Abstract
Borderline personality disorder (BPD) is a complex and debilitating psychiatric illness. Prior research in adults has shown that neurophysiological deficits in feedback processing and learning from rewards may be central to the development of BPD; however, little research has examined these markers in adolescents and young adults with BPD. The present study used event-related potentials (ERPs) and time-frequency decomposition analysis to probe neural responses to wins and losses in a guessing task among 68 females (13–23 years old) either with BPD (n = 35) or no history of mental disorders (Healthy Control [HC]; n = 33). Participants completed a guessing task wherein they won and lost money at equal frequencies while electroencephalogram (EEG) data were acquired. Adolescents and young adults with BPD showed a smaller differentiation between wins and losses in the Reward Positivity (RewP) relative to HCs. Using time-frequency decomposition, we isolated distinct frequency bands sensitive to wins (delta [< 3Hz]) and losses (theta [4–7 Hz]). Compared to BPD participants, HCs showed significantly larger delta power to wins, specifically. The groups did not differ in delta power to losses, nor theta power to wins or losses. Collectively, findings implicate altered reward processing in the pathophysiology of BPD and may inform early identification and targeted intervention.
Keywords: Borderline personality disorder, adolescents, reward positivity, P300, time-frequency decomposition
General Scientific Summary
The way in which individuals process rewards and losses may be central to the development and persistence of Borderline Personality Disorder (BPD); nonetheless, studies probing the neurophysiological correlates of feedback processing in adolescents and young adults with BPD are scarce. Relative to healthy controls, we found that female adolescents and young adults with BPD showed less differentiation in their neural responses to rewards versus losses—captured using event-related potentials. Further, our time-frequency decomposition analyses indicated that this lack of differentiation may be specifically due to a blunted response to rewards among individuals with BPD. Our findings clarify the nature of feedback processing deficits in BPD and lay the foundation for testing whether reward processing deficits detectable early in life confer vulnerability to developing BPD in late adolescence or adulthood.
Borderline personality disorder (BPD) is a complex psychiatric disorder characterized by pervasive disturbances in interpersonal relationships, emotion regulation, impulse control, and self-image (Crowell & Kaufman, 2016). Epidemiological research indicates that 0.7–3.0% of community adults (Gunderson, Herpetz, Skodol, Torgensen, & Zanirini, 2018)). Additionally, there is emerging evidence that reliable precursors of BPD may be evident in childhood (Hallquist, Hipwell, & Stepp, 2015), and it is estimated that 1–3% of adolescents in the general population meet BPD criteria (Zanarini et al., 2011). Theories propose that BPD is characterized by fundamental impairment in processing feedback (i.e., rewards or losses) critical to adapting to one’s environment (Crowell, Beauchaine, & Linehan, 2009). Further, research has identified neurophysiological indexes of feedback processing that predict the onset of other psychiatric illnesses (e.g., depression; Kujawa, Hajcak, & Klein, 2019; Nelson et al., 2018) and that elucidate individuals at risk for these disorders (e.g., Kujawa, Proudfit, & Klein, 2014). Despite implications for improving early identification and intervention, there is a dearth of research on neurophysiological responses to feedback among adolescents and young adults diagnosed with BPD.
A key reason for the lack of research in this area is that the diagnosis of BPD, until recently, has been controversial in adolescents (see Fonagy, Speranza, Luyten, Kaess, Hessels, & Bohus, 2015). Concerns have included difficulty differentiating typical adolescent experiences (e.g., greater risk-taking) from BPD symptoms and the incompleteness of personality development prior to adulthood (e.g., Laurenssen, Hutsebaut, Feenstra, Van Busschbach, & Luyten, 2013). Nonetheless, reviews have highlighted the convergent, concurrent, and predictive validity of BPD diagnoses (e.g., Fonagy et al., 2015; Kaess, Brunner, & Chanen, 2014). Specifically, the etiological features and psychopathological correlates of adolescent and adult BPD overlap substantially (Winsper et al., 2016). Further, BPD diagnoses shows moderate stability in adolescence, mirroring adult findings, and are as reliable among middle-to-late adolescents as adults (Sharp et al., 2018). As BPD in young people portends considerable negative sequelae (e.g., Winsper et al., 2015), it is critical to clarify neurophysiological correlates of BPD.
Theoretical Models of the Pathophysiology of BPD
Electroencephalography (EEG) studies have suggested that adults with BPD exhibit reduced neural responses to losses (i.e., losing money or points), indexed by an event-related potential (ERP) called the Feedback Negativity (FN) (Endrass, Schuermann, Roepke, Kessler- Scheil, & Kathmann, 2016; Schuermann, Kathmann, Stiglmayr, Renneberg, & Endrass, 2011). The FN is thought to be generated by activity in the anterior cingulate cortex (ACC; Gehring & Willoughby, 2002), a brain region involved in detecting outcomes that are negative or worse than expected (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). Further, a reduced FN was associated with a lack of behavioral adjustment in response to negative feedback. Collectively, these results have led some to contend that BPD is characterized by reduced sensitivity to negative consequences and/or punishments, and that this core deficit contributes to risky decision-making, impulsivity, and BPD features (Endrass et al., 2016; Schuermann et al., 2011).
Alternatively, other theories propose that the central dysfunction in BPD may lie in altered reward processing (Bandelow, Schmahl, Falkai, & Wedekind, 2010; Crowell & Kaufman, 2016). Specifically, hypodopaminergic functioning in the brain’s reward system, including pathways connecting the ventral tegmental area to regions of the ventral striatum that are critical to reward processing, is thought to confer vulnerability to BPD (Crowell & Kaufman, 2016). BPD symptoms like self-damaging, impulsive behaviors and affective lability may reflect efforts to stimulate a hypoactive or a hypo-responsive reward system (Bandelow et al., 2010). Adults with BPD show greater discounting of delayed rewards (Lawrence, Allen, & Chanen, 2010), reduced activation in reward regions during positive reinforcement (Vollm et al., 2007), and reduced ventral striatal activation when differentiating between rewards (versus non- rewards) in the presence of affective stimuli (Enzi et al., 2013). Taken together, parallel lines of theoretical and empirical work suggest that the pathophysiology of BPD may involve both deficits in the neural processing negative (i.e., losses or worse than expected feedback) and positive (i.e., rewards) outcomes.
In the present study, we use neurophysiological approaches to unpack whether feedback processing deficits observed in BPD are driven by altered neural responses to losses, rewards, or both. Thus, we arbitrate between the theories described above, and take steps towards clarifying the precise pathophysiology of BPD. The study harnesses a combination of ERPs elicited by feedback and time-frequency decomposition analysis (e.g., Bernat, Nelson, & Baskin-Sommers, 2015), an approach that isolates the spectral components (i.e., power at different frequency bands) that make up these ERPs. In prior research, this approach has been used to determine whether relative reductions in the FN/RewP among individuals with Major Depressive Disorder were due to blunted reward responsiveness or heightened punishment sensitivity (e.g., Nelson et al., 2018; Webb et al., 2017). We focus on the Reward Positivity (RewP) and the P300, two reliable and valid ERPs that have been previously examined in adults diagnosed with BPD.
