Abstract
Background
Leptospirosis is an important zoonotic disease with a global distribution, affecting a wide range of mammalian animals and humans. Japanese encephalitis (JE) virus is the major vector-borne zoonotic disease in the Asia-Pacific region. The main objective of this study was to evaluate the seroprevalence of serovar-specific Leptospira and JE in swine from 10 provinces in Vietnam.
Methods
Samples were initially collected for swine influenza surveillance from March to April 2017 at large-scale farms (with at least 50 sows and/or 250 fattening pigs) with pigs that tested positive for influenza in the previous surveillance period (2015–16).
Findings
A total of 2,000 sera samples were analyzed from 10 provinces. Overall, the seroprevalence of leptospirosis was 21.05% (95% CI: 19.28–22.90) using a cut-off titer of ≥ 1:100. The apparent prevalence of JE was 73.45% (95% CI: 71.46–75.37) while the true prevalence was slightly higher (74.46%, 95% credible interval: 73.73–86.41). We found a relatively high presence of leptospirosis and JE in pigs kept on large farms. Prevalence was comparable with other studies suggesting opportunistic testing of samples collected for other surveillance purposes can be a valuable tool to better understand and prevent the potential transmission of these zoonotic diseases from pigs to people in Vietnam.
Conclusion
Our study provides evidence to veterinarians and animal health professionals for evidence-based practice such as diagnosis, vaccination and zoonotic control. Further investigation into the possible role of different domestic animals, wildlife species or environmental factors is needed to identify the potential risk factors and transmission routes in Vietnam.
Introduction
Leptospirosis is an important zoonotic disease with a global distribution, affecting a wide range of mammalian animals and humans. Japanese encephalitis (JE) virus is the major vector-borne zoonotic disease in the Asia-Pacific region[1–5]. Leptospira spp. are maintained in a wide range of reservoir hosts, such as cattle, pigs, dogs, rats, raccoons, skunks and opossums[6–10]. Leptospirosis is caused by gram-negative bacteria, and more than 260 pathogenic serovars have been identified[11]. Pigs are considered to be reservoir hosts for serovars Bratislava, Pomona and Tarassovi Mitis, and also become infected with Icterohaemorrhagiae from rats and Hardjo from cattle [12–14]. JE virus is a flavivirus and the main cause of viral encephalitis in Asian countries, resulting in 13,600–20,400 deaths per year [15,16]. It is estimated that more than 3 billion people live in endemic areas [17]. JE virus circulates between Culex sp. mosquitoes and domestic/wild birds or pigs [18]. JE in pigs can cause abortion, stillbirth and infertility which may result in significant economic losses to producers [19,20]. Pigs are considered to be amplifying hosts and an important risk factor for transmission of JE to humans [21,22]. Bird migration may play an important role in the spread of JEV[15].
In Vietnam, leptospirosis is a notifiable disease in humans, but few cases (less than 20 cases per year) have been reported to the Ministry of Health. Despite the low numbers of reported cases, leptospirosis is considered to be endemic and serovar Bratislava is commonly observed [23–26]. A study in the Mekong delta reported a seroprevalence of 18.8% among 1,400 randomly selected people aged between 15 and 60 years[27]. A study in pigs, using titer ≥1:100 by the microscopic agglutination test (MAT), found a seroprevalence of 73% among 424 sows in the Mekong delta area[23]. The serovar Bratislava showed the highest followed by Autumnalis.
JE virus was first isolated in 1951 in Vietnam, and then it was detected in birds, pigs and in the late 1960s and 1970s[28,29]. A study to evaluate JE virus infection in mosquitoes in Vietnam from 2006 to 2008 showed that several JE virus genotype I populations were circulating across the country[30]. A recent study found a seroprevalence was 60.4% in 641 samples of pig sera[31]. A JE vaccine was introduced in 1997 and is used for children under five years of age as part of Vietnam’s national immunization program in 12 high-risk diseases in the northern region[4].
To our knowledge, only a few multi-site studies have been implemented to assess the seroprevalence of leptospirosis and JE in swine and these have covered in limited areas in Vietnam. Therefore, the main objective of this study was to evaluate the seroprevalence of serovar-specific Leptospira and JE in swine from 10 provinces in Vietnam.
Material and methods
Study locations and sampling
Samples were initially collected for swine influenza surveillance from March to April 2017 under the Department of Animal Health (DAH) and Food and Agriculture organization (FAO) of the United Nations. The Department selected 10 provinces (Ha Noi, Bac Ninh, Thai Binh, Bac Giang, Quang Ninh, Quang Ngai, Binh Duong, Dong Nai, Dong Thap and Soc Trang) (Fig 1). The sampling was targeted at large-scale farms (with at least 50 sows and/or 250 fattening pigs) with pigs that tested positive for influenza in the previous surveillance period (2015–16, 19 provinces: 3800 sera samples). In each province, 10 blood samples from piglets (4–8 weeks old) and fattening pigs (9–12 weeks old) and five blood samples from sows were collected from four-large scale farms, but the sex of the pigs was not recorded. Pigs with symptoms of influenza were prioritized for sampling.
Fig 1. Selected sampling provinces (in blue) for leptospirosis and JE in swine.
