Abstract
We investigated the hypothesis that endothelial cells activated by erythropoietin (EPO) promote the migration of neuroblasts. This hypothesis is based on observations in vivo that treatment of focal cerebral ischemia with EPO enhances the migration of neuroblasts to the ischemic boundary, a site containing activated endothelial cells and angiogenic microvasculature. To model the microenvironment within the ischemic boundary zone, we used a coculture system of mouse brain endothelial cells (MBECs) and neural progenitor cells derived from the subventricular zone of the adult mouse. Treatment of MBECs with recombinant human EPO (rhEPO) significantly increased secretion of matrix metalloproteinase 2 (MMP2) and MMP9. rhEPO-treated MBEC supernatant as conditioned medium significantly increased the migration of neural progenitor cells. Application of an MMP inhibitor abolished the supernatant-enhanced migration. Incubation of neurospheres alone with rhEPO failed to increase progenitor cell migration. rhEPO activated phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase (ERK1/2) in MBECs. Selective inhibition of the PI3K/Akt and ERK1/2 pathways significantly attenuated the rhEPO-induced MMP2 and MMP9, which suppressed neural progenitor cell migration promoted by the rhEPO-activated MBECs. Collectively, our data show that rhEPO-activated endothelial cells enhance neural progenitor cell migration by secreting MMP2 and MMP9 via the PI3K/Akt and ERK1/2 signaling pathways. These data demonstrate that activated endothelial cells can promote neural progenitor cell migration, and provide insight into the molecular mechanisms underlying the attraction of newly generated neurons to injured areas in brain.
Keywords: rhEPO, migration, MMP2, MMP9, neural progenitor cell, mouse brain endothelial cell
Introduction
Ischemic stroke increases neurogenesis in the subventricular zone (SVZ) of adult rodent brain, and newly generated neurons in the SVZ migrate to the ischemic boundary regions (Jin et al., 2001; R. Zhang et al., 2001, 2004; Arvidsson et al., 2002; Parent et al., 2002; Sun et al., 2003). Molecules that regulate migration of new neurons to the ischemic regions have not been fully investigated.
Angiogenesis and neurogenesis are coupled processes (Palmer et al., 2000; Louissaint et al., 2002; Taguchi et al., 2004). Coculture of endothelial cells with neural progenitor cells enhances neural progenitor cell proliferation and differentiation (Leventhal et al., 1999; Shen et al., 2004). In the adult rodent brain, neural progenitor cells are localized adjacent to endothelial cells in the SVZ and dentate gyrus (Palmer et al., 2000; Gotts and Chesselet, 2005). Erythropoietin (EPO), a hematopoietic cytokine, regulates neurogenesis (Shingo et al., 2001; Wang et al., 2004). Treatment of stroke with recombinant human EPO (rhEPO) enhances angiogenesis and increases numbers of new neurons in the SVZ and ischemic boundary regions (Wang et al., 2004). However, it is not known whether rhEPO-induced angiogenesis mediates migration of newborn neurons in the SVZ to the ischemic boundary regions.
Matrix metalloproteinases (MMPs) are a family of enzymes responsible for the proteolytic processing of extracellular matrix structural proteins, which regulate endothelial cell migration (Karagiannis and Popel, 2005; Segarra et al., 2005). Activated endothelial cells secrete MMP2 and MMP9 during angiogenesis (Arkell and Jackson, 2003). We hypothesized that endothelial cells activated by rhEPO secrete MMPs, which promote neural progenitor cell migration. In the present study, we tested this hypothesis using a coculture system of mouse brain endothelial cells (MBECs) and neural progenitor cells derived from the SVZ of the adult mouse. In addition, by selectively blocking individual signaling pathways with specific pharmacologic inhibitors, we investigated whether the phosphatidylinositol-3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK1/2) signaling pathways mediate the migration of neural progenitor cells to brain endothelial cells.
Materials and Methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and the Henry Ford Health System animal care committee.
Animal model.
Male Wistar rats weighing 320–380 g were used. The middle cerebral artery (MCA) was occluded by placement of an embolus at the origin of the MCA (Zhang et al., 1997).
Immunohistochemistry.
Single immunostaining and double immunofluorescent staining for brain tissue was performed, as previously described (Zhang et al., 2000; Wang et al., 2004; R. Zhang et al., 2004). Antibodies against doublecortin (DCX) (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and endothelial barrier antigen (1:1000; Sternberg Monoclonals, Lutherville, MD) were used for detecting migrating neuroblasts and vascular endothelial cells, respectively.
Neurosphere culture.
Neural progenitor cells isolated from the SVZ form neurospheres in the presence of growth factors (Reynolds and Weiss, 1992; Morshead et al., 1994; Gritti et al., 1996). Neural progenitor cells migrate out of neurospheres when neurospheres are placed in a Matrigel. This provides an in vitro model for studying neural progenitor cell migration (Wichterle et al., 1997). In the present study, neural progenitor cells were dissociated from the SVZ of transgenic mice expressing eGFP (enhanced green fluorescent protein) (The Jackson Laboratory, Bar Harbor, ME) or wild-type mice (C57B/6J; The Jackson Laboratory), as previously reported (De Marchis et al., 2004). The cells were plated at a density of 2 × 104 cells per milliliter in growth medium. Growth medium contains DMEM–F-12 medium (Invitrogen, Carlsbad, CA), 20 ng/ml EGF (epidermal growth factor) (R & D Systems, Minneapolis, MN) and bFGF (basic fibroblast growth factor) (R & D Systems). DMEM–F-12 medium contains l-glutamine (2 mm), glucose (0.6%), putrescine (9.6 μg/ml), insulin (0.025 mg/ml), progesterone (6.3 ng/ml), apo-transferrin (0.1 mg/ml), and sodium selenite (5.2 ng/ml). The generated neurospheres (primary sphere) were passaged by mechanical dissociation and reseeded as single cells at a density of 20 cells per microliter in growth media (passage 1 cells). Passage 1 cells were used in the present study.