The Reward Positivity (RewP) and Borderline Personality Disorder (BPD)
The RewP is a feedback-related ERP that has traditionally been called the FN. The FN is maximal over frontocentral scalp regions approximately 200–400 ms post-feedback and is more negative to losses relative to wins (Gehring & Willoughby, 2002; Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003). Often, the FN has been defined as the magnitude of the difference between feedback conditions (i.e., losses minus rewards) (Proudfit, 2015). In the context of gambling tasks in which participants choose high- or low-risk options, adults with BPD have shown a reduced FN (i.e., less differentiation is responses to losing versus winning money) compared to healthy controls (Andreou et al., 2015; Endrass et al., 2016; Schuermann et al., 2011; Vega et al., 2013). These studies conclude that BPD may involve reduced neural sensitivity to negative outcomes—in line with traditional descriptions of the FN (Holroyd et al., 2003).
However, mounting evidence indicates that the apparent negativity observed in the FN may actually reflect a positive deflection of the ERP in response to rewards that is absent following non-rewards (i.e., a RewP; Proudfit, 2015). Like the FN, the RewP is defined as the magnitude of the difference between conditions, but reversed (i.e., reward minus loss). The RewP is thought to reflect the early categorization of information as rewarding or not, regardless of other characteristics of the stimulus (e.g., feedback magnitude) (Glazer, Kelley, Pornpattananangkul, Mittal, & Nusslock, 2018; Proudfit, 2015). Greater RewP amplitude is associated with increased activation in the striatum (Carlson, Foti, Mujica-Parodi, Harmon- Jones, & Hajcak, 2011) and better performance on behavioral measures of reward sensitivity (Bress & Hajcak, 2013), underscoring its potential link with detecting reward.
These competing conceptualizations of the time-domain FN/RewP arise because this feedback-related component may be composed of neural activity from at least two overlapping processes: a negative deflection in the ERP waveform sensitive to losses but not rewards and/or a positive deflection sensitive to rewards but not losses (Bernat et al., 2015; Carlson et al., 2011 Foti, Weinberg, Dien, & Hajcak, 2011). Collectively, these components sum to produce the differentiation (or lack thereof) between wins and losses observed in the time domain (i.e., FN/RewP). Extant studies of feedback processing in BPD have only focused on the loss minus win difference score in the time domain. Thus, they indicate that adults with BPD show abnormalities in feedback processing relative to controls but cannot disentangle whether these are due to neural activity generated by rewards, losses, or both.
Recent studies have used time-frequency decomposition analyses to obtain a more fine-grained understanding of the neural activity that contributes to the RewP. In both adults (Bernat et al., 2015; Foti, Weinberg, Bernat, & Proudfit, 2015) and adolescents (Nelson et al., 2018; Webb et al., 2017), monetary losses elicit greater theta band activity (4–7 Hz) compared to gains, while delta activity (< 3Hz) is enhanced for wins versus losses; these signals have been localized to the ACC and striatum, respectively (Foti et al., 2015). Thus, theta and delta activity are dissociable components of the RewP in that they have separate neural generators, tend to be weakly associated (e.g., Bernat et al., 2015), and reflect distinct cognitive processes. While theta is sensitive to the occurrence of negative feedback or errors, delta specifically tracks reward delivery and may drive behavioral adjustments based on reward receipt (see Glazer et al., 2018; Weinberg, Ethridge, Ait Oumeziane, & Foti, under review). These separable loss- and reward- specific neural signals also have provided unique information about psychopathology not captured by time-domain ERPs. For example, in one study, reduced reward-related delta predicted the onset of MDD over and above the time-domain RewP and increased the sensitivity and positive predictive value of the models (Nelson et al., 2018). In another, anxiety and depression symptom severity showed dissociable correlations with punishment-related theta and reward-related delta, respectively, among depressed adults, a pattern not found with time-domain ERPs (Cavanagh, Bismark, Frank, & Allen, 2019).
Time-frequency decomposition has been applied in two studies of adults with BPD. In both cases, relative to controls, the BPD group showed reduced theta activity following losses in the FN/RewP time-window, suggesting that these individuals were devoting less attention and cognitive resources to processing and learning from negative feedback (Andreou et al., 2015; Vega et al., 2013). However, this work examined loss-related theta in isolation, neglecting the contribution of delta to the overall feedback-related ERP. The present study will extend these findings by measuring both delta and theta power to understanding feedback processing among adolescents and young adults with and without BPD.
The P300 and BPD
The P300 is a positive-going centro-parietal component that peaks between 300 and 600 ms post-feedback stimuli. In the context of losses and rewards, the P300 may reflect the elaborative processing of outcome-related information and updating working memory to maximize future rewards (Polich, 2007). In contrast to the RewP, the P300 is sensitive to feedback magnitude (e.g., enhanced for larger versus smaller rewards) and probability (e.g., enhanced for rare versus common outcomes), but may not be modulated by valence (e.g., positive versus negative). Potentiation of the P300 to positive versus negative feedback found in some prior studies can thus be challenging to interpret as it necessitates disentangling the contribution of valence from magnitude and probability (Glazer et al., 2018). Two studies have probed the P300 in adults with BPD using the Iowa Gambling Task (Bechara, Damasio, Damasio, & Anderson, 1994) and a simple two-choice gambling task (Gehring & Willoughby, 2002), respectively. In the former, rewards and losses occur with different probabilities (i.e., 80% versus 20% in some decks), while in the latter, outcome magnitude was varied. In both cases, the P300 was enhanced to losses compared to rewards overall, but the BPD group showed less differentiation in their responses compared to controls (Endrass et al., 2016; Schuermann et al., 2011). Since the P300 is sensitive to stimulus probability and magnitude, it is unclear whether loss feedback is less salient for adults with BPD, as some suggest (Endrass et al., 2016), or alternatively, that these individuals are less sensitive to stimulus parameters (e.g., probability) in general. To resolve this issue, the present study uses a task in which outcome magnitude is fixed and wins and losses occur on exactly 50% of the trials (i.e., the expected value of losses and rewards is 0).
Goals of the Present Study
Although BPD may be characterized by alterations in processing both negative and positive feedback, studies have not parsed the distinct neurophysiological correlates of rewards and losses and examined their potentially unique relations to BPD. In the present study, we used both time-domain (i.e., RewP) and time-frequency (i.e., delta, theta) components that provide dissociable information about feedback processing to elucidate the specific deficits that distinguish females with BPD from healthy controls. We extend prior work in this area by using a younger age range (i.e., adolescents and young adults); this is critical as, for many, BPD symptoms onset in adolescence (Zanarini et al., 2011), and little is known about the neurophysiological correlates of BPD early in the illness course. Further, we extended prior studies that probed theta power, a spectral component sensitive to loss, by additionally examining reward-related delta power. Finally, we used a task in which wins and losses occur at equal frequencies to clarify potential group differences in the P300, a time-domain component that is modulated by feedback probability.
