Abstract
Importance of the field
Increased localization of Zn, Fe, Cu and Al within the senile plaques (SP) exacerbates amyloid beta (Aβ)-mediated oxidative damage, and acts as catalyst for Aβ aggregation in Alzheimer’s disease (AD). Thus, disruption of aberrant metal-peptide interactions via chelation therapy holds considerable promise as a rational therapeutic strategy against Alzheimer’s amyloid pathogenesis.
Areas covered in this review
The complexities of metal-induced genesis of SP are reviewed. The recent advances in the molecular mechanism of action of metal chelating agents are discussed with critical assessment of their potential to become drugs.
What the reader will gain
Taking into consideration the interaction of metals with the metal-responsive elements on the Alzheimer’s amyloid precursor protein (APP), readers will gain understanding of several points to bear in mind when developing a screening campaign for AD-therapeutics.
Take home message
A functional iron-responsive element (IRE) RNA stem loop in the 5′ untranslated region (UTR) of the APP transcript regulates neural APP translation. Desferrioxamine, clioquinol, tetrathiolmolybdate, dimercaptopropanol, VK-28 and natural antioxidants, such as curcumin and ginko biloba need critical evaluation as AD therapeutics. There is a necessity for novel screens (related to metallobiology) to identify therapeutics effective in AD.
Keywords: APP, Aβ, iron responsive element, metal chelators, screening
1. Introduction
The two predominant pathological features of Alzheimer’s disease (AD) are the extracellular amyloid plaques and intracellular neurofibrillary tangles in the brain. The abnormal processing of the amyloid precursor protein (APP) is the initiating event in AD pathogenesis, subsequently causing aggregation of amyloid beta (Aβ), specifically Aβ42. Formation of neuritic plaques instigates the formation of neurofibrillary tangles, composed of hyperphosphorylated microtubule-associated tau protein, and results in significant loss of neurons and synapses leading to cognitive impairment and dementia [1–4].
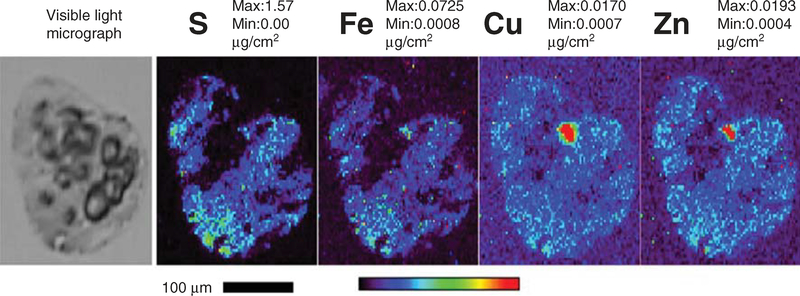
One of the physiologically relevant environmental factors able to affect the conformation of amyloidogenic proteins/peptides is metal ions. The ‘metal hypothesis’ of AD proposes that the interaction of APP and its proteolytic product ‘Aβ’ with specific metals drives Aβ-pathogenicity [5]. Increased concentrations of Fe, Cu and Zn [6] have been observed within the amyloid plaques (Figure 1) [7] that are released from glutamergic neurons at synapses [5]. The Fe and Zn levels have been reported to reach as high as 1 mM in the vicinity of amyloid plaques [8]. When compared with the surrounding tissue, levels of Zn, Cu, Fe and Ca inside the plaque were higher by 9.09 ± 0.20-fold, 11.72 ± 1.24-fold, 5.74 ± 3.31-fold and 98.40 ± 31.56-fold respectively [9]. Proton-induced X-ray emission, epifluorescence microscopy, immersion autometallography [10] and synchrotron X-ray fluorescence microprobes confirmed ‘hot spots’ of Fe, Cu, and Zn co-localized with Aβ in the rims and cores of the senile plaques [6,11,12]. Fe-rich Aβ plaques could be seen in AD-mouse brains in vivo through MRI and susceptibility-weighted MRI with significant increases in the basal ganglia cortex [13,14]. Furthermore, laser capture micro-dissection coupled with X-ray fluorescence microscopy could determine elemental profiles in Aβ amyloid plaques (Figure 1) [7].
Figure 1. Synchrotron X-ray fluorescence (XRF) microprobe images of human Alheimer’s disease AD plaque.
Elemental profiles (S, Fe, Cu, and Zn) in a typical Alzheimer’s amyloid beta (Aβ) amyloid plaque. The cryo-sectioned (10 μm thickness) AD brain tissues were stained with 0.1% Thioflavin-T for amyloid plaques. The amyloid plaque-bearing human brain tissues were procured by laser capture microdissection (LCM) (Arcturus Pixcell IIE platform) and mounted on Si3N4 membrane grids (2.0 × 2.0 mm). Guided by the optical amyloid plaque images, the samples were excited with incident synchrotron X-ray of 10 keV for elemental Kα characteristic emission lines. Elemental profiles (S, Fe, Cu, and Zn) were obtained using synchrotron scanning X-ray fluorescence microscopy (μ-XRF) at the Advanced Photon Source of the Argonne National Laboratory. Red depicts the hottest spot of the metals in plaques. (The significance of sulfur (S) element may reflect its high abundance in proteinaceous elemental composition and as an indicator for amyloid plaque-associated oxidative stress since protein S-glutathionylation is a salient feature of oxidative stress).
Reproduced from [7].
Metals play a key role in the aggregation of hyperphosphorylated tau into insoluble paired helical and straight filaments, which are involved in the pathogenesis of AD. Fe and Al were reported to accumulate in neurons within neurofibrillary tangles (NFTs) of AD brain [15], and, notably, tau pathology with hippocampal neurons was exacerbated in copper-exposed triple transgenic (Tg)-AD mice [16].
Thus chronic exposure to metal(s) (mainly Cu, Al, Fe and Zn) accelerates amyloid and tau pathology in AD, and trapping of these metals might be necessary to protect the brain from susceptible degeneration during AD.
2. Metals and AD
2.1. Fe-APP, Aβ and AD
A unique CAGA box, ‘amyloid’ (+83/+86), present only in the APP gene from amyloid plaque-forming species and absent in genes of APP-like-proteins (APLP1 and APLP2), plays a key role in APP gene regulation [17]. The functional iron responsive element (IRE)-Type II around the amyloid CAGA in the 146 nt-5′-untranslated region (UTR) of APP mRNA (+ 51 to + 94 from the 5′-cap site) [18] binds to the iron regulatory proteins (IRP1 and IRP2) and controls mRNA translation rate [8]. In normal human brain extracts, the IRP is detected as a double IRE-IRP complex but in two of six AD brain extracts a single IRE-IRP complex with decreased mobility was observed. Alterations in the IRP-IRE interaction possibly through elevated endogenous RNase activity could be the site in the AD brains at which Fe mismanagement occurs and the Fe regulatory system becomes dysfunctional [19].
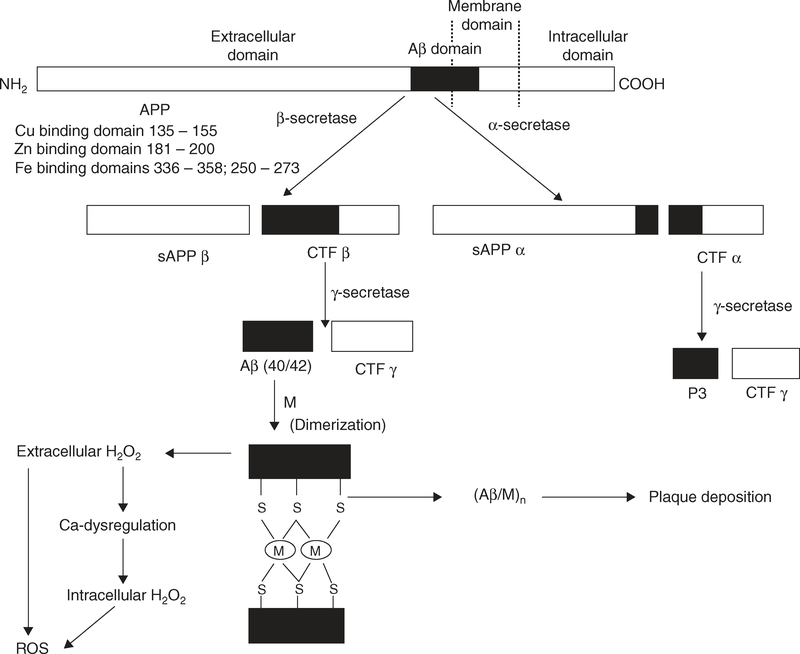
Fe enhances amyloidogenicity through different mechanisms. Firstly, Fe downregulates furin protein levels that promote non-amyloidogenic α-secretase activity [20]. Secondly or possibly most commonly Fe participates in the production of the reactive oxygen species (ROS) (Figure 2) that upregulate inhibition of matrix metalloprotease and shift aconitase to the IRP1 form.
Figure 2. The proteolytic processing of amyloid precursor protein (APP) to produce Aβ that coordinates the metal ions (M: Zn, Cu and Fe) to induce aggregation, and generation of ROS.
APP (695, 751, 770 amino acid isoforms that predominate in brain) can be processed in the plasma membrane as it travels from the intracellular origin to extracellular matrix through the non-amyloidogenic route that involves cleavage at the α-secretase site at amino acid 17 of the 40 – 42 amino acid Aβ domain resulting in two fragments, sAPPα and a C-terminal fragment (CTFα). Further proteolysis of the CTFα fragment by α-secretase generates the non-amyloidogenic peptide p3 and a C-terminal fragment CTFγ. When APP escapes processing at the α-site, it undergoes β-secretase cleavage at the beginning of Aβ domain, resulting in a C-terminal fragment CTFβ and sAPPβ. Next, the resultant β-stub becomes the substrate for γ-secretase cleavage, culminating in extracellular Aβ secretion. The hyper-metallated (by Zn, Fe and Cu) state of Aβ as a consequence of age-dependent elevations in tissue metal concentrations can induce Aβ aggregation. H2O2 can initiate a number of oxidative events, including Fenton reactions to form toxic hydroxyl radicals and calcium dysregulation, and subsequent reactive oxygen species (ROS) generation.
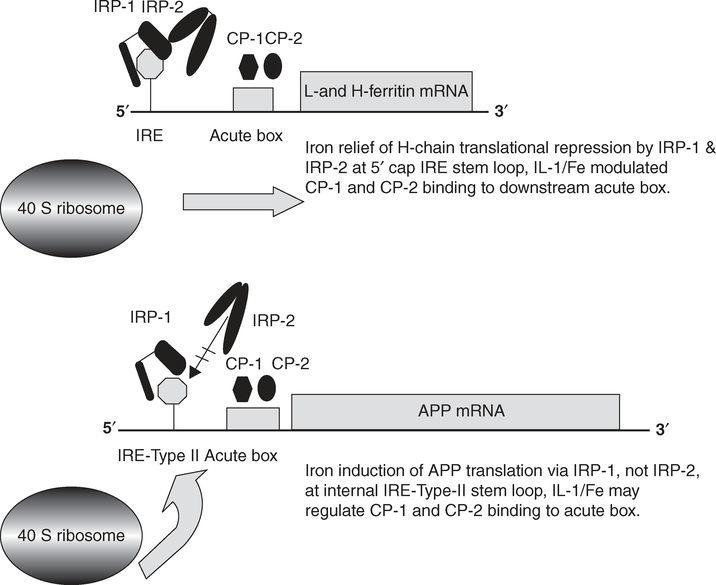
When Fe is scarce, the IRP1 binds to IREs that regulates translation or stability of IRE-mRNAs (Figure 3), whereas when Fe is abundant IRP1 forms a [4Fe–4S] cluster and is converted from an RNA-binding form to an aconitase [21]. Unlike IRP1, IRP2 is degraded by the proteasome by a process involving Fe-catalyzed oxidation. IRP-2 colocalizes with redox-active Fe, and change in IRP-2 has been reported to be directly linked to impaired Fe homeostasis in AD [22]. Such changes could modulate APP mRNA translation and APP synthesis in astrocytes [23] and neurons to significantly reduce expression of the neuroprotective secreted APP (APP(s)) [24]. In terms of AD pathology, the APP–IRE–RNA secondary structure might be disrupted in the presence of an adjacent 5′UTR-specific single nucleotide polymorphism that could be genetically linked to increased risk for spontaneous AD [25].
Figure 3. Model for the iron-induced change of iron regulatory protein (IRP) interaction with the APP/ferritin iron-responsive element (IRE) to modulate APP/ferritin translation.
The APP IRE is homologous with the canonical L-and H-ferritin IRE mRNA stem-loop that binds the iron regulatory proteins (IRP1 and IRP2), and modulates translation of ferritin to control intracellular iron homoeostasis [226]. Iron influx increases ferritin mRNA translation by releasing IRP1–IRP2 binding to the 5′ cap site of IRE stem–loop. The iron-induced change of IRP1 interaction with the APP-IRE activates either 5′cap translation or internal 40S ribosome entry and the onset of APP protein synthesis [227]. The IRE of APP interacts with IRP1, whereas the canonical H-ferritin IRE RNA stem-loop binds to IRP2 in neural cell lines, in human brain cortex tissue and human blood lysates. The canonical H-ferritin IRE RNA stem-loop binds also to IRP2. The APP mRNA acute box domain, as for H-ferritin mRNA, is located immediately upstream of the start codon and may well also interact with RNA poly(C)-binding proteins, CP-1 and CP-2 and control cytokine-induced APP mRNA translation.
