Abstract
The “toxin-resistant” R-type Ca2+ channels are expressed widely in the CNS and distributed mainly in apical dendrites and spines. They play important roles in regulating signal transduction and intrinsic properties of neurons, but the modulation of these channels in the mammalian CNS has not been studied. In this study we used whole-cell patch-clamp recordings and found that muscarinic activation enhances R-type, but does not affect T-type, Ca2+ currents in hippocampal CA1 pyramidal neurons after N, P/Q, and L-type Ca2+ currents selectively were blocked. M1/M3 cholinergic receptors mediated the muscarinic stimulation of R-type Ca2+ channels. The signaling pathway underlying the R-type enhancement was independent of intracellular [Ca2+] changes and required the activation of a Ca2+-independent PKC pathway. Furthermore, we found that the enhancement of R-type Ca2+ currents resulted in the de novo appearance of Ca2+ spikes and in remarkable changes in the firing pattern of R-type Ca2+ spikes, which could fire repetitively in the theta frequency. Therefore, muscarinic enhancement of R-type Ca2+ channels could play an important role in modifying the dendritic response to synaptic inputs and in the intrinsic resonance properties of neurons.
Keywords: acetylcholine, muscarinic, R-type calcium spikes, α1E calcium subunits, CaV2.3, T-type calcium currents, theta, epilepsy, oscillations, hippocampus
Introduction
R-type voltage-sensitive Ca2+ currents originally were identified as the high voltage-activated (HVA) Ca2+ currents that were resistant to the antagonists ω-conotoxin MVIIC, ω-conotoxin-GVIA, ω-agatoxin IVA, and the dihydropyridines (Zhang et al., 1993; Randall and Tsien, 1995). In hippocampal CA1 pyramidal neurons the R-type voltage-sensitive Ca2+ channels (VSCCs) are highly expressed in distal dendrites (Christie et al., 1995; Magee and Johnston, 1995) and are thought to be primarily responsible for Ca2+ influx in dendrites and spines (Sabatini and Svoboda, 2000; Yasuda et al., 2003). R-type Ca2+ currents are involved in generating action potential bursts and afterdepolarizations (Magee and Carruth, 1999; Metz et al., 2005) and in the induction of synaptic plasticity (Isomura et al., 2002; Breustedt et al., 2003; Dietrich et al., 2003; Yasuda et al., 2003).
Modulation of R-type Ca2+ currents could have profound impacts on dendritic excitability via modification of intrinsic firing patterns and the integrative properties of dendrites. Brief trains of backpropagating action potentials have been shown to depress Ca2+ entry through R-type VSCCs located in dendritic spines and thereby block theta burst-induced long-term potentiation (LTP) (Yasuda et al., 2003). In expression systems the R-type Ca2+ currents caused by recombinant CaV2.3 (α1E) VSCCs (Piedras-Renteria and Tsien, 1998; Sochivko et al., 2002; Bannister et al., 2004) are stimulated by the activation of coexpressed muscarinic (Meza et al., 1999; Melliti et al., 2000; Bannister et al., 2004) or metabotropic glutamate receptors (mGluRs) (Stea et al., 1995). However, the modulation of R-type Ca2+ currents by muscarinic or metabotropic receptors has not been examined in native neurons in brain slices or in vivo. Therefore, we used whole-cell recordings to examine whether Ca2+ currents caused by R-type VSCCs are enhanced in hippocampal brain slices by muscarinic activation. We show that both R-type Ca2+ currents and spikes are enhanced by the stimulation of muscarinic receptors in CA1 pyramidal neurons. This is in striking contrast to the extensively studied depression of N-, P-/Q-, and L-type Ca2+ currents by the activation of muscarinic receptors (Gahwiler and Brown, 1987; Shapiro et al., 1999, 2001; Stewart et al., 1999). Furthermore, initiation of dendritic Ca2+ spikes has been suggested to play a role in generating or shaping neuronal network oscillations (Kamondi et al., 1998; Buzsaki, 2002). Interestingly, we found that muscarinic stimulation leads to remarkable and novel changes in the R-type Ca2+ spike firing pattern. After muscarinic receptor stimulation the enhanced R-type Ca2+ spikes repetitively fired at theta frequencies (6–10 Hz), and blocking R-type VSCCs depressed carbachol-induced spontaneous field potential theta oscillations, suggesting that enhanced R-type calcium spikes play a role in dendritic bursting and network oscillations. Therefore, muscarinic receptor activation in hippocampal neurons will alter profoundly the dendritic integration and intrinsic resonance properties by shifting the normal pattern of Ca2+ entry from the slowly inactivating N-, P/Q-, and L-type VSCCs to domination by the HVA rapidly inactivating R-type VSCCs.
Materials and Methods
Hippocampal slice preparation.
Hippocampal slices were prepared from Sprague Dawley rats, aged postnatal days 13–16, according to standard procedures (Fraser and MacVicar, 1996). Our experiments were approved by the Canadian Council for Animal Care and the University of British Columbia Animal Care Committee. All experiments were conducted in strict accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, the rats were anesthetized deeply with halothane and decapitated rapidly. The brain was removed quickly, and horizontal hippocampal slices (∼400 μm) were cut with a vibratome (VT100, Leica, Willowdale, Ontario, Canada) in chilled (0–4°C) slicing solution containing the following (in mm): 75 sucrose, 87 NaCl, 25 NaHCO3, 25 d-glucose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, and 7.0 MgCl2, pH 7.3. Then the slices were transferred to a storage chamber with fresh artificial CSF (ACSF) containing the following (in mm): 126 NaCl, 2.5 KCl, 2.0 MgCl2, 2.0 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 d-glucose, pH 7.3, and were incubated at room temperature for >1 h before recording. All solutions were saturated with 95% O2/5% CO2.
Whole-cell patch-clamp recordings.
Whole-cell voltage-clamp or current-clamp recordings from CA1 neurons within hippocampal slices (Blanton et al., 1989) were obtained at room temperature (22–24°C). Individual slices were transferred to a recording chamber located on an upright microscope (Axioskop, Zeiss, Oberkochen, Germany) and perfused rapidly with oxygenated ACSF (2 ml/min). Patch electrodes (3–5 MΩ) were pulled from 1.5 mm outer diameter thin-walled glass capillaries (150F-4, World Precision Instruments, Sarasota, FL) in three stages on a Flaming-Brown micropipette puller (model P-97, Sutter Instruments, Novato, CA) and were filled with intracellular solution containing the following (in mm): 115 Cs-methanesulphonate, 25 TEA-Cl, 10 HEPES, 1.1 EGTA, 0.1 CaCl2, 4 Mg-ATP, and 0.5 Na-GTP, pH 7.2. Intracellular [Ca2+] was calculated to be 16 nm. In some experiments BAPTA was substituted for EGTA, as described. In the BAPTA experiments stable whole-cell recordings were maintained for >15–20 min before recording to ensure that the BAPTA diffused into dendritic regions. R-/T-type Ca2+ currents were isolated pharmacologically by preincubating the slices in a mixture containing ω-conotoxin MVIIC (2 μm), ω-conotoxin-GVIA (2 μm), and ω-agatoxin IVA (0.4 μm) to block N-, P-, and Q-type Ca2+ currents and cytochrome c (0.1 mg/ml) to block nonspecific toxin binding for >1 h at room temperature. Nifedipine (20 μm) and tetrodotoxin (TTX; 1.2 μm) were bath applied to block L-type Ca2+ currents and Na+ currents, respectively. Also, 2 mm CsCl and 1 mm 4-aminopyridine (4-AP) were bath applied to block residue K+ channels.
Membrane potentials and currents were monitored with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), acquired via a Digidata 1200 series analog-to-digital interface onto a Pentium computer with Clampex 9.0 software (Molecular Devices). Data were sampled at 10 kHz, and most were low-pass filtered (four-pole Bessel) at 1 kHz. Data were not filtered for tail current analysis. For voltage-clamp recordings the leakage and capacitive currents were subtracted by using a P/−4 protocol (four negative correction pulses, with amplitude one-fourth of that of the test pulse). In current-clamp recordings, bridge balance and capacitance compensation were performed. Access resistance was monitored continuously, and only cells with access resistance <20 MΩ were used.
Extracellular recordings.
For extracellular recordings the slices were transferred to an interface chamber (32–34°C; Fine Science Tools, Vancouver, British Columbia). We recorded extracellular potentials with glass micropipettes filled with ACSF (1–3 MΩ). Recording electrodes were positioned in the CA1 pyramidal cell layer, and signals were acquired via an A-M Systems amplifier (low filter, 1.0 Hz; high filter, 5.0 kHz; 1000×) (model 1800 Microelectrode, A-M Systems, Sequim, WA). Before spontaneous field activity was recorded, slice viability and stability were verified by the recording of evoked field EPSPs. Glass micropipettes filled with ACSF were used to stimulate electrically the Schaffer collateral pathway (0.03 Hz). Stable recordings (∼20 min) with a population spike that had peak-to-peak amplitudes of >10 mV were used.
Reagents.