We tested the following a priori hypotheses. First, we hypothesized that the RewP would be more negative for losses and more positive for wins overall. In line with prior research, we predicted that adolescents and young adults with BPD would show reduced differentiation between wins and losses (i.e., smaller RewP) compared to healthy controls. Second, we examined delta and theta power, reward- and loss-related components thought to underlie the RewP. We hypothesized that the BPD group would show reduced loss-related theta power relative to controls, consistent with prior work in older adults. As this was the first study to examine reward-related delta in BPD, we did not have a firm hypothesis, although reduced reward-related delta in BPD would be predicted by theories that implicate hypodopaminergic function in reward circuits in this disorder (Bandelow et al., 2010; Crowell & Kaufman, 2016). Last, we hypothesized that, relative to healthy controls, the BPD group would show a reduced differentiation in P300 amplitude to rewards relative to losses.
We also explored relations between neurophysiological correlates of feedback processing and psychiatric symptoms (BPD severity, depression, anxiety, and anhedonia) within BPD individuals. Although there is evidence that the RewP is associated with depression severity, there is substantial variability within the literature (e.g., many studies not finding this association) that may be a function of sample characteristics (e.g., clinical versus community; age) and the type of experimental tasks used (Keren et al., 2018). Further, although some studies show that RewP and P300 amplitudes are associated with impulsivity in adults with BPD (Schuermann et al., 2011; Vega et al., 2013), relations among these ERPs (and underlying spectral components) and psychiatric symptoms in this group have not been tested.
Methods
Participants
Sample characteristics are presented in Table 1. Participants were 68 females (Healthy Controls [HC] = 35; Borderline Personality Disorder [BPD] = 33) aged 13–23 (M = 17.59, SD = 1.91). HCs were recruited from the community while BPD participants were completing an intensive inpatient dialectical behavior therapy (DBT) program. All participants were right handed and had no history of neurological disorders. HC participants reported no lifetime DSM- IV-TR Axis I diagnosis, confirmed with the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID; Sheehan et al., 2010), and no lifetime psychotropic medication use. BPD participants all met criteria for BPD based on the Structured Clinical Interview for DSM-IV Axis-II Personality Disorders, BPD Module (SCID-II BPD; First, Spitzer, Gibbon, Williams, & Benjamin, 1994). Additional sample details are provided in the Supplemental Material.
Table 1.
Descriptive statistics for the sample, stratified by group
| Descriptive Statistics (M [SD] or n [%]) | |||||
|---|---|---|---|---|---|
| BPD (n = 33) | HC (n = 35) | t / χ2 | p | d / Φ | |
| Demographics | |||||
| Age | 17.21 (1.76) | 17.94 (3.42) | −1.12 | 0.270 | −0.27 |
| Race | 2.51 | 0.643 | 0.19 | ||
| White | 25 (75.76) | 24 (68.57) | |||
| Black | 0 (0.00) | 1 (2.86) | |||
| Asian | 4 (12.12) | 6 (17.14) | |||
| 2 or more races | 4 (12.12) | 3 (8.57) | |||
| Other | 0 (0.00) | 1 (2.86) | |||
| Household Income | 18.33 | 0.005 | 0.52 | ||
| Unknown | 2 (6.06) | 5 (14.29) | |||
| < $10,000 | 0 (0.00) | 3 (8.57) | |||
| $10,000 - $25,000 | 1 (3.03) | 0 (0.00) | |||
| $25,000 - $50,000 | 0 (0.00) | 3 (8.57) | |||
| $50,000 - $75,000 | 0 (0.00) | 6 (17.14) | |||
| $75,000 - $100,000 | 1 (3.03) | 2 (5.71) | |||
| > $100,000 | 29 (87.88) | 16 (45.71) | |||
| Psychiatric Symptoms | |||||
| BPD Symptoms | 14.21 (6.84) | 0.66 (0.91) | 11.29 | <0.001 | 2.78 |
| Depression | 30.64 (11.37) | 0.86 (1.85) | 14.86 | <0.001 | 3.66 |
| Anxious Arousal | 35.38 (14.00) | 18.26 (1.65) | 6.98 | <0.001 | 1.72 |
| Anhedonia | 30.48 (7.74) | 17.07 (4.57) | 8.63 | <0.001 | 2.11 |
Note. HC = Healthy Control; BPD = Borderline Personality Disorder.
Procedure
The Partners Institutional Review Board provided approval for the study. Participants aged 13–17 years provided assent while parents, legal guardians, and participants 18 years and older provided written consent. Assessment procedures were completed during two laboratory visits, with most (n = 47; 69.12%) separated by 3 days or fewer (M = 3.29, Mdn = 1.50, SD = 3.87). In the first session, participants completed the MINI-KID, SCID-II BPD, and questionnaires assessing psychiatric symptom severity. During the second visit, EEG data were acquired while participants completed a guessing task. Participants were remunerated $50 for attending both appointments. All interviews were administered by trained bachelor’s-level research assistants, graduate students, and postdoctoral fellows with 50 hours of training (e.g., didactics, reviewing past interviews, mock interviews). Further, clinical recalibration meetings were held to ensure reliability and accuracy of interviewers’ diagnoses.
Clinical Interviews
MINI-KID (Sheehan et al., 2010).
The MINI-KID is a brief, structured diagnostic interview that evaluates current Axis I psychopathology in adolescents. It has strong agreement with gold-standard diagnostic interviews and has good psychometric properties in adolescents (Sheehan et al., 2010). Adolescents and young adults with BPD reported more than 3 comorbid disorders (M = 3.12, SD = 1.39). Unipolar mood disorders (n = 24, 72.73%) were most common, followed by anxiety disorders (n = 21, 63.64%), substance use disorders (n = 20, 39.39%) and behavioral disorders (n = 10, 30.30%). Additional clinical characteristics are provided in the Supplemental Material.
SCID-II BPD (First et al., 1994).
The SCID-II BPD is a semi-structured interview assessment of the diagnostic criteria for BPD. Previous studies suggest that the SCID-II is a reliable and valid measure of BPD diagnoses in adolescents (i.e., 13- to 17-year-olds) (Chanen et al., 2008). Additionally, only participants with a SCID-II BPD diagnosis that was independently confirmed by a treating psychiatrist or psychologist from the DBT unit were included.
Self-Report Questionnaires
Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD; Zanarini, 2003).
The ZAN-BPD is a 9-item questionnaire that assesses the severity of each DSM-IV-TR symptom of borderline personality disorder. Items are scored from 0 to 4, with higher total scores indicating greater BPD symptom severity (range = 0–36). The reliability ZAN-BPD was excellent in our sample (α = 0.93).
Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996).
The BDI-II is a 21- item questionnaire assessment of depression symptom severity in the previous two weeks. Item scores range between 0 and 3, and higher scores indicated more severe depression. Total scores range from 0 to 63, and in our sample, the BDI-II had excellent internal consistency (α = 0.97).
Mood and Anxiety Symptom Questionnaire, Anxious Arousal Subscale (MASQ-AA; Watson & Clark, 1991).
The MASQ-AA is a 17-item questionnaire assessing symptoms that are relatively unique to anxiety disorders (e.g., somatic tension; hyperarousal). Participants rated how much they experienced each symptom in the past week on a scale from 1 (not at all) to 5 (extremely) and total scores ranged from 17 to 85. The internal consistency of the MASQ-AA items was excellent (α = 0.95).
Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995).
