Abstract
Hypophosphatasia is a rare inherited disease caused by a loss of function mutations in the gene that codes for the tissue-nonspecific alkaline phosphatase enzyme. It is autosomally inherited and at least 388 different genetic defects have been identified. The clinical presentation is variable from a severe perinatal form, that is fatal if untreated, to adult-onset disease. This review covers the pathophysiology, diagnosis and current management option including the recently licensed enzyme replacement therapy asfotase alfa.
Keywords: alkaline phosphatase, asfotase alfa, hypophosphatasia, osteomalacia, rickets
Introduction
Hypophosphatasia (HPP) is a rare inherited disease caused by a loss of function of the enzyme tissue-nonspecific alkaline phosphatase (TNSAP). There are a number of challenges for the clinician in treating patients with this condition. Firstly, there is great heterogeneity in the clinical presentation and severity of the disease among affected individuals. A milder form of the disease that presents in adulthood can be misdiagnosed as osteoporosis if the treating doctor is not familiar with this condition.1 Moreover, the imperfect correlation between the various genotypes and phenotypes makes genetic counselling and prognostication difficult.2 Lastly, until recently, there was no specific treatment for this disease that carries a poor prognosis in its severe forms. In 2015, asfotase alfa, a recombinant bone-targeted alkaline phosphatase (ALP) was approved by multiple medicine regulatory authorities, including the European Medicines Agency and United States Food and Drug Administration (US FDA) for use in paediatric-onset HPP.3
Pathophysiology
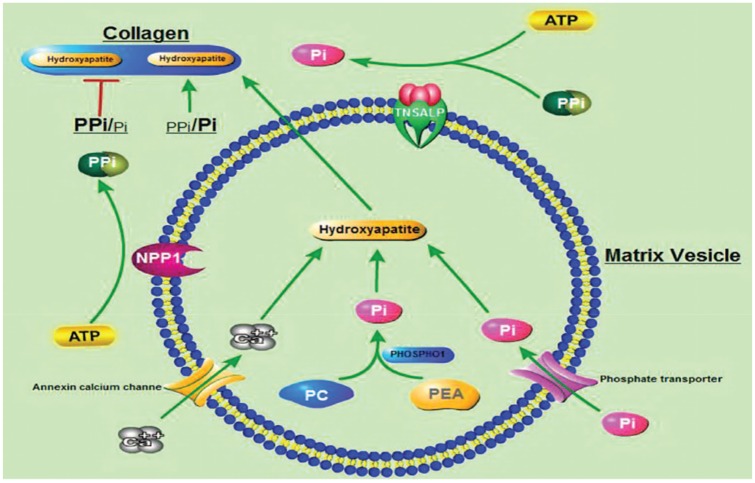
There are four isoenzymes of ALP (intestinal, placental, germ cell and TNSAP). TNSAP is found predominantly in the bone, liver and kidney, but also in other cell types.4 This homodimeric enzyme is located on the extracellular surface of the membrane and hydrolyses phosphates. In HPP, the reduced activity of TNSAP leads to the extracellular accumulation of inorganic pyrophosphate (PPi), pyridoxal 5′-phosphate (PLP) and phosphoethanolamine (PEA). The increased extracellular ratio of PPi/inorganic phosphate (Pi) acts as an inhibitor of skeletal mineralization.1,5 In the normal skeleton, there are two stages of mineralization. The first stage, that remains intact in HPP, involves the formation of hydroxyapatite (HA) crystals from calcium ions (Ca2+) and Pi within the matrix vesicles (MVs). The second stage is the release of HA from the MVs to the extracellular matrix that further elongates with the uptake of extracellular Pi and Ca2+. HA deposits in the collagen fibrils. The increased levels of PPi in HPP inhibit HA propagation (Figure 1).
Figure 1.
Schematic illustration of the process of mineralization. Mineralization begins with hydroxyapatite formation in the matrix vesicles budding from hypertrophic chondrocytes and osteoblasts. Hydroxyapatite is formed from Ca2+ incorporated by the annexin calcium channel and from Pi. The source of Pi involves two independent biochemical pathways: intravesicular Pi generation by the enzymatic action of PHOSPHO1 and the influx of Pi, generated in the perivesicular space by the activities of TNSAP and NPP1, via phosphate transporter. PPi which inhibits hydroxyapatite formation, is hydrolysed by TNSAP. The balance between PPi and Pi is crucial for mineralization.6
Source: www.ncbi.nlm.nih.gov/pmc/articles/PMC5584270PC, phosphocholine; PEA, phosphoethanolamine; Pi, inorganic phosphate; PPi, inorganic pyrophosphate; TNSAP, tissue-nonspecific alkaline phosphatase.
