaptic dense bodies, and its channel activity.
Footnotes
This work was supported by the Ligue Nationale Contre le Cancer, Fondation pour la Recherche Médicale, and Japan–France Integrated Action Program SAKURA (06980UF). We thank Martine Jodar for excellent technical assistance. S.T. received a postdoctoral fellowship from Fondation pour la Recherche Médicale. F.L. is the recipient of a “contrat d'interface” from the Institut National de la Santé et de la Recherche Médicale/Centre Hospitalier Universitaire, Unité de Neurologie, Hôpital Pasteur (Nice, France).
References
- Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Bearzatto B, Lesage F, Reyes R, Lazdunski M, Laduron PM. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. Neuroreport. 2000;11:927–930. doi: 10.1097/00001756-200004070-00006. [DOI] [PubMed] [Google Scholar]
- Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res. 2003;978:38–50. doi: 10.1016/s0006-8993(03)02758-6. [DOI] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Cáceres A, Payne MR, Binder LI, Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci U S A. 1983;80:1738–1742. doi: 10.1073/pnas.80.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honoré E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet V, Bichet D, Sandoz G, Marty I, Brocard J, Bourinet E, Mori Y, Villaz M, De Waard M. Multiple determinants in voltage-dependent P/Q calcium channels control their retention in the endoplasmic reticulum. Eur J Neurosci. 2002;16:883–895. doi: 10.1046/j.1460-9568.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem. 1999;274:30280–30287. doi: 10.1074/jbc.274.42.30280. [DOI] [PubMed] [Google Scholar]
- Decressac S, Franco M, Bendahhou S, Warth R, Knauer S, Barhanin J, Lazdunski M, Lesage F. ARF6-dependent interaction of the TWIK1 K+ channel with EFA6, a GDP/GTP exchange factor for ARF6. EMBO Rep. 2004;5:1171–1175. doi: 10.1038/sj.embor.7400292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M. Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol. 1997;138:1067–1075. doi: 10.1083/jcb.138.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Nachmanoff K, Halpain S, Williams RC., Jr Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry. 1996;35:12576–12586. doi: 10.1021/bi961135d. [DOI] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thümmler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Kashina AS, Semenova IV, Ivanov PA, Potekhina ES, Zaliapin I, Rodionov VI. Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr Biol. 2004;14:1877–1881. doi: 10.1016/j.cub.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Langnaese K, Seidenbecher C, Wex H, Seidel B, Hartung K, Appeltauer U, Garner A, Voss B, Mueller B, Garner CC, Gundelfinger ED. Protein components of a rat brain synaptic junctional protein preparation. Brain Res Mol Brain Res. 1996;42:118–122. doi: 10.1016/s0169-328x(96)00147-7. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honoré E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Rohács T, Czirják G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol. 2005;564:117–129. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000a;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000b;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Ozer RS, Halpain S. Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol Biol Cell. 2000;11:3573–3587. doi: 10.1091/mbc.11.10.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Sandoz G, Thümmler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, Guy N, Lazdunski M, Lesage F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Kress Y, Shafit-Zagardo B. Antisense MAP-2 oligonucleotides induce changes in microtubule assembly and neuritic elongation in pre-existing neurites of rat cortical neurons. Cell Motil Cytoskeleton. 1994;27:234–247. doi: 10.1002/cm.970270305. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings HC., Jr Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–945. doi: 10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Feng J, Yan Z. Microtubule regulation of N-methyl-d-aspartate receptor channels in neurons. J Biol Chem. 2005;280:29420–29427. doi: 10.1074/jbc.M504499200. [DOI] [PubMed] [Google Scholar]