Abstract
Parkinson's disease (PD), a common neurodegenerative disease, is caused by loss of dopaminergic neurons in the substantia nigra. Although the underlying cause of the neuronal loss is unknown, oxidative stress is thought to play a major role in the pathogenesis of PD. The amino acid methionine is readily oxidized to methionine sulfoxide, and its reduction is catalyzed by a family of enzymes called methionine sulfoxide reductases (MSRs). The reversible oxidation-reduction cycle of methionine involving MSRs has been postulated to act as a catalytic antioxidant system protecting cells from oxidative damage. Here, we show that one member of the MSR family, MSRA, inhibits development of the locomotor and circadian rhythm defects caused by ectopic expression of human α-synuclein in the Drosophila nervous system. Furthermore, we demonstrate that one way to enhance the MSRA antioxidant system is dietary supplementation with S-methyl-L-cysteine (SMLC), found abundantly in garlic, cabbage, and turnips. SMLC, a substrate in the catalytic antioxidant system mediated by MSRA, prevents the α-synuclein-induced abnormalities. Therefore, interventions focusing on the enzymatic reduction of oxidized methionine catalyzed by MSRA represent a new prevention and therapeutic approach for PD and potentially for other neurodegenerative diseases involving oxidative stress.
Keywords: oxidative stress, Parkinson's disease, methionine, methionine sulfoxide reductase, dietary supplement, synuclein
Introduction
Parkinson's disease (PD) is a common neurodegenerative disease; yet, a cure or an affordable and effective disease management strategy remains to be discovered (Lang and Lozano, 1998a; Koller and Cersosimo, 2004; Muller et al., 2004; Weber and Ernst, 2006). PD patients often exhibit motor symptoms such as resting tremor, rigidity, and bradykinesia (Lang and Lozano, 1998b) as well as nonmotor abnormalities (Chaudhuri et al., 2005), and the symptoms are caused by, in part, loss of dopaminergic neurons in the substantia nigra pars compacta (Moore et al., 2004). The neurons that survive in the PD brain often contain eosinophilic proteinacious Lewy bodies, which contain α-synuclein (Spillantini et al., 1997). Mutations in the α-synuclein gene are associated with familial PD (Polymeropoulos et al., 1997; Krüger et al., 1998), and the importance of α-synuclein in PD has been demonstrated in many model systems (Recchia et al., 2004; Cooper et al., 2006), including the fruit fly Drosophila melanogaster (Feany and Bender, 2000; Marsh and Thompson, 2006). For example, flies that overexpress human α-synuclein in the nervous system display the essential features of PD, a loss of dopaminergic neurons, and accelerated deterioration of the motor activity with age (Feany and Bender, 2000). This Drosophila PD model has been useful to explore pharmacological interventions for PD (Auluck and Bonini, 2002; Pendleton et al., 2002).
The molecular mechanism of PD remains elusive (Dawson and Dawson, 2003; Lee and Trojanowski, 2006; Litvan et al., 2007a), but one important factor in the disease pathogenesis is oxidative stress (Jenner, 2003; Litvan et al., 2007b), which can promote oxidation of cell constituents, including amino acids. Among them, cysteine and methionine are particularly susceptible to oxidation. Methionine is oxidized to methionine sulfoxide (met-O) by the addition of an oxygen atom to its reactive sulfur atom and the reduction reaction is catalyzed by the family of methionine sulfoxide reductases (MSRs) (Weissbach et al., 2005). The reversible oxidation and reduction cycle involving MSRs, such as the enzyme MSR A (MSRA), has been postulated to serve as a catalytic antioxidant mechanism against deleterious effects of oxidative stress (Kantorow et al., 2004; Yermolaieva et al., 2004; Weissbach et al., 2005) and to act as a determinant of lifespan (Moskovitz et al., 2001; Ruan et al., 2002). We therefore hypothesized that enhanced repair of met-O by MSRA may lead to a beneficial outcome in PD and have developed a doubly transgenic Drosophila line that overexpresses both human α-synuclein and MSRA. We show here that overexpression of MSRA prevents development of the locomotor and circadian rhythm defects and extends the lifespan in the α-synuclein Drosophila model of PD. Furthermore, dietary supplementation with S-methyl-L-cysteine (SMLC), which participates in the catalytic antioxidant mechanism involving MSRA, mimics the effect of overexpression of MSRA and prevents the development of the α-synuclein-induced dysfunctions. Manipulation of the MSRA system, such as that by dietary supplementation with SMLC, may represent a novel prevention strategy for PD and also for many other diseases where oxidative stress is implicated.
Materials and Methods
Construction of a doubly transgenic Drosophila line.
A UAS responder line designed to express both enhanced green fluorescent protein (EGFP)-bovine MSRA and human α-synuclein (UAS-EGFP-MSRA; UAS-α-synuclein) was generated by the standard genetic method using the UAS responder line UAS-EGFP-MSRAC (Ruan et al., 2002) and the UAS responder line UAS-α-synuclein (Feany and Bender, 2000). The mutant flies showed no detectable changes in the developmental time course up to eclosion. The larval animals were maintained at a density of 200 per 75 ml of stock vial.
Panneuronal expression of transgenes.
Expression of the transgenes primarily in the nervous system was achieved by crossing the UAS responder lines with the elavC155-GAL4 activator line. Overexpression of EGFP-MSRA was confirmed with fluorescence microscopy and Western blotting. Overexpression of α-synuclein was confirmed by Western blotting. Briefly, heads from 30 animals, 30 d of age, were homogenized in lysis buffer (Bio-Rad, Hercules, CA). The protein samples (30 μg in each group) were separated by SDS-PAGE (10%) and transferred to a nitrocellulose membrane. After overnight blocking at 4°C with skim milk powder, the membrane was incubated overnight at 4°C with the primary antibody and subjected to ECL detection. Antibodies used were a monoclonal antibody against human α-synuclein (1:500; BD Transduction Laboratories, San Jose, CA) and a polyclonal antibody against bovine MSRA (1:1000; Upstate Biotechnology, Waltham, MA). Blots were either probed concurrently or stripped and reprobed with an anti-β-actin antibody (1:1000; Abcam, Cambridge, UK). In some experiments, the transgenes were expressed preferentially in the dopaminergic neurons using the ddc-GAL4 activator line (Li et al., 2000).