The hypomineralization manifests clinically as a ‘rickets-like’ deformity and bone deformation in infants and children and with a similar clinical picture to osteomalacia once the growth plates close, along with muscle weakness.
One further feature of HPP can be seizures in infants. The deficiency in the dephosphorylation of extracellular PLP is thought to be the cause of seizures in HPP. PLP is a cofactor for the synthesis of important molecules in the central nervous system such as gamma-aminobutyric acid (GABA). However, PLP needs to be dephosphorylated and converted to pyridoxal (PL) in order to enter the cells.1,5 PLP is the active form of B6 and seizures in HPP can respond to the administration of vitamin B6 (pyridoxine).
Epidemiology
The ethnic group with the highest reported incidence of HPP is the Mennonites in Manitoba, Canada. This population is reported to have 1 in 25 individuals carrying a TNSAP mutation and around 1 in 25,000 newborns having lethal HPP.7 Data from the US suggests that HPP is more prevalent in white people than in black people8–10 and the majority of affected American children have a mild disease.8,11 The prevalence of severe forms of HPP was estimated to be 1 in 100,000 births in Canada.12 A molecular study has found the prevalence of severe HPP to be 1 in 300,000 in Europe, but the same study pointed that moderate forms of HPP are 50-times more frequent.13 In addition, two mutations have been reported to cause severe HPP in Japanese patients.14
Genetics
The alkaline phosphatase-liver (ALPL) gene coding the enzyme TNSAP is located in chromosome 1.1 HPP can either be autosomal dominantly (AD) inherited or it can be autosomal recessive (AR). The chances of a couple who had one child with perinatal HPP (AR) to have another pregnancy affected is 25%. The penetrance of the dominant mode is not 100% and therefore not all carriers of a dominant mutation will develop the disease.2 There is also variable expression of the disease within the same family.15
There are at least 388 mutations of ALPL described.16 Given the variety of genetic mutations, patients can be compound heterozygotes, which means carriers of two different pathologic alleles, a phenomenon that enhances the phenotypic variety.17 Some mutations have a dominant negative effect which is seen on heterozygotes with mild or moderate forms of HPP.2,18
Clinical types
There are six different subtypes of HPP as described in Table 1.
Table 1.
Clinical subtypes of HPP.
| Subtype | Inheritance | Clinical presentation |
|---|---|---|
| Perinatal lethal | AR | Death from respiratory failure, seizures, hypercalcaemia |
| Prenatal benign | AR or AD | Skeletal abnormalities in utero with spontaneous improvement during 3rd trimester or ex utero |
| Infantile (<6 months old) | Mostly AR | Failure to thrive, seizures, intracranial hypertension, symptoms from hypercalcaemia |
| Childhood (>6 months old) | AR or AD | Premature teeth loss, weakness, bowed legs, enlarged joints |
| Adult | AR or AD | Metatarsal fractures, femoral pseudofractures, pseudogout and CPPD arthritis |
| Odontohypophosphatasia | AR or AD | No skeletal or systemic manifestations. Premature exfoliation of deciduous teeth, severe dental caries |
AD, autosomal dominant; AR, autosomal recessive; CPPD, calcium pyrophosphate dihydrate crystal deposition disease; HPP, hypophosphatasia.