MBEC culture.
MBECs (American Type Culture Collection, Manassas, VA) were incubated in DMEM/10% fetal bovine serum (Invitrogen) and maintained at 37°C in 5% CO2/95% ambient mixed air. The culture media were changed every 48 h. Passage 10–12 MBECs were used in experiments.
Coculture of endothelial cells with neurospheres.
MBECs (1 × 104) were plated into 96 wells in medium with 10% FBS 24 h before coculture with neurosphere. To aid in cell identification during the coculture, MBECs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbo-cyanine perchlorate (DiI), a fluorescent marker (Vybrant DiI cell-labeling solution; Invitrogen). To protect against neurosphere contamination by excessive DiI from the MBECs, MBECs were washed three times in PBS 4 h before the coculture and then transferred to serum-free medium. Neurospheres of ∼100 μm diameter were overlaid onto MBECs in the growth medium.
Quantification of neurosphere motility on MBECs.
Images of green fluorescent protein (GFP)-positive neurospheres were captured directly after neurospheres were overlaid onto MBECs and then additionally every 24 h thereafter, for a total of 72 h. To consistently measure cell migration out of neurospheres, 12 measurements were taken per neurosphere and averaged (Robin et al., 2006). Measurements were taken every 30° emanating from the sphere center and extending radially to encompass the most distant identifiable neural progenitor cell migrating out of the neurosphere. This yielded an average value for neural progenitor cells displacement per neurosphere per time point (0, 24, 48, and 72 h) and was used to track cellular displacement throughout the course of the 72 h assay. For the purposes of calculating final neurosphere migration, only the 0 and 72 h measurements were used. Zero hour average radial measurements were subtracted from those obtained at 72 h, and an overall neurosphere displacement was calculated. Increases in overall cellular displacement were quantified as positive migration.
Image acquisition and quantification of vascular density and DCX-positive cells.
Measurements of cerebral vascular density and DCX-positive cells were performed according to our published methods (Zhang et al., 1999; L. Zhang et al., 2004). Briefly, endothelial barrier antigen (EBA)-positive vessels and DCX-positive cells on coronal sections (8 μm, at least 4 sections/rat) localized to bregma −0.2 to −2.8 mm (Paxinos and Watson, 1986) were digitized using a 20× objective via the microcomputer imaging device system. For measurements of vascular density, the numbers of EBA-positive vessels were counted throughout the ischemic boundary area and the total number of vessels was divided by the total boundary area to determine vascular density. For measurements of DCX-positive cells, areas with DCX-positive cells were measured throughout the SVZ and the ischemic boundary region. Data are presented as percentage of DCX-positive area. Double immunofluorescent images were acquired using Zeiss (Oberkochen, Germany) confocal microscopy (Zeiss LSM 510 NLO).
Blind-well chamber assay.
To further investigate cell migration, a blind-well chamber assay was performed (Robin et al., 2006). Briefly, 8 μm pore-size polycarbonate filters (PFA5; Neuroprobe, Gaithersburg, MD) were coated with BD Matrigel Matrix Basement Membrane (356231; BD Biosciences, San Jose, CA) and positioned between upper and lower chambers. The lower chamber contained supernatant collected from MBECs treated with rhEPO in the presence or absence of MMP inhibitor, hydroxamate derivative of oleic acid (OA HY) (Liao et al., 2003) (5 μm; Calbiochem, San Diego, CA) or N-[(2R)-2(hydroxamidocarbonylmethyl)-4-methylpantanoyl]-L-tryptophan methylamide (GM6001) (Webber et al., 2002) (10 μm; Chemicon, Temecula, CA), whereas the upper chamber included 50,000 neural progenitor cells suspended in growth media. Chambers were incubated for 20 h. Using cotton-tipped applicators, Matrigel was carefully wiped away from the filter, and filters were stained for 20 min at room temperature in 4% paraformaldehyde. Migrating cells caught in the membrane were then stained using Mayer's hematoxylin and eosin. The number of cells in the membrane were counted in 20 randomly selected field views under a 40× objective, and data are presented by fold changes compared with the control group, which was normalized to unity.
Experimental protocol.
