Abstract
Background
Burst suppression occurs in the EEG during coma and under general anaesthesia. It has been assumed that burst suppression represents a deeper state of anaesthesia from which it is more difficult to recover. This has not been directly demonstrated, however. Here, we test this hypothesis directly by assessing relationships between EEG suppression in human volunteers and recovery of consciousness.
Methods
We recorded the EEG of 27 healthy humans (nine women/18 men) anaesthetised with isoflurane 1.3 minimum alveolar concentration (MAC) for 3 h. Periods of EEG suppression and non-suppression were separated using principal component analysis of the spectrogram. After emergence, participants completed the digit symbol substitution test and the psychomotor vigilance test.
Results
Volunteers demonstrated marked variability in multiple features of the suppressed EEG. In order to test the hypothesis that, for an individual subject, inclusion of features of suppression would improve accuracy of a model built to predict time of emergence, two types of models were constructed: one with a suppression-related feature included and one without. Contrary to our hypothesis, Akaike information criterion demonstrated that the addition of a suppression-related feature did not improve the ability of the model to predict time to emergence. Furthermore, the amounts of EEG suppression and decrements in cognitive task performance relative to pre-anaesthesia baseline were not significantly correlated.
Conclusions
These findings suggest that, in contrast to current assumptions, EEG suppression in and of itself is not an important determinant of recovery time or the degree of cognitive impairment upon emergence from anaesthesia in healthy adults.
Keywords: anaesthetic, inhaled; burst suppression; isoflurane; electroencephalography; cognitive dysfunction; principal component analysis
Editor's key points.
-
•
In most clinical circumstances, general anaesthesia is regarded as being excessively deep when the EEG shows a burst suppression pattern.
-
•
Burst suppression has been associated with adverse outcomes such as delirium in post-surgical and intensive care settings.
-
•
The authors exposed 27 healthy volunteers to a minimum of isoflurane 1.3 minimum alveolar concentration anaesthesia for 3 h, and studied their recovery characteristics.
-
•
The duration of burst suppression was not correlated with either the speed of emergence from anaesthesia or with the quality of cognitive recovery.
As an experimental tool, general anaesthesia provides a valuable opportunity to study how a healthy brain recovers from a dramatic perturbation that disrupts consciousness.1 EEG features under general anaesthesia have been extensively studied and used to quantify anaesthetic depth for decades.2 Burst suppression is one well-known EEG activity pattern traditionally associated with deep anaesthesia. This pattern is characterised by periods of isoelectric EEG punctuated by bursts of electrical activity and is induced at high concentrations of various general anaesthetics with different mechanisms of action.3 During burst suppression, 95% of cortical neurons are hyperpolarised,4 excitatory transmission is greatly diminished,5 inhibitory postsynaptic potentials are completely abolished,5 and cerebral metabolic rate is reduced.6
Outside of the domain of anaesthesia, burst suppression is observed during states of profound cerebral dysfunction, including encephalopathy7 and coma.8 Furthermore, burst suppression has been hypothesised to be a poor prognostic indicator for recovery after brain injury.9 Burst suppression has been associated with adverse outcomes such as delirium and death in post-surgical10, 11, 12 and intensive care13 settings.14, 15 Yet, the fundamental physiology of burst suppression as a determinant of recovery from anaesthesia has not been established.
We hypothesised that greater duration of EEG suppression during anaesthesia would be associated with increased time to emergence, defined as time to recovery of consciousness after discontinuation of the anaesthetic. Contrary to our hypothesis, the addition of metrics of EEG suppression in a model built to predict time to emergence did not sufficiently improve the predictive ability of the model. We also hypothesised that longer durations of EEG suppression would be correlated with greater cognitive impairment at emergence from anaesthesia. The assumption that suppression would predict cognitive impairment also proved false. Thus, while burst suppression is thought to reflect the deepest plane of general anaesthesia, EEG suppression is not a good predictor of prolonged recovery time or degree of impairment of cognition in healthy human volunteers.
Methods
All data were collected as a part of the ReCCognition (NCT01911195) study after appropriate institutional review board approval: University of Pennsylvania, Philadelphia, PA, USA (Protocol #818401), University of Michigan, Ann Arbor, MI, USA (Protocol #HUM0071578), and Washington University in St. Louis, St. Louis, MO, USA (Protocol #201308073). To be eligible to participate, all subjects gave written informed consent in accordance with the Declaration of Helsinki. Details of the study design and eligibility and exclusion criteria are previously described.16
Anaesthetic administration
EEG was recorded in 30 healthy (ASA patient status 1 and 2)17 volunteers (12 women and 18 men, 22–39.5 yr), with 10 subjects recorded at each of three sites: University of Michigan, Washington University, and University of Pennsylvania. EEG was sampled at 500 Hz using the Electrical Geodesics, Inc. (Eugene, OR, USA) EEG system with either a 32 (20 subjects), 64 (one subject), or 128 (nine subjects) channel montage referenced to Cz. Sessions began with baseline cognitive testing. After pre-oxygenation using a face mask, anaesthesia was induced with a stepwise increasing infusion rate of propofol: 100 μg kg−1 min−1×5 min, increasing to 200 μg kg−1 min−1×5 min, and then to 300 μg kg−1 min−1×5 min. After 15 min of propofol administration, we administered isoflurane 1.3 age-adjusted MAC, delivered via a laryngeal mask airway, for 3 h. Subjects breathed spontaneously with pressure support adjusted to attain tidal volumes 5–8 ml kg−1.
During anaesthetic administration, vital signs and other data (ECG, noninvasive BP, pulse oximetry, end-tidal carbon dioxide and isoflurane concentration, and nasopharyngeal temperature) were monitored and recorded in an electronic anaesthetic record and analysed post hoc. Surface warming blankets were applied to maintain body temperature in the normal range. BP was maintained within 20% of starting values by intermittent boluses or infusions of phenylephrine. I.V. ondansetron (4 mg) was administered 30 min before discontinuation of isoflurane.
At the discontinuation of isoflurane, an audio command loop, issued every 30 s, asked the subject to squeeze either the right or left hand twice. Emergence was defined by the initial time at which participants responded correctly to two consecutive commands. Recovery time was defined as the period between isoflurane discontinuation and emergence.
Cognitive testing battery
Upon emergence, a cognitive testing battery was administered at 30-min intervals. Six tasks from the computerised Cognition test battery18 were used to serially assess cognitive task performance with high temporal resolution. Here, we focused on two robust cognitive tests in order to reduce the number of statistical comparisons. We first used the digit symbol substitution test (DSST), a test of cognitive throughput and visual scanning previously used to characterise cognitive recovery in surgical patients recovering from general anesthesia.19, 20, 21 The DSST requires participants to select keys based upon a menu of nine matching symbols and numbers displayed on the screen. We additionally used the psychomotor vigilance test (PVT), a measure of vigilance and attention resistant to learning effects with repeat administration.22 The PVT measures reaction time23 by requiring the space bar to be pressed as quickly as possible after the appearance of a counter with incrementing numbers.