A changed IRP-IRE binding modulates expression of the Fe-storage protein (Figure 3), transferrin and melanotransferrin in brain white matter [26–28] in a way that parallels the pathological lesions in AD [29,30]. Furthermore, genetic evidence implicates synergy between the C282Y allele of the hemochromatosis gene (HFE) and the C2 allele of transferrin as risk factors for developing AD [31]. It has also been reported that although each of the HFE variants alone had relatively little effect on Fe status, the combination of either HFE C282Y and HFE H63D or of HFE C282Y and transferrin C2 markedly raised transferrin saturation in those without dementia, but had little effect in those with mature AD [32].
The physiological relevance of Fe to Aβ is demonstrated by their concurrent accumulation in Aβ deposits in AD [29,33] as well as in transgenic mouse models [34]. Fe-binding to Aβ enhances its aggregation and facilitates the oxidative damage in the immediate vicinity of the senile plaques [35–37]. Dysfunction of ferritin with ferroxidase activity [38], particularly abundant in myelinated axons and oligodendrocyte processes, results in an increase of toxic brain Fe2+ ions. In support of this, null ferritin heterozygous mutants mice of mixed C57BL6/J × 129SvEv genetic background have also been found to develop oxidative features in the cortex that are reminiscent of AD and Parkinson’ disease (PD) [39].
In AD pathobiology, intracellular Aβ forms a complex with free heme that results in functional heme deficiency [40]. Regulation of cerebral heme biosynthesis is profoundly altered in AD and may contribute towards disease pathogenesis by affecting cell metabolism as well as Fe homeostasis [41]. A significant decrease in loosely bound Fe has been reported in the hippocampal white matter of mild–moderate and severe AD patients with a trend towards increased non-heme iron in the hippocampal gray matter of severe AD [42]. The AD brain has been observed to have 2.5-fold more heme-b and 26% less heme-a compared with controls, with a significant 2.9-fold decrease in the heme-a:heme-b ratio [43]. When the rate of production of Aβ exceeds the capacity of heme synthesis or there is an increase in free metals, Aβ reacts with free metals and forms aggregates [43]. The Aβ-heme complex, being a peroxidase, catalyzes the oxidation of serotonin and 3, 4-dihydroxyphenylalanine by H2O2 that leads to oxidative damage to macromolecules and depletes specific neurotransmitters. The intracellular oxidative stress-induction of the astroglial and neuronal hemoxygenase-1 gene through a ‘common pathway’ leads to pathological brain Fe2+ deposition. This further causes enhanced free radical generation [44], mitochondrial insufficiency, enhanced cytochrome c oxidase activity [45] and H2O2-generation that releases free radical hydroxyl (OH) via the Fenton reaction: Fe+2 + H2O2 → Fe+3 + OH− + OH or by virtue of hypervalent iron compounds, as observed in vitro [46,47]. Oxidative stress triggers activation and/or translocation of NF-κB, p53 and c-Jun transcription factors resulting in enhanced apoptosis [48]. This is accompanied by DNA damage, blood–brain barrier (BBB) disruption [49] and age-related myelin breakdown [50,51]. The toxicity of Aβ is mediated, at least in part via redox active Fe that precipitates lipid peroxidation and cellular oxidative stress [52].
2.2. Zn and AD
In AD, recurrent episodes of focal high Zn release are observed from the presynaptic vesicles to the postsynaptic neurons of the neocortex, hippocampus and amygdale in a Ca2+-and depolarization-dependent fashion [53]. This indicates prior injury with toxic ‘floods’ of free Zn in the brain as a major risk factor in AD-development [54]. A decreased concentration of astrocytic growth inhibitory factor that chelates Zn [55] causes the highest concentration of Zn in the hippocampus. This is associated with increased extracellular Zn metalloproteinase activities [56] due to cholinergic deafferentation of the hippocampus [57]. The sequestration of Zn in Aβ-Zn complexes further leads to reduced Zn availability at synaptic terminals and consequent loss of Zn modulatory activity at excitatory synapses [58].
The Zn transporter proteins (ZNTs, ZnT2 – 8), abundantly expressed in Aβ plaque, and cerebral amyloid angiopathic vesicles [59,60] induce an abnormality in the uptake or distribution of Zn in the AD brain [61]. The ZnT immunoreactions were detected in the amyloid plaques and amyloid angiopathic vessels of brains of APPswe/PS1dE9 transgenic mice. ZnT1 and ZnT4 are extensively expressed in all parts of the plaques. ZnT3, ZnT5, and ZnT6 are expressed most prominently in the degenerating neurites in the peripheral part of the plaques, while ZnT7 is present in the core of the plaques. The amyloid angiopathic vessels showed a strong ZnT3 immunoreactivity [59]. The disruption in neuronal Zn homeostasis triggers ZnT1 expression that caused increased efflux of Zn ions in the extracellular space. This might involve altered homeostasis of metallothionein and other Zn-binding proteins such as α2 macroglobulin, or pro-inflammatory cytokine polymorphism [62]. ZnT2 – ZnT8 are localized in the intracellular membranes and transport Zn ions into different intracellular compartments when the intercellular level of Zn ions is raised [63]. The synaptic ZnT3 activity promotes cerebral amyloid angiopathy by indirectly increasing exchangeable Zn concentrations in the perivascular spaces of the brain [59,64,65]. The increase in synaptic Zn correlates well with higher levels of insoluble Aβ and plaque loads in aging females, and these sex differences completely disappear in ZnT3−/− mice, suggesting the role for synaptic Zn in the sex differences in AD [65].
Zn interaction may play an important, evolutionary conserved role in APP function and metabolism. The sequence of APP harboring the Zn binding site (181 – 200 amino acids) is evolutionarily conserved with the coordination sites as Glu-185, Cys-188 and Cys-189, and a saturation binding KA of 750 nmol/l [66]. The obligatory motif for the ectodomain Zn binding region on APP is a novel sequence for a Zn binding site, GVEFVCCP that is highly conserved in APLP l and APLP2, as well as in the Drosophila and Caenorhabditis elegans APP-like protein [67]. The Zn binding domain may play a role in regulating the adhesiveness of APP through its control over the Kunitz-type protease inhibitory insert and heparin binding affinity of APP695 [66,68]. Disturbed homeostasis of extracellular Zn2+ in AD may interfere with the normal binding of APP to heparin-like molecules such as heparan sulfate moiety of proteoglycans (known to alter protein conformation, and the clearance and processing of bound proteins) [66]. It also modulates the binding of APP to extracellular matrix components such as laminin [69] that controls crucial cell–cell and cell–matrix interactions [70].
Indeed, Zn-binding may also influence APP processing and has been found to specifically inhibit the α-secretase cleavage of APP [71]. Exogenous Zn enhances the synthesis of presenilin in a dose-dependent manner. This further elevates γ-secretase activity that could result in increased production of Aβ and the formation of more senile plaques, which in turn could trap more Zn [72]. The Zn-dependent transcription factors NF-κB and specific protein-1 (Sp1) bind to the promoter region of the APP gene, and also inhibit enzymes that degrade APP to non-amyloidogenic peptides and degrade the soluble form of Aβ [73]. A reduction in Zn-stimulated protein tyrosine kinase activities in AD hippocampus indicates a possible connection of neuronal protein tyrosine kinase activity loss to severe memory and intellectual impairment that is characteristic of AD [74].
Human Aβ specifically and saturably manifests high-affinity (Ka =107 nM) binding [75] (concomitant with Zn-induced Aβ aggregation) with Zn in less than a millisecond [76] at a Zn2+: Aβ stoichiometry of 1:1 [77], and induces tinctorial amyloid formation [61]. A low-affinity binding with the second Zn ion at 2:1 stoichiometry seems to have a moderate effect on peptide conformation [77]. The 1 – 16 region represents a possible initiation site for the entire Aβ transconformation, and the minimal Aβ fragment required for Zn binding [77]. The Zn is coordinated i) at the N-terminal hydrophilic region of Aβ with the imidazole side chain of His6, His13, His14 [78–80], ii) with Asp1 either with the N-terminus or/and the carboxylate group and iii) with the carboxylate side chain of Glu11 [80–82]. The dissociation constant (Kd) of Zn for the fragment Aβ-peptide 1 – 28 (measured by fluorescence study) and that for Aβ-peptide 1 – 40 (through NMR) had values in the μM range at pH 7.2 and 286 K. Zn also has a second, weaker binding site involving residues between 23 and 28 [79]. Moreover, the apparent dissociation constant (Kd, app) of Zn binding to all forms of Aβ (soluble Aβ, Aβ fibrils, or Zn-induced Aβ aggregates) is in the low μM range (1 – 20 μM) [80,83]. Isomerization of Asp7 or the substitution of Asp7 with Asn (in Tottori–Japan mutation with higher susceptibility of spontaneous conversion to iso-aspartate) in Aβ promotes Zn-induced oligomerization of the Aβ–Zn binding domain [84]. The Zn-Aβ binding prevents the formation of the typical amyloid fibrils, the inhibitory effect (IC50 =1.8 μM) being three times stronger than that for Cu(II). It further induces the accumulation of large unorganized aggregates of smaller non-fibrillar pathogenic forms of Aβ [76,85]. The promotion of Aβ28 aggregation by Zn is based on the transformation of the partially α-helical conformer (intermediate) towards the Aβ-sheet amyloid [80] structure by destabilization of the α-helix in the intermediate [86].
With regard to the tau-protein-mediated AD pathology, the Zn-binding protein S100β has been identified as an interacting partner with tau. S100β-tau binding, promoted by Zn, may represent a key pathway for neurite development, possibly through S100β modulation of tau phosphorylation and/or functional stabilization of microtubules and process formation [87]. Interestingly, S100β-tau interaction may be disrupted by hyperphosphorylation and/or imbalances in Zn metabolism and this may contribute to the neurite dystrophy associated with AD. Zn has also been reported to enhance tau binding as an important factor in the internalization of S100β [87].
2.3. Cu and AD
The possibility that Cu may contribute to AD pathology is suggested by the ceruloplasmin fragmentation that indicates improper Cu transport, together with the ‘free’ Cu rise in AD [88]. Cu2+ levels in the brain increase with age in transgenic Tg2576 mice [89], and Cu along with ROS homeostasis are compromised in AD patients [6,89–93]. Even a small increase in the serum free Cu can be of significance, particularly over a long period of time [94]. The free Cu can cross the BBB in living patients and supply the brain with a continuous flux of noxious redox Cu. Cu2+ levels in the AD neuropil are 400% higher than in the neuropil of a healthy brain [6], and oxidative damage [95] involving Fenton cycling is the probable source of ROS [96].
Neurotoxicity of Aβ with Cu-induced dityrosine crosslinking of Aβ 1 – 28, Aβ 1 – 40, and Aβ 1 – 42 [97] has been linked to H2O2 production [98], with the generation of highly toxic hydroxyl radical species [99]. The oxidative coupling is initiated by interaction of H2O2 with a Cu2+ tyrosinate that induces Aβ aggregation under mildly acidic conditions (e.g., pH 6.8 – 7.0) [100].
Hyperlipidemia is a significant risk factor of an interaction between free copper and Aβ [94,101], and Cu alters the structure of lipid rafts through flotillin-2 lipid raft association that inhibits APP endocytosis. The primary products of lipid peroxidation are phospholipid hydroperoxides that are degraded by free Cu2+ in the presence of ascorbic acid to yield hydroxy-2-nonenal, a toxic factor in the pathogenesis of AD [102]. It has also been seen that total cellular Cu2+ is associated inversely with lipid raft Cu2+ levels, so that under intracellular Cu2+ deficiency Aβ–Cu complexes are more likely to form [103]. Diffusible Aβ oligomers concentrate Cu2+ in a toxic redox-active state at the membrane that in turn causes further oxidative stress and upregulation of Aβ. The Aβ accumulation causes altered kinase and phosphatase activities leading to neurofibrillar tangles of tau protein and dementia [104].
APP has many of the features of a Cu transporter, and the Cu2+ binding site at residues 135 – 155 at a Kd APP of approximately 10 nM can promote the reduction of bound Cu2+ leading to increased oxidative stress in neurons [105,106]. The APP-Cu binding domain consists of an α-helix (residues 147 – 159) packed against a triple-stranded β-sheet (residues 133 – 139, 162 – 167 and 181 – 188) through Cy- rich disulfide bonds [107]. The metal ligands are His147, His151, Tyr168 and two water molecules that are arranged in a square pyramidal geometry [108]. There is a clear link between APP processing and Cu2+ homoeostasis in the brain with the 24-residue peptide of the C-terminal domain of β-secretase 1 (BACE1) binding with high affinity to a single Cu1+ atom through Cys residues. Overproduction of BACE1 reduces superoxide dismutase (SOD)1 activity in cells through the interaction of cytoplasmic domain of APP with the Cu2+ chaperone for SOD 1 [109]. Perturbations to APP metabolism and in particular, its secretion or release from neurons may alter Cu homeostasis [110] resulting in increased Aβ accumulation and free radical generation [111].
