Abstract
High-quality evidence shows that MRI in biopsy-naive men can reduce the number of men who need prostate biopsy and can reduce the number of diagnoses of clinically insignificant cancers that are unlikely to cause harm. In men with prior negative biopsy results who remain under persistent suspicion, MRI improves the detection and localization of life-threatening prostate cancer with greater clinical utility than the current standard of care, systematic transrectal US-guided biopsy. Systematic analyses show that MRI-directed biopsy increases the effectiveness of the prostate cancer diagnosis pathway. The incorporation of MRI-directed pathways into clinical care guidelines in prostate cancer detection has begun. The widespread adoption of the Prostate Imaging Reporting and Data System (PI-RADS) for multiparametric MRI data acquisition, interpretation, and reporting has promoted these changes in practice. The PI-RADS MRI-directed biopsy pathway enables the delivery of key diagnostic benefits to men suspected of having cancer based on clinical suspicion. Herein, the PI-RADS Steering Committee discusses how the MRI pathway should be incorporated into routine clinical practice and the challenges in delivering the positive health impacts needed by men suspected of having clinically significant prostate cancer.
© RSNA, 2019
Summary
The Prostate Imaging Reporting and Data System MRI-directed biopsy pathway enables the delivery of key diagnostic benefits to men suspected of having cancer according to their clinical priorities.
Key Points
■ High-quality Prostate Imaging Reporting and Data System (PI-RADS)-compliant multiparametric MRI should be performed before prostate biopsy in most men who are suspected of having clinically significant disease and are likely to be offered active treatment.
■ A monitoring safety net must be in place for patients who decline immediate biopsy after MRI reveals a low likelihood of disease and should include clinical examination, laboratory assays, and imaging, as per local clinical practice and as consistent with clinical goals for individual patients; the roles and responsibilities of the participants and the circumstances that should trigger reinvestigations should be clearly defined.
■ For men proceeding to biopsy after MRI reveals intermediate or high likelihood of disease (ie, PI-RADS category 3 or higher), a combination of systematic and targeted biopsies should be performed in biopsy-naive men; only targeted biopsies are needed in men with persistent suspicion after prior negative systematic transrectal US-guided biopsy findings.
Introduction
The current version of the Prostate Imaging Reporting and Data System (PI-RADS) was formulated on experience gained from PI-RADS version 1, accumulated scientific evidence, and expert consensus (1). The release of PI-RADS version 2.1 is expected to further improve observer variability (2). A recent publication evaluated multiple clinical studies, systematic analyses, and professional guidelines on their use of multiparametric MRI in prostate cancer detection (3). It showed that the test performance of multiparametric MRI-directed biopsy in the detection of prostate cancer is superior to that of systematic transrectal US-guided biopsy. High-level evidence has now established multiple benefits of MRI-directed biopsy over systematic transrectal US-guided biopsy of the prostate (4). These benefits include (a) a reduction in the number of men who need to undergo biopsy (5–9); (b) a reduction in the number of diagnoses of clinically insignificant cancers that are unlikely to cause harm (4,10), with the potential to reduce overtreatment, treatment-related complications, and active surveillance rates (6); (c) improved detection of clinically significant prostate cancers, particularly in patients with prior negative transrectal US-guided biopsy findings (4,10); and (d) improved risk stratification of diagnosed cancers owing to greater precision in tumor grade and volume determinations, which helps direct disease management. All these advantages can be achieved with fewer targeted biopsy cores per patient, potentially reducing biopsy-related morbidity (6,8,11). The purpose of this article is to focus on how multiparametric MRI results can positively impact the health of men suspected of having clinically significant prostate cancer.
Who Should Undergo MRI before Biopsy?
Patients chosen for MRI before biopsy include biopsy-naive men with elevated serum prostate-specific antigen (PSA) levels, abnormal digital rectal examination findings, or both, and men who are deemed to have persistent elevated risk of harboring clinically significant cancers despite prior negative or nonexplanatory systematic transrectal US biopsy findings. Indications for MRI should be based on the recommendations for screening and early diagnosis of the National Comprehensive Cancer Network and the European Association of Urology (12,13). Accordingly, biopsy-naive men with lower than average risk of prostate cancer should not undergo prostate biopsy or MRI. However, limiting the options to prespecified PSA thresholds is not recommended, as there are many factors (eg, symptoms, age, race, family history, PSA kinetics, digital rectal examination findings) that inform the decision to perform MRI or biopsy. Many tools are being developed to decide on the need for biopsy. The emerging paradigm is to integrate clinical factors, risk calculators, molecular diagnostic results, and MRI findings (14,15).
Recently updated prostate cancer risk calculators (16,17) can be informative when determining who might not need biopsy based on lower than average predicted risk, as well as when identifying men with higher than average risk (13). However, risk calculators should not be the only tool used to determine the need for MRI or biopsy. Clinical judgment, intended purpose, and patient preference should be considered when deciding on the need for MRI or biopsy.
MRI results can be used in two distinct ways to improve the yield of prostate biopsy and reduce the number of overdiagnoses (10). The first is the combined biopsy pathway, in which patients with low-likelihood MRI findings undergo scheduled systematic biopsy and those with higher-likelihood MRI findings undergo both systematic and MRI-directed biopsy (18) and in so doing improve the diagnostic yield of clinically significant cancers (15,17,19–22). The second is the MRI pathway, which is distinct in that men with low-likelihood MRI findings do not undergo biopsy at all and men with higher-likelihood findings undergo only MRI-directed biopsy (without systematic cores). The advantage of the MRI pathway is to reduce the number of men who need biopsies and to reduce the total number of biopsy cores in men with high-likelihood MRI findings, thus helping reduce the overdiagnosis of clinically insignificant disease (15,23,24). A mixed approach can also be used.
There has been robust debate about how to best use MRI results: to increase yields of clinically significant cancers or reduce overdiagnoses of clinically insignificant cancers. In all circumstances, MRI interpretations and the need for biopsy should be assessed by multidisciplinary teams in the context of patient care priorities. In biopsy-naive men, there is a need to minimize overdiagnoses and detect clinically significant cancers (25). However, the priority in men with persistent suspicion of clinically significant cancers after negative findings at previous biopsy is to not miss potentially life-threatening cancers.
When systematic evaluations of multiparametric MRI using PI-RADS are undertaken in appropriately chosen men, an assessment of the likelihood of clinically significant cancer as low (PI-RADS category 1 or 2), intermediate (PI-RADS category 3), or high (PI-RADS category 4 or 5) can be made (1).