Lifespan trials.
In a typical lifespan trial, 100–200 adult flies, 20 animals in each vial, were used in each group. The control and experimental group trials were always performed concurrently. The animals were raised on standard cornmeal/yeast agar (University of Pennsylvania Department of Genetics Cell Center Facility) and transferred to fresh vials every 3 d without using any anesthetic. The animals were maintained at 25°C in 12 h light/dark cycle incubators. The humidity was ∼60%. Average lifespans were compared using resampling statistics (Simon, 1992). Survival distributions were fitted with a Gompertz distribution of the form H(t) = exp([A0/G]*[1 − exp(G*t)]), where t is adult age since eclosion in days, A0 is the initial mortality at t = 0 or intrinsic vulnerability, and G is the Gompertz mortality or failure rate coefficient. The analysis procedures were performed using Igor Pro (WaveMetrics, Lake Oswego, OR).
Locomotor assays.
A reactive climbing assay (Williamson, 1982) was used to infer the overall activity level of the animals. The animals were maintained as specified above, and 20 flies of a given age were transferred to an empty glass vial. The vial was then vortexed (Vortex Genie-2; VWR, West Chester, PA) at the maximum setting for 10 s, and the number of flies on the vial wall after a 10 s rest period was recorded. The active fraction is defined as the fraction of the animals on the vial wall away from the bottom. At each age, 60–100 animals were tested. Statistical significance was evaluated using resampling statistics (Simon, 1992). The results were fitted with the Gompertz distribution function (see above).
Spontaneous locomotive behavior was quantified using a commercially available system (Drosophila Activity Monitoring System; Trikinetics, Waltham, MA) (Rosato and Kyriacou, 2006) located inside a 12 h light/dark cycle incubator at 25°C. Briefly, an animal was placed inside a 10-cm-long glass tube with food, and the number of times that the animal interrupts a transverse infrared light beam was counted. Each measurement trial, typically with 32 animals, lasted for 7 d. The results were analyzed using Igor Pro, incorporating the analysis algorithms described previously (Dowse, 2007). The results from the first 2 d in each trial were excluded from analysis to allow the animals to adapt to the measurement chamber.
Dopaminergic neuron detection.
The ddc-GAL4 activator line (Li et al., 2000) was used to express human α-synuclein primarily in the dopaminergic neurons and analyzed in whole-mount brain preparations as described previously (Whitworth et al., 2005) using confocal microscopy (Zeiss LSM 510; University of Pennsylvania Biomedical Imaging Core). Adult heads were dissected in cold PBS and fixed immediately in PBS with formaldehyde (4%) for 30 min. After washing three times, 10 min each in PBS with Triton X-100 (0.1%), the samples were blocked for 1 h in a pH 7.5 solution with Tris-HCl (0.1 m), NaCl (0.15 m), Triton X-100 (0.1%), and heat-inactivated fetal bovine serum (10%). Rabbit polyclonal antibodies against tyrosine hydroxylase (TH) (Ab152, 1:100; Chemicon, Temecula, CA) were added to the blocking solution and incubated overnight at 4°C. The samples were washed again three times, 10 min each, in PBS with Triton X-100 (0.1%) and incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA). The samples were mounted between two glass coverslips using ProLong antifade reagent (Invitrogen). Whole-mount brain preparations were scanned with Zeiss LSM META 510 along the z-axis at 1 μm intervals. In each group, four to six different samples were analyzed. Such image analyses showed that the adult Drosophila brain contains numerous TH-containing cells throughout the brain, especially in the deep dorsal medial cluster (DMC), the ventral medial cluster (VMC) and the cluster near the mushroom body, confirming previous reports (Konrad and Marsh, 1987; Coulom and Birman, 2004; Wang et al., 2007). The large number of cells present in the aforementioned areas rendered cell counting impractical. However, the dorsolateral posterior protocerebral area (PPL1), lateral posterior protocerebral area (PPL2), dorsomedial posterior protocerebral area (PPM3), and dorsolateral anterior protocerebral area (PAL) (Friggi-Grelin et al., 2003) contained a much smaller number of TH-containing cells, and the total number of cells in these regions was estimated from the z-series images covering the whole thickness of the brain.
Food supplements.
A freshly prepared solution of SMLC was mixed with food to a final concentration of 10 mm. Food intake was visually confirmed by mixing the dye Brilliant Blue (1 mg/ml) in the food. The dye concentrations in the abdomen homogenates were also quantified with a spectrophotometer at 630 nm, and no detectable difference in the dye concentrations between the control and experimental groups was observed. It is not known how much SMLC each animal consumed in our trials.
Reduction of oxidized SMLC by MSRs.
A solution of SMLC (1 mg/ml) in 20 mm Tris, pH 8.0, was incubated overnight at room temperature (RT) with 400 μm H2O2 to produce SMLC sulfoxide (SMLC-O). Residual H2O2 was removed by incubation with catalase attached to agarose (Sigma, St. Louis, MO) for 5 min at RT. The agarose-linked catalase was sedimented by brief centrifugation, and the supernatant containing SMLC-O was collected. The supernatant (0.5 ml) was mixed with 0.5 ml of 2,4-dinitrofluorobenzene-ethanol (DNFB) (Sigma; 0.7% in 100% ethanol) and 0.1 ml reaction buffer (2 m KOH, 2.4 m KHCO3), and the reaction was incubated overnight at RT in the dark. As a control, SMLC was derivatized using the same procedure. The DNFB-derivatized amino acids were run through a Source 15RPC-ST4.6/100 reverse phase column (GE Healthcare, Pittsburgh, PA) using H2O plus 0.1% trifluoroacetic acid as the aqueous and 84% acetonitrile in H2O plus 0.1% trifluoroacetic acid as the organic phase. Absorption was monitored at 365 nm, and a single peak was observed at the end of a gradient from 0 to 84% acetonitrile. The yellow material corresponding to this peak was collected, vacuum-dried, and redissolved in 50 mm Tris buffer, pH 7.4. In control runs, the nonoxidized SMLC eluted 2 ml after SMLC-O, and separation to the baseline was observed when applying the two amino acids together. Recombinant human MSRA and Drosophila MSRA were incubated with aliquots of SMLC-O in 50 mm Tris, pH 7.4, with 10 mm DTT. As controls, two aliquots were incubated with either DTT or MSRA alone. The reactions were incubated at 37°C for 2 h and kept on ice until analysis. The samples were run through the Source 15RPC column as described above.
