Abstract
Paracoccidioides brasiliensis and P. lutzii cause human paracoccidioidomycosis. We have previously characterized the <200-nt RNA sub-populations contained in fungal extracellular vesicles (EVs) from P. brasiliensis Pb18 and other pathogenic fungi. We have presently used the RNA-seq strategy to compare the <200- and >200-nt RNA fractions contained in EVs isolated from culture supernatants of P. brasiliensis Pb18, Pb3, and P. lutzii Pb01. Shared mRNA sequences were related to protein modification, translation, and DNA metabolism/biogenesis, while those related to transport and oxidation-reduction were exclusive to Pb01. The presence of functional full-length mRNAs was validated by in vitro translation. Among small non-coding (nc)RNA, 15 were common to all samples; small nucleolar (sno)RNAs were enriched in P. brasiliensis EVs, whereas for P. lutzii there were similar proportions of snoRNA, rRNA, and tRNA. Putative exonic sRNAs were highly abundant in Pb18 EVs. We also found sRNA sequences bearing incomplete microRNA structures mapping to exons. RNA-seq data suggest that extracellular fractions containing Pb18 EVs can modulate the transcriptome of murine monocyte-derived dendritic cells in a transwell system. Considering that sRNA classes are involved in transcription/translation modulation, our general results may indicate that differences in virulence among fungal isolates can be related to their distinct EV-RNA content.
Keywords: Paracoccidiodes, extracellular vesicles, RNA-seq, mRNA, sRNA
1. Introduction
Paracoccidioides brasiliensis and P. lutzii cause human paracoccidioidomycosis (PCM), which is an endemic systemic mycosis prevalent in Latin American countries, but predominantly reported in Brazil [1]. The successful establishment of this fungal infection depends on the thermo-dependent dimorphic transition from environmental conidia to the yeast phase in the lung alveoli. P. lutzii isolates are responsible for the majority of PCM cases in the Central and Northern Brazil and are represented by the highly studied Pb01 isolate [2]. P. brasiliensis is a complex of several phylogenetic groups originating from Brazilian regions and other countries [3,4]. The Pb18 and Pb3 isolates represent the phylogenetic main species S1 and a cryptic PS2 group, respectively [5]. PS2 isolates cause a milder form of murine PCM when compared to S1 isolates, apparently by stimulating a predominant Th1-type of cellular immune response [6], but the fungal features that contribute to their distinct virulence are not known. A broad analysis of genome diversity in Paracoccidioides showed that the phenotypic differences among phylogenetic groups and species are not due to differences in large protein families, but probably due to unique genes with no orthologs in other lineages [7].
Extracellular vesicles (EVs) constitute an important cellular mechanism of non-conventional secretion across all kingdoms. EV is a general term used to define spherical bi-layered-membrane structures that group, according with their origin, (a) exosomes of 30–100 nm deriving from multivesicular bodies; (b) microvesicles or ectosomes of 100–1000 nm originating from either budding or invagination of the plasma membrane; (c) apoptotic bodies that are larger than 1000 nm [8,9,10,11,12]. EVs can safely transport to the extracellular environment and distant sites a vast number of proteins, including active enzymes and toxins, lipoproteins, DNA, RNA, polysaccharides, and pigments [9]. EVs are involved in a broad array of intercellular communication and active biomolecule transfer that have implications in physiological cellular processes, infection, and disease [13,14]. EVs from microorganisms can directly interact with cells of the immune system and affect the infection process [15]. Distinct RNA specimens can be transported inside EV-like structures or associated with RNA-binding proteins and high-density lipoprotein complexes; therefore, the standardization of EV isolation and RNA purification is essential step to evaluate those populations [12,16]. After the detection of mRNA and small RNAs (sRNA) within EVs [17], the role of micro RNA (miRNA) from EVs in the genetic regulation of recipient cells has been widely addressed [18,19]. EVs also contain different classes of long non-coding RNA (lncRNAs) that are potentially able to regulate the transcription by recruiting epigenetic modifiers in the recipient cells [20].