Low-Likelihood MRI Scans (PI-RADS Categories 1 and 2)
PI-RADS–compliant MRI has a powerful ability to assist physicians when ruling out clinically significant cancer, with the potential for biopsy avoidance in men who are unlikely to have clinically significant cancer based on prostate MRI findings (PI-RADS categories 1 and 2). The ability of MRI to enable a physician to rule out important cancers depends on many factors, including the histologic definition used for clinically significant cancer and the method or methods used to verify PI-RADS category 1 or 2 MRI results. Unfortunately, there has been no universal agreement among pathologists and urologists regarding a working definition of clinical importance. Recently, however, consensus has emerged around the International Society of Urological Pathology (ISUP) (26) recommendation that a Gleason score of at least 3+4 (ISUP grade group ≥2) should be used as the primary definition of significant cancer, and this standard has been adopted in this report. Currently, the ISUP consensus does not distinguish between microfocal and larger-volume tumors with a Gleason grade of 3+3 (ISUP grade group 1), instead assigning equal prognoses to both.
The most reliable studies providing information on the ability of multiparametric MRI to help rule out clinically significant cancer in biopsy-naive patients have used transperineal template-mapping biopsy (5,27) or transperineal targeting with 24-core systematic saturation biopsies (9,28) for histologic verification. The PROMIS study (PROstate MRI Imaging Study) used transperineal mapping in biopsy-naive men (5). Missed tumors included ISUP grade group 1 lesions, scattered microfocal ISUP grade group 2 cancers, and ISUP grade group 2 cancers with lower-volume Gleason pattern 4 histology findings. Patients with these types of lesions are often regarded as having favorable prognoses (29,30) and may undergo active surveillance (30,31). It is also important to consider the sensitivity of prostate MRI in the detection of cancers that are universally regarded as having an unfavorable prognosis and should not be missed; these include lesions with ISUP grade group 3 or higher and those with extraprostatic extension, seminal vesical invasion, or both. Mapping biopsy studies show high sensitivities exceeding 90% in most studies (5,32,33), suggesting that very few high-grade tumors are missed when MRI is used, unlike when systematic transrectal US-guided biopsy is used (5).
Patient Implications after MRI Reveals PI-RADS Category 1 or 2 Results
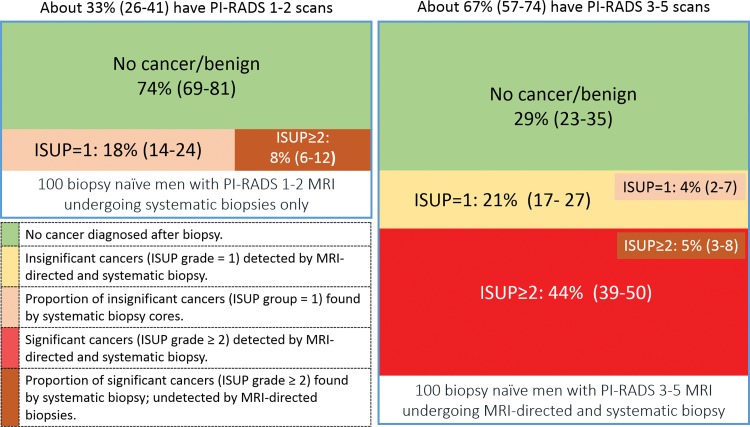
Because of the high negative predictive value of PI-RADS–compliant MRI protocols (5,9), a high proportion of men can avoid immediate biopsy after low-likelihood MRI findings without substantially affecting the detection rates of clinically significant cancers. The number of men with PI-RADS category 1 or 2 MRI findings is dependent on the prevalence of clinically significant cancers within a population (34). In prospective studies, the average proportion of biopsy-naive men with a low-likelihood multiparametric MRI finding is 33% (95% confidence interval [CI]: 26%, 41%) and ranges from 21% to 49% (5,6,8,9,35) (Fig 1). When deciding to perform histologic sampling, one should consider the risks and benefits in the context of (a) the likely yields of systematic transrectal US biopsy in the absence of an MRI-definable target or targets, (b) the impact of clinical biomarkers on biopsy decisions, and (c) the long-term safety of and follow-up monitoring regimens in men who chose the no immediate biopsy approach.
Figure 1:
Infographic shows approximate diagnostic yields of systematic biopsy in biopsy-naive men on the MRI pathway. The MRI pathway consists of no biopsy in men with Prostate Imaging and Reporting Data System (PI-RADS) category 1 or 2 findings and MRI-targeted biopsy in men with PI-RADS category 3–5 findings, without additional clinical factor input. Data are for guidance only and are based on a combination of the MRI pathway and systematic biopsy as the reference standards. Data do not total 100% because of rounding and aggregation of different data sources reported by Drost et al (10). Data in parentheses are 95% confidence intervals for the mean values. ISUP = International Society of Urological Pathology. This graphic was prepared with data kindly provided by Ivo Schoots, MD, PhD (Erasmus University Medical Center, Rotterdam, the Netherlands).
Yield of Systematic Transrectal US Biopsy after MRI Reveals PI-RADS Category 1 or 2 Results
To mitigate the missed cancer risk of the no immediate biopsy approach, some urologists advocate performing systematic transrectal US-guided biopsy in biopsy-naive men with PI-RADS category 1 or 2 imaging findings. Data show that the average detection rate of systematic transrectal US-guided biopsy in patients with ISUP grade group 2 or higher disease is 8% (95% CI: 6%, 12%) in biopsy-naive men (4,7,8,36–41) (Fig 1); this number is lower in men after prior negative biopsy results (3). In other words, on average, 12 biopsy-naive men with low-likelihood MRI findings need to undergo transrectal US-guided biopsy to find one man with an ISUP grade group 2 or higher cancer. In biopsy-naive men with low-likelihood imaging findings, the proportion of men with a diagnosis of ISUP grade group 3 or higher lesions at systematic transrectal US-guided biopsy is very low (approximately 2%) (4,7,8,36–41).
On the other hand, performing systematic transrectal US–guided biopsy after low-likelihood MRI findings in all men results in overdetection in about 18% (95% CI: 14%, 24%) of patients with ISUP grade group 1 disease (8,10,35–37,42–44). In other words, if systematic US-guided transrectal biopsies are performed after low-likelihood MRI, two men will be diagnosed with insignificant cancer for one man detected with an ISUP grade group 2 or greater cancer.
Combining MRI Results with Clinical Parameters
Emerging data suggest that many men can safely avoid immediate systematic transrectal US-guided biopsy after PI-RADS category 1 or 2 MRI findings when the findings are reinforced by clinical variables. The literature on the value of clinical variables for biopsy avoidance in most PI-RADS category 2 cases and in some PI-RADS category 3 cases suggests that PSA density, family history, other biomarkers (24,45), and risk calculator scores (15,20) may be helpful (46). Several investigators found that lower PSA density improves the negative predictive value of MRI (11,33,42,47–49). For example, Hansen et al noted that the negative predictive value for clinically significant cancer was better for patient subcohorts with lower PSA density (≤0.1 ng/mL/cm3), where the negative predictive value increased from 80% to 91% for ISUP grade group 2 or higher tumors (47). Conversely, the negative predictive value of MRI was only 66% when PSA density was greater than 0.2 ng/mL/cm3 (47), showing that the decision to perform biopsy after PI-RADS category 2 or 3 MRI findings requires integration with clinical variables.