Laboratory analysis
The sera were extracted after centrifugation and stored at -20°C until transportation in a cool box to the National Center for Veterinary Diagnosis (NCVD) in Hanoi. For leptospirosis, the MAT was used to assess the positivity of samples. Two-fold serial dilution was used after an initial 1:100 dilution, and the end-point (1:1600) was the highest dilution of serum that shows at least 50% agglutination of live leptospires compared to the control sample. We considered a sample leptospirosis positive if the MAT was ≥ 1:100 for at least one of the 16 serovars (Table 1). For JE, enzyme-linked immunosorbent assay (ELISA) was used to detect JE virus-specific antibodies in swine serum. We followed the manufacturer’s instructions of JE Ab ELISA protocol (VDPro JE Ab ELISA; Median, Chuncheonsi, Korea). A sample was determined as positive if the S/P ratio () is more than 0.25.
Table 1. List of Leptospira antigens used in the MAT.
| No. | Genomospecies | Serogroup | Serovar |
|---|---|---|---|
| 1 | L. interrogans | Australis | Australis |
| 2 | L. interrogans | Autumnalis | Autumnaliss |
| 3 | L. interrogans | Bataviae | Bataviae |
| 4 | L. interrogans | Australis | Bratislava |
| 5 | L. interrogans | Canicola | Canicola |
| 6 | L. kirschneri | Grippotyphosa | Grippotyphosa |
| 7 | L. interrogans | Hebdomadis | Hebdomadis |
| 8 | L. interrogans | Icterohaemorrhagiae | Icterohaemorrhagiae |
| 9 | L. borgpetersenii | Javanica | Javanica |
| 10 | L. noguchii | Panama | Panama |
| 11 | L. interrogans | Pomona | Pomona |
| 12 | L. interrogans | Pyrogenes | Pyrogenes |
| 13 | L. borgpetersenii | Sejroe | Hardjo |
| 14 | L. borgpetersenii | Sejroe | Saxkoebing |
| 15 | L. biflexa | Semaranga | Patoc |
| 16 | L. borgpetersenii | Tarassovi | Tarassovi |
Data analysis
We estimated the true prevalence (TP) of JE from apparent prevalence (AP) by adjusting for the diagnostic test sensitivity and specificity using a Bayesian framework [32]. The prior distribution for sensitivity [uniform (0.953–0.987)] and specificity [uniform (0.95–0.953)] were obtained from the company protocol. For the TP, a non-informative Jeffreys prior [beta = (0.5, 0.5)] was used to minimize the influence on the posterior [33]. Markov Chain Monte Carlo (MCMC) sampling in “truePrev” was conducted by JAGS through the rjags package [34,35]. The first 1,000 samples of the three MCMC chains were discarded as a burn-in period and the following 10,000 iterations were used for posterior inference. The AP was calculated based on the proportion of positive samples with a 95% Clopper-Pearson/Exact confidence interval (CI). The TP was estimated based on the posterior median value with a 95% credible interval. Statistical significance of differences between prevalence estimates was assessed by examining whether the two 95% CIs overlap. The outputs from the three chains were assessed visually using MCMC trace-plots, posterior density distribution plots, Brooks-Gelman-Rubin (BGR) plots, and auto-correlation plots using the CODA package. All data were entered in Microsoft Excel 2016 and analyzed using R (version 3.5.2). QGIS (Quantum GIS development Team 2018. QGIS version number 3.0.1) was used to generate the map.
Ethics approval and consent to participate
The study was approved by the Hanoi Medical University Institutional Review Board (HMU IRB: no. 00003121), Vietnam.
