Abstract
Crosslinked polyesters with Young’s moduli similar to that of certain soft biological tissues were prepared via bulk polycondensation of thiomalic acid and 1,8-octanediol alone, and with citric or maleic acid. The copolymers were converted to nitric oxide (NO)-releasing S-nitrosothiol (RSNO) analogues by reaction with tert-butyl nitrite. Additional conjugation steps were avoided by inclusion of the thiolated monomer during the polycondensation to permit thiol conversion to RSNOs. NO release at physiological pH and temperature (pH 7.4, 37 °C) was determined by chemiluminescence-based NO detection. The average total NO content for poly(thiomalic-co-maleic acid-co-1,8-octanediol), poly(thiomalic-co-citric acid-co-1,8-octanediol), and poly(thiomalic acid-co-1,8-octanediol) was 130 ± 39 μmol g−1, 200 ± 35 μmol g−1, and 130 ± 11 μmol g−1, respectively. The antibacterial properties of the S-nitrosated analogues were confirmed against Escherichia coli and Staphylococcus aureus. The hydrolytic degradation products were analyzed by time-of-flight mass spectrometry after a 10-week study to investigate their composition. Tensile mechanical tests were performed on the non-nitrosated polymers as well as their S-nitrosated derivatives and suggested that the materials have appropriate Young’s moduli and elongation values for biomedical applications.
Graphical apstract

1. Introduction
Biodegradable polyesters have been extensively studied in the field of materials research due to their applications in drug delivery therapies, tissue engineering, and wound dressings.1, 2 Such polymers are of particular interest given the broad range of processing methods that can be utilized for their preparation, their hydrolytic degradation, and the ability to create modulus-matched materials.3–5 Polyesters utilized for the preparation of biomaterials such as sutures and hernia meshes include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), their copolymer poly(lactic-co-glycolic acid) (PLGA), and poly(ε-caprolactone) (PCL).6 The limitations for their use as biomaterials, however, include lack of appropriate mechanical properties and adverse reactions at the material interface. The latter phenomena may be caused by the interaction of the polymer with the surrounding biological environment, which may result in bacterial infection or the foreign body response.7 An approach to mitigate or prevent the occurrence of these events is through the use of nitric oxide (NO) as a therapeutic agent.
Research in the field of biomedical devices has significantly advanced with the development of NO-releasing materials. This is due to the immense role that NO plays within biological systems, including antibacterial action, mitigation of the foreign body response, and prevention of platelet aggregation. Incorporation of NO donor groups such as N-diazeniumdiolates or S-nitrosothiols (RSNOs) into polymeric systems produce materials that release NO at physiological pH and temperature as a result of NO donor decomposition.8 Such processes facilitate the use of materials in a unique drug delivery therapy that harnesses the benefits of NO for its delivery at a localized site. RSNOs are the most commonly used NO sources given their low toxicity and natural occurrence in the form of S-nitrosoalbumin and S-nitrosoglutathione.9 RSNOs decompose through thermal, photolytic, or transition metal-catalyzed reactions to yield NO and the corresponding disulfide as expressed by the equation: 2RSNO → 2NO + RSSR.10 Previous studies have reported the thermal and photolytic decomposition of RSNOs as a twofold process. The initial process is generally understood to involve homolytic cleavage of the S–N bond to form the radical species nitric oxide and a thiyl radical (NO• and RS•). The formation of the thiyl radical is followed by the dimerization of RS• to yield the disulfide RSSR.11, 12 Contrary to RSNOs, N-diazeniumdiolates decompose to form secondary amines. Such compounds have been reported to produce carcinogenic secondary N-nitrosamines as byproducts under oxygenated conditions.13, 14 Therefore, the use of RSNOs as NO donor groups provides a release pathway without the health risks associated with N-nitrosamines.
Prior research has extensively explored the preparation and use of NO-releasing polyester materials. Seabra et al. have reported the synthesis of NO-releasing polyesters from poly(ethylene glycol) and thiomalic acid. NO was coupled to the thiolated polyester by bubbling a mixture of NO/synthetic air through the liquid polyester. The S-nitrosated polyester was blended with poly(methyl methacrylate) (PMMA) and used to coat PMMA plates and stainless steel intracoronary stents. The films were shown to reduce platelet adhesion after their direct contact with whole blood, suggesting that the strategy could be used to improve blood compatibility and thrombogenicity of different medical devices. NO release from these materials was indirectly measured by the Griess assay, which monitors the formation of nitrite (NO2−) by spectrophotometric analysis.15 The materials released 0.15 μmol g−1 of NO for approximately 24 h, at 37 °C.16 Yang et al. developed hyperbranched polyesters with bis(hydroxymethyl)propionic acid in a similar fashion as the materials reported in this study through a one-pot reaction. However, the conjugation of a thiolated moiety was reported as an additional reaction. The S-nitrosation procedure was performed using sodium nitrite in hydrochloric acid. The influence of the thiol structure and the polymer scaffold were studied in terms of NO release. The measured NO payloads (2 μmol g−1) for up to 20 h via chemiluminescence-based analysis were claimed to represent the largest level for a biodegradable material at the time of the study in 2016.17
Herein, we report the synthesis of S-nitrosated polyesters with NO-releasing properties. Three materials were prepared from the combination of various monomeric units such as thiomalic acid, citric acid, maleic acid, and 1,8-octanediol. The prepolymer was prepared via one-pot, melt phase polycondensation, with subsequent thermal curing to yield crosslinked products that can be processed into various morphologies for the desired application.18, 19 The synthetic route circumvents the use of additional coupling reactions for the covalent functionalization of the material to yield a thiolated polymer, which is the prevailing method for the preparation of such materials. The crosslinked polyesters and their S-nitrosated analogues were the subject of bacterial studies in order to assess their antibacterial properties. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were exposed to the S-nitrosated materials as well as the non-nitrosated polymers, which were used as controls. The materials were found to be efficacious antibacterial agents, in their ability to successfully kill Gram-negative and Gram-positive bacteria strains, characteristic of broad-spectrum therapies. The degradation profiles at physiological pH and temperature were investigated by gravimetric analysis in order to establish material degradation. The degradation products of the polymers were analyzed by mass spectrometry to study the outcome of hydrolysis within the polymer chains. Tensile mechanical tests were conducted to analyze the mechanical properties of the materials and explore their potential applications based on the Young’s modulus.
The optimization of mechanical properties for antibacterial polymeric substrates in conjunction with a more efficient synthetic route, have led to the development of materials with high tensile strain and promising applications for combating bacterial infection on damaged skin tissue.20, 21
2. Materials and methods
2.1. Materials
Thiomalic acid (98%), citric acid (99.5%), maleic acid (98%), 1,8-octanediol (98%), and triethylamine (TEA) were obtained from Alfa Aesar (Ward Hill, MA, USA). tert-Butyl nitrite (t-BuONO, 90%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tetrahydrofuran (THF) and anhydrous diethyl ether were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). Phosphate buffered saline (PBS) tablets and anhydrous N,N-dimethylformamide (DMF) were procured from EMD Chemicals (Gibbstown, NJ, USA). DL-Dithiothreitol (DTT, 99%) was obtained from AMRESCO (Solon, OH, USA). Oxoid™ nutrient broth media (NBM, OXCM0001B), Oxoid™ nutrient agar (NA, OXCM0003B), sodium chloride were purchased from Fisher Scientific (Fair Lawn, NJ, USA). 24-well tissue culture treated plates were obtained from Corning (Corning, NY, USA). E. coli (ATCC 25922) and S. aureus (ATCC 29213) were purchased from American Type Culture Collection (ATCC, USA). LC-MS grade methanol was purchased from VWR (Denver, CO, USA).
