1. Introduction
Asthma is one of the most common pediatric emergencies among children throughout the world, where an estimated 300 million individuals are affected [1]. The prevalence of asthma in Saudi Arabia is 23% according to Alfrayeh [2]. Most asthma is uncontrolled and Jahdali estimated that up to 64% of patients have uncontrolled asthma [3]. Morbidity related to asthma has also increased in recent years. In addition, hospitalization for asthma has increased [4]. However, mortality due to asthma is decreasing worldwide [5]. Asthma is one of the main causes of health care utilization and the costs related to asthma are increasing [6]. Approximately 50% of pediatric asthma cases are still uncontrolled in Saudi Arabia, even in tertiary centers [7]. Numerous guidelines are available online and the guidelines of the Saudi Initiative for Asthma (SINA), which were updated in 2016, are very useful for the pediatric age group [8]. The availability of asthma guidelines can improve the outcomes of asthma in children [9].
In general, asthma treatments that involve the administration of oxygen, inhaled bronchodilators, systemic steroids, and anticholinergic agents are common, and these agents are the main forms of therapy. Frequent assessments and monitoring the response to these therapies are crucial for tailoring medications to needs. In this study, we aimed to minimize the controversy regarding asthma exacerbation management by providing the best evidence for patient care according to the resources available at a specific institute [10].
1.1. Definition of exacerbation
Asthma exacerbation is recognized clinically based on the following features: progressive increase in shortness of breath, coughing, wheezing, or chest tightness, and a progressive reduction in lung function that requires medical intervention [5,11]. An exacerbation may include an increase in coughing, especially when the child is asleep, a decrease in exercise tolerance, impairment of daily activities, and acute or sub-acute increases in wheezing or shortness of breath [12,13].
1.2. Goal of therapies
-
•
Assessing the severity of an exacerbation.
-
•
Correcting hypoxemia/hypercarbia and rapidly reversing the airflow obstruction by using appropriate and prompt medical interventions.
-
•
Preventing complications that might occur, such as air leakage.
-
•
Ensuring the appropriate disposition of the patient after initial therapies are provided at the emergency department.
-
•
Reducing the possibility of recurrence by providing adequate baseline control therapies and arranging follow-up [8,[13], [14], [15]].
1.3. Initial assessment and severity
Acute asthma exacerbation is a medical emergency that should be diagnosed and managed immediately [11]. The treatment of asthma exacerbation depends on the severity of the exacerbation. The assessment of an asthma exacerbation is a continuous process with two different stages: (1) a static assessment to determine the severity of the attack, and (2) frequent assessments to evaluate the response to treatment. Overall, the assessment process requires the analysis of several factors [5]. Different scoring systems exist, such as the Asthma Scoring System or Pediatric Respiratory Assessment Measure (PRAM). The SINA group adopted the PRAM scoring system as a valid and reliable scoring system for assessing the severity of exacerbation in children aged 2–17 years [8]. The PRAM score is a 12-point score based on the oxygen saturation, suprasternal retractions, scalene muscle contractions, air entry, and wheezing [12]. The PRAM clinical pathway for inpatient management has been shown to decrease the length of stay in the emergency department and with no adverse outcomes. The SINA expert panel recommends determining the PRAM score for asthmatic patients in the emergency department to categorize the risk of hospital admission [8,16,17].
-
•
Total score of 1–3: Low risk (<10%) of hospital admission.
-
•
Total score of 4–7: Moderate risk (10–50%) of hospital admission.
-
•
Total score of 8–12: High risk (>50%) of hospital admission.
1.4. Medical history
A brief history should be obtained for any exacerbation. The objective of taking the history is to determine the duration of symptoms and the severity of the exacerbation. Therefore, it is necessary to determine precipitating factors, including medications the child has been given, any previous exacerbations such as previous admissions, number of bronchodilators used, courses of steroids, environmental triggers, compliance, psycho-social factors, and risk factors for death in intensive care unit (ICU), including previous pediatric ICU (PICU) admission, intubation, severe life-threatening conditions, and deterioration while taking systemic steroids [13,18].
1.5. Physical examination
A physician should inspect the patient thoroughly and focus on the patient's general appearance and vital signs. Is the child able to lie down, or does he or she prefer a sitting position? Is the child sweating, using accessory muscles to talk, or wheezing audibly? It should be noted that although the degree of wheezing is not correlated with the severity of obstruction, a silent chest is an ominous sign. Agitation, confusion, and mental drowsiness are extreme signs of cerebral hypoxia, which requires immediate intervention. Each patient should be screened for risk factors for fatal asthma (Table 11,12,13,15). Objective evaluations of disease severity and response to therapy are most important, and scores for objective measures should be obtained. Frequent assessment is crucial. The lung function test (spirometry) is difficult to perform in younger children age, especially in those aged less than 6 years during exacerbation, and the results may not correlate with the asthma scoring system. Therefore, spirometry is unreliable in children [19,20].
Table 1.
Risk factors for death from asthma.
| Asthma history |
| Previous severe exacerbation (e.g., intubation or ICU admission for asthma) |
| Two or more hospitalizations for asthma in the past year |
| Three or more emergency department visits for asthma in the past year |
| Hospitalization or emergency department visit for asthma in the past month |
| Use of >2 canisters of short-acting beta2-agonist per month |
| Difficulty perceiving asthma symptoms or the severity of exacerbations |
| Other risk factors: |
| Lack of a written asthma action plan |
| Social history |
| Low socioeconomic status or inner-city residence |
| Illicit drug use |
| Major psychosocial problems |
| Comorbidities |
| Cardiovascular disease |
| Other chronic lung disease |
| Chronic psychiatric disease |
ICU: Intensive Care Unit, Adapted from British Thoracic Society, Scottish Intercollegiate Guidelines Network. “British Guideline on the Management of Asthma,” Revised 2018.
Recommendation: The severity level of any patient with an exacerbation should be assessed using both clinical and objective evaluations, including pulse oximetry or blood gas in severe cases.
