Abstract
In cortical neurons, pore-forming α-subunits of the Kv4 subfamily underlie the fast transient outward K+ current (IA). Considerable evidence has accumulated demonstrating specific roles for IA channels in the generation of individual action potentials and in the regulation of repetitive firing. Although IA channels are thought to play a role in synaptic processing, little is known about the cell type- and synapse-specific distribution of these channels in cortical circuits. Here, we used immunolabeling with specific antibodies against Kv4.2 and Kv4.3, in combination with GABA immunogold staining, to determine the cellular, subcellular, and synaptic localization of Kv4 channels in the primary visual cortex of mice, in which subsets of pyramidal cells express yellow fluorescent protein. The results show that both Kv4.2 and Kv4.3 are concentrated in layer 1, the bottom of layer 2/3, and in layers 4 and 5/6. In all layers, clusters of Kv4.2 and Kv4.3 immunoreactivity are evident in the membranes of the somata, dendrites, and spines of pyramidal cells and GABAergic interneurons. Electron microscopic analyses revealed that Kv4.2 and Kv4.3 clusters in pyramidal cells and interneurons are excluded from putative excitatory synapses, whereas postsynaptic membranes at GABAergic synapses often contain Kv4.2 and Kv4.3. The presence of Kv4 channels at GABAergic synapses would be expected to weaken inhibition during dendritic depolarization by backpropagating action potentials. The extrasynaptic localization of Kv4 channels near excitatory synapses, in contrast, should stabilize synaptic excitation during dendritic depolarization. Thus, the synapse-specific distribution of Kv4 channels functions to optimize dendritic excitation and the association between presynaptic and postsynaptic activity.
Keywords: Kv4.2 subunit, Kv4.3 subunit, visual cortex, pyramidal cells, GABAergic neurons, excitatory synapses, inhibitory synapses, A-type K+ current
Introduction
In the peripheral and central nervous systems, voltage-gated K+ (Kv) channel pore-forming (α) subunits of the Kv4 subfamily underlie the fast transient outward K+ current (IA), an important determinant of action potential threshold, waveform, and firing frequency (Song et al., 1998; Malin and Nerbonne, 2000; Tkatch et al., 2000; Yuan et al., 2005). In hippocampal and neocortical pyramidal cells and in interneurons, IA is synaptically regulated and controls the backpropagation of action potentials into dendrites, which activates Ca2+, Na+, and NMDA receptor channels and influences the dendritic integration of synaptic inputs (Hoffman et al., 1997; Goldberg et al., 2003; Cai et al., 2004; Korngreen et al., 2005; Losonczy and Magee, 2006). Although progress has been made in defining the roles of IA in controlling neuronal excitability and synaptic plasticity (Frick and Johnston, 2005), little is known about the influence of IA on synaptic processing in neocortical circuits. A key question is how Kv4 channels are distributed in different cell types, dendrites, and at synapses of cortical circuits.
In situ hybridization studies in rat have shown that Kv4.2 and Kv4.3 mRNA expression varies in different areas and layers of neocortex (Serôdio and Rudy, 1998). Similar variations exist in the expression of the Kv4.2 and Kv4.3 proteins, except that in middle layers of rat neocortex, Kv4 immunostaining density is low relative to mRNA levels (Rhodes et al., 2004). Regardless, the laminar patterns suggest that Kv4 expression in neocortex is circuit specific. In rat parietotemporal cortex, antibodies against Kv4.2 label the apical dendrites of pyramidal cells, which lack Kv4.3 (Rhodes et al., 2004). In contrast, Kv4.3 antibodies label the somata and dendrites of nonpyramidal neurons, which lack Kv4.2 (Rhodes et al., 2004). Thus, it is thought that, similar to the hippocampus (Sheng et al., 1992; Maletic-Savatic et al., 1995; Rhodes et al., 2004), neocortical Kv4 channels are contained in somatodendritic membranes and that Kv4.2 is present only in pyramidal neurons, whereas Kv4.3 is expressed in interneurons (Trimmer and Rhodes, 2004).
Studies in mouse hippocampus and rat cerebellum showed that Kv4.2 and Kv4.3 immunoreactivities represent membrane-bound clusters (Jinno et al., 2005). Some of the clusters were found to be associated with subsets of synapses. In rat supraoptic nucleus, Kv4.2 clusters are concentrated at asymmetric synapses (Alonso and Widmer, 1997), whereas in rat cerebellar granule cells and mouse parasubiculum, Kv4.2 clusters are excluded from asymmetric synapses but instead are expressed at symmetric synapses (Jinno et al., 2005; Strassle et al., 2005). In contrast, in rat cerebellar climbing fiber/interneuron synapses and contacts between olfactory mitral cell dendrites, Kv4.2 and Kv4.3 are localized at novel nonsynaptic junctions (Kollo et al., 2006). Thus, it appears that whenever Kv4 clusters are associated with synapses, the association is circuit specific.
This study was undertaken to determine the expression patterns of Kv4.2 and Kv4.3 in mouse neocortex. The results show that Kv4.2 and Kv4.3 are expressed in membranes of somata, dendrites, and spines of pyramidal cells and GABAergic neurons. Both subunits are present in GABAergic synapses but are excluded from non-GABAergic synapses.
Materials and Methods
Experiments were performed on 6- to 8-week-old wild-type, transgenic yellow fluorescent protein (YFP)-expressing (H-line) (Feng et al., 2000), Kv4.2−/− (Guo et al., 2005), and Kv4.3−/− (J. M. Nerbonne, unpublished observation) C57BL6/J mice. All experimental protocols were approved in advance by the Washington University Animal Studies Committee.
Light microscopy.
Mice were anesthetized by intraperitoneal injection with a mixture of ketamine (87 mg/kg) and xylazine (13.4 mg/kg) and were perfused through the left ventricle with PBS containing heparin (100 U/ml), followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. The brains were postfixed in the same fixative for 2 h at 4°C and washed overnight in 0.1 m PB at 4°C. Vibratome sections were cut in the coronal plane at 25 μm. Sections were treated with 0.5% sodium borohydride, followed by 0.3% H2O2 and 50% ethanol in PB, washed, and blocked with 10% normal donkey serum (NDS) and 0.01% Triton X-100 in PB. After washing, the sections were incubated (overnight at 4°C) in primary antibodies in PB containing 5% NDS and 0.01% Triton X-100. For single immunostaining experiments, the following antibodies were used: mouse anti-Kv4.2 (K57/41.1, K57/1.1, 1:1000; NeuroMab, University of California Davis, Davis, CA), rabbit anti-Kv4.2 (APC-023, 1:1000–1:5000; Alomone Labs, Jerusalem, Israel), rabbit anti-Kv4.2 (AB5928, 1:1000–1:5000; Chemicon, Temecula, CA), goat anti-Kv4.2 (C-20, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Kv4.2 (x1038, x1505, 1:1000; Exalpha Biologicals, Watertown, MA), mouse anti-Kv4.3 (K75/41, 1:1000; NeuroMab), rabbit anti-Kv4.3 (APC-017, 1:1000–5000; Alomone Labs), rabbit anti-Kv4.3 (AB5194, 1:1000–1:5000; Chemicon), and goat anti-Kv4.3 (C-17, 1:200; Santa Cruz Biotechnology). Sections were washed and incubated (1:500, 1 h, 21°C) with one of the following fluorescently labeled secondary antibodies: donkey anti-mouse Cy3 (Jackson ImmunoResearch, West Grove, PA) or donkey anti-rabbit Cy3 (Chemicon); donkey anti-goat Cy3 (Jackson ImmunoResearch).