Safety of the No Immediate Biopsy Approach
Single- and multicenter studies have shown that it can be safe to use the no immediate biopsy strategy after low-likelihood MRI findings, provided adequate follow-up monitoring regimens are in place to enable detection of emerging clinically significant disease (8,50–52). For example, Panebianco et al (50) observed 1255 men with PI-RADS category 1 or 2 MRI findings (659 biopsy-naive men, 596 men with previous negative biopsy results). They found that the cancer-free survival rate at 2-year follow-up was 95% in biopsy-naive men and 96% in those with previous negative biopsy results. All emerging clinically significant ISUP grade group 2 or higher cancers (n = 60) were detected within 2 years of follow-up, with ISUP grade group 3 or higher disease found in 28 men; all emergent cancers were confined to the prostate gland and were potentially curable.
These considerations indicate that the yields of clinically significant cancers in biopsy-naive men with low-likelihood MRI findings are not high enough to justify the use of transrectal US-guided biopsy in all men, nor are they high enough to justify their medical discharge. The health care burden of monitoring men with low-likelihood MRI results who are not undergoing biopsy needs to be weighed against the potential for treatment-related harm from active treatments or the follow-up regimens of active surveillance in patients with a good prognosis and low-risk cancers brought on by nontargeted systematic transrectal US-guided biopsy. When multidisciplinary teams decide not to perform immediate biopsy after low-likelihood multiparametric MRI, the follow-up monitoring regimens need to be precisely defined (8,31,50,51). It is also important to record the characteristics of cancers that emerge during follow-up to audit the safety of the no immediate biopsy approach. Recommendations for PI-RADS category 1 or 2 cases can be found in Tables 1 and 2.
Table 1:
Patient Groups and Proposed MRI-directed Biopsy Strategies according to PI-RADS Categorization
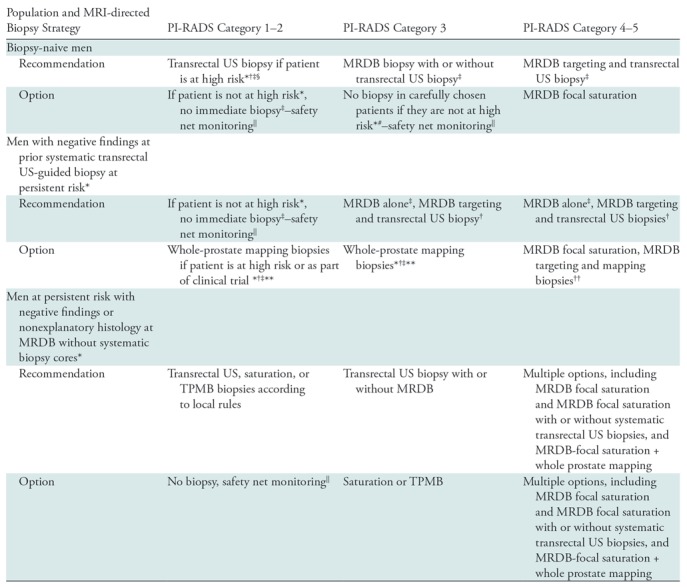
Note.—For transrectal US biopsy, 10–12 systematic transrectal US-guided core biopsies are performed, as per international standards. Saturation biopsy is performed by using transrectal or transperineal sampling (eg, Ginsburg approach). Focal saturation biopsy involves four or more cores for each MRI target, including surrounding sextants. MRDB = MRI-directed biopsy using US/MRI or the in-bore technique with two to four cores per lesion. PI-RADS = Prostate Imaging Reporting and Data System, TPMB = transperineal mapping biopsy.
*Higher risk based on clinical suspicion, family history, prior biopsy result, and biomarkers, including 4 K, prostate health index, prostate cancer gene 3, family history, prostate-specific antigen (PSA) density, and risk calculator scores alone or in combination. 4K incorporates a panel of four kallikrein protein biomarkers (total PSA level, free PSA level, intact PSA level, and human kallikrein-related peptidase 2 level) and other clinical information in an algorithm that provides a percentage risk for clinically significant (Gleason grade ≥7) cancer seen at transrectal US biopsy.
†American Urological Association/Society of Abdominal Radiology 2016 guidelines (79)
‡European Association of Urology 2019 prostate guidelines (https://uroweb.org/guideline/prostate-cancer/) (13).
#National Health Service in England, 2018 (83).
§National Comprehensive Cancer Network version 1.2019 Prostate Cancer Early Detection Recommendations (https://www.nccn.org/) (12).
||Safety net monitoring consists of prostate-specific antigen level monitoring and follow-up imaging, as per local clinical practice and consistent with clinical goals in individual patients, with roles and responsibilities defined by multidisciplinary management teams.
**NICE guideline proposal 2019 (https://www.nice.org.uk/guidance/indevelopment/gid-ng10057) (31).
††Dependent on the size and likely next step in management if clinically important prostate cancer is found.
Table 2:
PI-RADS Recommendations for Multiparametric MRI-directed Biopsies

Note.—EAU 2019 prostate cancer guidelines can be found at https://uroweb.org/guideline/prostate-cancer/ (13). NICE 2019 guidelines can be found at https://www.nice.org.uk/guidance/indevelopment/gid-ng10057 (31). NCCN Clinical Practice Guidelines in Oncology version 1.2019 can be found at https://www.nccn.org (12).
Intermediate- (PI-RADS Category 3) and High-Likelihood (PI-RADS Categories 4 and 5) MRI Results
Multiple patient- and lesion-level analyses have shown that higher levels of likelihood are associated with higher overall and higher clinically significant cancer detection rates, both in biopsy-naive patients and in those with prior negative biopsy results (5,8,9,11,35,53). The validation study by Hansen et al (9) used the Ginsburg saturation biopsy scheme and documented increases in the detection rate of ISUP grade group 2 or higher lesions for PI-RADS category 3 (31%; 95% CI: 25%, 38%) and PI-RADS category 4 or 5 (71%; 95% CI: 67%, 75%). Another study in 339 biopsy-naive men using transrectal US targeting and systematic transrectal US-guided biopsy of 737 targets found ISUP grade group 2 or higher cancers in 12%, 22%, and 72% of men for PI-RADS categories 3, 4, and 5, respectively (54). On average, the diagnostic yields of ISUP grade group 2 or higher for PI-RADS categories 3, 4, and 5 were 12%, 48%, and 72%, respectively, in a pooled analysis by Barkovich et al (55) and 21% (95% CI: 4%, 27%), 39% (95% CI: 31%, 52%), and 73% (95% CI: 61%, 86%), respectively, in the pooled analysis by Schoots (56).