The prenatal lethal form can be diagnosed during the second trimester with ultrasonography and radiography. Some of the discriminating ultrasound findings are narrow thorax, skeletal hypomineralization, skin-covered osteochondral spurs (which are pathognomonic). The differential diagnosis includes osteogenesis imperfecta type 2 and achondrogenesis.19
The prenatal benign form cannot always reliably be differentiated from the lethal form radiologically, nor with molecular analysis. There is spontaneous improvement during the third trimester or postnatally in this form of HPP. The course of the disease can vary from odontohypophosphatasia to the infantile form.20
The infantile form develops clinically the first 6 months of life. The presence of seizures or rib fractures and chest deformity are indicators of poor prognosis. Mortality is estimated around 50% for this type of HPP.3
The childhood form is commonly characterized by premature deciduous tooth loss, frequently affecting the incisors. Classic radiographic features of the disease are the ‘tongues’ of lucency that expand from the growth plates to the metaphyses.20 Other possible X-ray findings are craniosynostosis and enlarged metaphyses. Interestingly, the presence of bone marrow oedema on magnetic resonance imaging in some patients can mimic the picture of chronic recurrent multifocal osteomyelitis.4 Some children might enter complete remission, but the disease can relapse during adulthood.1
The adult form usually becomes apparent during the middle age. The condition should be suspected in patients with recurrent metatarsal fractures that can heal poorly. Femoral pseudofractures (often bilateral) that originate from the lateral cortex of the subtrochanteric region are another distinctive pathology of HPP. Other types of osteomalacia such as X-linked hypophosphataemia are also associated with femoral pseudofractures, but these are usually located in the medial cortex of the neck of the femur.21 Low bone mass may be noted although not necessarily, and these patients can be misdiagnosed with osteoporosis. There is a concern that inappropriate treatment with bisphosphonates can further undermine the defective mineralization and precipitate atypical femoral fractures in these patients.21 Therefore it is prudent that clinicians pay attention to the low ALP in patients with ‘osteoporosis’, especially in the presence of atypical femoral fractures and investigate the patients accordingly. The other interesting presentations of the disease in adults relate to the accumulation of extracellular PPi that can form calcium pyrophosphate in joints and cause calcium pyrophosphate dihydrate crystal deposition disease (CPPD). The patients can develop pseudogout attacks, calcific periarthritis and spinal hyperostosis.22 There is sometimes a history of premature loss of primary dentition, as mentioned in the childhood form, but there can also be premature loss of the mature dentition.
Odontohypophosphatasia is a form of HPP that by definition affects only the teeth. It causes premature deciduous teeth loss. These patients also have the biochemical abnormalities that are discussed below and can help in making the diagnosis.
Diagnosis
Along with recognising the radiographic and clinical characteristics of the disease, the laboratory abnormalities are crucial in making the diagnosis. ALP has different normal levels depending on age of the patient and it is therefore essential to use age-related reference ranges otherwise the diagnosis may be missed. The low levels of ALP that give the name to the disease are not pathognomonic as there may be other causes for reduced ALP. On the other hand, causes for raised ALP such as cholestasis can sometimes mask the disease. The increased plasma PLP levels are considered the most useful finding in association with the ALP, as it is a sensitive and specific test. The plasma and urine PPi levels will also be raised if measured, but these tests are not widely available. The plasma and urine PEA levels are expected to be high but these are not pathognomonic as they can be affected by urine excretion, diet and age.21
In adult patients presenting with stress fractures and low bone mineral density, HPP can easily be mistaken for osteoporosis. It is essential not to ignore a low ALP measurement in this group and to remember that after a fracture, ALP will be raised and therefore the diagnosis may be missed. If there is clinical suspicion of HPP due to the presentation, then ALP measurements should be repeated once the fracture has healed.
Genetic tests are not always necessary to make the diagnosis but can play an important role in managing a patient and their family. ALPL gene sequencing is used to confirm the diagnosis and to clarify the mode of inheritance.2 A genetic diagnosis will assist with genetic counselling, prenatal diagnosis in the presence of a previous pregnancy affected by severe HPP, and in cases where the diagnosis is unclear. In the second scenario, DNA from chorionic villi can be tested for the known mutation.
Management
Until recently, the management of HPP has been symptomatic and supportive only. Babies can require ventilation if severely affected, which can be technically difficult.23 Neonatal seizures can be treated with Vitamin B6 but may become refractory.24
Management of HPP needs a multidisciplinary team to provide best care to the patient and support to the family. Paediatric patients need input from paediatricians and older patients should be managed by adult physicians with an expertise in metabolic bone diseases (mostly commonly, rheumatologists and endocrinologists). As these cases are rare, care is best provided in specialist centres where teams will be managing more patients with the condition and therefore have more experience. If this is not possible due to geography, then a ‘hub and spoke model’ can be applied where a local team is supported by an expert team from a distance. Orthopaedic surgeons are needed in the team as well as dentists with an interest in the condition. Pain management services are also helpful to help manage pain. Specialist nurses are vital members of the team to provide support, information and help patients to access appropriate care. Psychological support may be required: either in a formal sense via a psychology service or informally by the support of the specialist nurse. Physiotherapy and occupational therapy may also be required.
