Abstract
The selective localization of protein synthetic machinery at postsynaptic sites makes it possible for the synthesis of particular proteins to be regulated by synaptic signals. Here we consider how the structure of the machinery constrains synthetic capacity and the evidence that mRNA translation is locally controlled by synaptic signals.
Since the discovery of protein synthetic machinery at synaptic sites on dendrites (Steward and Levy, 1982), substantial progress has been made in identifying dendritic mRNAs and in showing that dendritic protein synthesis is critical for persistent synaptic modifications like long-term potentiation (LTP) and long-term depression (LTD). Although many pieces of the puzzle have been identified, major questions remain. Here we focus on one of the unknowns: how translational activity at synapses is regulated and whether regulation involves upregulation or downregulation of overall translation or differential regulation of the translation of particular transcripts. It is useful to begin by considering constraints imposed by the nature of the protein synthetic machinery at synapses.
Keywords: protein synthesis, mRNA, ribosome, synapse, dendrite, synaptic plasticity
There are not many ribosomes at an individual synapse
The availability of ribosomes must constrain the overall capacity for protein synthesis at individual synapses. Quantitative electron microscopic studies reveal that the number of ribosomes at individual synapses is quite limited (Steward and Reeves, 1988; Ostroff et al., 2002). The number of ribosomes in a polyribosome at synapses in the hippocampus and dentate gyrus ranges from ∼3 to 28 with the average being 8. There is no quantitative information on the number of individual ribosomes in dendrites, but qualitatively, few individual ribosomes are seen. It is not known whether dissociated ribosomal subunits are present, and if so, how many.
How is this limited machinery allocated to the different mRNAs that are potentially available for translation? Is the translational machinery running at full capacity, or can it be modulated? The answers to these questions are unknown.
How many mRNAs can fit on the translational machinery beneath an individual synapse?
When ribosomes are present as polyribosomes, it is presumed that they are translating a single mRNA. Most spine synapses have no more than one to two polyribosomes, suggesting that one to two mRNAs are being translated at a given time. It is possible, although unlikely, that each individual ribosome is engaged with a different mRNA, which would allow a greater number of different proteins to be synthesized at the same time.
If most ribosomes at synapses are engaged in translation, then increasing initiation probability (for example, as a result of phosphorylation of initiation factors) would probably not increase overall translational capacity. Conversely, decreasing initiation could reduce translation. Thus, an important question is whether ribosome availability limits the extent to which alterations in initiation factors affect global translational capacity in dendrites [see the Mini-Review by Pfeiffer and Huber (2006) in this issue].
Can additional ribosomes be recruited to the translational machinery at a synapse? There is evidence for shifts in ribosomes from shafts to spines after LTP and increases in the number of synapses with ribosomes (Ostroff et al., 2002). To increase overall translational capacity at one synapse, however, the ribosomes would have to come from somewhere else. Thus, the overall translational capacity of small dendritic segments would presumably be stable unless there were substantial numbers of unengaged ribosomes or unassembled ribosomal subunits that are not apparent by electron microscopy, or a capacity to transport additional ribosomes from a distance.
Regarding the possibility of unassembled ribosomal subunits, recent studies have indicated that mRNAs for certain ribosomal proteins are present in neurites in Aplysia (Moccia et al., 2003) and in dendrites of mammalian neurons in vivo (Zhong et al., 2006). These ribosomal proteins tend to be located at or near the edge of the ribosome where the two subunits link up. Local synthesis of these critical proteins could promote assembly of the subunits into a ribosome, but the subunits would still have to be present.
Traffic jams at the translational machinery of the synapse?
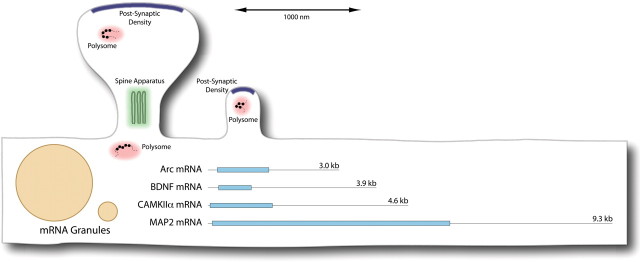
One additional point to consider is the size of the mRNAs that are present in dendrites. The length of an mRNA can be estimated as 0.34 nm/base times the number of bases. So, the mRNAs for calcium/calmodulin-dependent protein kinase II (CAMKII), Arc, and BDNF would be ∼1.57, 1.03, and 1.32 μm, respectively, and MAP2 would be a whopping 3.17 μm long (longer than the average spine!). Figure 1 illustrates how these sizes compare with the size of typical spine synapses. Presumably, mRNAs are configured in tangled loops and hairpins, but even in their three-dimensional form, they must occupy considerable space, especially if they are associated with mRNA-binding proteins that regulate translation [see the Mini-Review by Wells (2006) in this issue]. The actual physical sizes and configurations of dendritic mRNAs are not known, but these considerations indicate that the area beneath and within spines may be very crowded if more than one mRNA is being translated at any one time. How this constrains the movement of mRNA to and from the translational machinery is not known, but movement of mRNAs into spines has been reported after induction of LTP (Havik et al., 2003).