(1) To examine the effect of EPO on angiogenesis and neurogenesis, rhEPO (epoietin α; Amgen, Thousand Oaks, CA) was administered intraperitoneally at a dose of 5000 U/kg daily for 7 consecutive days starting at 24 h after MCA occlusion (n = 7). Ischemic rats (n = 6) treated with the same volume of saline were used as a control group. These rats were killed 28 d after MCA occlusion. Angiogenesis and neurogenesis were analyzed on brain tissues. (2) To examine whether rhEPO enhances neural progenitor cell migration in the presence of endothelial cells, neurospheres were overlaid onto MBECs in growth medium with different concentrations of rhEPO (1, 5, or 10 U/ml epoietin α; Amgen). The cells in the medium without rhEPO were used as a control group. Migration of neural progenitor cells out of neurospheres was recorded daily for 72 h. (3) To examine whether rhEPO stimulates MBECs to secrete MMP2 and MMP9, and whether MMP2 and MMP9 secreted by endothelial cells promote neural progenitor cell migration, we first measured MMP2 and MMP9 levels in endothelial cells. MBECs at 80% confluence in serum-free medium were treated with different concentrations of rhEPO (1, 5, or 10 U/ml epoietin α) for 24 or 48 h. The endothelial cells and supernatant of the culture were collected. mRNA levels and proteins of MMP2 and MMP9 were analyzed using real-time RT-PCR and zymography, respectively. We then used the supernatant as conditioned medium to treat neurospheres. Supernatant from MBECs treated with rhEPO (5 U/ml) were collected, centrifuged at 12,000 rpm for 10 min, and filtered through 0.54 μm pore-size filters (Costar, Cambridge, MA). Neurospheres were cultured in the growth medium containing 50% of the supernatant with or without an MMP inhibitor, OA Hy (5 μm) or GM6001 (10 μm) for 72 h, and migration of neural progenitor cell out of neurospheres was measured. Neurospheres cultured in the growth medium containing of 50% DMEM with rhEPO (1, 5, or 10 U/ml epoietin α) were used as control groups. (4) To further examine the effects of MMPs secreted by rhEPO-activated endothelial cells on neural progenitor cell migration, a blind-well chamber assay was performed. The lower chamber contained supernatant collected from MBECs treated with rhEPO in the presence or absence of MMP inhibitors OA Hy (5 μm) or GM6001 (10 μm), whereas the upper chamber included 50,000 neural progenitor cells suspended in growth media. (5) To examine whether the PI3K/Akt and ERK1/2 pathways are involved in the rhEPO action on endothelial cells, activation of Akt and ERK1/2 and the biological function of these two pathways were measured. MBECs at 80% confluence in serum-free medium were treated with rhEPO (5 U/ml epoietin α) in the presence or absence of the PI3K/Akt inhibitors, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) (10 μm; Calbiochem) and wortmannin (2 μm; Sigma-Aldrich, St. Louis, MO) (Wang et al., 2005), or the ERK1/2 inhibitors, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene (U0126) or 2-(2-diamino-3-methoxyphenyl-4H-1-benzopyran-4-one (PD98059) (10 μm; Calbiochem) (Sengupta et al., 1998). MBECs were collected 1 h after incubation. Activation of Akt and ERK1/2 was measured using Western blot analysis. For analysis of MMP2 and MMP9 levels, supernatants were collected 48 h after incubation and zymography was performed (Z. G. Zhang et al., 2001).
For dissolving OA Hy, GM6001, LY294002, and U0126, DMSO was used. Therefore, additional groups treated with the same volume of DMSO without inhibitors were used as vehicle control groups.
Real-time PCR.
Quantitative PCR was performed using SYBR Green real-time PCR method (Wang et al., 2004). Total RNA was isolated from neurosphere cultures using the Stratagene Absolutely RNA MicroRNA Isolation kit (Stratagene, La Jolla, CA). Quantitative RT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using three-stage program parameters provided by the manufacturer as follows: 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 15 s at 95°C and 1 min at 60°C. Specificity of the produced amplification product was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during PCR. PCR products were run on 2% agarose gels to confirm that correct molecular sizes were present. Each sample was tested in triplicate using quantitative RT-PCR, and samples obtained from three independent experiments were used for analysis of relative gene expression data using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
The following primers for real-time PCR were designed using Primer Express software (Applied Biosystems): glyceraldehyde-3-phosphate dehyrogenase (GAPDH) (forward, AGA GAG AGG CCC TCA GTT GCT; reverse, TTG TGA GGG AGA TGC TCA GTG T), MMP2 (forward, CAG GGA ATG AGT ACT GGG TCT ATT; reverse, ACT CCA GTT AAA GGC AGC ATC TAC), MMP9 (forward, AAT CTC TTC TAG AGA CTG GGA AGG AG; reverse, AGC TGA TTG ACT AAA GTA GCT GGA), β-III tubulin (forward, CTC CCA GGT TAA AGT CCT TCA GTA; reverse, GCA ACA TAA ATA CAG AGG TGG CTA), and GFAP (forward, ACC ATT CCT GTA CAG ACT TTC TCC; reverse, AGT CTT TAC CAC GAT GTT CCT CTT).
SDS-PAGE zymography.
Conditioned media were collected and concentrated using a centricon (Millipore, Bedford, MA). Protein concentrations were analyzed with a Bio-Rad (Hercules, CA) system. Equal amounts of protein (20 μg/lane) for each sample were mixed with 2× sample buffer and loaded on a 10% polyacrylamide gel incorporated with 0.1% gelatin for electrophoresis. MMP2 and MMP9 zymographic standards were used as positive controls (Chemicon). After electrophoresis, gels were washed in 2.5% Triton X-100 for 1 h, incubated for 18 h at 37°C in collagenase buffer, and stained for 1 h with 0.1% Coomassie brilliant blue. Gelatinolytic activity was visualized as a transparent band against a blue background. Zymography was measured for quantification analysis by spot density measurement using a digital imaging analysis system (Alpha Innotech, Mt. Prospect, IL).
Western blot analysis.
Western blots were performed according to published methods (Wang et al., 2005). Briefly, cells were washed twice with PBS and scraped into a lysis buffer. Protein concentrations were analyzed with a Bio-Rad system. Equal amounts of protein (20 μg/lane) for each sample were electrophoresed through a 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ), and the blots were subsequently probed with the following antibodies: phosphospecific Akt (Ser473; 1:1000), Akt (1:1000; Cell Signaling Technology, Beverly, MA), phosphospecific ERK1/2 (1:1000), and ERK1/2 (1:1000; Santa Cruz Biotechnology). For detection, horseradish peroxidase-conjugated secondary antibodies were used (1:2000) followed by enhanced chemiluminescence development (Pierce, Rockford, IL). Bands were scanned and semiquantified by densitometry. Total Akt and ERK1/2 were used as an internal control.
Statistical analysis.
A one-way ANOVA was performed and statistical significance was set at p < 0.05. All data are presented as mean ± SE.