TTX, ω-conotoxin-GVIA, and ω-agatoxin IVA were purchased from Alamone Labs (Jerusalem, Israel); ω-conotoxin MVIIC from Bachem (Torrance, CA); nickel (II) chloride from J.T. Baker (Paris, KY); pirenzepine, protein phosphatase 2 (PP2), and 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF 109203x) from Tocris (Ellisville, MO); BAPTA from Molecular Probes (Eugene, OR); 3-[1–3-(amidinothio)-propyl-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl)maleimide (Ro 31-8220), 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl-N-methyl-benzylamine) (KN-93), and 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Go 6976) from Calbiochem (La Jolla, CA); all other reagents were purchased from Sigma (St. Louis, MO).
Data analysis.
Data were analyzed with Clampfit 9.0 (Molecular Devices). In all cases Student's t tests were used for statistical comparisons, with p < 0.001 considered significant. Values are reported as the mean ± SEM.
Results
Carbachol enhances toxin-resistant HVA R-type currents but does not affect low voltage-activated T-type Ca2+ currents
We tested the hypothesis that muscarinic receptor stimulation enhances R-type VSCCs in CA1 pyramidal neurons similar to the muscarinic enhancement of recombinant CaV2.3 VSCCs described in human embryonic kidney (HEK) 293 cells (Melliti et al., 2000; Bannister et al., 2004). We performed voltage-clamp recordings to characterize the cholinergic modulation of the toxin-resistant Ca2+ currents, which were isolated with a mixture containing TTX, nifedipine, and VSCC toxins (as described in Materials and Methods). CA1 pyramidal neurons from hippocampal slices were patch clamped with a Cs+- and TEA+-based intracellular solution to suppress K+ channels and to minimize muscarinic-mediated effects on K+ channels. The ACSF contained Cs+ and 4-AP to block potassium current and to increase the space-clamp efficiency. Under these conditions we observed an enhancement of HVA Ca2+ currents. In the presence of the VSCC blocker mixture toxin-resistant low (T-type) and high (R-type) voltage-activated transient Ca2+ currents were recorded in all cells (Fig. 1A). The classification of currents was based on the potentials for activation, toxin resistance, and Ni2+ sensitivity. Only the HVA R-type current was enhanced dramatically after the application of carbachol (n = 13) (Fig. 1B). This carbachol-mediated stimulation was reversible (n = 6) (Fig. 1C), and the currents were sensitive to 50 μm Ni2+ (n = 5) (Fig. 1D). Strikingly, the I–V curve of the Ca2+ currents showed that the T-type component (observed as a shoulder in the I–V at –60 to –30 mV) was not affected, whereas the peak of the R-type current was enhanced dramatically in carbachol (Fig. 1E). The averaged I–V curve before and after carbachol treatment is shown in Figure 2A (n = 7). The peak amplitudes of T-type and R-type currents are plotted in Figure 2B, showing that the T-type component did not change (99.6 ± 2% of control; p = 0.76; n = 10), whereas the R-type component increased significantly (154 ± 4% of control; p < 0.0001; n = 13). The example traces for T-type and R-type are shown in Figure 2C. The enhanced amplitude of the R-type Ca2+ current was associated consistently with a significant shift of the voltage-dependent activation curve to negative potentials, with a change in the voltage for half-maximal activation (Vact) (ΔVact= –4.2 ± 0.7 mV; p < 0.001; n = 7) (Fig. 2D). The enhancement of R-type Ca2+ currents was reversible and blocked by atropine (1 μm), demonstrating that it was attributable to muscarinic receptor activation by carbachol (n = 6) (Fig. 2E). Pooled data for muscarinic modulation of R-type and T-type VSCCs are shown in Figure 2F (for each group, n ≥ 5). These results suggest that, in CA1 pyramidal neurons, muscarinic activation enhances only the HVA R-type Ca2+ currents, but not the low voltage-activated (LVA) T-type Ca2+ currents.
Figure 1.
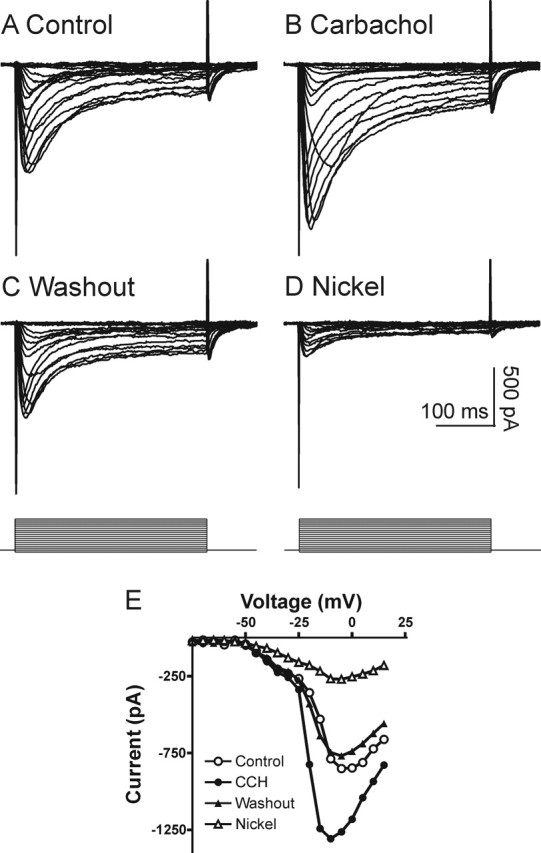
Carbachol enhances toxin-resistant HVA Ca2+ currents. Whole-cell patch-clamp recordings were performed with a Cs+- and TEA+-based intracellular solution. Slices were preincubated with toxins (as described in Materials and Methods) and perfused with TTX (1.2 μm) and nifedipine (20 μm). A, In voltage-clamp mode, a well clamped Ca2+ current was recorded. B, The current was enhanced by the application of carbachol (30 μm). This enhancement was reversible (C) and sensitive to Ni2+ (50 μm; D). E, I–V relationships of this cell before and after treatment, showing that the T-type component (observed as a shoulder at –60 to –30 mV in the I–V) was not affected, whereas the peak of the R-type current was enhanced dramatically in carbachol (CCH).
Figure 2.
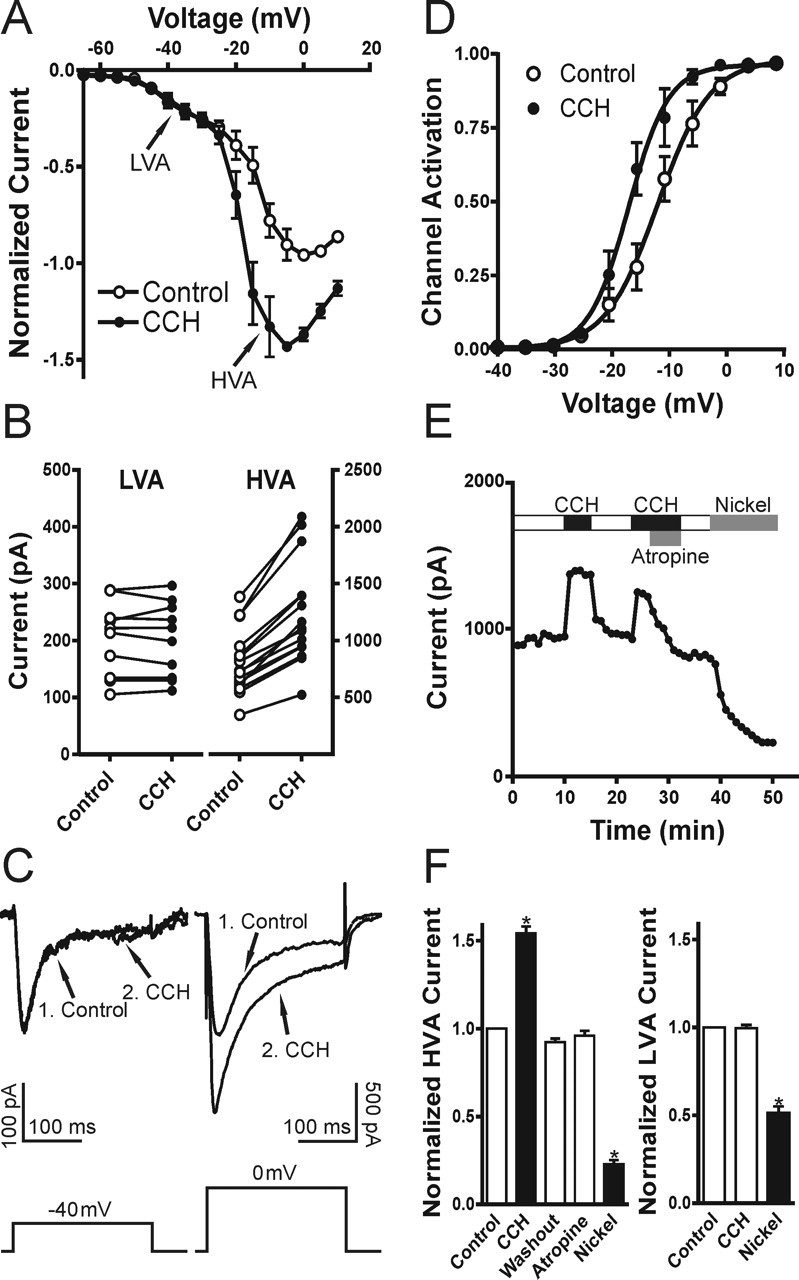
Muscarinic activation enhances R-type, but not T-type, Ca2+ current. A–C, HVA, but not LVA, component is stimulated by muscarinic activation. The mean I–V relationships before and after carbachol (CCH) treatment from seven cells are shown in A. The peak current amplitudes and sample traces are shown in B, C (1, Control; 2, CCH). D, The Ca2+ channel activation curve analyzed from peak currents and estimated conductance is shifted significantly to the left. The result was similar when tail currents were analyzed (data not shown). The result was fit with a Boltzmann equation. E, Time course of the peak HVA Ca2+ current shows that carbachol (CCH) stimulation of R-type VSCCs is reversible and is blocked by the muscarinic receptor antagonist atropine (1 μm). F, Mean data for the muscarinic modulation of the LVA and HVA Ca2+ current components. All recordings were obtained in TTX and VSCC blockers (as described in Materials and Methods). Error bars indicate the mean ± SEM. Asterisk indicates significant change (∗p < 0.001).