The yields of clinically significant cancers per likelihood category depend on multiple factors, including histologic definitions, with higher yields for definitions that incorporate both tumor volume and tumor grade (5,8). Yields also increase when systematic biopsy cores are combined with MRI-directed biopsy cores (8,35). Invariably, yields of ISUP grade group 3 or higher cancers are highest in patients with PI-RADS category 5 lesions (8,9,11,54).
The prevalence of the intermediate likelihood category (PI-RADS category 3) and the diagnostic yields of clinically significant cancers within this category are dependent on the population disease prevalence, quality of MRI, expertise of readers, and methods used to verify biopsy findings (8). Within reported clinical studies in biopsy-naive men, the mean prevalence of PI-RADS category 3 is 20% (95% CI: 13%, 35%) (8,9,24,37,44,57–60) but is higher in men with prior negative biopsy results (mean, 33%; 95% CI: 17%, 48%) (32,58,61,62). In a systematic analysis, Schoots (56) reported that the mean prevalence of ISUP grade group 2 or higher cancers in this group detected with transrectal systematic biopsy, targeted biopsy, or both was 20%; this was confirmed in two recent large prospective multicenter clinical studies (8,35).
Multiple studies have shown that expert review can decrease the percentage of intermediate likelihood category (PI-RADS category 3) scans (6,8,63). For example, in the PRECISION (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) study, central review reduced the percentage of PI-RADS category 3 scans from 20% to 6% (6). Published studies suggest that further refinements of MRI criteria could be used to identify men in this subgroup who are more likely to harbor cancers by including the number of sequences with which abnormalities are visible (64), by using patterns of contrast enhancement that are not currently codified within the PI-RADS system (65), or by using apparent diffusion coefficient measurements. As previously noted, multiple publications have shown that clinical factors, including PSA density (11,20,42,47,49), risk calculators (19,56), and other serum biomarkers (24), can help determine which men with PI-RADS category 3 findings have higher probability of having clinically significant cancers.
Patient Implications after Intermediate- or High-Likelihood MRI Findings
The prospective randomized PRECISION trial showed the utility of targeted biopsy alone (without accompanying systematic transrectal US-guided biopsy cores) in biopsy-naive men with intermediate- or high-likelihood MRI findings (6). The PRECISION investigators found that targeted biopsy alone in men with PI-RADS category 3–5 MRI results was superior to systematic transrectal US-guided biopsy in the detection of ISUP grade group 2 or higher cancers (+12%) and reduced the detection of clinically insignificant (ISUP grade group 1) cancers by 13% (6). These findings are countered by the consistent finding in the literature of additional ISUP grade group 2 or higher disease not being detected with MRI-directed biopsy-only cores but instead being found in accompanying systematic transrectal US-guided biopsy cores in 5% (95% CI: 3%, 8%) of biopsy-naive men (8,37,53,58,66) (Fig 1).
Recent data also show that most additional ISUP grade group 2 or higher tumors found at systematic biopsy are in sextants adjacent to MRI-identified lesions (8,35,67–69), suggesting that targeting errors and tumor heterogeneity contribute substantially to nondetection and undergrading. These studies also document low yields of sampling normal-appearing nonadjacent sextants that do not alter overall risk stratification in the majority of patients with a cancer diagnosis (Fig 2) (8,35,67). Multiple analyses indicate that when targeted biopsies are performed, additional cores (so-called focal saturation) increase biopsy yields (67,69–72); however, the optimal number of focal saturation biopsy cores remains undefined (8,69,70,73).
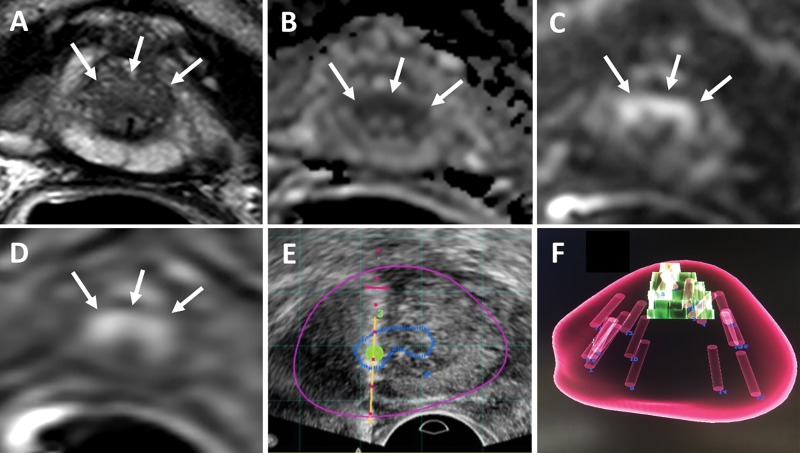
Figure 2:
Images in a 75-year-old man with a serum prostate-specific antigen level of 14.6 ng/mL. A, T2-weighted MRI, B, apparent diffusion coefficient map, C, diffusion-weighted MRI (b value, 2000 sec/mm2), and, D, dynamic contrast-enhanced MRI show a lesion (arrows) classified as Prostate Imaging Reporting and Data System category 4 in A and C and as positive in D in the midline anterior apical transition zone. E, Targeted biopsy was performed with transrectal US/MRI and revealed Gleason grade 3+4 cancer in three of the four cores sampled from this lesion. F, Volume-rendered MRI shows mapping of the four targeted cores and systematic 12 cores. One of the 12 systematic biopsy cores revealed Gleason grade 3+3 prostate adenocarcinoma (5% core involvement), which made no difference for risk stratification in this patient.
It is necessary to determine the optimal number of biopsy cores per lesion because of the link to cancer detection rates and for deciding on the need for repeat biopsy after negative results. Biopsy core numbers are also related to the accuracy of cancer risk stratification. Recently, Zhang et al (72) noted that more clinically significant prostate cancers were detected when the number of core biopsy samples per index lesion was increased from one to three and from three to five (6.4% and 2.4%, respectively) when performing cognitive MRI-targeted transrectal US biopsy. As the biopsy paradigm changes toward targeted biopsy, it seems paradoxical that acquisition of more cores per lesion is advocated to overcome targeting inaccuracies and tumor heterogeneity, particularly when a major MRI-directed biopsy claim is the improved detection of clinically significant cancer with fewer core samples.