Cell viability assay.
Rat A-10 cells were obtained from ATCC (Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum at 37°C with 5% CO2. Cells were plated onto 96-microwell plates at a density of 5000 cells/well and adenovirus particles (University of Iowa Gene Vector Core, Iowa City, IA) encoding the human MSRA with a deletion in the N terminus (Hansel et al., 2002) were applied at 300 multiplicity of infection. After incubation for 8 h, the virus particles were washed out, and SMLC (50 mm) was added to the medium. After 24 h of SMLC treatment, cells were thoroughly washed and incubated with the oxidant chloramine-T (1.5 mm; Sigma). MTT cell viability assay (ATCC) was used to measure cell viability 12 h later using absorbance at 540 nm. Six wells were analyzed in each condition.
Results
Overexpression of human α-synuclein primarily in the nervous system of Drosophila accelerates age-dependent loss of locomotor activity, recapitulating an essential feature of PD (Feany and Bender, 2000). One way to quantify the Drosophila motor activity is the reactive climbing assay, which measures the animal's ability to climb up the wall of a vial after tapped to the bottom. With advancing age, fewer animals manage to climb the vial wall (Ganetzky and Flanagan, 1978). Consistent with the original report (Feany and Bender, 2000), the animals expressing human α-synuclein in a panneuronal manner through the GAL4 bipartite expression system (Brand et al., 1994) (UAS-α-synuclein/elavC155-GAL4) showed a markedly accelerated age-dependent decline in the reactive climbing ability (Fig. 1A, open circles) compared with that in the control group without overexpression of α-synuclein (UAS-α-synuclein/+) (Fig. 1A, triangles). The active fraction, the fraction of the animals that retain the ability to climb the wall, decreased to 50% at ∼45 d in the control group, whereas in the α-synuclein group, the fraction decreased to 50% in only ∼20 d, more than twice as fast as that in the control group.
Figure 1.

Overexpression of MSRA delays the decline in the movement ability in the animals expressing human α-synuclein preferentially in the nervous system. A, Reactive climbing ability as a function of adult age in the female (left) and male (right) animals expressing α-synuclein (open circles) and α-synuclein and MSRA together (filled circles). The results from two heterozygous control groups that do not express α-synuclein or MSRA are also shown (triangles). Open circles, UAS-α-synuclein/elavC155-GAL4; filled circles, UAS-α-synuclein;UAS-EGFP-MSRA/elavC155-GAL4; triangles, UAS-α-synuclein/+; inverse triangles, UAS-α-synuclein;UAS-EGFP-MSRA/+. B, Reactive climbing ability as a function of adult age in the female (left) and male (right) animals expressing α-synuclein A30P and α-synuclein A30P and MSRA together. Open circles, UAS-α-synuclein A30P/elavC155-GAL4; filled circles, UAS-α-synuclein A30P;UAS-EGFP-MSRA/elavC155-GAL4; triangles, UAS-α-synuclein A30P/+; inverse triangles, UAS-α-synuclein A30P;UAS-EGFP-MSRA/+. C, Confirmation of the presence of EGFP-MSRA in the heads of the doubly transgenic animals by Western blot analysis. The left two lanes show the results from the doubly transgenic animals designed to express EGFP-MSRA and human α-synuclein (UAS-α-synuclein;UAS-EGFP-MSRA/elavC155-GAL4), and the right two lanes show those from the heterozygous control animals (UAS-α-synuclein;UAS-EGFP-MSRA/+). M, Male animals; F, female animals. The blot was stripped and reprobed against β-actin (not shown). D, Confirmation of the presence of human α-synuclein in the heads of the doubly transgenic animals by Western blot analysis. The lane information is as in C. E, Reactive climbing ability as a function of adult age in the female (left) and male (right) animals expressing α-synuclein (open circles) and α-synuclein and MSRA together (filled circles) preferentially in the dopaminergic neurons using the ddc-GAL4 activator line (Li et al., 2000). Open circles, UAS-α-synuclein/ddc-GAL4; filled circles, UAS-α-synuclein;UAS-EGFP-MSRA/ddc-GAL4; triangles, UAS-α-synuclein;UAS-EGFP-MSRA/+; inverse triangles, UAS-EGFP-MSRA/+. F, MSRA overexpression protects TH-immunoreactive neurons. Projected whole-mount brains of 70-d-old animals expressing MSRA and α-synuclein together (left) and α-synuclein alone (right) are stained with antibodies against TH. The transgenes were expressed using the ddc-GAL4 activator line (Li et al., 2000). Well-defined TH-immunoreactive neurons are visible when MSRA is overexpressed (left), but only a diffuse background staining is present in the animal overexpressing α-synuclein alone (right). Scale bars, 50 μm. G, Quantification of the protection of the dopaminergic neurons by MSRA. The total number of TH-immunoreactive neurons in select areas of the brain (PPL1, PPL2, PPM3, and PAL) (Friggi-Grelin et al., 2003) where cell counting is feasible (see Materials and Methods) is shown for the 60-d-old animals that express α-synuclein alone (left) and also for those that express both α-synuclein and MSRA (right). The transgenes were expressed using the ddc-GAL4 activator line (Li et al., 2000).
The age-dependent decline in the reactive climbing ability in Drosophila is markedly slowed by overexpression of MSRA primarily in the nervous system (Ruan et al., 2002) (supplemental information, available at www.jneurosci.org as supplemental material). To test whether MSRA could rescue the accelerated decline in the reactive climbing ability in the animals expressing α-synuclein, we constructed transgenic flies that concurrently express both bovine MSRA (Ruan et al., 2002) and human α-synuclein preferentially in the nervous system (UAS-α-synuclein;UAS-EGFP-MSRA/elavC155-GAL4). The presence of α-synuclein and EGFP-MSRA in the doubly transgenic line was confirmed by Western blotting (Fig. 1C,D) and fluorescence microscopy (data not shown). Overexpression of EGFP-MSRA primarily in the nervous system in the background of α-synuclein (UAS-α-synuclein;UAS-EGFP-MSRA/elavC155-GAL4) totally prevented the accelerated decline in the reactive climbing ability caused by α-synuclein (Fig. 1A). The age at which 50% of the animals fail to climb the wall was delayed dramatically from ∼20 d in the α-synuclein animals (Fig. 1A, open circles) to >60 d in the doubly transgenic α-synuclein/MSRA animals (Fig. 1A, filled circles) (p < 0.0001), a threefold increase. A qualitatively similar preventative effect of MSRA on the age-dependent decline in the reactive climbing was observed in both female and male animals.