To verify additionally that T-type Ca2+ currents were not modulated by muscarinic activation, we first blocked all HVA Ca2+ currents by perfusing 30 μm Cd2+ instead of the strategy used above with toxins and nifedipine. This is effective for isolating T-type Ca2+ currents, because the HVA Ca2+ currents are much more sensitive to low concentrations of Cd2+ (Ozawa et al., 1989; Mogul and Fox, 1991; Avery and Johnston, 1996; Huguenard, 1996). Under these conditions a LVA component was observed with no apparent HVA component (n = 7) (Fig. 3A). Application of Cd2+ (30 μm) only slightly decreased T-type Ca2+ currents (91 ± 6% of control; p = 0.68; n = 7) (Fig. 3B,C), whereas the R-type Ca2+ currents were abolished (11 ± 7% of control; p < 0.0001; n = 7) (Fig. 3B,D). We then tested the modulation of Cd2+-isolated T-type Ca2+ currents by muscarinic receptor activation. In agreement with our results described above, we did not observe any significant alteration of the isolated T-type Ca2+ currents by carbachol (104 ± 7% of control; p = 0.57; n = 6) (Fig. 3E–G). The averaged I–V curves for the effects of carbachol on T-type Ca2+ currents are shown in Figure 3F. Application of Ni2+ (50 μm) suppressed this current by ∼50% (n = 5; p < 0.0001) (Fig. 3E,G). Because low micromolar concentrations of Ni2+ block only α1H subunits, but not α1G or α1I (Klockner et al., 1999; Lee et al., 1999; Perez-Reyes, 2003), our results suggest the existence of multiple subunits of T-type VSCCs in CA1 pyramidal neurons, which is consistent with previous in situ hybridization work (Talley et al., 1999). These results confirmed that, in CA1 pyramidal neurons, muscarinic activation had no effect on T-type Ca2+ currents.
Figure 3.
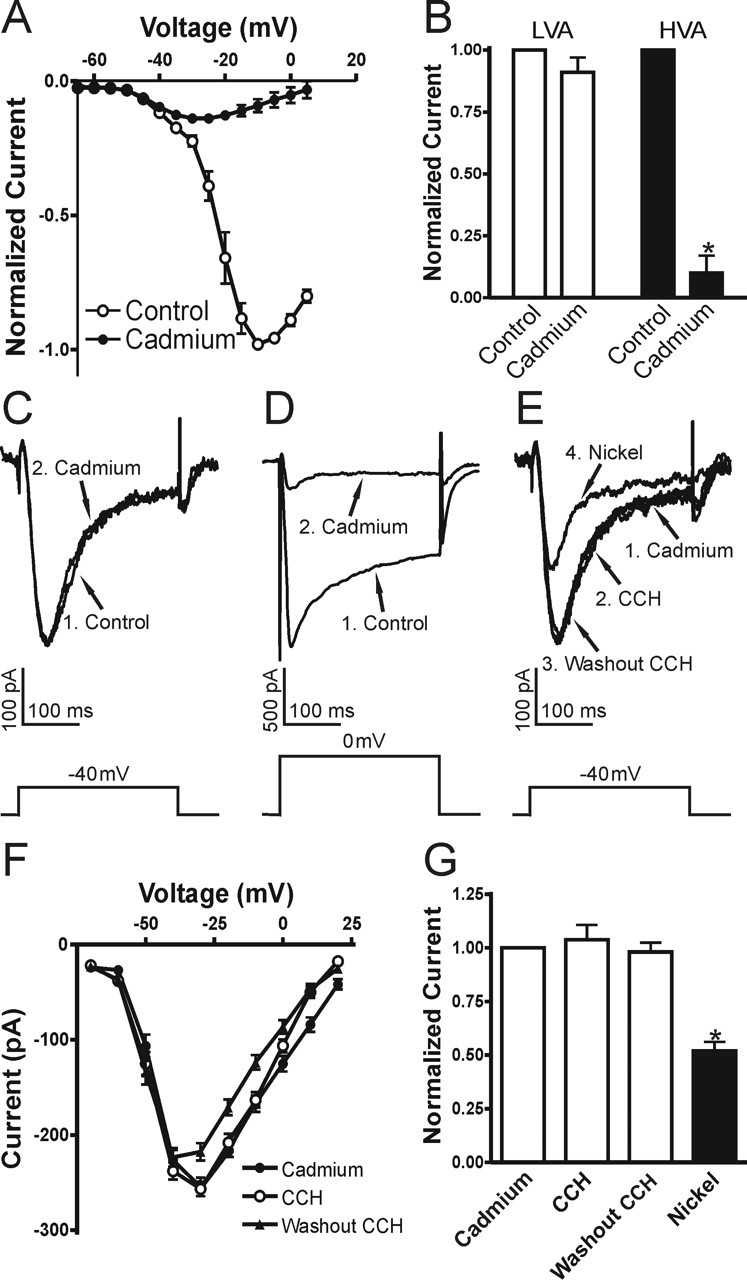
T-type Ca2+ current is not affected in carbachol. Cd2+ (30 μm) was used to isolate the LVA VSCCs. A, Mean I–V curve of Cd2+-isolated Ca2+ current (n = 7). Mean data for Cd2+ effects in B show that the LVA component was decreased slightly, whereas the HVA component was nearly diminished by Cd2+ (30 μm). C, D, Sample traces for LVA and HVA currents before (1, Control) and after (2, Cadmium) Cd2+ treatment are shown. E, The Cd2+-isolated LVA Ca2+ current (1, Cadmium) was not affected by carbachol (2, CCH) or during washout (3, Washout CCH) but was blocked partially by subsequent treatment with Ni2+ (4, Nickel; 50 μm). F, Mean I–V curves for effects of carbachol (CCH and Washout CCH) on the Cd2+-isolated T-type Ca2+ currents (n = 5). G, Mean data for muscarinic modulation of Cd2+-isolated T-type VSCCs (for each group, n ≥ 5). Error bars indicate the mean ± SEM. Asterisk indicates significant change (∗p < 0.001).
M1/M3 cholinergic receptors mediate muscarinic stimulation of R-type VSCCs
The ability of atropine (1 μm) to block the stimulation of carbachol in R-type Ca2+ currents demonstrates that muscarinic receptors mediate this enhancement. In the hippocampus four of the muscarinic subtypes (M1–M4) are expressed abundantly (Vilaro et al., 1993; Levey et al., 1995; Rouse et al., 1999). To determine further the muscarinic receptor subtypes involved in the enhancement of R-type Ca2+ currents, we tested the antagonists and agonists for M1–M4 cholinergic receptors. We found that both pirenzepine (1 μm; n = 5), a M1-specific antagonist, and 4-diphenylactoxy-N-methylpiperidine (4-DAMP; 1 μm; n = 4), an antagonist with equal affinity to both M1 and M3 receptors, could reverse the carbachol enhancement of R-type Ca2+ currents (Fig. 4A–C). In contrast, the M2/M4 antagonists methatropine (1 μm) and tropicamide (1 μm) had no effect (n = 5) (Fig. 4C). To confirm the involvement of M1/M3 subtypes, we tested the M1 agonist 4-N-[3-chlorophenyl]carbamoyloxy-2-butynyl-trimethylammonium chloride (McN-A-343; 100 μm). We found that McN-A-343 mimicked the carbachol-mediated enhancement of R-type Ca2+ currents (n = 6), and, when applied first, McN-A-343 occluded additional enhancement by the subsequent treatment of carbachol (30 μm; n = 4) (Fig. 4D–F). Thus the muscarinic enhancement of R-type Ca2+ currents is mediated by M1/M3 subtypes.
Figure 4.