There are discrepancies between histologic grading from MRI-directed biopsy and those from final pathologic examination after prostatectomy (both upgrading and downgrading) (69,74). These discrepancies are also common after diagnoses made with systematic transrectal US-guided biopsy (75). Readers should be aware of the potential for risk inflation with MRI-directed biopsies because of greater tumor core involvement (76). For these reasons, oncologic equivalence between selective sampling of a few MRI-directed biopsy cores and 10–12 cores obtained during systematic transrectal US-guided biopsy cannot be assumed.
The debate between using MRI-directed biopsy alone or in combination with systematic transrectal US-guided biopsy after intermediate- or high-likelihood MRI findings in biopsy-naïve men is evenly balanced. Since the estimated added value of systematic transrectal US biopsy to MRI-directed biopsy is approximately 5% (Fig 1), 20 combined biopsies are needed to detect one additional ISUP grade group 2 or higher cancer not detected with MRI-directed biopsy cores (8,37,58,66). This number is higher in men with prior negative biopsy results (4). Systematic US-guided biopsy cores also expose men to the diagnosis of ISUP grade group 1 cancers on top of those diagnosed with MRI-directed biopsy (Fig 1). Recommendations for PI-RADS category 3–5 cases can be found in Tables 1 and 2.
Recommendations for MRI-directed Biopsy
To our knowledge, there is no clear stepwise algorithm that will enable us to advise patients clinically suspected of having cancer to undergo blood or urine biomarker tests, MRI, and then biopsy and treatment. Clinical decision making in patients with a prostate cancer diagnosis incorporating MRI findings is inherently complex, and there is no optimal way to balance the gains (eg, correctly diagnosing clinically significant cancers or avoiding unnecessary biopsy) and losses (eg, missing clinically significant cancers or diagnosing clinically insignificant disease). Decision curve analyses (77) and prostate cancer risk calculators (16,17) can be helpful when managing the complex information for patient decision making regarding the need for biopsy after MRI (78).
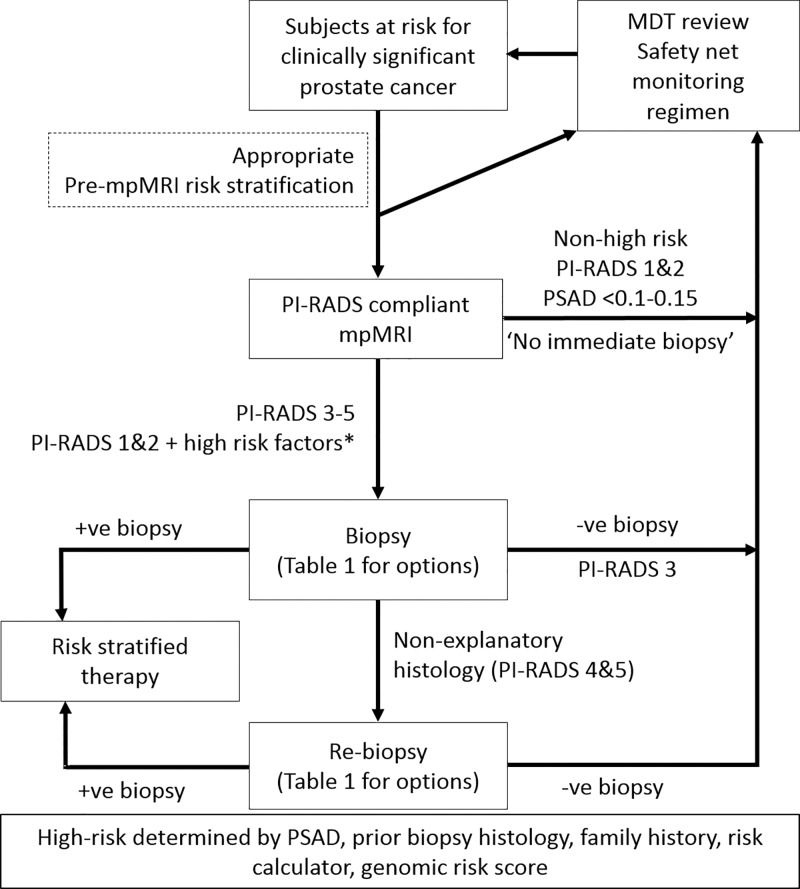
Diagnostic pathway benefits can only be accrued if MRI findings direct patient treatment. Decision making regrading biopsy needs and methods and follow-up strategies in men with high-, intermediate-, or low-likelihood MRI findings requires consensus within multidisciplinary teams. Radiologists need to be part of this conversation as new pathway paradigms incorporating MRI and MRI-directed biopsy emerge (Fig 3). Decisions are multifaceted depending on (a) the respective clinical priorities for biopsy-naive men and those with prior negative biopsy results, (b) the prevalence of clinically significant cancer within the examined population, (c) the ability of MRI to help rule-out clinically significant cancer according to local disease prevalence and diagnostic team expertise tailored to working definitions of clinically significant disease, (d) the likely diagnostic yields of nontargeted systematic transrectal US-guided biopsies for finding clinically significant and insignificant cancers in men with both low- and intermediate- or high-likelihood MRI findings, and (e) the number of negative biopsy results that urologists and, most importantly, patients are willing to accept to find one additional case of clinically significant cancer (17) (Fig 1).
Figure 3:
Diagram shows patient flow in the prostate cancer diagnostic pathway. Safety net monitoring consists of prostate-specific antigen monitoring and follow-up imaging as per local clinical practice and consistent with clinical goals for individual patients, with roles and responsibilities defined by multidisciplinary management teams (8,52). MDT = multidisciplinary team, mpMRI = PI-RADS-compliant multiparametric MRI, PI-RADS = Prostate Imaging Reporting and Data System, PSAD = prostate-specific antigen density.
There are limited guidelines on MRI-directed management actions in biopsy-naive patients, even though the use of MRI in these patients is increasing. If the clinical priority as expressed by the U.S. Preventative Services Task Force is to avoid overdiagnoses, especially in men older than 70 years (25), then the 2019 European Association of Urology recommendation to not perform systematic biopsy after low-likelihood MRI findings is appropriate (https://uroweb.org/guideline/prostate-cancer/) (Table 2). However, this advice is at odds with the Society of Abdominal Radiology and American Association of Urology statement, which recommends systematic biopsy in the absence of an MRI target (79), as does the 2019 National Comprehensive Cancer Network guideline (12). As stated by the European Association of Urology, consideration must be given to other risk factors that could override a recommendation not to perform biopsy, as discussed in the preceding section on patient selection.
Performing only targeted biopsies in men with intermediate- or high-likelihood images has been shown to be efficacious in an influential prospective randomized study (6). However, the 2019 European Association of Urology guidelines (13) suggest the use of combined systematic and targeted biopsies in biopsy-naive men with intermediate- or high-likelihood MRI findings and recommend omitting systematic cores only in those with prior negative biopsy results, which the PI-RADS steering committee endorses (Table 2).
