Abstract
Polysialic acid (PSA) regulates functions of the neural cell adhesion molecule (NCAM) during development and in neuroplasticity in the adult; the underlying mechanisms at different phases of learning and memory consolidation are, however, unknown. To investigate the contributions of PSA versus the extracellular domain of the NCAM glycoprotein backbone to synaptic plasticity, we applied NCAM, PSA-NCAM, and PSA to acute slices of the hippocampal CA1 region of NCAM-deficient mice and measured their effects on long-term potentiation (LTP). Remarkably, only PSA and PSA-NCAM, but not NCAM restored normal LTP. Application of these molecules to the dorsal hippocampus of wild-type mice showed that PSA-NCAM and PSA, but not NCAM, injected before fear conditioning, impaired formation of hippocampus-dependent contextual memory. Consolidation of contextual memory was affected by PSA-NCAM only when injected during its late, but not early phases. None of the tested compounds disturbed extrahippocampal-cued memory. Mice lacking the polysialyltransferase (ST8SialV/PST) responsible for attachment of PSA to NCAM in adulthood showed a mild deficit only in hippocampal contextual learning, when compared with NCAM-deficient mice that were disturbed in both contextual and cued memories. Contextual and tone memory in NCAM-deficient mice could be partially restored by injection of PSA-NCAM, but not of NCAM, into the hippocampus, suggesting that the impact of PSA-NCAM in synaptic plasticity and learning is not mediated by modulation of NCAM–NCAM homophilic interactions. In conclusion, our data support the view that polysialylated NCAM is involved in both formation and late consolidation of contextual memory.
Keywords: fear conditioning, LTP, NCAM, polysialic acid, hippocampus, freezing
Introduction
Considerable progress has been made in understanding the numerous functions of the neural cell adhesion molecule (NCAM) and its associated carbohydrate, polysialic acid, in ontogenetic development, synaptic plasticity, and learning and memory in the adult (for review, see Panicker et al., 2003; Kleene and Schachner, 2004). Perturbation of NCAM functions by ablation of NCAM or by disruption of NCAM-mediated interactions in vitro and in vivo impaired synaptic plasticity in the hippocampus, induced amnesia in a passive avoidance task, and caused spatial memory deficits (Doyle et al., 1992; Cremer et al., 1994, 1998; Luthi et al., 1994; Arami et al., 1996; Muller et al., 1996, 2000; Bukalo et al., 2004; Stoenica et al., 2006).
NCAM can carry in its fifth immunoglobulin-like domain long chains of sialic acid residues in an unusual α-2,8 linkage, forming a large negatively charged and hydrated glycan shell, called polysialic acid (PSA). PSA is thought to modulate homophilic and heterophilic binding of the NCAM glycoprotein backbone by steric interference, thus promoting plastic changes (Weinhold et al., 2005 and references therein). Removal of PSA by endosialidase-N, an enzyme which specifically cleaves PSA, disturbs neuronal migration and axonal sprouting, branching, and fasciculation (Yamamoto et al., 2000; Durbec and Cremer, 2001), synaptogenesis (Dityatev et al., 2000, 2004), synaptic plasticity (Becker et al., 1996; Muller et al., 1996) and spatial memory (Becker et al., 1996; Venero et al., 2006).
To evaluate the functional roles of PSA versus the NCAM glycoprotein backbone in vivo, we used Pavlovian fear conditioning that has emerged as a leading behavioral paradigm for studying the mechanisms through which emotional memories are formed and stored (LeDoux, 2000; Maren, 2001; Dityatev and Bolshakov, 2005). This paradigm has been chosen previously to show impairment in cued (amygdala-dependent) and contextual (hippocampus- and amygdala-dependent) fear memories in NCAM-deficient mice (Stork et al., 2000), in mice deficient for polysialyltransferase ST8SiaII/STX, which is important for polysialylation of NCAM during early phases of development (Angata et al., 2004), and in mice that transgenically secrete soluble extracellular NCAM fragments (Pillai-Nair et al., 2005).
In the present study, we addressed the following questions: (1) is PSA itself or PSA attached to NCAM (i.e., PSA-NCAM), required for CA1 LTP and learning; (2) at which stages of LTP, learning and memory consolidation do PSA and/or PSA-NCAM act; and (3) is the action of PSA caused by modulation of NCAM–NCAM homophilic interactions? To investigate the roles of PSA and NCAM, we injected the recombinantly produced extracellular domains of PSA-NCAM and NCAM fused to the Fc portion of human immunoglobulin (PSA-NCAM-Fc and NCAM-Fc, respectively) as well as free PSA into the hippocampi of wild-type and NCAM-deficient mice before and after induction of CA1 LTP or learning in a fear-conditioning paradigm. To control that injections into the hippocampus would not have unspecific effects on hippocampus-independent forms of learning, we used a fear-conditioning paradigm in which an animal was simultaneously subjected to hippocampus-independent learning of tone and hippocampus-dependent learning of context.
Materials and Methods
Mice.
Constitutive NCAM-deficient (NCAM−/−) mice were generously provided by Dr. Harold Cremer (Cremer et al., 1994) and were inbred for at least eight generations onto the C57BL/6J background. ST8SiaIV/PST-deficient (PST−/−) mice and their wild-type controls (PST+/+) were inbred for six generations onto the C57BL/6J background (Eckhardt et al., 2000). For behavioral experiments, 3- to 6-month-old males were used. For recording of LTP in vitro, 6- to 8-week-old mice of both sexes were used. C57BL/6J, NCAM−/− mice and their wild-type littermates (NCAM+/+) were bred in the animal facility of the Universitätsklinikum Hamburg-Eppendorf (Germany), whereas PST−/− mice and their wild-type controls were delivered from the Medizinische Hochschule Hannover (Germany). At least 1 week before the experiments, mice were transferred to a small vivarium where they were housed individually in type II Macrolon cages (25 × 19 × 15 cm) with food and water ad libitum on a reverse 12 h light/dark cycle (lights on at 8:00 P.M.) under constant temperature (22 ± 1°C) and humidity (55 ± 5%). Behavioral experiments were conducted during the dark part of the cycle. All surgical and behavioral procedures were approved by the Committee on Animal Health and Care of the local governmental body.
Slice preparation.
Transverse slices of hippocampi (350 μm thick) were prepared from 6- to 8-week-old NCAM+/+ and NCAM−/− mice as described previously (Eckhardt et al., 2000). Briefly, after halothane anesthesia, decapitation and removal of the brain, the hippocampi were cut with a Leica (Bensheim, Germany) VT 1000M vibratome, in ice-cold artificial CSF (ACSF) containing the following (in mm): 250 sucrose, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, pH 7.3, adjusted with NaOH. All of these reagents were from Sigma-Aldrich (Deisenhofen, Germany). The slices were kept at room temperature in carbogen-bubbled ACSF (composition as above except for 250 mm sucrose that was substituted by 125 mm NaCl) for at least 2 h before the start of recordings.
Recordings of long-term potentiation.
Slices were transferred to a submerged recording chamber and perfused with carbogen-bubbled ACSF at the rate of 2 ml/min. Recordings of field EPSPs (fEPSPs) were simultaneously performed using glass pipettes filled with ACSF and having a resistance of 2 MΩ at two sites in the stratum radiatum of the CA1 region of the hippocampus (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) near the injection pipette and in a control noninjected site, being ∼400 μm apart from the injection pipette (Strekalova et al., 2002). The recombinant proteins and PSA were diluted in ACSF and delivered using a nanoliter injector (World Precision Instruments, Berlin, Germany) at a rate of 2.3 nl every 3 s for 20 min at a concentration of 100 μg/ml. Two independent pathways were stimulated by a tripolar electrode made from three Teflon-insulated platinum wires (World Precision Instruments). Theta-burst stimulation (TBS) was applied to one of these pathways, whereas the second pathway was used to control for the stability of recordings at both recording sites. TBS consisted of four trains of 10 bursts of four stimuli, with 20 s, 200 ms, and 10 ms intervals between trains, burst, and stimuli, respectively (Eckhardt et al., 2000). The paired-pulse test was used to control the independence of the two pathways. The ratio S2/S1 was used as a measure of independence, where S1 and S2 denote slopes of fEPSPs evoked by a single-pulse stimulation of the control pathway and by stimulation of the control pathway which was followed a single-pulse stimulation of the TBS pathway with an interval of 50 ms. Only experiments without slow-developing changes in the control pathway (deviation from initial values <15%) and a ratio of S2/S1 smaller than 115% were taken for analysis. Results are presented as the relative changes produced in the TBS-applied pathway above the control pathway. Two TBSs were applied to measure immediate and later functional consequences of the compound injections: one immediately after the injection and a second one 30 min after the injection. Data acquisition and analysis were performed using AxoClamp 2B (Molecular Devices, Union City, CA), EPC9, and the PULSE software (HEKA, Lambrecht, Germany).
Surgical procedures.