2.2. Characterization techniques
1H NMR, 13C NMR, homonuclear correlation spectroscopy (COSY) 2D NMR, and heteronuclear single quantum correlation (HSQC) 2D NMR spectra were obtained in dimethylsulfoxide-d6 (DMSO-d6) using an Agilent (Varian) Inova 400 MHz FT-NMR (Agilent Technologies, Inc., Santa Clara, CA, USA). FTIR-ATR spectra were recorded in the range of 650–4000 cm−1 using a Nicolet 6700 FTIR spectrometer (Thermo Electron Corporation, Madison, WI, USA). UV-Vis absorption studies were performed using a Nicolet Evolution 300 UV-Vis spectrophotometer (Thermo Electron Corporation). Polymer molecular weight was characterized by gel permeation chromatography (GPC) in DMF using polystyrene standards with a Waters University 1500 GPC instrument (Waters, Milford, MA, USA). Thermal transitions were determined by differential scanning calorimetry (DSC) with a TA modulated 2920 DSC and the decomposition temperature was measured by thermogravimetric analysis (TGA) utilizing a TA thermogravimetric analyzer 2950 (TA Instruments, New Castle, DE, USA). Tensile mechanical tests were conducted based on ASTM standard D638 on an Instron 4442 mechanical tester equipped with a 50 N load cell (Instron, Norwood, MA, USA). Briefly, the dog-bone-shaped specimens were pulled at a rate of 100 mm/min. Values obtained from the stress-strain curves were used to calculate the Young’s modulus and crosslinking density of each materials.
Polymer degradation at physiological pH and temperature.
Gravimetric analysis was performed to assess changes in weight after incubating the materials at physiological pH and temperature, which in turn confirmed the occurrence of hydrolytic degradation. Three different types of polyester and their S-nitrosated analogues were incubated in 10 mM PBS (pH 7.4) at 37 °C for up to 10 weeks. Samples were collected every 7 days (n = 3), washed with Millipore water, and lyophilized for 24 h prior to measurement. For samples incubated longer than 1 week, the buffer solution was replaced at the end of each week.
Mass spectrometric identification of degradation products.
The degradation products from the polymer degradation studies were identified using time-of-flight mass spectrometry (TOF-MS). All mass spectrometric analyses were performed on an Agilent 6224 TOF LC/MS (Agilent Technologies, Palo Alto, CA, USA). The instrument was equipped with an Agilent multimode ion (MMI) source capable of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). For all experiments, mixed-mode ionization in negative polarity was employed with the range of mass analysis set at 100–3200 m/z. The collected sample solutions were directly introduced into the ion source by flow injection, without a pre-separation step, at a flow rate of 0.22 mL/min via the ESI nebulizer with methanol as the mobile phase (n ≥ 3). The ion source conditions were as follows: capillary voltage, 2500 V; fragmentor voltage, 120 V; skimmer voltage, 60 V; charge voltage, 2000 V; drying gas temperature, 310 °C; drying gas flow rate (N2), 10 L/min; and nebulizer pressure, 45 psig. All data were analyzed with Agilent MassHunter Qualitative Analysis B.07.00.
Chemiluminescence-based NO analysis.
NO release from S-nitrosated crosslinked polyesters was evaluated using Sievers chemiluminescence NO analyzers (NOA 280i, GE Analytical, Boulder, CO, USA) following our previously reported procedure.22 The instruments were calibrated prior to each analysis using nitrogen (zero gas) and 45 ppm NO/nitrogen. The nitrogen sweep gas flow during analysis was maintained at 200 mL min−1. Total NO content was obtained by heating the polymer samples (n ≥ 3) and appropriate controls at 150 °C in the absence of solvent followed by irradiation of light at 365 nm, which promoted the thermal and photodecomposition of the RSNO groups. The NO release from this process was used to quantify the amount of thermally and photo-releasable NO present in each material. In addition, NO release at physiological pH and temperature was determined. Polymer samples (n ≥ 3) were suspended in deoxygenated 10 mM PBS (pH 7.4) at 37 °C, and NO release was measured for 6 to 20 h, depending on the release properties of the material. For the latter experiments, the samples were shielded from direct exposure to light to prevent further photodecomposition of the RSNO.
2.3. Synthesis of materials
Poly(thiomalic-co-maleic acid-co-1,8-octanediol (PTMO) (1).
The polymer was prepared via melt-phase polycondensation reaction using thiomalic acid (4.31 g, 28.8 mmol), maleic acid (2.90 g, 25.0 mmol), and 1,8-octanediol (7.31 g, 50.0 mmol). The polymerization was achieved without an exogenous catalyst and in the absence of solvent. The reagents were added to a vented 500 mL flask equipped with a nitrogen inlet and outlet. The mixture was stirred under nitrogen flow for 30 min, then heated to 140 °C with constant stirring to initiate the polycondensation. The colorless melted mass was maintained at 140 °C for 38 min under nitrogen flow to obtain a viscous polymer. The crude polymer was dissolved in absolute ethanol (40 mL) by sonication then treated with DTT (0.203 g, 1.31 mmol) and TEA (183 μL, 1.31 mmol) to reduce disulfide bonds formed during the reaction. The solution was stirred for one hour then added over 30 min to Millipore water (200 mL) (18.2 MΩ·cm) containing Tween-80 to remove any unreacted materials. The mixture was stirred for 1 h at room temperature, then cooled at 4 °C for 1 h. The supernatant was decanted, and the polymer was washed twice with Millipore water (200 mL) and lyophilized for 7 days. After lyophilization, the prepolymer was characterized by various NMR techniques and GPC, to avoid solubility restraints caused by the following crosslinking procedure. A 20% (w/v) polymer solution in THF was prepared to cast films on a polytetrafluoroethylene (PTFE) mold, which was heated at 110 °C for 136 min under nitrogen flow to obtain a crosslinked material. The crosslinked polymer was used for the remaining characterization techniques stated previously. 1H NMR δH/ppm (400 MHz, DMSO-d6): 12.73 (–CO2H), 6.67–6.73 (–HC=CH–), 3.94–4.07 (–OCH2–), 3.72–3.89 (–S–CH–CO2–), 3.32–3.35 (–CH2OH), 2.58–2.91 (–CH2CO2–), 1.52–1.60 (–CH2–(CH2)4–CH2–), 1.34–1.39 (–CH2–CH2OH), 1.24 (–(CH2)4–). 13C NMR δC/ppm (100 MHz, DMSO-d6): 170.3–172.6 (–CO2–), 130.4–133.6 (–HC=CH–), 64.8–65.5 (–CH2OH), 61.2 (–OCH2–), 41.9–42.0 (–S–CH–CO2–), 35.9–37.1 (–CH2CO2–), 33.0 (–CH2–CH2OH), 29.0–29.3 (–(CH2)2–), 28.4–28.5 (–CH2–(CH2)4–CH2–), 25.7–25.9 (–(CH2)2–). IR υmax/cm−1: 3468–2400 (O–H), 2929–2856 (C–H), 1725 (C=O) and 1159 (C–O). A synthetic scheme that illustrates the synthesis of PTMO and its S-nitrosated analog is given in Fig. 1.
Fig. 1.
Synthesis of PTMO (1) and and S-nitrosated PTMO (1a). (i) 140 °C, 38 min, (ii), DTT, TEA, 1 h, (iii) 110 °C, 136 min, (iv) t-BuONO, EtOH, 20 min.
S-nitrosated poly(thiomalic-co-maleic acid-co-1,8-octanediol) (PTMO–NO) (1a).
A mixture of crosslinked PTMO/ethanol (1) (25 mg/mL) and t-BuONO (0.250 mL) was stirred at room temperature for 20 min in a vial protected from light. The solvent was evaporated under vacuum for 2 h to obtain an S-nitrosated crosslinked material. Given that RSNOs decompose through thermal and photolytic pathways, all materials reported herein were stored at −20 °C in sealed vessels protected from light until their use. IR υmax/cm−1: 3468–2400 (O–H), 2930–2856 (C–H), 1725 (C=O) and 1159 (C–O). UV-Vis λmax/nm: 335 (RSNO, π → π*) and 544 (RSNO, nN → π*).