Results
EPO enhances angiogenesis
Treatment of stroke with rhEPO significantly (p < 0.01) increased cerebral vascular density (4.25 ± 0.57%; n = 7) (Fig. 1B,D) around the ischemic boundary region compared with the density (1.83 ± 0.19%; n = 6) (Fig. 1E,G) in the control group. Stroke induced migrating neuroblasts identified by DCX-positive cells toward the ischemic boundary region (Fig. 1F,G), whereas DCX-positive cells were only localized to the SVZ in the contralateral hemisphere (Fig. 1I,J). Treatment with rhEPO further increased the number of DCX-positive cells in the ischemic boundary (Fig. 1C,D). Quantitative analysis revealed that treatment with rhEPO significantly (p < 0.01) increased the number of DCX-positive cells in the ischemic boundary regions (9.4 ± 0.01%) compared with the number in the control group (3.5 ± 0.01%). These results confirmed our previous findings that rhEPO enhances angiogenesis and neurogenesis in stroke brain (Wang et al., 2004), and suggest that angiogenesis is coupled to migration of neuroblasts.
Figure 1.
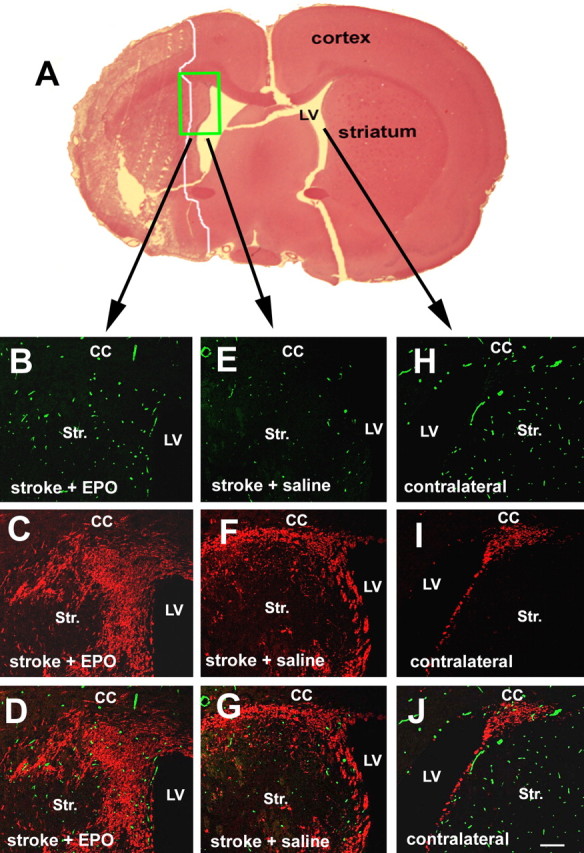
Effect of rhEPO on angiogenesis and neurogenesis. A is a hematoxylin and eosin (H&E)-stained coronal section showing ischemic core outlined by a white line. Three-dimensional confocal images (B–G) were acquired from a boxed area of the ipsilateral hemisphere in A, as indicated by arrows, whereas images H–J were from the homologous area of the contralateral hemisphere. Confocal images show EBA-immunoreactive cerebral microvessels (B, E, H) and DCX-positive migrating neuroblasts (C, F, I) of representative rats treated with rhEPO (B–D) or saline (E–J). D, G, and J are merged images of EBA-positive vessels (green) and DCX-positive cells (red). LV, Lateral ventricle; Str, striatum; CC; corpus callosum. Scale bar, 100 μm.
EPO promotes MBEC-coupled neural progenitor cell migration
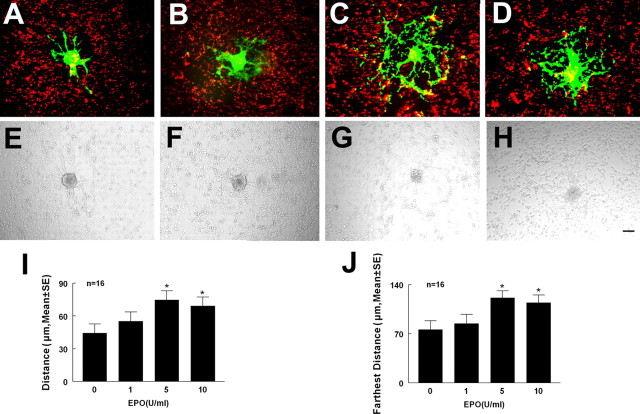
To examine whether rhEPO enhances neural progenitor cell migration in the presence of endothelial cells, we used a coculture system of MBECs and adult mouse SVZ cells that models the interaction of these cell populations in the microenvironment of the ischemic boundary region (Wang et al., 2004). Neurospheres expressing GFP were overlaid onto DiI-labeled MBECs for 72 h in the presence or absence of rhEPO. The distance of migration of neural progenitor cells out of neurospheres was measured (Robin et al., 2006). rhEPO significantly promoted MBEC-coupled neural progenitor cell migration, with maximum migration found at a dose of 5 U/ml compared with the control group (Fig. 2). These data indicate that rhEPO regulates MBEC-coupled neural progenitor cell migration.
Figure 2.
Effect of rhEPO on MBECs coupled neurosphere migration. Coculture of MBECs (red) and neurospheres (green) with rhEPO (epoietin α) at doses of 0 (A), 1 (B), 5 (C), and 10 U/ml (D). E–H are bright-field images corresponding to A–D. I and J are quantitative data of average distance and farthest distance of neural progenitor cell migration. Error bars indicate SEM. ∗p < 0.05 versus the control. Scale bar, 100 μm.