We have previously characterized the sRNA fraction contained in EVs isolated from P. brasiliensis (isolate Pb18), Cryptococcus neoformans, Candida albicans, and also from Saccharomyces cerevisiae [21]. The sRNA fraction included sequences of various sizes shorter than 250 nt, specifically, 114 small non-coding (nc)RNA sequences of the small nucleolar (sno)RNA and transporter (t)RNA classes. We also identified 1246 conserved miRNA-like sequences, from which 20 were common to all samples and 47 had differentially represented levels. There was a small percentage of mRNA (10%) that co-fractionated in the <200 nt-enriched fraction and was also characterized. Interestingly, these sequences were unique to EVs from each species [21].
The present work aimed at comparing the RNA populations carried by EVs from two isolates (Pb18 and Pb3) representative of P. brasiliensis lineages S1 and PS2 and P. lutzii (Pb01), since differences in RNA content could be valuable to partially explain differences in virulence among isolates. We also tested the functionality of EVs mRNA and if extracellular fractions containing fungal EVs can evoke transcriptional changes in dendritic cells.
2. Materials and Methods
2.1. Fungal Growth Conditions
For EVs isolation from culture supernatant, P. brasiliensis isolates Pb18, Pb3, and P. lutzii Pb01 were cultivated for 2 days at 37 °C, under shaking, in defined Ham’s F12 medium supplemented with 1.5% glucose (500 mL), as previously described [21].
2.2. EVs Isolation
Paracoccidioides EV preparations were performed as described [21,22]. In brief, culture supernatants from two 500-mL cultures were centrifuged for 15 min at 4000× g, then for 30 min at 15,000× g, followed by filtration through a 45 μm membrane and concentration using Amicon ultrafiltration membranes (100-kDa cutoff). The cell-free, debris-free concentrated supernatant was ultracentrifuged at 100,000× g for 1 h at 4 °C and the pellets containing EVs were washed in phosphate-buffered saline (PBS). This protocol for EV isolation results in EVs with size peaks between 40 to 80 nm (suggestive of exosomes), as verified by nanoparticle-tracking analysis (NTA) for Pb18; small peaks over 150 nm are also visible. The final pellets were lyophilized for RNA extraction.
2.3. Murine Monocyte-Derived CD11c+ Cells (MoDC)
The cells extracted from the C57BL/6 mice bone marrow were cultured in 6-well plates (2.5 × 105 cells/well) in 2 mL of complete DMEM high glucose (Gibco) supplemented with 10% FBS (fetal bovine serum, Gibco), glutamine (2 mM) (Gibco), 2-mercaptoethanol (0.05 mM) (Gibco), penicillin/streptomycin (100 U/mL; 100 μg/mL) (Gibco), and 20 ng/mL GM-CSF (Gibco). The plates were kept at 37 °C at 5% CO2 and, after four days of incubation, 1 mL of media was replaced by 1 mL of fresh DMEM supplemented with 40 ng/mL GM-CSF. By the seventh day, 500 µL of fresh medium without GM-CSF replaced the same volume of old medium. On day 9, non-adherent cells and loosely adherent cells were harvested, stained in ice-cold PBS containing 1% BSA using anti-CD11c monoclonal antibody-PerCP-Cyanine5.5 (eBioscience) and sorted by FACS.
2.4. Indirect Co-Culture of Murine Monocyte-Derived CD11c+ Cells (MoDC) and Pb18
Indirect co-culture of MoDC with Pb18 was performed using a transwell system (0.4-μm membrane porosity) in 1 mL DMEM supplemented with 10% FBS in each chamber. Pb18 yeast cells (1 × 106) were seeded in the upper chamber and 1 × 106 CD11c+ cells (dendritic cells, MoDC) were placed in the lower chamber of a six-well plate. The plates were incubated for 48 h at 37 °C, at 5% CO2, the MoDCs were harvested, washed and used for RNA extraction.