Mice were anesthetized with a ketamine-xylazine solution [ketamine 100 mg/kg (Albrecht, Aulendorf, Germany); xylazine (Rompun) 16 mg/kg (Bayer, Leverkusen, Germany); i.p., 0.01 ml/g body weight] with a dose to provide a surgical level of anesthesia lasting ∼1.5 h without additional reinjections. The skull was shaved and cleaned with 75% ethanol. Mice were placed in a stereotaxic frame (Narishige, Tokyo, Japan) with their eyes protected from drying by an eye gel. The scalp was incised and retracted. The skull was leveled to have lambda and bregma positions in the same horizontal plane. Three small holes (two for cannulas and one for a screw) were drilled using a microsurgery hand bore (Technobox; Bien Air, Bienne, Switzerland). The surface of the cranium bone was carefully cleaned with 75% ethanol and dried with a hot air gun (HG 2000E; Steinel, Herzebrock-Clarholz, Germany) at a temperature not higher than 40°C. Sterile stainless steel 23 gauge double guide cannulas (Plastic One, Roanoke, VA) were stereotaxically implanted bilaterally reaching the border of the dorsal hippocampus with the cortex (anteroposterior, −2 mm, mediolateral, ±1.5 mm from the bregma; dorsoventral, −1.5 mm from the skull surface) according to Paxinos and Franklin (2001). For NCAM −/− mice, the anteroposterior measure was −1.5 mm, because their brains are smaller than those of wild-types because of abnormal development of olfactory bulbs and ventricles (Cremer et al., 1994). Guide cannulas were fixed on the skull bone by dental Kallocryl cement (CPGM gelb; Speiko, Münster, Germany) and a small jeweler's screw (supplemental Fig. 2C,D, available at www.jneurosci.org as supplemental material). After implantation, the incision was closed and the wound was edged by Histoacryl tissue glue (Braun-Aesculap, Tuttlingen, Germany). The body temperature of mice was maintained at 37°C with a regulated homeothermic blanket control unit (HSE-Harvard, March-Hugstetten, Germany) throughout the surgery. After the operation, mice were placed back to their home cages and kept in a warm (∼37°C) room until awakening. Metamizol (Novaminsulfon; Ratiopharm, Ulm, Germany; 50 mg/kg body weight, i.p.) was used as a postoperative analgetic. All subsequent behavioral investigations were performed after mice had fully recovered during 5–7 d after surgery. Special care was taken to minimize both the suffering and the number of animals used in this study.
Conditions for behavioral tests.
All animals were handled and habituated to the experimental conditions in the unpaired context (CC−) of a tone cage for 5 min during two consecutive days before training (supplemental Fig. 2A,B,E, available at www.jneurosci.org as supplemental material). The unpaired context for a cued-memory test was represented by a transparent Plexiglas cage (185 × 150 × 210 mm) cleaned with 1% acetic acid and equipped with two loudspeakers (M10, 8 Ω; Visaton, Haan, Germany) in opposite sites of the box, a dark (3 lux) experimental room (a white noise of 40 dB) lighted with a red lamp (0.2 lux). Conditioning of mice was performed in a gray-brown aluminum conditioning chamber (ENV-307A; MED Associates, St. Albans, VT) that was tooled with a shock floor consisting of 3.5 mm stainless-steel rods spaced 7 mm apart and one loudspeaker on the back wall. The conditioned context (CC+) was represented by the above described conditioning chamber, which was cleaned with 75% ethanol before training of each animal, the day-lighted experimental room (80 lux), and the isolation cubicle (40 lux), and a noise (52 dB) produced by a fan. All experiments were performed in a double-walled sound-attenuated isolation cubicle. The conditioned stimulus (CS+) was a patterned tone (i.e., series of 20 brief tones; 50 ms, 7.5 kHz, 75 dB) presented every second. The neutral stimulus (CS−) that was not paired with a footshock was a 7.5 kHz continuous pure tone (75 dB, 60 s). Mice show a good discrimination between patterned and continuous tones in a fear-conditioning paradigm (Tang et al., 2003). A self-customized acoustic stimulator (Tang et al., 2001, 2003) produced both stimuli. The unconditioned stimulus (US) was a 1 s, 0.7 mA (alternating current) scrambled middle intensity shock delivered to the rod floor. Delivery of tones and shocks was controlled by customized software (Signal 1.88; CED, Cambridge, UK) using a PC computer (Pentium-II, 340 MHz, 128 megabytes of random access memory; CompuMent, Hamburg, Germany) through an analog-to-digital/digital-to-analog converter (Micro 1401 board; CED).
Fear-conditioning paradigm.
The procedure of fear conditioning was as follows (supplemental Fig. 2B, available at www.jneurosci.org as supplemental material): (1) a naive mouse was placed into the conditioning chamber for 100 s to record its baseline behavior before delivering the footshock, abbreviated as a trial “B” (baseline); (2) then, the animal received two CS–US pairings spaced apart by 60 s. For CS–US pairing, a 20 s patterned tone was coterminated with a 1 s footshock; the 60 s time period between the shocks was taken to evaluate freezing as a measure of the shock sensitivity of all mice. This period is labeled as interval “S” (sensitivity to a shock); (3) 30 s after the last CS–US pairing, the mouse was returned to its home cage.
Freezing behavior.
The behavior of animals was videotaped for each session and analyzed off-line by a trained observer blind to the identity of mice and to the applied tones (conditioned or neutral) for each of the 60 s uniform tone episodes (cued freezing). Freezing time, which served as a measure of fear-related memory, was quantified as described previously (Tang et al., 2001). Briefly, freezing was assigned if the animals remained motionless, except for respiratory movements, in a tense posture that was characterized by horizontal positioning of the mouse head, a stretched state of the body, and stiffening of the tail.
Injections.
To evaluate the effects of the infused compounds at the chosen two phases of learning (acquisition of task and consolidation of memory), mice were divided into three groups, with injections made 4 h before fear conditioning and 2 or 6 h after training. Compounds were injected into the hippocampus during very short CO2 anesthesia (∼2 min), with the same injection volume (0.4 μl per side) and infusion rate (0.4 μl/min) for all animals. The bilateral injection cannula (C235; Plastic One) was connected to a pump (TSE Systems, Bad Homburg, Germany) through a Tygon tubing system, with 2/3 of the tube length filled with sterile sesame oil (Sigma-Aldrich, Deisenhofen, Germany) as a pressure conductor, and 1/3 with the injected compound. The injection cannula was gently inserted into the bilateral guide cannula ∼10 s before injection, extending the tip of the guide cannula by 1 mm. The site of the injection was aimed to the hilus of both hippocampi. The injection cannula was left within the guiding cannula for at least 30 s before removing. Then, the guide cannula was closed by a dummy cannula and a screw cap. All animals recovered from this procedure within 2–3 min after transfer to their home cage.
Injected compounds.
Recombinantly produced proteins NCAM-Fc and PSA-NCAM-Fc, containing the extracellular portion of NCAM fused with a human Fc fragment of IgGs, were used for injections. Murine PSA-NCAM-Fc was produced as described previously (Vutskits et al., 2001) using a stably transfected TE671 cell line kindly provided by Genevieve Rougon (Institute of Developmental Biology of Marseille, Université de la Mediterranee, Marseille, France). Mouse NCAM-Fc was produced using stably transfected CHO cells as described previously (Chen et al., 1999) and tested for stimulation of neurite outgrowth in vitro. Polysialylation of PSA-NCAM-Fc and absence of PSA on NCAM-Fc were checked by Western blotting (Vaithianathan et al., 2004) using a monoclonal antibody to PSA (clone 735) (Frosch et al., 1985). As a control for NCAM-Fc and PSA-NCAM-Fc, we used the human Fc-fragment (Dianova, Hamburg, Germany). Concentration of all injected proteins was 0.5 mg/ml, corresponding approximately to 15 μm for Fc and 2 μm for NCAM-Fc and PSA-NCAM-Fc, which is similar to the effective concentration of NCAM antibodies and NCAM ligand peptide C3d in disruption of memory formation in different behavioral paradigms (Roullet et al., 1997; Foley et al., 2000; Cambon et al., 2003). PSA was administered as the bacterially produced PSA, known as colominic acid (#27698; Sigma-Aldrich). Another negatively charged carbohydrate, chondroitin sulfate C (C-4384; Sigma-Aldrich), served as a control in the behavioral experiments. Both carbohydrates were applied at a concentration of 1 mg/ml, corresponding approximately to 50 μm. All compounds were freshly prepared in a sterile ACSF (for composition, see recordings of LTP in acute hippocampal slices). Implantations, injections, and behavioral tests were performed in groups of 15–20 mice. Control mice receiving compounds such as chondroitin sulfate or Fc were always tested in parallel to mice receiving PSA and Fc-fused proteins, respectively.
Statistics.
All data are expressed as mean ± SEM. To analyze the effects of the injected compounds on contextual memory we used two-way ANOVA having treatment as the between-groups factor and trial as the within-groups factor, followed by the post hoc Fisher's least standard mean (FLSD) test if significant effects of factors were detected (for detailed statistical analysis, see supplemental Table 1, available at www.jneurosci.org as supplemental material). To analyze the effects of the injected compounds on cued memory we used three-way ANOVA for treatment and repeated measurements trial and tone. Additionally, statistical comparison between freezing responses to CS+ and CS− on a given day within each group was performed using the nonparametric Wilcoxon signed-rank test. Significant differences between groups were detected by the Wilcoxon test and the paired t test for the same groups and, therefore, we refer in the text only to p values provided by the Wilcoxon test. The levels of LTP recorded at two sites within the same slice were compared with the use of paired t test. Statistical significance of differences was accepted if p < 0.05.
Auditory and pain sensitivity.
Because acquisition of cued (tone) memory depends on how well mice process sound as CS, we inspected the auditory abilities of mice. We observed that after the first series of tones has been paired with a footshock, all mice exhibited a startle response to the second application of the tones, a phenomenon known as sensitization (Kamprath and Wotjak, 2004). We took this parameter as a confirmation of normal auditory sensitivity in all mice tested (data not shown).