Results
Leptospirosis
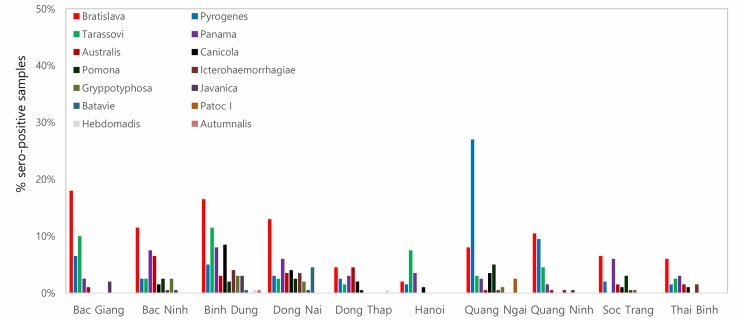
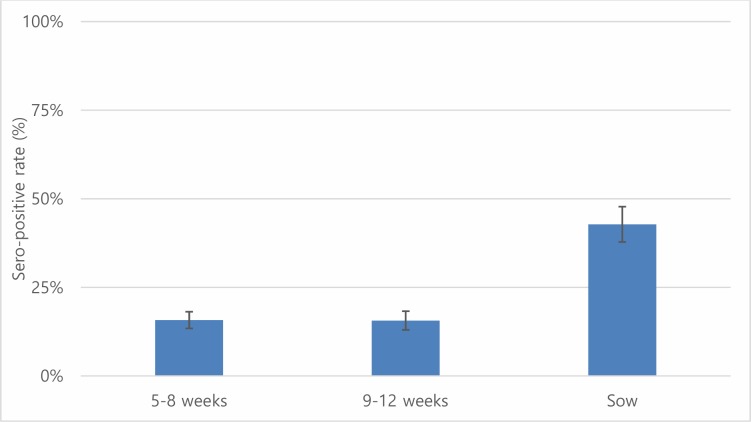
A total of 2,000 sera samples were analyzed from 10 provinces. Overall, the seroprevalence of leptospirosis was 21.05% (95% CI: 19.28–22.90) using a cut-off titer of ≥ 1:100 (Table 2). Quang Ngai showed the highest seroprevalence (37.5%) followed by Binh Duong (32.5%) whereas Soc Trang had the lowest sero-prevalence (10.0%), and the differences were statistically significant. By using a low cut-off titer (≥ 1:100), the most frequently observed presumptive infective serovar was Bratislava (9.65%), followed by Pyrogenes (6.1%) and Tarassovi (4.55%) (Table 3). Using a cut-off titer (≥ 1:200), Bratislava (1.95%) and Panama (0.75%) had the highest seroprevalences. By province, Bratislava had the highest sero-prevalence of the 8 provinces (except Hanoi and Quang Ngai) (Fig 2). Serovar Tarassovi and Pyrogenes had the highest seroprevalences in Hanoi and Quang Ngai, respectively. A total of 144 samples were positive with more than two serovars, which was accounting for 34.20% (95% CI: 29.68–38.95) among positive samples. Two samples from Binh Dung and Dong Nai were positive with maximum seven serovars. The sero-prevalence of sow (42.78%, 95% CI: 37.85–47.83%) was significantly higher than younger age groups (5–8 weeks and 9–12 weeks) (Fig 3).
Table 2. Sero-prevalence (%) with 95% CI for Leptospira serovars in pigs in Vietnam using MAT.
| Province (no.) | Sero-positive samples (a titer≥1:100 for any serovars) |
Sero-positive (%) with 95% CI |
|---|---|---|
| Bac Giang (200) | 61 | 30.5 (24.20–37.39) |
| Bac Ninh (200) | 45 | 22.5 (16.91–28.92) |
| Binh Duong (200) | 65 | 32.5 (26.06–39.47) |
| Dong Nai (200) | 38 | 19.0 (13.81–25.13) |
| Dong Thap (200) | 23 | 11.5 (7.43–16.75) |
| Hanoi (200) | 23 | 11.5 (7.43–16.75) |
| Quang Ngai (200) | 75 | 37.5 (30.77–44.61) |
| Quang Ninh (200) | 43 | 21.5 (16.02–27.85) |
| Soc Trang (200) | 20 | 10.0 (6.22–15.02) |
| Thai Binh (200) | 28 | 14.0 (9.51–19.59) |
| Total (2,000) | 421 | 21.05 (19.28–22.90) |
Table 3. MAT results for Leptospira serovars in pigs by using 3 cutoff titers.
| Serovar | Total samples | ≥ 1:100 | ≥ 1:200 | ≥ 400 |
|---|---|---|---|---|
|
N (%, 95% CI) |
N (%, 95% CI) |
N (%, 95% CI) |
||
| Australis | 2,000 | 45 (2.25, 1.65–3.00) | 4 (0.2, 0.005–0.05) | 0 |
| Autumnalis | 2,000 | 1 (0.05, 0.0001–0.28) | 0 | 0 |
| Bataviae | 2,000 | 10 (0.5, 0.24–0.92) | 1 (0.05, 0.0001–0.28) | 0 |
| Bratislava | 2,000 | 193 (9.65, 8.39–11.03) | 39 (1.95, 1.39–2.66) | 8 (0.4, 0.17–0.79) |
| Canicola | 2,000 | 45 (2.25, 1.65–3.00) | 2 (0.1, 0.01–0.36) | 0 |
| Grippotyphosa | 2,000 | 18 (0.9, 0.53–1.42) | 2 (0.1, 0.01–0.36) | 0 |
| Icterohaemorrhagiae | 2,000 | 22 (1.1, 0.69–1.66) | 3 (0.15, 0.03–0.44) | 0 |
| Javanica | 2,000 | 13 (0.65, 0.35–1.11) | 4 (0.2, 0.005–0.05) | 0 |
| Panama | 2,000 | 87 (4.35, 3.50–5.34) | 15 (0.75, 0.42–1.23) | 2 (0.1, 0.01–0.36) |
| Pomona | 2,000 | 31 (1.55, 1.06–2.19) | 3 | 0 |
| Pyrogenes | 2,000 | 122 (6.1, 5.09–7.24) | 8 (0.4, 0.17–0.79) | 0 |
| Hardjo | 2,000 | 0 | 0 | 0 |
| Sakoebing | 2,000 | 0 | 0 | 0 |
| Tarassovi | 2,000 | 91 (4.55, 3.68–5.56) | 8 (0.4, 0.17–0.79) | 1 (0.05, 0.0001–0.28) |
| Patoc | 2,000 | 5 (0.25, 0.08–0.58) | 1 (0.05, 0.0001–0.28) | 0 |
Fig 2. Proportion of sero-positive samples by serovar in each province using cutoff titer ≥ 1:100.