To test whether Kv4 subunits are expressed in astrocytes, pyramidal cells, or GABAergic interneurons, sections were exposed to a mixture of the mouse anti-Kv4.2 (K57/41.1, 1:1000; NeuroMab) or the mouse anti-Kv4.3 (K75/41, 1:1000; NeuroMab) antibody with a rabbit anti-GFAP primary antibody (G-9269, 1:2000; Sigma, St. Louis, MO), a rabbit anti-neurofilament (N-4142, 1:2000; Sigma), or a rabbit anti-GABA primary antibody (A2052, 1:2000; Sigma). Coexpression was detected with donkey anti-mouse Cy3 (1:500; Jackson ImmunoResearch) and donkey anti-rabbit Cy5 (1:500; Jackson ImmunoResearch) secondary antibodies. After washing, sections were mounted onto glass slides and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Selected sections were counterstained with cresyl violet to reveal cortical layers and areal borders. Microscopic analyses were performed with an epifluorescence microscope (Eclipse-800; Nikon, Tokyo, Japan) equipped with a cooled CCD camera (Optronics, Goleta, CA). High-resolution digital images were captured with a confocal microscope (FV500; Olympus, Tokyo, Japan) and assembled from stacks of images taken at 0.25 μm steps. Figures were assembled using Photoshop CS2 software (Adobe Systems, San Jose, CA).
For quantitative analyses of Kv4.2 and Kv4.3 expression in GABAergic neurons, GABA-only and GABA plus Kv4-labeled neurons were counted in 1-mm-wide strips across all layers of the primary visual cortex (area V1). Percentages were determined from nine sections derived from three different animals.
Electron microscopy.
Mice were anesthetized and perfused as described for light microscopic experiments, except that the fixative was a mixture of 4% paraformaldehyde, 0.5% glutaraldehyde, and 0.1% picric acid in PB, pH 7.4. Postfixation, sectioning, blocking treatment, and incubation in mouse anti-Kv4.2 (K57/41.1, 1:500) or mouse anti-Kv4.3 (75/41, 1:500) were as described for the light microscopic studies, except that the solutions contained normal horse serum without Triton X-100 and biotinylated horse anti-mouse (1:200; Vector Laboratories) secondary antibodies were used. Immunoperoxidase staining was performed with an ABC Elite kit (Vector Laboratories), 0.005% H2O2, and 0.5% diaminobenzidine. Sections were fixed in 1% OsO4, dehydrated in ethanol and propylene oxide, and flat embedded in Durcupan resin (Fluka, Ronkonkoma, NY). Tissue pieces containing layer 2/3 of the primary visual cortex were glued onto blocks of resin and resectioned on an ultramicrotome (Ultracut E; Reichert, Depew, NY). Thin sections were mounted on 300 mesh nickel grids and stained with 1% uranyl acetate and Reynolds lead citrate. Selected grids were used for postembedding GABA immunogold staining. For this purpose, sections were washed in Tris-buffered saline (TBS), pH 7.6, and treated in 2% fish gelatin, 1% bovine serum albumin (BSA), and 0.02% Triton X-100 in TBS. Sections were then incubated in rabbit anti-GABA antibody (A2052, 1:5000; Sigma) in 0.2% fish gelatin, 0.1% BSA, and 0.02% Triton X-100. After washing in TBS, pH 8.2, sections were treated with goat anti-rabbit secondary antibody conjugated to 15 nm gold particles (1:25; Amersham Biosciences, Arlington Heights, IL) in TBS, pH 8.2. Finally, sections were stained with uranyl acetate and Reynolds lead citrate. Ultrastructural analyses were performed in a Jeol-100 electron microscope.
Results
Specificity of Kv4.2 and Kv4.3 antibodies
To test the specificity of Kv4.2 and Kv4.3 antibodies, sections from mice in which the KCND2 [Kv4.2−/− (Guo et al., 2005)] or the KCND3 [Kv4.3−/− (Nerbonne, unpublished observation)] locus was deleted by homologous recombination were used. Despite the absence of the Kv4.2 and Kv4.3 proteins in the targeted deletion mice, we found that the polyclonal rabbit and goat antibodies from Alomone Labs, Chemicon, Exalpha Biologicals, and Santa Cruz Biotechnology used at many different concentrations labeled cell bodies and neuropil in the cerebral cortex, hippocampus, and cerebellum. This nonspecific staining is illustrated for the goat anti-Kv4.2 antibody (Santa Cruz Biotechnology), which intensely labeled cell bodies and weakly stained the neuropil in the visual cortex of Kv4.2−/− mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Similar results were obtained with the goat anti-Kv4.3 antibody (Santa Cruz Biotechnology), which produced punctate labeling of somata and dendrites in the cortex of Kv4.3−/− mice (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). In contrast, no staining was evident in Kv4.2−/− brain sections exposed to mouse monoclonal antibodies directed against an epitope in the S1–S2 extracellular loop of Kv4.2 (K57/1.1, K57/41.1; NeuroMab) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Similarly, Kv4.3−/− sections stained with a mouse monoclonal antibody against the intracellular C terminus of Kv4.3 (K75/41; NeuroMab) were blank (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), resembling results obtained in control experiments in which primary antibodies were omitted. The monoclonal mouse anti-Kv4.2 and anti-Kv4.3 antibodies therefore appear to reliably detect only the targeted Kv4 proteins. These monoclonal antibodies were used in all of the studies described here.
Kv4.2 and Kv4.3 are expressed in pyramidal and GABAergic visual cortical neurons
Using the monoclonal anti-Kv4.2 and anti-Kv4.3 antibodies, immunofluorescence was found in all layers of the primary visual cortex (Fig. 1A,B; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Both subunits are most strongly expressed in layers 1 and 4 and the bottom of layer 2/3. Staining is weaker at the top of layer 2/3 and in layers 5 and 6. However, the layer 5/6 border is typically marked by a narrow, slightly more intense band (Fig. 1B). A similar laminar distribution of Kv4.2 and Kv4.3 was found in the barrel field of the primary somatosensory cortex. In this area, however, staining in layer 4 is non-uniform and is much stronger in barrel centers than in the septa between barrels (Fig. 1C,D).
Figure 1.