After taking these variations into account, the PI-RADS steering committee proposes the PI-RADS multiparametric MRI and MRI-directed biopsy pathway (3) (Table 1, Fig 3), which details the acceptable actions for low-likelihood (PI-RADS categories 1 and 2), intermediate-likelihood (PI-RADS category 3), and high-likelihood (PI-RADS categories 4 and 5) MRI findings. When formulating these recommendations, available clinical guidelines have been incorporated to provide consistent advice for practice. When clinical guidelines are at odds with our view or for imaging matters that are in our purview, the PI-RADS steering committee recommendations stand apart. Clear annotations on the origin of recommendations are given in Tables 1 and 2, so as not to give widely variant advice.
Delivering Benefits to Patients
The PI-RADS steering committee acknowledges the considerable challenge presented by implementation of multiparametric MRI and MRI-directed biopsies for most men suspected of having clinically significant prostate cancer. We acknowledge that not all patients suspected of having cancer benefit from the multiparametric MRI approach because clinical studies have largely excluded men with clinically obvious locally advanced disease with a markedly elevated PSA level (80). Furthermore, we realize that the results of studies undertaken in high-volume expert centers, with the advantages of state-of-the-art equipment, optimized protocols, and highly experienced subspecialized radiologists, may not be applicable to clinical practice everywhere. For multiparametric MRI and MRI-directed biopsy to deliver the intended pathway benefits, the quality of the entire diagnostic process must be ensured by having robustly trained technologists, experienced radiologists, and practitioners who conduct MRI-directed biopsy while working within multidisciplinary teams.
Many centers struggle with image optimization, particularly with diffusion-weighted sequences, which are important in disease detection and characterization. The quality of diffusion-weighted images affects apparent diffusion coefficient map calculations and computed high-b-value images, both of which are important for MRI risk categorization. Unfortunately, there are no universally accepted technical quality criteria that encompass the necessary spatial resolution, signal-to-noise ratio, and diffusivity measurements for prostate MRI. The Radiological Society of North America Quantitative Imaging Biomarkers Alliance is addressing this issue. MRI manufacturers need to provide equipment that delivers high-quality diffusion-weighted sequences; this point cannot be overemphasized. Well-trained MRI technologists and real-time quality control are needed to obtain the best images possible with the equipment used.
Reader expertise is also a major issue contributing to variability in reported studies and can potentially affect clinical care. Multiple factors affect the learning curve of prostate MRI reading (81). These include the expertise of radiologists, the availability of histopathologic and urologic feedback during multidisciplinary meetings, and mentoring. Several groups have advocated training and certification for radiologists who supervise and interpret prostate multiparametric MRI (52,82), suggesting that performance measures should be considered to ensure quality throughout the process. These measures include (a) requiring MRI readers and those who perform MRI-guided biopsy to complete continuing medical education courses, (b) requiring that radiologists interpret and report on a minimum number of prostate MRI studies per year, (c) requiring that whomever performs biopsies performs a minimum number of biopsies per year, (d) establishing benchmarks for the percentage of MRI studies that result in PI-RADS category 3 findings, and (e) requiring feedback from pathology and urology colleagues so that results can be audited and improved. Expert panels working within accrediting organizations, such as the American College of Radiology and the European Society of Urogenital Radiology, are taking the lead in defining detailed quality criteria for these purposes.
Future Directions and Conclusions
The Prostate Imaging Reporting and Data System (PI-RADS) version 2.1 update was recently released (2). The next major revision of PI-RADS is anticipated to be a multiyear endeavor because it will require additional research data to emerge on the clinical use of MRI and MRI-directed biopsy. The international PI-RADS Steering Committee therefore encourages investigations into the areas highlighted within Table E1 (online); the strength of emerging evidence will inform future PI-RADS guidance developments.
Notwithstanding, there is already high-quality evidence showing that PI-RADS evaluations of multiparametric MRI can help detect and localize life-threatening prostate cancer with greater clinical utility than the current standard, which is systematic transrectal US biopsy. MRI is already transforming prostate cancer diagnosis internationally. However, additional work needs to be done before we know the clinical impact of the incorporation of prostate MRI on the health outcomes of men suspected of having prostate cancer.
SUPPLEMENTAL TABLES
Disclosures of Conflicts of Interest: A.R.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a lecture fee and travel compensation from Siemens Healthineers. Other relationships: disclosed no relevant relationships. J.B. disclosed no relevant relationships. G.V. disclosed no relevant relationships. A.B.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received royalties from Thieme Medical Publishers. Other relationships: disclosed no relevant relationships. D.J.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Blue Earth Diagnostics, institution received an in-kind contribution of pulse sequences from Siemens Healthineers. Other relationships: disclosed no relevant relationships. B.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has cooperative research and development agreements with NVIDIA and Philips Medical Systems; receives royalties from U.S. patents for MRI/US biopsy and computer-aided diagnosis software. Other relationships: disclosed no relevant relationships. H.C.T. disclosed no relevant relationships. F.C. disclosed no relevant relationships. M.A.H. disclosed no relevant relationships. K.J.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received grants from Siemens Healthineers, Profound Medical, and GlaxoSmithKline; receives royalties from Elsevier. Other relationships: disclosed no relevant relationships. C.M.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: developed a CME program for Medscape. Other relationships: disclosed no relevant relationships. S.V. disclosed no relevant relationships. J.C.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: is an unpaid volunteer on two American College of Radiology (ACR) committees, PI-RADS and LI-RADS, and has been involved in ACR discussions about other reporting and data system efforts.
