Abstract
The circadian clock modulates the induction of long-term sensitization (LTS) in Aplysia such that long-term memory formation is significantly suppressed when animals are trained at night. We investigated whether the circadian clock modulated core molecular processes necessary for memory formation in vivo by analyzing circadian regulation of basal and LTS-induced levels of phosphorylated mitogen-activated protein kinase (P-MAPK) and Aplysia CCAAT/enhancer binding protein (ApC/EBP). No basal circadian regulation occurred for P-MAPK or total MAPK in pleural ganglia. In contrast, the circadian clock regulated basal levels of ApC/EBP protein with peak levels at night, antiphase to the rhythm in LTS. Importantly, LTS training during the (subjective) day produced greater increases in P-MAPK and ApC/EBP than training at night. Thus, circadian modulation of LTS occurs, at least in part, by suppressing changes in key proteins at night. Rescue of long-term memory formation at night required both facilitation of MAPK and transcription in conjunction with LTS training, confirming that the circadian clock at night actively suppresses MAPK activation and transcription involved in memory formation.
The circadian clock appears to modulate LTS at multiple levels. 5-HT levels are increased more when animals receive LTS training during the (subjective) day compared with the night, suggesting circadian modulation of 5-HT release. Circadian modulation also occurred downstream of 5-HT release because animals treated with 5-HT to induce LTS exhibited significantly greater LTS when treated during the (subjective) day compared with the night. Together, our studies suggest that the circadian clock modulates LTS at multiple steps and locations during the formation of long-term memory.
Keywords: Aplysia, C/EBP, circadian rhythm, MAP kinase, learning and memory, serotonin
Introduction
Behavioral evidence for circadian modulation of long-term memory has been broadly observed across species. We discovered in Aplysia that the circadian clock strongly modulates non-associative long-term sensitization (LTS) and associative learning that food is inedible (LFI) (Fernandez et al., 2003; Lyons et al., 2005). Diurnal regulation of conditioned taste aversion occurs in snails (Wagatsuma et al., 2004). In mice, the circadian clock modulates contextual fear conditioning and hippocampal long-term potentiation (Valentinuzzi et al., 2001; Chaudhury and Colwell, 2002; Chaudhury et al., 2005). Some circadian modulation of memory also has been observed in rats (Valentinuzzi et al., 2004). Despite the behavioral evidence for circadian modulation of memory, little molecular evidence exists demonstrating an interaction between the circadian oscillator and downstream targets involved in memory formation.
Conserved steps exist during the induction and consolidation of memory between different types of learning as well as phylogenetically. For example, transcriptional dependence can be considered a hallmark of long-term memory. The transcription factors cAMP response element-binding protein (CREB) and CCAAT/enhancer binding protein (C/EBP) are important in long-term memory across species (Alberini et al., 1994; Taubenfeld et al., 2001a,b; Lonze and Ginty, 2002). Similarly, the mitogen-activated protein kinase (MAPK) cascade assumes a prominent role in learning in vertebrates and invertebrates (Sweatt, 2001; Sharma and Carew, 2004; Reissner et al., 2006). Given the broad circadian modulation of long-term memory, we hypothesized that the circadian clock modulated conserved steps necessary for the formation of long-term memory. Aplysia presents an excellent model to test this hypothesis because considerable cellular and molecular information regarding LTS is known (Kandel, 2001; Bailey et al., 2004). Thus, we investigated whether the circadian clock modulates MAPK activity or the transcription factor ApC/EBP.
No basal circadian regulation was seen for MAPK. In contrast, ApC/EBP protein was rhythmically expressed with peak levels during the night when memory formation is suppressed. Consequently, the rhythm in ApC/EBP cannot explain circadian modulation of LTS. However, we found that the circadian clock regulated the effect of LTS training on the activation of phosphorylated MAPK (P-MAPK) and the induction of ApC/EBP. Relatively small changes in these proteins occurred when animals were trained at night, but P-MAPK and ApC/EBP were significantly increased after training during the (subjective) day. Our results indicate that the circadian clock modulates LTS by modulating key proteins (P-MAPK and C/EBP) required for the formation of long-term memory. The suppression of MAPK and C/EBP during LTS training at night was confirmed by the “rescue” of memory formation, which required facilitation of both MAPK and transcription.
The cellular levels at which the circadian clock modulated long-term memory were also investigated. We examined 5-HT levels in the hemolymph before and after LTS training at different times. We found greater increases in 5-HT levels when LTS training was administered during the (subjective) day, suggesting circadian modulation of 5-HT release. We also found that inducing LTS via in vivo 5-HT treatments resulted in circadian modulation of memory, indicating circadian modulation of downstream events. Thus, circadian modulation of memory formation occurs at multiple cellular locations.
Materials and Methods
Animal maintenance
Aplysia californica (100–150 g; Charles Hollahan, Santa Barbara, CA; Alacrity, Redondo Beach, CA) were housed in artificial seawater (ASW) at 15°C with a 12 h light/dark (LD) cycle. Experiments in constant darkness (DD) were started on the second day of DD. In experiments in DD, CT refers to circadian time or the subjective free-running internal time of the animal with regard to the previous LD cycle. CT 0 refers to subjective dawn, and CT 12 refers to subjective dusk.
Hemolymph 5-HT
To analyze 5-HT levels in the hemolymph, 0.2–0.3 ml samples of hemolymph were withdrawn from each animal. Hemolymph was centrifuged (2 min, 4°C) and then immediately frozen at −80°C. 5-HT was measured using an ELISA (ICN Biochemicals, Costa Mesa, CA) and processed as described previously (Levenson et al., 1999).
Training via electrical stimulation
Long-term sensitization training was performed by electrical stimulation through an electrode placed on the skin on one side of the body wall. LTS training consisted of four 10 s blocks of 10 shocks (500 ms, 60 mA shocks delivered at 1 Hz) with 30 min intervals between blocks of shocks (Levenson et al., 2000; Fernandez et al., 2003).
As reported previously, no differences in behavior were observed in the immediate response of the animal to LTS training with respect to time of day (Fernandez et al., 2003). Training was conducted by multiple individuals in this study. Animals that inked at any time before training were not used in experiments. Batches of animals laying eggs were excluded.
Behavioral pretraining and testing
Animals had the posterior portion of both parapodia clipped 7 d before training to improve visualization of the siphon. Animals were fed to satiation with laver seaweed 5 d before training and then isolated from food (Fernandez et al., 2003). To elicit the reflex behavior in a standard manner, electrodes were implanted in the tail 3 d before baseline testing and training.
The threshold to elicit siphon withdrawal, measured as the minimal amount of current required to elicit the reflex, was determined for each side of the animal. Pretraining and posttraining siphon withdrawal durations were measured using one 20 ms shock at 2× threshold current. Siphon withdrawal was elicited by current from an alternating current stimulator (1504 Isolated Variable AC Line Supply; Global Specialties, Cheshire, CT) through the implanted electrodes. Five pretraining baseline measurements (interstimulus interval of 10 min) of siphon withdrawal duration were made on each side 30 min after threshold determination. The duration of withdrawal was measured from the time the siphon initially contracted to the first sign of siphon relaxation. The stimulus used to elicit pretraining siphon withdrawal did not cause habituation. Posttests for long-term memory were conducted 24 h after training.
Drug treatments and behavior
Animals received drug treatments after behavioral pretests. For trichostatin A (TSA) (Sigma, St. Louis, MO) treatments, animals were bathed in 1 μm TSA 1 h before and during the first hour of LTS training. For potassium bisperoxo(1,10-phenanthroline) oxovanadate V (bpV) (Calbiochem, La Jolla, CA; EMD Biosciences, San Diego, CA) treatments, animals were injected 30 min before training with 1 ml, 0.65 mm bpV in filtered ASW (Instant Ocean; Aquarium Systems, Mentor, OH) per 100 g of body weight to ∼10 μm final concentration in the animal based on the estimated 65% hemolymph of total body weight reported previously (Levenson et al., 1999). Control animals were injected with similar amounts of ASW.