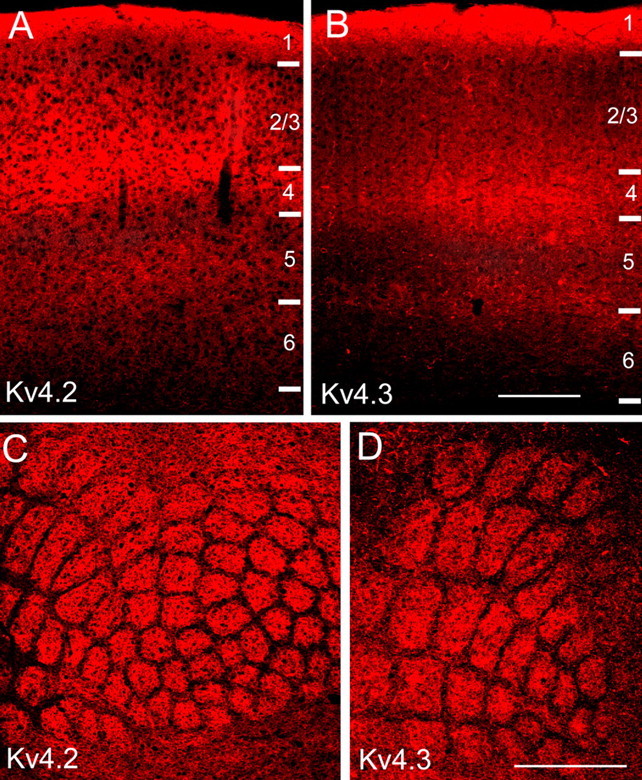
Expression levels of Kv4.2 and Kv4.3 in mouse primary visual and primary somatosensory cortex are layer specific. A, B, Immunofluorescence images of Kv4.2 and Kv4.3 expression in coronal sections through mouse primary visual cortex. Staining is densest in layers 1 and 4 and the layer 5/6 border. C, D, Kv4.2 and Kv4.3 immunofluorescence in tangential sections through mouse primary somatosensory cortex. Labeling is most intense in layer 4, in the center of barrels. Expression in septa between barrels is sparse. Scale bars: A, B, 0.2 mm; C, D, 1 mm.
In all layers, anti-Kv4.2 staining appears to be more highly concentrated in the neuropil than in cell bodies (Fig. 1A). At high magnification, Kv4.2 expression in the neuropil looks punctate and shows little organization, suggesting a preferential association with a complex network of thin processes (Fig. 2A,D). Double-labeling experiments with antibodies against Kv4.2 and GFAP showed that Kv4.2 is not expressed in astrocytes (data not shown), suggesting that Kv4.2 puncta are associated with neuronal elements. To identify the elements associated with Kv4.2 subunit clusters, experiments were performed on the visual cortices of transgenic mice (YFP-H) (Feng et al., 2000) that express YFP in a subset of layer 5 and 6 pyramidal cells, which are readily identified under epifluorescence illumination by the spine-covered dendrites (Fig. 2B,E). High-resolution confocal microscopic images of anti-Kv4.2-stained YFP-H sections revealed that Kv4.2 clusters are associated mainly with pyramidal cell dendrites, particularly with thin branches and spines (Fig. 2C,F). In contrast, the Kv4.2 labeling density of pyramidal cell somata is sparse (Fig. 2A,C). Dual immunofluorescence labeling with antibodies against GABA and Kv4.2 also showed that Kv4.2 is expressed in the somata and dendrites of GABAergic neurons and was detected in ∼mf30 % (24 of 62) of all GABA-labeled cells (Fig. 3).
Figure 2.
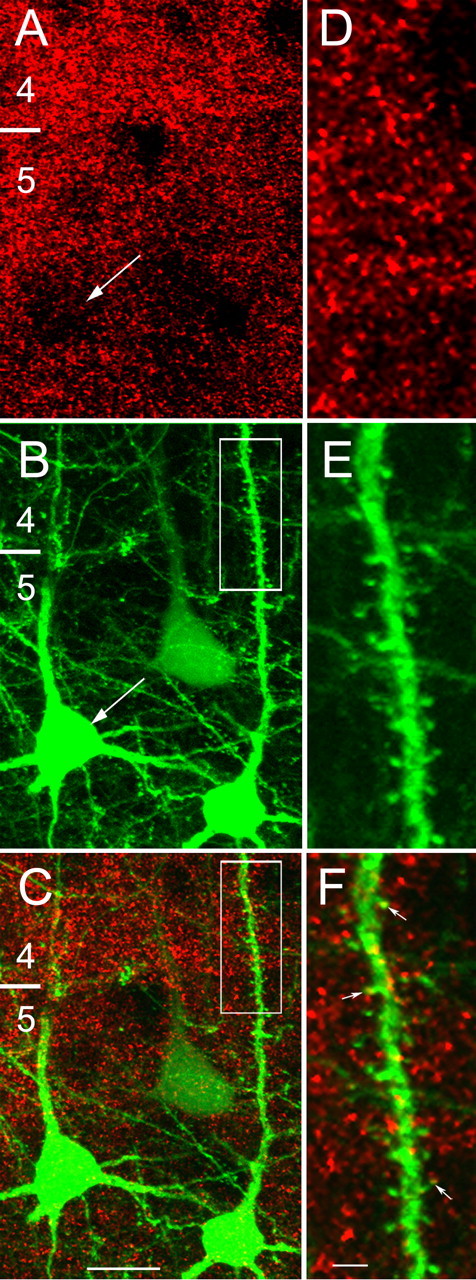
Kv4.2 clusters are expressed in pyramidal cell somata, dendrites, and spines. A, Confocal microscopic images of punctate Kv4.2 immunolabeling (red) in layers 4 and 5 of mouse primary visual cortex. The distribution of Kv4.2 clusters is non-uniform, showing a higher density over the neuropil than the pyramidal cell soma in the same focal plane (arrows; A, B). B, C, Kv4.2 clusters (red, yellow) are associated with YFP-labeled (green) pyramidal cell somata and with basal and apical dendrites. D–F, High-magnification images of the boxed apical dendrite of a YFP-labeled layer 5 pyramidal neuron (B, C), showing close association of Kv4.2-labeled clusters (red and yellow puncta marked with arrows) with dendritic shaft and spines. The numbers indicate layers. Scale bars: A–C, 10 μm; D–F, 2 μm.
Figure 3.
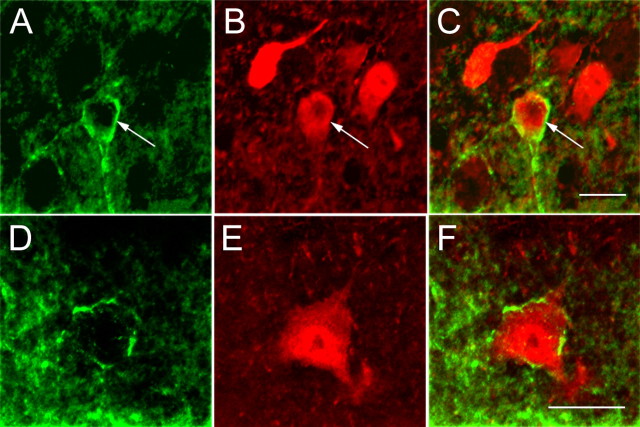
Kv4.2 expression in GABAergic neurons of mouse primary visual cortex. Confocal microscopic images of immunolabeled GABAergic neurons (red) in layer 2/3 (A–C) and layer 5 (D–F). Kv4.2 (green) is coexpressed in somata and dendrites of a subset of GABAergic neurons. Arrows in A–C mark Kv4.2-expressing GABAergic neuron. Scale bars, 10 μm.
