Abstract
Clathrin-coated vesicles (CCVs) are responsible for the endocytosis of multiple cargo, including synaptic vesicle membranes. We now describe a new CCV protein, termed connecdenn, that contains an N-terminal DENN (differentially expressed in neoplastic versus normal cells) domain, a poorly characterized protein module found in multiple proteins of unrelated function and a C-terminal peptide motif domain harboring three distinct motifs for binding the α-ear of the clathrin adaptor protein 2 (AP-2). Connecdenn coimmunoprecipitates and partially colocalizes with AP-2, and nuclear magnetic resonance and peptide competition studies reveal that all three α-ear-binding motifs contribute to AP-2 interactions. In addition, connecdenn contains multiple Src homology 3 (SH3) domain-binding motifs and coimmunoprecipitates with the synaptic SH3 domain proteins intersectin and endophilin A1. Interestingly, connecdenn is enriched on neuronal CCVs and is present in the presynaptic compartment of neurons. Moreover, connecdenn has a uniquely stable association with CCV membranes because it resists extraction with Tris and high-salt buffers, unlike most other CCV proteins, but it is not detected on purified synaptic vesicles. Together, these observations suggest that connecdenn functions on the endocytic limb of the synaptic vesicle cycle. Accordingly, disruption of connecdenn interactions with its binding partners through overexpression of the C-terminal peptide motif domain or knock down of connecdenn through lentiviral delivery of small hairpin RNA both lead to defects in synaptic vesicle endocytosis in cultured hippocampal neurons. Thus, we identified connecdenn as a component of the endocytic machinery functioning in synaptic vesicle endocytosis, providing the first evidence of a role for a DENN domain-containing protein in endocytosis.
Keywords: AP-2, clathrin, DENN domains, endocytosis, endophilin, intersectin, NMR, synaptic vesicle
Introduction
Clathrin-mediated endocytosis (CME) is the major route of endocytic entry for a broad array of cargo in most cell types. Highly specialized forms of CME also exist, most notably, the recapture and reformation of synaptic vesicles (SVs) after their collapse into the plasma membrane during exocytosis (Murthy and De Camilli, 2003; Ryan, 2003). CME of SVs is critical to prevent the depletion of the finite number of SVs that exist in the nerve terminal, especially during periods of sustained release (Wu and Betz, 1998; Moser and Beutner, 2000). Over the past decade, numerous protein components of the complex machinery regulating CME and the formation of clathrin-coated pits (CCPs) and vesicles (CCVs) have been identified. Many of these proteins have general functions common to all forms of CME and are found in all cell types. However, in many cases, isoforms or splice variants of the proteins exist that are expressed exclusively or at higher levels in neurons (McPherson and Ritter, 2005). These specializations are needed to meet the high demands placed on neurons for CME of SVs because it is estimated that ∼90% of all endocytic CCVs in neurons are dedicated to SV recycling (Girard et al., 2005a).
The proteins of the endocytic machinery form an interaction web in which the globular appendage domain of the α-adaptin (α-ear) subunit of the adaptor protein 2 (AP-2) complex serves as a key organizing hub (Slepnev and De Camilli, 2000; Ritter and McPherson, 2004; Traub, 2005). Interactions with the α-ear, which are mediated by short peptide motifs (McPherson and Ritter, 2005), allow for recruitment of accessory proteins to sites of CCV formation. Interestingly, a recent proteomic analysis of CCVs from brain revealed additional novel proteins (Blondeau et al., 2004), suggesting that our understanding of CCV formation is far from complete. One protein family identified was the NECAPs (adaptin ear-binding coat-associated proteins), which bind to the α-ear but do not contain DPF/W or FXDXF motifs, which until recently were the only known α-ear-binding motifs (Ritter et al., 2003, 2004). Analysis of the NECAPs led to a novel α-ear-binding motif, the WXXF-acidic motif (Ritter et al., 2003, 2004; Mishra et al., 2004; Praefcke et al., 2004; Walther et al., 2004). The presence of α-ear-binding motifs in previously uncharacterized proteins has proven to be predictive of a role in CME (Metzler et al., 2001; Mishra et al., 2001; Conner and Schmid, 2002). A search of the Prosite database for proteins containing these motifs identified an uncharacterized open-reading frame with the sequences DPF, FSDVF, and WETFE. At the N terminus, the protein contains a DENN (differentially expressed in neoplastic versus normal cells) domain. The DENN domain is readily detected in a National Center for Biotechnology Information Conserved Domain search (Marchler-Bauer and Bryant, 2004) and is found in a wide variety of proteins from diverse species. However, little information is available regarding the biological context of DENN domain function (Levivier et al., 2001). Based on the presence of α-ear-binding motifs, we hypothesized that the protein, which we named connecdenn, would function in CME. Our data reveal connecdenn as a new component of the endocytic machinery involved in SV endocytosis.
Materials and Methods
Antibodies and peptides.
Monoclonal antibodies against the following proteins were from the indicated commercial sources: α-adaptin [clone 8 (BD Transduction Laboratories, Lexington, KY) and clone AP.6 (Upstate, Charlottesville, VA)], dynamin, and Na+/K+ ATPase α-1 (Upstate), clathrin-heavy chain (CHC) (BD Transduction Laboratories), Flag (M2; Sigma, St. Louis, MO), postsynaptic density-95 (PSD-95) (mAb-045; Affinity BioReagents, Golden, CO), actin (Chemicon, Temecula, CA), and synaptophysin (Sigma). Polyclonal antibodies recognizing endophilin A1 (Micheva et al., 1997), intersectin-s and -l (2173) (Hussain et al., 1999), and NECAP 1 (Ritter et al., 2004) were previously described. A polyclonal antibody against synaptotagmin was described previously (Mundigl et al., 1993) and was a gift from Dr. Pietro De Camilli (Yale University, New Haven, CT). Polyclonal sera recognizing connecdenn were raised in rabbits (3775 and 3776) against a synthetic peptide containing amino acids 1002–1016 of the mouse connecdenn protein with an added N-terminal cysteine (CVEQLRRQWETFE) coupled to keyhole limpet hemocyanin (KLH). Polyclonal sera recognizing clathrin-light chain a and b (CLCa/b) were raised in rabbits (4045 and 4046) against a synthetic peptide encoding amino acids from the conserved domain with an added N-terminal cysteine (CEEDPAAAFLAQQESEIAGIEND) coupled to KLH. Peptides used for competition assays were as follows: DPW peptide from epsin 1, CSDPWGSDPWG; FXDXF peptide from amphiphysin 1, CSFFEDNFVPE; and WXXF-acidic peptide from NECAP 1, CQAPQPSNWVQF. All synthetic peptides were purchased from Howard Hughes Medical Institute/Keck Biotechnology Resource Laboratory (Yale University, New Haven, CT).
Expression constructs.
Bacterial expression constructs for the adaptin ear domains of adaptor protein 1 (AP-1) and AP-2 were described previously (Ritter et al., 2003). Glutathione S-transferase (GST) fusion proteins of the α-ear point mutations R707A, F740A, and Q782A were described previously (Ritter et al., 2004), and point mutation R707A was generated using the megaprimer approach (Barik, 1995) with the oligonucleotide GCGCGAATTCGGCTCTGAAGACAACTTTGCCGCGTTTGTTTGTAAAAATAATGGTG. GST fusion proteins of the various Src homology 3 (SH3) domains of intersectin (Yamabhai et al., 1998) and the SH3 domain of amphiphysin I (David et al., 1994) and II (Ramjaun et al., 1997), endophilin A1 (Micheva et al., 1997), and PKC and CK2 substrate in neurons 1 (PACSIN 1) (Wasiak et al., 2001) were described previously. The PACSIN 2 construct was a generous gift from Dr. Markus Plomann (University of Cologne, Cologne, Germany). Flag-tagged full-length connecdenn (residues 1–1016) was generated by PCR from the full-length mouse expressed sequence tag clone (gi31542026) using forward primer CGCGAATTCATGGGCTCCAGGATCAAG and reverse primer CGCGTCGACTCACTCAAAGGTCTCCCAC with subcloning into pCMV-Tag-2B. The C-terminal peptide motif domain (residues 372–1016) was made the same way using the forward primer CGCGAATTCCTAGACCTTCTCAATTCCG and the reverse primer from above with cloning into peGFP-C2. All constructs were verified by sequence analysis.
Binding studies.