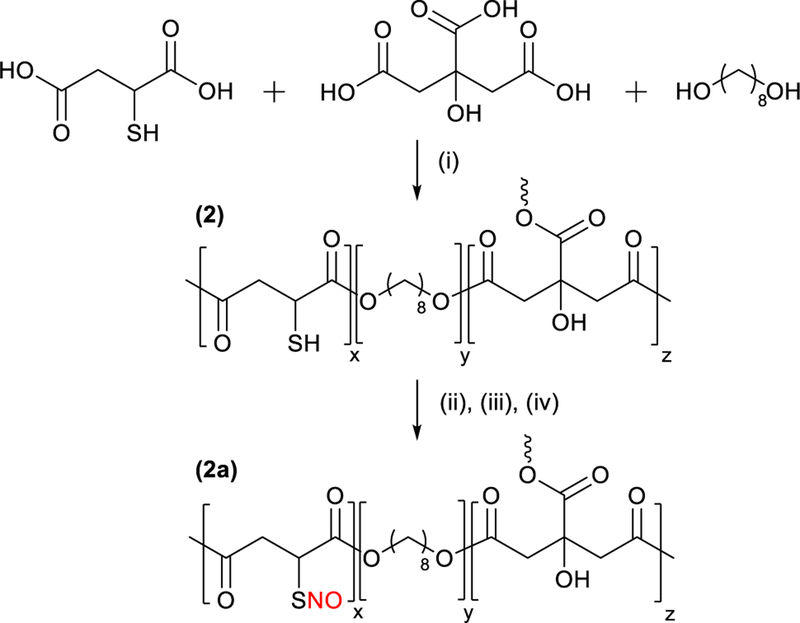
Poly(thiomalic-co-citric acid-co-1,8-octanediol) (PTCO) (2).
A polyester was prepared following the general method provided for material 1 using thiomalic acid (2.19 g, 14.3 mmol), citric acid (2.40 g, 12.5 mmol), and 1,8-octanediol (3.66 g, 25.0 mmol). The polycondensation reaction was performed at 140 °C for 1 h under nitrogen flow to obtain a viscous polymer. The viscous polymer was dissolved in absolute ethanol (20 mL) by sonication and treated with DTT (0.107 g, 0.69 mmol) and TEA (96.6 μL, 0.690 mmol). The prepolymer was characterized by NMR techniques and GPC given that the crosslinking procedure renders the material insoluble. Films were casted on PTFE molds using a 20% (w/v) polymer solution in THF. The film was dried at RT, then heated at 110 °C for 24 h under nitrogen flow to obtain a crosslinked material. The crosslinked polymer was used for the remaining characterization techniques. 1H NMR δH/ppm (400 MHz, DMSO-d6): 5.56 (–C–OH), 3.93–4.07 (–OCH2–), 3.58–3.69 (–S–CH–CO2), 3.32–3.36 (–CH2OH), 2.61–2.91 (–CH2CO2–), 1.51–1.53 (–CH2–(CH2)4–CH2–), 1.36–1.39 (–CH2–CH2OH), 1.25 (–(CH2)4–). 13C NMR δC/ppm (100 MHz, DMSO-d6): 169.6–174.9 (–CO2–), 72.9–73.3 (–C–OH), 64.4–65.3 (–OCH2–), 61.2 (–CH2OH), 41.9–42.0 (–CH2CO2–), 35.8–35.9 (–S–CH–CO2–), 33.0 (–(CH2)2–), 28.4–28.5 (–CH2–(CH2)4–CH2–), 25.6–25.9 (–(CH2)2–). IR υmax/cm−1: 3494–2400 (O–H), 2928–2855 (C–H), 1725 (C=O), 1167 (C–O). A synthetic scheme that depicts the synthesis of PTCO and S-nitrosated PTCO is given in Fig. 2.
Fig. 2.
Synthesis of PTCO (2) and S-nitrosated PTCO (2a). (i) 140 °C, 1 h, (ii), DTT, TEA, 1 h, (iii) 110 °C, 24 h, (iv) t-BuONO, EtOH, 20 min.
S-nitrosated poly(thiomalic-co-citric acid-co-1,8-octanediol) (PTCO–NO) (2a).
Crosslinked PTCO was S-nitrosated following the general procedure for 1a. PTCO/ethanol (2) (25 mg/mL) and t-BuONO (0.250 mL) was stirred for 20 min, followed by solvent evaporation for 2 h under vacuum. IR υmax/cm−1: 3468–2400 (O–H), 2930–2856 (C–H), 1727 (C=O), 1167 (C–O). UV-Vis λmax/nm: 331 (RSNO, π → π*) and 544 (RSNO, nN → π*).
Poly(thiomalic acid-co-1,8-octanediol) (PTO) (3).
The material was synthesized following the procedure for polymer 1 using thiomalic acid (3.99 g, 26.6 mmol) and 1,8-octanediol (3.89 g, 26.6 mmol). The reaction mass was maintained at 140 °C for 17 h under nitrogen flow to obtain a viscous polymer. The crude polymer was dissolved in ethyl ether anhydrous (20 mL) by sonication and treated with DTT (0.104 g, 0.68 mmol) and TEA (94.4 μL, 0.68 mmol). The prepolymer was characterized by NMR and GPC before the crosslinking step to avoid solubility restraints. Layers of a 20% (w/v) polymer solution in THF were casted on PTFE molds for the film preparation. After the solvent evaporated, the molds were heated at 110 °C for 82 h under nitrogen flow to obtain a flexible polyester. The crosslinked polymer was used for the remaining characterization techniques. 1H NMR δH/ppm (400 MHz, DMSO-d6): 3.94–4.06 (–OCH2–), 3.66–3.70 (–S–CH–CO2–), 2.71–2.91 (–CH2CO2–), 1.57 (–CH2–(CH2)4–CH2–), 1.25–1.30 (–(CH2)4–). 13C NMR δC/ppm (100 MHz, DMSO-d6): 170.4–173.7 (–CO2–), 64.7–65.2 (–OCH2–), 39.8 (–CH2CO2–), 35.9–36.1 (–S–CH–CO2–), 29.0 (–CH2–(CH2)4–CH2–), 28.4–28.5 (–(CH2)2–), 25.6–25.7 (–(CH2)2–). IR υmax/cm−1: 3544–2400 (O–H), 2930–2856 (C–H), 1727 (C=O), 1158 (C–O). The synthetic scheme for the synthesis of PTO and its S-nitrosated analog is given in Fig. 3
Fig. 3.
Synthesis of PTO (3) and S-nitrosated PTO (3a). (i) 140 °C, 17 h, (ii), DTT, TEA, 1 h, (iii) 110 °C, 82 h, (iv) t-BuONO, EtOH, 20 min.
S-nitrosated poly(thiomalic acid-co-1,8-octanediol) (PTO–NO) (3a).
The general method provided for material 1a was followed for the preparation of PTO–NO with a variation in solution concentration. PTO/ethanol (3) (12.5 mg/mL) and t-BuONO (0.250 mL) was stirred for 20 min followed by solvent removal under vacuum for 7.3 h. IR υmax/cm−1: 3544–2400 (O–H), 2927–2900 (C–H), 1728 (C=O), 1159 (C–O). UV-Vis λmax/nm: 333 (RSNO, π → π*) and 540 (RSNO, nN → π*).
2.4. Bacteria studies
E. coli and S. aureus bacteria culture.
Initial stock cultures of E. coli and S. aureus were obtained by streaking agar plates and inoculating the bacteria in nutrient broth. The stock culture was grown overnight in NBM to an O.D.600nm ~1.0. The bacterial solution was then combined with glycerol (30% v/v) in a 1:1 fashion to obtain a final glycerol concentration of 15% (v/v). These solutions were stored at −80 °C until further use. Prior to each bacterial assay, a 10 mL frozen culture was allowed to thaw at room temperature and centrifuged at 4700 rpm for 10 min. The supernatant was discarded and the pellet was resuspended in 5 mL warmed NBM. The solution was transferred to an additional 45 mL NBM and allowed to grow overnight in a 37 °C incubator under stirring conditions until the O.D600nm reached ~1.0. The following day, the culture was diluted using NBM to a working concentration corresponding to an O.D. 600nm ~0.35.
Antibacterial activity of polyesters.