1.6. Oxygen therapy
Hypoxia is the primary cause of death from exacerbation, and oxygen may help to decrease dyspnea, aid bronchodilation, support the myocardium, and minimize the risk of arrhythmia [21]. Oxygen should be titrated to achieve saturation ≥ 94% [8,12,15]. Oxygen will not suppress respiratory drive in the absence of preexisting CLD [20]. Oxygen saturation < 92% in room air can predict admission [8,13,22,23].
Recommendations: Oxygen supplements should be provided for patients with oxygen saturation levels below 92% in room air, and patients should be weaned from oxygen when the saturation level exceeds 94%.
Beta2 agonist bronchodilators: A bronchodilator trial should occur during an asthma exacerbation. Most researchers prefer metered-dose inhalers (MDIs) with a spacer to nebulizers. These inhalers are the first choice for pediatric asthma exacerbations, and strong evidence (evidence based medicine-A) supports their use for mild to moderate exacerbations [8,12,14,15,24]. The recommended doses for pediatric patients are 4–5 puffs for children < 20 kg and 6–10 puffs for those > 20 kg. Nebulization should be limited to: severe patients <5 years: 2.5 mg/3–4 ml, and >5 years: 5.0 mg/3–4 ml (see Table 2) [[25], [26], [27], [28], [29], [30]]. MDIs and nebulizers are equally effective methods for delivering beta2 agonists to children with acute asthma and mild to moderate exacerbations.
Table 2.
Summary recommendations for bronchodilator according to common international asthma guidelines.
| GINA 2018 | Mild: 2 puffs; Moderate: 6 puffs <5 years Moderate: 4–10 puffs >5 years |
<5 years Alternative: 2.5 mg over 20 min >5 year 5 mg/ml NS |
|---|---|---|
| SINA 2016 | 5 puffs vs. 10 puffs based on PRAM assessment Shift to nebulizer in severe cases |
<20 kg vs. >20 kg 2.5–5 mg in 3–5 ml NS |
| Cincinnati 2010 | 6–puff range (4–8 h) as effective as nebulizer for mild to moderate cases | <30 kg 2.5/3 ml, >30 kg 5 mg/3 ml NS Continuous nebulizer: 0.5 mg/kg/h (<15 mg/h) |
| SIGN 2016 | 2–6 puffs <5 years vs. 4–10 puffs >5 years Shift to nebulizer in third hour |
<20 kg vs. >20 kg 2.5 mg vs. 5 mg |
| NHLBI 2007 | Mild: 4–8 puffs × 3. Moderate: 4–8 puffs over 20 min for 1–4 times |
Nebulizer in severe cases |
| New Zealand 2017 |
6 puffs for mild and moderate cases | Provide 2.5–5 mg continuous nebulization |
GINA: Global Initiative National for Asthma; PRAM: Pediatric Respiratory Assessment Measure; SINA: Saudi Initiative for Asthma; SIGN: Scottish Intercollegiate Guidelines Network (British guide); NS: normal saline.
Recommendations: Inhaled short-acting beta2-agonist should be administered as the drug of choice for rapid reversal of airflow obstruction.
Recommendations: MDIs improve discharge from the emergency department and shorten the duration of stay, and they are either equally effective or superior to nebulizers for mild to moderate exacerbations.
Recommendations: Nebulization should be reserved for severe exacerbations or for patients who do not respond well to oxygen-driven MDIs.
Recommendations: Continuous nebulized salbutamol is more effective than intermittent treatment in severe cases.
1.7. Anticholinergic agents
The role of anticholinergics in acute asthma management is not well defined. However, ipratropium bromide is commonly added to an inhaled bronchodilator in the emergency department, which can lead to synergistic effects with the bronchodilator [31,32]. Ipratropium bromide is also often administered as a regular medication after admission [33]. The current best evidence for anticholinergic treatment confirms that multiple doses may be given in an emergency but that its use should be limited to moderate to severe cases. This treatment may reduce admission by 30–60% and with no apparent significant side effects. There are apparent benefits from the use of single doses of anticholinergic in asthma with mild exacerbation, or any added benefit due to administration after admission [34]. Anticholinergics are less effective than inhaled beta-agonists and they should not be used alone [35].
Recommendations: Anticholinergic treatment is recommended for moderate to severe exacerbations rather than for mild cases.
Recommendations: Anticholinergics should not be used alone or more than 24 h after admission.
1.8. Steroids
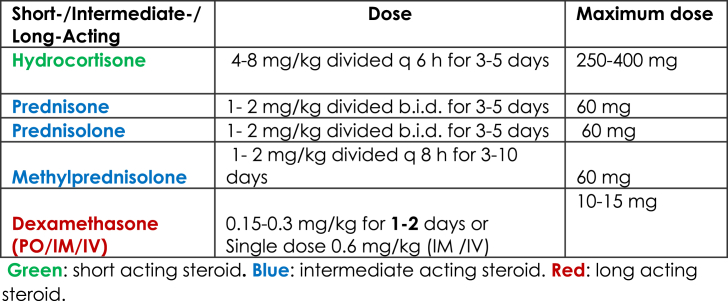
Steroid should be limited for children with moderate to severe asthma exacerbations (evidence based medicine-A) [8,12,23,36]. Different mechanisms allow steroids to interfere with leukotriene and prostaglandin synthesis, prevent cell migration, and upregulate airway b receptors, which can accelerate the resolution of the exacerbation, decrease hospitalization, and facilitate earlier discharge from the emergency department (evidence based medicine-A) [[37], [38], [39]]. Different steroid regimens are recommended (Table 3). Oral prednisone is the most convenient and least expensive steroid, and its effects are equivalent to those of intravenous methylprednisolone. However, methylprednisolone is the preferred medication when the patient is sick or unable to tolerate oral or IV medications. Oral dexamethasone is equivalent to several days of oral prednisolone [[40], [41], [42], [43], [44]]. Studies have shown that divided doses of oral steroids (AM and PM doses) minimize the symptoms late at night, and that tapering the steroid is not necessary if it is used for one week or less.