Because the freezing response is a function of the footshock intensity, it was necessary to control whether all compared groups of mice received footshocks of equal intensity. We thus measured the level of freezing that mice exhibited immediately after the first footshock during training (supplemental Fig. 3A, S period, available at www.jneurosci.org as supplemental material). This parameter reflects both pain sensitivity and reaction to it. All C57BL/6J mice injected before fear conditioning (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material) showed similar freezing during the S period at the level of 46.5 ± 2.4%. The one-way ANOVA evaluation did not detect any statistically significant difference among C57BL/6J mice injected with Fc, NCAM-Fc, PSA-NCAM-Fc, ACSF, chondroitin sulfate, and PSA (treatment: F(5,65) = 0.73, p = 0.6). The C57BL/6J mice that were injected after fear conditioning (regardless of the post-training time) and the unoperated C57BL/6J mice also exhibited comparable reactions to the first footshock. The levels of freezing were 28.62 ± 2.8 and 27.9 ± 2.9% when injections were performed 2 and 6 h after conditioning, respectively, and in unoperated mice freezing time was 20.8 ± 6.3%. By ANOVA, no statistically significant difference was detected among C57BL/6J mice injected with Fc, NCAM-Fc, PSA-NCAM-Fc, chondroitin sulfate, PSA, and unoperated C57BL/6J mice (treatment: F(5,37) = 0.56, p = 0.72; F(5,39) = 0.67, p = 0.64, for 2 and 6 h, respectively). It is noteworthy that freezing in C57BL/6J mice injected after fear conditioning or unoperated mice was significantly lower than in mice injected before training. This difference probably reflects that the procedure of injection is unpleasant and increases susceptibility to additional anxious stimuli such as the footshock. This difference, however, is not of importance because we did not compare freezing in mice injected before versus after memory acquisition, but rather, evaluated effects of different injections performed at one time point. PST−/− mice had a reaction to the footshock similar to PST+/+ mice (genotype: F(1,14) = 0.73, p = 0.4). PST+/+ mice on a predominantly (six backcrossings) C57BL/6J genetic background had similar sensitivity as C57BL/6J injected before fear conditioning, showing that the sensitivity in the latter group is not unusually high. Also NCAM−/− mice did not differ from NCAM+/+ mice in their reaction to the first footshock (genotype: F(1,48) = 1.11; p = 0.29). This analysis confirms that mice from all compared experimental groups equally perceived the footshock.
Furthermore, the behavior of all tested groups of mice in the CC+ context before they received a shock (Figs. 2B, 3B, 4B, 5B, 6B, column B) was not different from controls: mice showed no signs of anxiety or hyperactivity, and the episodes of freezing were not longer than 1–2 s and few in number, covering just 2–3% of the recorded times, which is normal for mice in novel contexts (Tang et al., 2003).
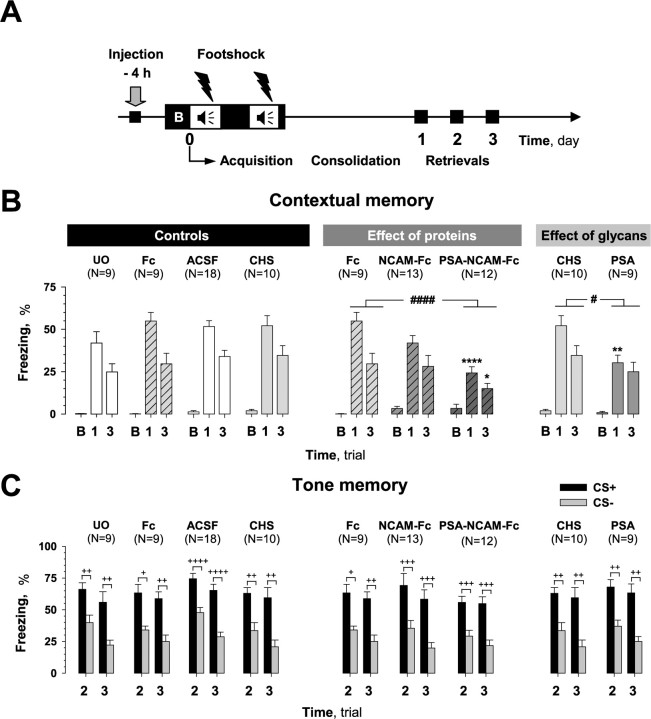
Figure 2.
Effects of pretraining injection of compounds on fear conditioning. A, Scheme showing manipulations performed in this series of experiments. Compounds were injected 4 h before training. A 100 s trial (B) was used to evaluate the baseline response to the conditioned context (CC+) before training. B, Pretraining injection of PSA-NCAM-Fc or PSA, but not NCAM-Fc, impaired contextual memory when tested on the first (both compounds) and third (PSA-NCAM-Fc only) days after training (trials 1 and 3, respectively). Mice injected with the Fc-fragment (Fc) and chondroitin sulfate (CHS) served as controls. Fc- and chondroitin sulfate-injected mice did not differ in contextual memory from unoperated (UO) and ACSF-injected mice, respectively. Fear memory was measured as the percentage of time that mice spent in the freezing state. Analysis of freezing during baseline trial B showed no difference between injected groups. C, Pretraining injection of tested compounds did not affect freezing time in response to paired (CS+) and unpaired (CS−) tones during trials 2 and 3. Discrimination between CS+ and CS− tones was also not different between injected groups. #p ≤ 0.05, ####p ≤ 0.001 (the effect of treatment by two-way ANOVA); *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.001 (post hoc FLSD test, as compared with corresponding controls, Fc or chondroitin sulfate, for corresponding days); +p < 0.05, ++p < 0.01, +++p < 0.005, ++++p < 0.001 (Wilcoxon test).
Histology.
To verify the injection sites and distribution of the injected Fc-fused recombinant proteins on a per animal basis, brains were investigated histologically on the fourth day (after behavioral tests). For evaluation of the distribution patterns of Fc-injected compounds in the living brain (supplemental Fig. 4, available at www.jneurosci.org as supplemental material), as well as for verification of exact place of injection, mice were reinjected with a given compound using the same injection procedure as on day 0. Reinjection could be used because, on the fourth day after the first injection, initially injected compound was no longer detectable. Four hours (that is the time interval between the first injection and fear conditioning) after reinjection, mice were deeply anesthetized with pentobarbital (100 mg/kg) and transcardially perfused with 4% paraformaldehyde in PBS, pH 7.4, during 20 min and brains were postfixed overnight in the same fixative at 4°C. Brains were then washed in PBS and embedded in 5% agar. Several mice were perfused 24 or 48 h after reinjection to assess the availability and distribution of injected compounds at these time points, corresponding to the first and second test days after the first injection. Coronal sections (50 μm thick, taken every 100 μm) were cut using a vibratome VT 1000S (Leica). Freely floating slices were treated for 1 h with blocking solution containing 1% BSA in PBS, followed by detection of the Fc portion by anti-human IgG Cy3-coupled antibody (Dianova) diluted 1:200 in 0.1% BSA/PBS solution and incubated for 60 min at room temperature. Sections were then washed three times for 10 min each in PBS and finally embedded in Aqua-Poly/Mount (Polyscience, Eppelheim, Germany) for visualization using Axiophot 2 (Zeiss, Jena, Germany).
Injections of NCAM-Fc, PSA-NCAM-Fc, or Fc-fragment alone resulted in prominent Fc immunoreactivity signal in the hilus and the molecular layer of the dentate gyrus when tested 4 h after injection. Diffusion of the injected proteins at this time point was quite extensive and the immunohistochemical signal was detectable across most of the dorsal hippocampus, covering also the CA1 and CA3 regions, although less prominently than the dentate gyrus. The immunohistochemical signal was limited to the hippocampus and was detectable at only very low levels in adjacent cortical and thalamic areas (supplemental Fig. 4C, asterisks, available at www.jneurosci.org as supplemental material). In ACSF-injected mice, no signal was detectable anywhere (data not shown), confirming the specificity of the immunofluorescence reaction. The Fc-fragment was detected in a larger area than NCAM-Fc and PSA-NCAM-Fc. However, the Fc-fragment was also never observed outside of the hippocampus. Immunoreactivity for the Fc-fragment was detectable in the dorsal hippocampus at 24 but not at 48 h after injection (data not shown).
To evaluate the positions of injection cannulas in the hippocampi injected with ACSF, PSA, and chondroitin sulfate, mice were reinjected on the fourth test day with methylene blue (0.4 μl per side with an infusion rate of 0.4 μl/min) and were killed by an overdose of CO2 20 min later. Brains were rapidly removed and frozen in the cryoprotective embedding medium Tissue-Tek (Sakura Finetek, Leiden, The Netherlands) in liquid nitrogen. Frozen 20 μm frontal sections were cut with a cryostat CM3050 (Leica), dried, and assessed at the light microscopic level to verify the positions of injection cannulas.
Only animals that had received bilateral injections into dorsal hippocampi were included in the analysis. Among the total of 229 animals, which were taken for the behavioral experiments, 216 were included in the analysis presented in this study, although 13 mice had to be excluded because of misplacement of cannulas or damage of guide cannulas after implantations during animal housing.
Results
Dissection of functional components of PSA-NCAM during LTP
Previous studies had shown an impairment of LTP in the CA1 region of the hippocampus after enzymatic removal of PSA and in PST−/− mice (Muller et al., 1996; Eckhardt et al., 2000), suggesting that polysialylated NCAM is required for CA1 LTP. Here, we aimed to verify whether the extracellular domain of PSA-NCAM or PSA alone is responsible for normal induction of LTP in CA1. To address this question, we applied these molecules in hippocampal slices derived from NCAM−/− mice.
In agreement with previous data (Muller et al., 1996) we found that LTP is reduced in NCAM−/− mice (for comparison of the two genotypes, see Figs. 1C,D, control sites). The mean level of LTP recorded in NCAM−/− mice 20–30 min after the first TBS was 117.9 ± 4.0%, whereas it was 132.7 ± 3.6% in NCAM+/+ controls. Mean levels of LTP recorded in NCAM−/− mice 20–30 min after the second TBS were also significantly lower than in slices from NCAM+/+ mice (122.6 ± 3.8 vs 139.2 ± 2.2%).