Figure 1.
Approximate sizes of representative dendritic mRNAs and translational elements at synaptic sites on dendrites. The drawing illustrates the approximate size range of spine synapses that would be found in rat forebrain structures such as the hippocampus and cerebral cortex. The lines represent the approximate length of representative dendritic mRNAs if they were straightened out. Shading indicates the length and position of the coding region.
These considerations provide the background for reviewing the evidence for two hypotheses. (1) That overall levels of dendritic protein synthesis are regulated by synaptic signals. (2) That overall levels of dendritic protein synthesis are not regulated, and instead, synaptic signals control the selection of particular mRNAs for translation.
Dendritic protein synthesis during the induction of LTP and LTD
Does physiological induction of LTP or LTD increase the overall level of dendritic protein synthesis? Few papers address this question directly, perhaps because the answer is negative. Autoradiographic assessment of incorporation of 3H-leucine after inducing LTP in the perforant path in vivo failed to reveal increases in labeling in dendritic laminas (O. Steward, unpublished observations). Electrical stimulation of hippocampal slices triggers increased global protein synthesis measured as 35S-met incorporation (Kelleher et al., 2004), but it was not established whether there were increases in dendritic protein synthesis. Slices may be physiologically and metabolically silenced in comparison to intact brain, and thus it is not clear whether increasing global protein synthesis reflects what would occur in vivo with physiological activation. As far as we are aware, the only study to report increases in overall dendritic protein synthesis used autoradiographic techniques to document small increases in incorporation in dendrites when patterned synaptic stimulation of hippocampal slices was paired with carbachol treatment to activate muscarinic ACh receptors (Feig and Lipton, 1993).
There is evidence that induction of LTP in hippocampal slices triggers synthesis of αCAMKII in dendrites (Ouyang et al., 1999) and the elongation factor EF1α (Tsokas et al., 2005). Also, induction of perforant path LTP in vivo leads to a dramatic increase in expression of Arc, causing massive increases in Arc mRNA and protein in dendrites, and also triggers an increase in immunostaining for CAMKII (Steward and Halpain, 1999) and EF1α (Huang et al., 2005). The latter is not blocked by inhibiting protein synthesis, however.
Pharmacological activation using neurotransmitter agonists and antagonists
Direct application of ionotropic glutamate receptor agonists (NMDA, AMPA, or glutamate) to cultured cortical neurons inhibits overall protein synthesis (Marin et al., 1997). Similarly, NMDA application decreases overall protein synthesis in the developing tectum (Scheetz et al., 2000) but increases the synthesis of αCAMKII protein. Similarly, treatment of subcellular fractions containing pinched off dendrites (synaptoneurosomes or synaptodendrosomes) with glutamate increases incorporation of 35S-methionine into select bands in fluorographs of Western blots from synaptodendrosomes (Leski and Steward, 1996) and increases the association of specific mRNAs with polysomes (Bagni et al., 2000) but does not significantly increase overall levels of protein synthesis (Leski and Steward, 1996).
Conversely, treatment of synaptoneurosomes with metabotropic glutamate receptor (mGluR) agonists increases the proportion of ribosomes associated with mRNA (Weiler and Greenough, 1993) and increases the translation of a reporter mRNA (Job and Eberwine, 2001). Also, bath application of the mGluR agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) to hippocampal slices, and injections of DHPG into the dendritic laminas of the hippocampus in vivo triggers a local synthesis of the elongation factor EF1α, whose mRNA is localized in dendrites (Huang et al., 2005; Tsokas et al., 2005). Experiments involving bath application used the same paradigm that has been shown to induce protein synthesis-dependent LTD, linking mGluR activation, increased synthesis of an elongation factor, and LTD. Because EF1α is an elongation factor, one might expect that increases in EF1α protein levels would cause increases in local protein synthesis, but assessments of 3H-leucine incorporation using autoradiographic techniques did not indicate increased labeling in dendritic laminas (Huang et al., 2005).