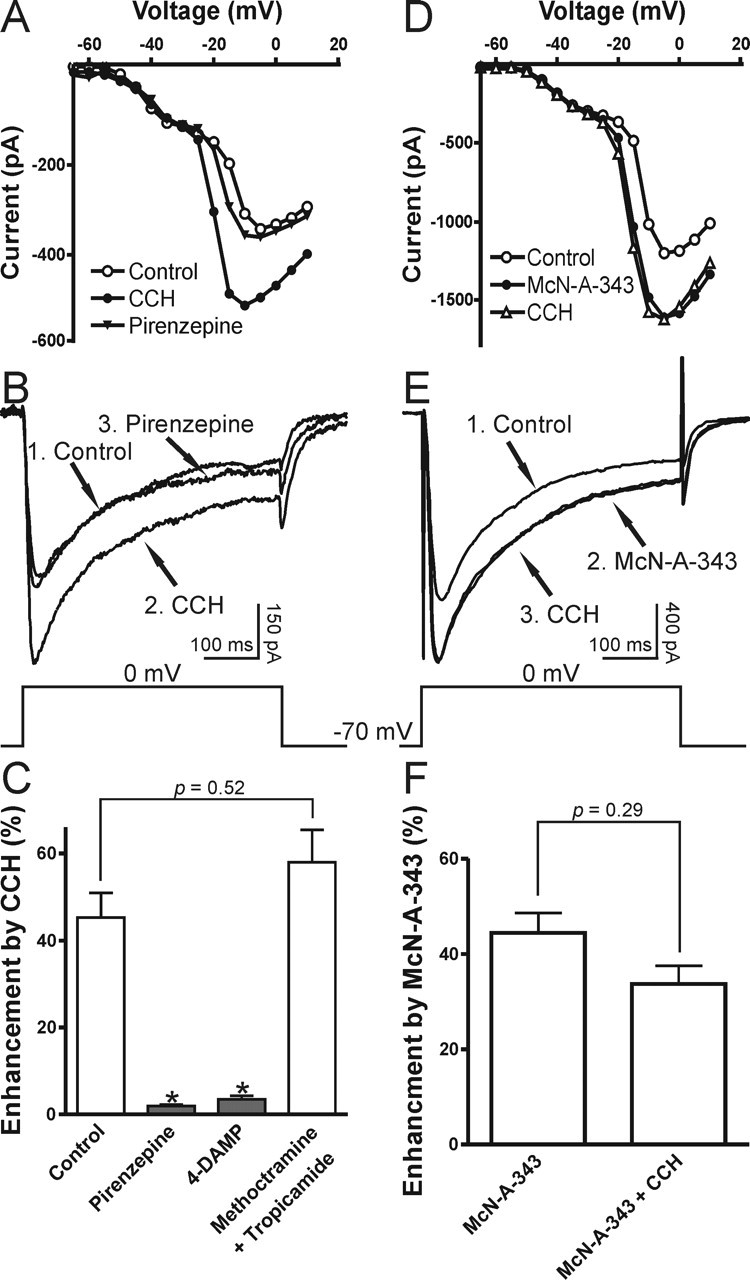
M1/M3 muscarinic subtypes mediate the stimulation of R-type Ca2+ current. A–C, The M1 antagonist pirenzepine (1 μm) reversed the stimulation of R-type VSCCs by carbachol (CCH). I–V curves and sample traces are shown in A, B (1, Control; 2, CCH; 3, Pirenzepine). C, Mean data for the effects of pirenzepine (1 μm), 4-DAMP (1 μm; M1/M3 antagonist), and methoctramine plus tropicamide (1 μm; M2 and M4 antagonists) on the enhancement of R-type VSCCs. D–F, M1 agonist McN-A-343 (100 μm) mimicked and occluded the stimulation of R-type VSCCs by carbachol (CCH). I–V curves and sample traces are shown in D, E (1, Control; 2, McN-A-343; 3, CCH). F, Mean data for the effects of McN-A-343 and the subsequent application of carbachol (CCH) on R-type VSCCs. All recordings were obtained in TTX and VSCC blockers (as described in Materials and Methods). Error bars indicate the mean ± SEM. Asterisk indicates significant change (∗p < 0.001).
Muscarinic modulation of R-type VSCCs requires a Ca2+-independent PKC pathway
In recombinant systems the stimulation of CaV2.3 Ca2+ currents is dependent on phosphorylation that is mediated by a pathway coupled to a pertussis toxin-insensitive Gα subunit (Gαq/11) (Bannister et al., 2004). M1/M3 receptors couple to Gαq subunits to stimulate phospholipase Cβ1 (PLCβ1), which initiates phosphatidylinositol 4,5-bisphosphate (PIP2) turnover. This leads to the production of diacylglycerol (DAG) and IP3-mediated Ca2+ release, which in turn activates protein kinase C (PKC). Basically, there are three groups of PKCs: the Ca2+- and DAG-dependent isoforms (group I), the Ca2+-independent but DAG-dependent isoforms (group II), and the atypical isoforms (group III). We examined the signaling mechanisms underlying the enhancement of R-type Ca2+ currents by first testing the Ca2+ dependence and then by examining the sensitivity to different PKC inhibitors. A high concentration of high-affinity Ca2+ chelator BAPTA (10 mm) was used in the pipette solution, which has proved to be sufficient to block completely the carbachol-induced plateau potentials or tail currents in our lab (Fraser and MacVicar, 1996; Kuzmiski and MacVicar, 2001). We found that 10 mm BAPTA could not prevent the carbachol enhancement of R-type VSCCs (n = 5) (Fig. 5A,D), suggesting it involves a Ca2+-independent pathway. To investigate the potential involvement of PKC in the enhancement of R-type VSCCs, we preincubated slices for >30 min with the broad spectrum PKC antagonists GF 109203x (10 μm; n = 5) or Ro 31-8220 (10 μm; n = 5). In the presence of these PKC antagonists carbachol did not enhance R-type Ca2+ currents (Fig. 5B,E), suggesting that PKCs are involved in this modulation. Interestingly, in the presence of PKC inhibitors (GF 109203x or Ro 8332) a significant suppression of R-type Ca2+ currents was observed by muscarinic stimulation (Fig. 5B,E). This inhibitory effect probably results from the activation of pertussis toxin-sensitive G-protein-coupled M2/M4 receptors and Gβγ subunit-mediated inhibition (Meza et al., 1999; Bannister et al., 2004). Because we observed a stable enhancement of R-type currents in normal conditions, we did not study this inhibitory effect further. To determine which group of PKCs is involved, we applied the specific group I (Ca2+-dependent) PKC inhibitor Go 6976 (10 μm; n = 5). Application of Go 6976 did not block the carbachol-mediated enhancement of R-type Ca2+ currents (Fig. 5C,E). Ca2+/calmodulin-dependent protein kinase II (CaMKII) and Src-family signaling pathways also have been reported to be involved in some muscarinic-activated pathways. However, we found that inhibitors of CaMKII (KN-93, 10 μm; n = 6) and Src-kinase (PP2, 10 μm; n = 5) had no effect on the muscarinic stimulation of R-type Ca2+ currents at concentrations that have been reported to be effective in brain slices (Fig. 5E) (Zhao et al., 2004; Grishin et al., 2005; Huang et al., 2005). This pattern of sensitivity to PKC antagonists and insensitivity to BAPTA suggests the involvement of Ca2+-independent PKCs in the muscarinic effect on R-type Ca2+ currents.
Figure 5.
