Abstract
Periodontitis is an infection-induced inflammatory disease that affects the tooth supporting tissues, i.e., bone and connective tissues. The initiation and progression of this disease depend on dysbiotic ecological changes in the oral microbiome, thereby affecting the severity of disease through multiple immune-inflammatory responses. Aggregatibacter actinomycetemcomitans is a facultative anaerobic Gram-negative bacterium associated with such cellular and molecular mechanisms associated with the pathogenesis of periodontitis. In the present review, we outline virulence mechanisms that help the bacterium to escape the host response. These properties include invasiveness, secretion of exotoxins, serum resistance, and release of outer membrane vesicles. Virulence properties of A. actinomycetemcomitans that can contribute to treatment resistance in the infected individuals and upon translocation to the circulation, also induce pathogenic mechanisms associated with several systemic diseases.
Keywords: Aggregatibacter actinomycetemcomitans, invasiveness, leukotoxin, cytolethal distending toxin, serum resistance, outer membrane vesicles
1. Introduction
Aggregatibacter actinomycetemcomitans is an opportunistic pathogen associated with aggressive forms of periodontitis that affect young individuals [1,2]. The bacterium colonizes the oral mucosa early in life and is inherited by vertical transmission from close relatives [3]. Colonization of A. actinomycetemcomitans on the mucosa is not associated with disease but is considered as a risk factor for translocation of the organism to the gingival margin [4]. Bacteria that colonize this ecological niche have the potential to initiate periodontal diseases if they are allowed to stay, proliferate, and express virulence factors [5,6]. A. actinomycetemcomitans is a facultative anaerobic Gram-negative bacterium with the capacity to produce a number of virulence factors, and it exhibits a large genetic diversity [2,7]. This bacterium is an early colonizer in the disease process, and resists oxygen and hydrogen peroxide, but is later often replaced by more strict anaerobes in the deep periodontal pocket [1]. In addition to colonizing the oral cavity, systemic translocation of this bacterium is frequently reported [8,9]. A. actinomycetemcomitans expresses adhesins that allow colonization of to the tooth surface and the oral epithelium, as well as to mature supragingival plaque [2]. The bacterium is described as an organism that utilizes the other inhabitants in the biofilm for its survival and utilizes metabolic products from other inhabitants of the biofilm for survival and growth [1]. In addition, it is suggested that A. actinomycetemcomitans can promote the overgrowth of other bacterial species, which can result in local host dysbiosis and susceptibility to infection [1]. The association of A. actinomycetemcomitans to systemic diseases includes endocarditis, cardiovascular diseases, diabetes, Alzheimer´s disease, and rheumatoid arthritis [10,11,12,13,14,15]. The mechanisms behind these associations are not known, but several virulence properties of A. actinomycetemcomitans, such as tissue invasiveness, exotoxin production, serum resistance, and outer membrane vesicle secretion are potential weapons [16,17,18,19]. In the sections below, we will further address and discuss the tools behind the ability of this bacterium to evade and suppress the host immune response. The aim of the present review is to identify and describe virulence mechanisms of A. actinomycetemcomitans, which are associated with immune subversion, as well as bacterial pathogenicity.
2. Invasive Properties
Invasion of periodontal tissues by different bacterial species has been reported in human periodontitis for several decades [20,21,22]. The study by Saglie and co-workers [22] observed the prevalence and gingival localization of A. actinomycetemcomitans in periodontal lesions of young patients. Transmission electron microscopic examination showed microcolonies of small Gram-negative rods in the connective tissue, as well as single bacterial cells between collagen fibers and in areas of cell debris [20]. In addition to these intra-tissue bacterial cells, bacteria were also found within phagocytic cells, which had invaded the gingival connective tissue. More recent studies have demonstrated invasion of A. actinomycetemcomitans into epithelial cells in vitro [23,24,25]. Interestingly, some A. actinomycetemcomitans genotypes have been suggested to have different tissue invasive-properties [23]. If this difference in invasive properties interferes with the ability of A. actinomycetemcomitans to cause various periodontal or systemic diseases is not known.
A number of A. actinomycetemcomitans factors that likely contribute to host cell invasion have been elucidated. These include the tad (tight adherence) gene locus, which mediates adhesion and is required for virulence in a rat model for periodontal disease [26]. OmpA1 (also known as Omp29) is associated with the entry of A. actinomycetemcomitans into gingival epithelial cells by up-regulating F-actin rearrangement via the FAK signaling pathway [27], and Omp100 (also known as ApiA) promotes adhesion of A. actinomycetemcomitans cells, and their invasion of human gingival keratinocytes [28,29]. It has also been described that bacteria that express a cytolethal distending toxin, such as A. actinomycetemcomitans, can cause disruption of the epithelial barrier and promote tissue invasion [30]. A role of A. actinomycetemcomitans invasion in immune modulation is supported by in vitro evidence of a subsequent induction of pro-inflammatory cytokine production, and/or apoptosis, in epithelial cells and macrophage-like cells, respectively [31,32].