Tests were administered on a 14″ LCD Dell Latitude E4530 laptop computer(Dell, Round Rock, TX, USA). Test order was randomised between participants. Alternate forms of cognitive tests were used at each administration to reduce practice effects. In considering a speed-accuracy trade-off,24 we analysed median response times and performance accuracy separately for each test.
EEG analysis
The analyses presented herein were performed on the EEG recorded during the 3 h of isoflurane administration at 1.3 MAC. Artifacts attributable to noise or movement were rejected manually. K-means clustering was performed on the number of minutes of clean data for all 30 subjects, resulting in the exclusion of three subjects. These three excluded subjects had only 92.2 (±12 min) (∼51% of recorded data) of clean data compared with the 158.8 (±9.8 min) (∼88% of recorded data) of clean data for the remaining 27 subjects (nine women, 22–39.5 yr). Thus, only the 27 subjects with longer clean EEG recordings were used in all subsequent analyses.
Data were high pass filtered at 0.1 Hz using a 4th order Butterworth filter. Spectrograms were computed on lead F3 for each subject using the Thomson's multitaper method25 implemented in MATLAB using the following parameters: 10 s window length, 1 s step size, time-bandwidth product NW=9, and number of tapers K=5. Power estimates were computed for frequencies up to 50 Hz. In order to expose fluctuations around the mean spectrogram, the temporal mean spectrogram was computed by averaging across all 10 s windows during the 3-h isoflurane administration. This mean spectrum was subtracted from the original spectrum obtained in each 10-s window, after a log transformation. For the group mean and median spectrograms, the individual spectrograms were not normalised. All subjects' spectrograms were truncated to the length of the shortest clean spectrogram, 124.35 min.
Many methods exist for detecting suppressed EEG (e.g.26, 27, 28). Here, we sought to separate periods of suppression in the EEG using the methodology similar to that described by Hudson and colleagues1 such that the EEG spectrum of the signal in each window is a vector. Each element of the vector specifies the power estimate at each frequency. To reduce the dimensionality of this vector, we subjected the matrix consisting of spectral estimates at each 10-s window to principal component analysis performed on each subject individually. To determine whether distribution of the data projected onto first principal component (PC1) was unimodal, we used Hartigan's dip statistic (1000 bootstraps).29 For every subject, if the null hypothesis of unimodality was rejected, k-means clustering was performed on their data projected onto the first two principal components. In the subjects with burst suppression, this procedure resulted in suppressed epochs grouped into a single cluster. Thus, each one of the 10-s windows of the spectrogram was effectively categorised as either EEG suppression or non-suppression.
Statistical analysis
To determine how time to emergence is related to experimental variables, we began our statistical analyses by constructing linear regression models for each of three isoflurane-derived measures and four suppression-derived measures with time to emergence. These isoflurane and suppression measures are described in further detail below.
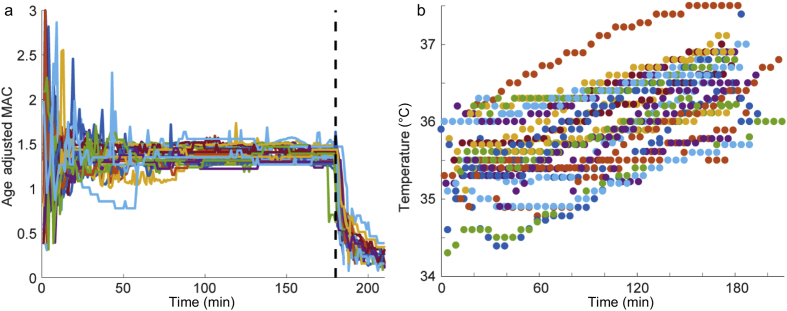
For all 27 subjects, end-tidal isoflurane values were collected and analysed (Fig. 1a). The three predictors derived from isoflurane measures were: 1) the summation from 1 to 30 min; 2) the mean value from 50 to 180 min; and 3) the rate constant of exponential decay of a curve fit to the measured isoflurane concentration after discontinuation of anaesthesia at 180 min until time of emergence. This rate constant was calculated for each subject individually using the following equation:
| (1) |
where t is the time in minutes since isoflurane was shut off. Parameters a and κ were fit to the data using the least-squares method. The only isoflurane-derived variable that had a significant relationship with time to emergence was the exponential time constant for isoflurane expiration, κ.
Fig. 1.
End-tidal isoflurane concentration and body temperature during anaesthetic exposure. (a) Age-adjusted isoflurane minimum alveolar concentration (MAC) from the start of isoflurane administration (time 0) to the discontinuation of isoflurane at 180 min (vertical dotted black line) for all 27 subjects. (b) Temperature is presented in °C over the 3 h of isoflurane administration.
Several measures of suppression were also considered in our initial analyses: fraction of total time spent in suppression, longest single episode of suppression, number of distinct suppression episodes, and the fraction of time spent in suppression during the last 15 min of isoflurane administration. Given the limited number of subjects included in this study, we chose to focus subsequent analyses on the fraction of total time spent in suppression and the fraction of time spent in suppression in the last 15 min at isoflurane 1.3 MAC.
To further investigate the relationship between suppression and time to emergence, we tested for independence between the rate of isoflurane expiration and durations of EEG suppression. To assess this, we constructed a pair of linear regression models with time to emergence as the dependent variable and independent variables κ and one of two suppression measures (either the fraction of total time spent in suppression or the fraction of suppression in the last 15 min of isoflurane exposure) in addition to an interaction term between κ and the suppression measure.
In order to test the hypothesis that the inclusion of information about the amount of EEG suppression improves model predictions of emergence time, we generated three additional models. Each model was built to estimate the emergence time based on measured and fitted parameters. The general intuition we used in construction of these models was that isoelectric EEG can be thought of as the deepest state of anaesthesia. Recovery of consciousness can then be conceptualised as the gradual evolution of the brain state from its starting point towards some threshold brain state at which emergence occurs. We modelled this trajectory as an exponential decay from the starting brain state at which anaesthetic was turned off to brain state at which emergence is observed. This model is mathematically expressed as follows:
| (2) |
where Ω is the threshold brain state at which emergence occurs, κ is the rate constant obtained by fitting decay of expired isoflurane to an exponential (Eq. 1) and A is the initial brain state at the time of isoflurane shut-off. Finally, twake is the time of emergence in minutes. The equation can be solved for twake and rewritten as:
| (3) |
In order to determine whether the amount of EEG suppression can predict twake, A was constructed in three different ways; models 1 and 2 each include a different measure of EEG suppression (σ), while model 3 does not contain any information concerning suppression of the EEG. The variable σ is percent of total time in suppression during the 3-h exposure, in model 1. In model 2, σ is percent of time in suppression during the last 15 min. In models 1 and 2, A=σ+ω where ω is an offset that ensures A is never equal to Ω. This offset allows us to deal with subjects that had no suppression. Model 3 does not include a suppression variable and A=ω.