Fig 3. Seropositive rates of leptospirosis with 95% confidence interval by age group in pigs using cut off titer ≥ 1:100.
Japanese encephalitis (JE)
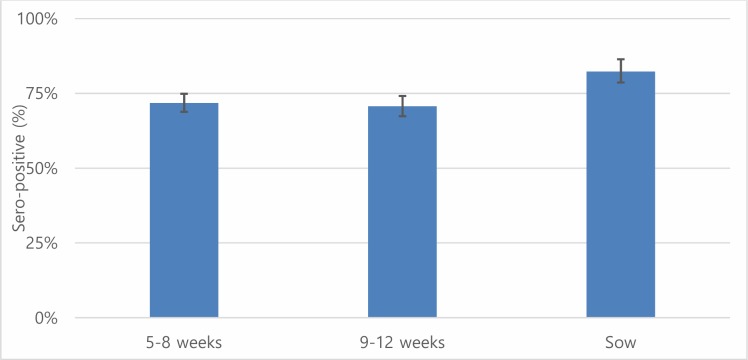
Overall, the AP of JE was 73.45% (95% CI: 71.46–75.37) while the TP was slightly higher (74.46%, 95% credible interval: 73.73–86.41) (Table 4). Dong Thap had the highest seroprevalence followed by Thai Binh whereas Binh Dung showed the lowest seroprevalence. Overall, the AP showed slightly higher than the TP except for Binh Duong province. The seroprevalence in sows (82.27%, 95% CI: 78.15–85.92) was significantly higher than that in animals of other age groups (Fig 4). The BGR plots showed that our chains have converged for TP, sensitivity and specificity, respectively (Fig 5).
Table 4. Apparent sero-prevalence with 95% CI and true sero-prevalence with 95% credible interval for Japanese encephalitis in pigs in Vietnam.
| Province (no.) | Sero-positive samples | Apparent sero-prevalence (%) with 95% CI |
True sero-prevalence (%)a with 95% credible interval |
|---|---|---|---|
| Bac Giang (200) | 158 | 79.0 (72.69–84.43) | 80.47 (73.73–86.41) |
| Bac Ninh (200) | 141 | 70.5 (63.66–76.72) | 71.21 (63.97–77.96) |
| Binh Duong (200) | 122 | 61.0 (53.87–67.80) | 60.92 (53.20–68.25) |
| Dong Nai (200) | 155 | 77.5 (71.08–83.09) | 78.83 (71.96–84.88) |
| Dong Thap (200) | 168 | 84.0 (78.17–88.79) | 85.93 (79.73–91.36) |
| Hanoi (200) | 125 | 62.5 (55.39–69.23) | 62.55 (55.14–69.70) |
| Quang Ngai (200) | 128 | 64.0 (56.93–70.65) | 64.22 (56.66–71.37) |
| Quang Ninh (200) | 161 | 80.5 (74.32–85.75) | 82.12 (75.58–87.89) |
| Soc Trang (200) | 145 | 72.5 (65.76–78.56) | 73.46 (66.30–79.94) |
| Thai Binh (200) | 166 | 83.0 (77.06–87.93) | 84.79 (78.46–90.39) |
| Total (2,000) | 1,469 | 73.45 (71.46–75.37) | 74.46 (71.84–77.06) |
a Median value was recorded.
Fig 4. Seropositive rates of Japanese encephalitis with 95% confidence interval by age group in pigs.
Fig 5. Brooks-Gelman-Rubin (BGR) plots for true prevalence (TP), sensitivity (SE) and specify (SP).
Discussion
A nation-wide study on seroprevalences of leptospirosis and JE in pigs was conducted in Vietnam. Overall, the seroprevalence (21.05%; 95% CI: 19.28–22.90) of leptospirosis was higher than that reported by Lee et al. (8.17%)[36]. A possible explanation is that pig samples from that study were randomly collected from slaughterhouses and it may be that healthy or less visually ill pigs were more likely to be sent for slaughter. Other studies conducted in the early 2000s reported higher seroprevalences (26%-73%) using a cut-off titer of ≥ 1:100 [23,37]. These study sites were mostly in the Mekong Delta, close to river areas, so that pigs in these areas may have more opportunities exposure. However, in our study, samples from three provinces located in the Mekong Delta had a lower seroprevalence [(Soc Trang (10.0%), Dong Thap (11.5%) and Dong Nai (19.0%)] than other provinces. Therefore, further investigation is necessary to assess the association between environmental factors and leptospirosis.