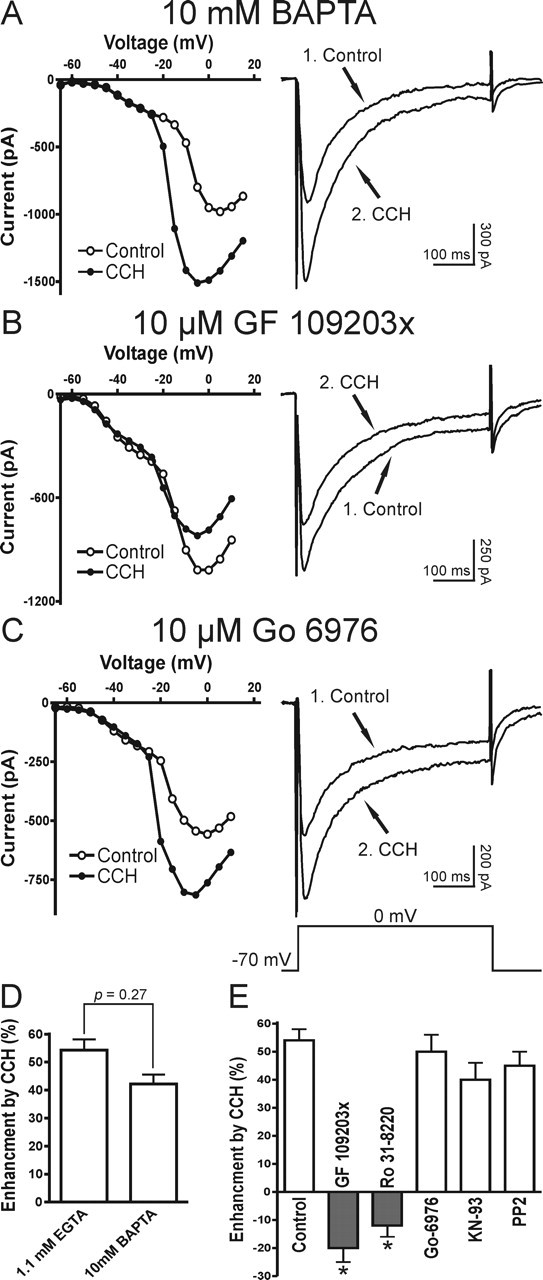
Mechanisms underlying the muscarinic stimulation of R-type VSCCs. A, Ca2+ independence of muscarinic enhancement of R-type VSCCs. In a CA1 pyramidal neuron recorded with 10 mm BAPTA-based intracellular solution, the R-type Ca2+ current is enhanced in carbachol (1, Control; 2, CCH). B, C, A Ca2+-independent PKC pathway is involved in the muscarinic modulation of R-type VSCCs (1, Control; 2, CCH). B, Preincubation of the broad spectrum PKC blocker GF 109203x (10 μm) abolishes the muscarinic stimulation of R-type Ca2+ currents and, conversely, results in current depression. C, After preincubation of the group I PKC (Ca2+-dependent isoforms) inhibitor Go 6976 (10 μm), the muscarinic stimulation of R-type VSCCs is not affected. D, Mean data for the effects of intracellular solutions containing either 1.1 mm EGTA or 10 mm BAPTA on the muscarinic enhancement of R-type VSCCs. E, Mean data for the effects of GF 109203x (10 μm), Ro 31-8220 (10 μm; broad spectrum PKC blocker), Go 6976 (10 μm), KN-93 (10 μm; CaMKII blocker), and PP2 (10 μm; Src tyrosine kinase inhibitor) on the muscarinic modulation of R-type VSCCs (for each group, n ≥ 5). All recordings were obtained in TTX and VSCC blockers (as described in Materials and Methods). Error bars indicate the mean ± SEM. Asterisk indicates significant change (∗p < 0.001).
Muscarinic enhancement of R-type Ca2+ spikes
Current-clamp recordings also were performed to characterize the cholinergic modulation of the toxin-resistant Ca2+ spikes. CA1 pyramidal neurons from hippocampal slices also were patch-clamped with a Cs+-based intracellular solution to suppress K+ channels and to minimize muscarinic-mediated effects on K+ channels. When recordings were obtained from CA1 neurons in TTX without a blocking of any Ca2+ currents, large-amplitude prolonged plateau Ca2+ spikes were evoked by injection of brief (40 ms) intracellular current pulses (n = 7) (Fig. 6A,B). Carbachol depressed the plateau Ca2+ spikes, consistent with a muscarinic depression of L-, N-, and P/Q-type VSCCs, leaving only a transient HVA Ca2+ spike. A significant portion of this calcium spike in carbachol could be attributable to R-type Ca2+ currents, because it was Ni2+-sensitive (n = 4). These results suggest that muscarinic receptor activation in hippocampal neurons could shift the normal pattern of Ca2+ entry from the slowly inactivating N-, P/Q-, and L-type VSCCs to domination by the HVA rapidly inactivating R-type VSCCs.
Figure 6.
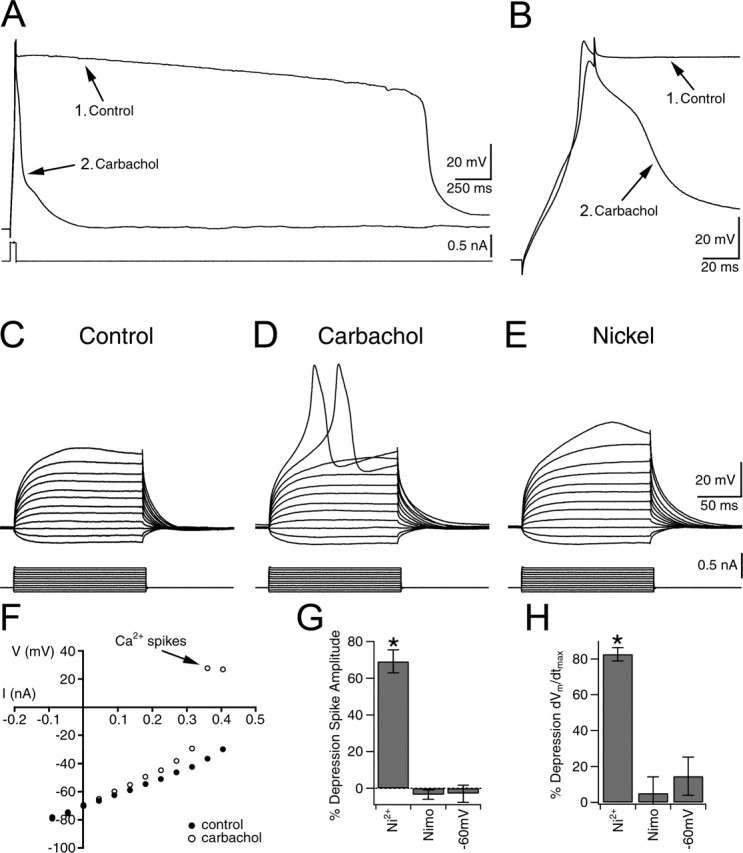
R-type VSCC-dependent spikes are enhanced by carbachol. A, Ca2+ spikes evoked by current injection before (1, Control) and after (2) carbachol application. B, Expanded view of Ca2+ spikes in A before (1, Control) and after (2) carbachol in TTX, but with no VSCC blockers. In carbachol, a HVA Ca2+ spike remains. C, Silent neuron in the presence of 1.2 μm TTX, 20 μm nifedipine, ω-conotoxin MVIIC, ω-conotoxin-GVIA, and ω-agatoxin IVA, as described in the voltage-clamp experiments. D, Activation of R-type VSCC-dependent spikes is observed only after the application of carbachol. E, Application of 50 μm Ni2+ (R-type VSCC blocker) depresses carbachol-enhanced R-type Ca2+ spikes. F, Plot of I–V relationship before and after carbachol. Note the activation of R-type Ca2+ spikes in carbachol. G, H, Effects of 50 μm Ni2+ (n = 20), 20 μm nimodipine (Nimo; L- and T-type VSCC blocker; n = 5), and membrane depolarization to –60 mV (n = 5) on the peak amplitude or the maximum rate of rise (dVm/dtmax) of the carbachol-enhanced R-type Ca2+ spikes as compared with spikes evoked in carbachol. Error bars indicate the mean ± SEM. Asterisk indicates significant depression (∗p < 0.001).
To delineate rigorously the direct action of carbachol on the toxin-resistant Ca2+ spikes, we isolated them with a mixture containing TTX, nifedipine, and toxins, as shown in previous voltage-clamp experiments. Under these conditions depolarization of neurons with intracellular current injection often led to the generation of a transient HVA Ca2+ spike (n = 50 of 69). The Ca2+ spike inactivated during the depolarizing command pulses, consistent with a Ca2+ spike mediated by rapidly inactivating HVA R-type Ca2+ currents (Randall and Tsien, 1997). However, even with strong current injection (≥0.5 nA) numerous cells (28%; n = 19 of 69) remained silent. As shown previously with current-clamp recordings (Kuzmiski et al., 2005), subsequent bath application of carbachol resulted in persistent enhancement of R-type Ca2+ spiking in both the spiking and initially silent neurons (Fig. 6C,D). The initially silent neurons exhibited transient Ca2+ spikes in the presence of carbachol (n = 19) (Fig. 6C,D). In neurons that initially showed an R-type Ca2+ spike, carbachol induced an increase in spike amplitude (ΔAPAMP = 5.7 ± 0.7 mV; p < 0.0001; n = 50), a decrease in the threshold for spike activation (ΔAPTH = –6.7 ± 0.8 mV; p < 0.0001; n = 50), and a reduction in the current injection required to evoke a spike (ΔI = –80 ± 12 pA; p < 0.0001; n = 50) (Fig. 6F). These enhanced spikes were blocked by the application of Ni2+ (50 μm; n = 20) (Fig. 6E,G,H), which at this concentration is selective for R- and T-type VSCCs. This enhancement was not caused by a muscarinic-mediated increase in input resistance (RIN) (control RIN, 115.7 ± 5.9 MΩ vs carbachol RIN, 118.0 ± 6.1 MΩ; p = 0.77; n = 69). The application of high concentrations of nimodipine (20 μm) sufficient to block partially the T-type VSCCs had no effect on the carbachol-enhanced HVA Ca2+ spikes (percentage of ΔAPAMP, 3.5 ± 2.6%; p = 0.25; percentage of ΔdVm/dtmax, –5.2 ± 9.1%; p = 0.38; n = 5) (Fig. 6G,H). In addition, the Ca2+ spikes were only weakly voltage dependent when held at depolarized voltages (from –70 to –60 mV) (percentage of ΔAPAMP, 2.9 ± 4.8%; p = 0.82; percentage of ΔdVm/dtmax, –14.6 ± 10.6%; p = 0.24; n = 5) (Fig. 6G,H). Consistent with our voltage-clamp results, we concluded that R-type, but not T-type, Ca2+ currents generated the toxin-resistant Ca2+ spikes.