We estimated Ω and A using least-squares fitting of Eq. 2 to the observed emergence times and measured parameters. Using the fitted parameters, we obtained three different sets of predictions for the time to emergence, : one from each model.
The ultimate question we aim to address in this study is whether incorporating a measure of EEG suppression (σ) into a model of emergence from anaesthesia improves predictions. To answer this question, we use a model selection strategy grounded in information theory—Akaike Information Criterion (AIC).30 The best model is not necessarily the one that minimises residuals. This is because models with more parameters will generally fit data better than models with fewer parameters, regardless of how relevant the additional parameters are. To ensure that there is not a bias for models with more parameters, AIC assigns a penalty for each additional parameter used in a model. With correction for a finite sample size, AIC was implemented according to the following equation:
| (4) |
where, n is the number of parameters in the model, L is the log likelihood of the residuals, and N is the total number of subjects. L is calculated using the following equation:
| (5) |
Models 1 and 2 include suppression and have two parameters (κ and σ). whereas model 3 has only one parameter (κ). AIC can be converted to the probability that the ith model, out of a set of considered models, is preferred. This probability is computed according to the following equation:31
| (6) |
In order to maximise reproducibility, we bootstrapped our calculation of AIC probabilities (1000 bootstraps).
Analysis of cognitive task performance
Accuracy and response time measures for the PVT and DSST were standardised as z-scores based upon the distribution of pre-intervention baseline scores from the study sample. The standardised scores were expressed as differences from individuals' baseline performance before anesthesia.16 Cognitive task performance as a function of time was fit using non-linear mixed effects model using a damped exponential function:
| (7) |
where i is the subject index and t represents time after recovery of consciousness. Φ1-2 were modelled as random effects that were fit for each subject independently, while Φ3 was treated as a fixed effect and accounted for differences relative to baseline. The sum of Φ1,i and Φ3 was used to model the change in cognitive performance at the time of emergence (t0). This accounted for the variability in the timing of DSST and PVT task administration and allowed cognitive task performance to be compared at a common time point across participants. As the modelled cognitive task measures at time of emergence were not normally distributed, Spearman's rank-order correlation was used to assess for relationships between measures of burst suppression and impairment in task performance at emergence.
Non-linear mixed effects modelling of cognitive task performance was performed in SAS (SAS Institute Inc., Cary, NC, USA). All further analyses were performed in R or in Matlab R2017a using customised scripts. All errors are shown in terms of standard deviation (SD) unless otherwise indicated.
Results
Individual variability in EEG characteristics during constant anaesthetic administration
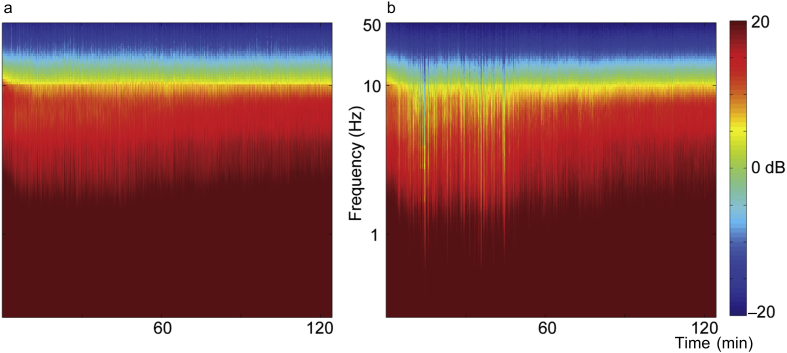
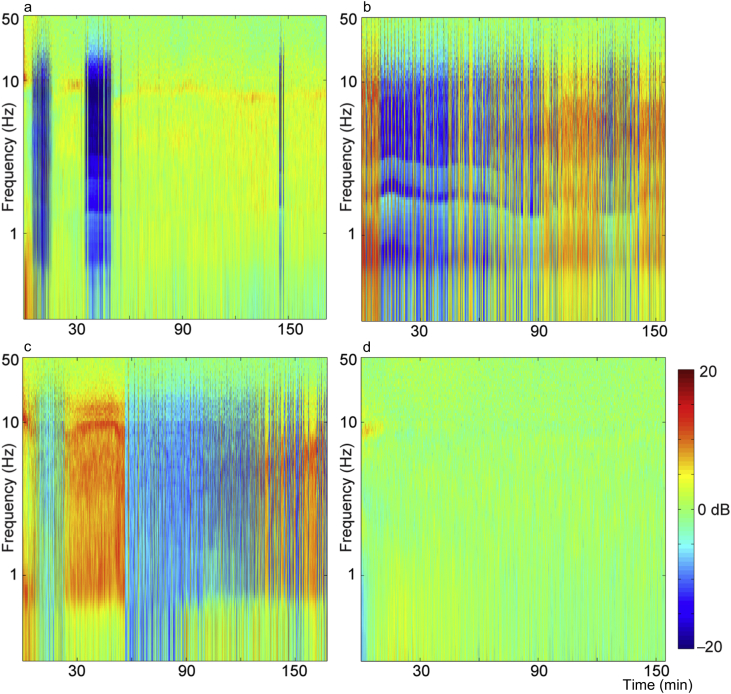
The end-tidal isoflurane concentration (Fig. 1a) was maintained near 1.3 age-adjusted MAC. Temperature (Fig. 1b) was maintained in the physiologic range. The population mean (Fig. 2a) and median (Fig. 2b) spectrogram exhibited a canonical high power of low frequency oscillations during isoflurane administration. Yet, both the mean and median spectrograms conceal two important sources of variability: inter- and intra-subject variability (Fig. 3). In the absence of surgical stimulus, and despite a constant isoflurane concentration, the spectra of EEG fluctuate as a function of time. Four examples of this variability are highlighted in Fig. 3. One subject (Fig. 3a) had two periods of prolonged isoelectric EEG in the first hour of isoflurane administration. During the remaining 2 h, no significant suppression was observed, but the frequency of α oscillations waxed and waned. In contrast, another subject (Fig. 3b) exhibited short periods of EEG suppression throughout the 3-h administration of isoflurane, and another (Fig. 3c) had EEG dominated by suppression interrupted by a consolidated period of non-suppressed period. Finally, a fourth subject (Fig. 3d) did not exhibit any appreciable EEG suppression at all. Thus, the population mean and median spectrograms do not reflect individual EEG dynamics under anaesthesia in healthy human volunteers undergoing constant isoflurane exposure. In the remainder of the manuscript, we will focus on one aspect of EEG activity—EEG suppression—to determine whether it is associated with a longer time to recovery of consciousness or greater impairment of cognition at emergence.
Fig. 2.