Table 3.
Recommendations: Steroids should be used in moderate or severe cases.
Recommendations: Steroids decrease admission and relapse if given during the first hour of an exacerbation.
Recommendations: Oral steroids are the most convenient and least expensive route of administration.
Recommendations: IV steroids should be administered to patients who do not respond to oral therapy.
Recommendations: One dose of dexamethasone is equal in efficacy to several days of prednisolone.
Recommendations: It is not necessary to wean patients from steroids if they are used for less than 7 days.
1.9. Inhaled corticosteroids for acute asthma
Multiple trials of different types of inhaled corticosteroids for asthma exacerbations have determined minimal improvements in the respiratory scores. However, no convincing evidence is available regarding their use as an add-on therapy for severe asthma exacerbations [23,[45], [46], [47], [48], [49], [50], [51]].
Recommendations: High-dose inhaled steroids are not recommended for acute asthma attacks.
1.10. Fluids
Patients are often dehydrated because of an increased basal metabolic rate and euvolemia is recommended. Syndromes resulting from inappropriate ADH are very rare but they may be considered in the presence of hyponatremia with markedly reduced urine output [14,52].
Recommendations: Euvolemia is recommended unless there are signs of dehydration or suspected SIADH.
Recommendations: Aggressive rehydration should be avoided during asthma exacerbation.
1.11. Antibiotics
The use of antibiotics for asthma exacerbation caused by viral illness is not necessary.
However, antibiotics may be considered if the patient has a high temperature and appears toxic, there is evidence of consolidation on the chest X-ray, or the patient expectorates purulent sputum with the presence of polymorphs [8,12,14].
Recommendations: Antibiotics should not be used to treat asthma exacerbation unless bronchopneumonia or another focus of infection is suspected.
1.12. Chest X-ray
Chest X-rays are obtained excessively in asthma exacerbation. Furthermore, the misinterpretation of peribronchial opacity as pneumonic infiltrate is common and can lead to the prescription of unnecessary medications [13,53].
Recommendations: Chest X-rays should be limited to the following conditions:
-
1.
Status asthmaticus (the patient is not responding to standard therapy);
-
2.
Presence of complications, e.g., barotrauma pneumothorax or pneumomediastinum;
-
3.
Suspected bronchopneumonia;
-
4.
Suspicion of a foreign body as the cause of wheezing and respiratory distress.
1.13. Serum electrolytes
The measurement of serum electrolytes is important in severe asthma, particularly for monitoring the serum potassium levels. The frequent use of ventolin to treat status asthmaticus may cause hypokalemia, and acidosis may result in transient hyperkalemia [8,14,15].
Recommendations: Electrolytes should be monitored in severe asthma exacerbation, especially potassium and lactate.
Serum glucose: Blood sugar levels are often affected in asthma exacerbation in children due to stress, infection, and the use of beta-agonists and corticosteroids. Hyperglycemia may be a predictor of longer admission [54]. However, younger children are more susceptible to hypoglycemia in response to infection because of their poor reserves.
Recommendations: Blood sugar should be monitored in severe asthma exacerbation.
1.14. Complete blood cell count (CBC)
Cell counts and differentials are commonly requested in the emergency department in the majority of cases of asthma exacerbation. Leukocytosis is common but neutrophilia should be interpreted with caution because beta-agonists and corticosteroids may result in the demargination of white cells and an increase in the peripheral white cell count with a predominant left shift [14,30]. Recommendations: CBC should not be performed routinely in cases of asthma exacerbation and leukocytosis could be a result of dermargination.
Viral study: It well known that viruses are the main trigger of asthma exacerbation [8].
Recommendations: Screening for viral illness should be limited to moderate or severe exacerbations or admitted cases, and it may aid in the discontinuation of unnecessary antibiotics [14,30].
1.15. Blood gas analysis
Recommendations: This analysis is not routinely required for asthma exacerbation. Asthma exacerbation is a clinical diagnosis and blood gas analysis should be limited to at least moderate exacerbations or patients who exhibit a suboptimal response to standard asthma therapy. [8,13,14].
1.16. Magnesium sulfate
Magnesium is an important cofactor in numerous enzymatic reactions, including the inhibition of calcium uptake in smooth muscles to cause muscle relaxation [55,56]. Magnesium may also inhibit cholinergic transmission, stimulate nitric oxide and prostacyclin synthesis, and stabilize mast cells and T-lymphocytes [57]. Meta-analyses and systematic reviews have demonstrated the effect of intravenous magnesium. Magnesium is administered in moderate to severe asthma exacerbations. The usual recommended dose is 25–75 mg/kg/20 min initially and then every 6 h as needed. IV magnesium is safe and beneficial for moderate to severe asthma exacerbations at doses of 4–6 mg/dl, but it exhibits toxicity at >12 mg/dl. Nausea and flushing are common side effects, and muscle weakness, hyporeflexia, and respiratory depression are manifestations of toxicity. Renal impairment and heart blockage are contraindications. Some studies obtained minimal evidence supporting the use of nebulized magnesium sulfate, especially in severe cases, depending on the scoring system or pulmonary function results [[58], [59], [60], [61], [62], [63]].
Recommendations: Magnesium sulfate may be given for moderate to severe asthma exacerbations in patients who exhibit minimal responses to bronchodilators and steroids. [[64], [65], [66], [67], [68], [69], [70]].
Recommendations: Insufficient data are available to support recommending the use of inhaled magnesium sulfate for severe asthma exacerbation.