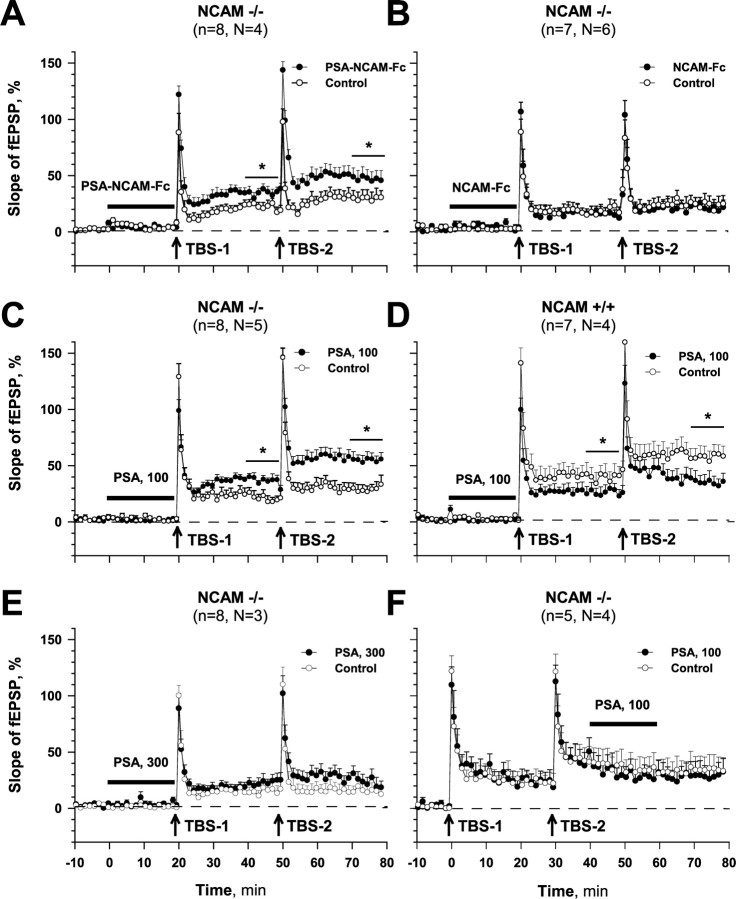
Figure 1.
In vitro LTP recording from the CA1 hippocampal region of NCAM deficient (NCAM−/−) and wild-type (NCAM+/+) mice. A, Injection of PSA-NCAM-Fc (100 μg/ml) into slice from NCAM−/− mice before first theta-bust stimulation (TBS-1) significantly increased LTP at the recording site adjacent to the injection site as compared with LTP recorded at the control site in the same slice. LTP induced by a second theta-bust stimulation (TBS-2) after PSA-NCAM-Fc injection was also higher at the injected than at the control site. B, Injection of NCAM-Fc (100 μg/ml) failed to rescue LTP induced by either TBS-1 or TBS-2 in NCAM−/− mice. C, Injection of PSA (100 μg/ml) restored LTP in NCAM−/− mice. D, Injection of PSA (100 μg/ml) impaired LTP in NCAM+/+ mice. E, PSA injected at a concentration of 300 μg/ml did not rescue LTP in NCAM−/− mice. F, Injection of PSA (100 μg/ml) starting 10 min after TBS-2 rather than 20 min before TBS-1 as in A–E did not affect LTP in NCAM−/− mice. All data are represented as mean slope + SEM of fEPSPs normalized to the baseline values. *p ≤ 0.05 (paired t test). Time intervals of injection are indicated by horizontal bars.
The deficit in LTP in NCAM−/− mice could be rescued by application of recombinant PSA-NCAM-Fc (100 μg/ml) (Fig. 1A), pointing to the possibility that the polysialylated extracellular domain of NCAM is responsible for induction of LTP. Injection of NCAM-Fc without PSA did not significantly increase LTP in NCAM−/− mice (Fig. 1B). Application of PSA (100 μg/ml) to slices of NCAM+/+ mice decreased levels of LTP measured 30 min after the first and second TBS (Fig. 1D), in agreement with data of Muller et al. (2000). Application of the same concentration of PSA to slices of NCAM−/− hippocampi significantly increased LTP as compared with the control site and reached levels comparable with those measured in NCAM+/+ hippocampi (Fig. 1C,D); mean levels of LTP recorded at the injected site after the first and second TBS were 134.0 ± 4.8 and 148.2 ± 7.2%, respectively. However, at a higher concentration (300 μg/ml), PSA did not normalize the levels of LTP in NCAM mutants; after the second TBS, LTP from both recording sites was only 117.4 ± 6.8 and 113.6 ± 7.6% (Fig. 1E), respectively. To test the effect of PSA on consolidation of LTP, we applied PSA after the second TBS. There was no LTP-promoting effect at the injection site, whereas the level of LTP was 126.4 ± 9.0 versus 126.2 ± 13.0% at the control site (Fig. 1F).
These results, together with previously published data demonstrate that (1) PSA is the functional entity of the PSA-NCAM that is necessary and sufficient for modulating the induction of LTP, and (2) there is an optimal PSA concentration that supports induction of LTP: absence or high concentrations of PSA are prohibitive for induction of LTP.
PSA and NCAM in acquisition of fear conditioning
Next, we tested how PSA-NCAM, nonpolysialylated NCAM, and PSA would affect learning in a fear-conditioning paradigm. As a first step, we addressed the question whether injection of control compounds (Fc-fragment, chondroitin sulfate, and vehicle ACSF) before training (−4 h) (Fig. 2A) would have an effect on acquisition of contextual memory and its subsequent retention as compared with unoperated C57BL/6J mice. As shown in Figure 2B, the level of freezing in the conditioned context CC+ was 50.5 ± 2.5% of the recorded times on the first day and 31.5 ± 2.4% on the third test day in control groups of mice that were unoperated or injected with ACSF, chondroitin sulfate, and Fc. There was no statistically significant difference in freezing between unoperated and ACSF, chondroitin sulfate, and Fc injected mice (treatment: F(3,42) = 1.01, p = 0.39; treatment × trial: F(3,42) = 0.54, p = 0.65). These results verify our injection procedure and the use of Fc-fragment and chondroitin sulfate as controls.
Injection of C57BL/6J mice with PSA-NCAM-Fc resulted in a strong impairment in contextual learning on both the first and third test days after training as compared with the control Fc-injected group (treatment: F(1,19) = 18.49, p = 0.001; FLSD: first day, p = 0.001; third day, p = 0.035). In contrast to PSA-NCAM-Fc, injection of NCAM-Fc affected contextual memory retrieval neither on the first nor third test days (treatment: F(1,20) = 1.0, p = 0.32). Acquisition of the contextual task was also disrupted in C57BL/6J mice injected with PSA (Fig. 2B) as compared with chondroitin sulfate (treatment: F(1,17) = 4.74, p = 0.04; FLSD: first day, p = 0.01; third day, p = 0.25) and ACSF (treatment: F(1,25) = 7.42, p = 0.01; FLSD: first day, p = 0.001; third day, p = 0.17).
Because contextual learning depends on both the hippocampus and amygdala our next question was whether the compounds injected into the dorsal hippocampi acted exclusively there or also affected other brain regions (e.g., the amygdala). To address this issue, we took advantage of the fact that mice were simultaneously exposed to both contextual and auditory stimuli during conditioning, and analyzed hippocampus-independent amygdala-dependent cued (tone) memory of conditioned mice. We found that intrahippocampal injection of either PSA-NCAM-Fc, NCAM-Fc, or PSA before acquisition of fear conditioning (−4 h) (Fig. 2C) did not affect the ability of mice to learn a cued task; three-way ANOVA did not reveal any differences between unoperated mice and three groups of mice that received control injections of ACSF, chondroitin sulfate, and Fc (treatment: F(3,42) = 1.26, p = 0.29), mice injected with recombinant proteins (treatment: F(2,31) = 0.36, p = 0.7), and mice injected with glycans (treatment: F(1,17) = 0.29, p = 0.59). Injection of PSA-NCAM-Fc, NCAM-Fc, or PSA also did not affect the ability of mice to differentiate the conditioned tone CS+ from unconditioned CS− on the second and third test days after training; three-way ANOVA did not reveal any significant interaction between treatment and tone among groups injected with recombinant proteins (tone × treatment: F(2,31) = 0.63, p = 0.53) or glycans (tone × treatment: F(1,17) = 0.01, p = 0.9). All mice showed significant discrimination of tones (at least p < 0.05, Wilcoxon test). Thus, hippocampus-targeted injections did not interfere with the amygdala-dependent form of learning in our experiments, and mice injected with PSA-NCAM-Fc and PSA had normal cued memory, although they showed robust impairment in contextual memory.
PSA and NCAM in consolidation of fear memories
Because pretraining injections of PSA-NCAM-Fc and PSA could affect either acquisition or consolidation of fear memories, we decided to differentiate between the two phases of learning by injection of compounds during the consolidation phase of learning. Injections were performed either 2 h after training (Fig. 3A), a time point used previously to study the early phase of consolidation (Doyle et al., 1992; Scholey et al., 1995), or 6 h after training (Fig. 4A), a time-window considered previously to interfere with NCAM functions (Cambon et al., 2003).
Figure 3.