Our study found that Bratislava and Tarassovi Mitis were more common, similar to the previous studies [23,36]. Mostly, pigs are considered to be the main host for serovars Tarassovi Mitis, Pomona, Bratislava and Muenchen [13,38,39]. Other studies found that Bratislava, Pomona and Tarassovi Mitis were commonly detected in wild boars [40,41]. Therefore, there is a possibility of leptospirosis transmission from wild boars to domesticated pigs. Further study is needed to better understand the role of wild animals in the transmission of leptospirosis in Vietnam. Serovars Pyogenes and Panama were newly detected in our study, which have been observed in cattle in other countries [42–44]. Interestingly, significantly higher prevalence of serovar Pyrogenes was detected in Quang Ngai. According to the Vietnam General statistics office, Quang Ngai has the third largest cattle population in the country (approximately 277,000), after Nghe An and Gia Lai (all central areas) [45], where was only included from the central region for our study. Therefore, it would be worthwhile to conduct an epidemiological investigation on the circulation of serovar Pyrogenes in the central region, where the largest cattle population is raised in Vietnam. Moreover, Icterohaemorrhagiae and Javanica were observed that rat may play an important role in the transmission to pigs [46]. One study suggests that older pigs have a higher chance of exposure to Letospira which is consistent with our result [23]. In humans, leptospirosis is one of 28 notifiable diseases in Vietnam. A total of 85 cases were officially recorded between 2008 and 2015. The low number of notifications is due to lack of public awareness and diagnostic facilities in rural areas resulting in under-reporting. Therefore, it is important to raise public awareness, especially among high-risk groups (e.g. agriculture/livestock farmers, mining workers, veterinarians and slaughterhouse workers) who are more likely to come in contact with infected animals and contaminated water or soil.
Overall, the seroprevalence (73.45%) of JE in pigs was similar to that in neighboring countries (Laos and Cambodia [65–75%]) [47,48], but higher than that in some other Asian countries (Indonesia, Nepal and Taiwan) [49–52]. In Vietnam, pigs were not vaccinated against JE virus mainly due to economic reasons, implying that positivity was due to natural infection. Furthermore, seroprevalence (82%) in pigs older than two months showed that detected antibodies were caused by virus infections rather than maternal immunity (one study found that maternal antibodies disappeared after two months [53]). Therefore, we can conclude that JE is widespread in the pig population across the country. In Southeast Asia, it is well documented that JE is endemic and a public health concern. Current pig production practices may pose a risk for JE, because people live very close to pigs, increasing exposure to mosquitoes with JE virus infection. In Vietnam, small-scale backyard pig production accounts for 70–75% of the total production [54].
The average seroprevalence from five northern provinces was 75.1% (95% CI: 72.42–77.78) which was significantly higher than the previous study (60.4% among 641 pig sera), sampling conducted in the northern part of Vietnam from 2009 to 2010 [31]. However, there is no evidence that JE is increasing over time in Vietnam. Over the last decade, in Vietnam, livestock production is undergoing a major change, a moving toward intensive systems and large production scales [55]. Therefore, a possible explain is the growth of intensive pig farms which provides more opportunities to encounter mosquitoes with JE virus infection.
The potential limitation is that sero-prevalences of leptospirosis and JE were likely to be higher than healthy pigs as our sampling population was targeted to large-scale farms with previously tested positive for influenza surveillance. Some studies have suggested that viral infections in animals and humans induce long-lasting immunosuppression in hosts [56,57]. Due to a random error with our measuring instrument, the TP of JE may be under/overestimated. In addition, our prior estimates for the sensitivity and specify of the JE virus test were obtained from the company protocol, and it is not clear to what extent these estimates are appropriate for the local, Vietnamese context.
In humans, most cases are in unvaccinated children under 14 years of age[58]. JE vaccine was first introduced in 1997 through the national immunization program for children aged 1–5 years and 85% of districts were covered in 2013. Currently, there are no routine pig national surveillance programs for JE virus. Therefore, it is difficult to assess the potential role of pigs in the transmission cycle to humans as well as the impact of the national immunization program.
We found less discrepancy between them as sensitivity and specificity are relatively high (> 95%) as well as a higher proportion of positive sample. A Bayesian approach provides an opportunity to combine prior information with investigated data and estimate values for both prevalence and diagnostic characteristics of tests[59]. If a diagnostic test with less than 100% sensitivity and specificity is used to estimate the prevalence of a disease, our results would be biased. Therefore, a Bayesian approach was used to estimate the TP from AP. In general, Bayesian models are sensitive to the selection of the priors, so we employed a Jeffreys prior to minimize the influence of prior to the posterior distribution as no prior information was available for JE prevalence in our study area. Our study was the first attempt to estimate the TP for JE in Vietnam, which showed how a Bayesian approach can be used to better estimate the prevalence of animal diseases.
This study shows the added value of opportunistic use of samples collected for one purpose in providing valuable information on other diseases. An advantage is the large sample size and wide cover. A disadvantage is potential bias linked with sample collection from large farms with previous influenza detection and preference given to apparently sick pigs.
Conclusions
We found a relatively high presence of leptospirosis and JE in pigs kept on large farms. Prevalence was comparable with other studies suggesting opportunistic testing of samples collected for other surveillance purposes can be a valuable tool to better understand and prevent the potential transmission of these zoonotic diseases from pigs to people in Vietnam. Our study provides evidence to veterinarians and animal health professionals for evidence-based practice such as diagnosis, vaccination and zoonotic control. Further investigation into the possible role of different domestic animals, wildlife species or environmental factors is needed to identify the potential risk factors and transmission routes in Vietnam.