1.17. Salbutamol
This treatment should be limited for patients with status asthmaticus who fail to respond to a continuous nebulized beta2-agonist. IV salbutamol improves lung function tests and gas exchange [71] but its toxicity increases as the concentration increases [72]. IV salbutamol should be limited to use in the pediatric ICU, where continuous cardiac monitors are available. Cardiac toxicity signs such as arrhythmia and significant tachycardia are common side effects of this medication. In an emergency, we recommend an initial dose of 15 μg/kg over 10 min under cardiac monitoring, and electrolyte measurement to avoid potential hypokalemia and increased lactate. Infusion should only occur in the ICU as indicated. Some researchers consider that there is no extra benefit of IV salbutamol compared with continuous salbutamol nebulization [73,74]. Recommendations: IV salbutamol may be administered under cardiac monitoring in refractory patients who do not respond to standard asthma therapy.
1.18. Aminophylline
This bronchodilator is unlikely to provide additional bronchodilatory effects compared with standard therapy (i.e., continuous inhaled beta2 agonist, ipratropium bromide, and IV steroids) and it should be reserved for children with severe asthma exacerbations who fail to improve despite maximized therapy after consulting a pediatric intensivist. The effects of aminophylline on patient oxygenation, duration of ventilation, and length of stay in PICU are still unknown. Side effects such as vomiting or dysrhythmia are common due to its narrow therapeutic range. Therefore, this medication should not be used without permission from the intensivist and it should be administered under patient cardiac monitoring. The general recommended dose is aminophylline infusion at 5 mg/kg over 20 min, followed by 0.9 mg/kg/h [75]. Aminophylline has a widely accepted therapeutic range of 10–20 mg/l, which determines dosing decisions in children [76].
Recommendation: Aminophylline should not be used for mild to moderate asthma exacerbation.
Recommendations: IV aminophylline should be reserved for severe asthma patients who fail to improve despite maximum therapy in the PICU, and its use may increase the frequency of adverse effects during an exacerbation [[77], [78], [79], [80]].
1.19. Inhaled heliox
A gas mixture of oxygen and helium (heliox) is commonly administered at a concentration ratio of 80:20 to decrease the progression of respiratory failure and prevent intubation. This mixture also increases laminar flow and reduces turbulent flow. Heliox should be reserved for patients with severe asthma exacerbation who fail to respond to maximum therapies in the ICU [13,14,23,[81], [82], [83], [84]].
Recommendation: Heliox-driven salbuterol nebulization might be considered in any patient with life-threatening exacerbation or with severe exacerbation that persist after intensive conventional adjunctive therapies.
1.20. Noninvasive PPV (NIPPV)
NIPPV is a safe and effective treatment for status asthmaticus in pediatric patients. It can prevent intubation as well as reducing mortality and treatment costs. Therefore, its use has become frequent. The main mechanism involves stenting the airway combined with the a bronchodilator, which induces alveolar recruitment [[85], [86], [87], [88], [89], [90]].
Recommendation: NIPPV use should be encouraged in refractory cases with the supervision of an intensivist.
Indications for ICU admission: The indications for admission to the ICU vary widely among centers [13]. The indications are summarized as follows [13,23].
-
•
Failure to exhibited a sustained clinical response to continuous bronchodilators and steroids;
-
•
Worsening of gas exchange;
-
•
Apparent fatigue or exhaustion;
-
•
Changes in the level of sensorium.
1.21. Endotracheal intubation and ventilation in asthmatic patients with respiratory failure
Intubation and mechanical ventilation are expected in less than 10% of cases admitted to the PICU, including lifesaving efforts for severe asthma attacks with respiratory failure. However, their use in children is commonly associated with significant complications, e.g., hypotension or pneumothorax, which occur in up to 50% of cases. Therefore, intubation should be avoided as much as possible [85,91−93]].
Recommended The indications under the supervision of a pediatric intensivist are as follows:
-
•
Respiratory or cardiac arrest;
-
•
Progressive hypoxemia despite O2 or NIPPV administration;
-
•
Progressive rising PaCo2 despite maximum therapy and/or NIPPV;
-
•
Deterioration of mental status;
-
•
Progressive exhaustion.
1.22. Recommendations
Intubation should be avoided as much as possible and only used as a last resort and under the supervision of a pediatric intensivist [13,85].
Intubation: Clinicians should follow the rapid sequence intubation protocol, where intubation should be conducted cautiously under cardio-respiratory monitoring by the most expert clinician in the unit [85]. Rapid ventilation during resuscitation should be avoided. Aim to ensure adequate oxygenation and sufficient gas exchange.
Setting: Controlled mechanical ventilation should be provided using the most familiar mode. However, PRVC is the optimum mode of ventilation for asthmatic patients. The goal is to save the child. Permissive hypercapnia may be beneficial using the following strategy: slow rate, low tidal volume (approximately 5 ml/kg), short inspiratory time ratio to allow time for exhalation and washout of PCO2, Fi O2 with 100% oxygen, and use fo physiological PEEP [[91], [92], [93]].
Disposition: Patients may be discharged when they have been extubated successfully with a stable hemodynamic status, have been weaned off continuous intravenous beta-agonists and are stable under intermittent beta-agonist aerosol therapy for more than 3 h [13,14]. Education has been proven to minimize the risk of re-admission or unscheduled visits to the emergency department. Follow-up should be conducted for any exacerbation to ensure that the patient is doing well after discharge.
Recommendations Education should be provided for any asthmatic patient and their families prior to discharge from the emergency department or inpatient unit. Education should be provided by an asthma educator or any staff trained in the field of asthma to ensure that the families understood the nature of asthma, the signs and symptoms of asthma, how to use asthma medications, how to recognize the risk factors, and when to ask for medical support [36,45]
Admission criteria [8,13,23,30]
-
-
An ongoing need for supplemental oxygen despite initial treatment.
-
-
A need for frequent beta2-agonist therapy (more than 3 h).
-
-
Any feature of life-threatening asthma.
-
-
Previous near-fatal event.
-
-
Exacerbation despite use of oral steroids.
-
-
Underlying psychosocial conditions.
-
-
Exacerbation despite adequate doses of oral steroids and presenting again.
-
-
Residence in a remote location or without transportation/communication.