Group spectrograms show canonical low frequency oscillations in the anaesthetised brain. Spectrograms measured from F3 combined over all 27 subjects. Spectra are computed for the last 2 h of isoflurane administration (starting 1 h into the administration). (a) The mean spectrogram computed over all subjects shows consistent power over all frequencies for the duration of anaesthetic exposure after equilibration. (b) The median spectrogram computed over all subjects shows similar results to the mean spectrogram. Both ways of combining the data across subjects show the well-known slow oscillations in the EEG under anaesthesia.
Fig. 3.
Individual spectrograms reveal the variability of EEG activity between subjects and across time. (a–d) Spectrograms of four individual subjects computed over the entire 3 h isoflurane administration. To better expose fluctuations in the spectral content, the mean of the spectrum across 3 h is subtracted. Periods in which there is suppressed EEG appear in the spectrogram as vertical blue lines, as these periods have decreased spectral activity across all frequencies. There is individual variability in the overall amount of suppression that occurred during the recording, the number of times a subject transitioned between suppressed and non-suppressed states, and the duration of a suppression period. (a) Suppression condensed into distinct periods. (b) Suppression occurring intermittently throughout the recording. (c) Suppression dominating the recording, but interrupted by a period without suppression. (d) No suppression. A lack of suppression was seen for two subjects (second subject in Supplementary Fig. S4c).
The first two principal components separate suppression from non-suppression
Periods of suppression are characterised as flat-lined, or isoelectric EEG, which is associated with a dramatic decrease in cortical activity. In the frequency domain, this is seen as a broadband decrease in power (blue regions in the spectrograms in Fig. 3).
Because EEG suppression is associated with a broadband decrease in EEG power, we reasoned that projecting the spectra onto the first principal component should separate periods of suppressed EEG into a distinct cluster. In 21 out of 25 subjects with burst suppression, assessed by visual inspection, the null hypothesis that the distribution is unimodal was rejected (P<0.05 after multiple comparison correction).
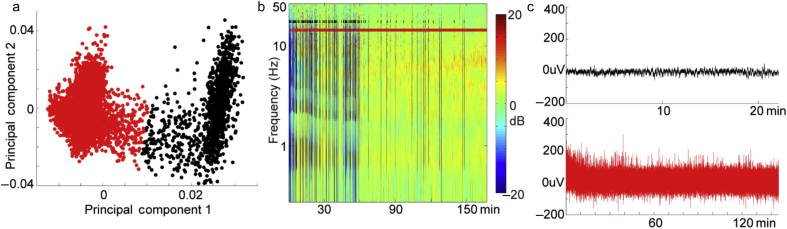
Consistent with the results of the Hartigan dip test, spectrograms projected onto a plane spanned by the first two PCs formed two clusters (Fig. 4a). Points were assigned to each cluster using a standard k-means algorithm and overlaid on the spectrogram to demonstrate the accuracy of cluster assignments (Fig. 4b).
Fig. 4.
Illustration of principal component analysis (PCA)-based method for isolating episodes of EEG suppression. (a) Projection of the spectrogram in (b) onto its first two principal components reveals two well-separated groups of points. These data were subjected to k-means clustering which revealed two distinct clusters (red and black). (b) Suppression-classified times (black) and non-suppression-classified (red) times are shown over the spectrogram. Suppression times do indeed correspond to periods of broadband power decreases. The overlaid spectrograms of all other subjects are plotted in Supplementary Figs. S1–S4. (c) To illustrate that PCA and k-means reliably separate periods of suppressed EEG from non-suppressed periods, all EEG time periods classified as suppressed (black) were concatenated. Note that almost no detectable voltage oscillations are observed. In contrast, EEG epochs classified as non-suppressed (red) exhibit sustained activity. Plots showing the concatenated suppressed and non-suppressed time series data for all other subjects exhibiting suppression are plotted in Supplementary Fig. S5.
Visual inspection suggested that all episodes of suppressed EEG were assigned to a single cluster. To verify that this was the case, we concatenated all epochs of the EEG assigned to the suppressed and the non-suppressed cluster (Fig. 4c). Additional examples of the robust separation of suppression from non-suppression using principal component analysis (PCA) can be found for the remaining subjects in Supplementary Figs. S1–S5.
EEG suppression is not independently associated with increased time to recovery of consciousness
As demonstrated in Fig. 3 and Supplementary Figs. S2–S4, the suppression patterns varied over all 27 subjects, including two subjects who failed to exhibit any EEG suppression. Variability occurred in the total amount of suppression, number of distinct suppression episodes, dwell time of suppression episodes, and the organisation of suppressed episodes during isoflurane administration.
Of the 25 subjects exhibiting suppression, the cumulative percentage of suppression ranged from 1.1 to 67.9% (average: 25%; SD: 21) of the recording. The maximum length of suppression ranged from 0.28 to 13 min (average: 3.5; SD: 3.3 min). The number of transitions between suppression and non-suppression ranged from six to 412 (average: 150.8; SD: 120.2). We also calculated the percentage of suppression during the last 15 min of isoflurane administration, as this might be a better indicator of whether a subject is likely to recover consciousness more quickly or more slowly than their suppression over the entire 3 h of isoflurane administration. Fourteen subjects showed no suppression during the last 15 min of isoflurane administration. In the remaining subjects, the average suppression duration was 2.9 min (2.3 SD).
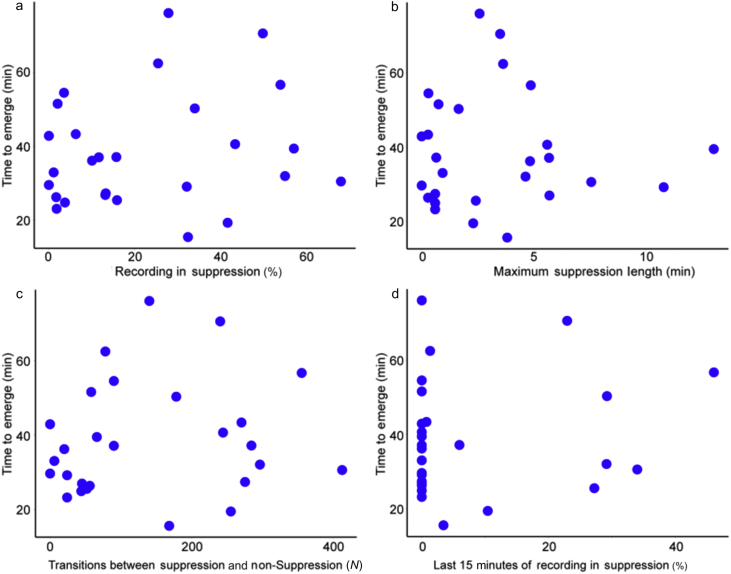
None of the four suppression-derived measures were individually correlated with time to emergence (percent suppression: R2=0.03, P=0.40, longest suppressed episode: R2=8×10−4, P=0.89, number of suppressed episodes: R2=0.02, P=0.51, percent suppression in the last 15 min: R2=0.02, P=0.44) (Fig. 5).
Fig. 5.