Patients should be signposted to patient support groups, who can provide information, education and support for those living with HPP. In the US the Soft Bones Foundation (www.softbones.org) and in the UK ‘Soft Bones UK’ (www.softbonesuk.co.uk) can provide support.
Genetic counselling is important for families who may consider having more children. In addition, this is important for patients with HPP as they reach adulthood and may wish to have their own children. At least 388 genetic mutations have been identified and sequencing of the ALPL gene is required. As discussed, the inheritance can be dominant or recessive and there is variable penetrance, so this makes the counselling complex and these discussions are best to be done in a specialist genetics service.
Pain management
Pain is a significant problem in this condition. Involvement of experts in pain management can be helpful and use of anti-inflammatories25 and neuropathic agents may be advised.
Fracture management
Orthopaedic management of fractures in HPP needs to be different to standard fracture treatment. Femoral fractures or pseudofractures should be rodded to provide stability. Pins and plates should be avoided as the bone is soft and complication rates are high with this approach.26 In addition to requiring orthopaedic intervention for fractures, patients may need neurosurgery for craniosynostosis.27
Teriparatide has been reported in case reports to help fracture healing in adults with HPP. Teriparatide is an analogue of the first 34 amino-acids of parathyroid hormone and cannot be used in children. It is licensed for treatment of osteoporosis in postmenopausal women and men at risk of fracture and for glucocorticoid induced osteoporosis. It is given as a 20 µg daily subcutaneous injection and has an anabolic effect on bone.10,28
There is also a case report of six postmenopausal women with HPP treated with teriparatide reducing pain, but no improvement in pain in one premenopausal woman.29
Avoid bisphosphonates
Bisphosphonates are structural analogues of pyrophosphate and are antiresorptive drugs, which work by their effect on osteoclasts. Bisphosphonates are licensed for osteoporosis, Paget’s disease, malignancy and hypercalcaemia. They are also used off-licence in other bone diseases, including osteogenesis imperfecta and bone marrow oedema syndrome. In HPP, pyrophosphate is one of the mineralization inhibitors that accumulates. Bisphosphonates may worsen the hypomineralization in HPP either directly or by binding zinc or magnesium and compromising any residual TNSAP activity30 and therefore there is no place for bisphosphonates in the management of HPP. Bisphosphonate-related atypical femoral fracture has been reported.31
Enzyme replacement therapy
In the 1980s ALP replacement therapy was trialled using infusions soluble ALP from Paget bone disease plasma32,33 or purified from human placenta.34 These studies showed transient hyperphosphatasia, but there was no clinical or radiographic improvement. There were two case reports of marrow and bone cell transplantation that appeared to rescue two girls with infantile HPP.35,36
Asfotase alfa (Stensiq) is an enzyme replacement therapy that was licensed by the US FDA in 2015 for use in paediatric-onset HPP. Asfotase alfa is a soluble glycoprotein that is composed of two identical polypeptide chains. Each of these chains is made up of the catalytic domain of human TNSAP, the human immunoglobulin G1 Fc domain and a deca-aspartate peptide used as a bone targeting domain.
In an animal model of infantile HPP using TNSAP knockout mice it was shown that the animals remained healthy if given asfotase alfa subcutaneously from birth.37 This study was followed by trials for infants or young children with perinatal or infantile HPP. A total of 11 patients were recruited into this study. The protocol was to receive one infusion of asfotase alfa intravenously and then three times a week, subcutaneous injections. Overall, one child was withdrawn by the parents due to an infusion reaction and then had skeletal deterioration. A second child died from sepsis, which was unrelated to the treatment. A total of nine children were treated and the results of this study were published in 2012. They showed improvements in muscle strength and skeletal mineralization as well as improved pulmonary, cognitive and motor function.27 These findings were replicated in a larger cohort of similar patients.38 At the same time, a study of 13 children who had survive infantile HPP or have severe childhood HPP were studied.39 They showed rapidly improved skeletal health, improved muscle strength and resolution of pain and disability that persisted after 5 years of treatment. In addition, a cohort of 37 patients with perinatal and infantile forms of HPP was compared with a natural history cohort. The treatment group had been treated for a median of 2.7 years and they showed mineralization of the HPP skeleton including the ribs, improved respiratory function and survival.40 Asfotase alfa treatment in adolescents and adults with HPP has indicated better mobility.41
The licensed indication for asfotase alfa is for patients of any age with perinatal/infantile- and juvenile-onset HPP. It is given as a subcutaneous injection at a dose of 6 mg/kg/week administered as 2 mg/kg given three times per week or 1 mg/kg given six times per week. Injection site reactions are common and may limit the tolerability of the six times per week regimen. For perinatal/infantile-onset HPP, the dose may be increased for lack of efficacy (e.g. no improvement in respiratory status, growth, or radiographic findings) up to 9 mg/kg per week administered subcutaneously as 3 mg/kg three time per week.