Samples of both non-nitrosated and S-nitrosated polymer (50 mg) were added to 24-well plates in triplicate before the addition of bacterial solution (1 mL). The plate was placed in a 37 °C incubator for 6 or 24 h under shaking conditions before 100 μL aliquots were removed from each well and 10-fold serial dilutions were performed in sterilized saline solution (0.85% w/v NaCl). After dilution, 50 μL aliquots were plated on agar before being placed in a 37 °C incubator. The number of colony-forming units (CFU) were counted the following day and converted to CFU/mL for comparison to positive control (bacteria solution in the absence of polymer samples). All samples were tested in replicate (n ≥ 6).23–25
3. Results and discussion
Three polyesters, PTMO, PTCO and PTO were synthesized from a combination of thiomalic acid, citric acid, maleic acid, and 1,8-octanediol following a modified literature protocol to yield crosslinked polyesters.16, 26 In particular, thiomalic acid was included in order to avoid the use of coupling reactions to incorporate the thiol moiety. These thiol pendant groups serve as functionalization sites to form S-nitrosothiols. The naturally-occurring monomer, citric acid, was utilized given its trifunctional connectivity, its well-studied hemocompatibility, and use in devices for drug delivery and cardiac-tissue engineering. Maleic acid was a relevant monomer to potentially increase the crosslinking density of the material through the alkene. Lastly, 1,8-octanediol enabled the formation of ester linkages and increased the hydrophobicity of materials.27
The structure of each material was independently confirmed by 1H NMR and 13C NMR. (Fig. S1–S6, ESI). The connectivity of the monomeric units was determined by two-dimensional NMR experiments such as COSY and HSQC spectroscopy (Fig. S7–S12, ESI). For all materials, the multiplets at 2.58–2.91 and 3.58–3.89 ppm correspond to thiomalic acid, while peaks at 1.24–1.60 and 3.93–4.07 ppm confirmed incorporation of 1,8-octanediol. For PTCO, the singlet at 5.56 ppm corresponds to citric acid. For PTMO, the doublets at 6.67–6.73 ppm represent the available alkenes of maleic acid and the occurrence of isomerization.
According to 2D NMR techniques, the carbons from 25.6–29.3 ppm have correlation with protons at 1.25–1.57 ppm. The atoms correspond to the inner carbons of 1,8-octanediol as they have correlations within each other and with protons at 3.96–4.06 ppm, which represent the outer 1,8-octanediol atoms adjacent to the ester linkages with carbons at 64.4–65.5 ppm. Thiomalic acid protons were detected at 2.71–2.91 ppm with homonuclear correlations at 3.66–3.70 ppm, and heteronuclear correlations at 35.8–42.0 ppm. The features are similar for all materials, with additional correlations for PTMO at 3.32–3.35 ppm with homonuclear correlations at 1.34–1.39 ppm and heteronuclear correlations at 64.8–65.5 ppm. Comparable correlations were found for PTCO at 3.32–3.36 ppm with homonuclear correlations at 1.36–1.39 ppm, and heteronuclear correlations at 61.2 ppm. The signals represent 1,8-octanediol protons on carbons bearing terminal hydroxyl groups, which indicate the presence of oligomers given that the step-growth polycondensation was stopped in order to characterize the prepolymers.28 Protons at 6.67–6.73 ppm represent sp2 hybridized maleic acid carbons, with heteronuclear correlations at 129–132 ppm. For PTCO, the multiplet at 2.61–2.91 ppm corresponds to protons from thiomalic and citric acid, with homonuclear correlations at 3.6 ppm and heteronuclear correlations at 40 and 44 ppm. The results and their interpretation were consistent with 1H NMR and 13C NMR spectra of similar polyesters.29–31 No features developed that could be attributable to the formation of thioester species, which typically result in features at 195 ppm.32 The FTIR-ATR spectra (shown in Fig. S13–S15, ESI) feature ester carbonyl stretches at approximately 1700 cm−1, which confirm the presence of ester linkages between the monomers. The contribution of carbonyl groups C=O stretching to the sharp feature at 1700 cm−1 supported the presence of ester linkages in the materials.33
GPC analysis with polystyrene standards indicated that the prepolymers had a number average molecular weight (Mn) higher than that of previously reported prepolymers (<1000 g mol−1) with similar characteristics such as NO release and degradability.34 This suggests that other prepolymers have been characterized at earlier stages of the polymerization, and the final properties of the materials such as Young’s modulus may be more informative for comparison. The glass transition temperature (Tg) of these materials is comparable to that of previously reported materials, except PTO, which reached lower values. A decrease in Tg can be attributed to the presence of oligomeric or monomeric components that function as plasticizers.35 Ideal Tg values for the applications intended would be below physiological temperature, the results confirm that this was achieved for all materials. In this case, the longer polymerization times increased the molecular weight of the material and reduced the Tg and the number of different sized oligomers, which can be deduced from the dispersity index (Đ).36 The prepolymers were characterized by GPC instead of the crosslinked polymers due to solubility restraints. Hence, the value for Đ deviates from unity given that the polymerization reaction had not reached completion at the stage of GPC analysis in order to obtain a soluble material. The crystallization temperature (Tc) and melting temperature (Tm) were identified, which denote that the materials are not completely amorphous and crystalline regions were detected.37 The values were similar for all materials where Tc ranged from 73.2–73.9 °C, and Tm ranged from 83.1–84.0 °C. All thiolated polyesters were flexible, colorless, transparent materials, which would be the preferred characteristics for the preparation of certain devices.
Thermal transitions, degradation profiles and the crosslinking density of the polyesters were influenced by the reaction time and the monomeric units employed for the synthesis of each material. The increased functionality of each monomer gave rise to more crosslinked polyesters. The crosslinking density and Young’s modulus was inversely proportional to the elongation at break of the specimens. The exception to the tendency occurred with PTMO, which had relatively high elongation at approximately 300% and high Young’s modulus and crosslinking density. Such finding suggests that the alkene-bearing monomer, maleic acid, increased the crosslinking density of the material without affecting the final elongation. The degradation time and thermal transitions were also proportional, as it was determined that the materials with lower Tg values were less prone to hydrolytic degradation. The results have comparable trends to those reported by Coneski et al.34
3.1. Synthesis and characterization of PTMO (1)
The catalyst-free polycondensation was carried out in the melt-phase at 140 °C for 38 minutes with a constant nitrogen flow to remove water, and the structure of PTMO was independently confirmed by 1H NMR, 13C NMR, COSY 2D NMR, and HSQC 2D NMR (Fig. S1, S4, S7, and S10, ESI), consistent with previous reports.29, 38 The 1H NMR spectrum shows multiplets at 2.58–2.91 (–CH2CO2–) and 3.72–3.89 ppm (–S–CH–CO2–) that correspond to the protons on thiomalate units, while peaks at 1.24 (–(CH2)4–), 1.34–1.39 (–CH2–CH2OH), 1.52–1.60 (–CH2–(CH2)4–CH2–), 3.32–3.35 (–CH2OH), and 3.94–4.07 (–OCH2–) ppm confirm the incorporation of 1,8-octanediol. The split alkenyl protons of the maleate group appeared at 6.67–6.73 (–HC=CH–) ppm. The broad feature at 12.73 (–CO2H) ppm is attributed to the remaining free carboxyl groups from thiomalate units. Assignment of peaks on the 1H NMR spectrum was confirmed by recording the proton correlations with COSY 2D NMR. 13C NMR spectrum represents the different carbon environments present in the polymer, which were assigned by noting the protons bonded to carbons using HSQC 2D NMR. The carbon spectrum demonstrates peaks at 25.7–25.9 (–(CH2)2–), 28.4–28.5 (–CH2–(CH2)4–CH2–), 29.0–29.3 (–(CH2)2–), 33.0 (–CH2–CH2OH), 61.2 (–OCH2–), and 64.8–65.5 (–CH2OH) ppm that correspond to 1,8–octanediol, while peaks at 35.9–37.1 (–CH2CO2–), 41.9–42.0 (–S–CH–CO2–), and 170.3–172.6 (–CO2–) ppm are attributed to thiomalate carbons. The sp2 hybridized carbons at 130.4–133.6 ppm (–CH=CH–) represent the incorporation of maleate units in the material. The FTIR-ATR spectrum features an ester carbonyl (C=O) stretching band at 1726 cm−1, which confirmed the ester linkages between the monomer. GPC analysis of the prepolymer demonstrated a Mn of 95.8 kDa and a Đ of 2.72. The dispersity index demonstrated that the material contains different sized oligomers. As determined by TGA, the decomposition temperature of the crosslinked polymer was 352.8 °C. Thermal transitions were analyzed by DSC, where it was established that the Tg was −4.18 °C.