Similar to Kv4.2, anti-Kv4.3 staining appears punctate, is highly concentrated in the neuropil, and is relatively sparse in cell bodies (Figs. 1 B, 4A,D). Kv4.3 immunostaining in the visual cortices of YFP-H mice (Feng et al., 2000) showed that many puncta are associated with the somata and dendrites of pyramidal cells (Fig. 4A–C). This association was further confirmed in double-immunolabeling experiments, which showed Kv4.3 expression in pyramidal cells that coexpressed neurofilament (Fig. 4D,E). In addition to pyramidal cells, we found that Kv4.3 is also expressed in GABAergic nonpyramidal cells (Fig. 5). In a subset of these GABAergic cells, Kv4.3 puncta are distributed throughout large parts of the dendritic arbor (Fig. 5A–C; supplemental Fig. 2, available at www.jneurosci.org as supplemental material). In most GABAergic neurons, however, detectable Kv4.3 expression is limited to somata and proximal dendrites (Fig. 5D–F). Quantitative analyses of the dual-labeling experiments revealed that ∼70% (60 of 85) of GABAergic neurons express Kv4.3.
Figure 4.
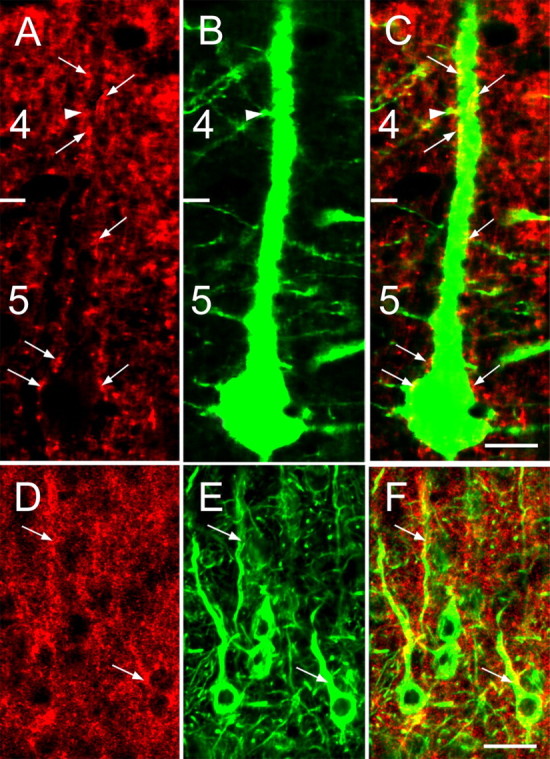
Confocal microscopic images of Kv4.3 immunolabeling in pyramidal neurons of mouse primary visual cortex. A–C, YFP-labeled pyramidal neuron with a soma in layer 5 and sparsely spiny apical dendrite in layers 4 and 2/3 that are decorated with Kv4.3 clusters (red and yellow puncta marked with arrows). The arrowhead indicates a spine, the neck of which contains a Kv4.3-positive cluster. D–F, Kv4.3 expression (red; D) in layer 2/3 pyramidal neurons that are stained with an antibody against neurofilament (green; E). Kv4.3 clusters (arrows) that are associated with somata and dendrites appear yellow (F). The numbers indicate layers. Scale bars: A–C, 10 μm; D–F, 20 μm.
Figure 5.
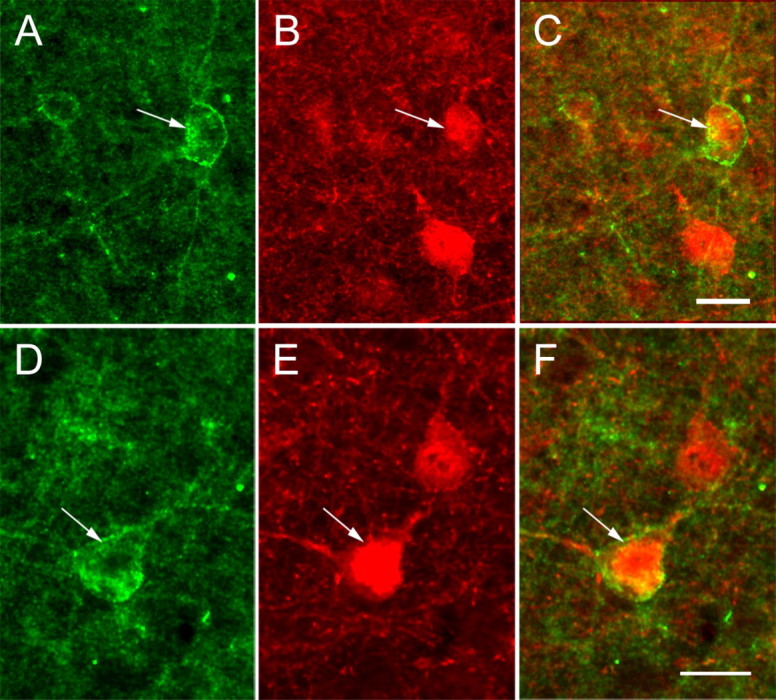
Confocal microscopic images of Kv4.3 immunolabeling in GABAergic neurons of mouse primary visual cortex. A–C, Coexpression (arrows) of Kv4.3 (green; A) and GABA (red; B) in a subset of layer 2/3 nonpyramidal cells (C) in which Kv4.3 labeling extends into distal branches of the dendritic tree. D–F, Coexpression (arrows) of Kv4.3 (green; D) and GABA (red; E) in a subset of layer 5 nonpyramidal neurons (F). Scale bars, 10 μm.