Endothelial cells activated by EPO enhance neural progenitor cell migration
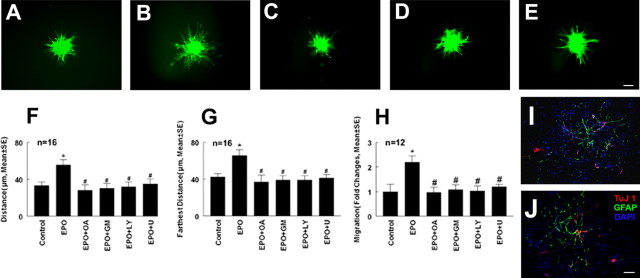
Endothelial cells and neural progenitor cells express the EPO receptor (Anagnostou et al., 1994; Wang et al., 2004). To examine whether rhEPO directly acts on neural progenitor cells to promote migration of these cells or rhEPO activates endothelial cells and consequently the endothelial cells enhance neural progenitor cell migration, additional experiments were performed. MBECs were cultured with or without rhEPO for 48 h and the supernatants were harvested. Neurospheres were cultured with the conditioned media for 72 h, and cell migration was measured. Compared with the conditioned medium collected from MBECs without treatment with rhEPO, the conditioned medium derived from rhEPO-treated MBECs resulted in a significant (p < 0.05) increase of neural progenitor cell migration (Fig. 3). In contrast, treatment of neurospheres with rhEPO for 72 h did not significantly enhance cell migration (n = 16; 32.5 ± 5.0 μm) compared with the control group (n = 16; 30 ± 5.8 μm). Endothelial cells promote neural stem cell differentiation into neurons (Shen et al., 2004). To examine whether the conditional medium harvested from rhEPO-activated endothelial cells promotes neural progenitor cell differentiation resulting in increases of cell migration, neuronal differentiation was measured. Real time RT-PCR analysis revealed that mRNA levels of β-III tubulin and GFAP were not significantly (p > 0.05) increased in neural progenitor cells treated with the conditional medium (n = 12; 1.3- ± 0.02-fold vs 1 in control for β-III tubulin and 0.8- ± 0.07-fold vs 1 in control for GFAP). Double immunofluorescent staining showed that few TuJ1- and GFAP-positive cells were present in neural progenitor cells cocultured with endothelial cells not treated with rhEPO (Fig. 3). Coculture of neural progenitor cells with endothelial cells treated with rhEPO did not increase the number of TuJ1- and GFAP-positive cells (Fig. 3), suggesting that, in the growth medium, endothelial cells activated by rhEPO do not increase neural progenitor cell differentiation. Together, these data suggest that soluble factors secreted by rhEPO-activated endothelial cells promote neural progenitor cell migration.
Figure 3.
Effect of conditioned medium on neurosphere migration. A–E are images of migration of neural progenitor cells out of neurospheres cultured with conditioned medium harvested from MBECs (A), MBECs and rhEPO (B), MBECs and rhEPO in the presence of OA Hy (C), LY294002 (D), or U0126 (E). F and G are quantitative data of average distance and farthest distance of neural progenitor cell migration. ∗p < 0.05 versus the control group and #p < 0.05 versus the rhEPO group. H shows quantitative data of fold changes of neural progenitor cell migration by blind-well chamber assay. ∗p < 0.01 and #p < 0.01 versus control and rhEPO groups, respectively. Error bars indicate SEM. I and J are images of double immunofluorescent staining showing TuJ1 (red) and GFAP (green)-positive cells in neural progenitor cells cocultured with endothelial cells treated with (I) and without rhEPO (J). OA, OA Hy; GM, GM6001; LY, LY294002; U, U0126. Scale bar, 100 μm.
MMP2 and MMP9 secreted by EPO-activated endothelial cells promote cell migration
EPO upregulates MMP2 expression in endothelial cells (Ribatti et al., 1999). MMP2 mediates endothelial cell migration (Karagiannis and Popel, 2005). To investigate whether rhEPO increases MMP2 and MMP9 levels secreted by the endothelial cells, the following experiments were performed. First, MBECs were treated with rhEPO at concentrations of 1, 5, and 10 U/ml for 24 h. MMP2 and MMP9 mRNA levels in the endothelial cells were analyzed using real-time RT-PCR. Treatment with rhEPO at concentrations of 5 and 10 U/ml significantly increased endothelial cell MMP2 and MMP9 mRNA levels compared with the control group (Fig. 4). Using gelatin zymography, MMP2 and MMP9 levels were measured in the supernatants collected from MBECs treated with or without rhEPO for 48 h. Zymography analysis revealed the presence of pro and active forms of MMP2 but not MMP9 in the supernatants without treatment with rhEPO (Fig. 5). However, the supernatants harvested from MBECs treated with rhEPO showed induction of pro and active forms of MMP9 and a significant increase of pro and active forms of MMP2 (Fig. 5). These data indicate that rhEPO stimulates endothelial cells to secrete MMP2 and MMP9. To investigate whether increased MMP2 and MMP9 regulate neural progenitor cell migration, neurospheres were cultured in the conditioned medium collected from MBECs treated with rhEPO in the presence or absence of an MMP inhibitor, OA Hy, or GM6001 abolished neural progenitor cell migration enhanced by rhEPO-treated MBECs (Fig. 3C,F,G). In parallel, the blind-well chamber assay revealed that condition medium collected from rhEPO-treated MBECs significantly (p < 0.01) increased the number of migrating neural progenitor cells compared with the number in the control group (Fig. 3H). Treatment with the MMP inhibitor OA Hy or GM6001 significantly (p < 0.01) blocked the number of migrating cells (Fig. 3H). These results indicate that MMP2 and MMP9 secreted by rhEPO-activated endothelial cells promote neural progenitor cell migration.
Figure 4.