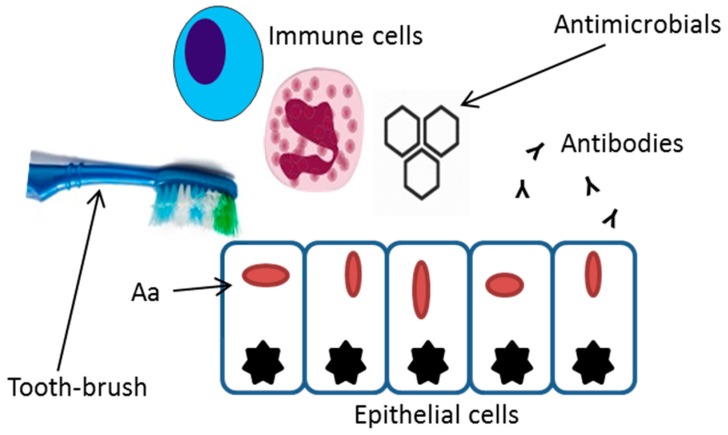
Severe extra-oral infections caused by A. actinomycetemcomitans include brain abscesses, meningitis, septicemia, urinary tract infections, osteomyelitis, and endocarditis [18,33,34,35]. Whether the systemic translocation through the epithelial barrier is due to an active invasive process, or a result of a passive leakage into the blood stream is not known [36,37]. Bacterial invasion of the periodontal tissues has been suggested as a relevant stage in the etiopathogenesis of periodontal disease, however, there is insufficient evidence to support or exclude this mechanism as a key step in periodontal disease [38,39]. Despite the lack of conclusive studies, the invasive properties of A. actinomycetemcomitans have the potential to help the bacterium to evade mechanical and chemical strategies immune responses, and mechanical or chemical eradication strategies [40] (Figure 1). If these properties of the bacterium contribute to systemic translocation and survival has not yet been studied. Evidently, major reasons for bacteremia caused by oral bacteria such as A. actinomycetemcomitans are gingival inflammation and mechanical manipulation [41].
Figure 1.
Invasion of A. actinomycetemcomitans into epithelial cells can protect the bacterial cells from mechanical removal, antibiotics, immune cell phagocytosis, and antibody binding.
3. Production of Exotoxins
A. actinomycetemcomitans is the only bacterium colonizing the oral cavity known to produce two exotoxins [42,43], leukotoxin (LtxA) that specifically induces killing of human leukocytes, and a cytolethal distending toxin (CDT), which is a genotoxin, causing growth arrest by affecting DNA in proliferating cells [16,44]. Both toxins are highly conserved and occur also in several other Gram-negative pathogens [45,46]. As a sign of the large genetic diversity of A. actinomycetemcomitans, there are strains, representing various genotypes, which produce highly different levels of these toxins [2,6,47]. All hitherto studied A. actinomycetemcomitans strains carry a complete ltxCABD gene locus, encoding for LtxA, activation and secretion [6]. Mutations, i.e., deletions and insertions in the ltx promoter region have been shown to influence leukotoxin production in A. actinomycetemcomitans [48,49,50]. The so called JP2 genotype, which harbors a 530-bp deletion in the ltx promoter region is well studied, and known to be strongly associated with disease risk in the individuals carrying it [5,6,51]. Its high LtxA production is considered to be an important factor for the enhanced pathogenicity of this genotype [52].
LtxA induces several pathogenic mechanisms in human leukocytes that can all be linked to the progression of periodontal disease [16]. A substantial humoral immune response against LtxA is initiated in all the infected individuals [53,54]. LtxA kills immune cells and protects the bacterium from phagocytic killing [55]. Neutrophils exposed to LtxA activate degranulation, concomitant with an extracellular release of proteolytic enzymes and metalloproteases, such as elastase and metallproteases [56,57]. LtxA can also affect human macrophages by activating the inflammasome complex, which results in the activation and secretion of pro-inflammatory enzymes (i.e., IL-1β and IL-18) [58]. In this context, an interesting observation was recently made that A. actinomycetemcomitans expresses an outer membrane lipoprotein, which binds host cytokines, including IL-1β [59]. The IL-1β binding protein was designated bacterial interleukin receptor I (BilRI) and has the ability to internalize IL-1β into the viable bacterial biofilm [60]. Taken together, the abilities of LtxA to cause a proteolytic environment that can degrade immunoproteins, internalize inflammatory proteins, and kill immune cells may all contribute to the survival of A. actinomycetemcomitans in the infected host.
The CDT is expressed by a majority of A. actinomycetemcomitans genotypes, even though some of them lack a complete gene operon for expression of an active holo-toxin [45]. In vitro and in vivo studies have shown that CDT affects cellular physiology involved in inflammation, immune response modulation, and causes tissue damage [61,62]. The holo-toxin consists of three subunits (CdtA, B, and C) and is transported to the nucleus of the mammalian target cells [63]. Cells exposed for CDT induce a growth arrest followed by apoptotic cell death [44,63]. In cultures of periodontal fibroblasts, CDT induces expression of cytokines and the osteoclast activating protein, receptor activator of nuclear factor kappa-Β ligand (RANKL) [64,65]. The role of CDT in the pathogenesis of periodontitis is not entirely clear, and the literature contains studies that demonstrate an association, as well as those reporting no correlation [42,66,67].
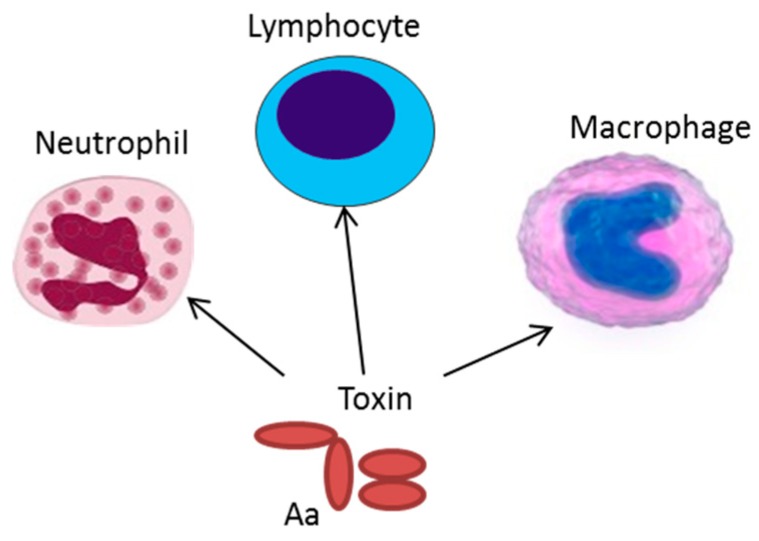
Together LtxA and CDT can act as strong weapons against the immune response raised against A. actinomycetemcomitans. They can cause an imbalance in the host response by activating inflammation, killing immune cells, affecting antigen presenting cells, and inhibiting lymphocyte proliferation (Figure 2). The impact of these exotoxins in the pathogenesis of periodontal disease is apparent, whereas their role in systemic diseases is not known. However, LtxA-exposed neutrophils do release net-like structures and express patterns of citrullinated proteins that are similar to those observed in synovial fluid from inflamed joints [12,68]. Moreover, antibiotic treatment of a periodontitis patient, infected with the highly leukotoxic JP2 genotype of A. actinomycetemcomitans, and suffering from rheumatoid arthritis, was also cured of the joint pain after the treatment [69]. These observations indicate that A. actinomycetemcomitans is an interesting organism in the etiopathogenesis of rheumatoid arthritis.