It is known that monomeric Aβ peptides bind Cu(II) ions in vitro [112], with concomitant acceleration of Aβ aggregation and precipitation [100,113,114]. The oxidative damage to Aβ amino acid side chains was profound in tests performed in vitro, and some of the modified chains, for example, 2-oxo-His, were also found in amyloid plaques [115].
Cu may participate in oxidative stress through redoxcycling between its + 2 and + 1 oxidation states to generate ROS that is governed by the binding mode. All of the ligands of the high affinity Cu(II) site are contained in the Aβ 16 N-terminal domain [112]. Two complexes (components I, at lower pH and component II, at higher pH), distinguishable by conventional 9 GHz electron paramagnetic resonance (EPR) spectroscopy are present near physiological pH values. Using pulse EPR techniques such as electron spin echo envelope modulation and hyperfine sublevel correlation spectroscopy (HYSCORE) Shin and Saxena proposed three His bound at pH 7.4 [116]. On the other hand Drew et al. proposed the binding of two His and Asp1 by Aβ16 N terminus and side chain carboxylate group at pH 6.3 – 6.9, and of three His and the carbonyl group of Ala2 at pH 8 [117,118]. Dorlet et al. further used a wide range of advanced EPR techniques in conjunction with specific isotopic labeling that were able to directly detect and thus identify the ligands of the Cu (II) ion coordinated to Aβ16 in the component I and component II forms. They proposed that in component I, the two His, the NH2 terminus, and the carbonyl group from Asp1 composed the equatorial coordination plane, while the side-chain carboxylate group of Asp1 occupied an axial position. In component II, the equatorial ligands were the NH2 terminus, the amide and carbonyl groups of Ala2 (due to deprotonation of the amide nitrogen atom of A2 upon pH increase), and one His. The side-chain carboxylate group of Asp1 was in an apical position [119]. The component I of Dorlet et al. [119] was in close agreement with Drew et al. [117,118] but component II differed significantly.
X-ray absorption (XAS) and EPR spectroscopy revealed that Cu(I) is ligated by the imidazole group of His13 and His14 in a linear coordination environment in Aβ and Cu-mediated oxidative damage of Aβ occurs over multiple redox cycles [120,121]. X-ray absorption fine structure spectroscopy and CO-binding studies demonstrated the preference of Cu I ions for two-coordinate geometry in binding to Aβ through a contiguous His13 – His14 motif [122]. Further, NMR studies revealed that structure is retained, even in the presence of three His residues (His6, His13, His14) [121] and additional potential donors (Tyr10, Asp7, Glu11, Ser8, backbone carbonyl O, amide N) [122].
The Cu interacts with Aβ to form simple reversible 1:1 complex [123], and at times a 2:1 complex [124]. At the Cu2+/peptide molar ratios > 0.3 Aβ coordinates a second Cu2+ atom in a highly cooperative manner [125]. But EPR spectroscopy indicated that both Cu have axial, Type II coordination geometry, square-planar or square-pyramidal, with nitrogen and oxygen ligands [124]. The EPR parameters are consistent with a Type 2 Cu2+ center with three nitrogen donor atoms and one oxygen donor atom in the coordination sphere of Cu2+, and this coordination in retained during organization of Aβ monomers into fibrils [126].
The rate constant for the reaction of superoxide with Cu2+-Aβ has been found to be much slower than that with SOD, and the His residues of Aβ 1 – 42 control the redox activity of transition metals present in senile plaques [127]. The addition of Cu2+ to Aβ in a negatively charged lipid environment caused a conformational change from β-sheet to α-helix, accompanied by peptide oligomerization and membrane penetration [128]. Buffer-independent conditional Kd for Cu(II)-αβ40 complex at pH 7.4 is equal to 0.035 μmol/l [129]. The dissociation constant of the Cu(αβ) complexes ranges from μM to pM values [113], with a preference for the region between 100 pM to 1 nM [79,80].
The modulation of oxidative stress related to Cu-dysfunction may also be one of the mechanisms that make apolipoprotein E4 gene a risk factor for AD [130–132].
2.4. Al and AD
High consumption of Al from drinking water has been epidemiologically reported to be a risk factor for AD [133–135]. Al neurotoxicity as a factor for AD onset or AD-like pathology has been observed in Al-dust exposed workers [136]. Although the role of Al in the etiology of AD remains controversial, energy-dispersive X-ray spectroscopy combined with transmission electron microscopy has detected Al colocalized with Aβ peptides in the cores of SP located in the hippocampus and the temporal lobe that facilitates iron-mediated oxidative reactions [137]. Aβ peptide (1 – 42) and Al have been found to induce helical transitions in supercoiled DNA, as a first step to AD neuropathology [138]. It further disturbs heme metabolism [139], perturbs neuronal [Ca2+]i homeostasis and mitochondrial respiration with enhanced Aβ accumulation and neurodegenerative damage [140].
A study in Tg2576 mice indicated that the Al-dietary supplementation increased soluble and insoluble Aβ levels and the levels of an isoprostane marker of oxidative stress in the hippocampus and iso-cortex [141]. This situation was associated with increased total number of proliferating neuronal cells in the dentate gyrus of hippocampus compatible with an accelerated neurodegeneration [142] and significant Al deposit in the cortex [143]. Al has also been suggested to interact with plasmin proteases involved in the degradation of Aβ and promotion of α-cleavage of the APP [144].
The NFTs observed in human neurons always developed in conjunction with cytoplasmic Al, suggesting that Al played an important role in their formation [145]. Al in brain bulk was observed to be colocalized with NFTs [146] probably as aluminosilicates [147]. In rat and mouse neurons Al was found to be accompanied by transition of both phosphorylated and unphosphorylated form of NFT peptides from α-helix to β-pleated sheet [148,149]. The NFTs were prone to dissolution by desferrioxamine (DFO) or EDTA [150].
3. Metal chelators as therapeutic agents
Disruption of aberrant metal–peptide interactions via metal chelation could play a crucial role in overcoming Alzheimer’s amyloid pathogenesis. The strategy of using chelators to block intracellular APP expression, Aβ fibrilization and Aβ-dependent metal oxidative stress-induced neurotoxicity could well produce a major new therapeutic effect on AD progression.
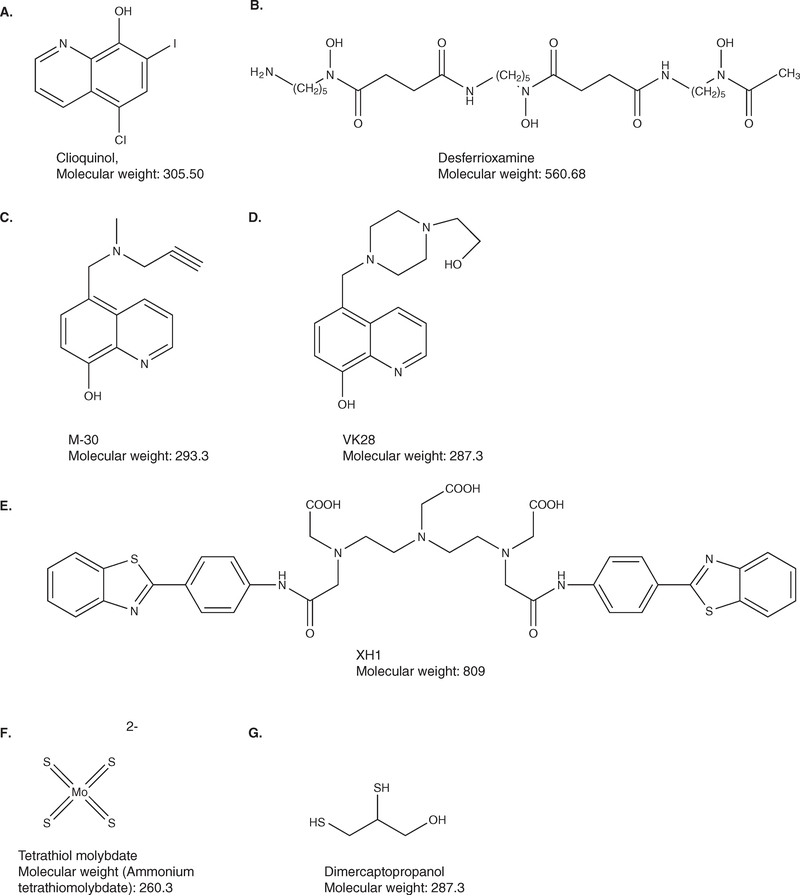
3.1. 5-chloro-7-iodo-8-hydroxyquinoline (CQ)
CQ (Figure 4A) has selective affinities for Cu and Zn and form relatively stable complexes [151]. Being hydrophobic CQ molecule crosses the BBB and inhibits homeostatic defects of brain metal ion metabolism in APP transgenics it redistributes Cu from plaques (as observed through NMR spectroscopy) to the cells, disaggregates the metal-ion-induced aggregates of Ap1 – 40 through metal chelation, retards the fibril growth along with Zn2+ [152] and dissolves Aβ with about 30 times greater efficiency and speed [153] than Aβ vaccine therapy [154]. CQ might work by a combined action that facilitates disaggregation of the Zn-mediated Aβ collections [100,155], while also inhibiting Cu- or Fe-mediated H2O2 production with increased Aβ clearance [96,156]. A paradoxical increase in Cu and Zn in CQ-treated APP2576 mice might be explained by CQ preventing Cu2+ and Zn2+ from sequestering with extracellular Aβ, and then diverting metal ions for uptake into metal-ion-deficient brain tissue [157]. The resolubilized Aβ may either be removed into the blood or degraded by intracellular uptake and hydrolysis. As observed in Caenorhabditis elegans and in mice, CQ chelates mitochondrial enzyme clock abnormal protein 1 that results in slowing down of the aging process in AD [158].
Figure 4. Important metal-chelators characterized as suppressors of APP and Aβ aggregation.
Chemical structures of A. Clioquinol, which inhibits Zn, Cu- or Fe-mediated oxidative stress and reduces clinically observed AD-induced cognition; B. Desferrioxamine, intracellular Fe3+ chelator that suppresses APP translation without changing α-secretase activity; C & D M-30 and VK-28. M-30 being derived from a prototype iron chelator, VK-28; both being developed for anti-amyloid efficacy and α-secreatase co-activation; E. Bifunctional XH1: [(4-benzothiazol-2-yl-phenylcarbamoyl)-methyl]-{2-[(2-{[(4-benzothiazol-2-yl-phenylcarbamoyl)methyl]-carboxymethyl-amino}-ethyl)-carboxymethyl-amino]-ethyl}-amino)-acetic acid, with amyloidtargeting metal chelating property; F. Tetrathiomolybdate, which shows excellent efficacy in animal AD models and is presently under clinical trial. G. Dimercaptopropanol, which has a significant effect on Aβ metabolism in vitro and/or in vivo.
CQ has been shown to reduce APP translation and Aβ production in vitro with reduced APP-5′UTR levels in APP Tg2576 [159]. Oral treatment with CQ caused 49% reduction in cerebral Aβ deposition and approximately 30 times greater reduction in absolute Aβ in APP 2576 Tg mice with its dissociation (1.45% elevated) into soluble form of Aβ [156] without affecting APP processing. It also showed a decrease in the amyloid plaque surface area without appreciable effects on weight loss or mean survival, and improved motor activity, alertness and general health. CQ was effective in liberating Aβ from postmortem brain samples of AD patients and may be the first credible drug candidate based on the amyloid hypothesis of AD [157]. A Phase II double-blind clinical trial on the effects of oral CQ has shown reduced cognitive decline and decreased plasma Aβ42 levels in moderate to severe AD patients [160].
In silico techniques that incorporate chelating properties into well-known intercalation compounds have designed new multifunctional agents for application in AD. The combination of main features of thioflavin-T, with strong affinity for fibrillar amyloid proteins, and CQ led to the three compounds 2-(2-hydroxyphenyl) benzoxazole, 2-(2-hydroxyphenyl)-benzothiazole, and 2-(2-aminophenyl)-1H-benzimidazole. They increased lipophilicity and suitability for crossing the BBB, obeyed Lipinski’s rules for pharmacokinetic properties, enhanced antioxidant properties and increased affinity for Aβ fibrils [161].
The second-generation 8-hydroxy quinoline analogue PBT2 is found to be superior to and a safer ionophore than CQ or CQ-PBT1 [162]. It promoted the transport and clearance of Cu, Zn and Fe across cell membranes, and prevented formation of Aβ aggregates through enhanced soluble Aβ formation. It also showed increased activities of the matrix metalloproteases such as neprilysin, insulin degrading enzyme and tissue plasminogen activator. PBT2 accelerated the degradation of soluble interstitial Aβ and reduced AD-like neuropathology and cognitive dysfunction in Tg models of AD [163]. AD patients (78 in number) who were treated with PBT2 (250 mg) in a Phase IIa double-blind, randomized and placebo-controlled trials showed a dose-dependent reduction in cerebrospinal fluid level of Aβ42 [164]. They demonstrated improvement in two executive function component tests of the neuropsychological test battery and in the executive factor z-scores without any serious adverse events [164,165].