The A30P mutation in α-synuclein is linked to an autosomal dominant early-onset form of PD (Krüger et al., 1998). Overexpression of the mutant A30P protein in the Drosophila nervous system recapitulates this human pathology and also causes a much more severe locomotor defect than that by wild-type α-synuclein (Fig. 1B, open circles) (Feany and Bender, 2000). Overexpression of MSRA also ameliorated the premature age-dependent decline in the reactive climbing ability in the animals caused by α-synuclein A30P (Fig. 1B, filled circles) (p < 0.0001).
The motor dysfunction in PD is associated with a selective loss of dopaminergic neurons in the substantia nigra (Moore et al., 2004). Overexpression of α-synuclein preferentially in the dopaminergic neurons accelerated the age-dependent decline in the reactive climbing ability (Fig. 1E). The decline in the climbing ability was associated with a loss of immunoreactivity to TH, a key enzyme in dopamine synthesis, especially in the deep DMC, VMC, and the cluster surrounding the mushroom body (Fig. 1F) (Feany and Bender, 2000; Auluck and Bonini, 2002). Overexpression of MSRA in the dopaminergic neurons abolished the accelerated decline in the reactive climbing ability caused by α-synuclein overexpression (Fig. 1E) and strikingly preserved the TH staining in the adult brain (Fig. 1F) (supplemental information, available at www.jneurosci.org as supplemental material). In 70-d-old animals expressing both α-synuclein and MSRA, TH-immunoreactive neurons were clearly visible throughout the brain (Fig. 1F). The total number of TH-immunoreactive neurons in the PPL1, PPL2, PPM3, and PAL regions was clearly greater in the MSRA animals (Fig. 1G).
The locomotor ability of the animals was also quantified by assessing their spontaneous movements over a 5 d span with an automated locomotion detector capable of revealing changes in motor activity level and circadian rhythm (Rosato and Kyriacou, 2006). This measurement is particularly relevant to PD, because one of its nonmotor symptoms is sleep disturbances (Chaudhuri et al., 2005). As found with the reactive climbing ability, the spontaneous locomotor activity level under the 12 h light/dark cycle in the control animals declined with age (Fig. 2A). The decline in the physical activity level was accompanied by a noticeable deterioration in the circadian rhythmicity as readily observed in the autocorrelation plots; older animals exhibited a much less defined circadian rhythmicity (Fig. 2B, top row). Overexpression of α-synuclein preferentially in the dopaminergic neurons significantly exacerbated the age-dependent decline in the spontaneous locomotor activity (Fig. 2A,B). In fact, the middle-age animals expressing α-synuclein (Fig. 2A,B, middle center) resemble the old control animals (Fig. 2A,B, top right) in their activity level and circadian pattern, and this is especially evident when the autocorrelation plots are compared (Fig. 2B). The 12 h periodicity was detectable in every group; however, the amplitude of the periodicity was noticeably smaller in the middle-age animals expressing α-synuclein (Fig. 2B, middle center). The observed adverse effects of α-synuclein were primarily obliterated by overexpression of MSRA. The activity level and the circadian rhythmicity in the old animals expressing both α-synuclein and MSRA were comparable with those of the corresponding control animals (Fig. 2A,B).
Figure 2.

MSRA overexpression slows decline of the spontaneous locomotor activity in the male animals overexpressing α-synuclein preferentially in the dopaminergic neurons. A, Frequency of movements over a 5 d span in the control animals (top), those expressing α-synuclein (middle), and those expressing α-synuclein and MSRA together (bottom). In each genotype, the results obtained at three different ages (young, immediately eclosion to 10 d; middle-age, 30d; old, 60 d at the start of the trials) are shown. Animals from the same crossings were measured at three different ages. Both MSRA and α-synuclein were expressed preferentially in the dopaminergic neurons using the ddc-GAL4 activator line (Li et al., 2000). The results for the “old” α-synuclein group are not available because of their short lifespan. The horizontal dark segments indicate dark periods. Each trial lasted 37 d, but the results from 5 d spans are shown. n = 16–32. B, Normalized autocorrelation plots of the results shown in A. The gray area represents SEM.
In addition to the locomotor defects quantified using both the reactive climbing and spontaneous activity assays, the animals overexpressing human α-synuclein in a panneuronal manner with the elavC155-GAL4 activator line had a shorter mean lifespan than the control animals (Fig. 3A) (p ≤ 0.0009). Overexpression of MSRA in the nervous system counteracted the lifespan-shortening effect of α-synuclein overexpression and extended the mean lifespan (p < 0.0001) by ∼50%. Using this lifespan information, we examined the deterioration of the reactive climbing ability normalized to the survival probability of each genotype. Such an analysis shows that α-synuclein overexpression disproportionately impaired the locomotor activity and that MSRA overexpression ameliorated the dysfunction even when normalized to the respective lifespan, especially in the male animals (Fig. 3B). Thus, the relief of the locomotor defect is more than a mere consequence of the longevity of the animals overexpressing MSRA; the enzyme overexpression preferentially restores the locomotor ability.
Figure 3.
MSRA overexpression extends the lifespan of the animals expressing human α-synuclein in the nervous system. A, Survival distributions of the female (left) and male (right) animals expressing α-synuclein (open circles) and α-synuclein and MSRA together (filled circles) in the nervous system. The survival distributions of the heterozygous control animals that do not express α-synuclein or MSRA are also shown (triangles and inverse triangles). Open circles, UAS-α-synuclein/elavC155-GAL4; filled circles, UAS-α-synuclein;UAS-EGFP-MSRA/elavC155-GAL4; triangles, UAS-α-synuclein+; inverse triangles, UAS-α-synuclein;UAS-EGFP-MSRA/+. The results from the female UAS-α-synuclein;UAS-EGFP-MSRA/+ group in this trial are not available. B, Decline in the locomotor function normalized to the survivorship. For each group, the age-dependent decline in the reactive climbing ability is plotted against the survival probability. The genotypes are as in A.