Figure 2.
Expression of exotoxins can result in resistance of A. actinomycetemcomitans to phagocytosis and neutrophil degranulation. The cytolethal distending toxin (CDT) can cause inhibited proliferation of stimulated lymphocytes, and LtxA induces an inflammatory cell death in the antigen presenting cells, macrophages, and monocytes.
4. Serum Resistance
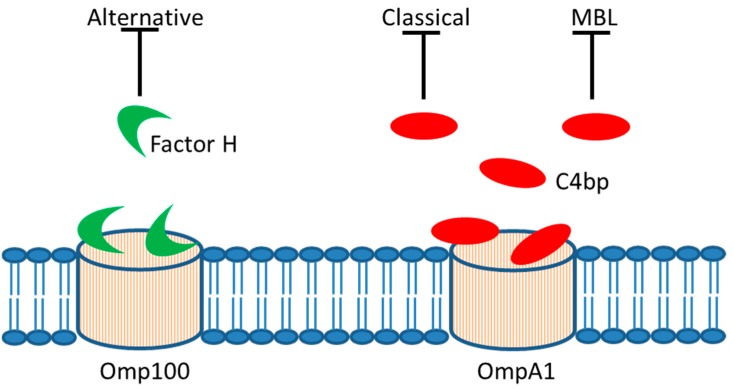
Serum resistance represents an important virulence factor of bacteria that enter into the bloodstream and cause infection, allowing the bacterial cells to evade the innate immune defense mechanisms present in serum, including the complement system and antimicrobial peptides [70,71,72]. The recognition of bacterial products mediating serum resistance, therefore, represents an approach to the vaccine and drug development [73,74]. Resistance to complement-mediated killing by human serum appears to be important for A. actinomycetemcomitans virulence, and is a common characteristic among strains of this species, although they typically do not form capsules [28,75]. The outer membrane protein, Omp100 (ApiA), was earlier demonstrated to be important for serum resistance in some serotype b and d strains and to physically interact with and trap the alternative complement pathway negative regulator, Factor H, in vitro [28,76] (Figure 3).
Figure 3.
Serum resistance. In vitro evidence supports that A. actinomycetemcomitans outer membrane proteins, Omp100 and OmpA1, may allow trapping of soluble repressors of complement activation, i.e., Factor H and C4 binding protein (C4bp), respectively. This would result in downregulation of the alternative, classical, and mannose-binding lectin (MBL) pathway of complement activation.
Evidently, Omps produced by A. actinomycetemcomitans strains are immuno-reactive in the human host [77]. As the presence of antibodies towards bacterial antigens such as Omps is a known trigger of classical complement activation [78], serum resistance of A. actinomycetemcomitans strains would be expected to also include mechanisms interacting with this activation. We recently presented evidence that the major outer membrane protein, OmpA1, is critical for serum survival in the A. actinomycetemcomitans serotype a model strain, D7SS [17]. Outer membrane integrity may be one mechanism behind OmpA-mediated serum resistance in Gram-negative bacteria [79]. Interestingly, serum resistant ompA1 mutants were fortuitously obtained, which expressed increased levels of the paralogue, OmpA2. Thus, OmpA2 can apparently operate as a functional homologue to OmpA1 in A. actinomycetemcomitans, and both proteins seemingly act, at least partly by binding and trapping of C4-binding protein [17] (Figure 3), which is an inhibitor of classical and mannose-binding lectin (MBL) complement activation [80]. Further to these activation pathways, alternative complement activation is needed to fully eliminate serum-sensitive ompA mutant A. actinomycetemcomitans derivatives [17]. It is plausible that serum resistance in this species, similar to in Acinetobacter baumanii [81], is highly complex and relies on a large number of gene products, including host factors. For example, whether cleavage of the complement molecule C3 by elastase [82], which release is triggered by leukotoxin [57], may contribute to A. actinomycetemcomitans serum resistance is not known. Moreover, albeit A. actinomycetemcomitans strains are ubiquitously serum resistant, strains not expressing their immunodominant, serotype-specific polysaccharide (S-PA) antigen are occasionally isolated [83]. As speculated previously [83], the lack of S-PA expression may represent a mechanism to evade from antibody-based host responses, which could be advantageous in blood circulation. However, an inconsistency with this notion is that the absence of S-PA expression in A. actinomycetemcomitans appears to be scarce.