Muscarinic enhancement of R-type Ca2+ spikes contributes to carbachol-induced theta burst oscillations
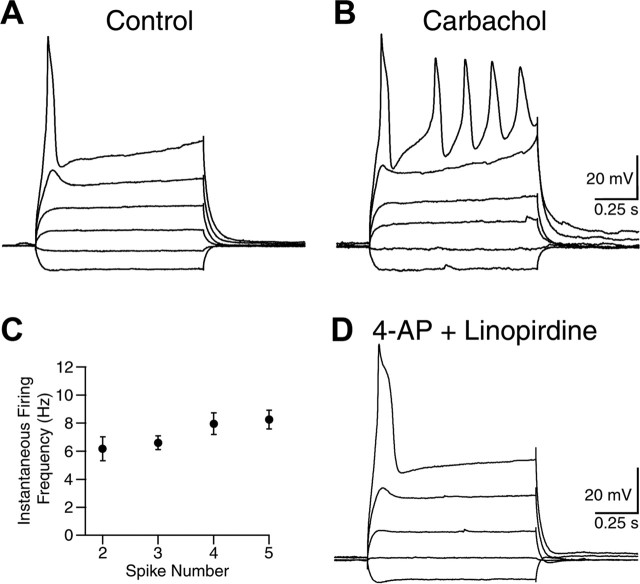
The cholinergic system is implicated in the generation of theta both in vitro and in vivo (Buzsaki, 2002). During theta in vivo the HVA Ca2+ spikes oscillate rhythmically at theta frequencies in the dendrites of CA1 pyramidal neurons and may contribute to current generation and amplification of theta (Kamondi et al., 1998; Buzsaki, 2002). However, the VSCC subtypes that contribute to dendritic oscillations during theta are unknown. We examined the possibility that muscarinic-enhanced R-type Ca2+ spikes can resonate at theta frequency and thereby contribute to the generation of dendritic oscillations. To evaluate the firing frequencies of R-type Ca2+ spikes, we injected long (0.5–1.0 s) depolarizing steps of current in neurons in the presence of TTX, VSCC toxins, and nifedipine as described above. Under these conditions either the cells were silent or only a single spike could be evoked even with strong current injection (Fig. 7A). After the addition of carbachol to the perfusate, R-type Ca2+ spiking was enhanced, and now multiple spikes could be evoked (Fig. 7B). Interestingly, repetitive R-type Ca2+ spiking displayed a regular rhythm in the theta frequency range (6–9 Hz; n = 11) (Fig. 7C). Application of K+ channel blockers (4-AP, 5 mm; linopirdine, 10 μm) failed to mimic this repetitive firing pattern (n = 3) (Fig. 7D), suggesting that the ability of the R-type Ca2+ spikes to fire at theta frequency is attributable to direct actions on R-type VSCCs. To avoid the generation of plateau potentials (Fraser and MacVicar, 1996), we limited our recordings to <1 s. These findings indicate that carbachol-enhanced R-type Ca2+ spiking could contribute to the intrinsic resonant depolarizations of CA1 pyramidal neurons in the theta frequency.
Figure 7.
Muscarinic-enhanced R-type Ca2+ spikes fire repetitively at theta frequency. A, R-type Ca2+ spike evoked by injection of a long (1.0 s) depolarizing current pulse. B, Repetitive R-type Ca2+ spiking evoked during a long current pulse after the application of carbachol. C, Instantaneous firing frequency of repetitive R-type Ca2+ spikes after carbachol treatment (n = 11). Error bars indicate the mean ± SEM. D, Application of 5 mm 4-AP (K+ channel blocker) and 10 μm linopirdine (M-current blocker) failed to induce the repetitive R-type Ca2+ spiking (n = 3).
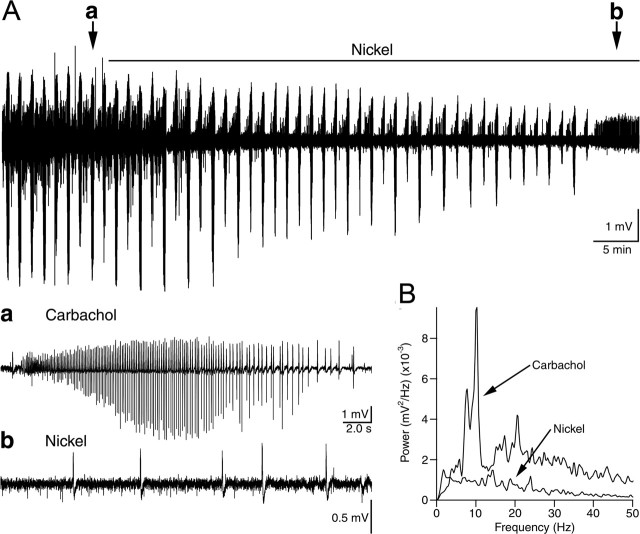
To examine the potential contribution of muscarinic-enhanced R-type VSCCs to theta burst oscillatory activity, we also recorded spontaneous extracellular events from area CA1. In the majority of naive hippocampal slices the theta frequency oscillations were generated after continuous bath application of carbachol (Fig. 8A). Field potential recordings showed a peak power spectra of 0.016 ± 0.0071 mV2/Hz at a mean frequency of 8.3 ± 0.6 Hz (n = 8) (Fig. 8B). The spontaneous oscillatory bursts occurred at regular intervals of 101.4 ± 10.9 s (n = 8) and lasted for 21.4 ± 2.6 s. Theta burst oscillations closely resembled those previously described (Bland et al., 1988). Subsequent application of a low concentration of Ni2+ depressed the oscillatory activity, as shown by an almost complete abolition of the power in the theta frequency range (n = 8 of 8) (Fig. 8A,B). In five of eight slices the oscillatory bursts were abolished completely. In the other three slices Ni2+ reduced the amplitude of the theta burst oscillations and increased the interburst interval without significant effects on burst durations (19.3 ± 2.8 s; n = 3) or the frequency of the oscillations (8.3 ± 0.4 Hz). Disruption of the theta bursts often was accompanied by a change in the field events to a slower, more interictal-like pattern. Because we demonstrated that T-type VSCCs are not affected by cholinergic stimulation, these results suggest that Ni2+ suppressed the carbachol-induced spontaneous field potential theta oscillations by depressing R-type VSCCs. Therefore, our results indicate that in CA1 pyramidal neurons the enhancement of R-type VSCCs could contribute to muscarinic-induced theta frequency oscillations.
Figure 8.
Carbachol-induced theta burst oscillations observed with extracellular recordings of field potentials are Ni2+sensitive. A, Extracellular recordings in area CA1 of spontaneous oscillatory bursts induced by perfusion with 40 μm carbachol before and after the application of 100 μm Ni2+. The solid horizontal bar indicates the application of Ni2+. a, Expanded trace from A, showing a spontaneous burst activated in carbachol. The arrow in A, labeled a, indicates the location of the burst. b, Expanded trace of the field potential recording after the application of Ni2+ indicated by the arrow in A, labeled b. B, Mean power spectra of the field potential oscillations in carbachol showing that the peak at theta frequency is abolished by Ni2+ (n = 8).
Discussion
Our results show that muscarinic activation stimulates R-type Ca2+ currents in hippocampal CA1 neurons that can lead to de novo activation of R-type dependent Ca2+ spikes. This enhancement likely is mediated via a Ca2+-independent PKC pathway because it is blocked by broad spectrum inhibitors to PKC, but not by an inhibitor of Ca2+-dependent PKC, and it is insensitive to chelation of intracellular Ca2+ by BAPTA. In contrast to the enhancement of the R-type currents, T-type Ca2+ currents were not modulated by muscarinic receptor activation. The muscarinic-mediated enhancement of transient R-type Ca2+ spikes resulted in remarkable changes in the firing pattern of R-type Ca2+ spikes and could contribute to theta oscillations.