Discharge criteria [8,13,23,30]
-
-
Need for beta2-agonists every 4–6 h.
-
-
Minimal signs of respiratory distress.
-
-
Saturation more than 92% in room air.
-
-
Good air entry.
-
-
No psychosocial illness.
2. Conclusion
The management of asthma exacerbation in children remains a challenge for public health systems, and thus we have provided updated guidelines in this review. Previous studies support MDI salbutamol as the best beta2 agonist of choice. Ipratropium bromide is effective in moderate to severe cases, and steroids are indicated for moderate and severe exacerbations. The first hour is the optimal treatment time, and medications should be adjusted according to progression, severity, and response to the asthma therapy. Inhaled steroids play no role in exacerbation. Magnesium sulfate administration is encouraged for patients who did not respond well to the initial therapy, and the use of aminophylline should be limited to severe cases in the PICU. Heliox and noninvasive ventilation may be used to prevent intubation. Intubation should be avoided, and clinicians should take great caution during intubation, which should always be performed by an expert. These guidelines require implementation and revision. It should be noted that exacerbation is a failure of the underlying regimen, which requires urgent revision and follow-up with a primary doctor after the parents have been fully educated.
Acknowledgments
Acknowledgment to the Saudi Pediatric Pulmonology Association (SPPA) for supporting this manuscript.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Al Frayh A.R., Shakoor Z., ElRab M.G., Hasnain S.M. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001 Mar 1;86(3):292–296. doi: 10.1016/s1081-1206(10)63301-7. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jahdali H.H., Al-Hajjaj M.S., Alanezi M.O., Zeitoni M.O., Al-Tasan T.H. Asthma control assessment using asthma control test among patients attending 5 tertiary care hospitals in Saudi Arabia. Saudi Med J. 2008;29(5):714–717. [PubMed] [Google Scholar]
- 4.Mannino D.M., Homa D.M., Pertowski C.A. vol. 47. 1998. pp. 1–27. (Surveillance for asthma- united state 1960–96. Morbidity and mortality weekly report CDC surveillance summary). [PubMed] [Google Scholar]
- 5.D'Amato . vol. 11. 2016. p. 37. (Multi-disciplinary respiratory medicine). [Google Scholar]
- 6.Weiss K.B., Gergen P.J., Hodgson T.A. An economic evaluation of asthma in the United States. N Engl J Med. 1992 Mar 26;326(13):862–866. doi: 10.1056/NEJM199203263261304. [DOI] [PubMed] [Google Scholar]
- 7.Alsahn B., Alshamrani A., Alzahrani A., Alsahmi O., Alqudhybi A. Asthma control assessment using asthma control test among pediatric patients attending a tertiary care hospital in Saudi Arabia. Egypt J Hosp Med. 2017 Jul 30;68(2) [Google Scholar]
- 8.Al-Moamary M.S., Alhaider S.A., Idrees M.M., Al Ghobain M.O., Zeitouni M.O., Al-Harbi A.S. The Saudi Initiative for Asthma-2016 update: guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2016 Jan;11(1):3. doi: 10.4103/1817-1737.173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham S., Logan C., Lockerbie L., Dunn M.J., McMurray A., Prescott R.J. Effect of an integrated care pathway on acute asthma/wheeze in children attending hospital: cluster randomized trial. J Pediatr. 2008;152(3):315–320. doi: 10.1016/j.jpeds.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Jones Brittany Pardue, Fleming Geoffrey M., Otillio Jaime Kaye, Asokan Ishan, Donald H. Arnold. Pediatric acute asthma exacerbations: evaluation and management from emergency department to intensive care unit. J Asthma. 2016;53(6):607–617. doi: 10.3109/02770903.2015.1067323. [DOI] [PubMed] [Google Scholar]
- 11.Reddel H.K., Taylor D.R., Bateman E.D., Boulet L.P., Boushey H.A., Busse W.W. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009 Jul 1;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 12.White J., Paton J.Y., Niven R., Pinnock H. British thoracic society, scottish intercollegiate guidelines Network. “British guideline on the management of asthma. Thorax. 2017;0:1–5. [Google Scholar]
- 13.Ortiz-Alvarez Oliva, Angelo Mikrogianakis. Managing paediatric patients with an acute asthma exacerbation. Paediatr Child Health. 2012;17(5):251–256. doi: 10.1093/pch/17.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Management of acute asthma exacerbation in children. Guideline, Cincinnati Children's Hospital Medical Center; 2012. [Google Scholar]
- 15.National heart, lung and blood institute. Expert panel report 3 (EPR-3) Guidelines for the diagnosis and Management of Asthma; N Y: 2007. 08–4051. [Google Scholar]
- 16.Chalut D.S., Ducharme F.M., Davis G.M. The preschool Respiratory Assessment Measure (PRAM): a retrospective index of acute asthma severity. J Pediatr. 2000;137:762–768. doi: 10.1067/mpd.2000.110121. [DOI] [PubMed] [Google Scholar]
- 17.Alnaji F., Zemek R., Barrowman N., Plint A. PRAM score as predictor of pediatric asthma hospitalization. Acad Emerg Med. 2014;21:872–878. doi: 10.1111/acem.12422. [DOI] [PubMed] [Google Scholar]
- 18.Belessis Y., Dixon S., Thomsen A., Duffy B., Rawlinson W., Henry R. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol. 2004 Mar;37(3):201–209. doi: 10.1002/ppul.70105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelick M.H., Stevens M.W., Schultz T., Scribano P.V. Difficulty in obtaining peak expiratory flow measurements in children with acute asthma. Pediatr Emerg Care. 2004;20(1):22–26. doi: 10.1097/01.pec.0000106239.72265.16. [DOI] [PubMed] [Google Scholar]