The SHAPS is a self- report assessment of hedonic capacity. Participants rated 14 items rated from 1 (strongly disagree) to 4 (strongly agree), with higher scores (range: 14 – 56) indicating greater anhedonia (i.e., diminished ability to experience pleasure). The internal consistency of SHAPS items was excellent (α = 0.96).
Experimental Task
While EEG data were acquired, participants completed a 60-trial guessing task (Carlson et al., 2011). In each trial, participants viewed a fixation cross for 500 ms, followed by 2 doors presented for 4000 ms. Participants were told that one door contained a prize (+$0.50) while the other contained a loss (-$0.25). Following their choice, participants saw a fixation cross for (500 ms) and then received feedback indicating whether they won (green arrow pointing upwards) or lost (red arrow pointing downwards) for 1000 ms. Wins (30 trials) and losses (30 trials) occurred in a predetermined, pseudorandom order regardless of participants’ responses.
EEG Recording, Data Reduction, and Analysis
EEG data were recorded using a 129-channel net from HydroCel GSN (Electrical Geodesics, Inc., EGI), and continuous EEG data were sampled at 250 Hz and referenced to Cz. Electrode impedances were kept below 50–75 kΩ. Offline analyses were performed using BrainVision Analyzer 2.1.1 software (Brain Products, Munich, Germany). EEG data were re-referenced to the average reference, and offline filters (0.1–30 Hz) were applied. Vertical and horizontal eye movement artifacts were identified and removed using an independent component analysis (ICA) transform using the following criteria: whole data, Classic PCA sphering, Infomax ICA, Energy ordering, and 512 convergence steps. In each trial, EEG data were segmented 200 ms before and 1,200 ms after stimulus onset. A semi-automated procedure to reject intervals for individual channels used the following criteria: (1) a voltage step greater than 50 μV between sample rates, (2) a voltage difference greater than 300 μV within a trial, and (3) a maximum voltage difference of less than 0.50 μV within a 100ms interval. All trials were inspected visually for manual artifact removal.
ERPs were computed time-locked to feedback presentation, and the average amplitude 200 ms pre-feedback served as the baseline. ERP amplitudes were examined at sensor locations equivalent to selected electrodes in the 10/10 system. Scalp location and time windows were consistent with previously published findings using the same gambling task (Bress, Meyer, & Hajcak, 2015; Proudfit, 2015). The RewP and P300 were calculated as the mean amplitude at FCz and Cz, respectively, for the following time windows: (1) RewP, 230ms-330ms and (2) P300, 250–450ms. In both, residualized scores were calculated by regressing losses onto wins and computing standardized residuals (i.e., residualized RewP; residualized P300), which range from approximately −3 to approximately 3.
For the time-frequency decomposition, we used a continuous wavelet transformation to isolate theta and delta power. Data were segmented using a larger time window (−1,500 ms to 1,500 ms) to discard edge effects (Bernat et al., 2015; Foti et al., 2015). Once the artifact rejection parameters discussed above were applied, a Morlet parameter c of 3.5 applied a complex Morlet wavelet transformation to the data from 0.5 to 20 Hz in 30 frequency steps distributed on a logarithmic scale. The baseline correction was scored from −500 to −300 ms pre- feedback. Wavelet transformations were averaged within subject and condition (e.g. wins and losses), providing a measure of total power. Wavelet layers corresponding to theta (central frequency: 5.6 Hz; spectral bandwidth: 3.2 Hz) and delta (central frequency: 1.07 Hz; spectral bandwidth: 1.32 Hz) were extracted. Similar to prior research (Bernat et al., 2015; Foti et al., 2015; Nelson et al., 2018; Webb et al., 2017), theta power was maximal at frontocentral electrodes and was calculated as the mean activity from 230–330 ms at FCz, and delta activity was maximal in centroparietal electrodes and was calculated as the mean activity from 100 to 450ms at Cz.
Data Analysis
For time-domain analyses of the RewP and P300, we used a Group (HC, BPD) X Condition (Win, Loss) repeated measures analysis of variance (RMANOVA) to test group differences in mean amplitude between 230 ms and 330 ms post-feedback at FCz and 250 ms to 450 ms post-feedback at Cz, respectively. In these models, Group was a between-subject variable and Condition was a within-subject variable. To further probe the components, we tested group differences in the residualized RewP and P300 scores using a t-test, in line with recent recommendations (Proudfit, 2015; Weinberg et al., under review). For time-frequency decomposition we conducted two additional Group X Condition RMANOVAs; in this case, the levels of the within-subject variable were mean theta power to wins and losses between 230 and 330 ms at FCz and mean delta power to wins and losses between 100 and 450 ms at Cz.
Finally, within the BPD group, we examined associations between neurophysiological responses to feedback (residualized RewP and P300; theta and delta power to wins and losses) and psychiatric symptom severity using Pearson product-moment correlations.
Results
Preliminary Analyses
We evaluated the internal consistency of ERPs by computing correlations among amplitudes on odd and even trials, corrected with the Spearman-Brown Prophecy Formula, in line with current recommendations (Hajcak, Meyer, & Kotov, 2017). The RewP and P300 each showed good/excellent internal consistency, r = 0.83 (corrected r = 0.91) and r = 0.88 (corrected r = 0.94), respectively. None of the ERP or time-frequency measures were related to participant age (−0.23 < rs < 0.16, ps > 0.064), race (Fs < 1.36, ps > 0.261, ηp2s < 0.08), or family income (Fs < 1.58, ps > 0.183, ηp2s < 0.13. Thus, no covariates were included in the RMANOVAs.
Time-domain.
RewP.
In the Group X Condition RMANOVA, we found a significant main effect of Condition, F(1, 66) = 33.47, p < 0.001, ηp2 = 0.34. Across groups, participants had greater mean amplitudes on win trials (M = 3.70, SEM = 0.51) compared to loss trials (M = 1.89, SEM = 0.48). However, the effect of Group was non-significant, F(1, 66) = 0.06, p = 0.804, ηp2 = 0.001.
The Condition effect was qualified by a significant Group X Condition interaction, F(1, 66) = 4.42, p = 0.039, ηp2 = 0.06. Both the HCs, F(1, 66) = 32.04, p < 0.001, ηp2 = 0.33, and BPD participants, F(1, 66) = 6.59, p < 0.013, ηp2 = 0.09, had higher amplitude responses to win relative to losses, but this effect was substantially larger in the HCs (Figure 1A). Indeed, the HC group (M = 0.60, SEM = 0.40) had a significantly greater residualized RewP than the BPD group (M = −0.64, SEM = 0.47), t(66) = 2.05, p = 0.044, d = 0.50. Critically, the groups did not differ in their response to wins or losses when these were analyzed separately (i.e., simple effects of Group were non-significant, Fs < 0.775 ps > 0.382).
Figure 1.