2.5. RNA Isolation and Sequencing
Total RNA extraction from Paracoccidioides EVs and fractionation were carried out as described [21]. Small and large RNA were fractionated using the miRNeasy mini kit (Qiagen, Germantown, MD, USA) and the RNeasy MinElute Cleanup Kit (Qiagen), according to the manufacturer’s protocol. We used an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to test the integrity of the RNA preparations before sequencing. Total RNA was extracted from 1 × 106 MoDC cells using the RNeasy kit (Qiagen). The RNA was eluted in RNAse-free water and stored at −70 °C. The RNA-seq was carried out as previously described [21].
2.6. Data Analysis
The RNA sequences were analyzed by CLC Genomics Workbench© v 7.0 (Qiagen), using both the corresponding Paracoccidioides genomes from NCBI as reference (Pb18-ABKI02000000, Pb3-ABHV02000000, and Pb01-ABKH02000000) and the Saccharomyces cerevisiae non-coding database (https://www.yeastgenome.org/). All the sequencing analyses were performed in triplicate, except for the < 200nt enriched-fraction from Pb18 EV that had a duplicate evaluated. The parameters used for the alignments were: mismatch cost (2), insertion cost (3), deletion cost (3), length fraction (0.8), and similarity fraction (0.8). The abundance values are presented in reads per kilobase of exon model per million mapped reads (RPKM) [23]. For the analysis of secondary structure, the RNA-seq reads obtained for the < 200 nt fraction were directly evaluated in a PPFold plugin in the CLC Genomics Workbench v.10.1, using default parameters [24]. The sequences were up to 50-nt long.
2.7. Data Access
The RNA-seq data have been deposited at the Sequence Read Archive (SRA) database under the accession number (SRA: SRP022849).
2.8. In Vitro Translation and Protein Analysis
The in vitro translation assay was performed according to the manufacturer’s instructions. Briefly, 0.6 µg total RNA from Pb18 EVs were incubated for 90 min at 30 °C with 35 µL Rabbit Reticulocyte Lysate (Promega) containing leucine/methionine (0.5 pM) and 40 U RNAseOUT (Invitrogen). The reaction product was filtered through a Millipore® membrane (0.45 µm), diluted 5 times in PBS-NaCl 0.3 M and incubated at 4 °C with 200 µL Ni-NTA (Amersham) until the resin became red due to hemoglobin binding [25]. The suspension was centrifuged (5 min at 16,000× g at 4 °C) and the proteins were precipitated overnight (−20 °C) from the supernatant by addition of 3 volumes of cold acetone. The sample was spun down for 30 min at 16,000× g (4 °C) and washed with cold acetone. The precipitated proteins were denatured, reduced, trypsinized, and desalted as described [25]. The tryptic peptides were suspended in 0.1% formic acid (FA) and loaded to an LTQ-VelosOrbitrap (Thermo Fisher Scientific, Waltham, MA, USA) through a coupled nanoHPLC (Proxeon, Odense, Denmark). The samples were desalted and concentrated in a pre-column (10-μm C18 beads, Phenomenex, 100 μm × 2 cm, Torrance, CA, USA) and separated at 200 nL/min in a reverse-phase capillary column (5-μm beads, Phenomenex 10 cm × 75 mm). The peptides were eluted by a linear gradient from 5% to 40% of solvent A [solvent A: 5% acetonitrile (ACN)/0.1% FA; solvent B: 100% ACN/0.1% FA] over 90 min and for an additional 15 min with up to 95% of solvent B. The eluted peptides were directly injected in the mass spectrometer via a nanoelectrospray set at 2.2 kV. All analyses were performed in the positive ionization mode at the 50–2000 m/z range. The mass spectrometer was operated in the data-dependent acquisition mode to automatically switch between one orbitrap full-scan and ten ion trap tandem mass spectra. The *.raw data files were processed at MaxQuant 1.3.0.5 and the searches performed at Andromeda against a merged database of P. brasiliensis Pb18 (https://www.ncbi.nlm.nih.gov/genome/?term=txid502780[Organism:noexp]) and Oryctolagus cuniculus of TrEMBL (downloaded at 2015/02) using a 1% false discovery rate (FDR). The search parameters included: (i) carbamidomethylation of cysteine residues as a fixed modification; (ii) oxidation of methionine residues as a variable modification; and (iii) 6 ppm and 0.5 Da for MS1 and MS2 tolerance, respectively. Proteins identified as contaminants at a reverse database and “only identified by site” were excluded from further analysis.