DP-109, a diester derivative of BAPTA (1,2-bis(2-aminophenyloxy) ethane-N,N,N′,N′-tetra acetic acid), selectively chelated transition metals such as Zn, Cu and Fe within membrane compartments [166]. DP-109 could be similar to CQ in attenuating Aβ plaque deposition, inflammation and neuronal damage in hippocampus. It reduced cerebral amyloid angiopathy and increased soluble:insoluble Aβ40:42 ratio. DP-109 effectively crossed the BBB neuronal and vesicular membranes to quench free Zn in synaptic vesicles to reach the brain of Tg2576 mice. However, with DP-109, possible chelating effects on other metals cannot be ignored [166].
1-(benzimidazole-2-ylmethyl)-1,4,7-triazacyclononane and 1,4-bis(benzimidazole-2-ylmethyl)-1,4,7-triazacyclonone), quite similar to CQ exhibited radical-scavenging potential with metal-protein-attenuating ability. The imidazole in the both compounds held sites that could be modified for preventing Aβ aggregation [167].
However, in contrast to previous studies, it has also been observed that CQ promoted the in vitro aggregation/fibrillogenesis of human Aβ in the presence of Cu and Zn with decreased viability in neuroblastoma cells [168]. There was an increased lethality of APP transgenics upon CQ treatment, which could be rescued by a co-treatment with Cu, confirming toxicity of CQ. Moreover, the exposure to Cu with CQ led to a modest but significant increase in cerebral Cu levels, most probably due to enhanced transport of CQ–Cu complexes with a secondary role as a chelator. Most conspicuously, until 1975 there were 10,000 cases of subacute myelo-optic neuropathy (SMON) associated with CQ administration. SMON resembled an accelerated form of sub acute combined degeneration due to vitamin B12 deficiency [156,169,170].
3.2. DFO
DFO (Figure 4B) has become the mainstay of iron- and Al-chelating therapy [171] (later on the beneficial effect was suggested to be due to the chelating of Cu and Zn also [172]) with probable inhibition of free radical formation and inflammation [173]. DFO either prevented the formation of β-pleated amyloidal fibrils or it initiated the dissolution of febrile amyloidal plaques [150]. In a clinical trial that involved the treatment of AD patients, the administration of DFO led to a significant reduction in the rate of decline of daily living skills as assessed by both group means and variances (p < 0.04) [171].
DFO is non-oral, rapidly metabolized and relatively unstable molecule, and is administered via painful intramuscular injections [172]. It is a highly costly drug with the need for parenteral administration. Systemic metal ion depletion (anemia) as a side effect of DFO are important reasons to search for an orally effective, cheaper and less toxic chelating agent than DFO [172].
3.3. Bifunctional metal chelators
New oral Fe chelators, 5-[N-methyl-N-propargylaminomethyl]-8-hydroxyquinoline (M-30) (Figure 4C) and the 8-hydroxyquinoline derivative, VK28 (Figure 4D), are being developed for the treatment of AD and other neurodegenerative diseases [174,175]. The neuroprotective activity of propargylamines led to the development of several novel bifunctional iron chelators, (5-[4-propargylpiperazin-1-ylmethyl]-8-hydroxyquinoline) HLA-20, M30 and M30A from the prototype brain-permeable iron chelator, VK-28. The chelators had cholinesterase and monoamine oxidase B inhibitory activities and retained the in vitro and in vivo neuroprotective activity of rasagiline. The N-propargyl moiety of the anti-PD drugs rasagiline (Azilect, Teva) and selegiline have been found to be the most effective that exerted Fe chelation potency, served as radical scavengers and inhibited Fe-induced membrane lipid peroxidation features [176].
Fe chelators of low molecular weight, minimal toxicity and satisfactory lipophilicity have added a new facet in the etiology of AD therapy. Fe-binding drugs may also stabilize hypoxia-inducible factor that in turn would transactivate the expression of established protective genes, including VEGF, erythropoietin, aldolase and p21.
The metal-complexing bifunctional molecule, XH1 (Figure 4E), ([(4-benzothiazol-2-yl-phenylcarbamoyl)-methyl]-{2-[(2-{[(4-benzothiazol-2-yl-phenylcarbamoyl)methyl]-carboxymethyl- amino}-ethyl)-carboxymethyl-amino]-ethyl}-amino)-acetic acid, which crosses the BBB and holds the potential of being an amyloid-targeting metal chelator for AD treatment [177] contains two identical amyloid-binding and one metal-chelating moiety that specifically targets amyloid. It i) binds to Aβ1 – 40 peptide putatively, ii) decreases Zn(II)-induced Aβ aggregation in vitro, iii) specifically reduces APP protein expression in human SH-SY5Y neuroblastoma cells and iv) attenuates cerebral Aβ amyloid pathology in PS1/APP transgenic mice without inducing apparent toxicity and behavior disturbances [177].
Due to the attached carbohydrate moiety responsible for increased tissue specificity at a physiological pH, and due to the phenolic moieties as suitable antioxidants the tetrahydrosalens such as (N,N′-bis[(5-β-D-glucopyranosyloxy-2-hydroxy)benzyl]-N, N′-dimethyl-ethane-1,2-diamine (H2GL1) and N,N′-bis[(5-β-D-glucopyranosyloxy-3-tert-butyl-2-hydroxy)benzyl]-N,N′-dimethyl-ethane-1, 2-diamine (H2GL2)) were developed [178]. Both H2GL1 and H2GL2 were found to reduce Zn-and Cu-induced aβ1 – 40 aggregation in vitro, with a higher affinity for Cu over Zn with H2GL1 displaying better coordinating ability at physiological pH [179]. The moderate affinity of H2GL1 and 2 for metal ions at physiological pH might obviate the toxicity commonly associated with chelating therapy [179].
3-hydroxy-4-pyridinones that contain phenol groups for antioxidant functionality are further elaborated with pendant glucose moieties for improved BBB targeting. Glycosidase removal of the carbohydrate substituents gives ligands that are ready to passivate excess metal ions, especially Cu and Zn, in the brain [180,181].
Curcumin and other polyphenols are anti-inflammatory and antioxidant agents, and structurally interfere with Aβ aggregation and metal dyshomeostasis [182]. The use of synthetic activity to rescue protein-aggregate-mediated cellular toxicity led to the synthesis of apocyclen attached to selective Aβ recognition motifs (KLVFF or curcumin). The resultant complexes interfere with Aβ aggregation and degrade Aβ into fragments, preventing H2O2 formation and toxicity in neuronal cell culture [183].
3.4. Al chelators
Tacrine, ascorbate plus Feralex-G have also been found to be particularly effective in removing Al(III) from the nuclear matrix in addition to enhancing cholinergic transmission [184]. Feralex-G disaggregated compacted paired helical filaments isolated from aged human brain [185]. Feralex could also dissociate binding of Al and Fe with hyperphosphorylated Tau of AD [185]. Simultaneous administration of two Al chelators, DFO and tacrine as a palliative treatment for AD patients has also been taken into consideration. Administration of N-(2-hydroxyethyl) EDTA, a potential antidote for Al overload, in combination with citric acid has been proposed as a chelation therapy for AD [186].
3.5. Nanoparticle chelator delivery
Chelator-delivery with the help of nanoparticles has been suggested to be a significantly improved method of chelation therapy with higher efficacy, reduced toxicity and substantial tissue-specific targeting. Covalent conjugates of Fe chelators with nanoparticles, such as prototype nanoparticle–chelator conjugate, could attenuate the lipophilic character of the chelator. They provided better BBB permeability and safe treatment with higher chelator bioavailability in AD, without affecting metal binding ability of chelators. The nanoparticle–chelator conjugates could effectively inhibit Aβ aggregate formation and, thereby, protect human brain cells from Aβ-related toxicity. Using in vitro studies, it was shown that chelator–nanoparticle system complexed with Fe. When incubated with human plasma it preferentially adsorbed apolipoprotein E and apolipoprotein A-I that would facilitate transport of chelators and chelator–metal complexes in both directions across the BBB. The system thus provided safer and more effective chelation treatment in AD and other neurodegenerative diseases [187–190].
4. Targeting neuronal signalling
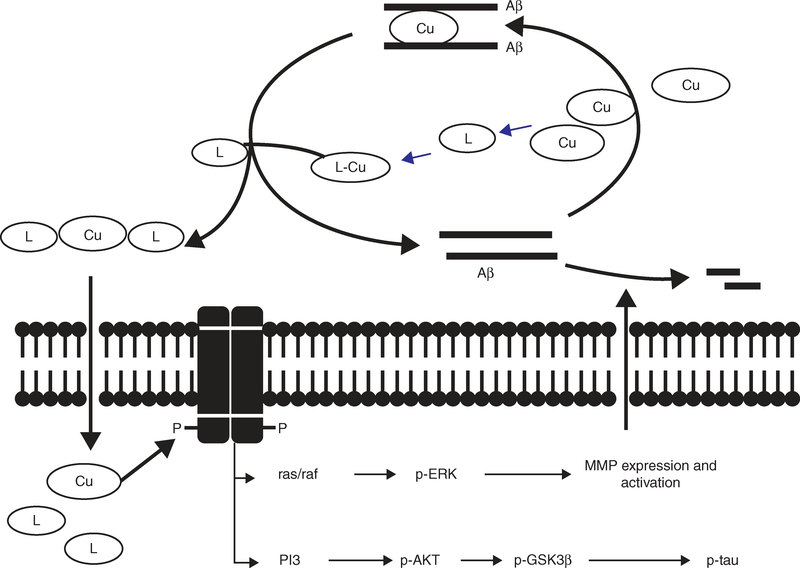
A novel neurotherapeutic approach involved the activation of neuronal cell signaling mechanisms using metallo-complexes. The metal ligand-Cu complexes as with CQ or PBT2 or alternative metal-complexes such as Cu/Zn-bis(thiosemicarbazone) (Figure 5A) complex entered brain cells and upregulated MMP. This involved activation of ERK signaling to cleave the monomeric Aβ (Figure 6) [90]. In another situation, inhibition of PI3K and C-JNK prevented depletion of Aβ by the metallo-complexes [191].
Figure 5. Metallo-complexes that target neuronal signaling.
Chemical structures of A. Cu-bis(thiosemicarbazone) that reduces tau phosphorylation through PI3K and ras/raf signalling; B. Lipoic acid that chelates metal ion and promotes pro-survival signaling pathways.
Figure 6. Proposed metal ligand action that targets neuronal cell signaling in treatment of AD.
Metal-free ligands (L) such as CQ or PBT2 may bind with Cu of the Aβ peptide-Cu complex resulting in dissolution of Aβ into Cu-free monomers. The metal ligand–Cu complexes or alternative metal complexes such as Cu-bis(thiosemicarbazone) then enter cells, activate PI3K followed by sequential phosphorylation of AKT and glycogen synthase kinase beta (GSK3β) that inhibits tau phosphorylation [228]. The complex-mediated activation of ras/raf signalling activates ERK, upregulates MMP activity, which cleaves the monomeric Aβ.
Adapted from [90].
Lipoic acid (LA) (Figure 5B) or dihydrolipoic acid (DHLA) exhibited the ability to chelate metal ion with the disulfide group of dithiolane ring [192]. They reduced liposomal peroxidation [193] and activated the pro-survival PI3K and ERK1/2 signaling pathways. LA/DHLA protected cultured hippocampal neurons against Aβ and Fe/H2O2 [194] and improved learning-memory in a Tg2576 mouse model for AD [195,196].
5. Natural antioxidants
Curcumin (Figure 7A) molecules chelated cations with the diketone and pairs of phenol and methoxy groups, reduced oxidative stress and prevented amyloid aggregation. Its two molecules bind the Cu and Fe on Aβ at Kd1 ~ 10 – 60 μM and Kd2 ~ 1.3 μM (for binding of the first and second curcumin molecules, respectively) [197,198]. But the antioxidants like curcumin and ginkgo (common name kaempferol) (Figure 7B) extracts fail to reach the target site in sufficient amounts during oxidative stress, and hence are best as prophylactics to complex the metals before the stress cascade starts [199].
Figure 7. Important metal-chelators characterized as natural antioxdants for AD.
Chemical structures of A. curcumin, a polyphenol that binds Fe and Cu on Aβ and prevents amyloid aggregation; B. Ginko biloba, inhibits a free radical scavenger that reduces clinically observed AD-induced cognition; C. (−)-epigallocatechin-3-gallate (EGCG) decreased Aβ levels and plaques via promotion of α-secretase activity.
Human epidemiology confirms that tea extract contains nutrients endowed with possible prospective neurobiological-pharmacological actions. They are beneficial to human health due to the natural antioxidant, catechin polyphenol constituent (–)-epigallocatechin-3-gallate (EGCG) (Figure 7C) [200]. EGCG targets APP 5′UTR and decreases Aβ levels [169] and plaques in the cingulate cortex, hippocampus, and entorhinal cortex. It was found to promote non-amyloidogenic α-secretase proteolytic pathway in ‘Swedish’ mutant APPsw and in Tg2576 mice with a disintegrin and metalloprotease (ADAM)-10 activation. EGCG administration also markedly suppressed sarkosyl-soluble phosphorylated tau isoforms with significant behavioral improvements [201,202].
Moderate consumption of wine and increased intake of fruits and vegetables is associated with a lower incidence of AD [203–205]. The antioxidant, resveratrol (a bioactive compound in red wine), through the protein kinase pathway could lower Aβ peptide formation, and promote Aβ degradation in 15-week-old male APP/PS1 transgenic mice (B6C3-Tg (APPswe, PSEN1dE9)85Dbo/J), The Jackson Laboratory [206].