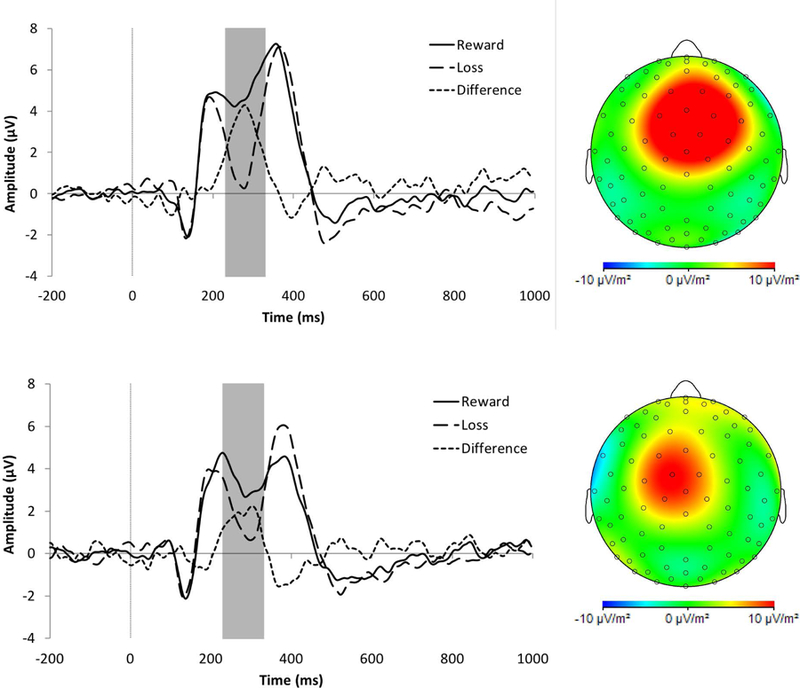
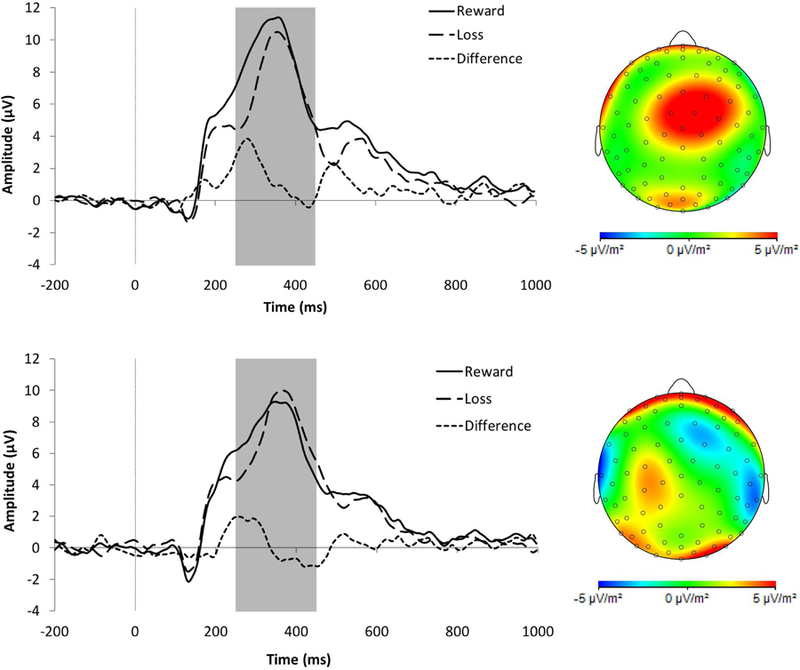
Event-related potentials evoked by monetary losses (large dashed lines) and rewards (solid lines), along with the gain minus loss difference score (small dashed lines). (A) The RewP (highlighted in the gray box) for healthy controls (HC; n = 35; top panel) and female adolescents and young adults with borderline personality disorder (BPD; n = 33; bottom panel) at electrode FCz. Scalp topographies for HC and BPD participants reflect the difference of wins – loss; (B) The P300 (gray box) for HCs (top panel) and BPD (bottom panel) at electrode Cz. Scalp topographies show wins minus losses.
P300.
There was a main effect of Condition, F(1, 66) = 8.65, p = 0.005, ηp2 = 0.12, and again, the P300 was potentiated for win trials (M = 8.15, SEM = 0.47) compared to loss trials (M = 7.50, SEM = 0.47). The main effect of Group was non-significant, F(1, 66) = 1.54, p = 0.219, ηp2 = 0.02.
The Group X Condition interaction was non-significant, F(1, 66) = 3.02, p = 0.087, ηp2 = 0.04; however, given the aims of the study, we probed the underlying simple effects in an exploratory fashion. The P300 was potentiated to wins versus losses among HCs, F(1, 66) = 11.27, p = 0.001, ηp2 = 0.15, but not in the BPD group, F(1, 66) = 0.70, p = 0.404, ηp2 = 0.01 (Figure 1B). Further, the HC group (M = 0.22, SEM = 0.18) had a greater residualized P300 than the BPD group (M = −0.24, SEM = 0.14), and the medium-sized effect approached statistical significance, t(66) = 1.95, p = 0.056, d = 0.47. Similar to RewP findings, the simple effects of Group on P300 amplitudes to wins and losses were non-significant, Fs < 2.59, ps > 0.111.
Time-Frequency Decomposition
Theta.
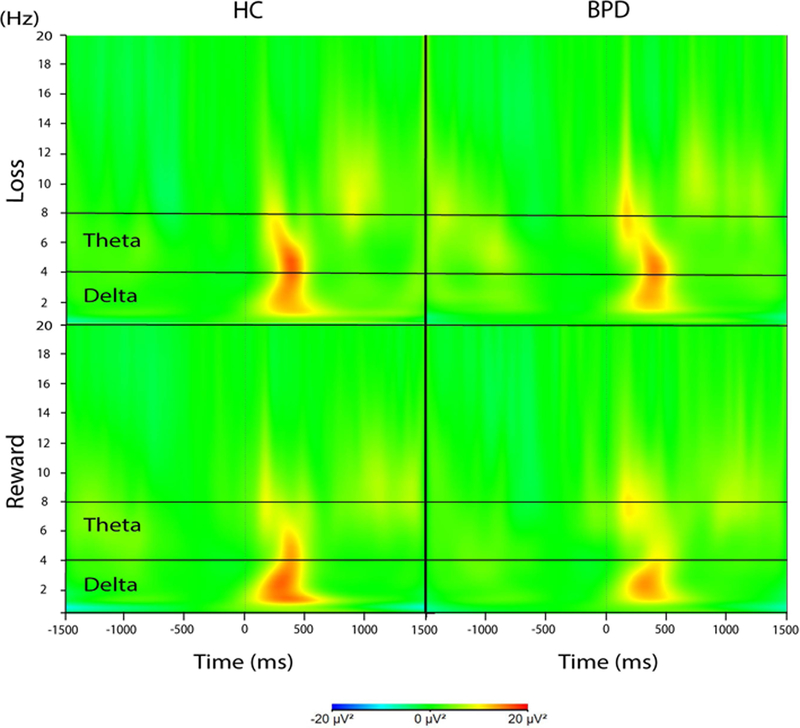
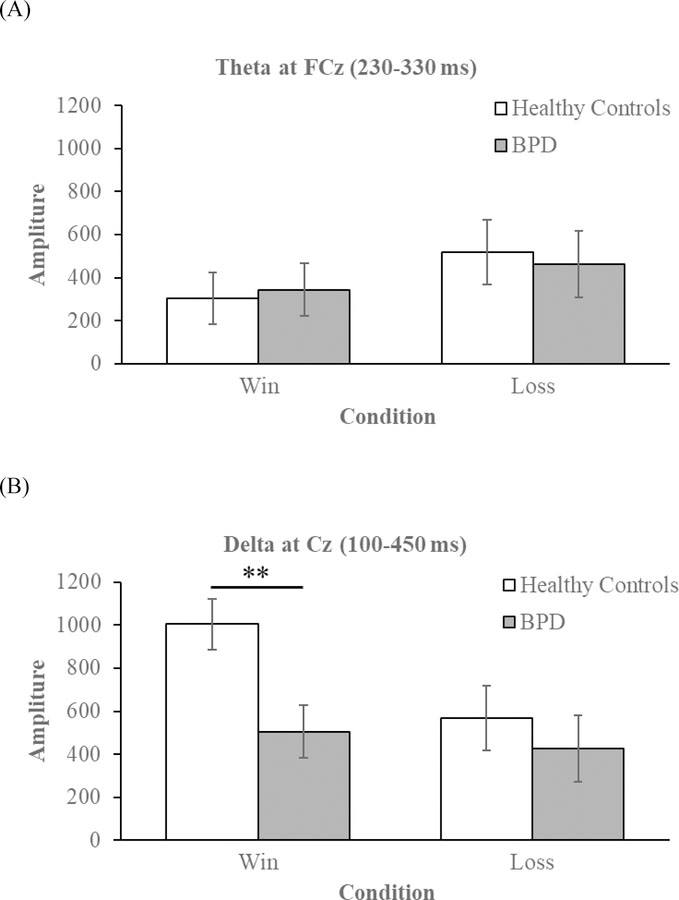
The RMANOVA yielded the expected main effect of Condition, F(1, 66) = 17.93, p < 0.001, ηp2 = 0.21. Across groups, theta power was greater in response to losses (M = 490.85, SEM = 46.38) versus wins (M = 323.94, SEM = 36.92) (Figures 2 and 3). The main effect of Group, F(1, 66) = 0.01, p = 0.915, ηp2 < 0.001, and the Group X Condition interaction, F(1, 66) = 1.47, p = 0.230, ηp2 = 0.02, were non-significant.
Figure 2.
Time-frequency plots for losses (top panel) and rewards (bottom panel) for healthy controls (HC; n = 35, left panels) and female adolescents and young adults with borderline personality disorder (BPD; n = 33, right panels). Theta activity was recorded at FCz and delta activity was recorded at Cz.
Figure 3.
Visual depictions of the mean theta (A) and delta (B) power to wins and losses among HC (white bars) and BPD (gray bars) participants. Error bars depict the standard error of the mean. Note: ** p < 0.01.
Delta.
We found an effect of Condition, F(1, 66) = 10.91, p = 0.002, , ηp2 = 0.14, such that delta was greater for wins (M = 754.10, SEM = 85.16) compared to losses (M = 498.09, SEM = 107.99) (Figures 2 and 3). The main effect of Group were non-significant, F(1, 66) = 3.24, p = 0.076, , ηp2 = 0.05. There was, however, a significant Group X Condition interaction, F(1, 66) = 5.28, p = 0.025, ηp2 = 0.07. Compared to the BPD group, HCs had significantly greater delta power to wins, t(66) = 2.93, p = 0.005, d = 0.71. In contrast, the groups did not differ in delta power to losses, p = 0.510, d = 0.16 (Figure 3).
Associations among Neurophysiological Indicators and Symptom Severity
The residualized P300 was associated with BPD symptoms within the BPD group; lower amplitude to wins versus losses was associated with worse symptoms, r(33) = −0.42, p = 0.015. None of the other time-domain or time-frequency variables was significantly associated with BPD symptoms, depression, anxiety, or anhedonia, all rs > −0.27 and < 0.18, ps > 0.13 (see Supplemental Table 1).