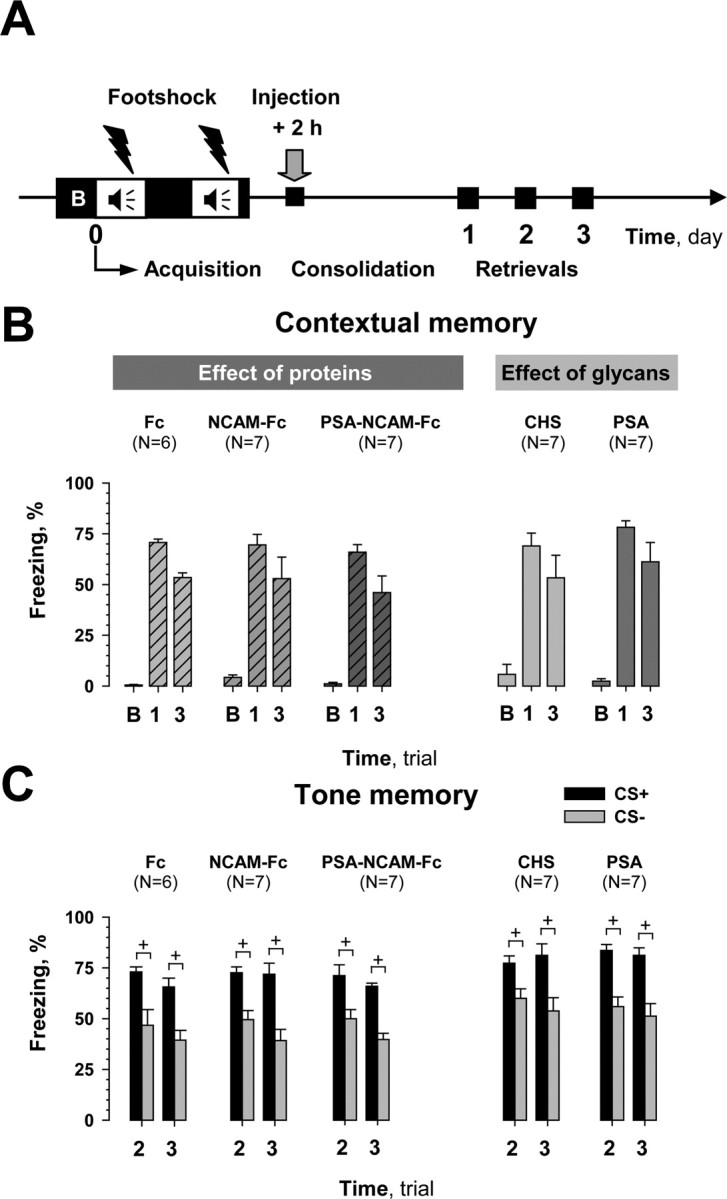
Effects of post-training injection of compounds during early consolidation phase of fear conditioning. A, Scheme showing manipulations performed in this series of experiments. A 100 s trial (B) was used to evaluate the baseline response to the conditioned context (CC+) before training. Compounds were injected 2 h after initiation of training. B, None of the mice showed any impairment in contextual memory when tested on the first and third days after training (trials 1 and 3, respectively) and during trial B. Mice injected with the Fc-fragment (Fc) and chondroitin sulfate (CHS) served as controls. C, Post-training injection of tested compounds did not affect cued memory during trials 2 and 3. Discrimination of paired (CS+) versus unpaired (CS−) tones also did not differ between groups. +p < 0.05 (Wilcoxon test).
Figure 4.
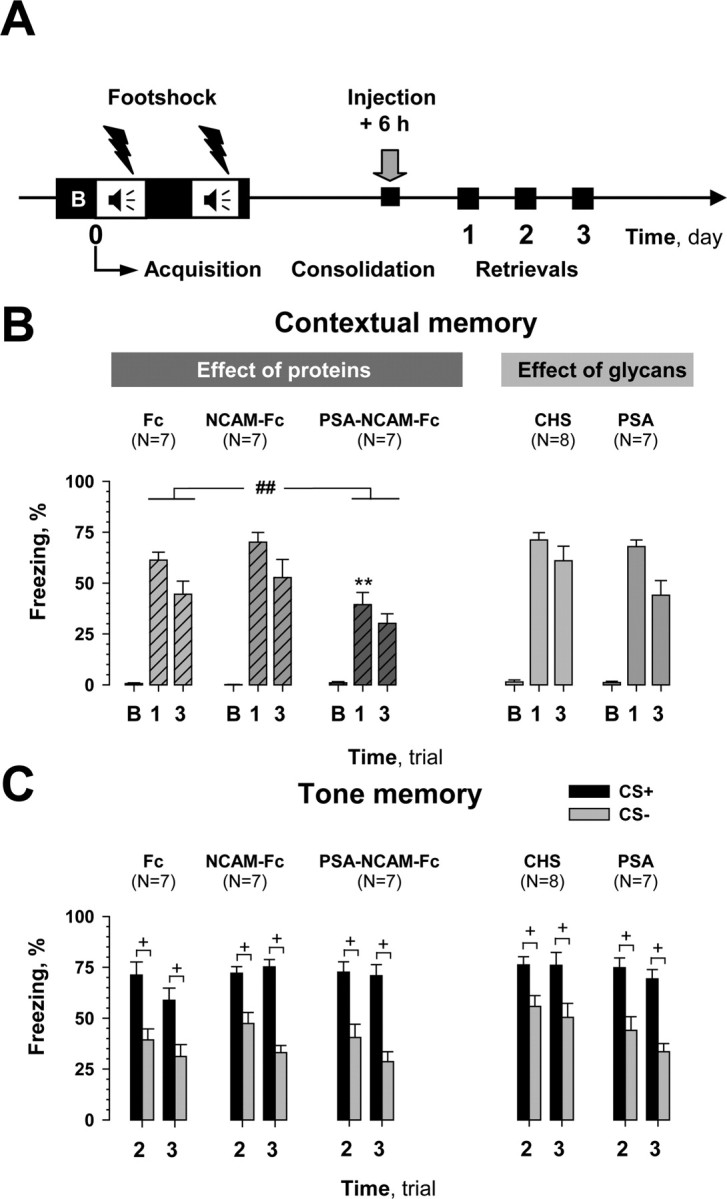
Effects of post-training injections of compounds during late consolidation phase of fear conditioning. A, Scheme showing manipulations performed in this series of experiments. A 100 s trial (B) was used to evaluate the baseline response to the conditioned context (CC+) before training. Compounds were injected 6 h after initiation of training. B, Among injected compounds, only PSA-NCAM-Fc impaired contextual memory tested in CC+ on the first and third days after training (trials 1 and 3, respectively). Mice injected with the Fc-fragment (Fc) and chondroitin sulfate (CHS) served as controls. There was no difference between injected groups during trial B. C, Post-training injection of compounds did not affect cued memory and discrimination between paired (CS+) versus unpaired (CS−) tones. ##p ≤ 0.01 (ANOVA); **p ≤ 0.01 (post hoc FLSD test as compared with Fc for day 1); +p < 0.05 (Wilcoxon test).
As shown in Figure 3B, PSA-NCAM-Fc had no effect when injected 2 h after training (treatment: F(1,11) = 0.93, p = 0.35), but significantly impaired contextual memory when administered 6 h after training (Fig. 4B) (treatment: F(1,12) = 11.19, p = 0.006; FLSD: first day, p = 0.010; third day, p = 0.09). Post-training NCAM-Fc injections, similar to the pretraining ones, had no effect on contextual memory when performed either 2 or 6 h after training (treatment: F(1,11) = 0.011, p = 0.91; treatment: F(1,12) = 1.19, p = 0.29, respectively). Although pretraining injection of PSA impaired contextual memory, it was as ineffective as chondroitin sulfate when injected either 6 h (treatment: F(1,13) = 2.04, p = 0.17) or 2 h (treatment: F(1,12) = 0.68, p = 0.42) after fear conditioning. Similar to the pretraining injection experiments, PSA-NCAM-Fc, NCAM-Fc, and PSA altered cued memory neither at 2 h (Fig. 3C) nor at 6 h after training (Fig. 4C); three-way ANOVA did not reveal any differences between groups injected with recombinant proteins (treatment: F(2,17) = 0.11, p = 0.89; treatment: F(2,18) = 0.71, p = 0.5, respectively to the time) or glycans (treatment: F(1,12) = 0.001, p = 0.98; treatment: F(1,13) = 1.8, p = 0.2, respectively to the time). Discrimination of CS+ and CS− tones in all mice that were injected after training was also normal (at least p < 0.05, Wilcoxon test). In summary, only post-training injection of PSA-NCAM-Fc at 6 h, but not 2 h reduced contextual memory, whereas PSA and NCAM-Fc were ineffective.
Impaired fear conditioning in PST-deficient mice
To obtain genetic evidence for the role of PSA in fear conditioning, we tested PST−/− mice that lack the polysialyltransferase important for polysialylation of NCAM in adult mice. PST−/− mice showed impaired contextual memory on all test days (Fig. 5A), as compared with their wild-type PST+/+ controls (genotype: F(1,14) = 11.02, p = 0.005; FLSD: first day, p = 0.004; third day, p = 0.045). However, cued memory in PST−/− mice was normal (genotype: F(1,14) = 0.02, p = 0.89) as well as discrimination between CS+ versus CS− (at least p < 0.05, Wilcoxon test) (Fig. 5B).
Figure 5.