Antioxidants, such as α-tocopherol (Vitamin E) and ascorbic acid had modest benefits in elderly subjects [207]. Gossypin (3,3′,4′,5,7,8-hexahydroxyflavone 8-glucoside) [208] and ginko biloba [209] protected cortical cell cultures from Aβ-induced toxicity. They exhibited neuroprotective effects in several mouse models and maintained and improved cognitive function in AD patients [208]. It was found that 6-hydroxy melatonin administration proved successful in reducing Fe2+-induced neurotoxicity, lipid peroxidation and necrotic cell damage in the rat hippocampus in vivo [210].
A pilot study examining the efficacy of the Cu-chelating agent D-penicillamine (treatment for Wilson’s disease) in AD patients showed decrease in oxidative damage with depletion of bio-available Cu and increased excretion of Cu in the urine [211]. But, the role of D-penicillamine in antioxidant balance remains inconclusive, possibly due to the action of vitamin B6 that was administered to placebo- and D-penicillamine-treated patients [212]. Further studies with larger cohorts of AD patients are needed to elucidate the real therapeutic efficacy of D-penicillamine.
A relatively new concept deals with possibility of using Zn compounds (used for Wilson’s disease treatment [213]) that could induce or maintain a state of Cu-malabsorption. Successful clinical trials with Zn could lead to safe, inexpensive and effective biological anti-Cu agent in AD [214,215].
6. Expert opinion
Over the last few decades, researchers have provided a wealth of information on the underlying nature of AD therapy. Although the amyloid-based pathogenic mechanisms that result in the onset and progression of AD are yet to be clearly understood, there are several growing bodies of evidence to support a central role for bio-metals in many critical aspects of the illness. The APP and Aβ still remain the current focus of AD research that is associated with their integral function in iron metabolism and homeostasis of metal stores in the brain. The deposition of metals in the plaque of AD patients and the demonstration of metal dependent translation of APP 5′UTR mRNA have distinctly indicated the involvement of metals in amyloid-associated characteristc pathological feature of AD. However, further research is necessary to fully understand the complex and interdependent pathways of biometal homeostasis and amyloid metabolism in AD.
In this review we have provided an update on the development of potential therapeutic agents for AD based on the modulation of metal bioavailability. The compounds could target the metal binding sites on APP and Aβ, deprive the biological systems of metal ions, or promote metal uptake into cells, and thus inhibit Aβ:metal-mediated redox activity.
The metal chelating drug, CQ, was well tolerated and did appear to produce some modest benefits in AD patients that could support the proof of concept in humans that drugtargeting metal-Aβ interactions can have a significant beneficial effect on the progression of AD. But the study only involved a small number of patients and required further observations in larger groups of patients.
The metal chelating drugs DFO (Figure 4B), tetrathiolmolybdate (Figure 4F), and dimercaptopropanol (Figure 4G) [47,216] have shown a significant effect on Aβ metabolism in vitro and/or in vivo. A transfection-based screen of a library of FDA drugs to identify compounds that limited APP luciferase reporter expression translated from the APP 5′UTR in neuroblastoma cells (SY5Y) cells identified the leads, paroxetine (Paxil™) and dimercaptopropanol. The compound limited Aβ peptide secretion from lens epithelial cells (B3 cells), the former probably through chelation of or change in distribution of interacellular iron [217]. Tetrathiolmolybdate (Cu2+ chelator), dimercaptopropanol (Pb2+ and Hg2+ chelator) [217] and XH1 (Figure 4E), with Aβ-binding and metal-chelating moieties [177], suppressed APP holoprotein expression and Aβ secretion; tetrathiolmolybdate also showed excellent efficacy in animal models [218]. In addition to these compounds, phenserine, which is a novel and highly selective acetylcholinesterase inhibitor, is being tested for the treatment of AD [219]. Phenserine was extremely efficient in blocking translation under conditions of intracellular Fe chelation with DFO, suggesting that the anticholinesterase operated through an Fe-dependent pathway at the APP 5′-UTR site. The FDA-preapproved drugs had the major advantage of being pharmacologically fully characterized, with respect to the toxicity, half-life, capacity for oral administration, and capacity to cross the BBB.
However, a major problem associated with the widespread clinical use of the available metal-complexing agents is their poor target specificity and consequent clinical safety. The long-term use of these agents is likely to perturb the homeostasis of many biometals and normal physiological functions of essential metal-requiring biomolecules. Thus, the development of metal passivating agents with necessary water solubility, efficacy, minimum toxicity and specific targeting is essential for new effective therapies for AD.
New screening approaches targeting APP 5′UTR could thus be very useful in identifying novel metal-complexing agents from world-wide drug libraries. We have performed such a high-throughput screen (HTS) of 110,000 compounds obtained from the library of the Laboratory for Drug Discovery on Neurodegeneration that yielded several non-toxic specific inhibitors of APP mRNA 5′UTR-driven luciferase in the stable SH-SY5Y transfectants [220]. The identified compounds could be expected to hold therapeutic promise as metal chelators at least for those retaining long-term bio-activity. Use of transgenic models of AD could be the next and required step for testing our APP-directed compound hits. We could test the efficacy of lead APP 5′UTR-directed drugs to limit amyloid burden in CRND8 mice wherein the human APP-695 transgene (London/Swedish double mutations in APP-695 cDNA) is expressed under the transcriptional control of the prion protein gene promoter and the translational control of the natural APP 5′UTR [221].
It has been observed that intracellular modifiers of levels of Zn [159] and Fe [222] adjust α-secretase to limit Aβ peptide. Thus, the screening of chemical compounds or antioxidants that could selectively target and promote metal-dependent catalysis of ADAM-protease and the decrease in Aβ levels could serve as a strategy for identifying anti-AD drugs [220,223,224]. Signaling events involved in the non-amyloidogenic, metalloprotease-α-secreatse activity could be the site of pharmacological intervention in AD. Activators of PKC, adenylate cyclase/protein kinase A system, phoslpholipase C (PLC) and the MAPK-signaling system could also claim to be potential and new anti-amyloid agents [225].
The selective expression of human metal-binding protein biomarker S100A7 in the brain of transgenic mice resulted in significant promotion of α-secretase activity. Furthermore, the promotion of S100A7 expression in the brain selectively promoted α-secretase activity in the brain of AD [225]. Thus upregulating S100A7 in the brain or cerebrospinal fluid could be developed as another strategy for promoting of ‘non-amyloidogenic’ α-secretase/ADAM-10 mediated responses.
Although development of drugs that target abnormal metal accumulation of Aβ is in process, we still need to explore new screening approaches and technologies to identify novel therapeutics that could promote neuroprotective signalling pathways in AD with minimum adverse effects.
Article highlights.
Metal dependent amyloid formation: Amyloid precursor protein (APP) is a Cu-, Zn- and Fe- binding protein, and these metals clearly provide one of the pathological requirements for polymerization of amyloid-beta (Aβ) peptide. Fe (perhaps Cu/Zn) controls iron-responsive element (IRE)-iron regulatory protein (IRP) binding to APP mRNA to reset and increase its translation rate thus worsening metal-associated pathological lesions in Alheimer’s disease (AD). Al is also a long-known risk factor for AD.
Chelation therapy: Metal chelation serves as a therapeutic strategy for treatment of AD. Clioquinol inhibits binding of Cu and Zn to Aβ and promotes Aβ clearance. Desferrioxamine (DFO) has been shown to be therapeutic after intramuscular injection. A new range of bifunctional metal chelators (M-30, VK-28, HLA-20, XH1, curcumin and polyphenols) are being assessed for AD treatment. Simultaneous administration of two chelators is being taken into consideration. Nanoparticle chelator delivery should significantly improve the efficacy and reduce the toxicity of chelation therapy.
Neuroprotective signaling: A promising future lies in the use of metallo-complexes to trigger neurotherapeutic signaling pathways with subsequent inhibition of tau phosphorylation and Aβ generation.
Anti-oxidants: Curcumin, kaempferol, epigallocatechin-3-gallate (EGCG), resveratrol, vitamin E and D-penicillamine are beneficial to counteract the toxicity of the redox interaction between metals and Aβ peptide.
Future directions: The challenge of a transfection-based screening approach will be to find new drugs that suppress APP holoprotein translation and thereby limit Aβ peptide. Use of transgenic models of AD is the next and required step for testing the novel APP directed lead compounds.
This box summarizes key points contained in the article.
Acknowledgments
We thank A Rai (Senior Research Fellow, Indian Institute of Toxicology Research) for helping in making Figures 1 and 6.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.Grill JD, Cummings JL. Current therapeutic targets for the treatment of Alzheimer’s disease. Expert Rev Neurother 2010;10:711–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Strooper B Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 2010;90:465–94 [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–6 [DOI] [PubMed] [Google Scholar]
- 4.Bettens K, Sleegers K, Van Broeckhoven C. Current status on Alzheimer disease molecular genetics: from past, to present, to future. Hum Mol Genet 2010;19(R1):R4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008;5:421–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovell MA, Robertson JD, Teesdale WJ, et al. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 1998;158:47–52 [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Huang W, Moir RD, et al. Metal exposure and Alzheimer’s pathogenesis. J Struct Biol 2006;155:45–51 [DOI] [PubMed] [Google Scholar]
- 8.Rogers JT, Bush AI, Cho HH, et al. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem Soc Trans 2008;36:1282–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leskovjan AC, Lanzirotti A, Miller LM. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer’s disease. Neuroimage 2009;47:1215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoltenberg M, Bruhn M, Sondergaard C, et al. Immersion autometallographic tracing of zinc ions in Alzheimer beta-amyloid plaques. Histochem Cell Biol 2005;123:605–11 [DOI] [PubMed] [Google Scholar]
- 11.Danscher G, Jensen KB, Frederickson CJ, et al. Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J Neurosci Methods 1997;76:53–9 [DOI] [PubMed] [Google Scholar]
- 12.Miller LM, Wang Q, Telivala TP, et al. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J Struct Biol 2006;155:30–7 [DOI] [PubMed] [Google Scholar]
- 13.McCrea RP, Harder SL, Martin M, et al. A comparison of rapid-scanning X-ray fluorescence mapping and magnetic resonance imaging to localize brain iron distribution. Eur J Radiol 2008;68:S109–13 [DOI] [PubMed] [Google Scholar]
- 14.Bartzokis G, Sultzer D, Mintz J, et al. In vivo evaluation of brain iron in Alzheimer’s disease and normal subjects using MRI. Biol Psychiatry 1994;35:480–7 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto A, Shin RW, Hasegawa K, et al. Iron (III) induces aggregation of hyperphosphorylated τ and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 2002;82:1137–47 [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa M, Cheng D, Laferla FM. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J Neurochem 2009;108:1550–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahiri DK, Ge YW, Maloney B. Characterization of the APP proximal promoter and 5′-untranslated regions: identification of cell type-specific domains and implications in APP gene expression and Alzheimer’s disease. FASEB J 2005;19:653–5 [DOI] [PubMed] [Google Scholar]
- 18.Maloney B, Ge YW, Greig N, Lahiri DK. Presence of a “CAGA box” in the APP gene unique to amyloid plaque-forming species and absent in all APLP-1/2 genes: implications in Alzheimer’s disease. FASEB J 2004;18:1288–90 [DOI] [PubMed] [Google Scholar]