Kv4.2 and Kv4.3 are expressed in somata, dendrites, and spines of pyramidal and GABAergic visual cortical neurons
Light microscopic analyses strongly suggested that Kv4 subunits are expressed mainly in dendrites. To directly demonstrate the subcellular distribution of Kv4 subunits, we performed electron microscopic analyses of Kv4.2 and Kv4.3 immunoreactivity in layer 2/3 neurons of mouse primary visual cortex. Figures 6 and 7 show that both Kv4.2 and Kv4.3 are present in somata, dendrites, and spines. In contrast, neither Kv4.2 nor Kv4.3 was observed in axon initial segments, axon cylinders, or presynaptic axon terminals. The staining patterns for both subunits appeared as dark clustered deposits in the plasma membrane and the adjacent cytoplasm. Of the total number of Kv4.2- and Kv4.3-immunoreactive clusters, only a small percentage was in the membranes of somata (3–5%) (Figs. 6E, 7D, 8C) and thick (>0.8 μm in diameter) dendrites (8–12%) (Fig. 8B; Table 1). Most of the Kv4.2 and Kv4.3 staining was in the membranes of thin (<0.8 μm in diameter) dendrites (72–77%) (Figs. 6A, 7A,B) and spines (11–12%) (Figs. 6B,C, 7C; Table 1). Expression in spines confirmed the light microscopic observations that both Kv4.2 and Kv4.3 are expressed in pyramidal cells. Furthermore, in agreement with the light microscopic analyses, we found that both Kv4.2 and Kv4.3 are expressed in interneurons. One example of a GABA immunogold-labeled Kv4.2-expressing dendrite is shown in Figure 6D. A Kv4.3-expressing interneuron with a typical mitochondria-rich cytoplasm and a nucleus with deep infoldings is shown in Figure 7D. From observations in anti-GABA immunogold and anti-Kv4 immunoperoxidase double-labeled material, we estimate that ∼20% (7 of 35) of Kv4.2-expressing and ∼30% (15 of 48) of Kv4.3-expressing dendrites are GABAergic.
Figure 6.
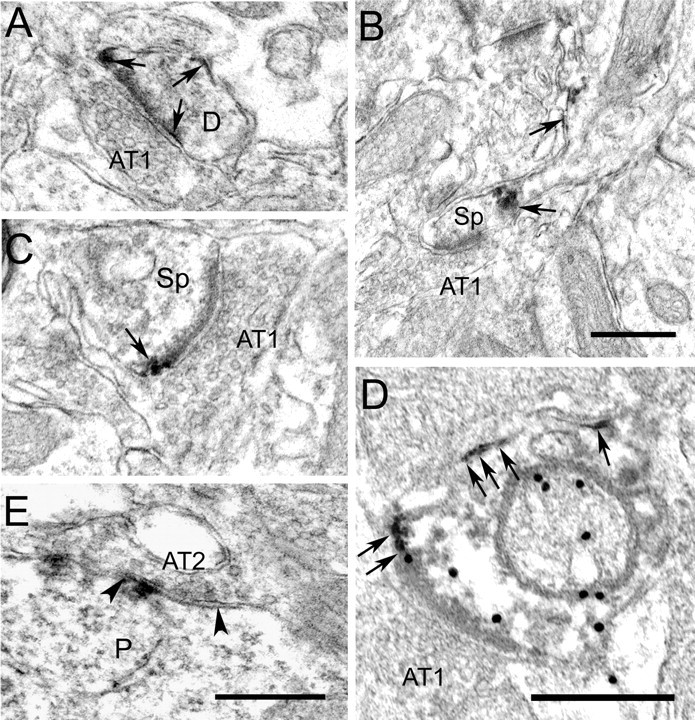
Electron micrographs showing that Kv4.2 is differentially expressed at putative inhibitory and excitatory synapses in mouse primary visual cortex. A, Extrasynaptic localization of Kv4.2 immunoperoxidase labeling (arrows) at an asymmetric synapse onto a thin dendrite (D). The dark immunoperoxidase reaction product is easily distinguished from the much lighter postsynaptic density. B, Kv4.2 immunoperoxidase at the base and neck of spine. Kv4.2 is absent from the asymmetric synapse. C, Extrasynaptic localization of Kv4.2 at an asymmetric synapse onto spine. D, Extrasynaptic localization of Kv4.2 (arrows) at an asymmetric synapse onto GABA immunogold (black dots)-labeled dendrite. E, Synaptic localization of Kv4.2 at a symmetric synapse (AT2) onto a pyramidal cell soma (P). Notice that Kv4.2 is contained in the postsynaptic membrane associated with the synaptic cleft, the margins of which are indicated by arrowheads. Scale bars, 0.5 μm. AT1, Asymmetric synapse; Sp, spine.
Figure 7.
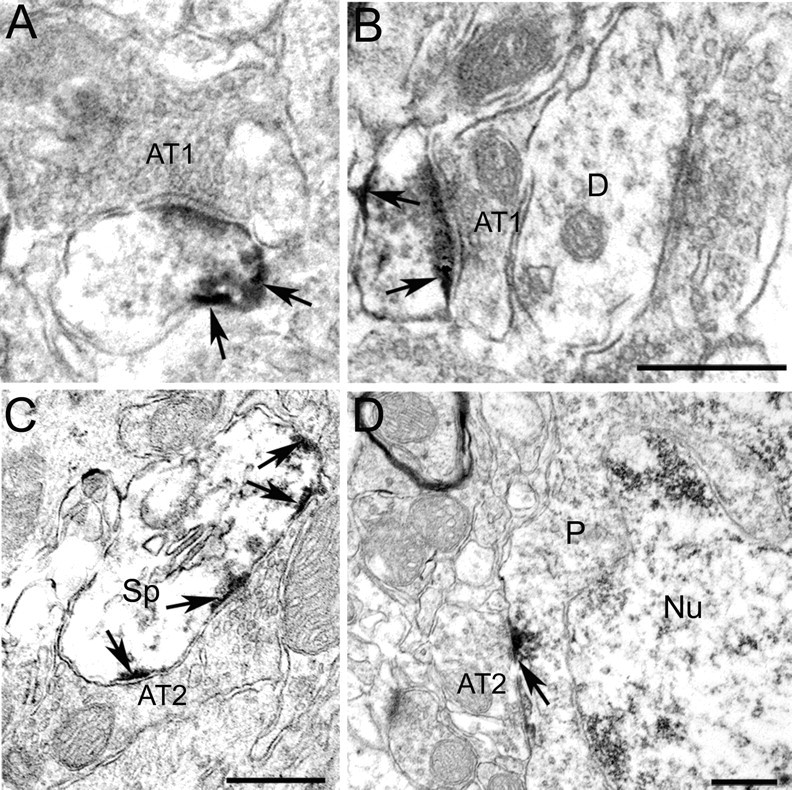
Electron micrographs showing that Kv4.3 is differentially expressed at putative inhibitory and excitatory synapses in mouse primary visual cortex. A, B, Kv4.3 immunoperoxidase labeling (dark reaction product marked by arrows) in thin dendrites is excluded from asymmetric synapses (AT1). The thick dendrite (D) does not express Kv4.3. C, D, Kv4.3 immunoperoxidase labeling (arrows) in postsynaptic membranes directly across Gray type 2 axon terminals (AT2) that contain flattened vesicles and form symmetric, putative inhibitory synapses with spine (Sp) and perikaryon (P) of a nonpyramidal cell [identified by infoldings of nuclear (Nu) membrane]. Scale bars, 0.5 μm.
Figure 8.