5. Outer Membrane Vesicles
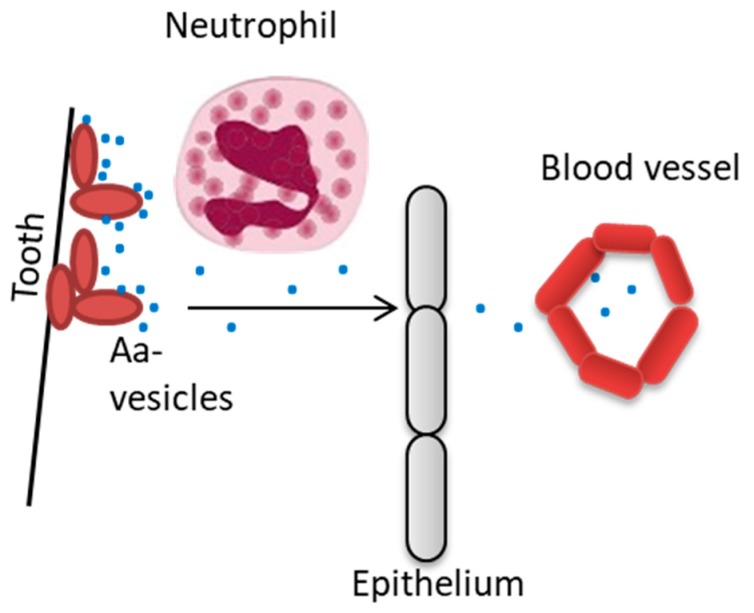
Outer Membrane Vesicles (OMVs) of Gram-negative bacteria are spherical membrane-enclosed nanostructures that are released from the outer membrane. They can operate as a fundamental mechanism for discharging proteins and additional bacterial components into the surrounding environment and to target host cells [84,85]. Evidence from in vitro experiments shows that A. actinomycetemcomitans OMVs can deliver an abundance of biologically active virulence factors to host cells, and which can modulate the immune response (Figure 4).
Figure 4.
Release of outer membrane vesicles may serve as protection of A. actinomycetemcomitans from phagocytic- and serum killing, and also as a means to transport virulence factors to tissues that not are in close contact with the infecting bacteria.
One such example is CDT, which is delivered into HeLa cells and human gingival fibroblasts via OMVs [86]. OMVs are also involved in the export of LtxA, peptidoglycan-associated lipoprotein (Pal), and the chaperonin GroEL to host cells [87,88,89,90]. Proteomics and Western blot analysis of A. actinomycetemcomitans OMVs has identified additional proteins that can contribute to evasion of the immune defense, including the IL1β-binding lipoprotein, BilRI, the outer membrane proteins Omp100, OmpA1, and OmpA2, and a Factor H-binding protein homologue [17,91,92]. A functional role in the interaction with complement by vesicles is supported by observations that A. actinomycetemcomitans OMVs in an OmpA1-dependent manner can bind to the classical and MBL complement inhibitor, C4-binding protein [17]. It has also been demonstrated that A. actinomycetemcomitans OMVs can carry small molecules, including lipopolysaccharide (LPS), which can interact with complement [93]. LPS may also play a role in the observed binding of A. actinomycetemcomitans OMVs to IL-8 [94]. Evidence that A. actinomycetemcomitans OMVs carry NOD1- and NOD2-active peptidoglycan, which can be internalized into non-phagocytic human cells including gingival fibroblasts [19], reveals a role of the vesicles as a trigger of innate immunity. Moreover, OMV-dependent release of microRNA-size small RNAs (msRNAs), may potentially represent a mechanism to transfer a novel class of bacterial signaling molecules into host cells [95]. It is not completely understood how A. actinomycetemcomitans OMVs may physically interact with and/or enter into human host cells to enhance bacterial evasion of the immune defense. The OMVs appear to enter into human cells via clathrin-mediated endocytosis [19,96], but can also fuse with host membranes in a process dependent on cholesterol [86]. Toxins exported via OMVs can function as adhesins in receptor-mediated endocytosis of the vesicles [97], albeit neither CDT nor leukotoxin are required per se for the OMV uptake into host cells [86,87]. Concomitantly, although LtxA has an apparent localization on the A. actinomycetemcomitans OMV surface, its receptor LFA-1 is not required for delivering the toxin into host cells [98].
6. Conclusions
We have summarized current knowledge regarding major attributes and strategies of A. actinomycetemcomitans, allowing this organism to evade the host response. Without doubt, the numerous virulence properties of A. actinomycetemcomitans can be linked to the pathogenesis of periodontal disease [99]. Utilization of these properties for systemic translocation of A. actinomycetemcomitans and its subsequent survival in this new environment has been excellently summarized and illustrated [1,100]. It is today hypothesized that the virulence characteristics of A. actinomycetemcomitans allow this organism to induce an immune subversion that tip the balance from homeostasis over to disease in oral and/or extra-oral sites [101]. Hence, in order to prohibit the negative systemic consequences that are associated with periodontitis, successful treatment in an early phase of the disease is fundamental. Development of specific diagnostic tools for assessment of periodontal pathogens and inflammatory components in the saliva of young individuals might make it possible to prevent the disease before its onset.
Author Contributions
Conceptualization, A.J., J.O. and, R.C.; writing—original draft preparation, A.J. and, J.O.; writing—review and editing, A.J., J.O., R.C., M.L., and C.H.Å.; funding acquisition, A.J., M.L. and, J.O.; supervision, C.H.Å., R.C., A.J. and, J.O.