3. Results
We characterized the ncRNA and mRNA sub-populations contained in EVs isolated from culture supernatant samples of the representative Paracoccidioides isolates Pb18, Pb3, and Pb01. Our results compile the analysis of three independent biological replicates for each fungal sample. In addition, we evaluated the effect of Pb18 extracellular fractions containing EVs on the transcription pattern of a murine monocyte-derived (CD11c+) cell line co-cultivated with Pb18.
3.1. Paracoccidioides EVs Carry Functional mRNA
In a previous study, we characterized mRNA sequences in fungal EVs that were co-purified in the < 200-nt RNA fraction [21]. We presently analyzed large RNA fractions to identify the most abundant mRNA sequences contained in Paracoccidioides EVs. The reads obtained from the mRNA libraries (>200 nt) were aligned with each isolate-specific genome at NCBI (Pb18-ABKI02000000, Pb3-ABHV02000000, and Pb01-ABKH02000000). For data validation, we only considered sequences with expression values of RPKM > 100 in all biological replicates. Those sequences were individually accessed and only mRNA transcripts with reads covering at least 50% of the CDS were validated (Table 1).
Table 1.
Proteins corresponding to validated mRNA sequences (RPKM > 100) found in Paracoccidioides EVs. The sequences are grouped according with their functions. Coverage used for validation is indicated, as well as the gene access codes for each isolate.
| Pb18 Feature ID | Pb3 Feature ID | Pb01 Feature ID | Sequence Description | Coverage | GO |
|---|---|---|---|---|---|
| Protein Modification | |||||
| PAAG_08003 | hsp70-like protein | 100% | ion binding | ||
| PAAG_05980 | ubiquitin-conjugating enzyme e2-16 kda | 80% | ligase activity | ||
| PADG_07715 | hsp90-like protein | 70% | protein folding, response to stress | ||
| PADG_01605 | PABG_03078 | PAAG_07080 | polyubiquitin | 70% | protein modification process |
| PADG_11111 | nuclear transport factor 2 | 75% | protein targeting | ||
| PAAG_05679 | ATP-dependent molecular chaperone hsc82 | 90% | nucleic acid binding transcription factor activity, protein folding | ||
| Carbohydrate Metabolism | |||||
| PADG_02145 | glycogen phosphorylase | 70% | carbohydrate metabolic process | ||
| Translation | |||||
| PADG_05025 | 60s ribosomal protein l26 | 70% | translation | ||
| PADG_00692 | PAAG_11418 | elongation factor 1-alpha | 100% | translation | |
| PADG_03326 | 40s ribosomal protein s9 | 80% | translation | ||
| PABG_05744 | pre-mrna splicing factor | 70% | translation | ||
| Oxidation-Reduction | |||||