- 20.Schneider W.V., Bulloch B., Wilkinson M., Garcia-Filion P., Keahey L., Hostetler M. Utility of portable spirometry in a pediatric emergency department in children with acute exacerbation of asthma. J Asthma. 2011 Apr 1;48(3):248–252. doi: 10.3109/02770903.2011.555036. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo G.J., Verde M.R., Peregalli V., Rodrigo C. Effects of short-term 28% and 100% oxygen on PaCO2 and peak expiratory flow rate in acute asthma: a randomized trial. Chest. 2003 Oct 1;124(4):1312–1317. doi: 10.1378/chest.124.4.1312. [DOI] [PubMed] [Google Scholar]
- 22.Geelhoed G.C., Landau L.I., Le Souef P.N. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23(6):1236–1241. doi: 10.1016/s0196-0644(94)70347-7. [DOI] [PubMed] [Google Scholar]
- 23.Indinnimeo L., Chiappini E. Del Giudice MM and the Italian Panel for the management of acute asthma attack in children. Ital J Pediatr. 2018;44:46. doi: 10.1186/s13052-018-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cates C.J., Welsh E.J., Rowe B.H. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD000052.pub3. CD000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camargo C.A., Spooner C.H., Rowe B.H. Continuous versus intermittent beta agonists for acute asthma. Cochrane Database Syst Rev. 2003;4 doi: 10.1002/14651858.CD001115. CD001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeronjanawong J., Manuyakorn W., Prapphal N., Harnruthakorn C., Sritippayawan S., Samransamruajkit R. Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler-spacer vs. jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39(5):466–472. doi: 10.1002/ppul.20204. [DOI] [PubMed] [Google Scholar]
- 27.Andrzejowski P., Carroll W. Salbutamol in paediatrics: pharmacology, prescribing and controversies. Arch Dis Child Educ Pract Ed. 2016;101:194–197. doi: 10.1136/archdischild-2014-307285. [DOI] [PubMed] [Google Scholar]
- 28.Mitselou N., Hedlin G., Hederos C.A. Spacers versus nebulizers in treatment of acute asthma - a prospective randomized study in preschool children. J Asthma. 2016;53:1059–1062. doi: 10.1080/02770903.2016.1185114. [DOI] [PubMed] [Google Scholar]
- 29.Travers A.H., Milan S.J., Jones A.P., Camargo C.A., Jr., Rowe B.H. Addition of intravenous beta (2)-agonists to inhaled beta (2)-agonists for acute asthma. Cochrane Database Syst Rev. 2012 Dec 12:12. doi: 10.1002/14651858.CD010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asthma and Respiratory Foundation NZ child and adolescent asthma guidelines. N Z Med J. 2017;130:1466. [PubMed] [Google Scholar]
- 31.Griffiths B., Ducharme F.M. Combined inhaled anticholinergics and short acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD000060.pub2. CD000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt E.L., Borland M.L., Doyle S.K., Geelhoed G.C. Metered-dose inhaler ipratropium bromide in moderate acute asthma in children: a single-blinded randomised controlled trial. J Paediatr Child Health. 2015 Feb;51(2):192–198. doi: 10.1111/jpc.12692. [DOI] [PubMed] [Google Scholar]
- 33.Vézina K., Chauhan B.F., Ducharme F.M. Inhaled anticholinergics and short acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD010283.pub2. CD010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigo G.J., Rodrigo C. The role of anticholinergics in acute asthma treatment: an evidence-based evaluation. Chest. 2002;121(6):1977–1987. doi: 10.1378/chest.121.6.1977. [DOI] [PubMed] [Google Scholar]
- 35.Teoh L., Cates C.J., Hurwitz M., Acworth J.P., van Asperen P., Chang A.B. Anticholinergic therapy for acute asthma in children. Cochrane Database Syst Rev. 2012;(4) doi: 10.1002/14651858.CD003797.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe B.H., Spooner C., Ducharme F., Bretzlaff J., Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1) doi: 10.1002/14651858.CD002178. [DOI] [PubMed] [Google Scholar]
- 37.Shefrin A.E., Goldman R.D. Use of dexamethasone and prednisone in acute asthma exacerbations in pediatric patients. Can Fam Physician. 2009 Jul 1;55(7):704–706. [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd M., Lasserson T.J., McKean M.C., Gibson P.G., Ducharme F.M., Haby M. Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database Syst Rev. 2009;15(2) doi: 10.1002/14651858.CD001290.pub2. CD001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camargo C.A., Jr., Rachelefsky G., Schatz M. Managing asthma exacerbations in the emergency department: summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. J Emerg Med. 2009;37(2l):6–17. doi: 10.1016/j.jemermed.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 40.Gordon S., Tompkins T., Dayan P.S. Randomized trial of single-dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. Pediatr Emerg Care. 2007;23:521. doi: 10.1097/PEC.0b013e318128f821. [DOI] [PubMed] [Google Scholar]
- 41.Cronin J.J., McCoy S., Kennedy U., an Fhailí S.N., Wakai A., Hayden J. A randomized trial of single-dose oral dexamethasone versus multidose prednisolone for acute exacerbations of asthma in children who attend the emergency department. Ann Emerg Med. 2016 May 1;67(5):593–601. doi: 10.1016/j.annemergmed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Keeney G.E., Gray M.P., Morrison A.K., Levas M.N., Kessler E.A., Hill G.D., Gorelick M.H., Jackson J.L. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014 Mar;133(3):493–499. doi: 10.1542/peds.2013-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paniagua N., Lopez R., Muñoz N. Randomized trial of dexamethasone versus prednisone for children with acute asthma exacerbations. J Pediatr. 2017;191:190. doi: 10.1016/j.jpeds.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Normansell R., Kew K.M., Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD011801.pub2. CD011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigo G., Rodrigo C. Inhaled flunisolide for acute severe asthma. Am J Respir Crit Care Med. 1998;157:698–703. doi: 10.1164/ajrccm.157.3.9704022. [DOI] [PubMed] [Google Scholar]