Funding
This work was supported by TUA grants from the County Council of Västerbotten, Sweden (to J.O. and A.J.), by funds from Insamlingsstiftelsen, Medical Faculty, Umeå University (to J.O. and A.J.), and from Svenska Tandläkare-sällskapet, Kempe Foundation, and Thuréus Foundation (to M.L.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Fine D.H., Patil A.G., Velusamy S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019;10:728. doi: 10.3389/fimmu.2019.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson B., Ward J.M., Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontol. 2000. 2010;54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x. [DOI] [PubMed] [Google Scholar]
- 3.Könönen E., Muller H.P. Microbiology of aggressive periodontitis. Periodontol. 2000. 2014;65:46–78. doi: 10.1111/prd.12016. [DOI] [PubMed] [Google Scholar]
- 4.Höglund Åberg C., Kelk P., Johansson A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence. 2015;6:188–195. doi: 10.4161/21505594.2014.982428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haubek D., Ennibi O.K., Poulsen K., Vaeth M., Poulsen S., Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet. 2008;371:237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 6.Höglund Åberg C., Kwamin F., Claesson R., Dahlen G., Johansson A., Haubek D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: A prospective cohort study of a young adolescent population. J. Clin. Periodontol. 2014;41:232–241. doi: 10.1111/jcpe.12209. [DOI] [PubMed] [Google Scholar]
- 7.Kittichotirat W., Bumgarner R.E., Chen C. Evolutionary Divergence of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2016;95:94–101. doi: 10.1177/0022034515608163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paju S., Carlson P., Jousimies-Somer H., Asikainen S. Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus in systemic and nonoral infections in Finland. APMIS. 2003;111:653–657. doi: 10.1034/j.1600-0463.2003.1110608.x. [DOI] [PubMed] [Google Scholar]
- 9.van Winkelhoff A.J., Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol. 2000. 1999;20:122–135. doi: 10.1111/j.1600-0757.1999.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 10.Demmer R.T., Jacobs D.R., Jr., Singh R., Zuk A., Rosenbaum M., Papapanou P.N., Desvarieux M. Periodontal Bacteria and Prediabetes Prevalence in ORIGINS: The Oral Infections, Glucose Intolerance, and Insulin Resistance Study. J. Dent. Res. 2015;94:201S–211S. doi: 10.1177/0022034515590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Zuniga J., Munoz Y., Melgar-Rodriguez S., More J., Bruna B., Lobos P., Monasterio G., Vernal R., Paula-Lima A. Serotype b of Aggregatibacter actinomycetemcomitans triggers pro-inflammatory responses and amyloid beta secretion in hippocampal cells: A novel link between periodontitis and Alzheimer’s disease? J. Oral Microbiol. 2019;11:1586423. doi: 10.1080/20002297.2019.1586423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konig M.F., Abusleme L., Reinholdt J., Palmer R.J., Teles R.P., Sampson K., Rosen A., Nigrovic P.A., Sokolove J., Giles J.T., et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laugisch O., Johnen A., Maldonado A., Ehmke B., Burgin W., Olsen I., Potempa J., Sculean A., Duning T., Eick S. Periodontal Pathogens and Associated Intrathecal Antibodies in Early Stages of Alzheimer’s Disease. J. Alzheimers Dis. 2018;66:105–114. doi: 10.3233/JAD-180620. [DOI] [PubMed] [Google Scholar]
- 14.Liljestrand J.M., Paju S., Pietiainen M., Buhlin K., Persson G.R., Nieminen M.S., Sinisalo J., Mantyla P., Pussinen P.J. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis. 2018;268:177–184. doi: 10.1016/j.atherosclerosis.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Revest M., Egmann G., Cattoir V., Tattevin P. HACEK endocarditis: State-of-the-art. Expert Rev. Anti-Infect. Ther. 2016;14:523–530. doi: 10.1586/14787210.2016.1164032. [DOI] [PubMed] [Google Scholar]
- 16.Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins. 2011;3:242–259. doi: 10.3390/toxins3030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindholm M., Min Aung K., Nyunt Wai S., Oscarsson J. Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter aphrophilus serum resistance. J. Oral Microbiol. 2019;11:1536192. doi: 10.1080/20002297.2018.1536192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raja M., Ummer F., Dhivakar C.P. Aggregatibacter actinomycetemcomitans—A tooth killer? J. Clin. Diagn. Res. 2014;8:ZE13–ZE16. doi: 10.7860/JCDR/2014/9845.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thay B., Damm A., Kufer T.A., Wai S.N., Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-kappaB activation. Infect. Immun. 2014;82:4034–4046. doi: 10.1128/IAI.01980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christersson L.A., Albini B., Zambon J.J., Wikesjo U.M., Genco R.J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J. Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- 21.Christersson L.A., Wikesjo U.M., Albini B., Zambon J.J., Genco R.J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J. Periodontol. 1987;58:540–545. doi: 10.1902/jop.1987.58.8.540. [DOI] [PubMed] [Google Scholar]
- 22.Saglie F.R., Marfany A., Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J. Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 23.Lepine G., Caudry S., DiRienzo J.M., Ellen R.P. Epithelial cell invasion by Actinobacillus actinomycetemcomitans strains from restriction fragment-length polymorphism groups associated with juvenile periodontitis or carrier status. Oral Microbiol. Immunol. 1998;13:341–347. doi: 10.1111/j.1399-302X.1998.tb00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer D.H., Sreenivasan P.K., Fives-Taylor P.M. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect. Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer D.H., Lippmann J.E., Fives-Taylor P.M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: A dynamic, multistep process. Infect. Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiner H.C., Sinatra K., Kaplan J.B., Furgang D., Kachlany S.C., Planet P.J., Perez B.A., Figurski D.H., Fine D.H. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc. Natl. Acad. Sci. USA. 2003;100:7295–7300. doi: 10.1073/pnas.1237223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kajiya M., Komatsuzawa H., Papantonakis A., Seki M., Makihira S., Ouhara K., Kusumoto Y., Murakami S., Taubman M.A., Kawai T. Aggregatibacter actinomycetemcomitans Omp29 is associated with bacterial entry to gingival epithelial cells by F-actin rearrangement. PLoS ONE. 2011;6:e18287. doi: 10.1371/journal.pone.0018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asakawa R., Komatsuzawa H., Kawai T., Yamada S., Goncalves R.B., Izumi S., Fujiwara T., Nakano Y., Suzuki N., Uchida Y., et al. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 2003;50:1125–1139. doi: 10.1046/j.1365-2958.2003.03748.x. [DOI] [PubMed] [Google Scholar]