Transfected HEK-293 cells were scraped in HEPES buffer (10 mm HEPES-OH, pH 7.4, 0.83 mm benzamidine, 0.23 mm phenylmethylsulphonyl fluoride, 0.5 μg/ml aprotinin, and 0.5 μg/ml leupeptin), incubated with 1% Triton X-100 and 150 mm NaCl for 30 min at 4°C, and then centrifuged for 30 min at 205,000 × g. Aliquots of the supernatant were incubated for 1 h at 4°C with GST fusion proteins precoupled to glutathione-Sepharose before washing in HEPES buffer with 1% Triton X-100 and 150 mm NaCl. Adult rat brain was homogenized in HEPES buffer and centrifuged at 800 × g for 5 min. The supernatant was incubated with 1% Triton X-100 and 33 mm NaCl for immunoprecipitation assays or 150 mm NaCl for GST pull-down assays for 30 min at 4°C and then centrifuged for 30 min at 205,000 × g. Aliquots of the supernatant (2 mg) were incubated for 1 h at 4°C with GST fusion proteins precoupled to glutathione-Sepharose before washing in HEPES buffer with 1% Triton X-100 and 150 mm NaCl. For competition experiments, extracts were incubated for 1 h at 4°C with 400 pmol of GST–α-ear in the presence of a 300- to 1000-fold molar excess of peptide as indicated. For coimmunoprecipitation studies, 2 mg aliquots of soluble brain extract were incubated with 5 μl of AP.6 antibody or 10 μl of 3775 or 2173 sera as well as protein G- or protein A-Sepharose beads, as appropriate. The samples were incubated for 2 h at 4°C before washing in HEPES buffer with 1% Triton X-100 and 33 mm NaCl.
Subcellular fractionation and extraction studies.
SVs were purified as described previously (Huttner et al., 1983). CCVs were purified from rat brain in buffer A (100 mm MES, pH 6.5, containing 1 mm EGTA and 5 mm MgCl2) as described previously (Girard et al., 2005b). For the extraction of coat proteins, 50–100 μg aliquots of CCVs were centrifuged at 200,000 × g for 15 min. The pellets were resuspended in 100 μl of buffer A, Tris buffer (1:1 mixture of buffer A and 1 m Tris, pH 9.5), buffer A with various concentrations of NaCl, or pH 11 buffer (25 mm Na2CO3, pH 11) and incubated for 30 min on ice. The samples were centrifuged at 200,000 × g, and the pellets were resuspended in buffer A and analyzed in parallel with the supernatant fraction. In other cases, CCVs in buffer A and CCVs extracted with Tris buffer were loaded on the top of linear 20–50% sucrose gradients prepared in buffer A and Tris buffer, respectively, and were centrifuged in a Sorvall (Newtown, CT) AH629 rotor at 145,000 × g for 1.5 h. The gradients were fractionated from the bottom, and gradient fractions were analyzed by Western blot.
Nuclear magnetic resonance spectroscopy and α-ear/peptide complex modeling.
The preparation of α-ear for nuclear magnetic resonance (NMR) was described previously (Denisov et al., 2004; Ritter et al., 2004). NMR spectra were acquired at 30°C on 800 MHz Varian (Palo Alto, CA) Unity Inova spectrometer. Detailed analysis of peptide binding to the α-ear was performed by comparison of chemical shifts for backbone amide signals in 1H-15N heteronuclear single quantum coherence (HSQC) spectra. HSQC spectra were recorded at 1:25, 1:5, 1:2, 1:1, and 2:1 peptide/protein ratios that were confirmed by UV concentration of both components and intensity of Hε1 (W) signals in one-dimensional NMR. The HADDOCK approach (Dominguez et al., 2003) was used to model the binding of the WETFE residues from the connecdenn peptide to the α-ear using the general protocol described previously (Ritter et al., 2004). Additional hydrogen bond restraint between the side-chain oxygen of E+1 in the peptide and NH3+ group of α-ear K727 was added based on x-ray structure of similar WVTFE/α-ear complex (Praefcke et al., 2004) and our α-ear mutagenesis data. The figure was generated using the MOLMOL program (Koradi et al., 1996).
Localization studies.
Immunofluorescence analysis of rat hippocampal neurons at 21 d in vitro (DIV) was performed as described previously (Burman et al., 2005). COS-7 were plated on poly-l-lysine-coated coverslips and transfected with Flag-tagged connecdenn using GeneJuice (Novagen, Madison, WI). After overnight expression, cells were fixed with 3% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100, and then incubated with primary antibodies for 1 h at room temperature. Cells were subsequently washed and incubated with appropriate secondary antibodies before visualization. Quantification of colocalization was performed with the RG2B colocalization plug-in of NIH ImageJ software, a public domain Java image processing and analysis program inspired by NIH Image for Macintosh (http://rsb.info.nih.gov/nih-image/about.html).
FM4-64 uptake studies.
For overexpression studies, hippocampal neurons were transfected after 7–8 DIV with green fluorescent protein (GFP) alone or GFP tagged to the peptide motif domain of connecdenn (residues 372–1016). Endocytosis of SVs was measured at 21–28 DIV using the fluorescent lipid membrane marker FM4-64 N-[3-triethylammoniumpropyl)-4-(6-(4-diethylamino)phenyl)hexatrienyl)pyridinium dibromide] (10 μm). SV labeling was performed by bath application of 40 mm KCl plus FM4-64 in HBSS for 90 s followed by 2 min in the presence of HBSS plus FM4-64. Background fluorescence was quenched with a 2 ml/min perfusion of HBSS plus ADVASEP-7 (1 mg/ml) for 10 min. To quantify SV endocytosis, we analyzed FM4-64 fluorescence images with NIH ImageJ software. The RG2B colocalization plug-in was used to select GFP-labeled varicosities that colocalized with FM4-64 staining. The colocalized regions were outlined, and the resulting regions of interest (ROIs) were saved and applied on original raw images for measurement of total FM4-64 fluorescence per ROI. The colocalized ROIs were then subtracted, and the remaining FM4-64 punctae, corresponding to active synapses from nontransfected neurons, were measured identically.
For loss-of-function studies, three small interfering RNAs (siRNAs) matching selected regions of mouse connecdenn, with dT overhangs already annealed, were synthesized by Qiagen (Hilden, Germany). siRNA#1 was not effective on transfected mouse connecdenn and was not used further. siRNA#2 (GGCCCAGGCTGCTTTCTTT) and siRNA#3 (GAGCTGCTTCTGTATCTTA) were effective in knocking down transfected mouse connecdenn, with siRNA#3 the most effective in knocking down endogenous connecdenn in COS-7 cells (data not shown). This sequence was thus selected for generation of short hairpin RNA (shRNA) packaged in a lentivirus delivery system, which was prepared as described previously (Harper et al., 2006). Briefly, PCR was used to amplify a mouse U6 promoter followed by a stem–loop–stem structure encoding the sequence of siRNA#3. The forward primer matching the mouse U6 promoter had the sequence GCGCAATTGCGGGAAAACTGAATAAGAG. The reverse primer, matching the 3′ end of the U6 promoter followed by the siRNA antisense strand, a loop, the siRNA sense strand, and an RNA Pol III termination sequence, had the sequence GCGCAATTGAAAAAAAGAGCTGCTTCTGTATCTTAT-GACAGGAAGTAAGATACAGAAGCAGCTCTTCAAAACAAGGCTTTTCTCCAAGG. The resulting PCR fragment was cloned into a shuttle vector that was used to generate feline immunodeficiency virus (FIV) expressing the connecdenn shRNA and GFP as described previously (Harper et al., 2006). Control virus expressing GFP alone was described previously (Harper et al., 2006). Hippocampal neurons were transduced with a multiplicity of infection (MOI) of 3 (GFP/connecdenn shRNA) or 10 (GFP alone) at 14 DIV, and cells were lysed at 21 DIV in SDS gel sample buffer to generate extracts for Western blot analysis. Alternatively, hippocampal neurons were transduced with one-fifth the MOI used for Western blot and were processed for FM4-64 uptake as described for transfection experiments.
Results
Identification of connecdenn
Connecdenn was identified through the presence of the sequences DPF, FSDVF, and WETFE (Fig. 1, dark gray ovals) that match consensus α-ear-binding motifs. Connecdenn also contains seven proline-rich stretches with potential SH3 domain-binding motifs (Fig. 1, light gray rectangles). At the N terminus, connecdenn contains a DENN domain composed of tandem upstream (u) DENN, DENN, and downstream (d) DENN modules (Fig. 1). Connecdenn is detected in multiple species with the mouse protein 27 and 21% identical throughout its length to fly and worm orthologs, respectively. Homology is found in small blocks throughout the proteins with the best conservation in the DENN domain (39 and 33% identity of the DENN domain of mouse with fly and worm orthologs, respectively). The annotated mouse connecdenn has been entered into GenBank under the accession number DQ448594.
Figure 1.