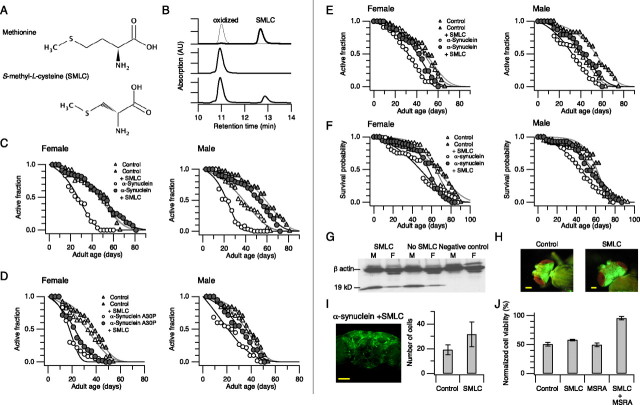
Although the results above suggest that gene therapy to overexpress MSRA may be a potential long-term therapeutic and prevention strategy for PD, conventional pharmacological interventions may have short-term advantages. However, levodopa (3,4-dihydroxy-L-phenylalanine), a traditional medication for PD, often loses its effectiveness with use, produces unwanted side effects, and does not slow the disease progression (Colosimo et al., 2006; Stocchi, 2006). Furthermore, no effective prevention strategy for PD is known (Weber and Ernst, 2006). We reasoned that the overall efficiency of the endogenous MSRA-mediated catalytic antioxidant mechanism (Levine et al., 1996) could be improved by increasing the availability of methionine to react with oxidants such that methionine acts as a “sink” for many reactive species, such as reactive nitrogen and oxygen species. Toward this goal, dietary supplementation with methionine itself may be considered. However, methionine is rapidly incorporated into protein, and it increases the plasma levels of homocysteine and cholesterol, which may be risk factors in cardiovascular diseases (Hirche et al., 2006). In fact, dietary methionine restriction, instead of supplementation, induces beneficial anti-aging effects (Malloy et al., 2006). Hence, the practical use of methionine supplementation in humans is probably doubtful. Thus, we selected a methionine analog, SMLC (Fig. 4A), which is found abundantly in cabbage, garlic, and turnips. As with methionine, SMLC is readily oxidized and reduced back to SMLC by purified recombinant Drosophila or human MSRA in the presence of DTT but not by DTT alone (Fig. 4B). Although the endogenous level of MSRA is not sufficient to protect against the cell toxicity caused by α-synuclein (Feany and Bender, 2000) (Fig. 1), the animals do express some MSRA as detected by Western blots (data not shown) and by transcript analysis (Cherbas et al., 1986). To examine whether the dietary supplementation with SMLC could ease the α-synuclein-mediated locomotor dysfunction, SMLC was added to the food mixture starting from eclosion and the age-dependent decline in the reactive climbing ability was monitored. Ingestion of SMLC by the animals was confirmed by mixing with the dye Brilliant Blue. We also verified that SMLC did not adversely affect the GAL4 expression system by monitoring elavC155-GAL4-induced expression of GFP (Fig. 4H). Furthermore, the presence of α-synuclein in the animals on the SMLC diet was confirmed by Western blotting (Fig. 4G).
Figure 4.
Supplementation of the food with SMLC alleviates the locomotor defect in the animals expressing human α-synuclein in the nervous system. A, Structure of methionine and SMLC. B, Oxidation of SMLC and its reduction by MSRA. The top HPLC chromatograph shows SMLC (thick sweep, right peak) and SMLC oxidized by peroxide (thin sweep, left peak). DTT alone does not reduce oxidized SMLC (middle chromatograph), but purified recombinant Drosophila MSRA with DTT reduces oxidized SMLC (bottom chromatograph). C, Reactive climbing ability as a function of age in the animals expressing human α-synuclein on the control diet (open circles), the α-synuclein animals on the SMLC diet (filled circles), the control animals on the normal diet (open triangles), and the control animals on the SMLC diet (filled triangles). The genotypes are as in Figure 1. The experimental group animals were placed on the SMLC diet starting from eclosion. D, Reactive climbing ability in the animals overexpressing human α-synuclein A30P. The genotypes are as in Figure 1. The experimental group animals were placed on the SMLC diet starting from eclosion. E, Reactive climbing ability as a function of age in the animals overexpressing human α-synuclein in dopaminergic neurons using the ddc-GAL4 activator line (Li et al., 2000). The genotypes are as in Figure 1. The experimental group animals were placed on the SMLC diet starting from eclosion. F, Survival distributions of the animals expressing α-synuclein in the nervous system on the normal diet (open circles), the animals expressing α-synuclein on the SMLC diet (filled circles), the control animals on the normal diet (open triangles), and the control animals on the SMLC diet (filled triangles). The genotypes are as in Figure 1. The experimental group animals were placed on the SMLC diet starting from eclosion. G, Confirmation of the presence of human α-synuclein in the heads of the transgenic animals on the SMLC diet by Western blot analysis. The experimental group animals were placed on the SMLC diet starting from eclosion. The left two lanes show the results from the animals designed to express α-synuclein (UAS-α-synuclein/elavC155-GAL4) on the SMLC diet, and the middle two lanes show the results from the same genotype animals on the normal diet without SMLC. The right two lanes show the results from the heterologous control animals that are not designed to express human α-synuclein (UAS-α-synuclein/+). M, Male animals; F, female animals. H, GFP fluorescence induced by the activator line elavC155-GAL4 in the animals on the control diet (left) and SMLC diet (right). Scale bars, 50 μm. I, Representative immunostaining of dopaminergic neurons of a 70-d-old animal fed with SMLC visualized with anti-TH antibodies (left) and quantification of the total number of TH-immunoreactive cells in selected areas of the brain in 60-d-old animals (PPL1, PPL2, PPM3, and PAL; right). Human α-synuclein was expressed using the ddc-GAL4 activator line (UAS-α-synuclein/ddc-GAL4) and confirmed by immunostaining as in Figure 1D. Scale bar, 50 μm. Overexpression of α-synuclein by the ddc-GAL4 activator line accelerates the development of locomotor defect, and the SMLC diet delays the onset (data not shown). The control data in the graph are the same as those in Figure 1F. J, Rat A-10 cell viability after treatment with the oxidant chloramine-T (1.5 mm). Human MSRA with a deletion in the N terminus (Hansel et al., 2002) was expressed in the cytoplasm using adenovirus particles. The results are normalized to the mean absorption value in the control group in the absence of the oxidant.