These studies indicate that the translation of particular mRNAs is regulated by neurotransmitter systems [see also the Mini-Review by Pfeiffer and Huber (2006) in this issue] but do not provide evidence for global increases in protein synthesis in dendrites.
Regulation of dendritic protein synthesis by neuromodulators and growth factors
BDNF can enhance synaptic transmission (Lohof et al., 1993; Kang and Schuman, 1995a; Messaoudi et al., 1998). Also, BDNF is required for some forms of LTP (Korte, 1995; Patterson, 1996), and this LTP requires dendritic protein synthesis (Kang and Schuman, 1996). BDNF can stimulate global protein synthesis of neurons in culture (Kelleher et al., 2004; Takei et al., 2004) and selectively stimulates the translation of certain proteins (Schratt et al., 2004).
Studies of protein synthesis in individual dendrites of neurons in culture using time-lapse imaging of a green fluorescent protein (GFP)-based protein synthesis reporter reveal that BDNF triggers increased translation in mechanically or optically isolated dendrites (Aakalu et al., 2001). In this case, the reporter was flanked by the 5′ and 3′ UTRs from CAMKII, implying that BDNF can stimulate the translation of CAMKII. It is not clear whether overall dendritic translation is enhanced, however.
Dopamine also stimulates dendritic protein synthesis, as demonstrated using a new protein synthesis reporter, a fluorescently tagged puromycin (F2P), and restricted microperfusion of dendrites (Smith et al., 2005). In addition, as highlighted in the accompanying Mini-Review by Pfeiffer and Huber (2006), dopamine agonists also cause a rapid, protein synthesis-dependent enhancement of mEPSC frequency and an increase in surface GluR1, revealing another form of plasticity that appears to require local protein synthesis. These studies involve dissociated neurons in culture, and it remains to be seen whether similar mechanisms operate in adult neurons in vivo.
Protein synthesis as a mechanism for maintaining homeostasis
Endogenous neurotransmitter release regulates the expression of different types of neurotransmitter receptors in a manner that maintains overall levels of excitability (that is, synaptic homeostasis). This has been demonstrated in experiments in which neural activity is either stimulated or reduced using antagonists to inhibitory or excitatory neurotransmitters.
The ability of neurons to respond to chronic changes in activity and initiate homeostatic responses is an area of intense investigation (Turrigiano et al., 1998). Can the protein synthesis machinery respond dynamically to acute changes in synaptic input? Sutton et al. (2004) used a GFP-based reporter to examine the sensitivity of local protein synthesis to changes in either action potential-dependent or action potential-independent synaptic events (a.k.a. miniature synaptic events or minis) in cultured hippocampal neurons. Although dendritic protein synthesis was modestly affected by blockade of action potential-dependent transmission, disruption of miniature synaptic transmission led to dramatic increases in local protein synthesis (Sutton et al., 2004) and surface synaptic GluR1 receptors (Sutton et al., 2006). Moreover, the protein synthesis-dependent increase in GluR1 leads to the insertion of a novel, Ca2+-permeable GluR channel. Thus, minis may serve as a signal for synaptic integrity at synapses; the presence of minis keeps the protein synthesis machinery in a repressed state, whereas a loss of minis results in a stimulation of protein synthesis. In a related study, the prolonged (3 d) blockade of activity (using TTX and APV) was reported to increase the levels of a transfected glutamate receptor in the dendrites (Ju et al., 2004). It is intriguing to imagine that the proteins synthesized may increase the sensitivity of postsynaptic neurons to compensate for net decreases in presynaptic input.
Together, these data suggest that dendritic protein synthesis is involved in plasticity of a fundamental nature: the moment-to-moment assessment of synaptic function and potentially the maintenance of synaptic connections. In addition to the immediate actions on synaptic transmission, intracellular signaling cascades initiated by neurotransmitters can also influence local protein synthesis, giving rise to enduring changes in synaptic function, which in turn play a role in local homeostatic responses that keep synaptic strength within a dynamic range.