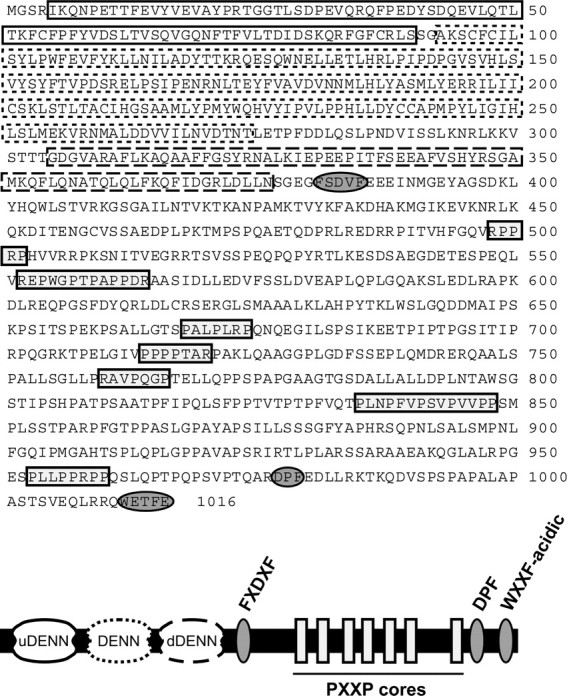
Identification of connecdenn. The amino acid sequence of mouse connecdenn (from gi31542027) and a domain/motif model are presented. The DENN domain is composed of uDENN, DENN, and dDENN modules indicated by solid, dotted, and dashed lines, respectively. Potential AP-2-binding motifs are indicated by ovals with dark shading, and proline-rich regions containing PXXP cores are indicated by boxes with light shading. The annotated mouse connecdenn has been entered into GenBank under the accession number DQ448594.
Connecdenn is an AP-2-binding partner
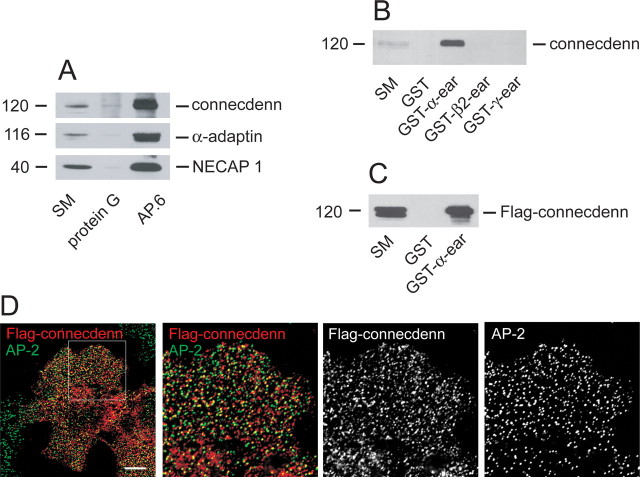
Immunoprecipitation of AP-2 from brain extracts leads to a robust coimmunoprecipitation of connecdenn (Fig. 2A). In pull-down assays, connecdenn from brain extracts binds to GST–α-ear but not to GST fusion proteins encoding the β2-ear or the ear of the γ-adaptin subunit of AP-1 (Fig. 2B). Flag-tagged connecdenn also binds to GST–α-ear (Fig. 2C). At low levels of expression in COS-7 cells, Flag-tagged connecdenn displays a punctate pattern that partially colocalizes with endogenous AP-2, a marker of plasma membrane CCPs (Fig. 2D). Quantification using the RG2B colocalization plug-in of NIH ImageJ software reveals that 44.7% of all connecdenn puncta are colocalized with AP-2 (20 cells from two separate experiments). Given the density of the immunopositive punctate, we rotated the relative orientation of the images by 2°, after which 16.4% of the punctae overlapped with AP-2. Thus, a significant fraction of Flag-tagged connecdenn is localized to CCPs.
Figure 2.
Interaction of connecdenn with AP-2. A, Soluble brain lysates were incubated with protein G beads alone or antibody AP.6 against the α-adaptin subunit of AP-2 and protein G beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. B, Soluble brain lysates were incubated with GST or GST fused to the ear domains of α-, β2-, and γ-adaptin, precoupled to glutathione-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibody against connecdenn. C, Lysates from HEK-293 cells transfected with Flag-tagged connecdenn were incubated with GST or GST–α-ear precoupled to glutathione-Sepharose beads, and proteins specifically bound to the beads were processed for Western blot with antibody against the Flag-epitope tag. D, COS-7 cells transfected at low levels with full-length Flag-tagged connecdenn and processed with a polyclonal Flag antibody (red channel) and a monoclonal antibody against AP-2 (AP.6, green channel). The blend of the two images is shown in the left panel, and a blend and individual images of the region indicated by the box is shown in the right three panels. Scale bar: lower-magnification image, 10 μm; higher-magnification images, 4.3 μm.
We next sought to identify the determinants within connecdenn responsible for α-ear interaction. The α-ear is a bi-lobed structure with FXDXF and DPF/W motifs binding to a site in the platform subdomain and WXXF-acidic motifs binding to a site in the sandwich subdomain (Ritter and McPherson, 2004). Mutation to alanine of sandwich subdomain residues involved in interactions with WXXF-acidic motifs, including K727, F740, and Q782 (Ritter et al., 2004), interferes with α-ear binding to connecdenn and NECAP 1, whereas mutation of R707, shown not to be involved in binding (Mishra et al., 2004), has no influence (Fig. 3A). These studies demonstrate an important contribution of the connecdenn WXXF-acidic motif to α-ear interaction. However, the residual binding of connecdenn to F740A and Q782A, in the face of no binding of NECAP 1 (Fig. 3A), which contains WXXF-acidic only (Ritter et al., 2003), suggests that connecdenn can use other α-ear-binding motifs. To address this issue, we performed peptide competition experiments. Addition of DPW, FXDXF, or WXXF-acidic peptides alone, at 300:1 or 1000:1 molar ratio to GST–α-ear fusion protein in pull-down assays, had little or no influence on the binding of the α-ear to connecdenn (Fig. 3B). Moreover, simultaneous incubation of DPW and FXDXF peptides also has little effect on connecdenn binding (Fig. 3B). In contrast, DPW or FXDXF peptides do strongly decrease binding when combined with the WXXF-acidic peptide, and a complete block in binding is observed when all three peptides are added simultaneously (900:1 total molar ratio to the fusion protein, whereas individual peptides were added as high as 1000:1 without effect) (Fig. 3B). Thus, WETFE is the predominant sequence mediating connecdenn binding to AP-2, but the DPF and FSDVF sequences also contribute.
Figure 3.
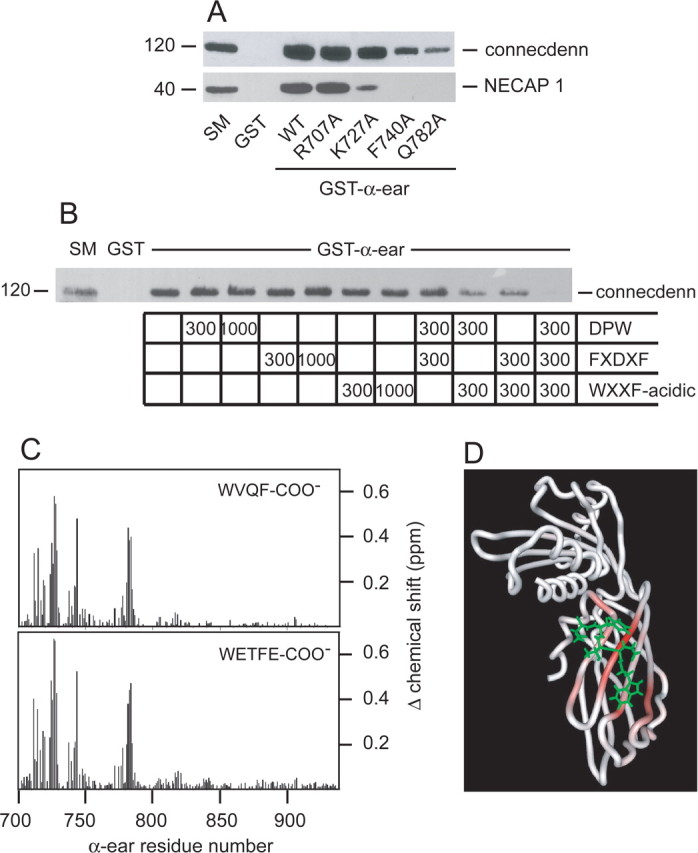
Identification of the connecdenn-binding site on AP-2. A, Soluble brain lysates were incubated with GST or GST fused to wild-type (WT) α-ear or a variety of α-ear point mutants as indicated, precoupled to glutathione-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. B, Soluble brain lysates were incubated with GST or GST–α-ear, precoupled to glutathione-Sepharose beads. Incubations were without or with a DPW peptide (CSDPWGSDPWG) from epsin 1, a FXDXF peptide (CSFFEDNFVPE) from amphiphysin1, or a WXXF-acidic peptide (CQAPQPSNWVQF-COO−) from NECAP 1 at the molar ratios of peptide to fusion protein indicated. Proteins specifically bound to the beads were processed for Western blot with antibody against connecdenn. For all above experiments, an aliquot of starting material (SM) equal to 1/10 of that added to the beads was run in parallel. C, Magnitude of the amide chemical shift changes [{(Δ1H shift)2 + (Δ15N shift × 0.2)2}1/2 in parts per million (ppm)] of the α-ear during binding CQAPQPSNWVQF-COO− (WVQF-COO−, NECAP 1; top) and CVEQLRRQWETFE (WETFE-COO−, connecdenn; bottom). The residue numbers correspond to mouse α-adaptin. D, HADDOCK modeled structure of WETFE/α-ear complex. The backbone trace of the α-ear is colored according to the size of the amide chemical shift changes during binding of the connecdenn peptide.