The time to emerge from isoflurane (time to recovery of consciousness) is not significantly correlated with different measures of suppression. (a) The cumulative percentage of the recording spent in suppression (R2=0.03, P=0.40); (b) the longest period of continuous suppression (R2=8×10−4, P=0.89); (c) the number of transitions between suppression and non-suppression (R2=0.02, P=0.51); and (d) the cumulative percentage of the last 15 min of the recording before isoflurane is discontinued spent in suppression (R2=0.02, P=0.44).
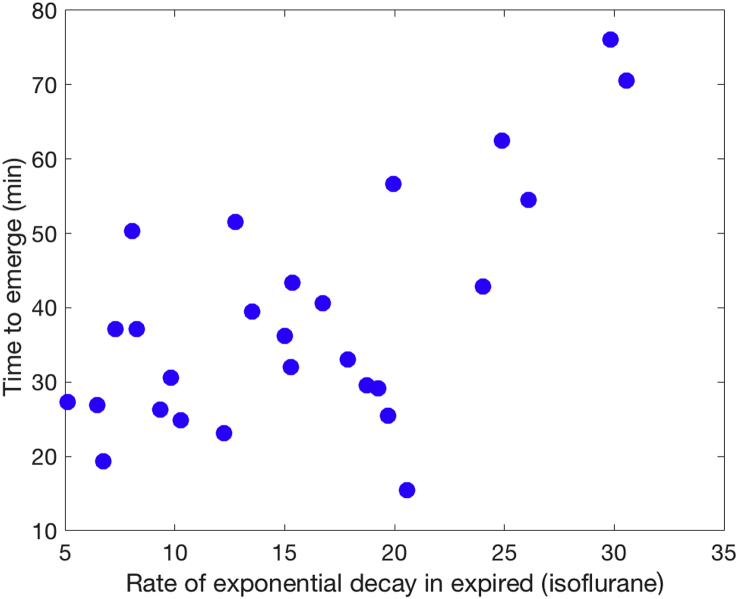
We investigated the relationship between time to emergence and three measures derived from the age-adjusted isoflurane MAC values. Two showed no relationship with time to emergence (MAC in the first 30 min: R2=9×10−5, P=0.96 and mean MAC from 50 to 180 min: R2=0.02, P=0.53). The third isoflurane-derived measure κ is the exponential decay rate for the end-tidal isoflurane starting from the moment isoflurane was shut off (Eq. 1). As expected, the decay rate constant was strongly correlated with time to emergence (R2=0.39, P=5×10−4), but accounted for less than 40% of the variance (Fig. 6). Therefore, the rate constant, κ, was included in all subsequent models that predict the time of emergence and impairment of cognition.
Fig. 6.
The time to emerge from isoflurane (time to recovery of consciousness) is significantly correlated with the rate of isoflurane expiration. The subjects that clear isoflurane more rapidly tend to be those that recover consciousness more quickly after discontinuation of isoflurane anaesthesia (R2=0.39, P=5×10−4).
We start with the conventional approach using linear regression. The regression models incorporated the isoflurane decay rate constant, κ, and either the percent of total time in suppression or percent of the last 15 min of isoflurane exposure spent in suppression. Both of these models demonstrated statistical significance (Table 1, Table 2). Yet, neither the fraction of total time spent in suppression nor the fraction of the last 15 min of isoflurane exposure spent in suppression showed a statistically significant association with time to emergence. Furthermore, no statistically significant interaction was found between the isoflurane decay rate constant and EEG suppression.
Table 1.
Results of linear regression model including decay time constant and overall percent suppression. Overall suppression is not significantly associated with time to recovery and fails to show statistically significant interaction with the isoflurane elimination time constant.
| Linear regression with elimination time constant and overall percent suppression | ||
|---|---|---|
| Overall significance (F-statistic: 6 on 3 and 23 DF) | Adjusted R2: 0.37 | P-value: 0.004* |
| Isoflurane elimination time constant (κ) | 0.79 (0.6) | P-value: 0.18 |
| Percent suppression (overall) | 0.23 (0.3) | P-value: 0.46 |
| Interaction | 0.02 (0.01) | P-value: 0.27 |
Asterisk denotes statistical significance at the level defined as p<0.05.
Table 2.
Results of linear regression model including decay time constant and percent of last 15 min of isoflurane exposure spent in EEG suppression. EEG suppression is not significantly associated with time to recovery and fails to show a statistically significant interaction with the isoflurane elimination time constant.
| Linear regression with elimination time constant and percent suppression in the last 15 min | ||
|---|---|---|
| Overall significance (F-statistic: 5.35 on 3 and 23 DF) | Adjusted R2: 0.33 | P-value: 0.006* |
| Isoflurane elimination time constant (κ) | 1.4 (0.4) | P-value: 0.003* |
| Percent suppression (overall) | 22.8 (51.8) | P-value: 0.66 |
| Interaction | −0.49 (2.9) | P-value: 0.87 |
Asterisk denotes statistical significance at the level defined as (p<0.05).
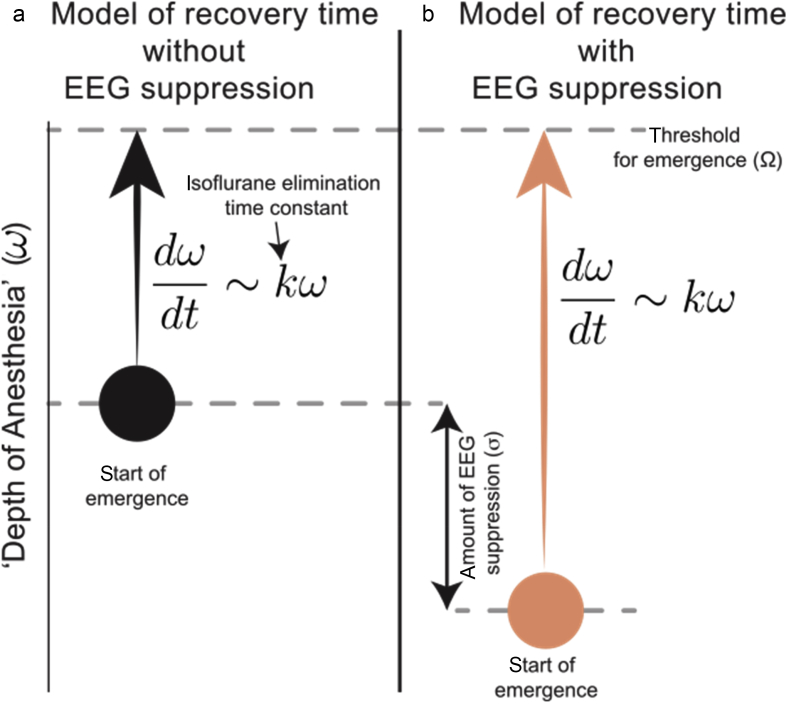
We, next, turn to a more physiologically motivated modelling approach graphically illustrated in Fig. 7. We model recovery of consciousness as a process that starts from some anaesthetic depth observed at the time when isoflurane is turned off (ω). As the anaesthetic is eliminated, the system gradually evolves towards a threshold (Ω) at the rate dictated by the elimination rate constant (κ). We model the percent of time spent in suppression as an additive anaesthetic depth (σ), as we expect that the brain with more EEG suppression should take longer to recover. To test this intuition, we determine whether the ability to predict the time of emergence is significantly improved by incorporating the fraction of time spent in suppression.