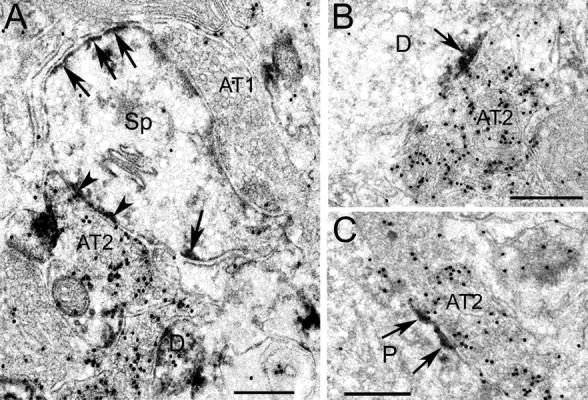
Electron micrographs showing differential localization of Kv4.2 and Kv4.3 at GABAergic and non-GABAergic synapses in mouse primary visual cortex. A, Kv4.2 immunoperoxidase (arrows and arrowheads) expression in a GABA immunogold (black dots)-labeled section. Notice that Kv4.2 (arrows) is expressed in the spine (Sp) membrane but is excluded from the asymmetric synapse formed by a non-GABAergic Gray type 1 axon terminal (AT1). In contrast, Kv4.2 is expressed at a symmetric synapse formed by GABA immunogold-labeled Gray type 2 axon terminal. B, C, Postsynaptic localization of Kv4.3 immunoperoxidase labeling (arrows) at symmetric synapses formed by GABAergic (black dots) Gray type 2 axon terminals onto dendrite (D) and perikaryon (P). Scale bars, 0.5 μm.
Table 1.
Percentage of Kv4.2- and Kv4.3-immunolabeled electron microscopically identified neuronal profiles in mouse visual cortex
| Dendrites |
|||||
|---|---|---|---|---|---|
| Antibody | Total number of stained profiles | Somata | Thina | Thickb | Spines |
| Kv4.2 | 129 | 3% (4 of 129) | 77% (99 of 129) | 8% (10 of 129) | 12% (16 of 129) |
| Kv4.3 | 116 | 5% (6 of 116) | 72% (83 of 116) | 12% (14 of 116) | 11% (13 of 116) |
aArea < 0.5 μm2.
bArea > 0.5 μm2.
Kv4.2 and Kv4.3 are localized inside of inhibitory synapses and outside of excitatory synapses
Clusters of Kv4.2 and Kv4.3 immunoreactivities were found to be distributed throughout the entire extent of the somatic and dendritic plasma membranes of pyramidal cells and interneurons, including the necks and heads of spines (Figs. 6B,C, 7C, 8A). In contrast, neither Kv4.2 nor Kv4.3 was ever found in presynaptic axon terminals. In addition, we found that the postsynaptic localization of Kv4 subunits in mouse visual cortex is synapse specific.
At asymmetrical (AT1) (Gray, 1959; Colonnier, 1968), round vesicle-containing, putative excitatory (Peters et al., 1991) synapses onto GABAergic as well as non-GABAergic neurons, both Kv4.2 and Kv4.3 were found perisynaptically (i.e., at or near the edge of the synaptic cleft). At all of these synapses, dark Kv4.2 and Kv4.3 immunoreaction product was readily distinguished from the much lighter postsynaptic density. Examples of this labeling pattern are shown for asymmetric synapses onto GABA immunogold-positive and -negative dendrites and spines (Figs. 6A,C,D, 7A,B, 8A). At each of these synapses, dark immunoperoxidase reaction product is present at the periphery of postsynaptic densities and is not directly associated with the dense postsynaptic material. At all asymmetric contacts examined, both Kv4.2 and Kv4.3 were located perisynaptically. Of the total number of asymmetric synapses in the tissue surrounding Kv4-immunostained profiles, ∼20% (13 of 70) showed perisynaptically localized Kv4.2, whereas slightly fewer (∼15%, 8 of 55) asymmetric synapses expressed perisynaptic Kv4.3.
In contrast, at symmetrical (AT2) (Gray, 1959; Colonnier, 1968), flat vesicle-containing, putative inhibitory (Peters et al., 1991) synapses onto the somata, dendrites, and spines of pyramidal cells and interneurons, both Kv4.2 and Kv4.3 are localized in the postsynaptic membrane (Figs. 6E, 7C,D). Double labeling by postembedding staining with an anti-GABA antibody and immunogold detection shows that the presynaptic terminals at Kv4-expressing, symmetrical synapses are GABAergic (Fig. 8). At these synapses, postsynaptic Kv4 subunit clusters appeared to be located preferentially within the confines of the synaptic region as defined by the limits of the synaptic cleft. Individual Kv4.2 and Kv4.3 clusters were often positioned directly opposite of presynaptic active zones, indicated by the high density of synaptic vesicles (Figs. 7C, 8). At all symmetric contacts examined, Kv4.2 and Kv4.3 were located inside the synapse. Of the total number of symmetric synapses in the tissue surrounding Kv4-immunostained profiles, ∼15% (9 of 57) showed synaptically localized Kv4.2, whereas Kv4.3 was evident in ∼25% (17 of 69) of symmetric synapses.
Discussion
Kv4.2 and K4.3 are expressed in cortical pyramidal cells and interneurons of mouse cerebral cortex
The results of the experiments described here demonstrate that both Kv4.2 and Kv4.3 are expressed in clusters throughout the somatodendritic membranes of pyramidal cells and GABAergic interneurons, and are absent in presynaptic axon terminals (Fig. 9). Similar subcellular distributions of Kv4 clusters have been described previously in rat hippocampus and mouse parasubiculum (Sheng et al., 1992; Maletic-Savatic et al., 1995; Jinno et al., 2005). In previous studies in rat neocortex, cell type-specific expression of Kv4.2 was inferred from images showing labeled pyramidal cell dendrites (Rhodes et al., 2004). Because no such dendrites were labeled with the Kv4.3 antibody, Rhodes et al. (2004) concluded that pyramidal cells in rat neocortex do not express Kv4.3. However, using double labeling of YFP-labeled pyramidal cells, we demonstrate here that, in mouse neocortex, pyramidal neurons express both Kv4.2 and Kv4.3.
Figure 9.