We next examined the interaction of a WETFE peptide from connecdenn with the α-ear by NMR. Interestingly, the chemical shift changes that occur on the α-ear in the presence of the connecdenn peptide, which demonstrate a direct interaction, resemble those seen with a WVQF-COO− peptide from NECAP 1 (Fig. 3C). Thus, the model of the connecdenn peptide docked to the α-ear (Fig. 3D) is similar to that seen previously for NECAP (Ritter et al., 2004).
Connecdenn interacts with endocytic SH3 domain proteins
Connecdenn contains a series of PXXP core sequences, suggesting that it is a binding partner for SH3 domains. We thus performed pull-down assays using GST–SH3 domains from multiple endocytic proteins. Flag-tagged connecdenn binds the SH3 domain of endophilin A1 and the SH3A and SH3C domains of the endocytic adaptor protein intersectin (Fig. 4A). The specificity of connecdenn interactions with SH3 domains is indicated by the lack of binding to the SH3 domains of amphiphysin I and II, PACSIN 1 and 2, and the SH3B, SH3D, and SH3E domains of intersectin (Fig. 4A). Dynamin demonstrates the expected pattern of binding in these experiments (Fig. 4A). Intersectin has two splice variants, intersectin-short (s) and intersectin-long (l), that share the five SH3 domains, with intersectin-l containing additional C-terminal sequence (Hussain et al., 1999, 2001). Endophilin A1 comprises an N-terminal BAR (Bin1/amphiphysin/Rvs167) domain coupled to the C-terminal SH3 domain (de Heuvel et al., 1997; Ringstad et al., 1997; Peter et al., 2004; Weissenhorn, 2005). Like connecdenn (Fig. 5), intersectin-l and endophilin A1 are enriched in brain and are present in the presynaptic compartment (Micheva et al., 1997; Ringstad et al., 1997; Hussain et al., 1999; Koh et al., 2004; Marie et al., 2004). Interestingly, connecdenn coimmunoprecipitates with intersectin-s/intersectin-l and endophilin A1 from brain extracts (Fig. 4B,C). The relatively low amount of endophilin recovered in the immunoprecipitated sample relative to the starting material likely results from the fact that endophilin A1 is much more abundant than connecdenn, and, as such, only a fraction of endophilin A1 in the sample can be coimmunoprecipitated. Thus, connecdenn is a component of an endocytic complex involving AP-2 and synaptic SH3 domain-bearing proteins.
Figure 4.
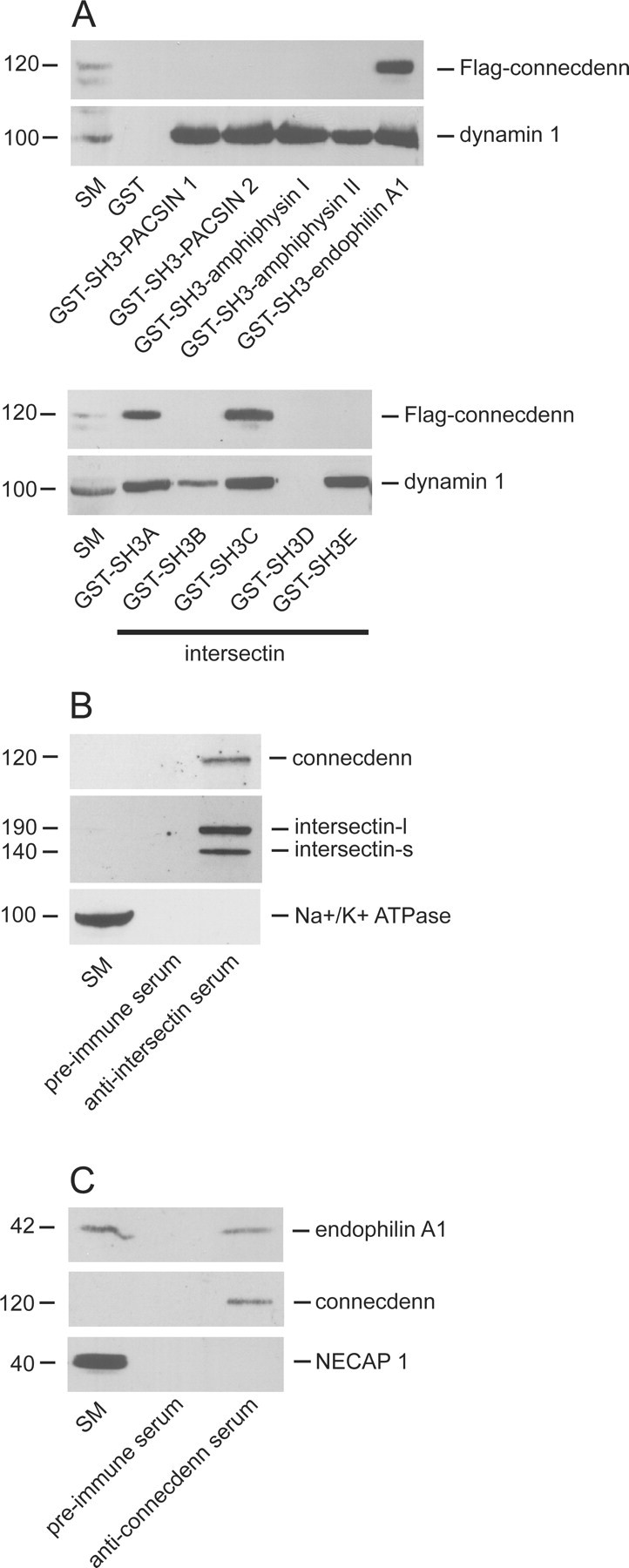
Connecdenn binds to SH3 domains from endocytic proteins. A, Soluble lysates from Flag–connecdenn-transfected HEK-293 cells were incubated with GST or GST fused to the SH3 domain of either amphiphysin I or II, PACSIN 1 or 2, endophilin A1, and the five individual SH3 domains of intersectin, precoupled to glutathione-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. B, Soluble brain lysates were incubated with anti-intersectin serum or preimmune serum from the same rabbit along with protein A-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. C, Soluble brain lysates were incubated with anti-connecdenn serum (3775) or preimmune serum from the same rabbit along with protein A-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. For B and C, Western blots with polyclonal antibodies were revealed with protein A-HRP. For all experiments, an aliquot of starting material (SM) equal to 1/10 of that added to the beads was run in parallel.
Figure 5.