Mechanisms underlying muscarinic enhancement of R-type VSCCs
R-type Ca2+ currents in hippocampal neurons are attributable mainly to CaV2.3 subunits (Sochivko et al., 2002, 2003), although there is still some controversy as to the proportion of R-type Ca2+ currents that remains in the CaV2.3 knock-out mouse (Wilson et al., 2000). In recombinant systems CaV2.3 subunit Ca2+ currents are enhanced by Gαq/11-coupled muscarinic receptor activation (Bannister et al., 2004; Kamatchi et al., 2004). Here we show a similar enhancement of R-type Ca2+ currents in hippocampal neurons also mediated by muscarinic M1/M3 receptor stimulation. Hippocampal pyramidal neurons express high levels of postsynaptic M1 and M3 receptors (Vilaro et al., 1993; Levey et al., 1995; Rouse et al., 1999). These receptors are Gαq/11 coupled, and their activation results in the generation of DAG and IP3 via PLC activation. DAG and IP3 in turn activate PKC and IP3 receptor pathways, respectively. Similar to previous work in HEK cells and Xenopus oocytes (Bannister et al., 2004; Kamatchi et al., 2004), the muscarinic stimulation of R-type VSCCs in hippocampal neurons was independent of intracellular Ca2+ and required the activation of Ca2+-independent group II PKCs. All three groups of PKCs are expressed in rat hippocampus (Naik et al., 2000), and PKCδ from among the group II PKCs (δ, ε, η, and θ) may be the isoform of PKC that is involved. PKCδ is highly expressed in rat hippocampal CA1 pyramidal neurons (McNamara et al., 1999; Tang et al., 2004), and in response to muscarinic stimulation PKCδ is activated and translocated to plasma membrane (Brown et al., 2005). In recombinant systems the coexpression of a dominant-negative PKCδ blocked the muscarinic stimulation of CaV2.3 subunits, whereas the blocker of PKCε could not (Bannister et al., 2004). However, we could not rule out definitively the contribution of other isoforms. In PKC inhibitors we observed a suppression of R-type Ca2+ currents by muscarinic stimulation, suggesting other inhibitory pathways. This inhibition may result from the activation of pertussis toxin-sensitive G-protein-coupled M2/M4 receptors and Gβγ subunit-mediated inhibition (Meza et al., 1999; Bannister et al., 2004).
R-type versus other VSCC types
Muscarinic enhancement of R-type VSCCs is strikingly opposite to muscarinic depression of all other HVA VSCCs (N-type, P-/Q-type, and L-type) (Gahwiler and Brown, 1987; Shapiro et al., 1999, 2001; Stewart et al., 1999). Christie and colleagues (1995) showed that in the soma and basal dendrites (≤ 50 μm) all of the HVA VSCCs as well as LVA VSCCs contribute to spike-triggered Ca2+ entry. In contrast, T-type and Ni2+-sensitive R-type VSCCs predominantly underlie spike-triggered Ca2+ entry in apical dendrites (≥100 μm) (Christie et al., 1995). Single channel analysis also shows that all VSCC types are expressed in the soma, whereas in dendrites T- and R-type VSCCs predominate (Magee and Johnston, 1995). Action potential or depolarization-induced Ca2+ influx in dendrites and spines also has been shown to be mediated mainly by R-type VSCCs (Sabatini and Svoboda, 2000; Yasuda et al., 2003). The distinct distribution in apical dendrites and the unique modulation of R-type VSCCs suggest that they play a different role and underlie distinct cellular functions from other types of VSCCs, such as synaptic integration and plasticity.
Despite the general suppression of Ca2+ currents, muscarinic activation paradoxically increases intracellular Ca2+ accumulations in the dendrites and spines from depolarization or synaptic stimulation (Muller and Connor, 1991, 1992; Tsubokawa and Ross, 1997; Beier and Barish, 2000). K+ conductance blockade and the IP3-mediated release of Ca2+ from intracellular stores are thought to contribute to this accumulation (Muller and Connor, 1991, 1992; Tsubokawa and Ross, 1997; Beier and Barish, 2000). However, our results suggest that the stimulation of R-type Ca2+ currents also might contribute to the muscarinic-mediated intracellular Ca2+ accumulations.
All three T-type VSCC subunits (α1G, α1H, and α1I) are expressed in hippocampal pyramidal neurons (Talley et al., 1999). However, their modulation and functional impact on hippocampal pyramidal neurons are not yet known. Compared with HVA channels, T-type VSCCs are more metabolically stable and are less likely to be modulated (Huang et al., 2005). Muscarinic activation has been reported to increase, decrease, or not affect the T-type Ca2+ currents, depending on the cell type and experimental conditions (Yunker, 2003). In the present study T-type Ca2+ currents in hippocampal pyramidal neurons were not affected by muscarinic stimulation.
Muscarinic stimulation is known to suppress K+ channels, such as M channels (Brown and Adams, 1980; Halliwell and Adams, 1982), thereby enhancing neuronal excitability. However, in the present study the enhancement of R-type Ca2+ currents is not attributable to K+ channel depression. High concentrations of potassium channel blockers were applied both intracellularly and extracellularly (see Materials and Methods), and most K+ currents were observed to be blocked. When the HVA Ca2+ currents were blocked first with Cd2+ (1 mm), muscarinic activation did not affect the residue K+ currents (data not shown). In addition, the repetitive R-type Ca2+ spike firing we observed in carbachol was not elicited with K+ channel inhibitors. Furthermore, T-type Ca2+ currents were found to be unaltered by carbachol application. Under normal physiological conditions it is likely that the suppression of K+ channels and enhancement of R-type VSCCs both contribute to the muscarinic stimulation of neuronal excitability.
A concern for this type of study is the possibility of poor voltage control during voltage-clamp experiments. To achieve well clamped currents, we applied high concentrations of K+ channel blockers both intracellularly and extracellularly (Colino and Halliwell, 1993), and we used younger animals (13–16 d) so that the calcium currents would be smaller and the dendritic processes less extensive. Also, large electrode tips were used (3–5 MΩ), and only cells with access resistances <20 MΩ were included in the study. The voltage clamp appeared to be sufficient in most experiments because there was a graded turn-on of the currents, and channel activation properties of the currents were similar to those observed in acutely isolated neurons (Foehring et al., 2000; Sochivko et al., 2003).
R-type spikes and theta oscillations
Muscarinic receptor stimulation in the hippocampus in vitro generates robust oscillations, which share a common frequency with theta rhythm observed in vivo. The activation of oscillatory intrinsic conductances contributes importantly to theta current generation (Buzsaki, 2002). Voltage-dependent oscillations have been described in the somata (Leung and Yim, 1991) and dendrites of pyramidal neurons (Kamondi et al., 1998). During theta induction the somatic membrane hyperpolarizes, whereas dendrites depolarize. When the dendritic depolarization is sufficiently strong, the resonant property of the membrane leads to a HVA Ca2+ spike-dependent self-sustained oscillation in the theta frequency range (Kamondi et al., 1998). Nickel ions selectively abolish dendritic calcium spikes via blocking R- and/or T-type VSCCs (Gillies et al., 2002; Isomura et al., 2002). Here we have demonstrated that carbachol-enhanced, Ni2+-sensitive R-type Ca2+ spikes can fire repetitively at theta frequencies. Given that T-type VSCCs are not affected by carbachol, our results suggest that R-type VSCCs contribute to the amplification of the theta oscillations.
Based on pharmacological sensitivity, two types of theta could be distinguished: atropine-sensitive and atropine-resistant (Kramis et al., 1975; Buzsaki, 2002). Our results show that Ni2+ blocked carbachol-induced spontaneous theta burst oscillations. This agrees with a previous study demonstrating that Ni2+ (100 μm) blocked mGluR-activated but atropine-resistant theta oscillations (Gillies et al., 2002). In this previous study Ni2+-dependent spikes were generated in the distal dendrites at theta frequencies. We found that the mGluR agonists trans-ACPD (trans-1-aminocyclopentane-1,3-dicarboxylic acid; 50 μm) and DHPG (dihydrophenylglycol; 50 μm) resulted in enhanced R-type Ca2+ spiking similar to that observed with carbachol (data not shown) (Kuzmiski and MacVicar, 2003). These results suggested that a similar contribution of R-type VSCCs to both atropine-sensitive and atropine-resistant forms of theta might exist.
In the hippocampus Ca2+ spikes are generated during highly synchronous excitatory input that is associated with behavior in vivo. The ability of carbachol-enhanced R-type Ca2+ spikes to resonate at theta frequencies suggests that it is an intrinsic property of the neuronal membrane that plays a key role in the generation of neuronal network oscillations. The resonance property of R-type VSCCs also may be important for synaptic plasticity induced by theta burst stimulation (Ito et al., 1995), the enhanced induction of theta during cholinergic stimulation (Huerta and Lisman, 1993), and the formation of accurate spatial memory (Kubota et al., 2001).
Footnotes
*C.T. and J.B.K. contributed equally to this work.
This work was supported by the Canadian Institutes of Health Research. B.A.M. is supported by a Canada Research Chair in Neuroscience and a Distinguished Scholar award from the Michael Smith Foundation for Health Research.