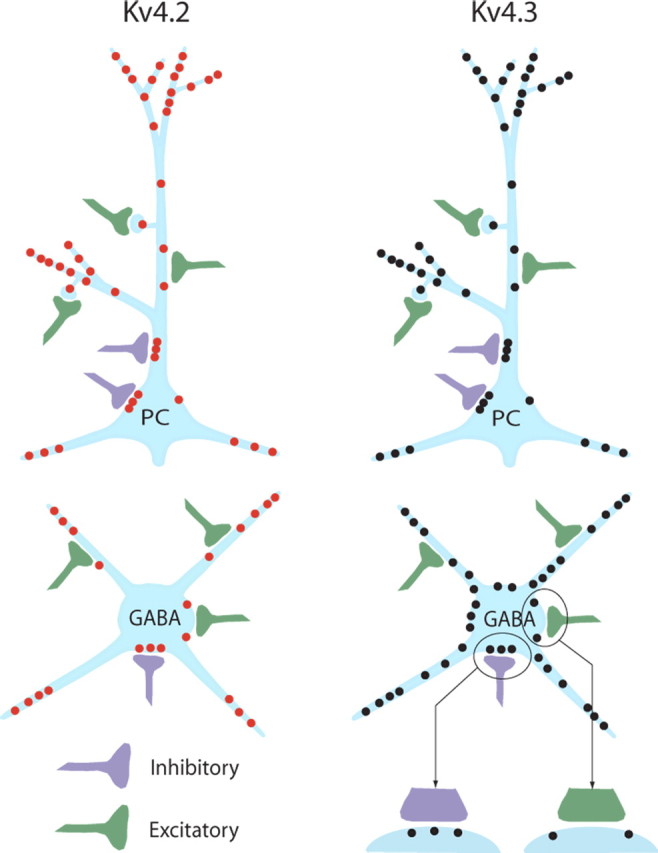
Schematic representation of Kv4.2 (red dots) and Kv4.3 (black dots) subunit distribution in neocortical pyramidal cells (top) and GABAergic interneurons (bottom). A close-up at the bottom right applies to both Kv4.2 and Kv4.3 and shows that Kv4 channels are excluded from excitatory synapses (green) but are present at GABAergic inhibitory synapses (purple). PC, Pyramidal cell.
Although the light microscopic evidence is highly suggestive, definitive proof that Kv4.2 and Kv4.3 are expressed in spiny neocortical neurons required electron microscopy. These analyses demonstrate that, unlike in rat parietotemporal cortex (Rhodes et al., 2004), Kv4 subunits are weakly expressed in somata and primary dendrites but are concentrated in distal dendrites and spines. The intense staining of layer 1 (Fig. 1A,B) also suggests that in many pyramidal cells, Kv4.2 and Kv4.3 are concentrated in the apical dendritic tufts. The robust expression of Kv4.2 and Kv4.3 in layer 1 appears in conflict with patch-clamp recordings in slices of rat somatosensory cortex, which show decreased IA density along the apical trunk of layer 5 pyramidal neurons (Korngreen and Sakmann, 2000). These recordings, however, did not include tuft dendrites of layer 1 (Korngreen and Sakmann, 2000). In addition, it has been demonstrated that IA density is increased in distal dendrites of CA1 hippocampal pyramidal neurons (Hoffman et al., 1997). Thus, in mouse neocortical pyramidal cell dendrites, Kv4 subunits may not be distributed in a simple proximal-to-distal gradient but may have a compartmental, input-specific distribution.
Based on the nonpyramidal cell morphology of immunolabeled cells, Rhodes and colleagues (Rhodes et al., 2004; Trimmer and Rhodes, 2004) suggested that Kv4.3 is concentrated in interneurons of the hippocampus and neocortex. The lack of labeled cells with readily identifiable nonpyramidal morphologies, however, made it difficult to know whether interneurons also express Kv4.2. Using double labeling with antibodies against GABA, we demonstrate here that Kv4.2 and Kv4.3 are expressed in the somatodendritic membranes of GABAergic interneurons. The percentages of Kv4.2- and Kv4.3-expressing GABAergic somata and dendrites, however, are not identical, suggesting interneuron type-specific (Markram et al., 2004) differences in Kv4 expression. The quantitative analyses, however, are based on counts of Kv4-expressing GABAergic somata, which do not include cells in which Kv4 subunits are concentrated in distal dendrites. Thus, it is likely that we have underestimated the total and relative percentages of Kv4.2- and Kv4.3-expressing GABAergic neurons. Regardless, recordings in interneurons of mouse visual cortex have shown that fast-spiking, irregular-spiking, and regular-spiking interneurons express A-type K+ currents that function to regulate the backpropagation of action potentials and the accumulation of Ca2+ in dendrites (Goldberg et al., 2003).
High density of Kv4.2 and Kv4.3 subunits in thalamocortical input layers
A striking finding of the present study is that the densities of the Kv4.2 and Kv4.3 subunits are highest in layer 1, the bottom of layer 2/3, in layer 4, and the layer 5/6 border. A similar laminar pattern was described in mouse somatosensory cortex, stained with antibodies against ERK (extracellular signal-regulated kinase)-phosphorylated Kv4.2 (Varga et al., 2000). The laminar pattern in mouse, however, differs from that in rat parietotemporal cortex, which shows extremely low Kv4.2 and Kv4.3 expression in layer 4 (Rhodes et al., 2004). Species and methodological differences may account for these discrepancies. Although layer 4, and to a lesser extent layer 1 and the layer 5/6 border, is a target of thalamocortical input (Antonini et al., 1999; Lu and Lin, 1999), structural analyses in different brain region-, lesion-, and channel-targeting studies suggest that Kv4.2 and Kv4.3 are not contained in presynaptic axons (Sheng et al., 1992; Rhodes et al., 2004; Strassle et al., 2005; Jinno et al., 2005; Chu et al., 2006) and therefore are not in thalamocortical terminals. Recordings in layer 4 of rat and mouse somatosensory cortex have shown that IA is present in spiny stellate cells, star pyramids, and fast-spiking interneurons (Massengill et al., 1997; Porter et al., 2001). Studies in cultured visual cortical neurons indicate that expression of Kv4.2 reduces the excitability of regular-spiking neurons and makes the mode (but not the rate) of repetitive firing and spike-frequency adaptation stimulus invariant (Yuan et al., 2005). Thus, Kv4 channels may play a role in gating of neuronal firing in thalamocortical input layers. In addition, thalamocortical synaptic input to proximal dendrites of layer 4 neurons (Staiger et al., 1996) may inactivate IA (Migliore et al., 1999), enable backpropagation of action potentials (Goldberg et al., 2003), allow pairing of dendritic Ca2+ spikes with inputs from recurrent axons of layer 6 corticothalamic projection neurons to distal layer 4 dendrites (McGuire et al., 1984), and contribute to an enhancement of thalamocortical responses in layer 4 (Ferster and Lindstrom, 1985).
In layer 1, Kv4 subunits are most likely expressed in dendritic tufts of layer 2/3 and 5 pyramidal cells and in the somata and dendrites of GABAergic interneurons (Lorente de No, 1992; Zhou and Hablitz, 1996; Chu et al., 2003). In rat somatosensory cortex, layer 1 dendrites and interneurons receive short-latency inputs from whiskers, which elicit Ca2+ action potentials (Larkum and Zhu, 2002; Zhu and Zhu, 2004). Similar responses were found after stimulation of excitatory inputs to thin distal dendrites of CA1 pyramidal neurons (Cai et al., 2004; Losonczy and Magee, 2006). Interestingly, the dendritic responses were increased by blocking of IA, which elevated the firing rate of somatic action potentials (Cai et al., 2004; Losonczy and Magee, 2006). Thus, Kv4 channels in layer 1 of the somatosensory cortex may have similar gating functions and may play a role in the association of synaptic inputs to different dendritic compartments and the control of spike output of layer 2/3 and 5 pyramidal neurons (Larkum et al., 2001).