Fig. 7.
Schematic of physiologically motivated model. Emergence occurs as the progression from initial brain state to a threshold brain state (Ω) at which consciousness is regained. The rate of this progression is defined by the rate of exponential decay in expired isoflurane (κ). Suppressed EEG (σ) is modelled as increased anaesthetic depth in (a). The motivating hypothesis for this model is that increased anaesthetic depth is expected to be correlated with increased time to emergence.
The ability of each model to predict time of recovery is shown in Table 3 as the Pearson correlation coefficient between the model and the time to emergence of each subject. Notice that each model has a similar correlation coefficient. To select the best one among the considered models, we computed the AIC for each. From the AIC, we computed the probability that each model minimises loss of information relative to the other models considered.31 From an information theoretic standpoint, the model with the highest probability is chosen as the most appropriate model. Using this approach, we conclude that the model without any measure of EEG suppression is the most appropriate model. In the interest of testing the consistency of the AIC and the associated relative probabilities, we bootstrapped our calculation of corrected AIC values. This was accomplished by randomly selecting (with replacement) a set of 27 subjects. Each model was then fit to this subset. The best model was selected for each such subset as the one that maximises relative probability. The model that did not include a measure of burst suppression was chosen in 86% of the 1000 bootstraps.
Table 3.
Statistical results of three exponential decay models. Pearson's R2 shows similar predictive ability of each model for time of emergence from anaesthesia. Probabilities based on Akaike information criteria indicate the probability that a single model is the best one to fit experimental data, given the set of models being considered. AIC, Akaike Information Criterion.
| Model with % suppression | Model with % suppression in last 15 min | Model with no suppression term | |
|---|---|---|---|
| R2 | 0.41 | 0.40 | 0.39 |
| p | 3×10−4 | 4×10−4 | 5×10−4 |
| No. of parameters | 2 | 2 | 1 |
| AIC probability | 0.24 | 0.19 | 0.58 |
EEG suppression is not associated with impairment in cognitive task performance at emergence.
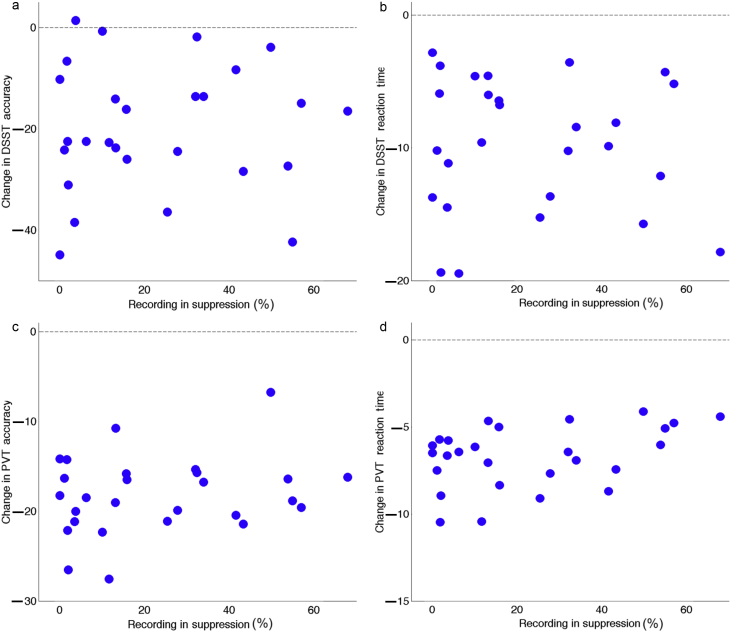
Having established that there is no statistically significant relationship between EEG suppression and recovery of consciousness, we asked whether increased EEG suppression is associated with greater impairment of cognitive function at this time point. For this purpose, we turned to estimates of changes in cognitive performance on the DSST and the PVT at emergence. Performances on both the PVT and DSST, in terms of both accuracy and response time, were impaired immediately upon recovery of consciousness. We hypothesised that subjects exhibiting more burst suppression would exhibit greater impairment relative to baseline. Additionally, we hypothesised that slower elimination kinetics of isoflurane would result in greater impairment. Yet, we found no evidence that the duration of EEG suppression is associated with greater impairment in accuracy or response time for both the PVT and DSST (Fig. 8 and Table 4, Table 5). Likewise, the time constant of isoflurane elimination was not correlated with estimates of impairment at emergence for either cognitive task, in terms of accuracy or response times (Table 6).
Fig. 8.
Association between amount of EEG suppression on performance speed and accuracy on both the psychomotor vigilance test (PVT) and digit symbol substitution test (DSST). (a–d) The dashed line at zero marks individuals' baseline performance. While most subjects demonstrated impaired performance upon emergence from anaesthesia, the degree of this impairment does not appear to be related to the amount of EEG suppression.
Table 4.
Analysis of correlation between the total time spent in EEG suppression and the degree of impairment in cognitive performance upon emergence. No significant relationship was observed between total duration of suppression and change in cognitive metrics relative to baseline. DSST, digit symbol substitution test; PVT, psychomotor vigilance test.
| Association between EEG suppression and cognitive performance | ||
|---|---|---|
| Spearman's Rho | P-value | |
| DSST reaction time | −0.0519 | 0.7971 |
| DSST accuracy | 0.036 | 0.8584 |
| PVT reaction time | 0.3444 | 0.078 |
| PVT accuracy | 0.1105 | 0.5832 |
Table 5.
Analysis of correlation between the fraction of the last 15 min of isoflurane exposure spent in EEG suppression and the degree of impairment in cognitive performance upon emergence. As with the measure of total duration of suppression, suppression in the last 15 min of anaesthetic exposure was not significantly correlated to initial cognitive task performance at emergence. DSST, digit symbol substitution test; PVT, psychomotor vigilance test.
| Association between EEG suppression in the last 15 min and cognitive performance | ||
|---|---|---|
| Spearman's Rho | P-value | |
| DSST reaction time | −0.1656 | 0.409 |
| DSST accuracy | −0.0165 | 0.9351 |
| PVT reaction time | 0.2754 | 0.1645 |
| PVT accuracy | 0.3007 | 0.1275 |
Table 6.