3.2. Synthesis and characterization of PTCO (2)
The 1H NMR spectrum shows multiplets at 2.61–2.91 (–CH2CO2–) attributed to the protons on the citrate and thiomalate units and at 3.58–3.69 (–S–CH–CO2) ppm that corresponds to the protons on the thiomalate units. The peaks at 1.25 (–(CH2)4–), 1.36–1.39 (–CH2–CH2OH), 1.51–1.53 (–CH2–(CH2)4–CH2–), 3.32–3.36 (–CH2OH), and 3.93–4.07 (–OCH2–) ppm confirm the incorporation of 1,8-octanediol. The singlet at 5.56 (–C–OH) ppm represents the hydroxyl group on the citrate unit (Fig. S2, ESI). 13C NMR spectrum (depicted in Fig. S5, ESI) represents the different carbon environments present in the polymer, which were assigned by noting the nuclear correlations using COSY and HSQC 2D NMR (Fig. S8 and S11, ESI). The carbon spectrum demonstrates peaks at 25.6–25.9 (–(CH2)2–), 28.4–28.5 (–CH2–(CH2)4–CH2–), 33.0 (–(CH2)2–), 61.2 (–CH2OH), and 64.4–65.3 (–OCH2–) ppm that correspond to 1,8–octanediol, while peaks at 35.8–35.9 (–S–CH–CO2–), 41.9–42.0 (–CH2CO2–), and 169.6–174.9 (–CO2–) ppm are attributed to thiomalate carbons. The signal at 72.9–73.3 (–C–OH) ppm represents the incorporation of citrate units in the material. The FTIR-ATR spectrum features an ester carbonyl C=O stretching band at 1726 cm−1, which confirmed the ester linkages between the monomer (Fig. S14, ESI). GPC analysis with polystyrene standards showed a Mn of 130 kDa and a Đ of 2.53. The crosslinked polymer was used for TGA and DSC where it was established that the decomposition temperature was 322.0 °C and the Tg was −23.7 °C. The Tg value was lower given that the material was able to reach a higher crosslinking density due to the trifunctional monomer, citric acid.36
3.3. Synthesis and characterization of PTO (3)
The material had higher linearity compared to the other polyesters due to the absence of functional groups that permit a higher crosslinking density to be reached.37 However, the thiol moiety on thiomalic acid provided some crosslinking sites, which increased the Young’s modulus of the material. The 1H NMR spectrum shows multiplets at 2.71–2.91 (–CH2CO2–) and 3.66–3.70 (–S–CH–CO2–) ppm that correspond to the protons on thiomalate units, while peaks at 1.25–1.25–1.30 (–(CH2)4–), 1.57 (–CH2–(CH2)4–CH2–) and 3.96–4.06 ppm (–OCH2–) confirm the incorporation of 1,8-octanediol (Fig. S3, ESI). 13C NMR spectrum (shown in Fig. S6, ESI) represents the different carbon environments present in the polymer, which were confirmed by COSY and HSQC 2D NMR experimental techniques (Fig. S9 and S12, ESI). The carbon spectrum demonstrates peaks at 25.6–25.7 (–(CH2)2–), 28.4–28.5 (–(CH2)2–), 29.0 (–CH2–(CH2)4–CH2–), and 64.7–65.2 (–OCH2–) ppm that correspond to 1,8–octanediol, while peaks at 35.9–36.1 (–S–CH–CO2–), 39.8 (–CH2CO2–), and 170.4–173.7 (–CO2–) ppm are attributed to thiomalate carbons. The FTIR-ATR spectrum features an ester carbonyl (C=O) stretching band at 1727 cm−1, which confirmed the ester linkages between the monomer, while the contribution of thiomalic acid (S–H) broad feature at 2557 cm−1 supported the preservation of free thiol groups for further functionalization into S-nitrosothiols (Fig. S15). Opposite to PTMO and PTCO, a resonance feature was observed for the thiol groups given that only two monomeric units were used for PTO. Therefore, the molar ratio of thiols to other functional groups is greater in this case. GPC analysis with polystyrene standards showed a Mn of 412 kDa and a Đ of 1.77. The larger molecular weight of this material is attributed to the longer reaction time, as well as the higher linearity that prevents crosslinking at various sites and elongates the polymer chains. The crosslinking procedure was performed for 82 h at 110 °C, which gave rise to a highly elastic material with a low crosslinking density. The crosslinked polymer was used to determine thermal properties using TGA and DSC. The decomposition temperature was 346.9 °C and the Tg was −33.5 °C. Minor degradation was measured over a 10-week experiment at physiological pH and temperature. The lack of degradation may be attributed to the increased hydrophobicity of the polymer chains due to the higher monomeric ratio of 1,8-octanediol to thiomalic acid in the material.
3.4. Synthesis and characterization of S-nitrosated PTMO, PTCO and PTO (1a, 2a, 3a)
S-nitrosation of the thiol-bearing polyesters was carried out in ethanol under anhydrous conditions using the alkyl nitrite, t-BuONO, as a nitrosating agent. The procedure exposes the material to milder conditions in comparison to other procedures. In addition, it avoids the use of sodium nitrite in aqueous acid that could lead to parallel hydrolysis of the polymeric ester linkages, and permits removal of unreacted t-BuONO and volatile products under vacuum. The conversion of thiol groups to the corresponding RSNO was supported by UV-Vis spectrophotometry in DMSO, where absorptions characteristic to RSNOs were observed at 335 (π → π*) and 544 nm (nN → π*) for PTMO–NO, at 331 and 544 nm for PTCO–NO, and at 333 and 540 nm for PTO (Fig. S16–S18, ESI). No features developed that could be attributable to the formation of undesirable alkyl nitrite species, which typically result in distinctive absorptions in the range of 260–400 nm.39 The FTIR-ATR of S-nitrosated materials indicated the preservation of the primary structural features of the polyester with bands at 3500–2400 (O–H), 2933–2857 (C–H), 1729 (C=O), and 1160 (C–O) cm−1 for PTMO–NO were consistent with those of the non-nitrosated polyester. These features appeared at 3500–2400 (O–H), 2933–2857 (C–H), 1729 (C=O), 1160 (C–O) cm−1 for PTCO–NO, and at 3500–2400 (O–H), 2989–2900 (C–H), 1727 (C=O), 1158 (C–O) cm−1 for PTO–NO (Fig. S13–S.15, ESI).
The total NO content of the materials was quantified using real-time chemiluminescence-based detection of NO. Samples were heated at 150 °C followed by irradiation of light at 365 nm in a custom glass vessel with constant flow of nitrogen.40 This process leads to the thermal and photo-decomposition of RSNO groups resulting in the quantifiable release of NO. The moles of NO released per gram of material were calculated using a calibration constant obtained from known concentrations of NO. The experiments continued until the NO release was equivalent to the baseline. (Table 1)
Table 1.
Summarized NO data for PTMO–NO, PTCO–NO and PTO–NO. All samples were tested in replicate (n ≥ 3) and results are reported as the mean ± standard deviation.
| Material | Cumulative NO releasea (μmol g1) | Total NO contentb (μmol g−1) |
|---|---|---|
| PTMO–NO (1a) | 90 ± 20 | 130 ± 39 |
| PTCO–NO (2a) | 80 ± 15 | 200 ± 35 |
| PTO–NO (3a) | 30 ± 6 | 130 ± 11 |
Release measured at pH 7.4, 37 °C in 10 mM PBS.
Values determined by NO analysis through thermal decomposition of the RSNO at 150 °C.
Experiments were performed in triplicate for all materials, and it was calculated that PTMO–NO had the highest cumulative NO release normalized by mass at physiological pH and temperature 90 ± 20 μmol g−1, followed by PTCO–NO 80 ± 15 μmol g−1, and PTO–NO 30 ± 6 μmol g−1. The materials released NO for different periods of time; PTCO–NO released for 20 h, PTO–NO for 12 h, and PTMO–NO for more than 6 h. A potential reason for the time variation of NO release is the degradation of the materials. As seen in Fig. 4, the NO release time follows a similar trend as the degradation at physiological pH and temperature, where PTCO–NO degraded the slowest followed by PTO–NO and PTMO–NO. Thus, the material susceptibility for hydrolytic degradation influenced the NO release time. Of note, the concentration of the solution used for the S-nitrosation reaction (tert-butyl nitrite in ethanol) could be decreased to obtain NO values that lie within the range for the intended application. The thiol-bearing polyesters produced by Seabra et al. have been reported to release NO at lower concentrations (0.15 μmol g−1) for approximately 24 h at 37 °C as calculated with the Griess assay. The S-nitrosation procedure varied in this case since NO gas was bubbled through the liquid polyester. The preparation of films using PMMA in acetonitrile after the S-nitrosation procedure may have lowered the amount of releasable NO, since the solvent was allowed to evaporate at 10 °C for 12 h.16 In a different study with thiolated polyesters, S-nitrosothiols where formed via exposure to aqueous solutions of acidified nitrite. The total NO release at near physiological conditions in CuBr2 supplemented PBS solution ranged from 1.60–1.97 μmol g−1.17 While the intended application for the latter materials may differ from those reported herein, it is noteworthy that higher NO payloads were achieved by our methods.