Supporting information
(ODS)
Acknowledgments
We thank the Department of Animal Health under the Vietnam Ministry of Agriculture and Rural Development and the Food and Agriculture Organization of the United Nations office in Vietnam for sharing samples. We also thank Tezira Lore of the International Livestock Research Institute for editing the manuscript.
Abbreviations
- JE
Japanese encephalitis
- CI
confidence interval
- TP
true prevalence
- AP
apparent prevalence
- ELISA
enzyme-linked immunosorbent assay
- MCMC
Markov Chain Monte Carlo
- BGR
Brooks-Gelman-Rubin
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We acknowledge the CGIAR Trust Fund, grant no. 1111 to DG (https://www.cgiar.org/funders), the Australian Centre for International Agricultural Research, Irish Aid, the European Union, the International Fund for Agricultural Development and the governments of the Netherlands, New Zealand, Switzerland, the United Kingdom, the United States of America and Thailand for funding to the CGIAR Research Program on Climate Change, Agriculture and Food Security. The study was also supported by the CGIAR Research Program on Agriculture for Nutrition and Health, led by the International Food Policy Research Institute.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. The Lancet infectious diseases. Elsevier; 2003;3: 757–771. [DOI] [PubMed] [Google Scholar]
- 2.Cachay ER, Vinetz JM. A global research agenda for leptospirosis. Journal of postgraduate medicine. NIH Public Access; 2005;51: 174. [PMC free article] [PubMed] [Google Scholar]
- 3.Faine S. Leptospira and leptospirosis CRC Press Inc.; 1994. [Google Scholar]
- 4.Yen NT, Duffy MR, Hong NM, Hien NT, Fischer M, Hills SL. Surveillance for Japanese encephalitis in Vietnam, 1998–2007. The American journal of tropical medicine and hygiene. ASTMH; 2010;83: 816–819. 10.4269/ajtmh.2010.10-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie JS, Williams DT, Smith DW. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. Perspectives in Medical Virology. Elsevier; 2006;16: 201–268. [Google Scholar]
- 6.Brown CA, Roberts AW, Miller MA, Davis DA, Brown SA, Bolin CA, et al. Leptospira interrogans serovar grippotyphosa infection in dogs. Journal of the American Veterinary Medical Association. 1996;209: 1265–1267. [PubMed] [Google Scholar]
- 7.Hanson LE. Leptospirosis in domestic animals: the public health perspective. Journal of the American Veterinary Medical Association. 1982;181: 1505–1509. [PubMed] [Google Scholar]
- 8.THIERMANN AB. The Norway rat as a selective chronic carrier of Leptospira icterohaemorrhagiae. Journal of wildlife diseases. Wildlife Dis Assoc; 1981;17: 39–43. [DOI] [PubMed] [Google Scholar]
- 9.Mikaelian I, Higgins R, Lequient M, Major M, Lefebvre F, Martineau D. Leptospirosis in raccoons in Quebec: 2 case reports and seroprevalence in a recreational area. The Canadian Veterinary Journal. Canadian Veterinary Medical Association; 1997;38: 440 [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan JE and, Karstad L. Field and laboratory studies of skunks, raccoons and groundhogs as reservoirs of Leptospira pomona. The Canadian Veterinary Journal. Canadian Veterinary Medical Association; 1965;6: 243 [PMC free article] [PubMed] [Google Scholar]
- 11.Levett PN. Leptospirosis. Clinical microbiology reviews. United States; 2001;14: 296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstein T, Baker JA. Leptospirosis in swine caused by Leptospira pomona. The Journal of infectious diseases. JSTOR; 1954; 53–64. 10.1093/infdis/94.1.53 [DOI] [PubMed] [Google Scholar]
- 13.Ryan TJ, Marshall RB. Isolation of a leptospire belonging to serogroup tarassovi Taylor & Francis; 1976; [DOI] [PubMed] [Google Scholar]
- 14.Bolin CA, Cassells JA, Hill HT, Frantz JC, Nielsen JN. Reproductive failure associated with Leptospira interrogans serovar bratislava infection of swine. Journal of Veterinary Diagnostic Investigation. SAGE Publications Sage CA: Los Angeles, CA; 1991;3: 152–154. 10.1177/104063879100300209 [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature medicine. Nature Publishing Group; 2004;10: S98 10.1038/nm1144 [DOI] [PubMed] [Google Scholar]
- 16.WHO. Japanese encephalitis. In: WHO [Internet]. Available: http://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis
- 17.Nations D of E and SAPDU. World urbanization prospects: the 2005 revision. United Nations New York; 2006.