Analysis of correlation between the time constant of elimination of isoflurane and the degree of impairment in cognitive performance upon emergence. The time constant of elimination was not significantly associated with any of the cognitive task measures. DSST, digit symbol substitution test; PVT, psychomotor vigilance test.
| Association between isoflurane elimination time constant (tau) and cognitive performance | ||
|---|---|---|
| Spearman's Rho | P-value | |
| DSST reaction time | −0.3291 | 0.0941 |
| DSST accuracy | −0.3065 | 0.1201 |
| PVT reaction time | −0.0122 | 0.9527 |
| PVT accuracy | 0.1099 | 0.5839 |
Discussion
The results of this study do not reveal an association between EEG suppression and time to recovery from general anaesthesia. Furthermore, we failed to find an association between the amount of EEG suppression and the degree of impairment in cognitive performance at emergence, as measured using two independent cognitive tests. Anaesthetic exposure profoundly impeded performance on both of these tests. Burst suppression, however, did not confer any additional decrement in cognitive function after anaesthesia.
Absence of evidence is clearly not evidence of absence. However, our findings reduce the probability of a strong relationship between EEG suppression and recovery of consciousness or impairment of cognition in healthy subjects. Here we defined recovery of consciousness as the time when the subject was first able to follow instructions to squeeze either the right or left hand twice. The ability to follow this command implies that the subject is capable of parsing a simple sentence, has an elementary conception of numbers, and the ability to communicate via the execution of a simple motor task. Thus, recovery of consciousness as defined herein, signifies return of at least rudimentary cognition. In an attempt to discover a relationship between the time it takes to recover consciousness and EEG suppression, we used several complementary modelling approaches. None reveals a significant association between EEG suppression and time to recovery of consciousness. This conclusion is further reinforced by the finding that subjects whose EEG exhibited significant suppression were no more impaired in performance time or performance accuracy of two cognitive tests that assess distinct neurobehavioral processes18, 23 than those without any suppression of the EEG. This is a counterintuitive observation, as isoelectric EEG is universally considered to be the deepest attainable state of anaesthesia.
Animal studies have established previously that EEG suppression is not necessarily associated with prolonged recovery.32 For example, Hambrecht-Wiedbusch and colleagues32 added ketamine to an isoflurane anaesthetic, which induced burst suppression but nevertheless accelerated emergence time. Our findings here are distinct from those of Hambrecht-Wiedbusch and colleagues32 as we demonstrated that burst suppression induced in humans with a single anaesthetic agent is not associated with longer time to recovery of consciousness.
Although the effect of various anaesthetics on the EEG has been extensively studied in humans,33, 34, 35, 36, 37 most studies present EEG characteristics averaged across subjects and time. When group averages (e.g. Fig. 2) and individual EEG recordings (e.g. Fig. 3) are compared, it becomes clear that the population and time averaging fail to represent the behaviour of any individual subject. We exploited this natural inter- and intra-subject variability to isolate the effect of EEG suppression on the timing of subsequent recovery of consciousness and impairment of cognition without the confounds of variably noxious surgical stimuli, changing anaesthetic concentrations, or complex polypharmacy commonly used in the operating room. Yet, we were unable to discover any statistically significant association between features of EEG suppression and 1) restoration of consciousness or 2) cognitive impairment at this time point.
A major limitation of the study was that only a single anaesthetic and a single concentration were evaluated. Full exploration of the possible activity patterns of the EEG and their dependence on the anaesthetic agent and concentration will require additional investigation. Further, it is likely that EEG activity patterns and the dynamics of switching among them could be dramatically altered by clinically relevant factors such as brain pathology, surgical stimulation, and the addition of opioids. Yet, this study in a healthy volunteer population establishes that, even when many variables are constrained, variability in EEG activity does not vanish in the anaesthetised brain.
The surprising results from this study indicate that no aspect of EEG suppression assessed here (cumulative time in suppressed EEG, longest epoch of suppressed EEG, percent of time in suppressed EEG during the penultimate 15 min before discontinuing the anaesthetic, and the frequency with which the anaesthetised brain transitions into and out of the suppressed state) correlate with the time to emerge from anaesthesia. Furthermore, features of EEG suppression were not significantly associated with estimates of cognitive impairment on the PVT and DSST at emergence. Such a result, while not definitive, is clearly consistent with the notion of a non-linear depth of anaesthesia, a dissociation between the dynamics governing the state of anaesthesia and recovery from anaesthesia. It also instructs the clinician that heretofore unrecognised factors underlie the variable fluctuation between states of EEG suppression and non-suppression in the presumptively healthy human brain.
In the clinical literature, burst suppression has received much attention because of its reported association with adverse outcomes in surgical and ICU patients. Monk and colleagues38 showed in a prospective study that increased cumulative deep anaesthesia time was associated with increased mortality after noncardiac surgery. While they did not directly study burst suppression, bispectral index below 45—defined as deep anaesthesia by Monk and colleagues38—is typically associated with burst suppression.39 Watson and colleagues15 found that burst suppression is associated with increased mortality in the ICU patient population. In addition to mortality, burst suppression has been reported to be associated with delirium in ICU13 and in surgical patients.10, 11, 12, 40 One interpretation of these findings is that administering excessive doses of anaesthetics may have deleterious effects on the brain. This view is consistent with the multiple lines of evidence demonstrating potentially neurotoxic effects of anaesthetics (reviewed by Vutskits and Xie41).
In studies on surgical and ICU patients, it is difficult to delineate why certain patients had more burst suppression than others. One possibility is that patients with more burst suppression received higher doses of anaesthetics and sedatives. Another possibility is that some patients are more sensitive to the effects of anaesthetics, and therefore are more likely to exhibit burst suppression at anaesthetic doses that do not typically elicit burst suppression in others. As all subjects in our study were exposed to similar anaesthetic concentrations, it is tempting to speculate that subjects who exhibit more suppression are more sensitive to the effects of isoflurane. If so, one would expect that more sensitive subjects should take a longer time to recover from anaesthesia and experience greater cognitive impediment. We find no evidence to support this hypothesis. Instead, we find that a parsimonious measure of deep anaesthesia—isoelectric EEG—is not useful in predicting the time to recovery of consciousness or subsequent decrement in cognitive function. While our findings do not invalidate the previously published association between suppressed EEG and cognitive outcomes,42 they illustrate that burst suppression per se may not be an important determinant of the recovery of consciousness and cognition. Indeed, none of the subjects in this cohort exhibited delirium based on the standard confusion assessment method for the intensive care unit (CAM-ICU) assessment. However, it is possible that EEG suppression is associated with subtle cognitive deficits upon recovery in normal subjects that were not captured in our study. This relationship will require further investigation, but results herein suggest that EEG suppression caeteris paribus is not associated with significantly greater impairment of cognition or increased time to recovery in healthy adults.
Authors' contributions
Designed experiment: MSA, GAM, MBK.
Collected data: VT, EJ, PP, SB-M, HRM, MRM, BJP, MSA, GAM, ARM-W, MBK.
Analysed data: LBH, RAM-M, BPS, CB, HU, BL, BJP, AP, WW, NL.
Wrote manuscript: LBH, RAM-M, BPS, AP.