- 19.Bandyopadhyay S, Huang X, Cho H, et al. Metal specificity of an iron-responsive element in Alzheimer’s APP mRNA 5′untranslated region, tolerance of SH-SY5Y and H4 neural cells to desferrioxamine, clioquinol, VK-28, and a piperazine chelator. J Neural Transm Suppl 2006;71:237–47 [DOI] [PubMed] [Google Scholar]
- 20.Silvestri L, Camaschella C. A potential pathogenetic role of iron in Alzheimer’s disease. J Cell Mol Med 2008;12:1548–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourley BL, Parker SB, Jones BJ, et al. Cytosolic aconitase and ferritin are regulated by iron in Caenorhabditis elegans. J Biol Chem 2003;278:3227–34 [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Wehr K, Harris PL, et al. Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res 1998;788:232–6 [DOI] [PubMed] [Google Scholar]
- 23.Rogers JT, Leiter LM, McPhee J, et al. Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J Biol Chem 1999;274:6421–31 [DOI] [PubMed] [Google Scholar]
- 24.Rogers JT, Randall JD, Cahill CM, et al. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 2002;277:45518–28 [DOI] [PubMed] [Google Scholar]
- 25.Athan ES, Lee JH, Arriaga A, et al. Polymorphisms in the promoter of the human APP gene: functional evaluation and allele frequencies in Alzheimer disease. Arch Neurol 2002;59:1793–9 [DOI] [PubMed] [Google Scholar]
- 26.Jefferies WA, Food MR, Gabathuler R, et al. Reactive microglia specifically associated with amyloid plaques in Alzheimer’s disease brain tissue express melanotransferrin. Brain Res 1996;712:122–6 [DOI] [PubMed] [Google Scholar]
- 27.Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res 1992;31:75–83 [DOI] [PubMed] [Google Scholar]
- 28.Grundke-Iqbal I, Fleming J, Tung YC, et al. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol 1990;81:105–10 [DOI] [PubMed] [Google Scholar]
- 29.Sayre LM, Perry G, Harris PL, et al. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J Neurochem 2000;74:270–9 [DOI] [PubMed] [Google Scholar]
- 30.Pinero DJ, Hu J, Connor JR. Alterations in the interaction between iron regulatory proteins and their iron responsive element in normal and Alzheimer’s diseased brains. Cell Mol Biol (Noisy-le-grand) 2000;46:761–76 [PubMed] [Google Scholar]
- 31.Robson KJ, Lehmann DJ, Wimhurst VL, et al. Synergy between the C2 allele of transferrin and the C282Y allele of the haemochromatosis gene (HFE) as risk factors for developing Alzheimer’s disease. J Med Genet 2004;41:261–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann DJ, Worwood M, Ellis R, et al. Iron genes, iron load and risk of Alzheimer’s disease. J Med Genet 2006;43(10):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 1997;94:9866–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MA, Hirai K, Hsiao K, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 1998;70:2212–5 [DOI] [PubMed] [Google Scholar]
- 35.Jiang D, Li X, Williams R, et al. Ternary complexes of iron, amyloid-beta, and nitrilotriacetic acid: binding affinities, redox properties, and relevance to iron-induced oxidative stress in Alzheimer’s disease. Biochemistry 2009;48:7939–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan A, Dobson JP, Exley C. Redox cycling of iron by Abeta42. Free Radic Biol Med 2006;40:557–69 [DOI] [PubMed] [Google Scholar]
- 37.Exley C. Aluminium and iron, but neither copper nor zinc, are key to the precipitation of beta-sheets of Abeta42. in senile plaque cores in Alzheimer’s disease. J Alzheimers Dis 2006;10:173–7 [DOI] [PubMed] [Google Scholar]
- 38.Collingwood JF, Chong RK, Kasama T, et al. Three-dimensional tomographic imaging and characterization of iron compounds within Alzheimer’s plaque core material. J Alzheimers Dis 2008;14:235–45 [DOI] [PubMed] [Google Scholar]
- 39.Thompson K, Menzies S, Muckenthaler M, et al. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 2003;71:46–63 [DOI] [PubMed] [Google Scholar]
- 40.Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci USA 2006;103:3381–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwyer BE, Smith MA, Richardson SL, et al. Down-regulation of aminolevulinate synthase, the rate-limiting enzyme for heme biosynthesis in Alzheimer’s disease. Neurosci Lett 2009;460:180–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magaki S, Raghavan R, Mueller C, et al. Iron, copper, and iron regulatory protein 2 in Alzheimer’s disease and related dementias. Neurosci Lett 2007;418:72–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atamna H, Frey WH II. A role for heme in Alzheimer’s disease: heme binds amyloid beta and has altered metabolism. Proc Natl Acad Sci USA 2004;101:11153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schipper HM, Gupta A, Szarek WA. Suppression of Glial HO-1 activitiy as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res 2009 published online October 1 2009. doi: 10.2174/156720509789207985 [DOI] [PubMed] [Google Scholar]
- 45.Perry G, Taddeo MA, Petersen RB, et al. Adventiously-bound redox active iron and copper are at the center of oxidative damage in Alzheimer disease. Biometals 2003;16:77–81 [DOI] [PubMed] [Google Scholar]
- 46.Schubert D, Chevion M. The role of iron in beta amyloid toxicity. Biochem Biophys Res Commun 1995;216:702–7 [DOI] [PubMed] [Google Scholar]
- 47.Rogers JT, Lahiri DK. Metal and inflammatory targets for Alzheimer’s disease. Curr Drug Targets 2004;5:535–51 [DOI] [PubMed] [Google Scholar]
- 48.Velez-Pardo C, Ospina GG, Jimenez del Rio M. Abeta[25–35] peptide and iron promote apoptosis in lymphocytes by an oxidative stress mechanism: involvement of H2O2, caspase-3, NF-kappaB, p53 and c-Jun. Neurotoxicology 2002;23:351–65 [DOI] [PubMed] [Google Scholar]
- 49.Ghribi O, Golovko MY, Larsen B, et al. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem 2006;99:438–49 [DOI] [PubMed] [Google Scholar]
- 50.Quintana C, Bellefqih S, Laval JY, et al. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol 2006;153:42–54 [DOI] [PubMed] [Google Scholar]
- 51.Bartzokis G Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 2004;25:5–18; author reply 49–62 [DOI] [PubMed] [Google Scholar]
- 52.Rottkamp CA, Raina AK, Zhu X, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med 2001;30:447–50 [DOI] [PubMed] [Google Scholar]
- 53.Suh SW, Jensen KB, Jensen MS, et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res 2000;852:274–8 [DOI] [PubMed] [Google Scholar]
- 54.Frederickson CJ, Cuajungco MP. Is zinc the link between compromises of brain perfusion (excitotoxicity) and Alzheimer’s disease? J Alzheimers Dis 2005;8:155–60; discussion 209–215 [DOI] [PubMed] [Google Scholar]
- 55.Uchida Y, Takio K, Titani K, et al. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 1991;7:337–47 [DOI] [PubMed] [Google Scholar]
- 56.Backstrom JR, Miller CA, Tokes ZA. Characterization of neutral proteinases from Alzheimer-affected and control brain specimens: identification of calcium-dependent metalloproteinases from the hippocampus. J Neurochem 1992;58:983–92 [DOI] [PubMed] [Google Scholar]
- 57.Stewart GR, Frederickson CJ, Howell GA, Gage FH. Cholinergic denervation-induced increase of chelatable zinc in mossy-fiber region of the hippocampal formation. Brain Res 1984;290:43–51 [DOI] [PubMed] [Google Scholar]
- 58.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci 2005;6:449–62 [DOI] [PubMed] [Google Scholar]
- 59.Zhang LH, Wang X, Zheng ZH, et al. Altered expression and distribution of zinc transporters in APP/PS1 transgenic mouse brain. Neurobiol Aging 2010;31:74–87 [DOI] [PubMed] [Google Scholar]
- 60.Zhang LH, Wang X, Stoltenberg M, et al. Abundant expression of zinc transporters in the amyloid plaques of Alzheimer’s disease brain. Brain Res Bull 2008;77:55–60 [DOI] [PubMed] [Google Scholar]
- 61.Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994;265:1464–7 [DOI] [PubMed] [Google Scholar]
- 62.Mocchegiani E, Costarelli L, Giacconi R, et al. Zinc homeostasis in aging: two elusive faces of the same “metal”. Rejuvenation Res 2006;9:351–4 [DOI] [PubMed] [Google Scholar]
- 63.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci 2004;61:49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedlich AL, Lee JY, van Groen T, et al. Neuronal zinc exchange with the blood vessel wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer’s disease. J Neurosci 2004;24:3453–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JY, Cole TB, Palmiter RD, et al. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci USA 2002;99:7705–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bush AI, Multhaup G, Moir RD, et al. A novel zinc(II) binding site modulates the function of the betaA4 amyloid protein precursor of Alzheimer’s disease. J Biol Chem 1993;268:16109–12 [PubMed] [Google Scholar]
- 67.Bush AI, Pettingell WH Jr, de Paradis M, et al. The amyloid beta-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. J Biol Chem 1994;269:26618–21 [PubMed] [Google Scholar]
- 68.Multhaup G, Mechler H, Masters CL. Characterization of the high affinity heparin binding site of the Alzheimer’s disease betaA4 amyloid precursor protein (APP) and its enhancement by zinc(II). J Mol Recognit 1995;8:247–57 [DOI] [PubMed] [Google Scholar]
- 69.Narindrasorasak S, Lowery DE, Altman RA, et al. Characterization of high affinity binding between laminin and Alzheimer’s disease amyloid precursor proteins. Lab Invest 1992;67:643–52 [PubMed] [Google Scholar]
- 70.Huang X, Cuajungco MP, Atwood CS, et al. Alzheimer’s disease, beta-amyloid protein and zinc. J Nutr 2000;130(5S Suppl):l488S–l492S [DOI] [PubMed] [Google Scholar]
- 71.Roberts SB, Ripellino JA, Ingalls KM, et al. Non-amyloidogenic cleavage of the beta-amyloid precursor protein by an integral membrane metalloendopeptidase. J Biol Chem 1994;269:3111–16 [PubMed] [Google Scholar]
- 72.Park IH, Jung MW, Mori H, Mook-Jung I. Zinc enhances synthesis of presenilin 1 in mouse primary cortical culture. Biochem Biophys Res Commun 2001;285:680–8 [DOI] [PubMed] [Google Scholar]
- 73.Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis 1997;4:137–69 [DOI] [PubMed] [Google Scholar]
- 74.Vener AV, Aksenova MV, Burbaeva G. Drastic reduction of the zinc- and magnesium-stimulated protein tyrosine kinase activities in Alzheimer’s disease hippocampus. FEBS Lett 1993;328:6–8 [DOI] [PubMed] [Google Scholar]
- 75.Bush AI, Pettingell WH Jr, Paradis MD, Tanzi RE. Modulation of Abeta adhesiveness and secretase site cleavage by zinc. J Biol Chem 1994;269:12152–8 [PubMed] [Google Scholar]
- 76.Noy D, Solomonov I, Sinkevich O, et al. Zinc-amyloid beta interactions on a millisecond time-scale stabilize non-fibrillar Alzheimer-related species. J Am Chem Soc 2008;130:1376–83 [DOI] [PubMed] [Google Scholar]
- 77.Kozin SA, Zirah S, Rebuffat S, et al. Zinc binding to Alzheimer’s Abeta(1–16) peptide results in stable soluble complex. Biochem Biophys Res Commun 2001;285:959–64 [DOI] [PubMed] [Google Scholar]
- 78.Smith DP, Smith DG, Curtain CC, et al. Copper-mediated amyloid-beta toxicity is associated with an intermolecular histidine bridge. J Biol Chem 2006;281:15145–54 [DOI] [PubMed] [Google Scholar]
- 79.Danielsson J, Pierattelli R, Banci L, Graslund A. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid beta-peptide. FEBS J 2007;274:46–59 [DOI] [PubMed] [Google Scholar]
- 80.Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans 2009;(7):1080–94 [DOI] [PubMed] [Google Scholar]
- 81.Gaggelli E, Janicka-Klos A, Jankowska E, et al. NMR studies of the Zn2+ interactions with rat and human beta-amyloid (1–28) peptides in water-micelle environment. J Phys Chem B 2008;112:100–9 [DOI] [PubMed] [Google Scholar]
- 82.Zirah S, Kozin SA, Mazur AK, et al. Structural changes of region 1–16 of the Alzheimer disease amyloid beta-peptide upon zinc binding and in vitro aging. J Biol Chem 2006;281:2151–61 [DOI] [PubMed] [Google Scholar]
- 83.Talmard C, Bouzan A, Faller P. Zinc binding to amyloid-beta: isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry 2007;46:13658–66 [DOI] [PubMed] [Google Scholar]
- 84.Tsvetkov PO, Popov IA, Nikolaev EN, et al. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid beta(1–16) peptide. Chembiochem 2008;9:1564–7 [DOI] [PubMed] [Google Scholar]
- 85.Tougu V, Karafin A, Zovo K, et al. Zn(II)- and Cu(II)-induced nonfibrillar aggregates of amyloid-beta (1–42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J Neurochem 2009;110:1784–95 [DOI] [PubMed] [Google Scholar]
- 86.Talmard C, Leuma Yona R, Faller P. Mechanism of zinc(II)-promoted amyloid formation: zinc(II) binding facilitates the transition from the partially alpha-helical conformer to aggregates of amyloid beta protein(1–28). J Biol Inorg Chem 2009;14:449–55 [DOI] [PubMed] [Google Scholar]