U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene] (Calbiochem) was injected (130 μl/100 g body weight of 7.5 mm DMSO stock) 30 min before LTS training. The concentration of U0126 was designed to approximate a 15 μm final concentration in the whole animal. Control animals were injected with 130 μl/100 g body weight of DMSO at the same time.
Training via in vivo 5-HT treatment
In some experiments, animals were trained with in vivo treatments of 500 μm 5-HT to produce LTS (Levenson et al., 2000). After pretraining testing, Aplysia were placed in 2 L of either 500 μm 5-HT (experimental group; Sigma) or seawater (control group) for 1.5 h at 15°C. Animals were returned to their home tank after treatment, and posttesting was performed 24 h later. The experimenter measuring siphon withdrawal did not know which animals received which treatment.
Western blotting
All molecular analysis was done using pleural ganglia (four pleural ganglia were pooled for each sample to minimize interanimal variability and provide sufficient tissue). It was technically not feasible to dissect out sensory clusters using only dim red light during dark time points or for experiments in constant dark conditions. Aplysia were anesthetized by injection of isotonic MgCl2, and ganglia were dissected 1 h after LTS training. Ganglia were immediately homogenized in lysis buffer (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 2% SDS, 1 mm EDTA, 1 mm EGTA, 1 mm sodium orthovanadate, 50 mm sodium fluoride, and a protease inhibitor mixture). Samples were boiled for 2 min and centrifuged at 16,000 × g, and the supernatant was stored at −80°C. Proteins were resolved via 12% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA).
C/EBP.
A polyclonal antibody to ApC/EBP (ADI, San Antonio, TX) was used to detect a 46 kDa band representing full-length ApC/EBP (Hattar, 2000). Commercially available antibodies were used for actin (Sigma) and for secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Binding of the antibodies was detected using chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ). Quantification was done using NIH Image software. Multiple exposures were used to determine linearity. Parallel gels were run and blotted with anti-actin.
MAPK.
Western blotting for P-MAPK and total MAPK was done as described previously (Sharma and Carew, 2002; Sharma et al., 2003). Antibodies for P-MAPK (Cell Signaling Technology, Danvers, MA) and total MAPK (ERK-1; Santa Cruz Biotechnology, Santa Cruz, CA) were used. Quantification of P-MAPK was normalized to total MAPK. As reported previously for Aplysia, P-MAPK from pleural ganglia extracts ran as a single band at 43 kDa (Sharma et al., 2003).
Statistical tests
Statistical analysis was by ANOVA with Tukey's post hoc analyses for parametric data. Nonparametric data were analyzed by Kruskal–Wallis test with Dunn's multiple comparison post hoc test. p values <0.05 were considered statistically significant.
Results
Circadian clock regulates basal ApC/EBP expression but not MAPK activity
Much of the evidence detailing the mechanisms underlying behavioral long-term sensitization centers on presynaptic facilitation and growth of the sensory neuron (Kandel, 2001; Bailey et al., 2004) and has been gathered from research using in vitro ganglia or cell culture assays. However, circadian modulation of memory has only been demonstrated at the behavioral level in Aplysia. Moreover, although the cellular location of the circadian oscillator that modulates long-term memory formation is known to be outside of the eye, it remains undetermined as to which cells or ganglia contain circadian oscillators in Aplysia (Lyons et al., 2006). Consequently, to test the hypothesis that the circadian clock modulates long-term memory formation by modulating core steps involved in memory formation, it was important to study memory formation in intact animals rather than analogs in in vitro assays.
We investigated whether the circadian clock modulated basal rhythms in two key molecules necessary for LTS, MAPK and C/EBP (Alberini et al., 1994; Martin et al., 1997; Michael et al., 1998), which could lead to circadian rhythms in memory formation. Circadian rhythms in MAPK activity have been reported in the suprachiasmatic nucleus of mammals and in Drosophila (Williams et al., 2001; Coogan and Piggins, 2003, 2004), whereas circadian rhythms in ApC/EBP gene expression have been shown in the Aplysia eye (Hattar et al., 2002). Basal circadian rhythms of protein levels of P-MAPK and total MAPK were analyzed in pleural ganglia, which contain the cell bodies of sensory neurons involved in LTS, of naive animals using Western blots. Whole pleural ganglia were used because it was technically not feasible to dissect out sensory clusters using only dim red light during dark time points. No time of day differences were seen in the levels of either P-MAPK or total MAPK in samples collected from animals maintained in LD cycles (four ganglia pooled per time point for each experiment, n = 4 experiments; p > 0.95) (Fig. 1A) or in DD (n = 4 each time point; p > 0.50) (Fig. 1B). The lack of diurnal or circadian rhythms in P-MAPK levels demonstrates that the circadian clock does not regulate the phosphorylation of MAPK in pleural ganglia. Consequently, circadian modulation of long-term memory formation in LTS does not occur through circadian regulation of basal levels of MAPK activity. However, we cannot rule out the possibility that a rhythm in the compartmentalization of P-MAPK exists, i.e., during the day, P-MAPK may be primarily in the nucleus.
Figure 1.
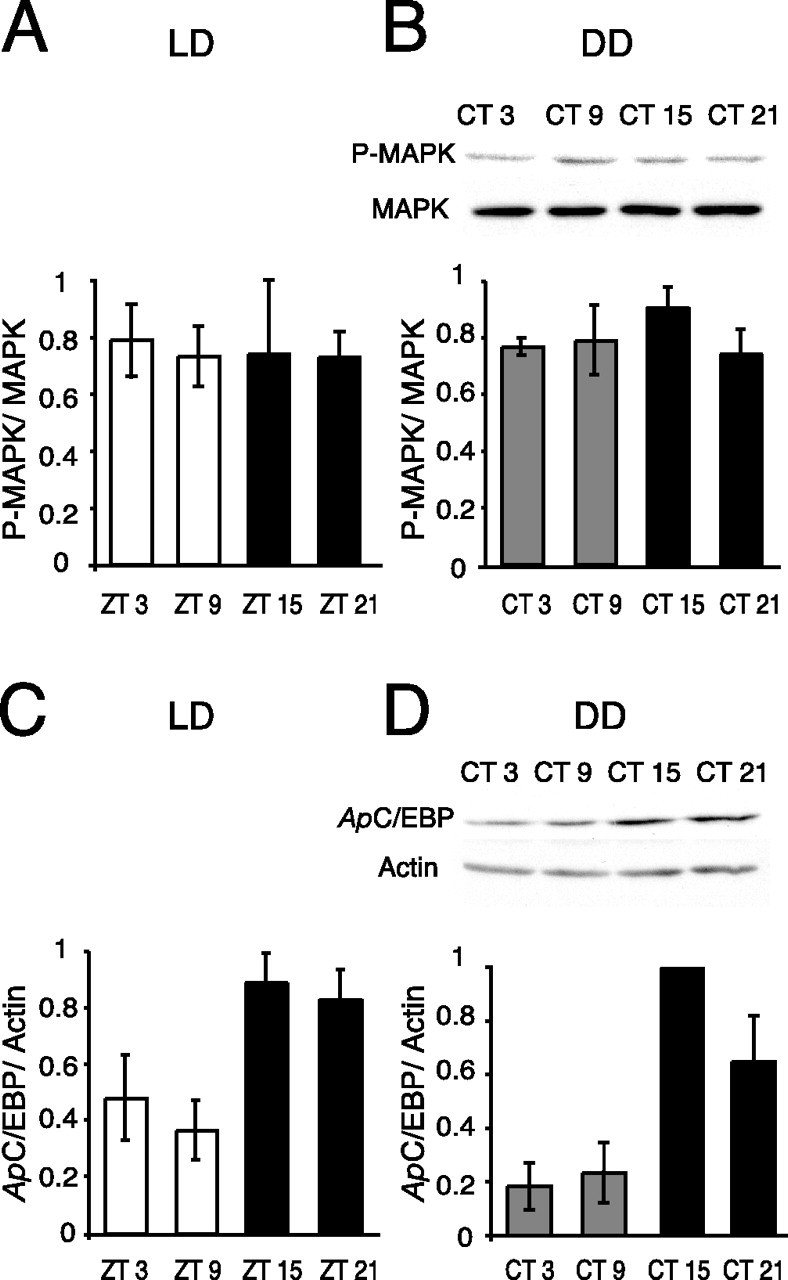
Circadian clock regulates basal ApC/EBP expression but not MAPK activity. To investigate whether the circadian clock regulates basal MAPK activity (P-MAPK levels) or C/EBP protein, animals were entrained to 12 h LD cycles and switched to DD, and pleural ganglia were dissected (4 ganglia per time point, each experiment; n = 4 experiments). A, No diurnal rhythm of MAPK activity in LD. Summary graph from Western blots of P-MAPK levels in pleural ganglia of naive animals. P-MAPK was normalized to total MAPK (p > 0.95; F(3,13) = 0.033). B, Representative Western blot and summary graph showing P-MAPK and total MAPK in pleural ganglia from naive animals in DD. No circadian rhythm was apparent in either P-MAPK or total MAPK levels in naive animals (p = 0.56; F(3,12) = 0.71). C, Summary graph from Western blots of ApC/EBP levels in pleural ganglia of naive animals at different times in LD. ApC/EBP was normalized to actin. A significant diurnal rhythm in the levels of ApC/EBP was apparent (p < 0.05; F(3,14) = 4.87; Tukey's post hoc analyses, p < 0.05 for ZT 9 vs ZT 15, ZT 9 vs ZT 21). D, Representative Western blot showing ApC/EBP and actin in pleural ganglia of naive animals in DD. ApC/EBP was rhythmically expressed with significantly higher levels in pleural ganglia observed during the subjective night (p < 0.01; F(3,12) = 11.62; Tukey's post hoc analyses, p < 0.05 for CT 3 vs CT 15, CT 9 vs CT 15).