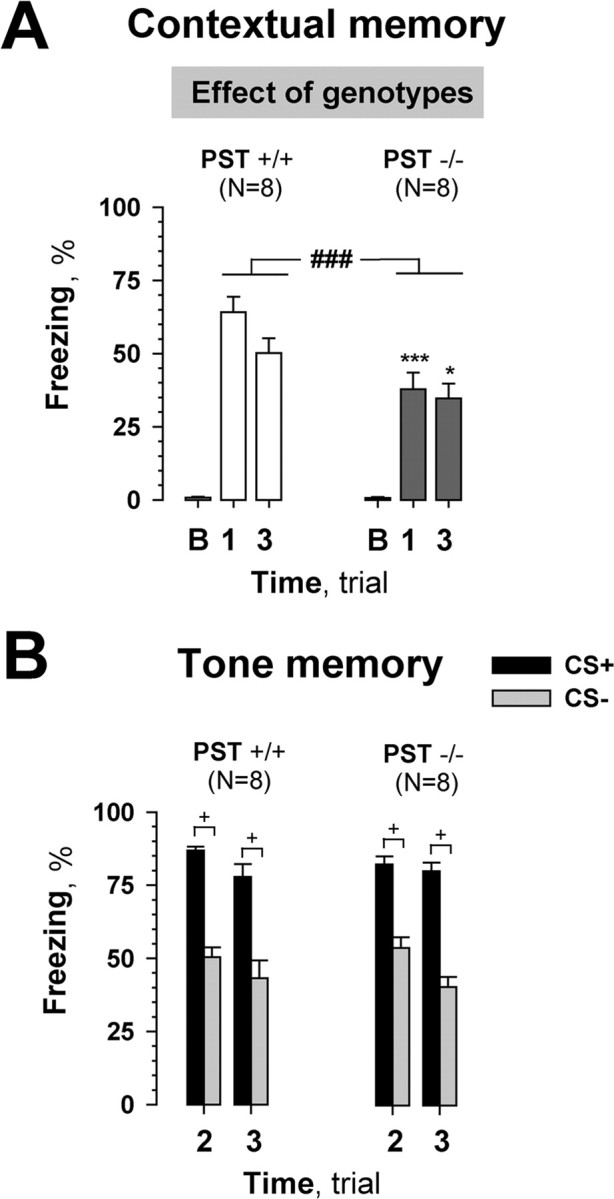
Fear conditioning of PST-deficient mice (PST−/−) and corresponding wild-type controls (PST+/+). A, PST−/− mice showed significantly less freezing in the conditioned context (CC+) compared with PST+/+ mice. A 100 s trial (B) was used to evaluate the baseline response to the conditioned context (CC+) before training. There was no difference between groups during trial B. B, Cued memory was normal on the second and third days after fear conditioning (trials 2 and 3) in PST−/− mice. Discrimination of paired (CS+) versus unpaired (CS−) tones also did not differ between genotypes. ###p ≤ 0.005 (ANOVA); *p ≤ 0.05, ***p ≤ 0.005 (post hoc FLSD test; as compared with PST+/+ for corresponding days); +p < 0.05 (Wilcoxon test).
Impaired fear conditioning in NCAM-deficient mice and its rescue by injection of PSA-NCAM-Fc
A previous study reported that NCAM−/− mice are impaired in both contextual and cued fear memories (Stork et al., 2000). In agreement, we found a difference in the freezing response between conditioned NCAM−/− and NCAM+/+ mice injected with the control Fc-fragment 4 h before training (Fig. 6B). We used Fc-injected rather than unoperated animals as had been done before (Stork et al., 2000) because Fc was found not to affect fear memory and we could use Fc-injected NCAM−/− mice as controls for injection of other recombinant proteins. A clear deficit in contextual memory in NCAM−/− mice was seen on the first and third post-training days (Fig. 6B) compared with Fc-injected NCAM+/+ mice (genotype: F(1,13) = 19.15, p = 0.001; FLSD: first day, p = 0.002; third day, p = 0.0001). NCAM−/− mice were also deficient in cued memory (Fig. 6C) (genotype: F(1,13) = 13.49, p = 0.003), but discriminated between CS+ and CS− on both test days (Wilcoxon test, p < 0.05).
Figure 6.
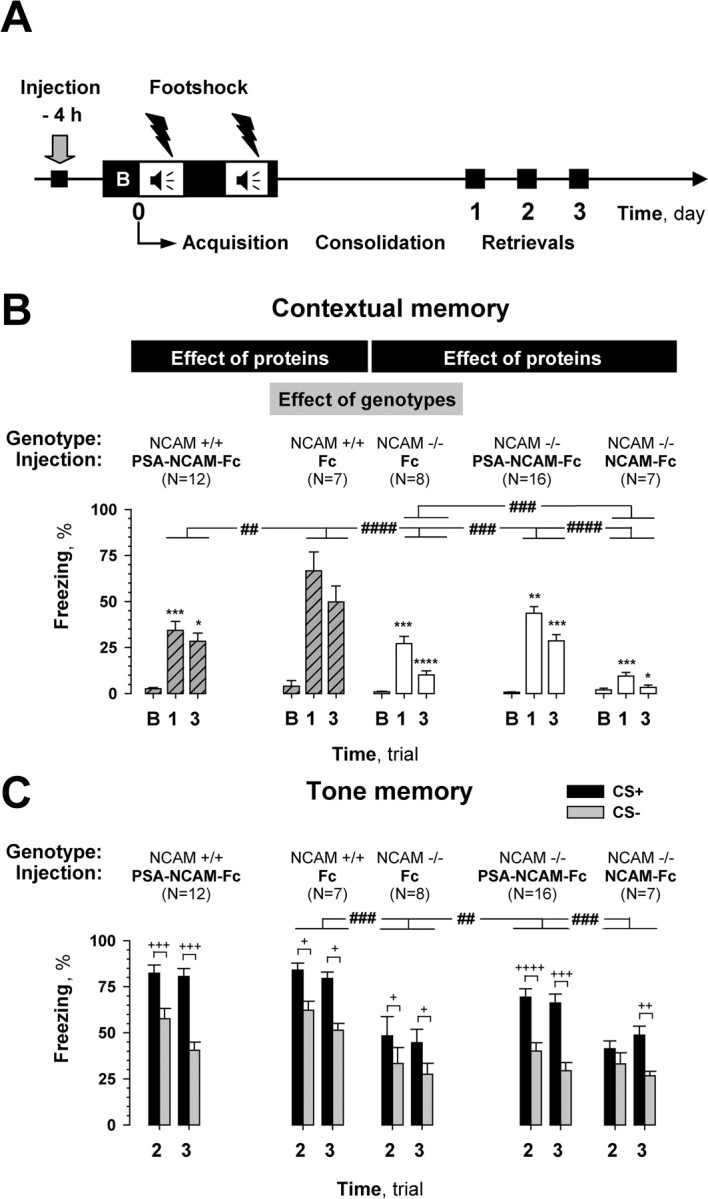
Effects of pretraining injection of compounds on fear conditioning in NCAM deficient (NCAM−/−) mice. A, Scheme showing manipulations performed in this series of experiments. Compounds were injected 4 h before training. A 100 s trial (B) was used to evaluate the baseline response to the conditioned context (CC+) before training. B, NCAM−/− mice showed robust impairment of contextual memory tested on the first and third days after training (trials 1 and 3, respectively) compared with their wild-type littermates, NCAM+/+. The deficit in contextual memory in NCAM−/− mice was partially rescued by pretraining injection of PSA-NCAM-Fc, but not by NCAM-Fc, as compared with Fc. Injections of PSA-NCAM-Fc into NCAM+/+ mice impaired contextual memory. All mice showed similar freezing responses during the baseline period (trial B). C, NCAM−/− mice were impaired in cued memory compared with NCAM+/+ mice. This deficit in NCAM−/− mice could be rescued by the pretraining injection of PSA-NCAM-Fc but not NCAM-Fc. Other groups showed no effect of treatment in cued memory processing as well as in discrimination of CS+ and CS− tones, as compared with Fc injected mice. #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.005, ####p ≤ 0.001 (ANOVA); *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005, ****p ≤ 0.001 (post hoc FLSD for comparisons indicated for ANOVA, for corresponding days); +p < 0.05, ++p < 0.01, +++p < 0.005, ++++p < 0.001 (Wilcoxon test).
Next, we asked whether PSA-NCAM-Fc could rescue the deficit in contextual memory in NCAM−/− mice. Indeed, pretraining injection of PSA-NCAM-Fc into NCAM−/− mice (Fig. 6B) resulted in a significant improvement of contextual learning as compared with Fc-injected NCAM−/− mice (treatment: F(1,22) = 11.49, p = 0.003; FLSD: first day, p = 0.010; third day, p = 0.002). This rescue, however, was not complete because there remained a significant difference between freezing responses for both test days of these mice and Fc-injected NCAM+/+ mice (treatment: F(1,21) = 8.029, p = 0.010; FLSD: first day, p = 0.014; third day, p = 0.012). Intrahippocampal injection of PSA-NCAM-Fc into NCAM−/− mice also improved cued learning as compared with Fc injected NCAM−/− mice (treatment × tone: F(1,22) = 8.48; p = 0.008). Furthermore, injection of PSA-NCAM-Fc into NCAM−/− mice highly improved their discrimination of CS+ versus CS− tones, as compared with Fc injected NCAM−/− mice (p < 0.001 for the second day; p = 0.001 for the third day, Wilcoxon test).
To analyze whether PSA-NCAM rescued normal learning and memory in NCAM-deficient mice via PSA attached to the NCAM glycoprotein backbone or to the NCAM glycoprotein backbone itself, we injected NCAM-Fc into NCAM−/− mice (Fig. 6B). NCAM-Fc inhibited contextual memory when compared with Fc injected into NCAM−/− mice (treatment: F(1,13) = 14.91, p = 0.002; FLSD: first day, p = 0.002; third day, p = 0.02). Tone memory (Fig. 6C) in these mice was neither improved nor inhibited as compared with NCAM−/− mice that had been injected with Fc (treatment: F(1,13) = 0.013, p = 0.91; treatment × tone: F(1,13) = 0.012, p = 0.91).