The diet supplemented with SMLC strikingly delayed the progression of the movement defect in the animals overexpressing α-synuclein in the nervous system (Fig. 4C, open and filled circles). The age at which 50% of the animals fail to climb the wall increased by ∼80% from ∼25–45 d in the female α-synuclein animals and by ∼130% from ∼22–51 d in the male α-synuclein animals. The age-dependent decline of the locomotor activity in the α-synuclein animals on the SMLC diet is similar to or even slower than that in the control animals without overexpression of α-synuclein on the normal diet. The motor ability of the control animals without α-synuclein overexpression was not adversely affected by the SMLC diet (Fig. 4C, filled triangles). In fact, in the male control animals, the SMLC diet noticeably preserved the reactive climbing ability (Fig. 4C, right, open and filled triangles), suggesting that, even in the wild-type animals, dietary supplementation with SMLC may be beneficial to maintain the motor ability. A similar protective effect of SMLC on the reactive climbing ability was observed when α-synuclein was preferentially expressed in the dopaminergic neurons (Fig. 4E).
Consistent with the preservation of the locomotor ability by the SMLC diet, the old α-synuclein animals on the SMLC diet retained many TH-immunoreactive neurons throughout the brain, for example, in the deep DMC area and the cluster surrounding the mushroom body (Friggi-Grelin et al., 2003) (Fig. 4I) (supplemental information, available at www.jneurosci.org as supplemental material). In the PPL1, PPL2, PPM3, and PAL regions where relatively a small number of TH-immunoreactive neurons are present, even in the young adult brain (Konrad and Marsh, 1987; Friggi-Grelin et al., 2003; Coulom and Birman, 2004), the difference in the total number of cells remaining was, however, not significant (Fig. 4I) (supplemental information, available at www.jneurosci.org as supplemental material) (p = 0.10).
The SMLC diet was also effective in delaying the age-dependent decline in the climbing ability in the animals expressing α-synuclein A30P (p < 0.023), albeit the effect was smaller (Fig. 4D). In addition to the alleviation of the locomotor defect, the mean lifespan was extended by the SMLC diet in the α-synuclein animals (Fig. 4F) (p < 0.017).
Although a systematic study was not performed, we found no sign of adverse or ill effect of the SMLC diet on the behavior of the control animals. The animals on the SMLC diet reproduced well when the male and female animals were housed together and the average body weight of the animals on the SMLC diet was indistinguishable from that of the animals on the control diet. These characteristics of the animals of the SMLC diet resemble those of the animals overexpressing MSRA on the normal diet reported previously (Ruan et al., 2002). We also excluded the possibility that SMLC simply acted as a general stimulant in Drosophila, akin to caffeine in humans. Caffeine (1–5 mg/ml) decreases the sleep-like duration and promotes activity in Drosophila (Shaw et al., 2000). Dietary supplementation with caffeine (2.2 mg/ml) did not induce any effects similar to those by SMLC. Generally, the caffeine diet shortens the lifespan of the control animals. We noticed, however, that those animals expressing α-synuclein were far more resistant to caffeine, but we cannot offer any explanation for this observation.
Dietary SMLC preserves the locomotor ability in the presence of α-synuclein. We suggest that SMLC exerts its protective effect by enhancing the efficacy of the endogenous MSRA catalytic system to confer greater oxidative stress resistance. This possibility is corroborated by a cell viability assay using a mammalian cell line (Fig. 4J). Rat A-10 cells normally do not express a significant level of MSRA in the cytoplasm as detected by reverse transcription-PCR or immunocytochemistry (data not shown). Pretreatment of A-10 cells with SMLC did not confer any greater resistance to oxidative stress induced by the oxidant chloramine-T (ChT), indicating that the ability of SMLC to work as a simple scavenger of reactive species is limited. Heterologous expression of cytoplasmic MSRA alone did not confer a noticeable protection against the oxidative stress caused by ChT. However, the cells expressing cytoplasmic MSRA and treated with SMLC exhibited a markedly greater resistance against the oxidative stress. MSRA typically derives its reducing power using electrons from the thioredoxin system in vivo or DTT in vitro (Moskovitz et al., 1996). SMLC itself is not an electron donor that the MSRA system can readily use to perform the reduction reaction (data not shown) as measured by the matrix-assisted laser desorption/ionization-based assay (Kuschel et al., 1999). Thus, the enhanced protection against oxidative stress only when cytoplasmic MSRA is present is consistent with the idea that SMLC enhances the efficacy of the MSRA catalytic antioxidant system by increasing the number of substrate molecules available.
The finding that dietary supplementation with SMLC starting immediately after eclosion is effective in preventing the locomotor defect and lifespan shortening caused by α-synuclein raises a practical and logical question: is the SMLC supplementation effective if it starts sometime after eclosion? We therefore examined whether dietary supplementation starting at day 21 after eclosion could lengthen the remaining lifespan in the animals expressing α-synuclein primarily in the dopaminergic neurons (Fig. 5). The supplementation lengthened the mean remaining span slightly in the male animals (p = 0.04). The increase observed in the female animals was, however, not significant (p = 0.1).
Figure 5.
Supplementation with SMLC starting at middle age. The animals expressing α-synuclein (UAS-α-synuclein/elavC155-GAL4) were placed on a diet containing SMLC (10 mm) after day 21 (filled circle). The control animals remained the normal diet (open circle). A, Survival distributions of the female and male animals. B, Conditional survival distributions of the animals given that they were alive after day 21.
Discussion
Parkinson's disease afflicts many individuals but an effective disease management or a prevention strategy is unavailable and the underlying molecular etiology is not clear (Dawson and Dawson, 2003; Lee and Trojanowski, 2006; Litvan et al., 2007a). Several molecular culprits of PD have been suggested, including α-synuclein, normally a presynaptic protein (Dawson and Dawson, 2003; Lee and Trojanowski, 2006). Indeed, overexpression of α-synuclein primarily in the nervous system of Drosophila causes a significant movement dysfunction (Feany and Bender, 2000). It has been reported that the effect of α-synuclein overexpression may not be fully penetrant under every experimental condition (Pesah et al., 2005). However, our study confirms that overexpression of wild-type α-synuclein and the mutant A30P α-synuclein associated with a familial early onset form of PD (Krüger et al., 1998) in the nervous system of Drosophila causes a clear movement defect as measured by the reactive climbing assay and an accelerated loss of dopaminergic neurons. Furthermore, our circadian rhythm analysis reveals that the α-synuclein overexpression profoundly accelerates the age-dependent deterioration of circadian rhythm. This finding further reinforces the usefulness of the Drosophila α-synuclein model to study the sleep problems commonly found in PD patients (Abbott et al., 2005).