Discussion
This study examined feedback processing in BPD and several key findings emerged. First, relative to healthy females, adolescents and young adults with BPD showed a reduced RewP (i.e., less differentiation between wins and losses). Second, the BPD group had significantly reduced delta power to wins compared to healthy controls. In contrast, the groups did not differ in delta power to losses, nor in theta to wins or losses. These time-frequency results suggest that the reduced differentiation between wins and losses (i.e., blunted RewP) observed in the BPD group was driven by a specific deficit in reward processing. Finally, healthy controls and females with BPD did not significantly differ in P300 amplitudes to wins and losses.
Reward Processing and BPD
In the present study, healthy adolescents and young adults, as well as those with BPD, showed significant differentiation in their neural responses to wins and losses. However, this differentiation was significantly reduced in the BPD group, extending findings from prior studies of individuals in middle-to-late adulthood (Andreou et al., 2015; Schuermann et al., 2011; Vega et al., 2013) to those earlier in their illness course. Importantly, prior work used either the two- choice gambling task (Gehring & Willoughby, 2002) or the Iowa Gambling Task (Bechara et al., 1994), both which vary the magnitude and probability of feedback, and the type of feedback received was related to participants’ choices. The fact that our findings using a much simpler feedback task converge with prior results underscore the replicability of this effect and suggest that feedback processing deficits indexed by the RewP may be central to BPD across age ranges and task types. Research shows that the RewP might index of how well individuals learn from feedback (e.g., Heydari & Holroyd, 2016), and ultimately, make decisions that lead to adaptive behavior (e.g., pursuing goals or relationships that are rewarding and avoiding those that are not). It has been proposed that a lack of differentiation between neural responses to rewards and losses may be linked to maladaptive behavior (e.g., Vega et al., 2013). The present study did not measure decision making or subsequent behavior; thus, testing the link between the RewP and these factors in individuals with BPD may clarify how feedback processing deficits are linked to functional outcomes in this population.
The RewP is remarkably stable from adolescence to adulthood (e.g., Ethridge et al., 2017; Lukie, Montazer-Hojat, & Holroyd, 2014), and its psychometric properties (e.g., reliability) are comparable at different stages of development (e.g., Ethridge & Weinberg, 2018). Further, the RewP demonstrates high test-retest correlations over periods as long as six years (e.g., Kujawa et al., 2018). Consistent with these studies, RewP amplitude was not correlated with age in our sample. The RewP stability across development supports its potential as an early objective marker of risk for BPD among non-affected individuals. At the same time, relative to children, adolescents experience more frequent and varied stressors (Rudolph, 2009), and adaptation to these and the normative developmental changes in early adulthood (e.g., beginning a career) may determine how and when BPD symptoms are manifested in individuals with feedback processing deficits. Thus, to build on our promising results, longitudinal studies of youth at elevated risk for BPD that repeatedly assess psychosocial factors are needed.