Similarly, ApC/EBP protein levels were examined in pleural ganglia of naive animals. ApC/EBP protein was normalized to actin, which did not vary with time of day. In contrast to P-MAPK or total MAPK levels, ApC/EBP protein levels exhibited a significant diurnal (n = 4 each time point; p < 0.05) (Fig. 1C) and circadian rhythm, with peak levels of protein seen during the night (n = 4 each time point; p < 0.01) (Fig. 1D). Additionally, ApC/EBP protein was also rhythmically expressed in cerebral ganglia with peak protein levels during the night (data not shown). These results demonstrate that mechanisms exist for the circadian oscillator to regulate ApC/EBP expression. The circadian rhythm in ApC/EBP protein levels in pleural ganglia occurs with the same phase as reported previously for circadian rhythms in ApC/EBP mRNA in the eye, with peak levels occurring during the subjective night (Hattar et al., 2002). However, the rhythm in levels of ApC/EBP protein in naive (untrained) animals is antiphase to the rhythm of learning of LTS (low levels of ApC/EBP during the day when LTS occurs) seen in trained animals.
Circadian modulation of MAPK activation occurs in the pleural ganglia after LTS training
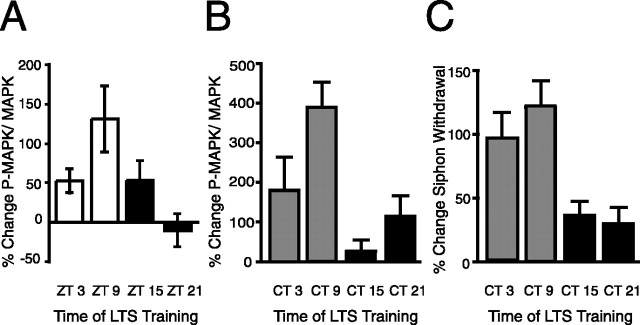
Our previous research demonstrated that circadian modulation of LTS occurred during the induction and consolidation of long-term memory (Fernandez et al., 2003). If the circadian clock modulates long-term memory formation by modulating the induction of key proteins by LTS training, we would predict that LTS training activates key proteins when animals are trained during the day but to a much lesser degree when animals are trained at night. Thus, MAPK activity after LTS training was examined at various phases. Animals were entrained to 12 h LD cycles and subjected to LTS training (electrical stimulation) at either Zeitgeber time 3 (ZT3), ZT 9, ZT 15, or ZT 21. Pleural ganglia were removed 1 h after LTS training, whereas the contralateral ganglia served as controls. A rhythm in activation of MAPK produced by LTS training was observed with peak levels of P-MAPK occurring after LTS training at ZT 9 and trough levels after LTS training at night at ZT 21 (p < 0.05) (Fig. 2A). Total MAPK levels did not significantly change with LTS training at any time point (percentage change between shocked and control ganglia: ZT 3, −6.0 ± 14.8; ZT 9, −2.6 ± 9.7; ZT 15, −16.3 ± 8.1; ZT 21, 5.3 ± 17.7; t test, p > 0.10 for each) as reported previously (Sharma et al., 2003).
Figure 2.
Diurnal and circadian modulation of P-MAPK activation in the pleural ganglia after LTS training. To investigate whether the circadian clock modulates activation of MAPK by LTS training, animals were entrained to 12 h LD cycles and then subjected to LTS training. Pleural ganglia from the shocked side of animals were pooled (4 animals per time point, each experiment), whereas the contralateral ganglia served as controls. P-MAPK levels were normalized to total MAPK abundance. A, In LD, LTS training caused a significant increase in P-MAPK levels during the day compared with LTS training during the night (n = 4–6 per time point; p < 0.05; F(3,15) = 3.82; Tukey's post hoc analyses, p < 0.05 for ZT 9 vs ZT 21). Total MAPK levels did not vary with LTS training or time of day. B, In DD, LTS training induced significantly greater increases in P-MAPK levels at CT 9 compared with other times (n = 3–8 per time point; p < 0.05; F(3,18) = 4.78; Tukey's post hoc analyses, p < 0.05 for CT 9 vs CT 15 and CT 9 vs CT 21). C, Animals were trained during the second day of DD (n = 8–18 for each time point), and then siphon withdrawal duration was measured 24 h later. Animals expressed a circadian rhythm in long-term sensitization in DD (p < 0.01; F(3,49) = 6.48; Tukey's post hoc analyses, p < 0.05 for CT 9 vs CT 15 and CT 9 vs CT 21). Means and SEs of changes in posttraining siphon withdrawal duration compared with pretraining baseline levels are graphed.
To determine whether the rhythmic induction of P-MAPK in LD was attributable to modulation of induction by the circadian clock, additional experiments were done in DD. Again, significantly greater induction of P-MAPK occurred when animals were trained during the subjective day at CT 9 compared with animals trained during the subjective night (p < 0.01) (Fig. 2B). As before, total MAPK levels did not vary with training (percentage change between shocked and control ganglia: CT 3, 0.3 ± 26.2; CT 9, −2.9 ± 8.3; CT 15, 14.4 ± 17.7; CT 21, −5.4 ± 14.5; t test, p > 0.50 for each). Therefore, LTS training of animals in LD and in constant conditions induced the greatest activation of MAPK at CT 9, the time when the highest amount of memory formation can be observed at the behavioral level (Fig. 2C). Circadian modulation of MAPK activity was correlated with the pattern of circadian modulation of LTS at the behavioral level. The Pearson's correlation coefficient was r = 0.89 (r2 = 0.79), which shows a strong correlation of the two variables (one-tailed, p = 0.05). These results suggest that circadian modulation of MAPK signaling may be responsible for much of the circadian modulation of LTS but that additional factors are involved. For example, moderate changes in P-MAPK are produced by LTS training at CT 21, but only minimal behavioral changes are observed at this time point.