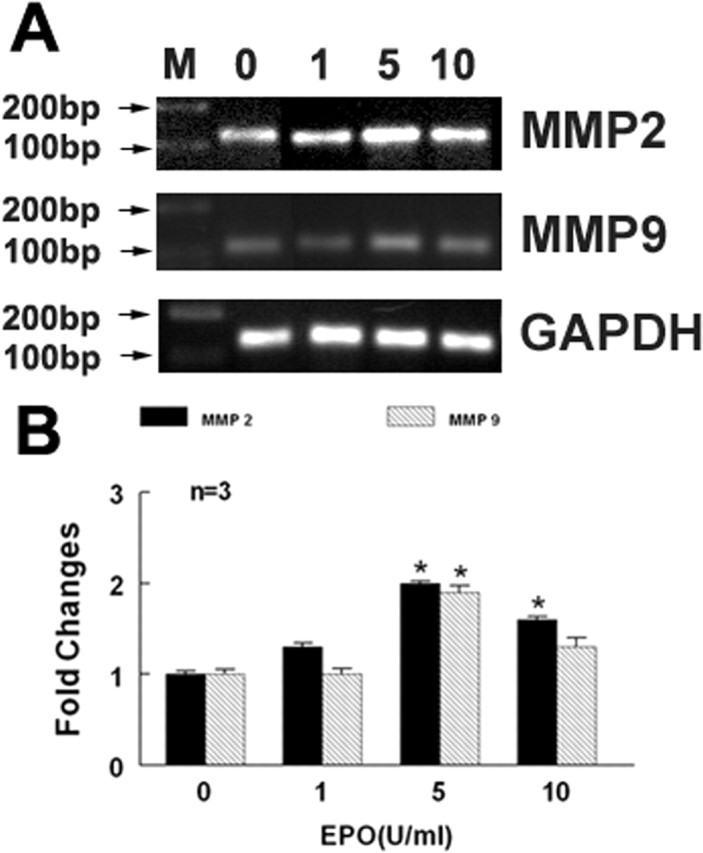
Effect of rhEPO on expression of MMP2 and MMP9. Real-time RT-PCR analysis (A) shows MMP2 and MMP9 mRNA levels in MBECs treated with rhEPO at concentrations of 0, 1, 5, and 10 U/ml. B shows quantitative data of MMP2 and MMP9 mRNA levels. GAPDH was used as an internal control. Error bars indicate SEM. ∗p < 0.05 versus the control group.
Figure 5.
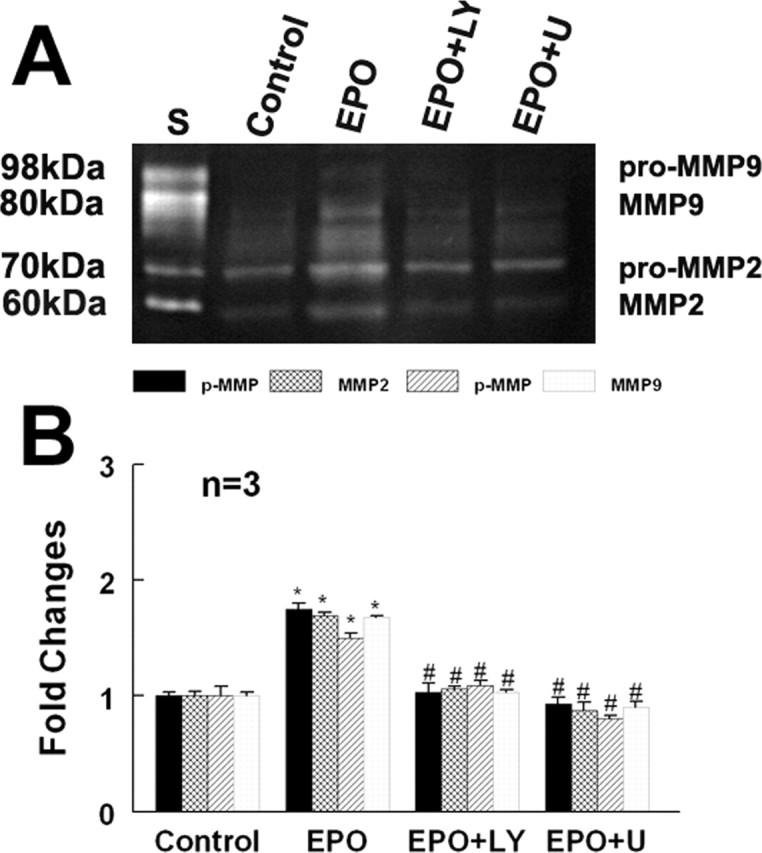
Effect of rhEPO on MMP2 and MMP9 secretion. Gelatin zymography analysis (A) shows MMP2 and MMP9 activities in the conditioned medium harvested from control, rhEPO (EPO), and rhEPO with LY294002 (EPO+LY) or U0126 (EPO+U). B shows quantitative analysis of MMP2 and MMP9. ∗p < 0.05 and #p < 0.05 versus control and rhEPO groups, respectively. Error bars indicate SEM.