Trial-averaged ERP waveforms like the RewP are limited in that the contributions of underlying processes that simultaneously occur in the same time window are difficult to isolate. Thus, we examined theta- and delta power hypothesized to reflect distinct loss- and reward-related activity that make up the time-domain RewP (Bernat et al., 2015; Glazer et al., 2018). Relative to controls, adolescents and young adults with BPD had significantly reduced delta power to rewards, but not to losses, and the groups did not differ with respect to theta power. Delta power is specifically responsive to rewards and may be generated in the basal ganglia, brain areas implicated in reward processing (e.g., Foti et al., 2015). Thus, findings provide preliminary support for theories suggesting that the pathophysiology of BPD is rooted in blunted responses to rewards, specifically. According to these theories, the symptoms and associated features of BPD (e.g., risky sexual behavior, aggression, frustration intolerance, substance abuse, self-injury) represent efforts to stimulate an underactive and/or under-responsive reward system (Bandelow et al., 2010; Crowell & Kaufman, 2016). Future research testing prospective associations between reward-related delta and BPD symptoms in clinical samples are indicated to further test these theoretical models.
An additional strength of time-frequency decomposition analyses is that spectral components may map clinical phenomena more strongly and/or specifically than time-domain components. For instance, in a recent study, reward-related delta prospectively predicted MDD onset over and above the significant effects of time-domain RewP (Nelson et al., 2018). Similarly, in our sample, reward-related delta, but not other time-frequency variables, significantly predicted group membership (BPD versus control), above the effects of time- domain ERPs (see Supplemental Material). Ultimately, reward-related delta is a promising neural marker of BPD that could be used to improve the positive predictive value of BPD screening measures. As Nelson and colleagues (2018) propose, neurophysiological measures of reward processing could be used following first-line screening tools (e.g., questionnaires) to further winnow risk status in those with higher likelihood of developing BPD.
Healthy and BPD participants did not significantly differ in loss- or reward-related theta, which is inconsistent with two prior studies (Andreou et al., 2015; Vega et al., 2013). Critically, theta activity is elicited not only by negative feedback, but also in tasks involving conflict and novelty, leading some to suggest that theta may be generally implicated in signaling the need for increased cognitive control (Cavanagh & Frank, 2014; Cavanagh & Shackman, 2015). Prior studies that found reduced theta among adults with BPD relative to controls used tasks in which participants had to choose between a smaller and larger value that they could potentially win or lose (Gehring & Willoughby, 2002). It is possible that, because we used a simpler guessing task, the lower cognitive demands meant we were not able to detect deficits in BPD individuals that may evident when more cognitive control is required.
P300 Amplitude and BPD
Healthy controls and adolescents and young adults with BPD did not differ in P300 amplitudes to wins relative to losses. P300 amplitude is generally thought to reflect top down elaborative information processing and memory encoding, particularly of motivationally salient information (Glazer et al., 2018; Polich, 2007). While the group effect was non-significant, the effect size for the analysis of the residualized P300 was similar to the RewP (d = 0.47 versus d = 0.50). Future inquiry aimed at understanding abnormalities in cognitive processes captured by the P300 should use tasks that are more traditionally used to unpack this ERP (e.g., oddball paradigms).
Associations with Symptom Severity
Generally, neurophysiological measures of feedback processing were not associated with psychiatric symptom severity in the BPD group. Importantly, these exploratory correlations were underpowered, so replication of these effects in larger samples is warranted. Anhedonia is a cardinal symptom of MDD (Kendler, 2016), and consequently, reward processing deficits are a fundamental feature of this disorder. Several studies have found that a blunted RewP, for example, is associated with a diagnosis of depression (e.g., Nelson et al., 2018) or more severe depressive symptoms (e.g., Bress et al., 2015) but others do not (e.g., Ait Oumeziane & Foti, 2016) and effects are generally heterogeneous (see Keren et al., 2018 for review). This study is the first to test relations among neurophysiological correlates of feedback processing and depression/anhedonia in BPD patients and highlights a need for additional work in this area. In contrast, the RewP is consistently not associated with anxiety symptoms (e.g., Bress et al., 2015; Cavanagh et al., 2018), supporting its conceptually specific link to depression (e.g., Proudfit, 2015), and our results are in line with this prior work. Finally, less differentiation in P300 amplitudes to wins and losses was associated with more severe BPD symptoms; however, this effect was in the context of a non-significant group difference and a small sample of adolescents and adults with BPD. Thus, these findings warrant further scrutiny in larger samples.
Limitations and Conclusions
There are several limitations in the present study. First, adolescents and young adults with BPD reported pronounced comorbidity, particularly with depression. This raises the possibility that group effects were driven by depression, a disorder strongly linked to altered reward processing, rather than BPD. To address this issue, future research should examine the neural correlates of feedback processing among depressed adolescents and young adults with and without comorbid BPD. Further, most BPD participants were using psychotropic medication, which may have influenced neurophysiological activity. Second, although the SCID-II has demonstrated adequate inter-rated reliability in adolescent samples (e.g., Chanen et al., 2008), we did not assess inter-rater reliability in our sample. We did, however, conservatively restricted the BPD sample to those adolescents and young adults who also were diagnosed with BPD by their treating clinician. Third, we only recruited females and the majority were White, limiting the generalizability of our findings to males and non-White individuals. Fourth, our findings require replication in larger samples of adolescents and young adults as we may have failed to detect true effects due to our modest sample size. Last, although we used a well-established guessing task (Proudfit, 2015), outcomes were fixed. Future research should examine how the pathophysiology of BPD using tasks where participant behavior influences outcomes.
In summary, our findings provide evidence for altered reward processing among adolescents and young adults with BPD. Identifying discrete neurophysiological patterns in BPD may, ultimately, enhance our ability to predict the onset of BPD and provide new targets for early interventions to curb a long-term course of psychosocial impairment.
Supplementary Material
Acknowledgments
Ethical approval for this study, “Identifying behavioral and neural mechanisms underlying adolescent borderline personality disorder” was obtained from the Partners Human Research Ethics Committee (2013P001870). This research was funded through awards from the National Institutes of Health (K23MH097786), Simches Fund, Rolfe Fund, and Warner fund, all awarded to Randy Auerbach. Additionally support was provided by the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant, 25040), the American Foundation for Suicide Prevention (PRG-1–140-15) and the Kaplen Fellowship on Depression from Harvard Medical School, all awarded to Jeremy Stewart.