Reported adverse effects include: injection site reactions (46–90%), lipodystrophy (18–70%), ectopic calcifications (5–55%), vomiting/emesis (3–10%), and systemic hypersensitivity reactions (2–10%).
It is yet to be seen what impact use of asfotase alfa will have in adults with paediatric-onset HPP. Further studies are ongoing and required in this patient group. In the UK, the National Health Service England have commissioned expert centres for the management of HPP. A managed access agreement is in place that allows patients to be treated if they meet certain clinical criteria (Table 2). This is only for patients with a confirmed paediatric onset of disease. Patients have to sign up to the managed access agreement that allows data on their response to be collected. Asfotase alfa treatment can only be initiated and managed via the expert centres. Strict start and stop criteria are laid out and patients have to be regularly assessed to ensure they are only treated if they are benefitting from the treatment. This agreement is currently in place for 5 years and will then be reviewed using the data that has been collected.42
Table 2.
National Institute for Health and Care Excellence managed access agreement starting criteria for asfotase alfa in the United Kingdom.
| Under age of 1 year | Aged 1–4 years (one of the following required) | Aged 5–18 years (one of the following required) | Aged 18 years and over (two of three following required) |
|---|---|---|---|
| Patients under 1 year of age with symptoms and signs of HPP should be initiated on asfotase alfa therapy as soon as is possible | Have not achieved expected developmental gross motor milestones for age as demonstrated by the BAMF (brief assessment of motor function) scale or | Continuing or recurring musculoskeletal pain where there is significant pain that affects daily activities which: • Affects quality of life • Hasn’t got better with two different types of painkiller which have been recommended by a national pain specialist | Current fractures with a history of nontraumatic, recurrent or non-/poorly-healing fractures (e.g. inability to remove fixation devices due to risk of recurrent fracture) |
| Continuing or recurring musculoskeletal pain where there is significant pain that affects daily activities which: • Affects quality of life • Hasn’t got better with two different types of painkiller which have been recommended by a national pain specialist | Limited mobility assessed by a specialist according to the modified Bleck Ambulation Efficiency Scoring and with a Bleck score between 1 and 6 | Continuing or recurring musculoskeletal pain where there is significant pain that affects daily activities which: • Affects quality of life • Hasn’t got better with two different types of painkillers which have been recommended by a national pain specialist | |
| Limited mobility assessed by a specialist according to the modified Bleck Ambulation Efficiency Scoring and with a Bleck score between 1 and 6 |
HPP, hypophosphatasia.
Asfotase alfa is currently not available in the UK for patients with adult-onset HPP.
Conclusion
HPP remains a complex disease with a variable phenotype. The availability of enzyme replacement therapy is transforming care and outcomes for those patients with perinatal and infantile-onset disease. It is yet to be seen how this treatment will affect outcomes in the longer term and whether it will reduce morbidity from the condition in adulthood. Exciting as this development is, there remain challenges in treating this disease. Asfotase alfa therapy is currently limited to those with the most severe disease. As this is a new treatment, we are not yet aware of any long-term complications of its use. There remains untreated morbidity in those patients who do not qualify for treatment. This continues to require a multidisciplinary team approach. In addition, we do not currently have a licensed treatment for those patients with adult-onset disease. There is ongoing research in this group.