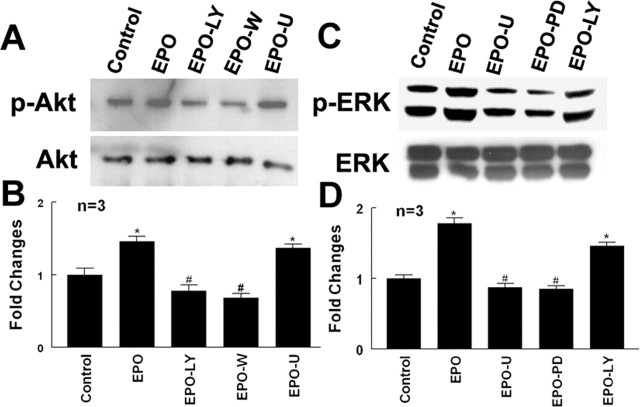
EPO regulates MMP2 and MMP9 secretion via PI3K/Akt and ERK 1/2 signaling pathways
EPO regulates cell function by acting through many signaling pathways including the PI3K/Akt and ERK1/2 pathways (Okkenhaug and Vanhaesebroeck, 2003). To investigate whether the PI3K/Akt and ERK1/2 pathways promote secretion of MMP2 and MMP9 by rhEPO-activated endothelial cells, phosphorylation of Akt and ERK1/2 was measured. Incubation of MBECs with rhEPO significantly activated Akt and ERK1/2 compared with MBECs without treatment with rhEPO (Fig. 6). Activation of Akt and ERK1/2 by rhEPO was significantly reduced when MBECs were incubated with the specific PI3K/Akt inhibitors LY294002 or wortmannin and the specific ERK1/2 inhibitors U0126 or PD98059 (Fig. 6). In addition, inhibition of PI3K/Akt and ERK1/2 pathways significantly attenuated secretion of MMP2 and MMP9 by rhEPO-activated endothelial cells (Fig. 5). Furthermore, blocking the PI3K/Akt and ERK1/2 pathways suppressed neural progenitor cell migration enhanced by rhEPO-activated MBECs (Fig. 3D–H) compared with the vehicle control rhEPO-treated groups (Fig. 3). These data indicate that the PI3K/Akt and ERK1/2 pathways regulate MMP2 and MMP9 secretion by rhEPO-activated endothelial cells.
Figure 6.
Effects of rhEPO on the PI3K/Akt and ERK1/2 signaling pathways. Western blot analysis shows phospho-Akt, total Akt (A), phospho-ERK1/2 and total ERK1/2 (C) in control, rhEPO (EPO), rhEPO with LY294002 (EPO+LY) or wortmannin (EPO+W), and rhEPO with U0126 (EPO+U) or PD9321 (EPO+PD). B and D show quantitative analysis of phospho-Akt and phospho-ERK1/2, respectively. ∗p < 0.05 and #p < 0.05 versus control and rhEPO groups, respectively. Error bars indicate SEM.
Discussion
Our results show the following: (1) in vivo, treatment of ischemic stroke with rhEPO substantially increases angiogenesis and recruitment of neuroblasts into the ischemic boundary regions; (2) in vitro, rhEPO stimulates endothelial cells to secrete MMP2 and MMP9; (3) MMP2 and MMP9 promote neural progenitor cell migration; (4) the PI3K/Akt and ERK1/2 signaling pathways control the rhEPO-induced endothelial cell secretion of MMP2 and MMP9. Therefore, the present study demonstrates that endothelial cells activated by rhEPO enhance neural progenitor cell migration. These data provide a molecular mechanism underlying the ability of the angiogenic niche adjacent to brain-injured areas to attract newly generated neurons.
EPO regulates angiogenesis and neurogenesis (Shingo et al., 2001; Kertesz et al., 2004; Wang et al., 2004). We previously demonstrated that rhEPO enhances angiogenesis and neurogenesis in stroke brain when rhEPO is administered 24 h after ischemic stroke (Wang et al., 2004). Treatment of stroke with rhEPO substantially increases the number of new neurons around the ischemic boundary regions where angiogenesis occurs (Wang et al., 2004). In vivo data of the present study confirmed our previous findings. EPO induces MBEC proliferation and promotes capillary-like tube formation, whereas blocking EPO with an anti-EPO neutralizing antibody abolishes EPO-induced capillary tube formation (Gotts and Chesselet, 2005). This in vitro assay was used to investigate the molecular basis of the migration of new neurons toward the angiogenic ischemic boundary. We found that treatment of MBECs with rhEPO increased MMP2 and MMP9 levels in the MBECs supernatant. When neural progenitor cells were cultured in the conditioned medium containing the supernatant, migration of neural progenitor cells out of neurospheres was significantly enhanced. The conditioned medium-promotion of cell migration was blocked by an MMP inhibitor, and direct treatment of neural progenitor cells with rhEPO alone did not significantly increase neural progenitor cell migration, although neural progenitor cells express the EPO receptor (Shingo et al., 2001; Wang et al., 2004). Furthermore, the condition medium did not enhance neuronal differentiation. Collectively, these data demonstrate that MBECs activated by rhEPO secrete MMP2 and MMP9, which enhance neural progenitor cell migration. Our data are consistent with previous findings that EPO stimulates MMP2 production in endothelial cells, and endothelial cells during angiogenesis secrete MMP2 and MMP9 (Ribatti et al., 1999; Stetler-Stevenson, 1999; Vacca et al., 1999). Therefore, in stroke brain, rhEPO-enhanced angiogenesis could attract neural progenitor cells via increased MMP2 and MMP9, which may partially explain in vivo findings that rhEPO increases the number of new neurons around the ischemic boundary (Wang et al., 2004). Although the MMP inhibitor used in the present study has been shown to block MMP2 activity (Liao et al., 2003; Aye et al., 2004), our results cannot rule out a role for MMP9 in mediating neural progenitor cell migration, and additional studies are warranted.