Kv4.2 and Kv4.3 are expressed at GABAergic synapses but are excluded from excitatory synapses
The present electron microscopic analyses demonstrate that in mouse neocortical pyramidal cells and GABAergic neurons, Kv4.2 and Kv4.3 clusters are situated at both synaptic and extrasynaptic locations (Fig. 9). In contrast, in rat cerebellum, Kv4.2 is located exclusively extrasynaptically at mossy fiber/granule cell synapses (Strassle et al., 2005). A recent study in rat also suggested that at cerebellar climbing fiber/interneuron contacts, Kv4.3 is associated with specialized nonsynaptic contacts (Kollo et al., 2006). Similar Kv4.2-expressing nonsynaptic junctions were found at contacts between dendrites of mitral cells in the rat olfactory bulb (Kollo et al., 2006). However, because we found in Kv4.2−/− and Kv4.3−/− mice that the goat anti-Kv4.2 and -Kv4.3 antibodies from Santa Cruz Biotechnology used by Kollo et al. (2006) do not exclusively detect Kv4 proteins, the identity of the proteins expressed at the proposed novel nonsynaptic junctions remains unclear. Synaptically localized Kv4.2 was previously found in rat supraoptic nucleus and at symmetric, putative inhibitory synapses onto pyramidal cells in mouse parasubiculum (Alonso and Widmer, 1997; Jinno et al., 2005). Together with the present findings, these observations suggest that the specific association of Kv4 with synapses depends on the neuronal circuit.
Because A-type K+ currents will function to counteract membrane depolarization, it is reasonable that Kv4 channels are excluded from excitatory synapses. But how then is IA modulated by synaptic inputs, as was suggested in a functional model of hippocampal pyramidal neurons (Migliore et al., 1999)? Imaging of miniature synaptic calcium transients has shown that the depolarization of the postsynaptic membrane spreads beyond the synaptic junction (Murthy et al., 2000). The spread of subthreshold depolarization may inactivate extrasynaptic Kv4 channels (Migliore et al., 1999) and enable backpropagation of action potentials into distal dendrites (Goldberg et al., 2003).
What then is the likely role of Kv4 channels at GABAergic synapses? One possibility is that IA channels are modulated by synaptically released GABA or other transmitters to allow short- or long-term modifications of inhibitory synapses. Another possibility is that dendritic Ca2+ spikes during coincident activation by backpropagating action potentials and synaptic inputs (Larkum et al., 2001) result in the activation of IA at GABAergic synapses, which clamps the postsynaptic membrane at or near the resting potential and weakens inhibition. Alternatively, IA may shift the membrane potential below the GABA reversal potential (Yuan et al., 2005) into a range at which GABA acts as an excitatory transmitter (Gulledge and Stuart, 2003). In both scenarios, IA at GABAergic synapses will function to augment dendritic excitation, either by attenuating inhibition or by amplifying excitation. Thus, both the absence of Kv4 channels at excitatory synapses and their presence at inhibitory synapses optimizes dendritic excitation and facilitates the association between presynaptic and postsynaptic activity, influencing the plasticity of synaptic connections.
Footnotes
This work was supported by National Institutes of Health Grants RO1-NS-030676 (J.M.N.) and RO1-EY-05935 (A.B.). We thank Ben Gerber for assistance with some of the previous immunostaining experiments and Rick Wilson for maintaining the mouse colony.
References
- Alonso G, Widmer H. Clustering of Kv4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. doi: 10.1016/s0306-4522(96)00561-1. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JPY, Tang C-M, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Chu P-J, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns of different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Ferster D, Lindstrom S. Augmenting responses evoked in area 17 of the cat by intracortical axon collateral of the cortico-geniculate cells. J Physiol (Lond) 1985;367:217–232. doi: 10.1113/jphysiol.1985.sp015821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity and excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: IA-type K+ channels control action potential backpropagation. J Physiol (Lond) 551. 2003;1:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses in the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston F. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Jinno S, Jeromin A, Kosaka T. Postsynaptic and extrasynaptic localization of Kv4.2 channels in the mouse hippocampal region, with special reference to targeted clustering at GABAergic synapses. Neuroscience. 2005;134:483–494. doi: 10.1016/j.neuroscience.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol (Lond) 525. 2000;3:621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen A, Kaiser MM, Zilberter Y. Subthreshold inactivation of voltage-gated K+ channels modulates action potential in neocortical bitufted interneurons from rats. J Physiol (Lond) 2005;562:421–437. doi: 10.1113/jphysiol.2004.077032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potential in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2002;22:6991–7005. doi: 10.1523/JNEUROSCI.22-16-06991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling between the dendritic with the axonal action potential initiation zone of adult layer 5 pyramidal neurons. J Physiol (Lond) 2001;533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. The cerebral cortex of the mouse. Somatosens Mot Res. 1992;9:3–36. doi: 10.3109/08990229209144760. [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1999;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampus neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Elimination of the fat transient in superior cervical ganglion neurons with expression of Kv4.2W36F: molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Massengill JL, Smith MA, Son DI, O'Dowd DK. Differential expression of K4-AP currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. J Neurosci. 1997;17:3136–3147. doi: 10.1523/JNEUROSCI.17-09-03136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Hornung J-P, Gilbert CD, Wiesel TN. Patterns of synaptic input to layer 4 of cat striate cortex. J Neurosci. 1984;4:3021–3033. doi: 10.1523/JNEUROSCI.04-12-03021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci. 1999;7:5–15. doi: 10.1023/a:1008906225285. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Dynamics of dendritic calcium transients evoked by quantal release at excitatory hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:901–906. doi: 10.1073/pnas.97.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. New York: Oxford UP; 1991. The fine structure of the nervous system. [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Dolveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 α subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serôdio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur M-L, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central nervous system. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kirai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in ray neostriatal cholinergic interneurons ate predominately of the A-type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger JF, Zilles K, Freund TF. Distribution of GABAergic elements postsynaptic to ventroposteromedial thalamic projections in layer IV of rat barrel cortex. Eur J Neurosci. 1996;8:2273–2285. doi: 10.1111/j.1460-9568.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Strassle BW, Menegola M, Rhodes KJ, Trimmer JS. Light and electron microscopic analysis of KChIP and Kv4 localization in rat cerebellar granule cells. J Comp Neurol. 2005;484:144–155. doi: 10.1002/cne.20443. [DOI] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Varga AW, Anderson AE, Adams JP, Vogel H, Sweat JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn Mem. 2000;7:321–332. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Burkhalter A, Nerbonne JM. Functional role of the fast transient outward K+ current IA in pyramidal neurons in (rat) primary visual cortex. J Neurosci. 2005;25:9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F-M, Hablitz JJ. Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J Comp Neurol. 1996;376:198–213. doi: 10.1002/(SICI)1096-9861(19961209)376:2<198::AID-CNE3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhu JJ. Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci. 2004;24:1272–1279. doi: 10.1523/JNEUROSCI.4805-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]