- 29.Yue G., Kaplan J.B., Furgang D., Mansfield K.G., Fine D.H. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and Old World primates. Infect. Immun. 2007;75:4440–4448. doi: 10.1128/IAI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiRienzo J.M. Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease. Cells. 2014;3:476–499. doi: 10.3390/cells3020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickinson B.C., Moffatt C.E., Hagerty D., Whitmore S.E., Brown T.A., Graves D.T., Lamont R.J. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol. Oral Microbiol. 2011;26:210–220. doi: 10.1111/j.2041-1014.2011.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okinaga T., Ariyoshi W., Nishihara T. Aggregatibacter actinomycetemcomitans Invasion Induces Interleukin-1beta Production Through Reactive Oxygen Species and Cathepsin B. J. Interferon. Cytokine Res. 2015;35:431–440. doi: 10.1089/jir.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahamat-Langendoen J.C., van Vonderen M.G., Engstrom L.J., Manson W.L., van Winkelhoff A.J., Mooi-Kokenberg E.A. Brain abscess associated with Aggregatibacter actinomycetemcomitans: Case report and review of literature. J. Clin. Periodontol. 2011;38:702–706. doi: 10.1111/j.1600-051X.2011.01737.x. [DOI] [PubMed] [Google Scholar]
- 34.Stepanovic S., Tosic T., Savic B., Jovanovic M., K’Ouas G., Carlier J.P. Brain abscess due to Actinobacillus actinomycetemcomitans. APMIS. 2005;113:225–228. doi: 10.1111/j.1600-0463.2005.apm1130312.x. [DOI] [PubMed] [Google Scholar]
- 35.Tang G., Kitten T., Munro C.L., Wellman G.C., Mintz K.P. EmaA, a potential virulence determinant of Aggregatibacter actinomycetemcomitans in infective endocarditis. Infect. Immun. 2008;76:2316–2324. doi: 10.1128/IAI.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doran K.S., Banerjee A., Disson O., Lecuit M. Concepts and mechanisms: Crossing host barriers. Cold Spring Harb. Perspect. Med. 2013;3 doi: 10.1101/cshperspect.a010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribet D., Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17:173–183. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Ji S., Choi Y.S., Choi Y. Bacterial invasion and persistence: Critical events in the pathogenesis of periodontitis? J. Periodontal Res. 2015;50:570–585. doi: 10.1111/jre.12248. [DOI] [PubMed] [Google Scholar]
- 39.Mendes L., Azevedo N.F., Felino A., Pinto M.G. Relationship between invasion of the periodontium by periodontal pathogens and periodontal disease: A systematic review. Virulence. 2015;6:208–215. doi: 10.4161/21505594.2014.984566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eick S., Pfister W. Efficacy of antibiotics against periodontopathogenic bacteria within epithelial cells: An in vitro study. J. Periodontol. 2004;75:1327–1334. doi: 10.1902/jop.2004.75.10.1327. [DOI] [PubMed] [Google Scholar]
- 41.Hirschfeld J., Kawai T. Oral inflammation and bacteremia: Implications for chronic and acute systemic diseases involving major organs. Cardiovasc. Hematol. Disord. Drug Targets. 2015;15:70–84. doi: 10.2174/1871529X15666150108115241. [DOI] [PubMed] [Google Scholar]
- 42.Höglund Åberg C., Antonoglou G., Haubek D., Kwamin F., Claesson R., Johansson A. Cytolethal distending toxin in isolates of Aggregatibacter actinomycetemcomitans from Ghanaian adolescents and association with serotype and disease progression. PLoS ONE. 2013;8:e65781. doi: 10.1371/journal.pone.0065781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Höglund Åberg C., Haubek D., Kwamin F., Johansson A., Claesson R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS ONE. 2014;9:e104095. doi: 10.1371/journal.pone.0104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belibasakis G.N., Mattsson A., Wang Y., Chen C., Johansson A. Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. APMIS. 2004;112:674–685. doi: 10.1111/j.1600-0463.2004.apm1121006.x. [DOI] [PubMed] [Google Scholar]
- 45.Fais T., Delmas J., Serres A., Bonnet R., Dalmasso G. Impact of CDT Toxin on Human Diseases. Toxins. 2016;8:220. doi: 10.3390/toxins8070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linhartova I., Bumba L., Masin J., Basler M., Osicka R., Kamanova J., Prochazkova K., Adkins I., Hejnova-Holubova J., Sadilkova L., et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010;34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson A., Claesson R., Hoglund Aberg C., Haubek D., Oscarsson J. The cagE gene sequence as a diagnostic marker to identify JP2 and non-JP2 highly leukotoxic Aggregatibacter actinomycetemcomitans serotype b strains. J. Periodontal. Res. 2017;52:903–912. doi: 10.1111/jre.12462. [DOI] [PubMed] [Google Scholar]
- 48.Brogan J.M., Lally E.T., Poulsen K., Kilian M., Demuth D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994;62:501–508. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claesson R., Gudmundson J., Höglund Åberg C., Haubek D., Johansson A. Detection of a 640-bp deletion in the Aggregatibacter actinomycetemcomitans leukotoxin promoter region in isolates from an adolescent of Ethiopian origin. J. Oral Microbiol. 2015;7:26974. doi: 10.3402/jom.v7.26974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He T., Hayashi J., Yamamoto M., Ishikawa I. Genotypic characterization of Actinobacillus actinomycetemcomitans isolated from periodontitis patients by arbitrarily primed polymerase chain reaction. J. Periodontol. 1998;69:69–75. doi: 10.1902/jop.1998.69.1.69. [DOI] [PubMed] [Google Scholar]