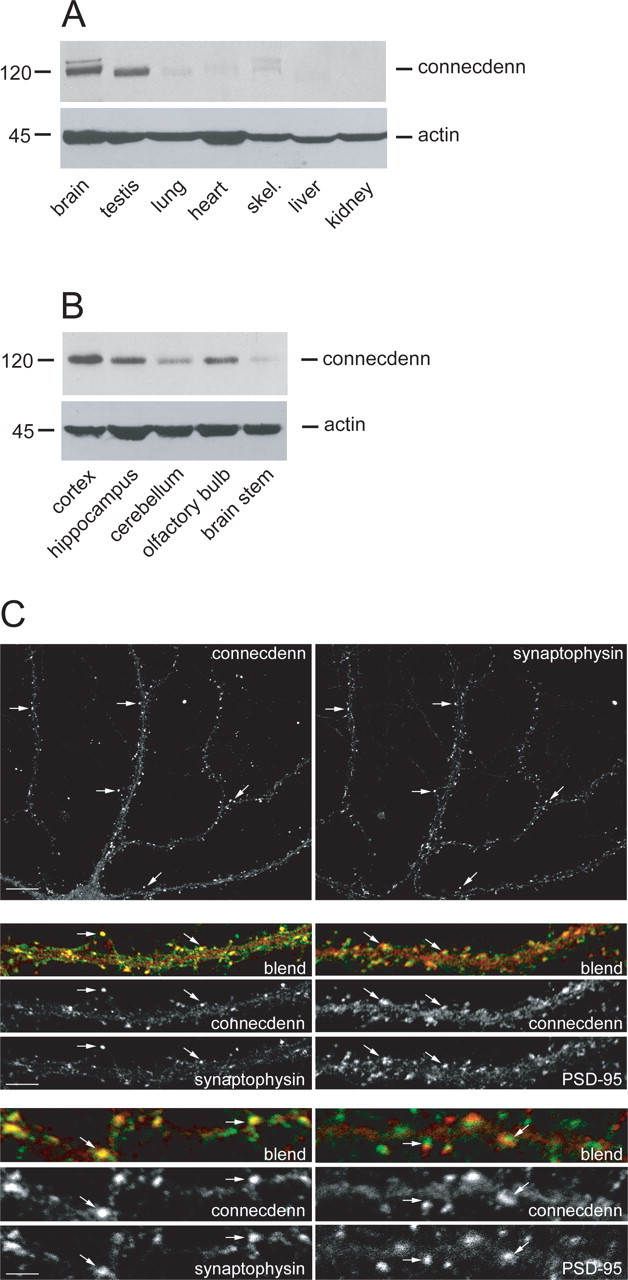
Connecdenn is brain enriched and is detected at the synapse of hippocampal neurons in culture. Equal protein extracts from various rat tissues including skeletal muscle (skel.) (A) and rat brain regions (B) were blotted with antibodies against connecdenn and actin. C, Rat hippocampal neurons at 21 DIV were processed for indirect immunofluorescence with a polyclonal antibody against connecdenn (3775) and monoclonal antibodies against either synaptophysin or PSD-95. For the bottom 12 panels, the blend of connecdenn (red) and synaptophysin or PSD-95 (green) or the individual images are shown. Scale bars: top two panels, 10 μm; middle six panels, 3.33 μm; bottom six panels, 2.33 μm. The percentage of connecdenn-positive punctate that colocalize with synaptophysin-positive punctae is 71% in C, above the average of 45.0% for all cells examined.
Connecdenn is a brain-enriched protein at the synapse
When equal protein amounts of tissue extracts are analyzed, connecdenn is detected at higher levels in brain and testis than in other tissues (Fig. 5A). Within brain, connecdenn is detected in all brain regions examined with variable expression levels (Fig. 5B). To examine the localization of connecdenn, we performed confocal immunofluorescence microscopy of mature hippocampal neurons at 21 DIV. Connecdenn is detected in neuronal cell bodies and can also be seen to extend into dendrites (Fig. 5C). In addition, brighter connecdenn punctae are detected that are situated along dendrites and that colocalize in part with synaptophysin, a presynaptic marker (Fig. 5C). Connecdenn punctae also partially colocalize with PSD-95, a marker of the postsynapse, but more often are found adjacent to PSD-95-positive structures (Fig. 5C). Quantification reveals that 45.0% of all synaptophysin-positive puncta contain connecdenn (23 cells from five experiments), whereas only 19.4% of PSD-95-positive puncta are colocalized with connecdenn (16 cells from three experiments). These data demonstrate that a pool of connecdenn is present at the synapse, with a proportion of the synaptic pool in the presynaptic compartment.
Connecdenn has a stable association with CCV membranes
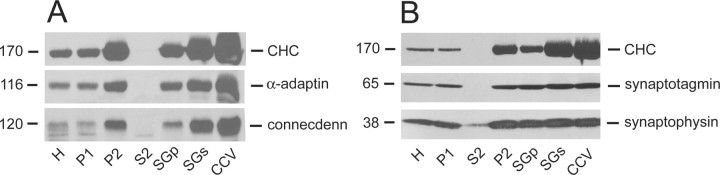
The presence of connecdenn in the presynaptic compartment and its binding to the α-ear of AP-2 prompted us to test whether the protein was present on CCVs. Interestingly, connecdenn is enriched on CCVs to the same extent as CHC and AP-2 (Fig. 6A). Moreover, it shows the same distribution pattern as these two key CCV components throughout the various fractions of the subcellular fractionation procedure (Fig. 6A). In contrast, the abundant SV membrane proteins synaptotagmin and synaptophysin are enriched on CCVs but only by approximately twofold to threefold (Fig. 6B), similar to what has been reported previously (Walch-Solimena et al., 1995; Takei et al., 1998).
Figure 6.
Connecdenn is enriched on CCVs. Equal protein aliquots of the successive fractions of a procedure leading to highly enriched CCVs were analyzed by Western blot with antibodies against CHC, α-adaptin, and connecdenn (A) and CHC, synaptotagmin, and synaptophysin (B). H, Homogenate; P, pellet; S, supernatant; SG, sucrose gradient.
The majority of peripheral membrane proteins of CCVs are stripped from the vesicles under conditions that remove the clathrin coat. Interestingly, connecdenn resists extraction with 0.5 m Tris, which removes clathrin coats (Fig. 7A) (Keen et al., 1979). Connecdenn is stripped from CCVs by pH 11.0, confirming that it is not an integral membrane protein like synaptophysin (Fig. 7A). The association of connecdenn with CCVs stripped of the clathrin coat is stable through a range of NaCl concentrations up to 1 m, indicating that the interaction is likely hydrophobic in nature (Fig. 7B). It is conceivable that, under the stripping conditions used, connecdenn is removed from the membrane but pellets as a result of aggregation. We thus ran stripped CCVs on sucrose gradients to separate the coats from the vesicles. When CCVs are run in control conditions (buffer A), CHC, synaptophysin, and connecdenn comigrate and move deeply into the gradient (Fig. 7C). In stripped CCVs, there is significantly less CHC, and the pool that remains migrates at the top of the gradient, likely attributable to the fact that it comes off the vesicles during the centrifugation step. In contrast, connecdenn and synaptophysin comigrate into deeper gradient fractions, although they do not migrate as fast as intact CCV, likely attributable to the loss of density after removal of the clathrin coat (Fig. 7C). These data support the conclusion that connecdenn remains associated with the vesicles after stripping of the clathrin coat. Interestingly, connecdenn is absent from highly purified SVs (Fig. 7D). Together, these data demonstrate that connecdenn is a CCV protein, suggesting that it is involved in endocytic traffic in the synapse.
Figure 7.
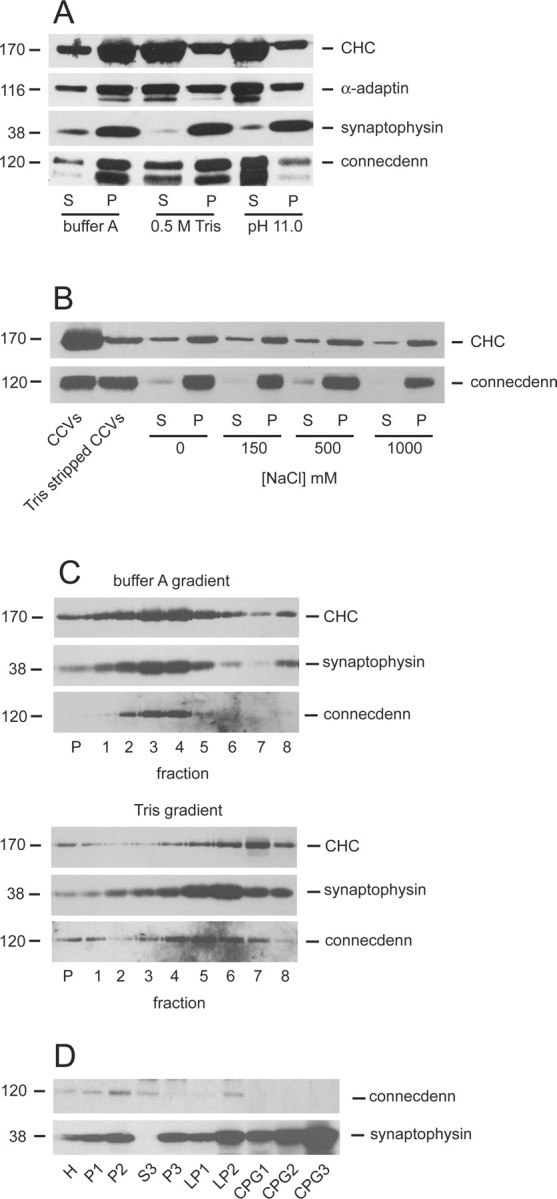
Connecdenn is stably associated with CCV membranes. A, CCVs isolated from rat brain were resuspended in buffer A, 0.5 m Tris buffer, or sodium carbonate buffer at pH 11.0. The samples were centrifuged at high g, and proteins in the supernatant (S) and pellet (P) fractions were analyzed by Western blot with antibodies against the indicated proteins. B, CCVs were stripped of their coats with two successive rounds of incubation with 0.5 m Tris. The resulting vesicles were split in five aliquots, pelleted at high g, and resuspended in Laemmlli sample buffer (Tris-stripped CCVs) or in 10 mm HEPES, pH 7.4, containing 0, 150, 500, or 1000 mm NaCl. Samples were again centrifuged, and the pellet (P) and supernatant (S) fractions were analyzed by Western blot with antibodies against the indicated proteins. C, CCVs in buffer A and CCVs extracted with Tris buffer were loaded on the top of linear 20–50% sucrose gradients prepared in buffer A and Tris buffer, respectively, and were centrifuged at 145,000 × g for 1.5 h. The gradients were fractionated from the bottom, and equal volume aliquots of each fraction along with the pellet (P) were analyzed by Western blot with the indicated antibodies. D, Equal protein aliquots of the successive fractions of a procedure leading to highly enriched SVs were analyzed by Western blot with the indicated antibodies. H, Homogenate; P, pellet; S, supernatant; LP, lysed pellet; LS, lysed supernatant; CPG, controlled pore glass. CPG3 contains purified SVs.