| PAAG_03216 | thiol-specific antioxidant | 90% | oxidoreductase activity | ||
| Transport | |||||
| PAAG_11262 | hsp7-like protein | 100% + MR | transmembrane transport | ||
| PAAG_03058 | high-affinity methionine permease | 100% + 5′R | transmembrane transport | ||
| PAAG_07634 | gtp-binding protein rhoa | 70% | vesicle-mediated transport | ||
| DNA Metabolism or Biogenesis | |||||
| PADG_00873 | PABG_02444 | PAAG_07099 | histone h3 | 100% | chromosome organization |
| PABG_05588 | PAAG_08917 | histone h2a | 90% | chromosome organization | |
| PAAG_08918 | histone h2b | 70% | chromosome organization | ||
| PADG_06568 | tctp family protein | 50% | cell differentiation | ||
| PABG_03449 | PAAG_08247 | calmodulin | 70% | ion binding | |
| Other/Unknown Function | |||||
| PADG_02280 | hypothetical protein | 75% | Unknown | ||
| PADG_02399 | calcium-binding protein | 70% | Unknown | ||
| PADG_04049 | hypothetical protein | 60% | Unknown | ||
| PADG_08402 | hypothetical protein | 100% | Unknown | ||
| PADG_12385 | ser thr protein phosphatase family protein | 60% | Unknown | ||
| PAAG_00340 | conserved hypothetical protein | 85% | unknown | ||
| PAAG_12435 | hypothetical protein | 80% | unknown | ||
| PAAG_12692 | ATP synthase subunit beta | 95% reverse | unknown | ||
| PAAG_12694 | plant senescence-associated protein | 100% reverse | unknown | ||
| PAAG_02087 | kelch-like protein 38 | 70% | unknown | ||
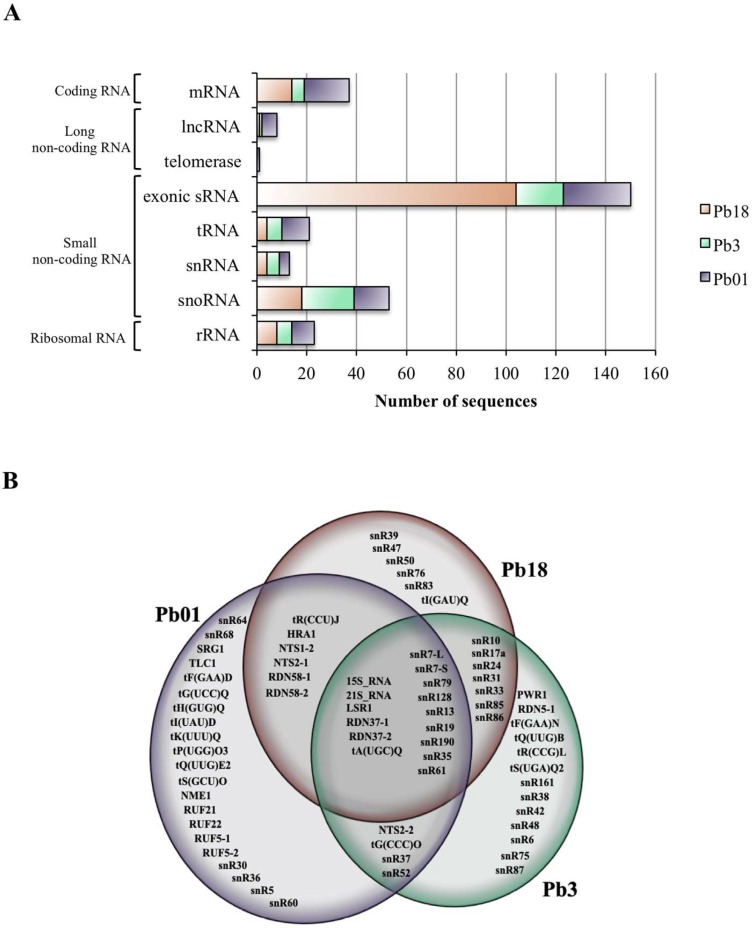
According to the aforementioned parameters, we validated a total of 30 mRNA sequences in EV samples from Pb18 (14), Pb3 (5), and Pb01 (18), as seen in Table 1 and Figure 1A. The sequences coding for polyubiquitin and histone H3 were found in EVs from all isolates, while those coding for calmodulin, elongation factor 1-alpha, and histone H2a were detected in two isolates (Table 1). The remaining sequences were exclusive to either Pb18 or Pb01. For Pb3, there was only one exclusive mRNA coding for a pre-mRNA splicing factor. In general, the mRNA sequences were mainly related to protein modification, translation, and DNA metabolism/biogenesis (Table 1). The latter function grouped most of the overlapping transcripts among the three samples. On the other hand, sequences related to transport and oxidation-reduction were only detected in Pb01. Twenty-seven out of the 30 validated sequences had over 69% of CDS coverage. Considering that we only validated mRNAs with over 50% coverage of each CDS, comparisons with our previous work were unfruitful because the validation parameters were distinct [21].