Circadian clock modulated changes in ApC/EBP expression in the pleural ganglia after LTS training
The transcription factor C/EBP has been shown to be necessary for long-term facilitation (LTF) in Aplysia, and at least two mammalian isoforms of C/EBP have been implicated in long-term memory formation (Alberini et al., 1994; Taubenfeld et al., 2001a,b). Additionally, as an immediate early gene whose transcription is CREB dependent, C/EBP also provides a readout of CREB1 transcriptional activity. However, injury, light, and 5-HT induce rapid increases in the levels of ApC/EBP mRNA (Alberini et al., 1994; Bartsch et al., 2000; Guan et al., 2002; Hattar et al., 2002). By training intact animals under both LD and DD conditions and examining C/EBP protein levels after LTS training, we avoided complications associated with the injury or light induction of ApC/EBP mRNA. Animals were entrained to LD cycles and subjected to LTS training at various times during the LD cycle or on the second day of DD. Pleural ganglia were removed 1 h after training. LTS training during the day induced significant increases in ApC/EBP protein levels (ZT 3 and ZT 9) in contrast to the effect of LTS training of animals at night (ANOVA, p < 0.05) (Fig. 3A). To confirm that this modulation of ApC/EBP expression after LTS training was attributable to the circadian clock, similar experiments were done with animals in DD. During the subjective day, LTS training induced significant increases in ApC/EBP protein levels in the pleural ganglia, whereas little or no increases in protein levels were observed when LTS training was administered during the subjective night (Kruskal–Wallis, p < 0.01) (Fig. 3B). Actin levels after LTS training at CT 3 and CT 9 demonstrated consistent, albeit not significant, decreases between the trained side of the animal and the contralateral side (CT 3, −26.8 ± 8.7%; CT 9, −21.9 ± 11.4%). Consequently, in these experiments, ApC/EBP levels were not normalized to actin, although the results were similar when actin normalization was used. A Pearson's correlation coefficient between LTS and the percentage change in C/EBP levels gave an r value of 0.99 (r2 = 0.99), which demonstrates a very strong correlation between changes in C/EBP levels (used to represent transcription) and long-term memory formation (p < 0.01). The combination of circadian modulation of MAPK activation and C/EBP induction after LTS training indicates that circadian modulation of LTS occurs through the suppression of the induction or activation of key proteins involved in memory consolidation when LTS training occurs at night.
Figure 3.
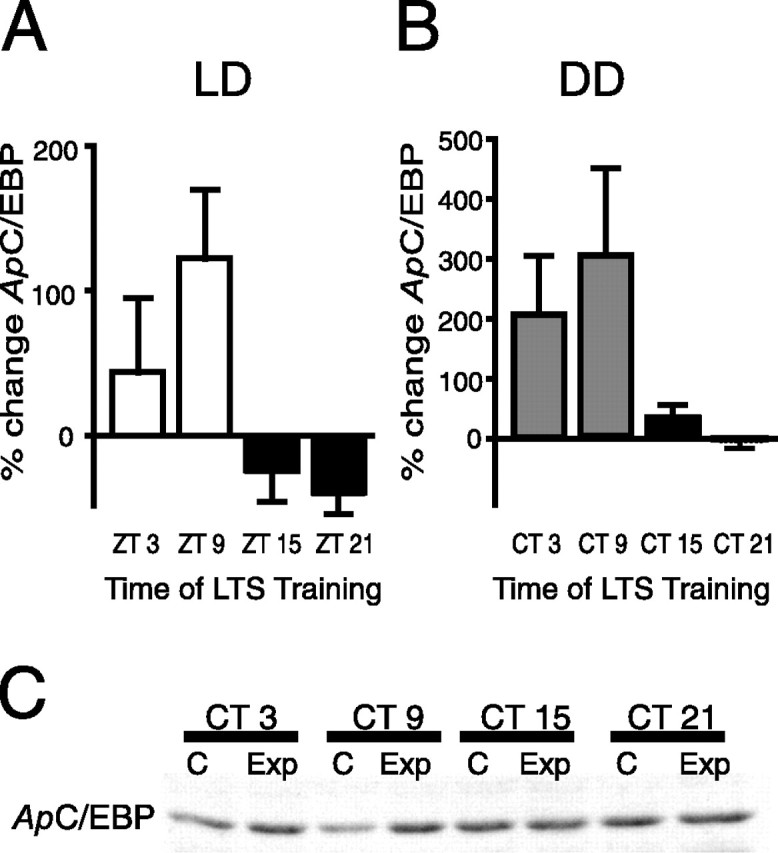
Circadian clock modulated expression of ApC/EBP induced by LTS training. A, In LD, LTS training caused a significant increase in ApC/EBP protein levels during the day compared with LTS training of animals at night (n = 3–4 experiments per time point; p < 0.05; F(3,10) = 4.39; Tukey's post hoc analyses, p < 0.05 for ZT 9 vs ZT 21). B, To determine whether the circadian clock modulated ApC/EBP levels after LTS training, animals were subjected to LTS training on the second day of DD. LTS training during the subjective day resulted in significantly greater increases in ApC/EBP protein than LTS training during the night (n = 3–8 per time point, Kruskal–Wallis = 12.3; p < 0.01; Dunn's multiple comparison, p < 0.01 for CT 9 vs CT 21). C, Representative Western blot showing induction of ApC/EBP after LTS training. Ganglia from the shocked side (Exp) of animals were pooled (4 animals per time point, each experiment), whereas the contralateral ganglia served as controls (C).
Presumably, because ApC/EBP is downstream of MAPK signaling, the very strong correlation seen between changes in ApC/EBP expression and LTS represents circadian modulation of MAPK activity as well as additional factors that result in the circadian modulation of ApC/EBP levels. We tested whether increased MAPK activity was necessary for the increases observed in C/EBP protein levels after LTS training during the subjective day. Animals were injected with the MAP kinase kinase (MEK) inhibitor U0126 (130 μl/100 g body weight of 7.5 mm stock in DMSO) 30 min before LTS training at CT 9. U0126 has been used previously to inhibit MAPK activity in Aplysia ganglia and in semi-intact preparations (Chin et al., 2002; Sharma et al., 2003; Khabour et al., 2004). Inhibiting the MAPK signaling pathway prevented significant increases in C/EBP protein levels. Animals were given U0126 before LTS training, and pleural ganglia were dissected 1 h after training. U0126 blocked the LTS-induced increase in P-MAPK (−9.8 ± 20.6% change between the shocked side and the control side of the animals; four pleural ganglia pooled for each experiment, n = 4). In these same animals, the change in ApC/EBP protein levels was −9.4 ± 26.3% between the shocked and control sides. Control animals injected with DMSO that received LTS training showed significant increases in both P-MAPK and C/EBP protein levels in pleural ganglia between the ipsilateral and contralateral sides (P-MAPK, 305.8 ± 136.5%; ApC/EBP, 159.1 ± 31.4%). Thus, it appears that increases in MAPK activity induced by LTS training are necessary for increases in ApC/EBP expression during the day. These results are consistent with the hypothesis that MAPK phosphorylation of CREB2 relieves transcriptional repression and allows transcription of C/EBP during the induction and consolidation of memory (Michael et al., 1998).
Removing the suppression of long-term memory at night
The experiments described above strongly suggest that the circadian clock suppresses the activation of MAPK as well as the induction of C/EBP during LTS training at night. To confirm these results, we investigated whether it was possible to rescue long-term memory formation at night by facilitating LTS training at night through pharmacological amplification of MAPK activity and transcription. Previously in Aplysia, a tyrosine phosphatase inhibitor, bpV, has been used to enhance tyrosine kinase activity and convert a subthreshold training paradigm into an effective protocol that induces LTS through increased MAPK signaling (Purcell et al., 2003). The histone deacetylase inhibitor TSA has also been used in Aplysia to facilitate LTF (Guan et al., 2002).
Using bpV and TSA either individually or together with LTS training, we asked whether facilitating MAPK activity or transcription during LTS training at night resulted in long-term memory formation. After behavioral pretests were performed on the second day of DD, animals were bathed in 1 μm TSA for 1 h before and during LTS training and/or injected with bpV 30 min before LTS training at CT 15 or CT 21. Control animals were injected with ASW. Animals were tested for LTS 24 h after training. TSA treatments in conjunction with LTS training did not result in the formation of long-term memory at night at either CT 15 (Fig. 4A) or CT 21 (Fig. 4B). LTS training plus bpV also did not induce long-term memory formation and was not significantly different from ASW-injected controls that received LTS training (Fig. 4A,B). In contrast, when animals received both TSA and bpV treatments with LTS training, animals showed robust unilateral LTS at both CT 15 and CT 21 on testing 24 h after training (compare shocked and control sides in Fig. 4A,B). Because LTS training was delivered to one side of the animal, the contralateral side acted as a control for the effect of the drug alone. TSA and/or bpV did not produce significant changes in siphon withdrawal duration on the contralateral side of the animal. These results suggest that individually facilitating transcription or MAPK activity is insufficient to overcome circadian modulation of memory formation at night and that the circadian clock suppresses both of these processes. Only when both MAPK activity and transcription are facilitated pharmacologically does LTS training at night induce long-term memory formation. These results provide strong support for the hypothesis that the circadian clock suppresses long-term memory formation at night at multiple steps.