To continue to develop this, it is probably best that patients are managed by the UK National Health Service at one of the commissioned highly specialized centres to allow access to asfotase alfa for those who need it and to allow ongoing research in this group. The future is certainly looking brighter for patients with HPP, but more work is needed.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: JS Bubbear  https://orcid.org/0000-0001-7576-250X
https://orcid.org/0000-0001-7576-250X
Contributor Information
V. Choida, Metabolic Unit, Royal National Orthopaedic Hospital, Stanmore, UK
J. S. Bubbear, Metabolic Unit, Royal National Orthopaedic Hospital, Brockley Hill, Stanmore, HA7 4LP, UK.
References
- 1. Bianchi ML. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int 2015; 26: 2743–2757. [DOI] [PubMed] [Google Scholar]
- 2. Mornet E. Genetics of hypophosphatasia. Archives de Pédiatrie 2017; 24: 5S51–5S56. [DOI] [PubMed] [Google Scholar]
- 3. Whyte MP. Hypophosphatasia: enzyme replacement therapy brings new opportunities and new challenges. JBMR 2017; 43: 667–675. [DOI] [PubMed] [Google Scholar]
- 4. Millan JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int 2016; 98: 398–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orimo H. Pathophysiology of hypophosphatasia and the potential role of Asfotase Alfa. Ther Clin Risk Manag 2016; 12: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan J, Zhang L, Liu T, et al. Genetic evaluations of Chinese patients with odontohypophosphatasia resulting from heterozygosity for mutations in the tissue-nonspecific alkaline phosphatase gene. Oncotarget 2017; 8: 51569–51577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenberg CR, Taylor CL, Haworth JC, et al. A homoallelic Gly317 → Asp mutation in ALPL causes the perinatal (lethal) form of hypophosphatasia in Canadian mennonites. Genomics 1993; 17: 215–217. [DOI] [PubMed] [Google Scholar]
- 8. Whyte MP, Zhang F, Wenkert D, et al. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25. Bone 2015; 75: 229–239. [DOI] [PubMed] [Google Scholar]
- 9. Whyte MP, Essmyer K, Geimer M, et al. Homozygosity for TNSALP mutation 1348c>T (Arg433Cys) causes infantile hypophosphatasia manifesting transient disease correction and variably lethal outcome in a kindred of black ancestry. J Pediatr 2006; 148: 753–758. [DOI] [PubMed] [Google Scholar]
- 10. Camacho PM, Mazharil AM, Wilczynskil C, et al. Adult Hypophosphatasia treated with teriparatide: report of two patients and review of the literature. Endocr Pract 2016; 22: 941–950. [DOI] [PubMed] [Google Scholar]
- 11. Mumm S, Wenkert D, Zhang X, et al. Hypophosphatasia: the c.1133A. J Bone Miner Res 2006; 21: S115. [Google Scholar]
- 12. Fraser D. Hypophosphatasia. Am J Med 1957; 22: 730–746. [DOI] [PubMed] [Google Scholar]
- 13. Mornet E, Yvard A, Taillandier A, et al. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet 2011; 75: 439–445. [DOI] [PubMed] [Google Scholar]
- 14. Ozono K, Michigami T. Hypophosphatasia now draws more attention of both clinicians and researchers: a commentary on prevalence of c. 1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasias in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet 2011; 56: 174–175. [DOI] [PubMed] [Google Scholar]
- 15. Hofmann C, Girschick H, Mornet E, et al. Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur J Hum Genet 2014; 22: 1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mornet E. The Tissue Nonspecific Alkaline Phosphatase Gene Mutations Database, www.sesep.uvsq.fr/03_hypo_mutations.php (accessed 4 February 2019). Last updated 08 July 2019.