Disruption of connecdenn function inhibits SV endocytosis
To address whether the interaction of connecdenn with its endocytic protein-binding partners is necessary for CME of SVs, we assessed SV uptake using the styryl dye FM4-64 in cultured hippocampal neurons. Neurons were transfected with GFP or GFP–connecdenn peptide motif domain, and FM4-64 uptake was performed and quantified as described in detail in Materials and Methods. FM4-64 uptake in GFP–connecdenn peptide motif domain expressing boutons was 45.9 ± 6.3% of that seen in nontransfected boutons from the same fields (10 fields from three independent cultures), whereas for GFP alone, uptake was 88.2 ± 13.3% of that seen in nontransfected boutons (12 fields from three independent cultures). The effect of the GFP–connecdenn peptide motif domain was significantly greater than that of GFP alone as determined by an ANOVA test followed by a Tukey's post hoc test (p < 0.05). A representative image of neurons transfected with GFP–connecdenn peptide motif domain shows that some GFP–connecdenn-positive boutons had reduced KCl-induced FM4-64 uptake compared with nontransfected neighboring processes, whereas others had no detectable uptake (Fig. 8A). Thus, overexpression of the peptide motif domain of connecdenn is sufficient to disrupt the endocytic machinery required for CME of SVs.
Figure 8.
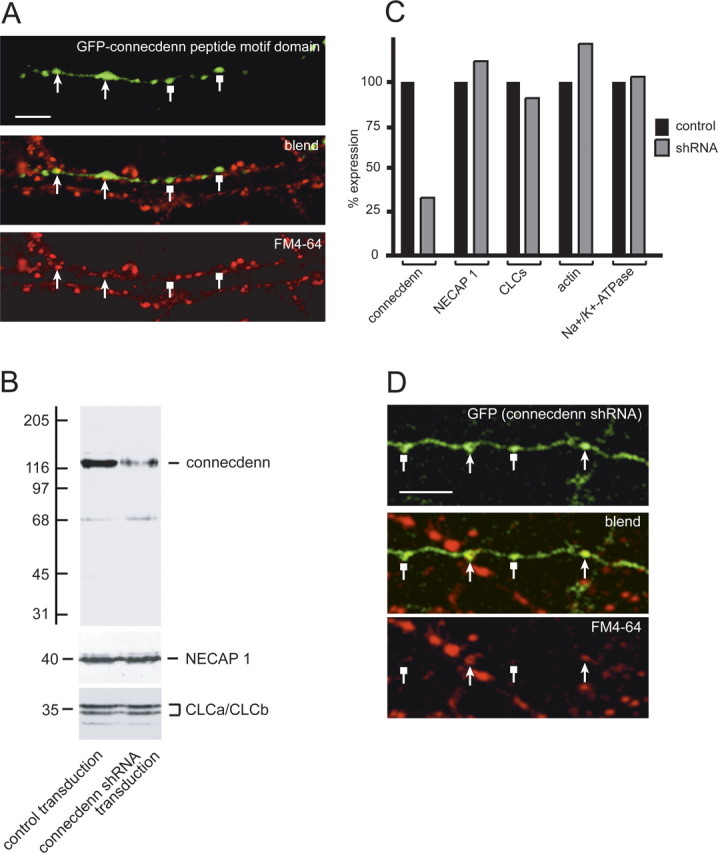
Disruption of connecdenn function reduces CME of SVs in neurons. A, An axon labeled with GFP-connecdenn peptide motif domain is seen to run along a dendrite from a nonexpressing cell. KCl-induced endocytosis of FM4-64 is indicated in red. Note the punctate staining pattern, indicative of FM dye uptake into nonlabeled axons. Note the reduced (arrowheads) or lack of (square heads) uptake in varicosities of the axon expressing GFP–connecdenn peptide motif domain. Scale bars, 5 μm. B, Hippocampal neurons at 14 DIV were transduced with FIV expressing GFP alone (control transduction) or GFP and an shRNA specific for connecdenn. At 21 DIV, protein extracts were made, and equal protein aliquots were analyzed by Western blot with antibodies against NECAP 1 and CLCa/b as well as actin and Na+/K+-ATPase (data not shown). C, Blots were scanned, and the relative amounts of the indicated proteins in control versus shRNA transduced neurons was plotted. D, A representative image of an axon from a neuron transduced with an FIV virus driving expression of GFP and an shRNA specific for connecdenn. The GFP-labeled axon is seen to cross an axon from a nonexpressing cell. KCl-induced endocytosis of FM4-64 is indicated in red. Note the punctate staining pattern, indicative of FM dye uptake into nonlabeled axons. Note the reduced (arrowheads) or lack of (square heads) uptake in varicosities of the axon expressing GFP. Scale bars, 5 μm.
Because the overexpression experiments suggested a role for connecdenn in SV endocytosis, we sought to examine the effect of knocking down the endogenous protein. Lentiviral delivery of shRNA is an emerging approach to perform loss-of-function experiments in nondividing cells such as hippocampal neurons (Janas et al., 2006). We thus generated FIV vectors driving the expression of GFP alone or the parallel expression of GFP and shRNA specific for connecdenn as described in Materials and Methods. Hippocampal cultures were transduced at 14 DIV, and the level of connecdenn was determined at 21 DIV. Connecdenn expression was strongly reduced in neurons transduced with connecdenn shRNA relative to control (Fig. 8B). Quantification of multiple blots revealed a 67% reduction, whereas the levels of other proteins, including NECAP 1, CLCa/b, actin, and Na+/K+-ATPase, were not affected (Fig. 8B,C). FM4-64 uptake was then assessed as described for the dominant-negative experiments using an MOI that led to ∼20% transduction rates. FM4-64 uptake in boutons from neurons transduced with GFP and connecdenn shRNA was 55.4 ± 4.5% of that seen in nontransduced boutons from the same fields (17 fields from three independent cultures), whereas for GFP alone, uptake was 82.4 ± 5.4% of that seen in nontransduced boutons (11 fields from three independent cultures). The effect of connecdenn shRNA expression on FM4-64 uptake was significantly greater than control transduction as determined by an ANOVA test followed by a Tukey's post hoc test (p < 0.05). A representative image of neurons transduced with GFP and connecdenn shRNA shows that GFP-positive boutons had reduced or undetectable KCl-induced FM4-64 uptake compared with nontransduced neighboring processes (Fig. 8D). Together with the observations that connecdenn is highly enriched on CCVs and binds to multiple components of the endocytic machinery, our data demonstrate that connecdenn is a new component of the endocytic machinery for SVs.
Discussion
CME is achieved using a network of enzymatic, mechanical, and scaffolding proteins held together by low-affinity protein–protein and protein–lipid interactions. Hallmark features of the endocytic machinery are the multiple modular protein domains that mediate recognition of small peptide motifs and/or specific phospholipid head groups, such that each single element is interconnected with multiple other components of the machinery. This creates a Velcro-like arrangement in which multiple low-affinity interactions lead to a stable overall structure. This also allows for a dynamic situation in which minor changes in interaction affinities can lead to rapid assembly and disassembly of the machinery. Such a process would be highly cooperative, and, in fact, assembly of the clathrin coat proceeds with a Hill coefficient >6 (Moskowitz et al., 2005).