Figure 4.
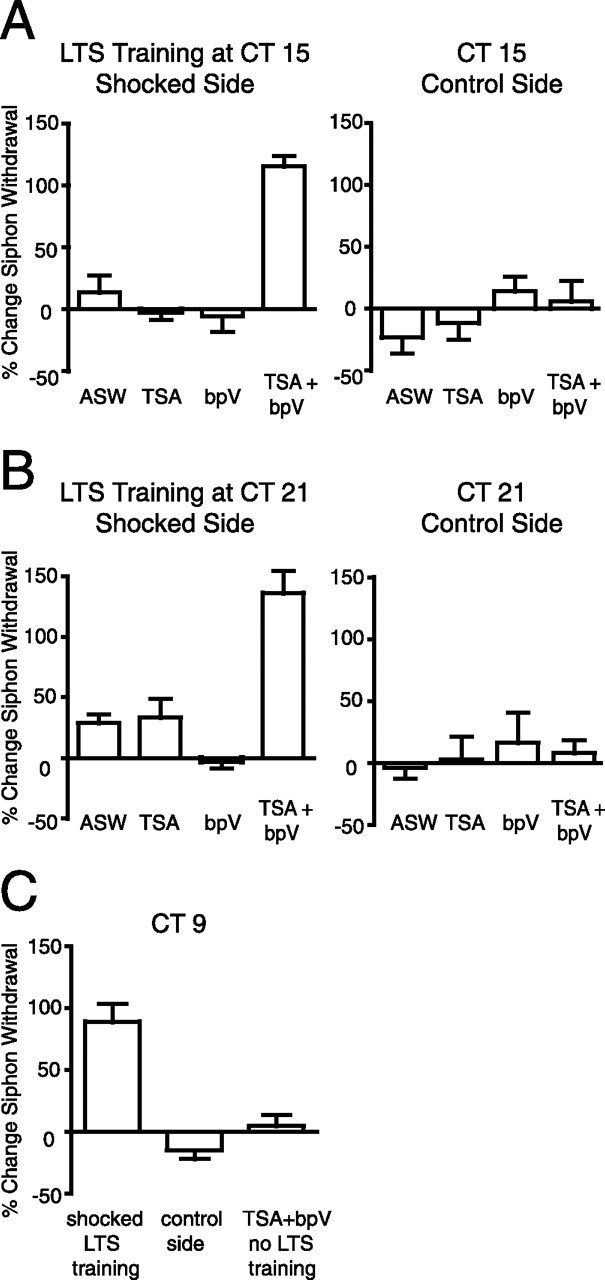
Facilitating both MAPK signaling and transcription concurrent with LTS training was necessary for the formation of long-term memory at night. To investigate whether the observed circadian suppression of MAPK activity and C/EBP expression after LTS training at night was responsible for the inhibition of long-term memory at the behavioral level, we used pharmacological facilitation of MAPK signaling and transcription to rescue long-term memory formation at night. Animals were either bathed in 1 μm TSA for 1 h before and during training and/or injected with 1 ml/100 g body weight, 0.65 mm bpV in ASW 30 min before training. At CT 15 (A) and at CT 21 (B), neither TSA nor bpV individually facilitated LTS training sufficient for the formation of long-term memory (CT 15, t tests, p > 0.6; CT 21, t tests, p > 0.15). Only when animals received both TSA and bpV treatments did LTS training rescue long-term memory formation at night (CT 15, ANOVA, p < 0.001, F(3,13) = 31.48; Tukey's post hoc analyses, p < 0.001 for TSA plus bpV treatment compared with all other groups; CT 21, ANOVA, p < 0.001, F(3,11) = 23.97; Tukey's post hoc analyses, p < 0.001 for TSA plus bpV treatment compared with all other groups). Drugs alone did not cause sensitization on the control side of the animal (n = 3–5 for each treatment). C, LTS training of animals during the subjective day at CT 9 resulted in significant unilateral sensitization (t test, p < 0.001). bpV and TSA treatments alone were not sufficient to induce LTS in the absence of training (n = 5–7 for each group).
One potential difficulty in using bpV to activate or facilitate MAPK signaling is that other signaling pathways may also be affected by bpV. To confirm that the robust long-term memory at night was induced by pathways known to be necessary for the induction of LTS, we tested whether the bpV and TSA facilitation of LTS training at night occurred through MAPK signaling by using the MEK inhibitor U0126. Importantly, U0126 has been shown previously not to affect baseline siphon withdrawal responses in control animals (Sharma et al., 2003). Behavioral pretests were performed to determine baseline siphon withdrawal durations before LTS training of animals at CT 15. After behavioral pretests, animals were bathed in 1 μm TSA for 1 h before and during LTS training and were also injected with bpV and U0126 (130 μl/100 g body weight of 7.5 mm stock in DMSO) 30 min before LTS training. Posttests were performed 24 h later. We found that inhibiting MAPK signaling in intact animals completely blocked the pharmacological rescue of long-term memory at night previously achieved by TSA and bpV with LTS training. No significant difference was observed for siphon withdrawal duration between the trained side of the animal and the control side (percentage change in siphon withdrawal duration between pretests and posttests: shocked side, −12.7 ± 6.5%; control side, −14.0 ± 6.0%; t = 0.15; p > 0.85; n = 6). These results demonstrate that the bpV (and TSA) facilitation of LTS training occurs through MAPK signaling.
Although the combination of TSA and bpV did not result in LTS on the side of the animal that did not receive electrical stimulation at night, we investigated whether the combination of bpV and TSA treatments when given to animals during the subjective day was sufficient to induce LTS in the absence of electrical stimulation. After behavioral pretests, animals were bathed in 1 μm TSA for a total of 2.5 h during the subjective day at CT 9. Animals received injections of bpV after the first 30 min of TSA treatment. We found that the combination of TSA and bpV treatment was insufficient to induce LTS without electrical stimulation (Fig. 4C). In control animals, training at CT 9 induced significant unilateral LTS when animals were tested 24 h later (Fig. 4C). The fact that TSA plus bpV did not induce LTS lends support to the premise that the circadian clock does not regulate basal MAPK activity and that no cellular or compartmentalization rhythm in P-MAPK exists. If P-MAPK was actually higher during the day, one would expect that providing an additional boost to MAPK activity with bpV and facilitating transcription with TSA would be sufficient to induce LTS.
Changes in 5-HT levels in the hemolymph after LTS training are regulated by the circadian clock
The previous experiments were designed as a first step to identify conserved steps through which the circadian clock impacts long-term memory formation and found that the circadian clock modulates key molecular processes necessary for long-term memory formation. However, for long-term sensitization in Aplysia, there are also cellular questions. At the cellular level, long-term sensitization involves (1) facilitatory neurons that release 5-HT after noxious stimulation, (2) sensory neurons in which 5-HT triggers transcription- and translation-dependent events that result in long-term presynaptic facilitation and subsequent increased neurotransmitter release and growth, and (3) motor neurons in which LTS is expressed as an increase in the amplitude of EPSPs. Interneurons are also involved in the siphon withdrawal reflex circuit, but their contribution to LTS has not been established. The circadian clock could regulate LTS by modulating the early steps of memory formation in facilitatory neurons such as serotonin release or modulating molecular processes within the sensory neurons farther downstream.