Abbreviations:
- CI
- confidence interval
- ISUP
- International Society of Urological Pathology
- PI-RADS
- Prostate Imaging Reporting and Data System
- PSA
- prostate-specific antigen
References
- 1.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging Reporting and Data System: 2015, version 2. Eur Urol 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol 2019 Mar 18 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Padhani AR, Weinreb J, Rosenkrantz AB, Villeirs G, Turkbey B, Barentsz J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 status update and future directions. Eur Urol 2019;75(3):385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stabile A, Giganti F, Emberton M, Moore CM. MRI in prostate cancer diagnosis: do we need to add standard sampling? a review of the last 5 years. Prostate Cancer Prostatic Dis 2018;21(4):473–487. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389(10071):815–822. [DOI] [PubMed] [Google Scholar]
- 6.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378(19):1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesen L, Nørgaard N, Løgager V, et al. Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy-naive men: the biparametric MRI for detection of prostate cancer (BIDOC) study. JAMA Netw Open 2018;1(2):e180219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 2019;75(4):570–578. [DOI] [PubMed] [Google Scholar]
- 9.Hansen NL, Barrett T, Kesch C, et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naïve men with suspicion of prostate cancer. BJU Int 2018;122(1):40–49. [DOI] [PubMed] [Google Scholar]
- 10.Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. The Cochrane database of systematic reviews. 2019;4:Cd012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venderink W, van Luijtelaar A, Bomers JG, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol 2017 Feb 28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology V1 .2019. Prostate cancer early detection recommendations. J Natl Compr Canc Netw 2019. [Google Scholar]
- 13.Mottet N, van den Bergh RC, Briers E, et al. 2019 EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer, Vol 53. Arnhem, the Netherlands: EAU Guidelines Office, 2019. [Google Scholar]
- 14.Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol (Dordr) 2016;39(2):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberts AR, Schoots IG, Bokhorst LP, van Leenders GJ, Bangma CH, Roobol MJ. Risk-based patient selection for magnetic resonance imaging-targeted prostate biopsy after negative transrectal ultrasound-guided random biopsy avoids unnecessary magnetic resonance imaging scans. Eur Urol 2016;69(6):1129–1134. [DOI] [PubMed] [Google Scholar]
- 16.Ankerst DP, Straubinger J, Selig K, et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. Eur Urol 2018;74(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts AR, Roobol MJ, Verbeek JFM, et al. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculators. Eur Urol 2019;75(2):310–318. [DOI] [PubMed] [Google Scholar]
- 18.Giganti F, Moore CM. A critical comparison of techniques for MRI-targeted biopsy of the prostate. Transl Androl Urol 2017;6(3):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehralivand S, Shih JH, Rais-Bahrami S, et al. A magnetic resonance imaging-based prediction model for prostate biopsy risk stratification. JAMA Oncol 2018;4(5):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radtke JP, Wiesenfarth M, Kesch C, et al. Combined clinical parameters and multiparametric magnetic resonance imaging for advanced risk modeling of prostate cancer: patient-tailored risk stratification can reduce unnecessary biopsies. Eur Urol 2017;72(6):888–896. [DOI] [PubMed] [Google Scholar]
- 21.Perlis N, Al-Kasab T, Ahmad A, et al. Defining a cohort that may not require repeat prostate biopsy based on PCA3 score and magnetic resonance imaging: the dual negative effect. J Urol 2018;199(5):1182–1187 [Published correction appears in J Urol 2018;200(3):660.]. [DOI] [PubMed] [Google Scholar]
- 22.Bjurlin MA, Rosenkrantz AB, Sarkar S, et al. Prediction of prostate cancer risk among men undergoing combined MRI-targeted and systematic biopsy using novel pre-biopsy nomograms that incorporate MRI findings. Urology 2018;112:112–120. [DOI] [PubMed] [Google Scholar]
- 23.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MGM. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68(3):438–450. [DOI] [PubMed] [Google Scholar]
- 24.Grönberg H, Eklund M, Picker W, et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol 2018;74(6):722–728. [DOI] [PubMed] [Google Scholar]
- 25.Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;319(18):1914–1931. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016;40(2):244–252. [DOI] [PubMed] [Google Scholar]
- 27.Valerio M, Anele C, Bott SRJ, et al. The prevalence of clinically significant prostate cancer according to commonly used histological thresholds in men undergoing template prostate mapping biopsies. J Urol 2016;195(5):1403–1408. [DOI] [PubMed] [Google Scholar]
- 28.Kuru TH, Wadhwa K, Chang RTM, et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int 2013;112(5):568–577. [DOI] [PubMed] [Google Scholar]
- 29.Bokhorst LP, Valdagni R, Rannikko A, et al. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol 2016;70(6):954–960. [DOI] [PubMed] [Google Scholar]
- 30.Chen RC, Rumble RB, Loblaw DA, et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2016;34(18):2182–2190. [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence (NICE) . Prostate cancer: diagnosis and management (update). 2019. https://www.nice.org.uk/guidance/indevelopment/gid-ng10057. Accessed January 2019. [PubMed] [Google Scholar]
- 32.Simmons LAM, Kanthabalan A, Arya M, et al. The PICTURE study: diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br J Cancer 2017;116(9):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Distler FA, Radtke JP, Bonekamp D, et al. The value of PSA density in combination with PI-RADS for the accuracy of prostate cancer prediction. J Urol 2017;198(3):575–582. [DOI] [PubMed] [Google Scholar]
- 34.Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? a systematic review and meta-analysis from the European Association of Urology prostate cancer guidelines panel. Eur Urol 2017;72(2):250–266. [DOI] [PubMed] [Google Scholar]
- 35.Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019;20(1):100–109. [DOI] [PubMed] [Google Scholar]
- 36.Itatani R, Namimoto T, Atsuji S, et al. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 2014;83(10):1740–1745. [DOI] [PubMed] [Google Scholar]
- 37.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66(1):22–29. [DOI] [PubMed] [Google Scholar]
- 38.Wysock JS, Mendhiratta N, Zattoni F, et al. Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int 2016;118(4):515–520. [DOI] [PubMed] [Google Scholar]
- 39.Wang RS, Kim EH, Vetter JM, et al. Determination of the role of negative magnetic resonance imaging of the prostate in clinical practice: is biopsy still necessary? Urology 2017;102:190–197. [DOI] [PubMed] [Google Scholar]
- 40.Lu AJ, Syed JS, Nguyen KA, et al. Negative multiparametric magnetic resonance imaging of the prostate predicts absence of clinically significant prostate cancer on 12-core template prostate biopsy. Urology 2017;105:118–122. [DOI] [PubMed] [Google Scholar]
- 41.Washino S, Okochi T, Saito K, et al. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int 2017;119(2):225–233. [DOI] [PubMed] [Google Scholar]
- 42.Boesen L, Nørgaard N, Løgager V, et al. Prebiopsy biparametric magnetic resonance imaging combined with prostate-specific antigen density in detecting and ruling out Gleason 7–10 prostate cancer in biopsy-naïve men. Eur Urol Oncol 2018 Sep 26 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol 2017;72(2):282–288. [DOI] [PubMed] [Google Scholar]
- 44.Bryant RJ, Hobbs CP, Eyre KS, et al. Comparison of prostate biopsy with or without prebiopsy multiparametric magnetic resonance imaging for prostate cancer detection: an observational cohort study. J Urol 2019;201(3):510–519. [DOI] [PubMed] [Google Scholar]