- 51.Ennibi O.K., Claesson R., Akkaoui S., Reddahi S., Kwamin F., Haubek D., Johansson A. High salivary levels of JP2 genotype of Aggregatibacter actinomycetemcomitans is associated with clinical attachment loss in Moroccan adolescents. Clin. Exp. Dent. Res. 2019;5:44–51. doi: 10.1002/cre2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haubek D., Johansson A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 2014;6 doi: 10.3402/jom.v6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brage M., Holmlund A., Johansson A. Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J. Periodontal. Res. 2011;46:170–175. doi: 10.1111/j.1600-0765.2010.01325.x. [DOI] [PubMed] [Google Scholar]
- 54.Johansson A., Buhlin K., Sorsa T., Pussinen P.J. Systemic Aggregatibacter actinomycetemcomitans Leukotoxin-Neutralizing Antibodies in Periodontitis. J. Periodontol. 2017;88:122–129. doi: 10.1902/jop.2016.160193. [DOI] [PubMed] [Google Scholar]
- 55.Johansson A., Sandstrom G., Claesson R., Hanstrom L., Kalfas S. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 2000;108:136–146. doi: 10.1034/j.1600-0722.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 56.Claesson R., Johansson A., Belibasakis G., Hanstrom L., Kalfas S. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 2002;37:353–359. doi: 10.1034/j.1600-0765.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 57.Johansson A., Claesson R., Hänström L., Sandström G., Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 2000;35:85–92. doi: 10.1034/j.1600-0765.2000.035002085.x. [DOI] [PubMed] [Google Scholar]
- 58.Kelk P., Abd H., Claesson R., Sandström G., Sjöstedt A., Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2011;2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahlstrand T., Tuominen H., Beklen A., Torittu A., Oscarsson J., Sormunen R., Pollanen M.T., Permi P., Ihalin R. A novel intrinsically disordered outer membrane lipoprotein of Aggregatibacter actinomycetemcomitans binds various cytokines and plays a role in biofilm response to interleukin-1beta and interleukin-8. Virulence. 2017;8:115–134. doi: 10.1080/21505594.2016.1216294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paino A., Ahlstrand T., Nuutila J., Navickaite I., Lahti M., Tuominen H., Valimaa H., Lamminmaki U., Pollanen M.T., Ihalin R. Identification of a novel bacterial outer membrane interleukin-1Beta-binding protein from Aggregatibacter actinomycetemcomitans. PLoS ONE. 2013;8:e70509. doi: 10.1371/journal.pone.0070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belibasakis G.N., Bostanci N. Inflammatory and bone remodeling responses to the cytolethal distending toxins. Cells. 2014;3:236–246. doi: 10.3390/cells3020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamoto D., Ando-Suguimoto E.S., Bueno-Silva B., DiRienzo J.M., Mayer M.P. Alteration of Homeostasis in Pre-osteoclasts Induced by Aggregatibacter actinomycetemcomitans CDT. Front. Cell. Infect. Microbiol. 2016;6:33. doi: 10.3389/fcimb.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DiRienzo J.M. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins. 2014;6:3098–3116. doi: 10.3390/toxins6113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belibasakis G.N., Johansson A., Wang Y., Chen C., Kalfas S., Lerner U.H. The cytolethal distending toxin induces receptor activator of NF-kappaB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect. Immun. 2005;73:342–351. doi: 10.1128/IAI.73.1.342-351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belibasakis G.N., Johansson A., Wang Y., Chen C., Lagergard T., Kalfas S., Lerner U.H. Cytokine responses of human gingival fibroblasts to Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cytokine. 2005;30:56–63. doi: 10.1016/j.cyto.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Ando E.S., De-Gennaro L.A., Faveri M., Feres M., DiRienzo J.M., Mayer M.P. Immune response to cytolethal distending toxin of Aggregatibacter actinomycetemcomitans in periodontitis patients. J. Periodontal. Res. 2010;45:471–480. doi: 10.1111/j.1600-0765.2009.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan K.S., Song K.P., Ong G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J. Periodontal. Res. 2002;37:268–272. doi: 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 68.Hirschfeld J., Roberts H.M., Chapple I.L., Parcina M., Jepsen S., Johansson A., Claesson R. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J. Oral Microbiol. 2016;8:33070. doi: 10.3402/jom.v8.33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee A., Jantsch V., Khan R., Hartung W., Fischer R., Jantsch J., Ehrenstein B., Konig M.F., Andrade F. Rheumatoid Arthritis-Associated Autoimmunity Due to Aggregatibacter actinomycetemcomitans and Its Resolution With Antibiotic Therapy. Front. Immunol. 2018;9:2352. doi: 10.3389/fimmu.2018.02352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abreu A.G., Barbosa A.S. How Escherichia coli Circumvent Complement-Mediated Killing. Front. Immunol. 2017;8:452. doi: 10.3389/fimmu.2017.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berends E.T., Kuipers A., Ravesloot M.M., Urbanus R.T., Rooijakkers S.H. Bacteria under stress by complement and coagulation. FEMS Microbiol. Rev. 2014;38:1146–1171. doi: 10.1111/1574-6976.12080. [DOI] [PubMed] [Google Scholar]