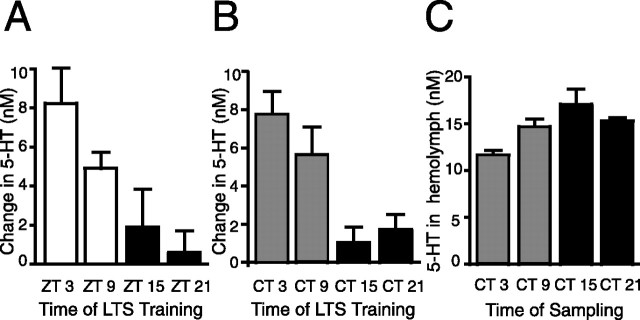
During and after LTS training, levels of 5-HT increase in the hemolymph (Levenson et al., 1999) and provide an indirect method for estimating 5-HT release. To determine whether the 5-HT release during and after LTS training was regulated by the circadian clock, hemolymph samples were taken from individual animals 15 min before LTS training and after LTS training. LTS training caused greater increases in 5-HT levels in the hemolymph when animals were trained during the day at ZT 3 and ZT 9 compared with animals trained during the night at ZT 15 and ZT 21 (Fig. 5A). Data are reported as the change in nanomolar between pretraining and posttraining levels of 5-HT for each animal. Sampling procedures themselves did not cause an increase in 5-HT levels between two samples spaced 1.5 h apart (LD, 1.30 ± 7.0% change in 5-HT levels as a result of sampling; n = 11; t test = 0.61; p = 0.56). These results suggest that 5-HT release produced by LTS training is diurnally modulated.
Figure 5.
LTS training induces a diurnal and circadian rhythm in increases in 5-HT levels in the hemolymph. A, In LD, LTS training resulted in larger increases in the amount of 5-HT (reported as nanomolar change in 5-HT) released into the hemolymph when animals were trained during the day compared with when animals were trained at night (n = 6–17 per time point, p < 0.01; F(3,40) = 5.56; Tukey's post hoc analyses, p < 0.05 for CT 3 vs CT 21 and CT 9 vs CT 21). B, In DD, LTS training during the subjective day resulted in significantly greater increases in 5-HT levels in the hemolymph compared with LTS training at night (n = 8–12 per time point, p < 0.01; F(3,33) = 6.47; Tukey's post hoc analyses, p < 0.05 for CT 3 vs CT 15, CT 3 vs CT 21 and CT 9 vs CT 15). C, 5-HT levels in the hemolymph varied with time of day under constant conditions with peak levels at CT 15 (n = 10–13 per time point, p < 0.05; F(3,41) = 4.16; Tukey's post hoc analyses, p < 0.01 for CT 3 vs CT 15).
To determine whether 5-HT release after LTS training was regulated by the circadian clock, animals were entrained to LD cycles and then switched to constant dark conditions. LTS training was done at different times on the second day of DD. As previously, animals were sampled 15 min before LTS training and 5 min after LTS training. Repeat sampling alone did not cause increases in 5-HT levels (−1.99 ± 3.3% change in 5-HT levels as a result of sampling; n = 7; t = 0.18; p = 0.86). LTS training during the subjective day induced a greater increase in the nanomolar amount of 5-HT in the hemolymph compared with LTS training of animals during the night (n = 8–12 for each time point) (Fig. 5B). These results demonstrate that the circadian clock modulates changes in 5-HT levels in the hemolymph produced by LTS training and strongly suggest that one way in which the circadian clock modulates LTS at the behavioral level is by modulating 5-HT release. However, these studies cannot anatomically pinpoint the site of 5-HT release, and previous research has shown that sensitization training results in widespread serotonin release (Marinesco and Carew, 2002; Marinesco et al., 2004).
Basal 5-HT levels in the hemolymph have been reported previously as being diurnally regulated (Levenson et al., 1999). Under the somewhat different conditions of our experiments in which the animals were removed from appetitive stimuli for a period of 5 d before LTS training, the variations seen in the basal levels of 5-HT in the hemolymph did not appear to be diurnally regulated, although 5-HT levels were slightly elevated at ZT 15 (LD: ZT 3, 13.83 ± 1.90 nm; ZT 9, 10.68 ± 0.82 nm; ZT 15, 16.25 ± 0.42 nm; ZT 21, 12.66 ± 0.7 nm). In contrast to previous research (Levenson et al., 1999), we found a small, but significant, circadian rhythm in 5-HT in the hemolymph with peak levels of 5-HT at CT 15 (ANOVA, p < 0.05; Tukey's post hoc analyses, p < 0.01 for CT 3 vs CT 15) (Fig. 5C). These fluctuations in basal 5-HT levels were too small to account for the diurnal or circadian rhythms in 5-HT release seen after LTS training. For example, basal 5-HT levels in the hemolymph were similar at CT 9 and CT 21 (CT 9, 14.66 ± 0.88 nm; CT 21, 15.29 ± 0.42 nm), and yet a clear difference in the magnitude of 5-HT release during LTS training existed between animals trained at these two times. During the subjective day, LTS training results in higher levels of 5-HT in the hemolymph than the basal levels at night, suggesting that the nighttime level of 5-HT does not represent a physiological ceiling and that the low-amplitude basal rhythm does not affect the induction of LTS. Thus, it appears that the circadian clock does strongly modulate 5-HT release after LTS training in addition to regulating basal 5-HT levels to a small degree.
Induction of LTS by 5-HT results in diurnal and circadian modulation of LTS
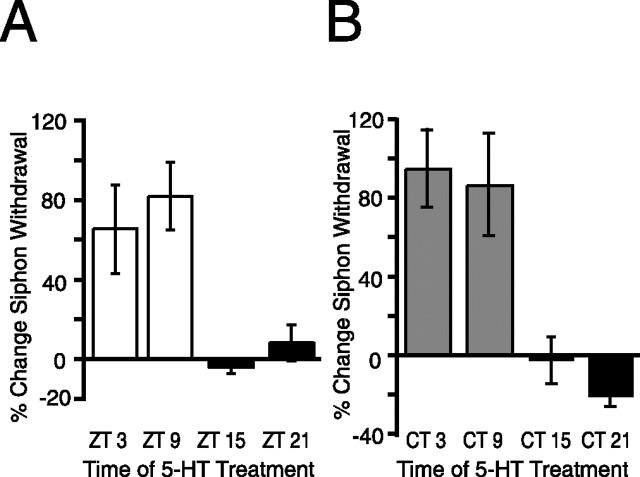
It is possible that circadian modulation of memory formation also occurs downstream of 5-HT release (in sensory or motor neurons) as well as 5-HT release itself. To test this, we investigated whether in vivo treatment of animals with the same concentration of 5-HT would produce a diurnal rhythm in LTS. Animals maintained in LD cycles were treated with 5-HT (500 μm, 1.5 h) at either ZT 3, ZT 9, ZT 15, or ZT 21 (n = 6, 8, 5, and 6 respectively). In vivo treatments of 500 μm 5-HT have been shown previously to induce LTS that lasts for at least 24 h (Levenson et al., 2000). LTS varied significantly depending on when animals were treated during the LD cycle (p < 0.01) (Fig. 6A). The change in the duration of siphon withdrawal was significantly higher for animals exposed to 5-HT during the day (ZT 3 and ZT 9) than for animals treated during the night (ZT 15 and ZT 21). Baseline expression for threshold and duration of siphon withdrawal before treatment did not vary with time of day (data not shown). These results suggest that the circadian clock modulates events in sensory neurons during formation of long-term memory.
Figure 6.
Diurnal and circadian modulation of long-term memory formation of LTS produced by 5-HT. To determine whether the circadian clock modulates LTS formation downstream of 5-HT release from facilitatory neurons, animals were treated with 5-HT (500 μm, 1.5 h) to induce LTS at different times in the LD cycle (A) or in DD (B). Siphon withdrawal duration was measured 24 h later. A, 5-HT induced significantly greater increases in siphon withdrawal when animals were treated during the day than when they were treated at night (n = 4–7 animals per time point, p < 0.01; F(3,21) = 6.91; Tukey's post hoc analyses, p < 0.05 for ZT 3 vs ZT 15, ZT 3 vs ZT 21, ZT 9 vs ZT 15, and ZT 9 vs ZT 21). B, 5-HT induced significantly greater LTS when animals in DD were treated during the subjective day compared with animals treated during the subjective night (n = 5–8 animals per time point, p < 0.01; F(3,19) = 8.23; Tukey's post hoc analyses, p < 0.05 for CT 3 vs CT 15, CT 3 vs CT 21, CT 9 vs CT 15, and CT 9 vs CT 21).