To verify that the effect of injected PSA-NCAM-Fc depends on the presence of endogenous NCAM, PSA-NCAM-Fc was injected into NCAM+/+ mice (rather than C57BL/6J as before) in parallel with injections into NCAM−/− mice (Fig. 6B). In agreement with our results for C57BL/6J mice, injection of PSA-NCAM-Fc impaired contextual memory in NCAM+/+ mice as compared with Fc injected NCAM+/+ mice (treatment: F(1,17) = 9.10, p = 0.008; FLSD: first day, p = 0.005; third day, p = 0.02). Injection of PSA-NCAM-Fc into NCAM+/+ mice, as in C57BL/6J, did not affect cued memory (Fig. 6C) or discrimination between CS+ and CS− (at least p < 0.05, Wilcoxon test). Thus, PSA-NCAM-Fc impairs fear learning in wild-type mice but restores it in NCAM−/− mice.
Discussion
The present study has analyzed the functions in learning and synaptic plasticity of three related molecules, the glycan PSA and the nonpolysialylated and polysialylated extracellular domains of NCAM, and identified PSA and PSA-NCAM to affect different phases of fear conditioning. We found that PSA-NCAM is unique in its properties in that it is active during consolidation of contextual memory more than the PSA or the NCAM glycoprotein backbone when applied individually. However, PSA alone mimics the action of PSA-NCAM during acquisition of fear learning and induction of LTP in the CA1 region of the hippocampus. The importance of PSA in formation of hippocampus-dependent contextual memory is further underscored by memory deficits in polysialyltransferase deficient (PST−/−) mice and restoration of memory and LTP deficits in NCAM−/− mice by application of PSA-NCAM, although not by NCAM. PSA appears to be less important for amygdala-dependent cued memory because it is not affected in PST−/− mice.
Effects of exogenous PSA-NCAM and PSA on LTP and contextual memory in wild-type mice
A novel finding of our study is that pretraining injections of PSA (alone or attached to the extracellular glycoprotein backbone of NCAM in the form of soluble PSA-NCAM-Fc) disrupt the formation of contextual memory in the fear-conditioning paradigm in wild-type mice. These effects of PSA and PSA-NCAM-Fc could be explained by different mechanisms. For instance, excess of PSA has been shown to interfere with induction of LTP (Muller et al., 2000; present study) and brain-derived neurotrophic factor signaling (Muller et al., 2000). PSA and PSA-NCAM-Fc may also function as competitive antagonists abolishing PSA-NCAM-driven synaptogenesis (Dityatev et al., 2004) at postsynaptic sites at which PSA-NCAM expression is upregulated by a learning event (Fox et al., 1995; Murphy et al., 1996; O'Connell et al., 1997; Sandi et al., 2003).
Different molecular mechanisms may also be involved during the consolidation phase of fear conditioning, because post-training injection of PSA-NCAM-Fc, but not PSA, impairs consolidation of contextual memory. Previous experiments using injection of NCAM antibodies and a synthetic peptide (C3d) that affects homophilic interaction with NCAM revealed the existence of a short post-training time window in which consolidation can be disrupted via alterations in NCAM functions (Doyle et al., 1992; Cambon et al., 2003). In addition, during memory consolidation, a transient, time-dependent learning-induced increase in expression and polysialylation of NCAM has been observed in the hippocampus, and entorhinal and perirhinal cortices throughout different behavioral paradigms and species (Doyle et al., 1992; Scholey et al., 1993; Fox et al., 1995; Arami et al., 1996; Murphy et al., 1996; O'Connell et al., 1997; Sandi et al., 2003). Taking these data together with our results, there is a time window at 6 h post-training that depends on PSA-NCAM, rather than on the individual components, PSA or NCAM.
Rescue of contextual fear memory and LTP in NCAM-deficient mice by PSA-NCAM
In this study, we show for the first time that deficits in contextual memory in NCAM-deficient mice are partially restored via delivery of exogenous PSA-NCAM-Fc, thus, highlighting the importance of PSA and its receptors in learning and memory. The fact that there is a reduction rather than restoration of contextual memory after NCAM-Fc injection highlights even more the differences in mechanisms underlying effects of polysialylated versus nonpolysialylated NCAM. Because NCAM-deficient mice lack detectable levels of the NCAM molecule, injected PSA-NCAM-Fc must restore contextual memory by other mechanisms than NCAM–NCAM homophilic interactions. These interactions are known to be important for early developmental events, including neurite outgrowth of cerebellar and cerebral cortical neurons (Saffell et al., 1997; Niethammer et al., 2002). Thus, mechanisms mediating acquisition of learning by NCAM may be less dependent of homophilic NCAM–NCAM interactions than neurite outgrowth during development. Our results are supported by the observation that the peptide C3d, which stimulates neurite outgrowth (Ronn et al., 1999), did not affect acquisition of contextual learning in rats (Cambon et al., 2003).
In addition to improving learning, we found that either PSA-NCAM-Fc or PSA rescued impaired CA1 LTP in NCAM-deficient mice. Because both a loss and excess of PSA leads to impairment of CA1 LTP (Becker et al., 1996; Muller et al., 1996; Eckhardt et al., 2000; Muller et al., 2000; present study), it is likely that higher levels of PSA may act as a negative competitor between interacting partners and that lower levels are not potent enough to induce a response.
It is noteworthy in this context that the heavily hydrated steric volume of PSA inhibits adhesion of other cell adhesion molecules independently of NCAM, among which are cadherins, L1, and integrins (Fujimoto et al., 2001). PSA may also potentiate signaling via receptors to fibroblast growth factor, platelet-derived neurotrophic factor, and brain-derived neurotrophic factor, and a subset of AMPA glutamate receptors (Muller et al., 2000; Dityatev et al., 2004; Vaithianathan et al., 2004; Zhang et al., 2004). Soluble PSA and PSA-NCAM-Fc also inhibit activation of NR2B subunit-containing NMDA receptors by low micromolar concentrations of glutamate (Hammond et al., 2006). Concentration of synaptically released glutamate at the synaptic cleft peaks above 100 μm, whereas micromolar concentrations of glutamate are present in the extrasynaptic space, suggesting that PSA inhibits extrasynaptic receptors rather than receptors in the synaptic cleft. Activation of extrasynaptic NMDA receptors depresses synaptic responses and reduces the activity of down-stream mechanisms, such as extracellular signal-regulated kinases (Massey et al., 2004; Ivanov et al., 2006; Li et al., 2006). Because of these effects, our working hypothesis is that inhibition of extrasynaptic NR2B-containing NMDA receptors by endogenous soluble PSA/PSA-NCAM favors induction of LTP in normal brains. This would explain why LTP is impaired in NCAM-deficient brains and exogenous PSA/PSA-NCAM-Fc restores LTP and fear learning.
Rescue of cued memory in NCAM-deficient mice by injection of PSA-NCAM-Fc
It is widely accepted that contextual memory in the fear-conditioning paradigm is hippocampus-dependent, whereas cued memory is stored extrahippocampally, and relies mostly on the amygdala (for review, see LeDoux, 2000; Dityatev and Bolshakov, 2005). However, recent studies support the view that the hippocampus is not only involved in contextual fear conditioning, but also in cued fear conditioning to a tone (Bast et al., 2003; Seidenbecher et al., 2003; Tang et al., 2003). Available data suggest that there is an exchange of fear-related information between the amygdala and the hippocampus during conditioned tone presentation. Thus, rescue of cued memory in NCAM−/− mice by intrahippocampal PSA-NCAM-Fc injection can be explained such that a relatively weak deficit in the processing of emotional information by the amygdala in NCAM−/− mice can be compensated via cross-talk between the hippocampus and amygdala. In other words, improvement of hippocampal function after injection of PSA-NCAM-Fc in NCAM−/− mice, which we detect as improvement in contextual memory, may result in an increased input from the hippocampus and better retrieval of amygdala-dependent memories. Thus, NCAM−/− mice are instrumental in obtaining insights into these aspects of representation and/or retrieval of cued memory. Another noteworthy observation is that amygdala-dependent cued memory was impaired in NCAM−/− but not PST−/− mice, whereas hippocampus-dependent contextual memory was reduced in both mutants, suggesting that polysialylation of NCAM by PST in the amygdala is less important for fear memory than that in the hippocampus.
Dysfunctions of the CNS mediated by abnormalities in expression of PSA and NCAM
The hippocampal formation is one of the most vulnerable regions during psychiatric disorders. Previous studies have shown abnormally increased levels of soluble NCAM in the cerebrospinal fluid of patients suffering from schizophrenia (Poltorak et al., 1995) and dementia (Strekalova et al., 2006). Also, total expression of NCAM is normal, while levels of soluble NCAM are increased and the number of PSA-immunoreactive cells is reduced in the hippocampal hilus of patients affected with schizophrenia (Barbeau et al., 1995; Vawter et al., 2001). The potential mechanisms of generation of different soluble NCAM isoforms in the extracellular space and CSF involve shedding of membrane NCAM or secretion of intact isoforms (Kalus et al., 2006). These alterations in soluble NCAM and abnormal PSA-NCAM expression are noteworthy in light of cognitive deficits in schizophrenia and other psychiatric diseases. The present study draws an important parallel between conditions in humans and animals, showing that an excess of soluble PSA and PSA-NCAM in the hippocampus of wild-type mice leads to deficits in contextual memory. Furthermore, in agreement with our observations on PSA-NCAM-Fc injected in wild-type mice, transgenic mice that overexpress soluble NCAM under the control of the late acting neuron-specific enolase promoter also exhibit deficits in contextual fear conditioning (Pillai-Nair et al., 2005). These and our results strongly suggest that upregulation of soluble NCAM, with a yet-unknown extent of polysialylation, as found under pathological conditions may lead to cognitive deficits. Thus, elucidation of the molecular and cellular mechanisms by which soluble PSA and PSA-NCAM affect synaptic functions may clarify the pathological process of brain malfunctions and guide the development of therapeutic strategies to prevent or compensate these deficits.