EPO interacts with its receptor and activates many signaling pathways including the PI3K/Akt and ERK1/2 pathways (Arcasoy and Jiang, 2005). We previously demonstrated that rhEPO activates the PI3K/Akt pathway in neural progenitor cells (Wang et al., 2006). Using pharmacological inhibitors, in the present study, we found that rhEPO-stimulated secretion of MMP2 and MMP9 by endothelial cells was mediated via the activation of the PI3K/Akt and ERK1/2 pathways. Blocking PI3K/Akt with LY294002 and wortmannin and blocking ERK1/2 with U0126 and PD98059 attenuated activation of Akt and ERK1/2 by rhEPO and suppressed MMP2 and MMP9 production, indicating that both pathways are required for rhEPO action on endothelial cells. Our in vitro data are consistent with in vivo findings that brain-derived EPO protects from focal cerebral ischemia by dual activation of PI3K/Akt and ERK1/2 pathways (Kilic et al., 2005). The PI3K/Akt and ERK1/2 pathways stimulate MMP2 and MMP9 production, which have been shown to regulate angiogenesis (Chung et al., 2004; D. Zhang et al., 2004). Thus, these pathways could mediate EPO-enhanced angiogenesis observed in stroke brain (Wang et al., 2004).
Migration of new neurons from the SVZ to the ischemic boundary region requires active navigation, which is a coordinated multistep process. We and others demonstrated that stromal cell-derived factor 1α (SDF-1α) and its receptor, CXCR4, regulate neural progenitor cell motility after ischemic stroke (Imitola et al., 2004; Robin et al., 2006), and it is possible that SDF-1α and other factors also contribute to EPO-induced neural progenitor cell migration. Although additional in vivo studies are warranted, the present study shows that MMP2 and MMP9 secreted by rhEPO-activated endothelia cells also promote neural progenitor cell migration, indicating that activated endothelial cells provide a permissive niche for enhancing recruitment of new neurons toward the injured brain, which could foster the restoration of neurological function (Zhang et al., 2005). Therefore, in vivo, multiple molecules likely regulate neural progenitor cell migration.
In conclusion, we demonstrated that MMP2 and MMP9 secreted by rhEPO-activated endothelial cells promote neural progenitor cell migration and rhEPO-mediated MMP2 and MMP9 induction is regulated by the PI3K/Akt and ERK1/2 signaling pathways in cultured MBECs. These data suggest that rhEPO activated endothelial cells are coupled with neural progenitor cell migration.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants PO1 NS23393, PO1 NS42345, RO1 NS43324, and RO1 HL64766.
References
- Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT (1994). Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA 91:3974–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcasoy MO, Jiang X (2005). Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol 130:121–129. [DOI] [PubMed] [Google Scholar]
- Arkell J, Jackson CJ (2003). Constitutive secretion of MMP9 by early-passage cultured human endothelial cells. Cell Biochem Funct 21:381–386. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. [DOI] [PubMed] [Google Scholar]
- Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J (2004). Ethanol-induced in vitro invasion of breast cancer cells: the contribution of MMP-2 by fibroblasts. Int J Cancer 112:738–746. [DOI] [PubMed] [Google Scholar]
- Chung TW, Lee YC, Kim CH (2004). Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J 18:1123–1125. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC (2004). GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci 20:1307–1317. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF (2005). Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol 194:139–150. [DOI] [PubMed] [Google Scholar]
- Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL (1996). Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci 16:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ (2004). Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 101:18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA (2001). Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA 98:4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis ED, Popel AS (2005). Distinct modes of collagen type I proteolysis by matrix metalloproteinase (MMP) 2 and membrane type I MMP during the migration of a tip endothelial cell: Insights from a computational model. J Theor Biol 238:124–145. [DOI] [PubMed] [Google Scholar]
- Kertesz N, Wu J, Chen TH, Sucov HM, Wu H (2004). The role of erythropoietin in regulating angiogenesis. Dev Biol 276:101–110. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM (2005). Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19:2026–2028. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA (1999). Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 13:450–464. [DOI] [PubMed] [Google Scholar]
- Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, Li B (2003). Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology 144:1656–1663. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Louissaint A Jr, Rao S, Leventhal C, Goldman SA (2002). Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D (1994). Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13:1071–1082. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B (2003). PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 3:317–330. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002). Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52:802–813. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell'Era P, Nico B, Roncali L, Dammacco F (1999). Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo Blood 93:2627–2636. [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M (2006). Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab 26:125–134. [DOI] [PubMed] [Google Scholar]
- Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, Urbano-Marquez A, Yamada KM, Cid MC (2005). Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. FASEB J 19:1875–1877. [DOI] [PubMed] [Google Scholar]
- Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB (1998). Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA 95:11107–11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304:1338–1340. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S (2001). Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 21:9733–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG (1999). Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103:1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003). VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T (2004). Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F (1999). Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 94:4143–4155. [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M (2004). Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 35:1732–1737. [DOI] [PubMed] [Google Scholar]
- Wang L, Gang Zhang Z, Lan Zhang R, Chopp M (2005). Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab 25:1150–1158. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Jiao ZX, Wang Y, Pourabdollah-Nejad DS, Letourneau Y, Gregg SR, Chopp M (2006). Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab 26:556–564. [DOI] [PubMed] [Google Scholar]
- Webber CA, Hocking JC, Yong VW, Stange CL, McFarlane S (2002). Metalloproteases and guidance of retinal axons in the developing visual system. J Neurosci 22:8091–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A (1997). Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18:779–791. [DOI] [PubMed] [Google Scholar]
- Zhang D, Bar-Eli M, Meloche S, Brodt P (2004). Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem 279:19683–19690. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M (2004). Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke 35:2890–2895. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M (2001). A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol 50:602–611. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M (2004). Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab 24:441–448. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR (1997). A rat model of focal embolic cerebral ischemia. Brain Res 766:83–92. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M (2005). Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist 11:408–416. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Davies K, Prostak J, Fenstermacher J, Chopp M (1999). Quantitation of microvascular plasma perfusion and neuronal microtubule-associated protein in ischemic mouse brain by laser-scanning confocal microscopy. J Cereb Blood Flow Metab 19:68–78. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M (2000). VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Goussev A, Powers C, Ho KL, Morris D, Smyth SS, Coller BS, Chopp M (2001). Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res 912:181–194. [DOI] [PubMed] [Google Scholar]