- 46.Schuh S., Dick P.T., Ftephens D. High-dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics. 2006;118(2):644. doi: 10.1542/peds.2005-2842. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigo G.J., Rodrigo C. Triple inhaled drug protocol for the treatment of acute severe asthma. Chest. 2003;123:1908–1915. doi: 10.1378/chest.123.6.1908. [DOI] [PubMed] [Google Scholar]
- 48.Upham B.D., Mollen C.J., Scarfone R.J., Seiden J., Chew A., Zorc J.J. Nebulized budesonide added to standard pediatric emergency department treatment of acute asthma: a randomized, double-blind trial. Acad Emerg Med. 2011 Jul;18(7):665–673. doi: 10.1111/j.1553-2712.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- 49.Alangari A.A., Malhis N., Mubasher M., Al-Ghamedi N., Al-Tannir M., Riaz M. Budesonide nebulization added to systemic prednisolone in the treatment of acute asthma in children: a double-blind, randomized, controlled trial. Chest. 2014 Apr 1;145(4):772–778. doi: 10.1378/chest.13-2298. [DOI] [PubMed] [Google Scholar]
- 50.McKeever T., Mortimer K., Wilson A., Walker S., Brightling C., Skeggs A. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med. 2018 Mar 8;378(10):902–910. doi: 10.1056/NEJMoa1714257. [DOI] [PubMed] [Google Scholar]
- 51.Jackson D.J., Bacharier L.B., Mauger D.T., Boehmer S., Beigelman A., Chmiel J.F., Fitzpatrick A.M., Gaffin J.M., Morgan W.J., Peters S.P., Phipatanakul W. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018 Mar 8;378(10):891–901. doi: 10.1056/NEJMoa1710988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nievas I.F., Anand K.J. Severe acute asthma exacerbation in children: a stepwise approach for escalating therapy in a pediatric intensive care unit. J Pediatr Pharmacol Ther. 2013;18(2):88–104. doi: 10.5863/1551-6776-18.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch B.A., Fenta Y., Jacobson R.M., Li X., Juhn Y.J. Impact of delay in asthma diagnosis on chest X-ray and antibiotic utilization by clinicians. J Asthma. 2012 Feb 1;49(1):23–28. doi: 10.3109/02770903.2011.637596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mobaireek K.F., Alshehri A., Alsadoun A., Alasmari A., Alashhab A., Alrumaih M. Hyperglycemia in children hospitalized with acute asthma. Adv Exp Med Biol. 2018;1070:19–25. doi: 10.1007/5584_2018_152. [DOI] [PubMed] [Google Scholar]
- 55.Rowe B.H., Bretzlaff J.A., Bourdon C., Bota G.W., Camargo C.A., Jr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD001490. CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowe B.H., Bretzlaff J.A., Bourdon C., Bota G.W., Camargo C.A., Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000;36(3):181. doi: 10.1067/mem.2000.105659. [DOI] [PubMed] [Google Scholar]
- 57.Elkady G.A., GabAllah R.R., Mansour A.Z. Magnesium in intensive care unit: a review. Egypt J Hosp Med. 2017;68(3):1497–1504. [Google Scholar]
- 58.Blitz M. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2005;3 doi: 10.1002/14651858.CD003898.pub3. CD003898. [DOI] [PubMed] [Google Scholar]
- 59.Hossein S., Pegah A., Davood F., Said A., Babak M., Mani M. The effect of nebulized magnesium sulfate in the treatment of moderate to severe asthma attacks: a randomized clinical trial. Am J Emerg Med. 2016;34(5):883–886. doi: 10.1016/j.ajem.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 60.Powell C., Dwan K., Milan S.J., Beasley R., Hughes R., Knopp-Sihota J.A. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12(12) doi: 10.1002/14651858.CD003898.pub5. CD003898. [DOI] [PubMed] [Google Scholar]
- 61.Powell C., Dona R.K., Lowe J., Boland A., Petrou S., Doull L. Magnesium sulfate in acute severe asthma in children (MAGNETIC): a randomized, placebo-controlled trial. Respir Med. 2013;1(4):301–308. doi: 10.1016/S2213-2600(13)70037-7. [DOI] [PubMed] [Google Scholar]
- 62.Powell C.V.E., Kolamunnage-Dona R., Lowe J., Boland A., Petrou S., Doull I. MAGNEsium Trial in Children (MAGNETIC): a randomized, placebo-controlled trial and economic evaluation of nebulized. Health Technol Assess. 2013;17(45):1–216. doi: 10.3310/hta17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knightly R., Milan S.J., Hughes R., Knopp-Sihota J.A., Rowe B.H., Normansell R. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2017;28(11) doi: 10.1002/14651858.CD003898.pub6. CD003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silverman R.A., Osborn H., Runge J., Gallagher E.J., Chiang W., Feldman J. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002 Aug 1;122(2):489–497. doi: 10.1378/chest.122.2.489. [DOI] [PubMed] [Google Scholar]
- 65.Cheuk D.K., Chau T.C., Lee S. A meta-analysis on intravenous magnesium sulfate for treating acute asthma. Arch Dis Child. 2005;90(1):74. doi: 10.1136/adc.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shan Z., Rong Y., Yang W., Wang D., Yao P., Xie J., Liu L. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321–330. doi: 10.1016/j.rmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths B., Kew K.M. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011050.pub2. CD011050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassan T., Gandhi A. 2012. Evidence review; what is the best second line treatment for acute severe asthma in children? Salbutamol, aminophylline or magnesium sulfate. [Google Scholar]