Fig. 4.
Representative real-time NO release profiles for PTMO–NO, PTCO–NO, PTO–NO under aqueous conditions (pH 7.4, 37 °C).
3.5. Polymer degradation at physiological pH and temperature
The potential biodegradability of the polymers was assessed by immersing samples (n = 3) of PTMO, PTMO–NO, PTCO, PTCO–NO, PTO, and PTO–NO in 10 mM PBS buffer (pH 7.4) at 37 °C in the absence of light for up to 10 weeks (Fig. 5). The results indicated a fast degradation for PTMO and PTMO–NO during the 10-week experiment. PTMO had a remaining mass percent of 33 ± 18 % and PTMO–NO had 7 ± 4 %. PTCO and PTCO–NO demonstrated a slower degradation under these conditions, where the mass percent remaining for PTCO was 90 ± 1 % and PTCO–NO had 86 ± 3 % mass remaining. For the previously mentioned polymers, hydrolytic degradation lead to the fragmentation of crosslinked polymer chains. The polymer with the higher linearity, PTO, was not significantly degraded after the experiment concluded with a remaining mass percent of 97 ± 0.05 %. However, PTO–NO degraded slowly after the first week, but retained 88 ± 0.9 % of its mass at the end of the experiment. In all cases, the S-nitrosated material had a more rapid degradation that the unmodified material. The results suggest that S-nitrosated polymers have a lower molecular weight caused by the S-nitrosation methodology. It was not possible to measure the molecular weight of S-nitrosated polymers due to their impaired solubility after the functionalization procedures. The outcome indicates that, even though disulfide bonds were formed as byproducts of NO release, the formation of such bonds did not improve the robustness of the materials in aqueous conditions. However, potential crosslinking from disulfide formation does not offset the cleavage of ester linkages. The finding suggests that the process of disulfide formation is not particularly relevant for the stability of these systems. Also, it insinuates that the reaction conditions used for the S-nitrosation reaction mildly modify the polymer backbone, which leads to a faster degradation.
Fig 5.
Degradation profiles of PTMO, PTCO, PTO and S-nitrosated derivatives at physiological pH and temperature (pH 7.4, 37 °C). All samples were tested in replicate (n = 3) and the mean and standard deviations are displayed.
The alkene bearing polymer, PTMO and PTMO–NO, demonstrated the highest degradation rates of the materials that were the subject of these experiments. PTCO and its S-nitrosated analogue had a slower degradation than PTMO and PTMO–NO. This behavior can be attributed to the trifunctional monomer, citric acid, which provides additional reaction sites and allows for a higher crosslinking density. PTO and PTO–NO had the slowest degradation, according to the gravimetric techniques utilized. Degradation time was determined to be proportional to the polymerization and crosslinking time for each material as well as the Tg. Hence, the materials that underwent longer polymerization times did not readily degrade during the 10-week experiment. As studied using GPC, the materials contain oligomers of various molecular weights that allow the polymer chains to produce smaller fragmented sections. The most significant mass loss occurred during the first week, which suggests that the bulk material readily releases oligomeric species upon immersion in PBS solution. The slow degradation combined with NO release at physiologically relevant levels suggest that the polymers may have potential applications as materials for wound dressings. The initial release of NO would prevent the risk of bacterial infection, while the slow degradation of the material would allow the materials to remain in place and provide moisture to the regenerating skin tissue.41
Prior studies with non-nitrosated polyesters report degradation profiles that indicate mass remaining percentages from 0% to 90% with various combinations of the monomeric units such as adipic acid, glutaric acid, glycerol and pentaerythritol.34 The composition of the materials based on the functionality of the monomers and crosslinking times and temperature were related to the degradation profiles. The study suggests that materials synthesized at higher temperatures and with a certain monomer combination, generally demonstrate slower hydrolytic degradation, which is in agreement with our findings.
3.6. TOF-MS identification of polymer degradation products
The degradation products found for PTO and PTO–NO were PTO-P1 (C12H20O5), PTO-P2 (C12H22O5S), PTO-P3 (C16H26O9S) and PTO-P4 (C24H42O10S). The degradation products found for PTMO and PTMO-NO were PTMO-P1 (C12H20O5), PTMO-P2 (C12H22O5S), PTMO-P3 (C16H26O9S) and PTMO-P4 (C24H42O10S). The degradation products for PTCO and PTCO–NO were PTCO- P1 (C12H20O5), PTCO-P2 (C12H22O5S), PTCO-P3 (C14H24O8), PTCO-P4 (C16H26O8S2), PTCO-P5 (C18H28O11S), PTCO-P6 (C26H44O12S), and PTCO-P7 (C12H22O5). The measured masses and formulas for each degradation product are shown in Table 2.
Table 2.
TOF-MS identification of polymer degradation products
| Label | Formula | Molecular weight [M-H]− |
|---|---|---|
| PTMO-P1*‡ | C12H20O5 | 243.1254 |
| PTMO-P2‡ | C12H22O5S | 277.1138 |
| PTMO-P3* | C16H26O9S | 393.1246 |
| PTMO-P4* | C24H42O10S | 521.2452 |
| PTCO-P1‡ | C12H20O5 | 243.1245 |
| PTCO-P2‡ | C12H22O5S | 277.1131 |
| PTCO-P3 | C14H24O8 | 319.1285 |
| PTCO-P4 | C16H26O8S2 | 409.1006 |
| PTCO-P5 | C18H28O11S | 451.1290 |
| PTCO-P6 | C26H44O12S | 579.2487 |
| PTCO-P7*† | C12H22O5 | 245.1407 |
| PTO-P1*‡ | C12H20O5 | 243.1253 |
| PTO-P2‡ | C12H22O5S | 277.1120 |
| PTO-P3* | C16H26O9S | 393.1246 |
| PTO-P4* | C24H42O10S | 521.2450 |
Ions also found as byproducts from S-nitrosated polymers (PTMO–NO, PTCO–NO and PTO–NO).
Ions common to all polymers (PTMO, PTMO–NO, PTCO, PTCO–NO, PTO and PTO–NO) corresponding to dimers composed of the bonded monomers.
Ion only found as byproduct from the S-nitrosated polymer PTCO–NO.
The collected spectra indicated that most of the ions identified correspond to a combination of the monomer units used for the synthetic procedure. All six polymers formed the ions m/z 243 and 277, which correspond to products formed from 1,8-octanediol – maleic acid, and 1,8-octanediol – thiomalic acid, respectively. A major difference specific to PTCO was the presence of the ion m/z 319, which corresponds to a conjugate formed from 1,8-octanediol – citric acid (Fig. S19, ESI). Identification of the majority of the degradation products as esters was encouraging, since the starting monomers (citric acid, maleic acid, thiomalic acid) were not prevalent.
3.7. Tensile mechanical test of crosslinked polyesters
The tensile modulus of the materials was measured using a tensiometer equipped with a 50 N load cell. The materials were elongated to failure in order to calculate the maximum extension as well as the Young’s modulus from the initial slope of the stress-strain curves. The crosslink density of the polyesters, expressed by n (moles of active network chains per unit volume) was calculated using the equation derived from rubber elasticity theory: n=E0/3RT, where E0 is Young’s modulus, R is the universal gas constant (8.314 J mol−1 K−1), and T is the temperature in K (Table 3).42
Table 3.