One of the most important interaction hubs within the endocytic network is AP-2, which participates in clathrin coat assembly and is recruited in a phosphatidylinositol(4,5)P2-dependent manner to the plasma membrane in which its timely recognition of cargo prevents catastrophic disassembly of CCPs (Ehrlich et al., 2004; Honing et al., 2005). In nerve terminals, AP-2 in a complex with stonin 2 interacts with phosphatidylinositol(4,5)P2 and synaptotagmin, allowing for coats to nucleate on SV membranes after SV fusion with the plasma membrane (Haucke and De Camilli, 1999; Diril et al., 2006). AP-2 also serves as a scaffolding platform for a battery of endocytic accessory proteins, including epsin, huntingtin interacting protein 1, AP180, amphiphysin, disabled 2, autosomal recessive hypercholesteremia gene product, and NECAP 1 (Slepnev and De Camilli, 2000; McPherson and Ritter, 2005; Traub, 2005). A common feature of these proteins is the presence of N-terminal globular domains, including E/ANTH (epsin/AP180 N-terminal homology) domains and BAR domains, which function in generating and/or sensing membrane curvature, and PTB (phosphotyrosine binding) domains, which serve as binding modules for endocytic cargo proteins (Traub, 2003; Legendre-Guillemin et al., 2004; McMahon and Gallop, 2005). Our data are the first to indicate that a DENN domain-containing protein is a component of the endocytic machinery. DENN domains are composed of uDENN and dDENN modules flanking a DENN module. These modules form a unit that is well conserved in diverse species, including humans, Caenorhabditis elegans, and Schizosaccharomyces pombe (Levivier et al., 2001). Although the function of the DENN domain is unknown, it is noteworthy that several DENN domain-bearing proteins are implicated in vesicle trafficking including myotubularin-related proteins 5 and 13, Rab6-interacting protein, and DENN/MADD/Rab3GEP (Levivier et al., 2001; Laporte et al., 2003). Interestingly, DENN/MADD/Rab3GEP is a guanine-nucleotide exchange factor for the SV protein Rab3, and knock-out of this protein leads to mice that have decreased numbers of SVs, suggesting a possible alteration in SV reformation (Miyoshi and Takai, 2004). Our data provide a direct link of a DENN domain protein to vesicle trafficking and will allow for a more detailed analysis of the role of DENN domains in membrane trafficking and CME in particular.
In addition to globular N-terminal domains, endocytic accessory proteins generally contain C-terminal peptide motif domains, which are presumably weakly structured and contain multiple sites for interactions with endocytic proteins, including clathrin and AP-2 (Kalthoff et al., 2002; Owen, 2004; Schmid et al., 2006). Connecdenn shares this common topology. Following from the globular N-terminal domain, the tail is presumably poorly structured and harbors three sequences, FSDVF, DPF, and WETFE, that match three distinct motifs that target the platform and sandwich subdomains of the α-ear. In fact, all three motifs contribute to α-ear binding, although the WETFE is the predominant sequence, consistent with the observation that WXXF-acidic motifs generally have higher affinity than DPF/W or FXDXF motifs (Praefcke et al., 2004). Because all three motifs are involved in binding, connecdenn may engage both α-ear subdomains, creating avidity effects that allow for a more stable interaction, consistent with the ability to coimmunoprecipitate connecdenn with AP-2 from brain extracts. Based on co-crystallization of the α-ear with a peptide containing a WXXF-acidic motif from synaptojanin, it was suggested that small, uncharged residues are preferred in the position following the invariant W attributable to their proximity to F740 in the α-ear (Praefcke et al., 2004). Interestingly, NMR analysis demonstrates that the WXXF-acidic motif from connecdenn (WETFE-COO−) interacts with α-ear in the same manner as that from NECAP (WVQF-COO−). Because connecdenn contains a glutamic acid in this position, this indicates that acidic residues are also permissible. Thus, searches for WXXF-acidic motifs need not be limited by small hydrophobic amino acids following the W, broadening the potential scope of proteins that contain the motif. Connecdenn also carries multiple PXXP core sequences, and, in brain, it forms complexes with the endocytic SH3 domain-containing proteins endophilin A1 and intersectin. Although the precise role of connecdenn interactions with AP-2, intersectin, and endophilin remain unknown, disruption of these interactions through overexpression of the peptide motif domain leads to a significant decrease in SV endocytosis.
Although the architecture of connecdenn is similar to classical AP-2-binding accessory proteins, its biochemical association with CCVs is strikingly different. Most accessory proteins are not enriched on CCVs, suggesting that they function relatively early in the formation of a CCP (McPherson and Ritter, 2005). Interestingly, it has been demonstrated recently that the ear domain of the β2-adaptin subunit of the AP-2 complex (β2-ear) plays an important role in the recruitment of accessory proteins (Edeling et al., 2006; Schmid et al., 2006). CHC interacts with the β2-ear, as well as with the region that links the β2-ear with the β2-adaptin core (Owen et al., 2000; Knuehl et al., 2006). It is thought that proteins that are dependent on interactions with β2-ear are lost from CCVs as clathrin assembles and competes with the accessory proteins for the β2-ear-binding site (Edeling et al., 2006; Schmid et al., 2006). In this manner, CHC assembly controls the temporal organization of accessory protein function such that proteins that are required late in the endocytic process are recruited through direct or indirect interactions with the α-ear (Brett and Traub, 2006). Because connecdenn interacts exclusively with the α-ear and is retained on the vesicles, it likely functions later in CCP formation. Moreover, connecdenn remains on CCVs after chemical stripping of clathrin coats. Thus, in addition to functioning in endocytosis, connecdenn could have a post-uncoating role in endocytic vesicle transport or in endosomal function. An example of such a dual function has been reported for clathrin assembly lymphoid myeloid leukemia protein (CALM), a homolog of AP180 that binds to AP-2 (Meyerholz et al., 2005). CALM function is required for CME of epidermal growth factor receptor (Huang et al., 2004) and the protein is enriched in synapses, consistent with a role in CME of SVs (Yao et al., 2005). Interestingly, knocking down CALM leads to a failure of transferrin to accumulate in perinuclear recycling endosomes (Meyerholz et al., 2005), suggesting that CALM knockdown disrupts endosome traffic or fusion.
Given that connecdenn is enriched in brain, is found in the presynaptic terminal, and is a component of the machinery for CME, it most likely functions in the CME of SVs. We thus sought to test for a role for connecdenn in SV endocytosis using a loss of function approach. Knocking down connecdenn by lentiviral delivery of shRNA led to a significant decrease in CME of SVs as measured by FM4-64 dye uptake. Given the complexity and redundancy of the machinery for SV endocytosis, it is perhaps surprising that knockdown of a single component leads to a significant effect on the process. This would suggest that connecdenn is an important component of the machinery. After uncoating, SVs are reloaded with neurotransmitter and are made available for additional rounds of exocytosis, either directly or after additional sorting through an endosomal intermediate (Wenk and De Camilli, 2004). Because connecdenn is absent from SVs, there must be a mechanism to remove the protein from endocytic vesicles before the exocytosis step. Thus, any potential effect of connecdenn on exocytosis would result from indirect effects mediated by its involvement in endocytosis. In summary, our data reveal connecdenn as a novel component of the synaptic endocytosis machinery.
Footnotes
This research was supported by Canadian Institutes of Health Research (CIHR) Grants MOP-13461 (P.S.M.) and MOP-43967 (K.G.). B.R., S.T., and J.L.B. were supported by fellowships from CIHR, the Killam Foundation/Montreal Neurological Institute (MNI), and McGill University, respectively. We acknowledge support from the Québec/Eastern Canada High Field Nuclear Magnetic Resonance Facility, funded by grants from the Canada Foundation for Innovation, the Québec Ministry of Research, Science and Technology, and McGill University. A grant from the Genome Quebec/Genome Canada project Réseau Protéomique de Montréal, Montreal Proteomics Network financially supported this work. P.S.M. is a Fonds de la Recherché en Santé Québec Senior Scholar, a McGill University William Dawson Scholar, and a Killam Scholar of the MNI. We thank Drs. Markus Plomann and Pietro De Camilli for reagents and Dr. Michael Fronda for discussion. We also thank Maria Scheel, Jacynthe Philie, Patrizio Delli Fraine, and Lyne Bourbonniere for excellent technical assistance.