The results from the above experiments in LD suggest that the circadian clock modulates memory formation downstream of 5-HT release. However, to confirm that this downstream rhythmic modulation was attributable to the circadian clock, similar experiments were performed on animals starting on the second day of constant darkness. As shown previously (Fernandez et al., 2003), no time of day differences were seen for any baseline component, i.e., threshold current necessary to elicit siphon withdrawal or duration of siphon withdrawal, from animals maintained in constant darkness [threshold (in mA): CT 3, 1.71 ± 0.17; CT 9, 1.79 ± 0.10; CT 15, 1.98 ± 0.25; CT 21, 2.11 ± 0.12; p = 0.35; pretest siphon withdrawal duration (in s): CT 3, 2.91 ± 0.16; CT 9, 3.14 ± 0.30; CT 15, 2.59 ± 0.26; CT 21, 3.06 ± 0.39; p = 0.53]. After pretests, animals were treated with 5-HT (500 μm, 1.5 h) at CT 3, CT 9, CT 15, or CT 21 (n = 6, 7, 6, and 4, respectively). Significant differences in LTS were seen between animals trained by 5-HT during the subjective day (CT 0–CT 12) and animals trained by 5-HT during the subjective night (CT 12–CT 24; p < 0.01) (Fig. 6B). These experiments (LD and DD) demonstrate that, when animals were treated with the same levels of 5-HT at different phases, the circadian clock modulates the induction of LTS downstream of 5-HT release.
Although the animals were exposed to the same high level of 5-HT, it is possible that the concentration of 5-HT in the hemolymph was different at different times of day because of, for example, the diurnal modulation of skin permeability. To investigate this possibility, we measured the concentration of 5-HT in the hemolymph after the exposure of the animal to 5-HT at different times of the day. Hemolymph samples were taken immediately after 5-HT treatments. No significant differences in the levels of 5-HT in the hemolymph were observed between animals exposed to 5-HT during the day (1.63 ± 0.36 μm; n = 5) and animals exposed to 5-HT during the night (1.95 ± 0.62 μm; n = 5; p = 0.67). Thus, it appears that rhythmic levels of 5-HT in the hemolymph were not necessary to generate the circadian modulation of LTS we observed.
Discussion
Circadian modulation of long-term memory formation occurs at multiple steps during LTS. LTS training induced greater increases in 5-HT levels in the hemolymph when animals were trained during the (subjective) day compared with animals trained at night (Fig. 5A,B), which suggests circadian modulation of serotonin release, including release from facilitatory neurons, during LTS. Circadian modulation of LTS also occurs downstream of 5-HT release. Treatment of animals with the same concentration of 5-HT at different times of day resulted in circadian rhythms in LTS (Fig. 6A,B). These results demonstrate that circadian modulation downstream of serotonergic facilitatory neurons and 5-HT release is sufficient to produce circadian modulation of long-term memory. Multiple sites of modulation in a behavioral circuit strongly suggest that modulation plays an important role in the behavior generated by the circuit.
The circadian clock modulates long-term memory for sensitization of the tail siphon withdrawal reflex and for LFI, such that time of training determines whether long-term memory formation occurs (Fernandez et al., 2003; Lyons et al., 2005). Given the modulation of different types of learning in numerous species by the circadian clock, we hypothesized that the circadian clock modulated conserved processes necessary for long-term memory formation such as MAPK signaling and transcription. We analyzed ApC/EBP protein as a reporter for transcription because ApC/EBP functions as both a transcription factor and a molecular readout of CREB activity. To investigate circadian modulation of changes in MAPK activity or ApC/EBP expression produced during memory formation, we trained animals with electrical stimulation. Electrical stimulation at the behavioral level represents the most appropriate level for these studies. Intact animals must be used to study circadian modulation because no in vitro Aplysia preparation, except the eye, has been shown to have a circadian clock.
The circadian clock could modulate LTS by regulating training-induced increases in MAPK activity or ApC/EBP expression. We found diurnal and circadian rhythms in MAPK activity and ApC/EBP expression after LTS training such that training resulted in peaks of P-MAPK and ApC/EBP protein levels when animals were trained during the subjective day and troughs when animals received training at night (Figs. 2, 3). The circadian modulation of MAPK activity and C/EBP expression during memory formation is in phase with the circadian modulation of LTS behaviorally and hence may be sufficient to explain a large portion of the circadian modulation of LTS.
We also investigated whether increased MAPK activity was necessary for increases in ApC/EBP levels using the MEK inhibitor U0126 in vivo. U0126 given before LTS training blocked the increases in C/EBP protein normally associated with the induction of LTS. Thus, it appears that MAPK signaling is necessary for increased ApC/EBP expression during long-term memory formation.
In addition to circadian modulation specific to the induction of LTS, the circadian clock could regulate basal levels of MAPK and ApC/EBP. We observed no diurnal or circadian rhythms of MAPK activity or total MAPK in pleural ganglia from naive animals (Fig. 1A,B), suggesting that circadian regulation of MAPK activity does not occur. Possibly, MAPK may be rhythmic in subsets of cells or intracellularly. In contrast, ApC/EBP protein exhibited significant diurnal and circadian rhythms with peak levels observed at night (Fig. 1C,D), consistent with our previous research demonstrating circadian rhythms of ApC/EBP mRNA (Hattar et al., 2002). Although the function of this basal rhythm remains unknown, identification of rhythmic ApC/EBP expression in pleural ganglia provides insight into a potential entry point for circadian modulation of memory.
It is perhaps unexpected that LTS training at night is ineffective given the high levels of ApC/EBP. Although it is difficult to compare behavioral experiments using animals with functional clocks with research using in vitro preparations, such comparisons may provide insight into circadian suppression of long-term memory. Lee et al. (2001) demonstrated that overexpression of ApC/EBP in cultured cells did not result in LTF, although overexpression facilitated subthreshold 5-HT treatments. This suggests that ApC/EBP alone is insufficient for induction of long-term memory and that ApC/EBP requires either additional modification (e.g., phosphorylation) or a binding partner that can be activated by 5-HT. In our experiments, using intact animals at night, high levels of ApC/EBP also are insufficient for LTS even with LTS training. This lack of LTS induction at night is presumably attributable to circadian suppression of additional factors or a binding partner that can modulate the activity of ApC/EBP.
Our results suggest that the circadian clock suppresses long-term memory formation at night through repression of training-induced increases in MAPK and ApC/EBP. To further test this, we used bpV, a tyrosine phosphatase inhibitor, to facilitate tyrosine kinase activity and subsequent MAPK signaling (Purcell et al., 2003), and a histone deacetylase inhibitor, TSA, to facilitate transcription at night (Guan et al., 2002). Animals received either a separate or combined drug treatment, in conjunction with LTS training at night. Increasing MAPK signaling via bpV and LTS training was insufficient to induce long-term memory at night (Fig. 4), suggesting that additional steps downstream of MAPK signaling remained suppressed by the circadian clock. Also, using TSA, to relax the threshold for transcription, with LTS training at night did not result in long-term memory. However, when combined bpV and TSA treatments were given with LTS training at night, animals exhibited robust LTS 24 h after training. The rescue of long-term memory at night achieved by facilitation of LTS training with bpV and TSA was completely blocked by the MEK inhibitor U0126. Thus, it appears that the circadian clock suppresses both MAPK signaling and transcriptional activity after LTS training at night, subsequently inhibiting the formation of long-term memory.
Our research suggests that the circadian clock actively suppresses the formation of long-term memory at night, whereas during the day, LTS training results in activation of MAPK and increases in ApC/EBP because of a lack of repression by the circadian clock. Thus, we propose that the formation of long-term memory normally represents a permissive state of regulation or lack of inhibition by the circadian clock. Indeed, recent studies using mutant mice and flies found that the lack of a functional circadian clock did not impair learning (Sakai et al., 2004; Zueger et al., 2006).