- 18.Scherer WF, Buescher EL, Flemings MB, Noguchi A, Scanlon J. Ecologic studies of Japanese encephalitis virus in Japan. The American journal of tropical medicine and hygiene. ASTMH; 1959;8: 665–677. [PubMed] [Google Scholar]
- 19.Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. The Lancet. Elsevier; 2011;377: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarantola A, Goutard F, Newton P, De Lamballerie X, Lortholary O, Cappelle J, et al. Estimating the burden of Japanese encephalitis virus and other encephalitides in countries of the mekong region. PLoS neglected tropical diseases. Public Library of Science; 2014;8: e2533 10.1371/journal.pntd.0002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon T. Control of Japanese encephalitis—within our grasp? New England Journal of Medicine. Mass Medical Soc; 2006;355: 869–871. 10.1056/NEJMp058263 [DOI] [PubMed] [Google Scholar]
- 22.Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Reviews Microbiology. Nature Publishing Group; 2004;2: 789 10.1038/nrmicro1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boqvist S, Chau BL, Gunnarsson A, Engvall EO, Vågsholm I, Magnusson U. Animal-and herd-level risk factors for leptospiral seropositivity among sows in the Mekong delta, Vietnam. Preventive Veterinary Medicine. Elsevier; 2002;53: 233–245. [DOI] [PubMed] [Google Scholar]
- 24.Boqvist S, Thi VTH, Magnusson U. Annual variations in Leptospira seroprevalence among sows in southern Vietnam. Tropical animal health and production. Springer; 2005;37: 443–449. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Khong NV, Xuan HN, Nghia VB, Nguyen-Viet H, Grace D. tnamSero-prevalence of specific Leptospira serovars in fattening pigs from 5 provinces in Vie. BMC veterinary research. BioMed Central; 2017;13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laras K, Van Cao B, Bounlu K, Nguyen TKT, Olson JG, Thongchanh S, et al. The importance of leptospirosis in Southeast Asia. The American journal of tropical medicine and hygiene. ASTMH; 2002;67: 278–286. 10.4269/ajtmh.2002.67.278 [DOI] [PubMed] [Google Scholar]
- 27.Van CTB, Thuy NTT, San NH, Hien TT, Baranton G, Perolat P. Human leptospirosis in the Mekong delta, Viet Nam. Transactions of the Royal Society of Tropical Medicine and Hygiene. Elsevier; 1998;92: 625–628. 10.1016/s0035-9203(98)90787-8 [DOI] [PubMed] [Google Scholar]
- 28.Tam NH, Yen NT. Japanese encephalitis in Vietnam 1985–93. Southeast Asian J Trop Med Public Health. 1995;26: 47–50.8629140 [Google Scholar]
- 29.Okuno T. An epidemiological review of Japanese encephalitis. World health statistics quarterly Rapport trimestriel de statistiques sanitaires mondiales. 1978;31: 120–133. [PubMed] [Google Scholar]
- 30.Kuwata R, Nga PT, Yen NT, Hoshino K, Isawa H, Higa Y, et al. Surveillance of Japanese encephalitis virus infection in mosquitoes in Vietnam from 2006 to 2008. The American journal of tropical medicine and hygiene. ASTMH; 2013;88: 681–688. 10.4269/ajtmh.12-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruget A, Beck C, Gabassi A, Trevennec K, Lecollinet S, Chevalier V, et al. Japanese encephalitis circulation pattern in swine of northern Vietnam and consequences for swine’s vaccination recommendations. Transboundary and emerging diseases. Wiley Online Library; 2018; [DOI] [PubMed] [Google Scholar]
- 32.Messam LLM, Branscum AJ, Collins MT, Gardner IA. Frequentist and Bayesian approaches to prevalence estimation using examples from Johne’s disease. Animal Health Research Reviews. Cambridge University Press; 2008;9: 1–23. 10.1017/S1466252307001314 [DOI] [PubMed] [Google Scholar]
- 33.Jeffreys H. An invariant form for the prior probability in estimation problems. Proc R Soc Lond A. The Royal Society; 1946;186: 453–461. [DOI] [PubMed] [Google Scholar]
- 34.Plummer M. JAGS: Just another Gibbs sampler. 2004.
- 35.Devleesschauwer B, Torgerson P, Charlier J, Levecke B, Praet N, Roelandt S, et al. Package ‘prevalence.’ 2015;
- 36.Lee HS, Khong NV, Xuan HN, Nghia VB, Nguyen-Viet H, Grace D. Sero-prevalence of specific Leptospira serovars in fattening pigs from 5 provinces in Vietnam. BMC veterinary research. BioMed Central; 2017;13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boqvist S, Montgomery JM, Hurst M, Thu HTV, Engvall EO, Gunnarsson A, et al. Leptospira in slaughtered fattening pigs in southern Vietnam: presence of the bacteria in the kidneys and association with morphological findings. Veterinary microbiology. Elsevier; 2003;93: 361–368. [DOI] [PubMed] [Google Scholar]
- 38.Rocha T. Isolation of Leptospira interrogans serovar mozdok from aborted swine fetuses in Portugal. Veterinary Record. 1990;126. [PubMed] [Google Scholar]
- 39.Ellis WA, McParland PJ, Bryson DG, Cassells JA. Prevalence of Leptospira infection in aborted pigs in Northern Ireland. The Veterinary Record. 1986;118: 63–65. 10.1136/vr.118.3.63 [DOI] [PubMed] [Google Scholar]