Edited manuscript: LBH, RAM-M, BPS, AP, MSA, GAM, MBK, BJP.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
James S. McDonnell Foundation (PIs: Mashour, Avidan, Kelz); 1R01GM124023 to AP; Mak-McCully was funded by 2T32HL007713-26A1; Hickman was funded by TL1TR002344.
Editorial decision: 08 March 2019
Handling editor: A.R. Absalom
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.03.046.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hudson A.E., Calderon D.P., Pfaff D.W., Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci U S A. 2014;111:9283–9288. doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mashour G.A. Monitoring consciousness: EEG-based measures of anesthetic depth. Semin Anesth Perioper Med Pain. 2006;25:205–210. [Google Scholar]
- 3.Akrawi W.P., Drummond J.C., Kalkman C.J., Patel P.M. A comparison of the electrophysiologic characteristics of EEG burst-suppression as produced by isoflurane, thiopental, etomidate, and propofol. J Neurosurg Anesthesiol. 1996;8:40–46. doi: 10.1097/00008506-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M., Amzica F., Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90:1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 5.Ferron J.-F., Kroeger D., Chever O., Amzica F. Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neurosci. 2009;29:9850–9860. doi: 10.1523/JNEUROSCI.5176-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodcock T.E., Murkin J.M., Farrar J.K., Tweed W.A., Guiraudon G.M., McKenzie F.N. Pharmacologic EEG suppression during cardiopulmonary bypass: cerebral hemodynamic and metabolic effects of thiopental or isoflurane during hypothermia and normothermia. Anesthesiology. 1987;67:218–224. [PubMed] [Google Scholar]
- 7.Ohtahara S., Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J Clin Neurophysiol. 2003;20:398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cloostermans M.C., Van Meulen F.B., Eertman C.J., Hom H.W., Van Putten M.J.A.M. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeijer J., Tjepkema-Cloostermans M.C., van Putten M.J.A.M. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol. 2014;125:947–954. doi: 10.1016/j.clinph.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F.M., Franck M., Lendner J., Krüger S., Wernecke K.D., Spies C.D. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 11.Fritz B.A., Kalarickal P.L., Maybrier H.R. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. doi: 10.1213/ANE.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soehle M., Dittmann A., Ellerkmann R.K., Baumgarten G., Putensen C., Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andresen J.M., Girard T.D., Pandharipande P.P., Davidson M.A., Wesley Ely E., Watson P.L. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014;42:2244–2251. doi: 10.1097/CCM.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sessler D.I., Sigl J.C., Kelley S.D. Hospital stay and mortality are increased in patients having a triple low of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–1203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]
- 15.Watson P.L., Shintani A.K., Tyson R., Pandharipande P.P., Pun B.T., Ely E.W. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–3177. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier K.L., McKinstry-Wu A.R., Palanca B.J.A. Protocol for the reconstructing consciousness and cognition (ReCCognition) study. Front Hum Neurosci. 2017;11:284. doi: 10.3389/fnhum.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vacanti C.J., Van Houten R.J., Hill R.C. A statistical analysis of the relationship of physical status to postoperative mortality in 68.368 cases. Anesth Analg. 1970;49:564–566. [PubMed] [Google Scholar]
- 18.Basner M., Savitt A., Moore T.M. Development and validation of the cognition test battery for spaceflight. Aerosp Med Hum Perform. 2015;86:942–952. doi: 10.3357/AMHP.4343.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLeod D.R., Griffiths R.R., Bigelow G.E., Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- 20.Larsen B., Seitz A., Larsen R. Recovery of cognitive function after remifentanil-propofol anesthesia: a comparison with desflurane and sevoflurane anesthesia. Anesth Analg. 2000;90:168–174. doi: 10.1097/00000539-200001000-00035. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim A.E., Ghoneim M.M., Kharasch E.D. Speed of recovery and side-effect profile of sevoflurane sedation compared with midazolam. Anesthesiology. 2001;94:87–94. doi: 10.1097/00000542-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Basner M., Dinges D.F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basner M., Mollicone D., Dinges D.F. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–959. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickelgren W.A. Speed-accuracy tradeoff and information processing dynamics. Acta Psychol (Amst) 1977;41:67–85. [Google Scholar]
- 25.Thomson D.J. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–1096. [Google Scholar]
- 26.Chemali J., Ching S., Purdon P.L., Solt K., Brown E.N. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J Neural Eng. 2013;10 doi: 10.1088/1741-2560/10/5/056017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Särkelä M., Mustola S., Seppänen T. Automatic analysis and monitoring of burst suppression in anesthesia. J Clin Monit Comput. 2002;17:125–134. doi: 10.1023/a:1016393904439. [DOI] [PubMed] [Google Scholar]
- 28.Leistritz L., Jäger H., Schelenz C. New approaches for the detection and analysis of electroencephalographic burst-suppression patterns in patients under sedation. J Clin Monit Comput. 1999;15:357–367. doi: 10.1023/a:1009990629797. [DOI] [PubMed] [Google Scholar]
- 29.Hartigan J.A., Hartigan P.M. The dip test for unimodality. Ann Stat. 1985;13:70–84. [Google Scholar]
- 30.Bozdogan H. Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- 31.Burnham K.P., Anderson D.R. Second ed. Springer Science & Business Media; New York: 2003. Model selection and multimodel inference: a practical information-theoretic approach. [Google Scholar]
- 32.Hambrecht-Wiedbusch V.S., Li D., Mashour G.A. Paradoxical emergence: administration of subanesthetic ketamine during isoflurane anesthesia induces burst suppression but accelerates recovery. Anesthesiology. 2017;126:482–494. doi: 10.1097/ALN.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cimenser A., Purdon P.L., Pierce E.T. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci U S A. 2011;108:8832–8837. doi: 10.1073/pnas.1017041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbs F.A., Gibbs E.L., Lennox W.G. Effect on the electro-encephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:54–166. [Google Scholar]
- 35.John E.R., Prichep L.S., Kox W. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- 36.Blain-Moraes S., Lee U., Ku S., Noh G., Mashour G.A. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci. 2014;8:114. doi: 10.3389/fnsys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee U., Ku S., Noh G., Baek S., Choi B., Mashour G.A. Disruption of frontal–parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monk T.G., Saini V., Weldon B.C., Sigl J.C. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 39.Bruhn J., Bouillon T.W., Shafer S.L. Bispectral index (BIS) and burst suppression: revealing a part of the BIS algorithm. J Clin Monit Comput. 2000;16:593–596. doi: 10.1023/A:1012216600170. [DOI] [PubMed] [Google Scholar]
- 40.Chan M.T.V., Cheng B.C.P., Lee T.M.C., Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2012;25:1. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 41.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 42.Hesse S., Kreuzer M., Hight D., Gaskell A., Devari P., Singh D., Taylor N.B., Whalin M.K., Lee S., Sleigh J.W., García P.S. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2019;122:622–634. doi: 10.1016/j.bja.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.