How does circadian suppression of MAPK activation and increased ApC/EBP expression occur during LTS? Perhaps circadian regulation of 5-HT release contributes to modulation of MAPK activity. However, the circadian clock must also regulate molecules downstream of 5-HT release (i.e., neuropeptide release, extracellular growth factors, etc.), because circadian modulation of LTS still occurs when induced by 5-HT. The absence of increased ApC/EBP expression after LTS training at night could be attributable to basal circadian regulation of ApC/EBP (Fig. 1), if the high levels of ApC/EBP protein at night represent ceiling levels. However, ApC/EBP activity also may be regulated through its binding partner(s), which include the transcriptional activator Aplysia activating factor (ApAF) and the repressor form of Aplysia CREB, ApCREB2 (Bartsch et al., 1995, 2000; Choi et al., 2003). The ApC/EBP–ApAF complex has been suggested as a necessary and sufficient transcriptional complex downstream of CREB for LTF (Bartsch et al., 2000; Park et al., 2005). Additionally, PKA phosphorylation of ApAF appears necessary (Park et al., 2005). Consequently, circadian modulation of LTS also may occur by regulating ApAF through its expression or phosphorylation. Alternatively, ApC/EBP–CREB2 dimers may function to actively repress transcription of genes involved in LTS at night and thus inhibit long-term memory. Then again, ApC/EBP protein at night may function in the transcriptional regulation of nonlearning-related genes.
Circadian suppression of the induction of MAPK signaling and transcription provides a framework to explain circadian modulation of LTS. However, the circadian clock probably modulates memory formation through multiple mechanisms. Circadian modulation at several steps could amplify the rhythm observed in long-term memory and/or produce variations in the pattern (waveform) of circadian modulation, depending on the stimuli that induced memory formation. Circadian modulation of additional molecules involved in memory formation that are specific to a particular species or type of learning may also occur, i.e., sensorin in Aplysia (Hu et al., 2004) or BDNF in mammals (Yamada and Nabeshima, 2003; Rattiner et al., 2005). Future molecular studies and the examination of circadian modulation of other types of learning will provide insight into additional steps through which circadian signaling connects with memory formation.
In the past decade, tremendous progress has been made in identifying cellular and molecular components of circadian systems. To regulate physiological outputs, core oscillators must convey temporal information to downstream targets. The results presented in this paper significantly contribute to our knowledge of circadian regulation of behavior by demonstrating rhythms at the molecular level that underlie the modulatory effect of the circadian clock on a well characterized long-term learning behavior. This identification of steps representing convergence between the circadian clock and memory formation will facilitate future research investigating the modulation of memory and may provide potential targets to improve memory and alleviate memory disorders.
Footnotes
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS050589. We thank Raymond Fernandez and Oliver Rawashdeh for assistance in implanting electrodes in the animals.
References
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Casadio A, Giustetto M, Karl KA, Zhu H, Kandel ER. Enhancement of memory-related long-term facilitation by ApAF, a novel transcription factor that acts downstream from both CREB1 and CREB2. Cell. 2000;103:595–608. doi: 10.1016/s0092-8674(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH. Transforming growth factor beta1 alters synapsin distribution and modulates synaptic depression in Aplysia. J Neurosci. 2002;22:RC220. doi: 10.1523/JNEUROSCI.22-09-j0004.2002. (1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee JA, Yim SW, Lim CS, Lee CH, Lee YD, Bartsch D, Kandel ER, Kaang BK. Using an Aplysia two-hybrid system to examine the interactions between transcription factors involved in long-term facilitation in the nervous system of Aplysia. Learn Mem. 2003;10:40–43. doi: 10.1101/lm.55303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, Piggins HD. Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. J Neurosci. 2003;23:3085–3093. doi: 10.1523/JNEUROSCI.23-07-03085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, Piggins HD. MAP kinases in the mammalian circadian system—key regulators of clock function. J Neurochem. 2004;90:769–775. doi: 10.1111/j.1471-4159.2004.02554.x. [DOI] [PubMed] [Google Scholar]
- Fernandez RI, Lyons LC, Levenson J, Khabour O, Eskin A. Circadian modulation of long-term sensitization in Aplysia. Proc Natl Acad Sci USA. 2003;100:14415–14420. doi: 10.1073/pnas.2336172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Hattar S. Dissertation, Department of biology and biochemistry. Houston: University of Houston; 2000. Role of the transcription factor ApC/EBP in the ocular rhythm of Aplysia; p. 172. [Google Scholar]
- Hattar S, Lyons LC, Eskin A. Circadian regulation of a transcription factor, ApC/EBP, in the eye of Aplysia californica. J Neurochem. 2002;83:1401–1411. doi: 10.1046/j.1471-4159.2002.01249.x. [DOI] [PubMed] [Google Scholar]
- Hu JY, Glickman L, Wu F, Schacher S. Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron. 2004;43:373–385. doi: 10.1016/j.neuron.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Khabour O, Levenson J, Lyons LC, Kategaya LS, Chin J, Byrne JH, Eskin A. Coregulation of glutamate uptake and long-term sensitization in Aplysia. J Neurosci. 2004;24:8829–8837. doi: 10.1523/JNEUROSCI.2167-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Kim HK, Kim KH, Han JH, Lee YS, Lim CS, Chang DJ, Kubo T, Kaang BK. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Byrne JH, Eskin A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Endo S, Kategaya LS, Fernandez RI, Brabham DG, Chin J, Byrne JH, Eskin A. Long-term regulation of neuronal high-affinity glutamate and glutamine uptake in Aplysia. Proc Natl Acad Sci USA. 2000;97:12858–12863. doi: 10.1073/pnas.220256497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lyons LC, Rawashdeh O, Katzoff A, Susswein AJ, Eskin A. Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proc Natl Acad Sci USA. 2005;102:12589–12594. doi: 10.1073/pnas.0503847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Rawashdeh O, Eskin A. Non-ocular circadian oscillators and photoreceptors modulate long-term memory formation in Aplysia. J Biol Rhythms. 2006;21:245–255. doi: 10.1177/0748730406289890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia. I. Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol. 2004;92:2468–2486. doi: 10.1152/jn.00209.2004. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc Natl Acad Sci USA. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee SH, Lee JA, Lee C, Chang DJ, Lee Y, Ko HG, Bartsch D, Kandel ER, Kaang BK. PKA-activated APAF–APC/EBP heterodimer is a key downstream effector of APCREB and is necessary and sufficient for the consolidation of long-term facilitation. Soc Neurosci Abstr. 2005;31:504.3. [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. The Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Shobe JL, Carew TJ. Molecular nodes in memory processing: insights from Aplysia. Cell Mol Life Sci. 2006;63:963–974. doi: 10.1007/s00018-006-6022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci USA. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Carew TJ. Inclusion of phosphatase inhibitors during Western blotting enhances signal detection with phospho-specific antibodies. Anal Biochem. 2002;307:187–189. doi: 10.1016/s0003-2697(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Carew TJ. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learn Mem. 2004;11:373–378. doi: 10.1101/lm.81104. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci. 2003;23:3899–3907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nat Neurosci. 2001a;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δ colocalizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J Neurosci. 2001b;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Kolker DE, Vitaterna MH, Ferrari E, Takahashi JS, Turek FW. Effect of circadian phase on context and cued fear conditioning in C57BL/6J mice. Anim Learn Behav. 2001;29:133–142. [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms. 2004;19:312–324. doi: 10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A, Sugai R, Chono K, Azami S, Hatakeyama D, Sadamoto H, Itoi E. The early snail acquires the learning. Comparison of scores for conditioned taste aversion between morning and afternoon. Acta Biol Hung. 2004;55:149–155. doi: 10.1556/ABiol.55.2004.1-4.18. [DOI] [PubMed] [Google Scholar]
- Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Zueger M, Urani A, Chourbaji S, Zacher C, Lipp HP, Albrecht U, Spanagel R, Wolfer DP, Gass P. mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J Neural Transm. 2006;113:347–356. doi: 10.1007/s00702-005-0322-4. [DOI] [PubMed] [Google Scholar]