References
- Avery RB, Johnston D (1996). Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci 16:5567–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister RA, Melliti K, Adams BA (2004). Differential modulation of CaV2.3 Ca2+ channels by Gαq/11-coupled muscarinic receptors. Mol Pharmacol 65:381–388. [DOI] [PubMed] [Google Scholar]
- Beier SM, Barish ME (2000). Cholinergic stimulation enhances cytosolic calcium ion accumulation in mouse hippocampal CA1 pyramidal neurones during short action potential trains. J Physiol (Lond) 526[Pt 1]:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Colom LV, Konopacki J, Roth SH (1988). Intracellular records of carbachol-induced theta rhythm in hippocampal slices. Brain Res 447:364–368. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR (1989). Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods 30:203–210. [DOI] [PubMed] [Google Scholar]
- Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D (2003). α1E-containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci USA 100:12450–12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR (1980). Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283:673–676. [DOI] [PubMed] [Google Scholar]
- Brown SG, Thomas A, Dekker LV, Tinker A, Leaney JL (2005). PKC-δ sensitizes Kir3.1/3.2 channels to changes in membrane phospholipid levels after M3 receptor activation in HEK-293 cells. Am J Physiol Cell Physiol 289:C543–C556. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2002). Theta oscillations in the hippocampus. Neuron 33:325–340. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D (1995). Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol 73:2553–2557. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV (1993). Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro Eur J Neurosci 5:1198–1209. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Kirschstein T, Kukley M, Pereverzev A, von der Brelie C, Schneider T, Beck H (2003). Functional specialization of presynaptic CaV2.3 Ca2+ channels. Neuron 39:483–496. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Mermelstein PG, Song WJ, Ulrich S, Surmeier DJ (2000). Unique properties of R-type calcium currents in neocortical and neostriatal neurons. J Neurophysiol 84:2225–2236. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA (1996). Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 16:4113–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler BH, Brown DA (1987). Muscarine affects calcium currents in rat hippocampal pyramidal cells in vitro Neurosci Lett 76:301–306. [DOI] [PubMed] [Google Scholar]
- Gillies MJ, Traub RD, LeBeau FE, Davies CH, Gloveli T, Buhl EH, Whittington MA (2002). A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol (Lond) 543:779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishin AA, Benquet P, Gerber U (2005). Muscarinic receptor stimulation reduces NMDA responses in CA3 hippocampal pyramidal cells via Ca2+-dependent activation of tyrosine phosphatase. Neuropharmacology 49:328–337. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR (1982). Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250:71–92. [DOI] [PubMed] [Google Scholar]
- Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY (2005). Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 123:105–118. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE (1993). Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364:723–725. [DOI] [PubMed] [Google Scholar]
- Huguenard JR (1996). Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 58:329–348. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Fujiwara-Tsukamoto Y, Imanishi M, Nambu A, Takada M (2002). Distance-dependent Ni2+ sensitivity of synaptic plasticity in apical dendrites of hippocampal CA1 pyramidal cells. J Neurophysiol 87:1169–1174. [DOI] [PubMed] [Google Scholar]
- Ito K, Miura M, Furuse H, Zhixiong C, Kato H, Yasutomi D, Inoue T, Mikoshiba K, Kimura T, Sakakibara S (1995). Voltage-gated Ca2+ channel blockers, omega-AgaIVA and Ni2+, suppress the induction of theta-burst induced long-term potentiation in guinea-pig hippocampal CA1 neurons. Neurosci Lett 183:112–115. [DOI] [PubMed] [Google Scholar]
- Kamatchi GL, Franke R, Lynch 3rd C, Sando JJ (2004). Identification of sites responsible for potentiation of type 2.3 calcium currents by acetyl-β-methylcholine. J Biol Chem 279:4102–4109. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzs↔ ki G (1998). Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8:244–261. [DOI] [PubMed] [Google Scholar]
- Klockner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T (1999). Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H, and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci 11:4171–4178. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH (1975). Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol 49:58–85. [DOI] [PubMed] [Google Scholar]
- Kubota M, Murakoshi T, Saegusa H, Kazuno A, Zong S, Hu Q, Noda T, Tanabe T (2001). Intact LTP and fear memory but impaired spatial memory in mice lacking CaV2.3 (αIE) channel. Biochem Biophys Res Commun 282:242–248. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, MacVicar BA (2001). Cyclic nucleotide-gated channels contribute to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 21:8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiski JB, MacVicar BA (2003). Muscarinic and metabotropic glutamate receptor enhanced R-type calcium spikes underlie theta oscillations in CA1 pyramidal neurons. Soc Neurosci Abstr 29:258.11. [Google Scholar]
- Kuzmiski JB, Barr W, Zamponi GW, MacVicar BA (2005). Topiramate inhibits the initiation of plateau potentials in CA1 neurons by depressing R-type calcium channels. Epilepsia 46:481–489. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E (1999). Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J 77:3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LW, Yim CY (1991). Intrinsic membrane potential oscillations in hippocampal neurons in vitro Brain Res 553:261–274. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995). Expression of M1–M4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15:4077–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Carruth M (1999). Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol 82:1895–1901. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D (1995). Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol (Lond) 487 [Pt 1]:67–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Wees EA, Lenox RH (1999). Differential subcellular redistribution of protein kinase C isozymes in the rat hippocampus induced by kainic acid. J Neurochem 72:1735–1743. [DOI] [PubMed] [Google Scholar]
- Melliti K, Meza U, Adams B (2000). Muscarinic stimulation of α1E Ca channels is selectively blocked by the effector antagonist function of RGS2 and phospholipase C-β1. J Neurosci 20:7167–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AE, Jarsky T, Martina M, Spruston N (2005). R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci 25:5763–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza U, Bannister R, Melliti K, Adams B (1999). Biphasic, opposing modulation of cloned neuronal α1E Ca channels by distinct signaling pathways coupled to M2 muscarinic acetylcholine receptors. J Neurosci 19:6806–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul DJ, Fox AP (1991). Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol (Lond) 433:259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Connor JA (1991). Cholinergic input uncouples Ca2+ changes from K+ conductance activation and amplifies intradendritic Ca2+ changes in hippocampal neurons. Neuron 6:901–905. [DOI] [PubMed] [Google Scholar]
- Muller W, Connor JA (1992). Ca2+ signaling in postsynaptic dendrites and spines of mammalian neurons in brain slice. J Physiol (Paris) 86:57–66. [DOI] [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Dow-Edwards D, Osman M, Sacktor TC (2000). Distribution of protein kinase Mζ and the complete protein kinase C isoform family in rat brain. J Comp Neurol 426:243–258. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Tsuzuki K, Iino M, Ogura A, Kudo Y (1989). Three types of voltage-dependent calcium current in cultured rat hippocampal neurons. Brain Res 495:329–336. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E (2003). Molecular physiology of low-voltage-activated T-type calcium channels [review]. Physiol Rev 83:117–161. [DOI] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Tsien RW (1998). Antisense oligonucleotides against α1E reduce R-type calcium currents in cerebellar granule cells. Proc Natl Acad Sci USA 95:7760–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AD, Tsien RW (1995). Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci 15:2995–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AD, Tsien RW (1997). Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology 36:879–893. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI (1999). Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci 64:501–509. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Svoboda K (2000). Analysis of calcium channels in single spines using optical fluctuation analysis. Nature 408:589–593. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Loose MD, Hamilton SE, Nathanson NM, Gomeza J, Wess J, Hille B (1999). Assignment of muscarinic receptor subtypes mediating G-protein modulation of Ca2+ channels by using knockout mice. Proc Natl Acad Sci USA 96:10899–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Gomeza J, Hamilton SE, Hille B, Loose MD, Nathanson NM, Roche JP, Wess J (2001). Identification of subtypes of muscarinic receptors that regulate Ca2+ and K+ channel activity in sympathetic neurons. Life Sci 68:2481–2487. [DOI] [PubMed] [Google Scholar]
- Sochivko D, Pereverzev A, Smyth N, Gissel C, Schneider T, Beck H (2002). The CaV2.3 Ca2+ channel subunit contributes to R-type Ca2+ currents in murine hippocampal and neocortical neurones. J Physiol (Lond) 542:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochivko D, Chen J, Becker A, Beck H (2003). Blocker-resistant Ca2+ currents in rat CA1 hippocampal pyramidal neurons. Neuroscience 116:629–638. [DOI] [PubMed] [Google Scholar]
- Stea A, Soong TW, Snutch TP (1995). Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron 15:929–940. [DOI] [PubMed] [Google Scholar]
- Stewart AE, Yan Z, Surmeier DJ, Foehring RC (1999). Muscarine modulates Ca2+ channel currents in rat sensorimotor pyramidal cells via two distinct pathways. J Neurophysiol 81:72–84. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA (1999). Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19:1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FR, Lee WL, Gao H, Chen Y, Loh YT, Chia SC (2004). Expression of different isoforms of protein kinase C in the rat hippocampus after pilocarpine-induced status epilepticus with special reference to CA1 area and the dentate gyrus. Hippocampus 14:87–98. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Ross WN (1997). Muscarinic modulation of spike backpropagation in the apical dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 17:5782–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Mengod G, Palacios G, Palacios JM (1993). Receptor distribution in the human and animal hippocampus: focus on muscarinic acetylcholine receptors. Hippocampus 3[Spec No]:149–156. [PubMed] [Google Scholar]
- Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ (2000). The status of voltage-dependent calcium channels in α1E knock-out mice. J Neurosci 20:8566–8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Sabatini BL, Svoboda K (2003). Plasticity of calcium channels in dendritic spines. Nat Neurosci 6:948–955. [DOI] [PubMed] [Google Scholar]
- Yunker AM (2003). Modulation and pharmacology of low voltage-activated (“T-type”) calcium channels. J Bioenerg Biomembr 35:577–598. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW (1993). Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology 32:1075–1088. [DOI] [PubMed] [Google Scholar]
- Zhao W, Bianchi R, Wang M, Wong RK (2004). Extracellular signal-regulated kinase 1/2 is required for the induction of group I metabotropic glutamate receptor-mediated epileptiform discharges. J Neurosci 24:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]