- 45.Punnen S, Nahar B, Soodana-Prakash N, et al. Optimizing patient’s selection for prostate biopsy: a single institution experience with multi-parametric MRI and the 4Kscore test for the detection of aggressive prostate cancer. PLoS One 2018;13(8):e0201384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oishi M, Shin T, Ohe C, et al. Which patients with negative magnetic resonance imaging can safely avoid biopsy for prostate cancer? J Urol 2019;201(2):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen NL, Barrett T, Koo B, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7-10 prostate cancer in a repeat biopsy setting. BJU Int 2017;119(5):724–730. [DOI] [PubMed] [Google Scholar]
- 48.Niu XK, He WF, Zhang Y, et al. Developing a new PI-RADS v2-based nomogram for forecasting high-grade prostate cancer. Clin Radiol 2017;72(6):458–464. [DOI] [PubMed] [Google Scholar]
- 49.Bhat NR, Vetter JM, Andriole GL, Shetty AS, Ippolito JE, Kim EH. Magnetic resonance imaging-defined prostate-specific antigen density significantly improves the risk prediction for clinically significant prostate cancer on biopsy. Urology 2019;126:152–157. [DOI] [PubMed] [Google Scholar]
- 50.Panebianco V, Barchetti G, Simone G, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: what’s next? Eur Urol 2018;74(1):48–54. [DOI] [PubMed] [Google Scholar]
- 51.De Visschere PJL, Naesens L, Libbrecht L, et al. What kind of prostate cancers do we miss on multiparametric magnetic resonance imaging? Eur Radiol 2016;26(4):1098–1107. [DOI] [PubMed] [Google Scholar]
- 52.Brizmohun Appayya M, Adshead J, Ahmed HU, et al. National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection: recommendations from a UK consensus meeting. BJU Int 2018;122(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofbauer SL, Maxeiner A, Kittner B, et al. Validation of Prostate Imaging Reporting and Data System version 2 for the detection of prostate cancer. J Urol 2018;200(4):767–773. [DOI] [PubMed] [Google Scholar]
- 54.Mehralivand S, Bednarova S, Shih JH, et al. Prospective evaluation of PI-RADS version 2 using the International Society of Urological Pathology prostate cancer grade group system. J Urol 2017;198(3):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barkovich EJ, Shankar PR, Westphalen AC. A systematic review of the existing Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) literature and subset meta-analysis of PI-RADSv2 categories stratified by Gleason scores. AJR Am J Roentgenol 2019;212(4):847–854. [DOI] [PubMed] [Google Scholar]
- 56.Schoots IG. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol 2018;7(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borkowetz A, Platzek I, Toma M, et al. Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy. BJU Int 2016;118(2):213–220. [DOI] [PubMed] [Google Scholar]
- 58.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016;122(6):884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jambor I, Boström PJ, Taimen P, et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD trial). J Magn Reson Imaging 2017;46(4):1089–1095. [DOI] [PubMed] [Google Scholar]
- 60.Gómez Rivas J, Giganti F, Álvarez-Maestro M, et al. Prostate indeterminate lesions on magnetic resonance imaging-biopsy versus surveillance: a literature review. Eur Urol Focus 2018 Mar 7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61.Hansen N, Patruno G, Wadhwa K, et al. Magnetic resonance and ultrasound image fusion supported transperineal prostate biopsy using the Ginsburg protocol: technique, learning points, and biopsy results. Eur Urol 2016;70(2):332–340. [DOI] [PubMed] [Google Scholar]
- 62.Hansen NL, Kesch C, Barrett T, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 2017;120(5):631–638. [DOI] [PubMed] [Google Scholar]
- 63.Luzzago S, Petralia G, Musi G, et al. Multiparametric magnetic resonance imaging second opinion may reduce the number of unnecessary prostate biopsies: time to improve radiologists’ training program? Clin Genitourin Cancer 2018 Oct 23 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64.Gaur S, Harmon S, Mehralivand S, et al. Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018;48(5):1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI-RADS version 2 decision rules: impact on prostate cancer detection. Radiology 2017;283(1):119–129. [DOI] [PubMed] [Google Scholar]
- 66.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313(4):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bryk DJ, Llukani E, Taneja SS, Rosenkrantz AB, Huang WC, Lepor H. The role of ipsilateral and contralateral transrectal ultrasound-guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging-ultrasound fusion-targeted prostate biopsy. Urology 2017;102:178–182. [DOI] [PubMed] [Google Scholar]
- 68.Muthigi A, George AK, Sidana A, et al. Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/transrectal ultrasound fusion biopsy. J Urol 2017;197(2):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calio BP, Sidana A, Sugano D, et al. Risk of upgrading from prostate biopsy to radical prostatectomy pathology: does saturation biopsy of index lesion during multiparametric magnetic resonance imaging-transrectal ultrasound fusion biopsy help? J Urol 2018;199(4):976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamid S, Donaldson IA, Hu Y, et al. The SmartTarget biopsy trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for frostate cancer risk stratification. Eur Urol 2019;75(5):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons LAM, Kanthabalan A, Arya M, et al. Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J Urol 2018;200(6):1227–1234. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M, Milot L, Khalvati F, et al. Value of increasing biopsy cores per target with cognitive MRI-targeted transrectal US prostate biopsy. Radiology 2019;291(1):83–89. [DOI] [PubMed] [Google Scholar]
- 73.Porpiglia F, De Luca S, Passera R, et al. Multiparametric magnetic resonance/ultrasound fusion prostate biopsy: number and spatial distribution of cores for better index tumor detection and characterization. J Urol 2017;198(1):58–64. [DOI] [PubMed] [Google Scholar]
- 74.Beksac AT, Cumarasamy S, Gupta A, et al. MRI fusion biopsy is associated with a higher rate of pathologic downgrading at radical prostatectomy. 18th Annual Meeting of the Society of Urologic Oncology, 2017; 186. [Google Scholar]
- 75.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012;61(5):1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mesko S, Marks L, Ragab O, et al. Targeted prostate biopsy Gleason score heterogeneity and implications for risk stratification. Am J Clin Oncol 2018;41(5):497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol 2018;74(6):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schoots IG, Roobol MJ. Multivariate risk prediction tools including MRI for individualized biopsy decision in prostate cancer diagnosis: current status and future directions. World J Urol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenkrantz AB, Verma S, Choyke P, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 2016;196(6):1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morote J, Celma A, Roche S, et al. Who benefits from multiparametric magnetic resonance imaging after suspicion of prostate cancer? Eur Urol Oncol 2018 Dec 14 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 81.Gaziev G, Wadhwa K, Barrett T, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int 2016;117(1):80–86. [DOI] [PubMed] [Google Scholar]
- 82.Puech P, Randazzo M, Ouzzane A, et al. How are we going to train a generation of radiologists (and urologists) to read prostate MRI? Curr Opin Urol 2015;25(6):522–535. [DOI] [PubMed] [Google Scholar]
- 83.NHS England . Implementing a timed prostate cancer diagnostic pathway. NHS Cancer Programme Operations Information. NHS England, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.