- 40.Jansen A, Luge E, Guerra B, Wittschen P, Gruber AD, Loddenkemper C, et al. Leptospirosis in urban wild boars, Berlin, Germany. Emerging infectious diseases. Centers for Disease Control and Prevention; 2007;13: 739 10.3201/eid1305.061302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vale-Gonçalves HM, Cabral JA, Faria MC, Nunes-Pereira M, Faria AS, Veloso O, et al. Prevalence of Leptospira antibodies in wild boars (Sus scrofa) from Northern Portugal: risk factor analysis. Epidemiology & Infection. Cambridge University Press; 2015;143: 2126–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins G, Loureiro AP, Hamond C, Pinna MH, Bremont S, Bourhy P, et al. First isolation of Leptospira noguchii serogroups Panama and Autumnalis from cattle. Epidemiology & Infection. Cambridge University Press; 2015;143: 1538–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel JM, Vihol PD, Prasad MC, Kalyani IH, Raval JK, Patel KM, et al. Seroepidemiological pattern of leptospirosis in bovine of South Gujarat, India. Veterinary World. Veterinary World; 2014;7. [Google Scholar]
- 44.Feresu SB, Bolin CA, Korver H, Terpstra WJ. Classification of leptospires of the pyrogenes serogroup isolated from cattle in Zimbabwe by cross-agglutinin absorption and restriction fragment length polymorphism analysis. International Journal of Systematic and Evolutionary Microbiology. Microbiology Society; 1994;44: 541–546. [DOI] [PubMed] [Google Scholar]
- 45.GSO. General Statistics Office of Vietnam [Internet]. 2018. Available: https://www.gso.gov.vn/default_en.aspx?tabid=774%3E
- 46.Loan HK, Cuong N Van, Takhampunya R, Kiet BT, Campbell J, Them LN, et al. How Important Are Rats As Vectors of Leptospirosis in the Mekong Delta of Vietnam? VECTOR-BORNE AND ZOONOTIC DISEASES. 140 HUGUENOT STREET, 3RD FL, NEW ROCHELLE, NY 10801 USA: MARY ANN LIEBERT, INC; 2015;15: 56–64. 10.1089/vbz.2014.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conlan J V, Vongxay K, Jarman RG, Gibbons R V, Lunt RA, Fenwick S, et al. Serologic study of pig-associated viral zoonoses in Laos. The American journal of tropical medicine and hygiene. ASTMH; 2012;86: 1077–1084. 10.4269/ajtmh.2012.11-0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duong V, Sorn S, Holl D, Rani M, Deubel V, Buchy P. Evidence of Japanese encephalitis virus infections in swine populations in 8 provinces of Cambodia: implications for national Japanese encephalitis vaccination policy. Acta tropica. Elsevier; 2011;120: 146–150. 10.1016/j.actatropica.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 49.Pant GR. A serological survey of pigs, horses, and ducks in Nepal for evidence of infection with Japanese encephalitis virus. Annals of the New York Academy of Sciences. Wiley Online Library; 2006;1081: 124–129. 10.1196/annals.1373.013 [DOI] [PubMed] [Google Scholar]
- 50.Chang KJ. Seasonal prevalence of anti-Japanese encephalitis virus antibody in pigs in different regions of Taiwan. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2002;35: 12–16. [PubMed] [Google Scholar]
- 51.Thakur KK, Pant GR, Wang L, Hill CA, Pogranichniy RM, Manandhar S, et al. Seroprevalence of Japanese encephalitis virus and risk factors associated with seropositivity in pigs in four mountain districts in Nepal. Zoonoses and public health. Wiley Online Library; 2012;59: 393–400. 10.1111/j.1863-2378.2012.01456.x [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka A, Mulyatno KC, Susilowati H, Hendrianto E, Utsumi T, Amin M, et al. Prevalence of antibodies to Japanese encephalitis virus among pigs in Bali and East Java, Indonesia, 2008. Jpn J Infect Dis. 2010;63: 58–60. [PubMed] [Google Scholar]
- 53.Scherer WF, Moyer JT, Izumi T. Immunologic studies of Japanese Encephalitis Virus in Japan: V. Maternal antibodies, antibody responses and viremia following infection of swine. The Journal of Immunology. Am Assoc Immnol; 1959;83: 620–626. [PubMed] [Google Scholar]
- 54.Lapar MLA. Identifying barriers to entry to livestock input and output markets in South-East Asia: the case of Vietnam. ILRI (aka ILCA and ILRAD); 2003. [Google Scholar]
- 55.Dinh XT, Cassou E, Cao BT. An Overview of Agricultural Pollution in Vietnam: The Livestock Sector World Bank Group: Washington, DC, USA: 2017; [Google Scholar]
- 56.English R V, Johnson CM, Gebhard DH, Tompkins MB. In vivo lymphocyte tropism of feline immunodeficiency virus. Journal of virology. Am Soc Microbiol; 1993;67: 5175–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trautwein G, Hewicker-Trautwein M. Immunopathogenesis of virus diseases of cats and dogs. Tierarztliche Praxis. 1994;22: 63–72. [PubMed] [Google Scholar]
- 58.WPRO. Questions and Answers on Japanese Encephalitis [Internet]. 2019. Available: http://www.wpro.who.int/vietnam/topics/immunization/faq_japanese_encephalitis/en/
- 59.Dorny P, Phiri IK, Vercruysse J, Gabriël S, Willingham Iii AL, Brandt J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. International journal for parasitology. Elsevier; 2004;34: 569–576. 10.1016/j.ijpara.2003.11.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ODS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.