Summarized tensile mechanical test results of PTMO, PTCO, PTO, and S-nitrosated derivatives. All samples were tested in replicate (n ≥ 4) and results are reported as the mean ± standard deviation.
| Material | Young’s Modulusa (MPa) | Elongation at Breaka (%) | Crosslinking Densityb (mol m−3) |
|---|---|---|---|
| PTMO (1) | 0.6 ± 0.2 | 300 ± 60 | 80 ± 22 |
| PTMO–NO (1a) | 0.24 ± 0.08 | 240 ± 60 | 30 ± 11 |
| PTCO (2) | 1.00 ± 0.06 | 220 ± 24 | 140 ± 8 |
| PTCO–NO (2a) | 1.1 ± 0.1 | 97 ± 5 | 150 ± 17 |
| PTO (3) | 0.18 ± 0.02 | 800 ± 54 | 24 ± 3 |
| PTO–NO (3a) | 0.22 ± 0.03 | 330 ± 37 | 30 ± 4 |
Values determined by tensile mechanical tests on a tensiometer with a 50 N load cell.
Values calculated from equation derived from rubber elasticity theory.
Non-linear stress-strain curves are typical for crosslinked materials, similar to those of ligament, especially PTCO and its S-nitrosated derivative (Fig. 6).43–45 The tensile Young’s modulus of the materials was similar to other polyesters made from glycerol and sebacic acid 0.282 ± 0.025 MPa.46 The material with the highest Young’s modulus was PTCO–NO, followed by PTCO. Such materials had the lowest elongation due to the high crosslinking density of the polymer chains that afforded crosslinked networks through the trifunctional monomer, citric acid. The value of the Young’s modulus of the materials is akin to that of ligaments, in the kPa scale, which contain mostly elastin and some collagen. The values are also in the range of tendons, which is in the GPa scale given their high collagen content.47–49 Furthermore, material modulus may be matched to that of soft tissue by varying their monomeric constitution based on the desired degree of crosslinking. Compared to soft tissue with higher moduli, the values are also in the range of human meniscal tissue, which was reported to have an instantaneous modulus at 1 MPa, equilibrium values approx. 0.2 MPa, and dynamic modulus values 0.7–0.8 MPa.50 Additionally, the instantaneous or dynamic moduli of ovine meniscus at 0.6 MPa is also within range of PTCO and PTCO–NO.51, 52 In all cases, except for PTMO–NO, the crosslinking density increased for the S-nitrosated polymers. These crosslinking events may be the result of disulfide formation through the thiol-bearing, thiomalic acid, as NO is released from the sample. Uniquely, PTO and PTO–NO had the lowest crosslinking densities due to the linearity of the structure, where chain entanglement gave rise to a crosslinked network. The crosslinking densities of the materials were comparable to previously reported polyesters with values that ranged from 16 ± 3 to 207 ± 3 mol m−3. The elongation of such materials was lower, with a range from 51–327%.30 The elongation at break is similar to that of arteries and veins, which is up to 260%, and larger than that of tendons at 18%.53, 54 PTO and PTO–NO showed the highest elongation, where PTO reached approximately 800% elongation at break. PTO had the longest reaction times, highest molecular weight, and showed minor degradation at physiological pH and temperature, which suggests that the applications for this polymer may differ from those of the rest of the materials reported herein. The high elongation values suggest that the materials could be used as bandages for wound dressings given that they could be placed at a site that is at continuous movement without damaging the films. In addition, the incorporation of NO to materials with similar modulus as that of soft biological tissue may expand the potential application of the materials given the occurrence of infection in implantable devices.7, 55
Fig. 6.
Representative stress-strain curves of PTMO, PTCO, PTO and S-nitrosated derivatives.
3.8. Bacteria studies
Although NO has been shown to participate in numerous biological functions, its use as a broad-spectrum antibacterial agent has been widely exploited for biomedical applications. The mechanism of antibacterial action is both from NO itself, which is thought to cause intracellular DNA damage, as well as from NO byproducts, which may cause oxidative and nitrosative stress on the viable bacteria. The potential antibacterial activity from the S-nitrosated polymers was tested against two bacteria strains (Gram-negative and Gram-positive) at two time points (6 and 24 h) using an agar plating assay. The S-nitrosated polymers were exposed to a solution of bacteria in NBM and maintained at 37 °C to ensure the integrity of the bacteria were not compromised by improper growing conditions. Additionally, use of the agar plating method enables high sensitivity in order to achieve extremely large log-reductions obtained between the controls and the S-nitrosated polymers. Two clinically relevant bacteria strains were chosen (E. coli and S. aureus) to demonstrate the importance of these materials against strains associated with hospital-acquired infections and to highlight the broad-spectrum action of these NO-releasing materials. The activity was evaluated after a 6 h exposure period, as this is considered the critical time period associated with infection between a material and biological interface, but again at 24 h in order to ensure there was no regrowth of the bacteria after initial exposure. The lack of bacterial regrowth after NO had been depleted from the polymers suggests that diminished NO release capabilities would not necessarily diminish the antibacterial performance of the materials.56
Table 4 displays the results from the bacteria studies, where the log-reductions in bacterial viability based on the agar plating method after 6 and 24 h exposure to the S-nitrosated polymers can be seen (raw CFU values can be found in Table S1, ESI). In the first 6 h of exposure to E. coli, a significant reduction in planktonic bacteria is observed for all three S-nitrosated polymers, with PTMO resulting in an astounding log-8 reduction, representing the limit of detection (LOD) for this technique. After 24 h exposure to E. coli, PTCO also reaches the LOD with a log-8 reduction, a reduction that is retained for PTMO. Interestingly, the PTO species shows slight regrowth of the bacteria from a log-5 to log-4 reduction in planktonic, however this still represents a significant reduction in planktonic bacteria to attain and is ultimately considered greater antibacterial efficacy than the current industry standard (log-3). Interestingly, an even greater reduction is observed when the polymers are exposed to S. aureus, yielding a log-7 reduction (LOD) within the first 6 h of exposure for all S-nitrosated polymers. This substantial reduction is ultimately maintained over the full 24 h exposure period. Indeed, these reductions using ~50 mg of material are extremely noteworthy, given the current industry standard of a log-3 (equivalent to 99.9%) reduction in viable bacteria. To ensure that the observed antibacterial action was a direct result of the S-nitrosation and not the parent compound itself, the non-nitrosated polymers were also tested under identical conditions. The results from this control study indicated no decrease in cellular viability for either bacterial strain in the presence of the non-nitrosated polymers, suggesting that the S-nitrosation itself is responsible for the observed effect. Taken together, these results suggest that all three S-nitrosated polymers are capable of effectively killing planktonic bacteria of multiple strains within a 24 h period, ultimately presenting an ideal material for combating bacterial infections.57
Table 4.
Log-reductions in viable bacteria obtained using the agar plating method after 6 and 24 h exposure to S-nitrosated polymers. All reductions are relative to a positive control containing bacteria in the absence of polymer (n ≥ 6).
| Log-Reduction in Bacteria | ||||
|---|---|---|---|---|
| E. coli | S. aureus | |||
| 6 h | 24 h | 6 h | 24 h | |
| PTMO–NO (1a) | 8 | 8 | 7 | 9 |
| PTCO–NO (2a) | 2 | 8 | 7 | 9 |
| PTO–NO(3a) | 5 | 4 | 7 | 9 |
Conclusions
The work presented here is the first, to our knowledge, that reports the synthesis of thiolated polyesters via one-pot reactions that were S-nitrosated in organic conditions. The method for the production of S-nitrosated analogues was optimized to reduce the reaction time and increase NO loading. All materials demonstrated hydrolytic degradation after a 10-week study at physiological pH and temperature, as quantified by gravimetric analysis. The hydrolytic degradation products of the materials at physiological temperature demonstrated the presence of esterified conjugates. Furthermore, the antibacterial activity of S-nitrosated materials was assessed against E. coli and S. aureus and resulted in a log-8 reduction in cellular viability with the majority of the materials, representing broad-spectrum therapies. As determined by tensile mechanical tests, the materials have appropriate tensile modulus for the preparation of medical devices or wound dressings that require particular elongation. In addition, the materials have Young’s moduli and elongation values akin to those of soft biological tissue such as meniscus, tendon, and arteries. The combination of therapeutic NO release for antibacterial applications, and similar mechanical properties as soft biological tissue, make this system a relevant platform for biomedical applications.