References
- Barik S. Site-directed mutagenesis by double polymerase chain reaction. Mol Biotechnol. 1995;3:1–7. doi: 10.1007/BF02821329. [DOI] [PubMed] [Google Scholar]
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Burman JL, Wasiak S, Ritter B, de Heuvel E, McPherson PS. Aftiphilin is a component of the clathrin machinery in neurons. FEBS Lett. 2005;579:2177–2184. doi: 10.1016/j.febslet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, Solimena M, De Camilli P. Autoimmunity in stiff-Man syndrome with breast cancer is targeted to the C-terminal region of human amphiphysin, a protein similar to the yeast proteins, Rvs167 and Rvs161. FEBS Lett. 1994;351:73–79. doi: 10.1016/0014-5793(94)00826-4. [DOI] [PubMed] [Google Scholar]
- de Heuvel E, Bell AW, Ramjaun AR, Wong K, Sossin WS, McPherson PS. Identification of the major synaptojanin-binding proteins in brain. J Biol Chem. 1997;272:8710–8716. doi: 10.1074/jbc.272.13.8710. [DOI] [PubMed] [Google Scholar]
- Denisov AY, Ritter B, McPherson PS, Gehring K. 1H, 15N and 13C resonance assignments and 15N-1H residual dipolar couplings for the alpha-adaptin ear-domain. J Biomol NMR. 2004;29:441–442. doi: 10.1023/B:JNMR.0000032518.06803.e7. [DOI] [PubMed] [Google Scholar]
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. doi: 10.1016/j.devcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Girard M, Allaire PD, McPherson PS, Blondeau F. Non-stoichiometric relationship between clathrin heavy and light chains revealed by quantitative proteomics of clathrin-coated vesicles from brain and liver. Mol Cell Prot. 2005a;4:1145–1154. doi: 10.1074/mcp.M500043-MCP200. [DOI] [PubMed] [Google Scholar]
- Girard M, Allaire PD, Blondeau F, McPherson PS. Isolation of clathrin-coated vesicles by differential and density gradient centrifugation. In: Bonifacino J, Lippincott-Schwartz J, Dasso M, Harford J, Yamada K, editors. Current protocols in cell biology, Unit 3.13, Subcellular fractionation and isolation of organelles. New York: Wiley; 2005b. pp. 3.13.1–3.13.20. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, Beck CR, Fineberg SK, Stein C, Ochoa D, Davidson BL. Optimization of feline immunodeficiency virus vectors for RNA interference. J Virol. 2006;80:9371–9380. doi: 10.1128/JVI.00958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, De Camilli P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 1999;285:1268–1271. doi: 10.1126/science.285.5431.1268. [DOI] [PubMed] [Google Scholar]
- Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O'Bryan JP, Der CJ, Kay BK, McPherson PS. Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem. 1999;274:15671–15677. doi: 10.1074/jbc.274.22.15671. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas J, Skowronski J, Van Aelst L. Lentiviral delivery of RNAi in hippocampal neurons. Methods Enzymol. 2006;406:593–605. doi: 10.1016/S0076-6879(06)06046-0. [DOI] [PubMed] [Google Scholar]
- Kalthoff C, Alves J, Urbanke C, Knorr R, Ungewickell EJ. Unusual structural organization of the endocytic proteins AP180 and epsin 1. J Biol Chem. 2002;277:8209–8216. doi: 10.1074/jbc.M111587200. [DOI] [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Knuehl C, Chen CY, Manalo V, Hwang PK, Ota N, Brodsky FM. Novel binding sites on clathrin and adaptors regulate distinct aspects of coat assembly. Traffic. 2006;7:1688–1700. doi: 10.1111/j.1600-0854.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Laporte J, Bedez F, Bolino A, Mandel JL. Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet. 2003;12:R285–R292. doi: 10.1093/hmg/ddg273. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- Levivier E, Goud B, Souchet M, Calmels TP, Mornon JP, Callebaut I. uDENN, DENN, and dDENN: indissociable domains in Rab and MAP kinases signaling pathways. Biochem Biophys Res Commun. 2001;287:688–695. doi: 10.1006/bbrc.2001.5652. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–W331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Ritter B. Peptide motifs: building the clathrin machinery. Mol Neurobiol. 2005;32:73–87. doi: 10.1385/MN:32:1:073. [DOI] [PubMed] [Google Scholar]
- Metzler M, Legendre-Guillemin V, Gan L, Chopra V, Kwok A, McPherson PS, Hayden MR. HIP1 functions in clathrin-mediated endocytosis through binding to clathrin and adaptor protein 2. J Biol Chem. 2001;276:39271–39276. doi: 10.1074/jbc.C100401200. [DOI] [PubMed] [Google Scholar]
- Meyerholz A, Hinrichsen L, Groos S, Esk PK, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J Biol Chem. 1997;272:27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Agostinelli NR, Brett TJ, Mizukami I, Ross TS, Traub LM. Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J Biol Chem. 2001;276:46230–46236. doi: 10.1074/jbc.M108177200. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hawryluk MJ, Brett TJ, Keyel PA, Dupin AL, Jha A, Heuser JE, Fremont DH, Traub LM. Dual engagement regulation of protein interactions with the AP-2 adaptor alpha appendage. J Biol Chem. 2004;279:46191–46203. doi: 10.1074/jbc.M408095200. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Dual role of DENN/MADD (Rab3GEP) in neurotransmission and neuroprotection. Trends Mol Med. 2004;10:476–480. doi: 10.1016/j.molmed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz HS, Yokoyama CT, Ryan TA. Highly cooperative control of endocytosis by clathrin. Mol Biol Cell. 2005;16:1769–1776. doi: 10.1091/mbc.E04-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundigl O, Matteoli M, Daniell L, Thomas-Reetz A, Metcalf A, Jahn R, De Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axons and dendrites. J Cell Biol. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Owen DJ. Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem Soc Trans. 2004;32:1–14. doi: 10.1042/bst0320001. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Praefcke GJ, Ford MG, Schmid EM, Olesen LE, Gallop JL, Peak-Chew SY, Vallis Y, Babu MM, Mills IG, McMahon HT. Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 2004;23:4371–4383. doi: 10.1038/sj.emboj.7600445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun AR, Micheva KD, Bouchelet I, McPherson PS. Identification and characterization of a nerve terminal-enriched amphiphysin isoform. J Biol Chem. 1997;272:16700–16706. doi: 10.1074/jbc.272.26.16700. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci USA. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, McPherson PS. Molecular mechanisms in clathrin-mediated membrane budding. In: Keränen S, Jantti J, editors. Topics in current genetics, Vol 10 Regulatory mechanisms of intracellular membrane transport. Berlin: Springer; 2004. pp. 9–37. [Google Scholar]
- Ritter B, Philie J, Girard M, Tung EC, Blondeau F, McPherson PS. Identification of a family of endocytic proteins that define a new alpha-adaptin ear-binding motif. EMBO Rep. 2003;4:1089–1095. doi: 10.1038/sj.embor.7400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Denisov AY, Philie J, Deprez C, Tung EC, Gehring K, McPherson PS. Two WXXF-based motifs in NECAPs define the specificity of accessory protein binding to AP-1 and AP-2. EMBO J. 2004;23:3701–3710. doi: 10.1038/sj.emboj.7600378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA. Kiss-and-run, fuse-pinch-and-linger, fuse-and-collapse: the life and times of a neurosecretory granule. Proc Natl Acad Sci USA. 2003;100:2171–2173. doi: 10.1073/pnas.0530260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak-Chew SY, Mills IG, Benmerah A, McMahon HT. Role of the AP2 beta-appendage hub in recruiting partners for clathrn-coated vesicle assembly. PloS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Diril MK, Jung N, Haucke V. Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc Natl Acad Sci USA. 2004;101:964–969. doi: 10.1073/pnas.0307862100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Quinn CC, Ritter B, de Heuvel E, Baranes D, Plomann M, McPherson PS. The Ras/Rac guanine nucleotide exchange factor mammalian Son-of-sevenless interacts with PACSIN 1/syndapin I, a regulator of endocytosis and the actin cytoskeleton. J Biol Chem. 2001;276:26622–26628. doi: 10.1074/jbc.M100591200. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W. Crystal structure of the endophilin-A1 BAR domain. J Mol Biol. 2005;351:653–661. doi: 10.1016/j.jmb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Kinetics of synaptic depression and vesicle recycling after tetanic stimulation of frog motor nerve terminals. Biophys J. 1998;74:3003–3009. doi: 10.1016/S0006-3495(98)78007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Petralia RS, Bushlin I, Wang Y, Furukawa K. Synaptic distribution of the endocytic accessory proteins AP180 and CALM. J Comp Neurol. 2005;481:58–69. doi: 10.1002/cne.20362. [DOI] [PubMed] [Google Scholar]