- 17. Mornet E. Molecular genetics of hypophosphatasia and phenotype-genotype correlations. Subcell Biochem 2015; 76: 25–43. [DOI] [PubMed] [Google Scholar]
- 18. Fauvert D, Brun-Heath I, Lia-Baldini AS, et al. Mild forms of hypophosphatasia mostly result from dominant negative effect of severe alleles or from compound heterozygosity for severe and moderate alleles. BMC Medical Genetics 2009; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guguloth A, Aswani Y, Anandpara KM. Prenatal diagnosis of hypophosphatasia congenita using ultrasonography. Ultrasonography 2016; 35: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whyte MP. Hypophosphatasia: an overview for 2017. Bone 2017; 102: 15–25. [DOI] [PubMed] [Google Scholar]
- 21. Marini F, Brandi ML. Atypical femur fractures: a distinctive tract of adult hypophosphatasia. Clin Cases Miner Bone Metab 2017; 14: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chuck AJ, Pattrick MG, Hamilton E, et al. Crystal deposition in hypophosphatasia: a reappraisal. Ann Rheum Dis 1989; 48: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriquez E, Bober MD, Davey L, et al. Respiratory mechanics in an infant with perinatal lethal hypophosphatasia treated with human recombinant enzyme replacement therapy. Pediatr Pulmonol 2012; 119: 14–19. [DOI] [PubMed] [Google Scholar]
- 24. Plecko B, Stockler S. Vitamin B6-dependent seizures. Can J Neurol Sci 2009; 36(Suppl. 2): S73–S77. [PubMed] [Google Scholar]
- 25. Whyte MP, Wenkert D, McAlister WH, et al. Chronic recurrent multifocal osteomyelitis mimicked in childhood hypophosphatasia. J Bone Miner Res 2009; 64: 1493–1505. [DOI] [PubMed] [Google Scholar]
- 26. Coe JD, Murphy WA, Whyte MP. Management of femoral fractures and pseudofractures in adult hypophosphatasia. J Bone Joint Surg (Am) 1986; 68: 981–990. [PubMed] [Google Scholar]
- 27. Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme replacement therapy in life-threatening hypophosphatasia. N Engl J Med 2012; 366: 904–913. [DOI] [PubMed] [Google Scholar]
- 28. Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab 2007; 92: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 29. Gagnon C, Sims NA, Mumm S, et al. Lack of sustained response to teriparatide in a patient with adult hypophosphatasia. J Clin ENdocrinol Metab 2010; 95: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 30. Otero JE, Gottesman GS, McAlister WH, et al. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res 2013; 28: 419–430. [DOI] [PubMed] [Google Scholar]
- 31. Sutton RA, Mumm S, Coburn SP, et al. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Miner Res 2012; 27: 987–994. [DOI] [PubMed] [Google Scholar]
- 32. Whyte MP, Valdes R, Jr, Ryan LM, et al. Infantile hypophosphatasia: enzyme replacement therapy by intravenous infusion of alkaline phosphatase-rich plasma from patients with Paget bone disease. J Pediatr 1982; 101: 379–386. [DOI] [PubMed] [Google Scholar]
- 33. Whyte MP, McAllister WH, Patton LS, et al. Enzyme replacement therapy for infantile hypophosphatasia attempted by intravenous infusions of alkaline phosphatase-rich Paget plasma: results in three additional patients. J Pediatr 1984; 105: 926–933. [DOI] [PubMed] [Google Scholar]
- 34. Whyte MP, Habib D, Coburn SP, et al. Failure of hyperphosphatasemia by intravenous infusion of purified placental alkaline phosphatase (ALP) to correct severe hypophosphatasia: evidence against a role of circulating ALP in skeletal mineralization. J Bone Miner Res 1992; 7: S155. [Google Scholar]
- 35. Whyte MP, Kurtburg J, McAlister WH, et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res 2003; 18: 624–636. [DOI] [PubMed] [Google Scholar]
- 36. Cahill RA, Wenkert D, Perlman SA, et al. Infantile hypophosphatasia trial of transplantation therapy using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab 2007; 92: 2923–2930. [DOI] [PubMed] [Google Scholar]
- 37. Milan JL, Narisawa S, Lemire I, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res 2008; 23: 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liese J, Hofmann C, Harmatz P, et al. Efficacy and safety of asfotase alfa in patients with infantile hypophosphatasia treated for up to 3.5 years: results from a phase II, open-label, uncontrolled study {abstract}. Proc., 98th Annual Endocrine Society Meeting, Boston, Mass, 2016. [Google Scholar]
- 39. Whyte MP, Madson KL, Phillips D, et al. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight 2016; 1: e85971: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whyte MP, Rockman-Greenberg C, Ozono K, et al. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab 2016; 101: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kishnani PS, Rockman-Greenberg C, Madson KL, et al. Ambulatory performance in adolescents and adults with hypophosphatasia treated with asfotase alfa: data from a phase II, randomized, dose-ranging open-label, multi-center study. J Bone Miner Res 2016; 31(Suppl. 1): S108. [Google Scholar]
- 42. National Institute for Health and Care Excellence. Asfotase alfa for treating paediatric-onset hypophosphatasia, www.nice.org.uk/guidance/hst6 (accessed 22 June 2019).