Supplementary Material
Acknowledgements
The authors thank Dr. Alec Lutzke for his thoughtful feedback during the preparation of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Bettinger CJ, Pure Appl. Chem, 2010, 83, 9–24. [Google Scholar]
- 2.Dhand C, Venkatesh M, Barathi VA, Harini S, Bairagi S, Goh Tze Leng E, Muruganandham N, Low KZW, Fazil MHUT, Loh XJ, Srinivasan DK, Liu SP, Beuerman RW, Verma NK, Ramakrishna S and Lakshminarayanan R, Biomaterials, 2017, 138, 153–168. [DOI] [PubMed] [Google Scholar]
- 3.Hubbell JA, Nat. Biotechnol, 1995, 13, 565. [Google Scholar]
- 4.Nair LS and Laurencin CT, Prog. Polym. Sci, 2007, 32, 762–798. [Google Scholar]
- 5.Li S, J. Biomed. Mater. Res, 1999, 48, 342–353. [DOI] [PubMed] [Google Scholar]
- 6.Makadia HK and Siegel SJ, Polymers, 2011, 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuijer R, Jansen EJ, Emans PJ, Bulstra SK, Riesle J, Pieper J, Grainger DW and Busscher HJ, Biomaterials, 2007, 28, 5148–5154. [DOI] [PubMed] [Google Scholar]
- 8.Lutzke A, Pegalajar-Jurado A, Neufeld BH and Reynolds MM, J. Mater. Chem. B, 2014, 2, 7449–7458. [DOI] [PubMed] [Google Scholar]
- 9.Williams DLH, Chem. Commun, 1996, 1085–1091.
- 10.Singh RJ, Hogg N, Joseph J and Kalyanaraman B, J. Biol. Chem, 1996, 271, 18596–18603. [DOI] [PubMed] [Google Scholar]
- 11.Marazzi M, López-Delgado A, Fernández-González MA, Castaño O, Frutos LM and Temprado M, J. Phys. Chem. A, 2012, 116, 7039–7049. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-González MA, Marazzi M, López-Delgado A, Zapata F, García-Iriepa C, Rivero D, Castaño O, Temprado M and Frutos LM, J. Chem. Theory Comput, 2012, 8, 3293–3302. [DOI] [PubMed] [Google Scholar]
- 13.Keefer LK, ACS Chem. Biol, 2011, 6, 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmähl D and Habs M, Oncology, 1980, 37, 237–242. [DOI] [PubMed] [Google Scholar]
- 15.Tsikas D, Gutzki FM, Rossa S, Bauer H, Neumann C, Dockendorff K, Sandmann J and Frölich JC, Anal. Biochem, 1997, 244, 208–220. [DOI] [PubMed] [Google Scholar]
- 16.Seabra AB, Da Silva R, De Souza GF and De Oliveira MG, Artif. Organs, 2008, 32, 262–267. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Lu Y, Soto RJ, Shah A, Ahonen MJR and Schoenfisch MH, Polym. Chem, 2016, 7, 7161–7169. [PMC free article] [PubMed] [Google Scholar]
- 18.Flory PJ, Chem. Rev, 1946, 39, 137–197. [DOI] [PubMed] [Google Scholar]
- 19.Pang K, Kotek R and Tonelli A, Prog. Polym. Sci, 2006, 31, 1009–1037. [Google Scholar]
- 20.Jones M, Ganopolsky JG, Labbé A, Gilardino M, Wahl C, Martoni C and Prakash S, Int. Wound J, 2012, 9, 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vercelino R, Ferreira ES and de Oliveira MG, Nitric Oxide, 2012, 27, S33–S34. [Google Scholar]
- 22.Damodaran VB and Reynolds MM, J. Mater. Chem, 2011, 21, 5870–5872. [Google Scholar]
- 23.Sutton S, J. Validation Tecnhol, 2011, 17, 42. [Google Scholar]
- 24.Sutton S, J. GXP Compliance, 2012, 16, 74. [Google Scholar]
- 25.Hamilton MA, The log reduction (LR) measure of disinfectant efficacy, MSU Center for Biofilm Engineering: Montana, 2010.
- 26.Yapor JP, Lutzke A, Pegalajar-Jurado A, Neufeld BH, Damodaran VB and Reynolds MM, J. Mater. Chem. B, 2015, 3, 9233–9241. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M, Liu Q, Zhang Q, Ye C and Zhou G, J. Polym. Sci. A, 2012, 50, 1026–1036. [Google Scholar]
- 28.Žagar E and Žigon M, Prog. Polym. Sci, 2011, 36, 53–88. [Google Scholar]
- 29.Wang Y, Kibbe MR and Ameer GA, Biomater. Sci, 2013, 1, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran RT, Thevenot P, Gyawali D, Chiao J-C, Tang L and Yang J, Soft Matter, 2010, 6, 2449–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seebacher W, Simic N, Weis R, Saf R and Kunert O, Magn. Reson. Chem, 2003, 41, 636–638. [Google Scholar]
- 32.Lütke-Eversloh T, Bergander K, Luftmann H and Steinbüchel A, Microbiology, 2001, 147, 11–19. [DOI] [PubMed] [Google Scholar]
- 33.Seabra AB, da Silva R and de Oliveira MG, Biomacromolecules, 2005, 6, 2512–2520. [DOI] [PubMed] [Google Scholar]
- 34.Coneski PN, Rao KS and Schoenfisch MH, Biomacromolecules, 2010, 11, 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snejdrova E and Dittrich M, in Recent advances in plasticizers, InTech, 2012.
- 36.Shibayama K and Suzuki Y, J. Polym. Sci. A, 1965, 3, 2637–2651. [Google Scholar]
- 37.Malmström E, Johansson M and Hult A, Macromolecules, 1995, 28, 1698–1703. [Google Scholar]
- 38.Barrère M and Landfester K, Polymer, 2003, 44, 2833–2841. [Google Scholar]
- 39.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T and Janczuk AJ, Chem. Rev, 2002, 102, 1091–1134. [DOI] [PubMed] [Google Scholar]
- 40.Damodaran VB, Place LW, Kipper MJ and Reynolds MM, J. Mater. Chem, 2012, 22, 23038–23048. [Google Scholar]
- 41.Schairer DO, Chouake JS, Nosanchuk JD and Friedman AJ, Virulence, 2012, 3, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling LH, Introduction to physical polymer science, John Wiley & Sons, New York, 2005. [Google Scholar]
- 43.Yamaguchi S, Arch. Oral Biol, 1992, 37, 439–444. [DOI] [PubMed] [Google Scholar]
- 44.Chiba M and Komatsu K, J. Biomech, 1993, 26, 561–570. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu K and Chiba M, Arch. Oral Biol, 1993, 38, 369–375. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Ameer GA, Sheppard BJ and Langer R, Nat. Biotechnol, 2002, 20, 602. [DOI] [PubMed] [Google Scholar]
- 47.Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H and Bernstorff S, J. Struct. Biol, 1998, 122, 119–122. [DOI] [PubMed] [Google Scholar]
- 48.Wang JL, Parnianpour M, Shirazi-Adl A and Engin AE, Theor. Appl. Fract. Mec, 1997, 27, 1–12. [Google Scholar]
- 49.Misof K, Rapp G and Fratzl P, Biophys. J, 1997, 72, 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danso EK, Mäkelä JTA, Tanska P, Mononen ME, Honkanen JTJ, Jurvelin JS, Töyräs J, Julkunen P and Korhonen RK, J. Biomech, 2015, 48, 1499–1507. [DOI] [PubMed] [Google Scholar]
- 51.Fischenich KM, Boncella K, Lewis JT, Bailey TS and Haut Donahue TL, J. Biomed. Mater. Res. A, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galley NK, Gleghorn JP, Rodeo S, Warren RF, Maher SA and Bonassar LJ, Clin. Orthop. Relat. Res, 2011, 469, 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee MC and Haut RC, J. Biomech, 1992, 25, 925–927. [DOI] [PubMed] [Google Scholar]
- 54.Haut RC, J. Biomech. Eng, 1985, 107, 166–174. [DOI] [PubMed] [Google Scholar]
- 55.Donlan RM, Emerging Infect. Dis, 2001, 7, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones ML, Ganopolsky JG, Labbé A, Wahl C and Prakash S, Appl. Microbiol. Biotechnol, 2010, 88, 401–407. [DOI] [PubMed] [Google Scholar]
- 57.Kenawy ER, Worley SD and Broughton R, Biomacromolecules, 2007, 8, 1359–1384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.