Clearly, there does appear to be some disparity in the conclusions from studies in vivo or in acutely prepared reduced preparations such as slices and synaptoneurosomes versus studies involving young neurons growing in culture. In the former case, there is no definitive evidence for extensive modulation of the overall level of dendritic protein synthesis. Instead, the results suggest that overall translational capacity in dendrites is stable and that regulation involves the selection of mRNA for translation and perhaps competition between mRNAs for limited translational machinery. In young neurons in culture, there is evidence that overall levels of protein synthesis can be regulated over a wide dynamic range. It is possible that the disparities reflect differences in methods of measurement. It seems more likely, however, that the greater “plasticity” of the translational machinery in developing dendrites is parallel to, and perhaps a cause of, a greater potential for synaptic growth and plasticity during the developmental period. Clearly, there is much work to be done in establishing the boundary conditions for both dendritic protein synthesis and synaptic plasticity across development.
Footnotes
O.S. was supported by National Institutes of Health (NIH) Grant NS12333. E.M.S. is an investigator of Howard Hughes Medical Institute and was also supported by NIH Grant MH65537.
References
- Aakalu G, Smith WB, Jiang C, Nguyen N, Schuman EM (2001). Dynamic visualization of dendritic protein synthesis in hippocampal neurons. Neuron 30:489–502. [DOI] [PubMed] [Google Scholar]
- Bagni C, Mannucci L, Dotti CG, Amaldi F (2000). Chemical stimulation of synaptosomes modulates a-CAMKII mRNA association to polysomes. J Neurosci 20:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig S, Lipton P (1993). Pairing the cholinergic agonist carbachol with patterned Schaffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J Neurosci 13:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havik B, Rokke H, Bardsen K, Davanger S, Bramham C (2003). Bursts of high-freqency stimulation trigger rapid delivery of pre-existing alpha-CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats. Eur J Neurosci 17:2679–2689. [DOI] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O (2005). The mRNA for EF1alpha is localized in dendrites and translated in response to treatments that induce long-term depression (LTD). J Neurosci 25:7199–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C, Eberwine J (2001). Identification of sites for exponential translation in living dendrites. Proc Natl Acad Sci USA 98:13037–13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC (2004). Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 7:244–253. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM (1995). Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267:1658–1662. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM (1996). A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273:1402–1406. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ III, Govindarajan A, Jung HY, Kang H, Tonegawa S (2004). Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116:467–479. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995). Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92:8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leski ML, Steward O (1996). Synthesis of proteins within dendrites: Ionic and neurotransmitter modulation of synthesis of particular polypeptides characterized by gel electrophoresis. Neurochem Res 21:681–690. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM (1993). Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363:350–353. [DOI] [PubMed] [Google Scholar]
- Marin P, Nastiuk KL, Daniel N, Girault JA, Czernik AJ, Glowinski J, Nairn AC, Premont J (1997). Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J Neurosci 17:3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR (1998). Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol 79:496–499. [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya E, Yaping E, Kalachikov S, Spahn CM, Frank J, Kandel ER, Barad M, Martin KC (2003). An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci 23:9409–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM (2002). Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35:535–545. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB (1999). Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci 19:7823–7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996). Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16:1137–1145. [DOI] [PubMed] [Google Scholar]
- Pfeiffer EM, Huber KM (2006). Current advances in local protein synthesis and synaptic plasticity. J Neurosci 26:7147–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz AJ, Nairn AC, Constantine-Paton M (2000). NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci 3:211–216. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004). BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24:7366–7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM (2005). Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron 45:765–779. [DOI] [PubMed] [Google Scholar]
- Steward O, Halpain S (1999). Lamina-specific synaptic activation causes domain-specific alterations in dendritic immunostaining for MAP2 and CAM kinase II. J Neurosci 15:7834–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Levy WB (1982). Preferential localization of ribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci 2:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Reeves TM (1988). Protein synthetic machinery beneath postsynaptic sites on CNS neurons: association between polyribosomes and other organelles at the synaptic site. J Neurosci 8:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Aakalu GN, Wall N, Schuman EM (2004). Miniature synaptic events regulate local protein synthesis in the dendrites of hippocampal neurons. Science 304:1979–1983. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito H, Cressy P, Kempf C, Woo J, Schuman EM (2006). Miniature synaptic transmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125:785–799. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H (2004). Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24:9760–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD (2005). Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci 25:5833–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zhang T, Bloch LM (2006). Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391:892–896. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT (1993). Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA 90:7168–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DG (2006). RNA-binding proteins: a lesson in repression. J Neurosci 26:7135–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]