- 69.Magnesium sulphate in acute severe asthma in children Health Technology Assessment. NIHR J Libr. 2013;17(45):1–234. doi: 10.3310/hta17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohammed S., Goodacre S. Intravenous and nebulized magnesium sulfate for acute asthma: systematic review and metaanalysis. Emerg Med J. 2007;24(12):823–830. doi: 10.1136/emj.2007.052050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberts G., Newsom D., Gomez K., Raffles A., Saglani S., Begent J. Intravenous salbutamol bolus compared with an aminophylline infusion in children with severe asthma: a randomised controlled trial. Thorax. 2003 Apr 1;58(4):306–310. doi: 10.1136/thorax.58.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norfolk and Norwich University Hospitals . vol. 1. 2017. pp. 1–7. (Guideline for the use of IV salbutamol in severe asthma). [Google Scholar]
- 73.Sellers F.S. Inhaled and intravenous treatment in acute severe and life –threatening asthma. British J Anesth. 2013;110(2):183–190. doi: 10.1093/bja/aes444. [DOI] [PubMed] [Google Scholar]
- 74.Starkey E.S., Mulla H., Sammons H.M., Pandya H.C. Intravenous salbutamol for childhood asthma: evidence-based medicine. Arch Dis Child. 2014;99(9):873–877. doi: 10.1136/archdischild-2013-304467. [DOI] [PubMed] [Google Scholar]
- 75.Roberts G., Newsom D., Gomez K., Raffles A., Saglani S., Begent J. Intravenous salbutamol bolus compared with an aminophylline infusion in children with severe asthma: a randomized controlled trial. Thorax. 2003;58(4):306–310. doi: 10.1136/thorax.58.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooney L., McBride A., Lilley A., Sinha I., Johnson T.N., Hawcutt D.B. Using pharmacokinetic modelling to improve prescribing practices of intravenous aminophylline in childhood asthma exacerbations. Pulm Pharmacol Therapeut. 2017 Apr 1;43:6–11. doi: 10.1016/j.pupt.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Mitra A., Bassler D., Goodman K., Lasserson T.J., Ducharme F.M. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;18(2) doi: 10.1002/14651858.CD001276.pub2. CD001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.British Thoracic Society British guideline on the management of asthma. Thorax. 2014;69(1):1–192. [Google Scholar]
- 79.Paediatric Formulary Comittee . Pharmaceutical Press; London: 2014. BNF for children (BNFC) 2014–2015. [Google Scholar]
- 80.Neame M., Aragon O., Fernandes R.M., Sinha I. Salbutamol or aminophylline for acute severe asthma: how to choose which one, when and why? Arch Dis Child Educ Pract. 2015 Aug 1;100(4):215–222. doi: 10.1136/archdischild-2014-306186. [DOI] [PubMed] [Google Scholar]
- 81.Kim I.K., Phrampus E., Venkataraman S. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: a randomized, controlled trial. Pediatrics. 2005;116(5):1127–1133. doi: 10.1542/peds.2004-2136. [DOI] [PubMed] [Google Scholar]
- 82.Kim I.K., Phrampus E., Venkataraman S., Pitetti R., Saville A.L., Corcoran T. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: a randomized, controlled trial. Pediatrics. 2005 Nov 1;116(5):1127–1133. doi: 10.1542/peds.2004-2136. [DOI] [PubMed] [Google Scholar]
- 83.Wong J.J., Lee J.H., Turner D.A., Rehder K.J. A review of the use of adjunctive therapies in severe acute asthma exacerbation in critically ill children. Expert Rev Respir Med. 2014 Aug 1;8(4):423–441. doi: 10.1586/17476348.2014.915752. [DOI] [PubMed] [Google Scholar]
- 84.Rodrigo G.J., Castro-Rodriguez J.A. Heliox-driven β2-agonists nebulization for Children and adults with acute asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;112:29–34. doi: 10.1016/j.anai.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 85.Carroll C.L., Schramm C.M. Noninvasive positive pressure ventilation for the treatment of status asthmaticus in children. Ann Allergy Asthma Immunol. 2006;96(3):454–980. doi: 10.1016/S1081-1206(10)60913-1. [DOI] [PubMed] [Google Scholar]
- 86.Carson K.V., Usmani Z.A., Smith B.J. Noninvasive ventilation in acute severe asthma: current evidence and future perspectives. Curr Opin Pulm Med. 2014;20(1):118–123. doi: 10.1097/MCP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 87.Silva P.D., Barreto S.S. Noninvasive ventilation in status asthmaticus in children: levels of evidence. Revista Brasileira de Terapia Intensiva. 2015 Dec;27(4):390–396. doi: 10.5935/0103-507X.20150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marohn K., Panisello J.M. Noninvasive ventilation in pediatric intensive care. Curr Opin Pediatr. 2013;25(3):290–296. doi: 10.1097/MOP.0b013e328360dbdf. [DOI] [PubMed] [Google Scholar]
- 89.Basnet S., Mander G., Andoh J., Klaska H., Verhulst S., Koirala J. Safety, efficacy, and tolerability of early initiation of noninvasive positive pressure ventilation in pediatric patients admitted with status asthmaticus: a pilot study. Pediatr Crit Care Med. 2012;13(4):393–398. doi: 10.1097/PCC.0b013e318238b07a. [DOI] [PubMed] [Google Scholar]
- 90.Williams A.M., Abramo T.J., Shah M.V., Miller R.A., Burney-Jones C., Rooks S. Safety and clinical findings of BiPAP utilization in children 20 kg or less for asthma exacerbations. Intensive Care Med. 2011;37(8):1338–1343. doi: 10.1007/s00134-011-2238-9. [DOI] [PubMed] [Google Scholar]
- 91.Van Den Bosch G.E., Merkus P.J., Buysse C.M., Boehmer A.L., Vaessen-Verberne A.A., Van Veen L.N. Risk factors for pediatric intensive care admission in children with acute asthma. Respir Care. 2012 Sep 1;57(9):1391–1397. doi: 10.4187/respcare.01325. [DOI] [PubMed] [Google Scholar]
- 92.Belessis Y., Dixon S., Thomsen A., Duffy B., Rawlinson W., Henry R. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol. 2004;37(3):201. doi: 10.1002/ppul.70105. 20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brenner B., Corbridge T., Kazzi A. vol. 124. 2009. pp. S19–S28. (Intubation and mechanical ventilation of the asthmatic patient in respiratory failure). (2) [DOI] [PubMed] [Google Scholar]