References
- Ait Oumeziane B, & Foti D (2016). Reward‐related neural dysfunction across depression and impulsivity: A dimensional approach. Psychophysiology, 53, 1174–1184. [DOI] [PubMed] [Google Scholar]
- Andreou C, Kleinert J, Steinmann S, Fuger U, Leicht G, & Mulert C (2015). Oscillatory responses to reward processing in borderline personality disorder. The World Journal of Biological Psychiatry, 16, 575–586. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, & Wedekind D (2010). Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychological Review, 117, 623–636. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bernat EM, Nelson LD, & Baskin-Sommers AR (2015). Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology, 52, 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50, 610–616. [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, & Hajcak G (2015). Dfferentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. Journal of Clinical Child and Adolescent Psychology, 44, 238–249. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage, 57, 1608–1616. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Bismark AW, Frank MJ, & Allen JJB (2019). Multiple dissociations between comorbid depression and anxiety on reward and punishment processing: Evidence from computationally informed EEG. Computational Psychiatry, 3, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. Journal of Physiology-Paris, 109, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen AM, Jovev M, Djaja D, McDougall E, Yuen HP, Rawlings D, & Jackson HJ (2008). Screening for borderline personality disorder in outpatient youth. Journal of Personality Disorders, 22, 353–364. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, & Linehan MM (2009). A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychological Bulletin, 135, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, & Kaufman EA (2016). Borderline personality disorder and the emerging field of developmental neuroscience. Personality Disorders, 7, 324–333. [DOI] [PubMed] [Google Scholar]
- Ethridge P, Kujawa A, Dirks MA, Arfer KB, Kessel EM, Klein DN, & Weinberg A (2017). Neural responses to social and monetary reward in early adolescence and emerging adulthood. Psychophysiology, 54, 1786–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge P, & Weinberg A (2018). Psychometric properties of neural responses to monetary and social rewards across development. International Journal of Psychophysiology, 132, 311–322. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Roepke S, Kessler-Scheil S, & Kathmann N (2016). Reduced risk avoidance and altered neural correlates of feedback processing in patients with borderline personality disorder. Psychiatry Research, 243, 14–22. [DOI] [PubMed] [Google Scholar]
- Enzi B, Doering S, Faber C, Hinrichs J, Bahmer J, & Northoff G (2013). Reduced deactivation in reward circuitry and midline structures during emotion processing in borderline personality disorder. The World Journal of Biological Psychiatry, 14, 45–56. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW, & Benjamin L (1994). Structured clinical interview for DSM-IV Axis II personality disorders (SCID-II, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Fonagy P, Speranza M, Luyten P, Kaess M, Hessels C, & Bohus M (2015). ESCAP Expert Article: Borderline personality disorder in adolescence: An expert research review with implications for clinical practice. European Child and Adolescent Psychiatry, 24, 1307–1320. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, & Proudfit GH (2015). Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology, 126, 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event‐related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32, 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, & Willoughby AR (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279–2282. [DOI] [PubMed] [Google Scholar]
- Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, & Nusslock R (2018). Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International Journal of Psychophsyiology, 132, 184–202. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, & Zanarini MC (2018). Borderline personality disorder. Nature Reviews Disease Primers, 4, 18029. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Meyer A, & Kotov R (2017). Psychometrics and the neuroscience of individual differences: Internal consistency limits between-subjects effects. Journal of Abnormal Psychology, 126, 823–834. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hipwell AE, & Stepp SD (2015). Poor self-control and harsh punishment in childhood prospectively predict borderline personality symptoms in adolescent girls. Journal of Abnormal Psychology, 124, 549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari S, & Holroyd CB (2016). Reward positivity: Reward prediction error or salience prediction error? Psychophysiology, 53, 1185–1192. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, & Cohen JD (2003). Errors in reward prediction are reflected in the event-related brain potential. Neuroreport, 14, 2481–2484. [DOI] [PubMed] [Google Scholar]
- Kaess M, Brunner R, & Chanen A (2014). Borderline personality disorder in adolescence. Pediatrics, 134, 782–793. [DOI] [PubMed] [Google Scholar]
- Kendler KS (2016). The phenomenology of major depression and the representativeness and nature of DSM criteria. American Journal of Psychiatry, 173, 771–780. [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, ... & Pine DS (2018). Reward processing in depression: A conceptual and meta-analytic review across FMRI and EEG studies. American Journal of Psychiatry, 175, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Carroll A, Mumper E, Mukherjee D, Kessel EM, Olino T, ... & Klein DN (2018). A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. International Journal of Psychophysiology, 132, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, & Klein DN (2019). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: Evidence across levels of analysis. Journal of Child Psychology and Psychiatry, 60, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenssen EMP, Hutsebaut J, Feenstra DJ, Van Busschbach JJ, & Luyten P (2013). Diagnosis of personality disorders in adolescents: A study among psychologists. Child and Adolescent Psychiatry and Mental Health, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KA, Allen JS, & Chanen AM (2010). Impulsivity in borderline personality disorder: reward-based decision-making and its relationship to emotional distress. Journal of Personality Disorders, 24, 786–799. [DOI] [PubMed] [Google Scholar]
- Lukie CN, Montazer-Hojat S, & Holroyd CB (2014). Developmental changes in the reward positivity: An electrophysiological trajectory of reward processing. Developmental Cognitive Neuroscience, 9, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Infantolino ZP, Klein DN, Perlman G, Kotov R, & Hajcak G (2018). Time-Frequency Reward-Related Delta Prospectively Predicts the Development of Adolescent-Onset Depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52, 449–459. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, & Nieuwenhuis S (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–447. [DOI] [PubMed] [Google Scholar]
- Rudolph KD (2009). The interpersonal context of adolescent depression. In Nolen- Hoeksema S & Hild LM (Eds.), Handbook of depression in adolescents (pp. 377–418). New York, NY: Routledge. [Google Scholar]
- Schuermann B, Kathmann N, Stiglmayr C, Renneberg B, & Endrass T (2011). Impaired decision making and feedback evaluation in borderline personality disorder. Psychological Medicine, 41, 1917–1927. [DOI] [PubMed] [Google Scholar]
- Sharp C, Steinberg L, Michonski J, Kalpakci A, Fowler C, Frueh BC, & Fonagy P (2018). DSM borderline criterion function across age-groups: A cross-sectional mixed- method study. Assessment 10.1177/1073191118786587 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, . . . Wilkinson B (2010). Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). Journal of Clinical Psychiatry, 71, 313–326. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone: the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry, 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Vega D, Soto A, Amengual JL, Ribas J, Torrubia R, Rodriguez-Fornells A, & Marco-Pallares J (2013). Negative reward expectations in borderline personality disorder patients: Neurophysiological evidence. Biological Psychology, 94, 388–396. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, McKie S, Elliott R, Dolan M, & Deakin B (2007). Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Research, 156, 151–167. [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1991). The mood and anxiety symptom questionnaire Unpublished manuscript. University of Iowa, Department of Psychology, Iowa City, IA. [Google Scholar]
- Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, & Pizzagalli DA (2017). Abnormal neural responses to feedback in depressed adolescents. Journal of Abnormal Psychology, 126, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Ethridge P, Ait Oumeziane B, & Foti D (under review). Time-frequency analyses in event-related potential methodologies. In Gable P., Miller M, & Bernat E (Eds.), Oxford Handbook of Human EEG Frequency Analysis New York: Oxford University Press. [Google Scholar]
- Winsper C, Lereya ST, Marwaha S, Thompson A, Eyden J, & Singh SP (2016). The aetiological and psychopathological validity of borderline personality disorder in youth: A systematic review and meta-analysis. Clinical Psychology Review, 44, 13–24. [DOI] [PubMed] [Google Scholar]
- Winsper C, Marwaha S, Lereya ST, Thompson A, Eyden J, & Singh SP (2015). Clinical and psychosocial outcomes of borderline personality disorder in childhood and adolescence: A systematic review. Psychological Medicine, 45, 2237–2251. [DOI] [PubMed] [Google Scholar]
- Zanarini MC (2003). Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. Journal of Personality Disorders, 17, 233–242. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Horwood J, Wolke D, Waylen A, Fitzmaurice G, & Grant BF (2011). Prevalence of DSM-IV borderline personality disorder in two community samples: 6,330 English 11-year-olds and 34,653 American adults. Journal of Personality Disorders, 25, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.