The accelerated loss in both the reactive climbing ability and circadian rhythmicity is effectively prevented by concurrent overexpression of MSRA. Although the MSRA system may not address the underlying cause of PD, potentially a defect in intracellular vesicle trafficking (Cooper et al., 2006), this methionine-based catalytic antioxidant system effectively alleviates the movement and circadian rhythm defects in the Drosophila model of PD. Therefore, manipulations of the catalytic antioxidant system involving MSRA may represent a novel prevention and therapeutic strategy for the motor and nonmotor symptoms in PD (Abbott et al., 2005; Chaudhuri et al., 2005). Our results also show that one practical way to dramatically bolster the efficacy of the MSRA system is to supplement the diet with the methionine analog SMLC, which acts as an MSRA substrate when oxidized and can be reversibly reduced. The greater substrate availability competes with other easily oxidized cellular molecules and protects the dopaminergic neurons from excessive oxidative stress.
The MSRA catalytic system has been postulated to confer greater oxidative stress resistance in two complementary ways: by repairing specific functionally critical methionine residues that have been oxidized and by maintaining a critical level of methionine residues important for scavenging reactive species (Levine et al., 1996; Hansel et al., 2005). The discovery that SMLC supplementation is effective in Drosophila and in mammalian cells suggests that the MSRA system contributes at least in part as a catalytic scavenging system for reactive species. Whether oxidation and reduction of methionine and oxidized methionine in α-synuclein itself contributes to the movement defect in our system remains unresolved (Glaser et al., 2005).
The MSRA system plays a fundamental role in oxidative damage control in numerous species, from bacteria, yeast, and flies to mice (Weissbach et al., 2005). In these model systems, disruptions of the MSRA system dramatically decrease the resistance to oxidative stress and often shorten the organism's lifespan (Moskovitz et al., 2001; Kantorow et al., 2004; Koc et al., 2004). Conversely, overexpression of MSRA by genetic manipulations confers a greater resistance to oxidative stress, such as that caused by paraquat (Moskovitz et al., 1998; Ruan et al., 2002), and leads to lifespan extension (Ruan et al., 2002; Kantorow et al., 2004). In addition to its role in normal aging, the therapeutic potential of the MSRA catalytic antioxidant system in many diseases has been frequently speculated as the disease states are often accompanied by inflammation and oxidative stress (Gabbita et al., 1999; Weissbach et al., 2002). Our study provides a convincing example of such potential.
The finding that MSRA has a protective effect on the Drosophila circadian rhythmicity is likely to be relevant to human sleep disorders, a common problem among the elderly (Pandi-Perumal et al., 2002) and PD patients (Chaudhuri et al., 2005). In one mouse model of sleep disorder caused by oxidative stress, MSRA in the forebrain has been implicated as an endogenous protective mechanism of normal sleep pattern (Veasey et al., 2004).
The results of this study suggest that dietary supplementation of SMLC to bolster the MSRA system might be successful in preventing PD or delaying the disease progression. Naturally, before testing this intriguing possibility, many issues need to be resolved first. For example, a systematic study to establish the effective oral intake level is in order. Another practical and important issue that needs to be addressed is whether dietary supplementation with SMLC is effective in preserving the movement function once the PD-like symptoms become noticeable. Our Drosophila study does not provide an unequivocal conclusion whether SMLC supplementation after the animals reach middle age may have a lifespan extension effect (Fig. 5), and the optimal condition needs to be explored further. It must be stressed that, in our study, the constitutive SMLC supplementation starting immediately after eclosion was clearly more effective, underscoring the importance of prevention of oxidative damage.
Whether routine dietary supplementation with SMLC in humans is desirable remains to be rigorously tested. SMLC, a naturally occurring compound, has been tested for short-term toxicity in mammals, including humans, and no adverse effect has been reported (Takada et al., 1997; Yeh and Liu, 2001; Hsu et al., 2004a,b). In fact, the effects reported by the previous studies may be considered “beneficial”: lowering of the serum cholesterol level (Yeh and Liu, 2001), an inhibition of cancer growth (Takada et al., 1997), and a delay in diabetes pathogenesis (Hsu et al., 2004b). Therefore, SMLC in conjunction with MSRA may not only prevent and/or alleviate PD symptoms, but this naturally occurring inexpensive compound may also promote longevity by alleviating other senescence-associated symptoms. It will be important to examine the effectiveness of dietary SMLC supplementation in other models of PD.
Footnotes
This work was supported in part by the National Institutes of Health, National Parkinson Foundation, and Parkinson's Disease Foundation. We thank Dr. M. Feany for the α-synuclein Drosophila lines.