- 87.Yu WH, Fraser PE. S100beta interaction with tau is promoted by zinc and inhibited by hyperphosphorylation in Alzheimer’s disease. J Neurosci 2001;21:2240–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Squitti R, Quattrocchi CC, Salustri C, Rossini PM. Ceruloplasmin fragmentation is implicated in ‘free’ copper deregulation of Alzheimer’s disease. Prion 2008;2:23–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maynard CJ, Bush AI, Masters CL, et al. Metals and amyloid-beta in Alzheimer’s disease. Int J Exp Pathol 2005;86:147–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bica L, Crouch PJ, Cappai R, White AR. Metallo-complex activation of neuroprotective signalling pathways as a therapeutic treatment for Alzheimer’s disease. Mol Biosyst 2009;5:134–42 [DOI] [PubMed] [Google Scholar]
- 91.Hureau C, Faller P. Abeta-mediated ROS production by Cu ions: structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 2009;91:1212–17 [DOI] [PubMed] [Google Scholar]
- 92.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 2010;15:61–76 [DOI] [PubMed] [Google Scholar]
- 93.Moreira PI, Santos MS, Oliveira CR, et al. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets 2008;7:3–10 [DOI] [PubMed] [Google Scholar]
- 94.Squitti R, Bressi F, Pasqualetti P, et al. Longitudinal prognostic value of serum “free” copper in patients with Alzheimer disease. Neurology 2009;72:50–5 [DOI] [PubMed] [Google Scholar]
- 95.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med 2007;43:658–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Opazo C, Huang X, Cherny RA, et al. Metalloenzyme-like activity of Alzheimer’s disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2. J Biol Chem 2002;277:40302–8 [DOI] [PubMed] [Google Scholar]
- 97.Atwood CS, Perry G, Zeng H, et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry 2004;43:560–8 [DOI] [PubMed] [Google Scholar]
- 98.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994;77:817–27 [DOI] [PubMed] [Google Scholar]
- 99.Barnham KJ, Haeffner F, Ciccotosto GD, et al. Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer’s disease beta-amyloid. FASEB J 2004;18:1427–9 [DOI] [PubMed] [Google Scholar]
- 100.Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer Abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 1998;273:12817–26 [DOI] [PubMed] [Google Scholar]
- 101.Sparks DL, Petanceska S, Sabbagh M, et al. Cholesterol, copper and Abeta in controls, MCI, AD and the AD cholesterol-lowering treatment trial (ADCLT). Curr Alzheimer Res 2005;2:527–39 [DOI] [PubMed] [Google Scholar]
- 102.Hayashi T, Shishido N, Nakayama K, et al. Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-beta peptide. Free Radic Biol Med 2007;43:1552–9 [DOI] [PubMed] [Google Scholar]
- 103.Hung YH, Robb EL, Volitakis I, et al. Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease. J Biol Chem 2009;284:21899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nadal RC, Rigby SE, Viles JH. Amyloid beta-Cu2+ complexes in both monomeric and fibrillar forms do not generate H2O2 catalytically but quench hydroxyl radicals. Biochemistry 2008;47:11653–64 [DOI] [PubMed] [Google Scholar]
- 105.Hesse L, Beher D, Masters CL, Multhaup G. The betaA4 amyloid precursor protein binding to copper. FEBS Lett 1994;349:109–16 [DOI] [PubMed] [Google Scholar]
- 106.Multhaup G, Schlicksupp A, Hesse L, et al. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I). Science 1996;271:1406–9 [DOI] [PubMed] [Google Scholar]
- 107.Kong GK, Miles LA, Crespi GA, et al. Copper binding to the Alzheimer’s disease amyloid precursor protein. Eur Biophys J 2008;37:269–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kong GK, Adams JJ, Harris HH, et al. Structural studies of the Alzheimer’s amyloid precursor protein copper-binding domain reveal how it binds copper ions. J Mol Biol 2007;367:148–61 [DOI] [PubMed] [Google Scholar]
- 109.Dingwall C A copper-binding site in the cytoplasmic domain of BACE1 identifies a possible link to metal homoeostasis and oxidative stress in Alzheimer’s disease. Biochem Soc Trans 2007;35:571–3 [DOI] [PubMed] [Google Scholar]
- 110.Bellingham SA, Lahiri DK, Maloney B, et al. Copper depletion down-regulates expression of the Alzheimer’s disease amyloid-beta precursor protein gene. J Biol Chem 2004;279:20378–86 [DOI] [PubMed] [Google Scholar]
- 111.White AR, Reyes R, Mercer JF, et al. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res 1999;842:439–44 [DOI] [PubMed] [Google Scholar]
- 112.Karr JW, Akintoye H, Kaupp LJ, Szalai VA. N-Terminal deletions modify the Cu2+ binding site in amyloid-beta. Biochemistry 2005;44:5478–87 [DOI] [PubMed] [Google Scholar]
- 113.Rozga M, Bal W. The Cu(II)/Abeta/ human serum albumin model of control mechanism for copper-related amyloid neurotoxicity. Chem Res Toxicol 2010;23:298–308 [DOI] [PubMed] [Google Scholar]
- 114.Rauk A The chemistry of Alzheimer’s disease. Chem Soc Rev 2009;38:2698–715 [DOI] [PubMed] [Google Scholar]
- 115.Metodiewa D Molecular mechanisms of cellular injury produced by neurotoxic amino acids that generate reactive oxygen species. Amino Acids 1998;14:181–7 [DOI] [PubMed] [Google Scholar]
- 116.Shin BK, Saxena S. Direct evidence that all three histidine residues coordinate to Cu(II) in amyloid-betal–16. Biochemistry 2008;47:9117–23 [DOI] [PubMed] [Google Scholar]
- 117.Drew SC, Noble CJ, Masters CL, et al. Pleomorphic copper coordination by Alzheimer’s disease amyloid-beta peptide. J Am Chem Soc 2009;131:1195–207 [DOI] [PubMed] [Google Scholar]
- 118.Drew SC, Masters CL, Barnham KJ. Alanine-2 carbonyl is an oxygen ligand in Cu2+ coordination of Alzheimer’s disease amyloid-beta peptide – relevance to N-terminally truncated forms. J Am Chem Soc 2009;131:8760–1 [DOI] [PubMed] [Google Scholar]
- 119.Dorlet P, Gambarelli S, Faller P, Hureau C. Pulse EPR spectroscopy reveals the coordination sphere of copper(II) ions in the 1–16 amyloid-beta peptide: a key role of the first two N-terminus residues. Angew Chem Int Ed Engl 2009;48:9273–6 [DOI] [PubMed] [Google Scholar]
- 120.Shearer J, Szalai VA. The amyloid-beta peptide of Alzheimer’s disease binds CuI in a linear bis-his coordination environment: insight into a possible neuroprotective mechanism for the amyloid-beta peptide. J Am Chem Soc 2008;13:17826–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hureau C, Balland V, Coppel Y, et al. Importance of dynamical processes in the coordination chemistry and redox conversion of copper amyloid-beta complexes. J Biol Inorg Chem 2009;14:995–1000 [DOI] [PubMed] [Google Scholar]
- 122.Himes RA, Park GY, Siluvai GS, et al. Structural studies of copper(I) complexes of amyloid-beta peptide fragments: formation of two-coordinate bis (histidine) complexes. Angew Chem Int Ed Engl 2008;47:9084–90877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.da Silva GF, Tay WM, Ming LJ. Catechol oxidase-like oxidation chemistry of the 1–20 and 1–16 fragments of Alzheimer’s disease-related beta-amyloid peptide: their structure-activity correlation and the fate of hydrogen peroxide. J Biol Chem 2005;280:16601–9 [DOI] [PubMed] [Google Scholar]
- 124.Syme CD, Nadal RC, Rigby SE, Viles JH. Copper binding to the amyloid-beta (Abeta) peptide associated with Alzheimer’s disease: folding, coordination geometry, pH dependence, stoichiometry, and affinity of Abeta-(1–28): insights from a range of complementary spectroscopic techniques. J Biol Chem 2004;279:18169–77 [DOI] [PubMed] [Google Scholar]
- 125.Tickler AK, Smith DG, Ciccotosto GD, et al. Methylation of the imidazole side chains of the Alzheimer disease amyloid-beta peptide results in abolition of superoxide dismutase-like structures and inhibition of neurotoxicity. J Biol Chem 2005;280:13355–63 [DOI] [PubMed] [Google Scholar]
- 126.Karr JW, Kaupp LJ, Szalai VA. Amyloid-beta binds Cu2+ in a mononuclear metal ion binding site. J Am Chem Soc 2004;126:13534–8 [DOI] [PubMed] [Google Scholar]
- 127.Nakamura M, Shishido N, Nunomura A, et al. Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry 2007;46:12737–43 [DOI] [PubMed] [Google Scholar]
- 128.Curtain CC, Ali F, Volitakis I, et al. Alzheimer’s disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J Biol Chem 2001;276:20466–73 [DOI] [PubMed] [Google Scholar]
- 129.Tougu V, Karafin A, Palumaa P. Binding of zinc(II) and copper(II) to the full-length Alzheimer’s amyloid-beta peptide. J Neurochem 2008;104:1249–59 [DOI] [PubMed] [Google Scholar]
- 130.Zappasodi F, Salustri C, Babiloni C, et al. An observational study on the influence of the APOE-e4 allele on the correlation between ‘free’ copper toxicosis and EEG activity in Alzheimer disease. Brain Res 2008;1215:183–9 [DOI] [PubMed] [Google Scholar]
- 131.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet 1996;14(1):55–61 [DOI] [PubMed] [Google Scholar]
- 132.Moir RD, Atwood CS, Huang X, et al. Mounting evidence for the involvement of zinc and copper in Alzheimer’s disease. Eur J Clin Invest 1999;29:569–70 [DOI] [PubMed] [Google Scholar]
- 133.Gauthier E, Fortier I, Courchesne F, et al. Aluminum forms in drinking water and risk of Alzheimer’s disease. Environ Res 2000;84:234–46 [DOI] [PubMed] [Google Scholar]
- 134.Neri LC, Hewitt D. Aluminium, Alzheimer’s disease, and drinking water. Lancet 1991;338:390. [DOI] [PubMed] [Google Scholar]
- 135.Rondeau V, Jacqmin-Gadda H, Commenges D, et al. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol 2009;169:489–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Polizzi S, Pira E, Ferrara M, et al. Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology 2002;23:761–74 [DOI] [PubMed] [Google Scholar]
- 137.Yumoto S, Kakimi S, Ohsaki A, Ishikawa A. Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer’s disease. J Inorg Biochem 2009;103:1579–84 [DOI] [PubMed] [Google Scholar]
- 138.Hegde ML, Anitha S, Latha KS, et al. First evidence for helical transitions in supercoiled DNA by amyloid beta peptide (1–42) and aluminum: a new insight in understanding Alzheimer’s disease. J Mol Neurosci 2004;22:19–31 [DOI] [PubMed] [Google Scholar]
- 139.Atamna H, Killilea DW, Killilea AN, Ames BN. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc Natl Acad Sci USA 2002;99:14807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodella LF, Ricci F, Borsani E, et al. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histol Histopathol 2008;23:433–9 [DOI] [PubMed] [Google Scholar]
- 141.Pratico D, Uryu K, Sung S, et al. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J 2002;16:1138–40 [DOI] [PubMed] [Google Scholar]
- 142.Ribes D, Colomina MT, Vicens P, Domingo JL. Effects of oral aluminum exposure on behavior and neurogenesis in a transgenic mouse model of Alzheimer’s disease. Exp Neurol 2008;214:293–300 [DOI] [PubMed] [Google Scholar]
- 143.Drago D, Cavaliere A, Mascetra N, et al. Aluminum modulates effects of betaamyloid1–42 on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Res 2008;11:861–71 [DOI] [PubMed] [Google Scholar]
- 144.Korchazhkina OV, Ashcroft AE, Kiss T, Exley C. The degradation of Abeta (25–35) by the serine protease plasmin is inhibited by aluminium. J Alzheimers Dis 2002;4:357–67 [DOI] [PubMed] [Google Scholar]
- 145.Walton JR. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 2006;27:385–94 [DOI] [PubMed] [Google Scholar]
- 146.Perl DP, Brody AR. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980;208:297–9 [DOI] [PubMed] [Google Scholar]
- 147.Candy JM, McArthur FK, Oakley AE, et al. Aluminium accumulation in relation to senile plaque and neurofibrillary tangle formation in the brains of patients with renal failure. J Neurol Sci 1992;107:210–18 [DOI] [PubMed] [Google Scholar]
- 148.Bondy SC, Truong A. Potentiation of beta-folding of beta-amyloid peptide 25–35 by aluminum salts. Neurosci Lett 1999;267:25–8 [DOI] [PubMed] [Google Scholar]
- 149.Fasman GD, Moore CD. The solubilization of model Alzheimer tangles: reversing the beta-sheet conformation induced by aluminum with silicates. Proc Natl Acad Sci USA 1994;91:11232–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.House E, Collingwood J, Khan A, et al. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis 2004;6:291–301 [DOI] [PubMed] [Google Scholar]
- 151.Di Vaira M, Bazzicalupi C, Orioli P, et al. Clioquinol, a drug for Alzheimer’s disease specifically interfering with brain metal metabolism: structural characterization of its zinc(II) and copper (II) complexes. Inorg Chem 2004;43:3795–7 [DOI] [PubMed] [Google Scholar]
- 152.Raman B, Ban T, Yamaguchi K, et al. Metal ion-dependent effects of clioquinol on the fibril growth of an amyloid beta peptide. J Biol Chem 2005;280:16157–62 [DOI] [PubMed] [Google Scholar]
- 153.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999;400:173–7 [DOI] [PubMed] [Google Scholar]
- 154.Treiber C, Simons A, Strauss M, et al. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of Alzheimer’s disease. J Biol Chem 2004;279:51958–64 [DOI] [PubMed] [Google Scholar]