Footnotes
This work was supported by German Research Foundation Grants DI 702/5-1,2,3 (A.D.) and GE 801/3-3 (R.G.S.), and the Roechling Foundation (M. Schachner) and German Academic Exchange Service (M. Sun). We thank Harold Cremer for NCAM-deficient mice, Achim Dahlmann and Eva Kronberg for genotyping and animal care, Genevieve Rougon for PSA-NCAM-Fc-producing cells, Suzhen Chen for NCAM-Fc-producing cells, Galina Dityateva, Tatjana Makhina, and Helen Strekalova for production and purification of NCAM-Fc and PSA-NCAM-Fc, Fabio Morellini for his comments on this manuscript, and Andreas Engel, Gerhard Engler, Andreas Hämisch, and Stefanie Petow for their help.
References
- Angata K, Long JM, Bukalo O, Lee W, Dityatev A, Wynshaw-Boris A, Schachner M, Fukuda M, Marth JD. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem. 2004;279:32603–32613. doi: 10.1074/jbc.M403429200. [DOI] [PubMed] [Google Scholar]
- Arami S, Jucker M, Schachner M, Welzl H. The effect of continuous intraventricular infusion of L1 and NCAM antibodies on spatial learning in rats. Behav Brain Res. 1996;81:81–87. doi: 10.1016/s0166-4328(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA. 1995;92:2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT, Schweizer M, Dityatev A, Schachner M. Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci. 2004;24:1565–1577. doi: 10.1523/JNEUROSCI.3298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Venero C, Berezin V, Bock E, Sandi C. Post-training administration of a synthetic peptide ligand of the neural cell adhesion molecule, C3d, attenuates long-term expression of contextual fear conditioning. Neuroscience. 2003;122:183–191. doi: 10.1016/s0306-4522(03)00597-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Mantei N, Dong L, Schachner M. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J Neurobiol. 1999;38:428–439. doi: 10.1002/(sici)1097-4695(19990215)38:3<428::aid-neu10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev AE, Bolshakov VY. Amygdala, long-term potentiation, and fear conditioning. Neuroscientist. 2005;11:75–88. doi: 10.1177/1073858404270857. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26:207–217. doi: 10.1016/s0896-6273(00)81151-4. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle E, Nolan PM, Bell R, Regan CM. Intraventricular infusions of anti-neural cell adhesion molecules in a discrete posttraining period impair consolidation of a passive avoidance response in the rat. J Neurochem. 1992;59:1570–1573. doi: 10.1111/j.1471-4159.1992.tb08477.x. [DOI] [PubMed] [Google Scholar]
- Durbec P, Cremer H. Revisiting the function of PSA-NCAM in the nervous system. Mol Neurobiol. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AG, Hartz BP, Gallagher HC, Ronn LC, Berezin V, Bock E, Regan CM. A synthetic peptide ligand of neural cell adhesion molecule (NCAM) IgI domain prevents NCAM internalization and disrupts passive avoidance learning. J Neurochem. 2000;74:2607–2613. doi: 10.1046/j.1471-4159.2000.0742607.x. [DOI] [PubMed] [Google Scholar]
- Fox GB, O'Connell AW, Murphy KJ, Regan CM. Memory consolidation induces a transient and time-dependent increase in the frequency of neural cell adhesion molecule polysialylated cells in the adult rat hippocampus. J Neurochem. 1995;65:2796–2799. doi: 10.1046/j.1471-4159.1995.65062796.x. [DOI] [PubMed] [Google Scholar]
- Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82:1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto I, Bruses JL, Rutishauser U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J Biol Chem. 2001;276:31745–31751. doi: 10.1074/jbc.M104525200. [DOI] [PubMed] [Google Scholar]
- Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. NCAM associated polysialic acid inhibits NR2B-containing NMDA receptors and prevents glutamate-induced cell death. J Biol Chem. 2006 doi: 10.1074/jbc.M602568200. in press. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol (Lond) 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus I, Bormann U, Mzoughi M, Schachner M, Kleene R. Proteolytic cleavage of the neural cell adhesion molecule NCAM by ADAM17/TACE is involved in neurite outgrowth. J Neurochem. 2006;98:78–88. doi: 10.1111/j.1471-4159.2006.03847.x. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li S, Tian X, Hartley DM, Feig LA. Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci. 2006;26:1721–15729. doi: 10.1523/JNEUROSCI.3990-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci USA. 2000;97:4315–4320. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, O'Connell AW, Regan CM. Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J Neurochem. 1996;67:1268–1274. doi: 10.1046/j.1471-4159.1996.67031268.x. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol. 2002;157:521–532. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell AW, Fox GB, Barry T, Murphy KJ, Fichera G, Foley AG, Kelly J, Regan CM. Spatial learning activates neural cell adhesion molecule polysialylation in a corticohippocampal pathway within the medial temporal lobe. J Neurochem. 1997;68:2538–2546. doi: 10.1046/j.1471-4159.1997.68062538.x. [DOI] [PubMed] [Google Scholar]
- Panicker AK, Buhusi M, Thelen K, Maness PF. Cellular signalling mechanisms of neural cell adhesion molecules. Front Biosci. 2003;8:d900–d911. doi: 10.2741/1014. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Gilmore KL, Demyanenko GP, Huang JZ, Wetsel WC, Maness PF. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak M, Khoja I, Hemperly JJ, Williams JR, el-Mallakh R, Freed WJ. Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp Neurol. 1995;131:266–272. doi: 10.1016/0014-4886(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Ronn LC, Olsen M, Ostergaard S, Kiselyov V, Berezin V, Mortensen MT, Lerche MH, Jensen PH, Soroka V, Saffell JL, Doherty P, Poulsen FM, Bock E, Holm A. Identification of a neuritogenic ligand of the neural cell adhesion molecule using a combinatorial library of synthetic peptides. Nat Biotechnol. 1999;17:1000–1005. doi: 10.1038/13697. [DOI] [PubMed] [Google Scholar]
- Roullet P, Mileusnic R, Rose SP, Sara SJ. Neural cell adhesion molecules play a role in rat memory formation in appetitive as well as aversive tasks. NeuroReport. 1997;8:1907–1911. doi: 10.1097/00001756-199705260-00023. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Kruyt ND, Murphy KJ, Regan CM. Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol Psychiatry. 2003;54:599–607. doi: 10.1016/s0006-3223(03)00182-3. [DOI] [PubMed] [Google Scholar]
- Scholey AB, Rose SP, Zamani MR, Bock E, Schachner M. A role for the neural cell adhesion molecule in a late, consolidating phase of glycoprotein synthesis six hours following passive avoidance training of the young chick. Neuroscience. 1993;55:499–509. doi: 10.1016/0306-4522(93)90519-l. [DOI] [PubMed] [Google Scholar]
- Scholey AB, Mileusnic R, Schachner M, Rose SP. A role for a chicken homolog of the neural cell adhesion molecule L1 in consolidation of memory for a passive avoidance task in the chick. Learn Mem. 1995;2:17–25. doi: 10.1101/lm.2.1.17. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Stoenica L, Senkov O, Gerardy-Schahn R, Weinhold B, Schachner M, Dityatev A. In vivo synaptic plasticity in the dentate gyrus of mice deficient in the neural cell adhesion molecule NCAM or its polysialic acid. Eur J Neurosci. 2006;23:2255–2264. doi: 10.1111/j.1460-9568.2006.04771.x. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Wolfer D, Schuster T, Mantei N, Stork S, Hoyer D, Lipp H, Obata K, Schachner M. Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. Eur J Neurosci. 2000;12:3291–3306. doi: 10.1046/j.1460-9568.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- Strekalova H, Buhmann C, Kleene R, Eggers C, Saffell J, Hemperly J, Weiller C, Muller-Thomsen T, Schachner M. Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol Aging. 2006;27:1–9. doi: 10.1016/j.neurobiolaging.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Sun M, Sibbe M, Evers M, Dityatev A, Gass P, Schachner M. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol Cell Neurosci. 2002;21:173–187. doi: 10.1006/mcne.2002.1172. [DOI] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res. 2001;919:232–241. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Tang J, Wagner S, Schachner M, Dityatev A, Wotjak CT. Potentiation of amygdaloid and hippocampal auditory-evoked potentials in a discriminatory fear-conditioning task in mice as a function of tone pattern and context. Eur J Neurosci. 2003;18:639–650. doi: 10.1046/j.1460-9568.2003.02758.x. [DOI] [PubMed] [Google Scholar]
- Vaithianathan T, Matthias K, Bahr B, Schachner M, Suppiramaniam V, Dityatev A, Steinhauser C. Neural cell adhesion molecule-associated polysialic acid potentiates alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J Biol Chem. 2004;279:47975–47984. doi: 10.1074/jbc.M407138200. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Usen N, Thatcher L, Ladenheim B, Zhang P, VanderPutten DM, Conant K, Herman MM, van Kammen DP, Sedvall G, Garver DL, Freed WJ. Characterization of human cleaved N-CAM and association with schizophrenia. Exp Neurol. 2001;172:29–46. doi: 10.1006/exnr.2001.7790. [DOI] [PubMed] [Google Scholar]
- Venero C, Herrero AI, Touyarot K, Cambon K, Lopez-Fernandez MA, Berezin V, Bock E, Sandi C. Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur J Neurosci. 2006;23:1585–1595. doi: 10.1111/j.1460-9568.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, Kiss JZ. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci. 2001;13:1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Inui K, Matsuyama Y, Harada A, Hanamura K, Murakami F, Ruthazer ES, Rutishauser U, Seki T. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J Neurosci. 2000;20:9145–9151. doi: 10.1523/JNEUROSCI.20-24-09145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci. 2004;117:93–103. doi: 10.1242/jcs.00827. [DOI] [PubMed] [Google Scholar]