- 72.Hovingh E.S., van den Broek B., Jongerius I. Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion. Front. Microbiol. 2016;7:2004. doi: 10.3389/fmicb.2016.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jongerius I., Schuijt T.J., Mooi F.R., Pinelli E. Complement evasion by Bordetella pertussis: Implications for improving current vaccines. J. Mol. Med. 2015;93:395–402. doi: 10.1007/s00109-015-1259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vila-Farres X., Parra-Millan R., Sanchez-Encinales V., Varese M., Ayerbe-Algaba R., Bayo N., Guardiola S., Pachon-Ibanez M.E., Kotev M., Garcia J., et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci. Rep. 2017;7:14683. doi: 10.1038/s41598-017-14972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sundqvist G., Johansson E. Bactericidal effect of pooled human serum on Bacteroides melaninogenicus, Bacteroides asaccharolyticus and Actinobacillus actinomycetemcomitans. Scand. J. Dent. Res. 1982;90:29–36. doi: 10.1111/j.1600-0722.1982.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 76.Ramsey M.M., Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc. Natl. Acad. Sci. USA. 2009;106:1578–1583. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komatsuzawa H., Asakawa R., Kawai T., Ochiai K., Fujiwara T., Taubman M.A., Ohara M., Kurihara H., Sugai M. Identification of six major outer membrane proteins from Actinobacillus actinomycetemcomitans. Gene. 2002;288:195–201. doi: 10.1016/S0378-1119(02)00500-0. [DOI] [PubMed] [Google Scholar]
- 78.Aung K.M., Sjostrom A.E., von Pawel-Rammingen U., Riesbeck K., Uhlin B.E., Wai S.N. Naturally Occurring IgG Antibodies Provide Innate Protection against Vibrio cholerae Bacteremia by Recognition of the Outer Membrane Protein U. J. Innate. Immun. 2016;8:269–283. doi: 10.1159/000443646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Confer A.W., Ayalew S. The OmpA family of proteins: Roles in bacterial pathogenesis and immunity. Vet. Microbiol. 2013;163:207–222. doi: 10.1016/j.vetmic.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 80.Suankratay C., Mold C., Zhang Y., Lint T.F., Gewurz H. Mechanism of complement-dependent haemolysis via the lectin pathway: Role of the complement regulatory proteins. Clin. Exp. Immunol. 1999;117:442–448. doi: 10.1046/j.1365-2249.1999.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez-Larrayoz A.F., Elhosseiny N.M., Chevrette M.G., Fu Y., Giunta P., Spallanzani R.G., Ravi K., Pier G.B., Lory S., Maira-Litran T. Complexity of Complement Resistance Factors Expressed by Acinetobacter baumannii Needed for Survival in Human Serum. J. Immunol. 2017;199:2803–2814. doi: 10.4049/jimmunol.1700877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Claesson R., Kanasi E., Johansson A., Kalfas S. A new cleavage site for elastase within the complement component 3. APMIS. 2010;118:765–768. doi: 10.1111/j.1600-0463.2010.02655.x. [DOI] [PubMed] [Google Scholar]
- 83.Kanasi E., Dogan B., Karched M., Thay B., Oscarsson J., Asikainen S. Lack of serotype antigen in A. actinomycetemcomitans. J. Dent. Res. 2010;89:292–296. doi: 10.1177/0022034509358865. [DOI] [PubMed] [Google Scholar]
- 84.Deatherage B.L., Cookson B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jan A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017;8:1053. doi: 10.3389/fmicb.2017.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rompikuntal P.K., Thay B., Khan M.K., Alanko J., Penttinen A.M., Asikainen S., Wai S.N., Oscarsson J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect. Immun. 2012;80:31–42. doi: 10.1128/IAI.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demuth D.R., James D., Kowashi Y., Kato S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 2003;5:111–121. doi: 10.1046/j.1462-5822.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 88.Goulhen F., Hafezi A., Uitto V.J., Hinode D., Nakamura R., Grenier D., Mayrand D. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 1998;66:5307–5313. doi: 10.1128/iai.66.11.5307-5313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karched M., Ihalin R., Eneslätt K., Zhong D., Oscarsson J., Wai S.N., Chen C., Asikainen S.E. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 2008;8:18. doi: 10.1186/1471-2180-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kato S., Kowashi Y., Demuth D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- 91.Kieselbach T., Zijnge V., Granstrom E., Oscarsson J. Proteomics of Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles. PLoS ONE. 2015;10:e0138591. doi: 10.1371/journal.pone.0138591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kieselbach T., Oscarsson J. Dataset of the proteome of purified outer membrane vesicles from the human pathogen Aggregatibacter actinomycetemcomintans. Data Brief. 2017;10:426–431. doi: 10.1016/j.dib.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iino Y., Hopps R.M. The bone-resorbing activities in tissue culture of lipopolysaccharides from the bacteria Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Capnocytophaga ochracea isolated from human mouths. Arch. Oral Biol. 1984;29:59–63. doi: 10.1016/0003-9969(84)90043-8. [DOI] [PubMed] [Google Scholar]
- 94.Ahlstrand T., Kovesjoki L., Maula T., Oscarsson J., Ihalin R. Aggregatibacter actinomycetemcomitans LPS binds human interleukin-8. J. Oral Microbiol. 2019;11:1549931. doi: 10.1080/20002297.2018.1549931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi J.W., Kim S.C., Hong S.H., Lee H.J. Secretable Small RNAs via Outer Membrane Vesicles in Periodontal Pathogens. J. Dent. Res. 2017;96:458–466. doi: 10.1177/0022034516685071. [DOI] [PubMed] [Google Scholar]
- 96.O’Donoghue E.J., Krachler A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016;18:1508–1517. doi: 10.1111/cmi.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kesty N.C., Mason K.M., Reedy M., Miller S.E., Kuehn M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23:4538–4549. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nice J.B., Balashova N.V., Kachlany S.C., Koufos E., Krueger E., Lally E.T., Brown A.C. Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles. Toxins. 2018;10:414. doi: 10.3390/toxins10100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gholizadeh P., Pormohammad A., Eslami H., Shokouhi B., Fakhrzadeh V., Kafil H.S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. Pathog. 2017;113:303–311. doi: 10.1016/j.micpath.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johansson A., Dahlén G. Bacterial virulence factors that contribute to periodontal pathogenesis. In: Bostanci N., Belibasakis G., editors. Pathogenesis of Periodontal Diseases. Springer Nature; Cham, Switzerland: 2018. pp. 31–49. [DOI] [Google Scholar]