References
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, Curb JD, Petrovitch H. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–1186. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Yates L, Martinez-Martin P. The non-motor symptom complex of Parkinson's disease: a comprehensive assessment is essential. Curr Neurol Neurosci Rep. 2005;5:275–283. doi: 10.1007/s11910-005-0072-6. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Schulz RA, Koehler MM, Savakis C, Cherbas P. Structure of the Eip28/29 gene, an ecdysone-inducible gene from Drosophila. J Mol Biol. 1986;189:617–631. doi: 10.1016/0022-2836(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Fabbrini G, Berardelli A. Drug insight: new drugs in development for Parkinson's disease. Nat Clin Pract Neurol. 2006;2:600–610. doi: 10.1038/ncpneuro0340. [DOI] [PubMed] [Google Scholar]
- Cooper A, Gitler A, Cashikar A, Haynes C, Hill K, Bhullar B, Kangning L, Xu K, Strathearn K, Liu F, Songsong Cao, Caldwell K, Caldwell G, Marsischky G, Kolodner R, LaBaer J, Rochet J-C, Bonini N, Lindquist S. α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dowse HB. Statistical analysis of biological rhythm data. Methods Mol Biol. 2007;362:29–45. doi: 10.1007/978-1-59745-257-1_2. [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Flanagan JR. On the relationship between senescence and age-related changes in two wild-type strains of Drosophila melanogaster. Exp Gerontol. 1978;13:189–196. doi: 10.1016/0531-5565(78)90012-8. [DOI] [PubMed] [Google Scholar]
- Glaser CB, Yamin G, Uversky VN, Fink AL. Methionine oxidation, alpha-synuclein and Parkinson's disease. Biochim Biophys Acta. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hansel A, Kuschel L, Hehl S, Lemke C, Agricola H-J, Hoshi T, Heinemann SH. Mitochondrial targeting of the human peptide methionine sulfoxide reductase (MSRA), an enzyme involved in the repair of oxidized proteins. FASEB J. 2002;16:911–913. doi: 10.1096/fj.01-0737fje. [DOI] [PubMed] [Google Scholar]
- Hansel A, Heinemann SH, Hoshi T. Heterogeneity and function of mammalian MSRs: enzymes for repair, protection and regulation. Biochim Biophys Acta. 2005;1703:239–247. doi: 10.1016/j.bbapap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hirche F, Schröder A, Knoth B, Stangl GI, Eder K. Methionine-induced elevation of plasma homocysteine concentration is associated with an increase of plasma cholesterol in adult rats. Ann Nutr Metab. 2006;50:139–146. doi: 10.1159/000090635. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Huang CN, Hung YC, Yin MC. Five cysteine-containing compounds have antioxidative activity in Balb/cA mice. J Nutr. 2004a;134:149–152. doi: 10.1093/jn/134.1.149. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004b;134:3245–3249. doi: 10.1093/jn/134.12.3245. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, Reddy VN, Hejtmancik JF. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc A, Gasch AP, Rutherford JC, Kim HY, Gladyshev VN. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc Natl Acad Sci USA. 2004;101:7999–8004. doi: 10.1073/pnas.0307929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Cersosimo MG. Neuroprotection in Parkinson's disease: an elusive goal. Curr Neurol Neurosci Rep. 2004;4:277–283. doi: 10.1007/s11910-004-0052-2. [DOI] [PubMed] [Google Scholar]
- Konrad KD, Marsh JL. Developmental expression and spatial distribution of dopa decarboxylase in Drosophila. Dev Biol. 1987;122:172–185. doi: 10.1016/0012-1606(87)90343-5. [DOI] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuschel L, Hansel A, Schönherr R, Weissbach H, Brot N, Hoshi T, Heinemann SH. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456:17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998a;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998b;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D, Jr, Price DL. The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol. 2007a;66:251–257. doi: 10.1097/nen.0b013e3180415e42. [DOI] [PubMed] [Google Scholar]
- Litvan I, Chesselet MF, Gasser T, Di Monte DA, Parker D, Jr, Hagg T, Hardy J, Jenner P, Myers RH, Price D, Hallett M, Langston WJ, Lang AE, Halliday G, Rocca W, Duyckaerts C, Dickson DW, Ben-Shlomo Y, Goetz CG, Melamed E. The etiopathogenesis of Parkinson disease and suggestions for future research. Part II. J Neuropathol Exp Neurol. 2007b;66:329–336. doi: 10.1097/nen.0b013e318053716a. [DOI] [PubMed] [Google Scholar]
- Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Thompson LM. Drosophila in the study of neurodegenerative disease. Neuron. 2006;52:169–178. doi: 10.1016/j.neuron.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2004;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Weissbach H, Brot N. Cloning and expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc Natl Acad Sci USA. 1996;93:2095–2099. doi: 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci USA. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Hefter H, Hueber R, Jost WH, Leenders KL, Odin P, Schwarz J. Is levodopa toxic? J Neurol. 2004;251(Suppl 6):VI/44–46. doi: 10.1007/s00415-004-1610-x. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Seils LK, Kayumov L, Ralph MR, Lowe A, Moller H, Swaab DF. Senescence, sleep, and circadian rhythms. Ageing Res Rev. 2002;1:559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Parvez F, Sayed M, Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J Pharmacol Exp Ther. 2002;300:91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- Pesah Y, Burgess H, Middlebrooks B, Ronningen K, Prosser J, Tirunagaru V, Zysk J, Mardon G. Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of α-synuclein in Drosophila. Genesis. 2005;41:154–159. doi: 10.1002/gene.20106. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18:617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- Rosato E, Kyriacou C. Analysis of locomotor activity rhythms in Drosophila. Nat Protoc. 2006;1:559–568. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Simon JL. Belmont: Duxbury; 1992. Resampling: the new statistics. [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stocchi F. The levodopa wearing-off phenomenon in Parkinson's disease: pharmacokinetic considerations. Expert Opin Pharmacother. 2006;7:1399–1407. doi: 10.1517/14656566.7.10.1399. [DOI] [PubMed] [Google Scholar]
- Takada N, Yano Y, Wanibuchi H, Otani S, Fukushima S. S-methylcysteine and cysteine are inhibitors of induction of glutathione S-transferase placental form-positive foci during initiation and promotion phases of rat hepatocarcinogenesis. Jpn J Cancer Res. 1997;88:435–442. doi: 10.1111/j.1349-7006.1997.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Wang C, Lu R, Ouyang X, Ho MW, Chia W, Yu F, Lim KL. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J Neurosci. 2007;27:8563–8570. doi: 10.1523/JNEUROSCI.0218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CA, Ernst ME. Antioxidants, supplements, and Parkinson's disease. Ann Pharmacother. 2006;40:935–938. doi: 10.1345/aph.1G551. [DOI] [PubMed] [Google Scholar]
- Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Weissbach H, Etienne F, Hoshi T, Heinemann SH, Lowther WT, Matthews B, St John G, Nathan C, Brot N. Peptide methionine sulfoxide reductase: structure, mechanism of action and biological function. Arch Biochem Biophys. 2002;397:172–178. doi: 10.1006/abbi.2001.2664. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci USA. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RL. Lithium stops hereditary shuddering in Drosophila melanogaster. Psychopharmacology. 1982;76:265–268. doi: 10.1007/BF00432558. [DOI] [PubMed] [Google Scholar]
- Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131:989S–993S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci USA. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]