- 155.Huang X, Atwood CS, Moir RD, et al. Zinc-induced Alzheimer’s Abeta1–40 aggregation is mediated by conformational factors. J Biol Chem 1997;272:26464–70 [DOI] [PubMed] [Google Scholar]
- 156.Cherny RA, Atwood CS, Xilinas ME, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001;30:665–76 [DOI] [PubMed] [Google Scholar]
- 157.Bush AI. Metal complexing agents as therapies for Alzheimer’s disease. Neurobiol Aging 2002;23:1031–8 [DOI] [PubMed] [Google Scholar]
- 158.Wang Y, Branicky R, Stepanyan Z, et al. The anti-neurodegeneration drug clioquinol inhibits the aging-associated protein CLK-1. J Biol Chem 2009;284:314–23 [DOI] [PubMed] [Google Scholar]
- 159.Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci 2003;26:207–14 [DOI] [PubMed] [Google Scholar]
- 160.Ritchie CW, Bush AI, Mackinnon A, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot Phase 2 clinical trial. Arch Neurol 2003;60:1685–91 [DOI] [PubMed] [Google Scholar]
- 161.Rodriguez-Rodriguez C, Sanchez de Groot N, Rimola A, et al. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J Am Chem Soc 2009;131:1436–51 [DOI] [PubMed] [Google Scholar]
- 162.Sampson E, Jenagaratnam L, McShane R. Metal protein attenuating compounds for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev 2008;(1):CD005380. [DOI] [PubMed] [Google Scholar]
- 163.Adlard PA, Cherny RA, Finkelstein DI, et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 2008;59:43–55 [DOI] [PubMed] [Google Scholar]
- 164.Faux NG, Ritchie CW, Gunn A, et al. PBT2 rapidly improves cognition in Alzheimer’s disease: additional Phase II analyses. J Alzheimers Dis 2010;20:509–16 [DOI] [PubMed] [Google Scholar]
- 165.Lannfelt L, Blennow K, Zetterberg H, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2008;7:779–86 [DOI] [PubMed] [Google Scholar]
- 166.Lee JY, Friedman JE, Angel I, et al. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging 2004;25:1315–21 [DOI] [PubMed] [Google Scholar]
- 167.Ji HF, Zhang HY. A new strategy to combat Alzheimer’s disease. Combining radical-scavenging potential with metal-protein-attenuating ability in one molecule. Bioorg Med Chem Lett 2005;15:21–4 [DOI] [PubMed] [Google Scholar]
- 168.Avramovich-Tirosh Y, Amit T, Bar-Am O, et al. Physiological and pathological aspects of Abeta in iron homeostasis via 5′UTR in the APP mRNA and the therapeutic use of iron-chelators. BMC Neurosci 2008;9(Suppl 2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yassin MS, Ekblom J, Xilinas M, et al. Changes in uptake of vitamin B12 and trace metals in brains of mice treated with clioquinol. J Neurol Sci 2000;173:40–4 [DOI] [PubMed] [Google Scholar]
- 170.Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriatr Soc 2003;51:1143–8 [DOI] [PubMed] [Google Scholar]
- 171.Crapper McLachlan DR, Dalton AJ, Kruck TP, et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991;337:1304–8 [DOI] [PubMed] [Google Scholar]
- 172.Cuajungco MP, Faget KY, Huang X, et al. Metal chelation as a potential therapy for Alzheimer’s disease. Ann NY Acad Sci 2000;920:292–304 [DOI] [PubMed] [Google Scholar]
- 173.Domingo JL. Aluminum and other metals in Alzheimer’s disease: a review of potential therapy with chelating agents. J Alzheimers Dis 2006;10:331–41 [DOI] [PubMed] [Google Scholar]
- 174.Avramovich-Tirosh Y, Amit T, Bar-Am O, et al. Therapeutic targets and potential of the novel brain- permeable multifunctional iron chelator–monoamine oxidase inhbitor drug, M-30, for the treatment of Alzheimer’s disease. J Neurochem 2006;100:490–502 [DOI] [PubMed] [Google Scholar]
- 175.Avramovich-Tirosh Y, Bar-Am O, Amit T, et al. Up-regulation of hypoxia-inducible factor (HIF) −1beta and HIF-target genes in cortical neurons by the Novel Multifunctional Iron Chelator Anti-Alzheimer Drug, M30. Curr Alzheimer Res 2010;7:300–6 [DOI] [PubMed] [Google Scholar]
- 176.Youdim MB, Bar-Am O, Yogev-Falach M, et al. Rasagiline: neurodegeneration, neuroprotection, and mitochondrial permeability transition. J Neurosci Res 2005;79:172–9 [DOI] [PubMed] [Google Scholar]
- 177.Dedeoglu A, Cormier K, Payton S, et al. Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer’s amyloidogenesis. Exp Gerontol 2004;39:1641–9 [DOI] [PubMed] [Google Scholar]
- 178.Storr T, Scott LE, Bowen ML, et al. Glycosylated tetrahydrosalens as multifunctional molecules for Alzheimer’s therapy. Dalton Trans 2009;28:3034–43 [DOI] [PubMed] [Google Scholar]
- 179.Storr T, Merkel M, Song-Zhao GX, et al. Synthesis, characterization, and metal coordinating ability of multifunctional carbohydrate-containing compounds for Alzheimer’s therapy. J Am Chem Soc 2007;129:7453–63 [DOI] [PubMed] [Google Scholar]
- 180.Green DE, Bowen ML, Scott LE, et al. In vitro studies of 3-hydroxy-4-pyridinones and their glycosylated derivatives as potential agents for Alzheimer’s disease. Dalton Trans 2010;39:1604–15 [DOI] [PubMed] [Google Scholar]
- 181.Schugar H, Green DE, Bowen ML, et al. Combating Alzheimer’s disease with multifunctional molecules designed for metal passivation. Angew Chem Int Ed Engl 2007;46:1716–8 [DOI] [PubMed] [Google Scholar]
- 182.Cavalli A, Bolognesi ML, Minarini A, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem 2008;51:347–72 [DOI] [PubMed] [Google Scholar]
- 183.Wu WH, Lei P, Liu Q, et al. Sequestration of copper from beta-amyloid promotes selective lysis by cyclen-hybrid cleavage agents. J Biol Chem 2008;283:31657–64 [DOI] [PubMed] [Google Scholar]
- 184.Sattin A, Muhoberac BB, Aprison MH, Schauf CL. Tetrahydroaminoacridine (THA) as a pharmacological probe in Alzheimer’s disease (AD) and other neurodegenerative disorders. Med Hypotheses 1989;29:155–9 [DOI] [PubMed] [Google Scholar]
- 185.Shin RW, Kruck TP, Murayama H, Kitamoto T. A novel trivalent cation chelator Feralex dissociates binding of aluminum and iron associated with hyperphosphorylated tau of Alzheimer’s disease. Brain Res 2003;96:139–46 [DOI] [PubMed] [Google Scholar]
- 186.Flora SJS, Mehta A, Satsangi K, et al. Aluminum-induced oxidative stress in rat brain: response to combined administration of citric acid and HEDTA. Comparative Biochemistry and Physiology Part C: Toxicol Pharmacol 2003;134:319–28 [DOI] [PubMed] [Google Scholar]
- 187.Liu G, Men P, Kudo W, et al. Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease. Neurosci Lett 2009;455:187–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu G, Men P, Harris PL, et al. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett 2006;406:189–93 [DOI] [PubMed] [Google Scholar]
- 189.Liu G, Garrett MR, Men P, et al. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim Biophys Acta 2005;1741:246–52 [DOI] [PubMed] [Google Scholar]
- 190.Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol 2010;610:123–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Donnelly PS, Caragounis A, Du T, et al. Selective intracellular release of copper and zinc ions from bis (thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide. J Biol Chem 2008;283:4568–77 [DOI] [PubMed] [Google Scholar]
- 192.Sigel H, Prijs B, McCormick DB, Shih JC. Stability and structure of binary and ternary complexes of alpha-lipoate and lipoate derivatives with Mn2+, Cu2+, and Zn2+ in solution. Arch Biochem Biophys 1978;187:208–14 [DOI] [PubMed] [Google Scholar]
- 193.Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol 1995;50:123–6 [DOI] [PubMed] [Google Scholar]
- 194.Lovell MA, Xie C, Xiong S, Markesbery WR Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J Alzheimers Dis 2003;5:229–39 [DOI] [PubMed] [Google Scholar]
- 195.Salinthone S, Yadav V, Bourdette DN, Carr DW. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets 2008;8:132–42 [DOI] [PubMed] [Google Scholar]
- 196.Quinn JF, Bussiere JR, Hammond RS, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging 2007;28:213–25 [DOI] [PubMed] [Google Scholar]
- 197.Rossi L, Mazzitelli S, Arciello M, et al. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem Res 2008;33:2390–400 [DOI] [PubMed] [Google Scholar]
- 198.Lim GP, Chu T, Yang F, et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001;21:8370–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim Biophys Acta 2007;1768:1976–90 [DOI] [PubMed] [Google Scholar]
- 200.Mandel SA, Amit T, Weinreb O, et al. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci Ther 2008;14:352–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rezai-Zadeh K, Arendash GW, Hou H, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res 2008;1214:177–87 [DOI] [PubMed] [Google Scholar]
- 202.Obregon DF, Rezai-Zadeh K, Bai Y, et al. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem 2006;281:16419–27 [DOI] [PubMed] [Google Scholar]
- 203.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 2002;156:445–53 [DOI] [PubMed] [Google Scholar]
- 204.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–92 [PubMed] [Google Scholar]
- 205.Truelsen T, Thudium D, Gronbaek M. Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology 2002;59:1313–19 [DOI] [PubMed] [Google Scholar]
- 206.Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 2010;285:9100–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 2004;61:82–8 [DOI] [PubMed] [Google Scholar]
- 208.Yoon I, Lee KH, Cho J. Gossypin protects primary cultured rat cortical cells from oxidative stress- and beta-amyloid-induced toxicity. Arch Pharm Res 2004;27:454–9 [DOI] [PubMed] [Google Scholar]
- 209.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 1998;55:1409–15 [DOI] [PubMed] [Google Scholar]
- 210.Maharaj DS, Maharaj H, Daya S, Glass BD. Melatonin and 6-hydroxymelatonin protect against iron-induced neurotoxicity. J Neurochem 2006;96:78–81 [DOI] [PubMed] [Google Scholar]
- 211.Squitti R, Lupoi D, Pasqualetti P, et al. Elevation of serum copper levels in Alzheimer’s disease. Neurology 2002;59:1153–61 [DOI] [PubMed] [Google Scholar]
- 212.Squitti R, Rossini PM, Cassetta E, et al. D-penicillamine reduces serum oxidative stress in Alzheimer’s disease patients. Eur J Clin Invest 2002;32:51–9 [DOI] [PubMed] [Google Scholar]
- 213.Hill GM, Brewer GJ, Juni JE, et al. Treatment of Wilson’s disease with zinc. II. Validation of oral 64copper with copper balance. Am J Med Sci 1986;292:344–9 [DOI] [PubMed] [Google Scholar]
- 214.Brewer GJ. Copper toxicity in the general population. Clin Neurophysiol 2010;121:459–60 [DOI] [PubMed] [Google Scholar]
- 215.Squitti R, Zito G. Anti-copper therapies in Alzheimer’s disease: new concepts. Recent Pat CNS Drug Discov 2009;4:209–19 [DOI] [PubMed] [Google Scholar]
- 216.Amit T, Avramovich-Tirosh Y, Youdim MB, Mandel S. Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 2008;22:1296–305 [DOI] [PubMed] [Google Scholar]
- 217.Morse LJ, Payton SM, Cuny GD, Rogers JT. FDA-preapproved drugs targeted to the translational regulation and processing of the amyloid precursor protein. J Mol Neurosci 2004;24:129–36 [DOI] [PubMed] [Google Scholar]
- 218.Brewer GJ. The use of copper-lowering therapy with tetrathiomolybdate in medicine. Expert Opin Investig Drugs 2009;18:89–97 [DOI] [PubMed] [Google Scholar]
- 219.Greig NH, Ruckle J, Comer P, et al. Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects. Curr Alzheimer Res 2005;2:483–92 [DOI] [PubMed] [Google Scholar]
- 220.Bandyopadhyay S, Ni J, Ruggiero A, et al. A high-throughput drug screen targeted to the 5′untranslated region of Alzheimer amyloid precursor protein mRNA. J Biomol Screen 2006;11:469–80 [DOI] [PubMed] [Google Scholar]
- 221.Chishti MA, Yang DS, Janus C, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 2001;276:21562–70 [DOI] [PubMed] [Google Scholar]
- 222.Bodovitz S, Falduto MT, Frail DE, Klein WL. Iron levels modulate alpha-secretase cleavage of amyloid precursor protein. J Neurochem 1995;64:307–15 [DOI] [PubMed] [Google Scholar]
- 223.Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer’s disease. Curr Med Chem 2007;14:2848–64 [DOI] [PubMed] [Google Scholar]
- 224.Bandyopadhyay S, Hartley DM, Cahill CM, et al. Interleukin-1alpha stimulates non-amyloidogenic pathway by alpha-secretase (ADAM-10 and ADAM-17) cleavage of APP in human astrocytic cells involving p38 MAP kinase. J Neurosci Res 2006;84:106–18 [DOI] [PubMed] [Google Scholar]
- 225.Qin W, Ho L, Wang J, et al. S100A7, a novel Alzheimer’s disease biomarker with non-amyloidogenic alpha-secretase activity acts via selective promotion of ADAM-10. PLoS One 2009;4(1):e4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cho HH, Cahill CM, Vanderburg CR, et al. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 2010: published online 29 June 2010, doi: 10.1074/jbc.M110.149161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Beaudoin ME, Poirel VJ, Krushel LA. Regulating amyloid precursor protein synthesis through an internal ribosomal entry site. Nucleic Acids Res 2008;36:6835–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 1996;109:1537–43 [DOI] [PubMed] [Google Scholar]