Figure 1.
(A) Graph showing the number of sequences in each class of RNA identified in EV preparations from Pb18, Pb3 and Pb01. (B) Venn diagram showing all ncRNA sequences found in EV preparations from Pb18, Pb3 and Pb01.
To confirm the presence of functional full-length mRNA in Paracoccidioides EVs, we carried out an in vitro translation assay. The Pb18 mRNA EV sample was translated using a rabbit reticulocyte lysate. Thereafter, the contaminating hemoglobin was removed by incubation with NI-NTA agarose and the remaining translated proteins were analyzed by LC-MS/MS. We found five proteins that were translated from EV mRNA (Table 2). Among them, only the transcript for a heat shock protein (PADG_07715) was included in Table 1 (RPKM > 100). Together, our results show that the Paracoccidiodes EVs contain functional full-length mRNAs and that they are differentially represented in Pb18, Pb3, and Pb01.
Table 2.
Protein sequences detected by in vitro translation of total mRNA extracted from Pb18 EVs. The mean RPKM corresponds to that found in the RNA-seq analysis.
| Feature ID | Name | RPKM Mean |
|---|---|---|
| PADG_00648 | conserved hypothetical protein | 7 |
| PADG_04056 | 14-3-3 protein epsilon | 25 |
| PADG_04810 | GTP-binding nuclear protein GSP1/Ran | 37 |
| PADG_06159 | sulfate transporter | 12 |
| PADG_07715 | Hsp90-like protein | 127 |
3.2. sRNA Sequences Aligning to mRNA Exons (Exonic sRNA)
The EV RNA reads detected in the < 200-nt fraction were initially aligned with each Paracoccidioides isolate-specific genome at NCBI. We noticed that this fraction was composed mostly of short 25-nt sequences in average that aligned to a specific region of a particular mRNA exon. In our previous work we observed that over 80% of the reads found in the <200-nt RNA fraction from EVs mapped to exons not only in P. brasiliensis Pb18, but also in C. albicans and S. cerevisiae, however not in C. neoformans [21]. In fungi, exonic short interfering RNAs (ex-siRNA) have been described to match to unique exon sites in either the reverse or the forward directions. They originate directly from single-strand RNA via an RNA-dependent RNA polymerase to generate double-stranded RNA (dsRNA) and are then converted to interfering RNA by Dicer [26]. Therefore, we presently deepened our investigation of the reads that had high depth of coverage in exonic regions. Each high-coverage coding sequence was manually accessed to evaluate the alignment position of the reads. Considering the reads generated by the sequencing of both < and > 200-nt fractions, 160 mRNA sequences showed high values of depth of coverage. Among them, only 30 had reads covering more than 50% of the entire mRNA, as detailed earlier. The remaining 130 were represented by small sequences that aligned at unique sites. We therefore called them exonic sRNA. We found a total of 104 (Pb18), 19 (Pb3), and 27 (Pb01) exonic sRNA in EVs that corresponded to a specific exonic region (5′, 3′or middle) of a single mRNA (Table 3 and Figure 1A). For Pb18 EVs, 53% of those sequences were only in the forward orientation (F), while 68% and 56% were only in the reverse (R) direction in Pb3 and Pb01 EVs, respectively.
Table 3.
Target mRNA of exonic sRNA sequences found in EVs from Pb18, Pb3, and Pb01 isolates. The position of sRNA in the exon is indicated as 5′, 3′, and M (middle), as well as the direction (F, forward; R, reverse). The sequences are grouped according with their functions.
| Pb18 Feature ID |
Alignment | Pb3 Feature ID |
Alignment | Pb01 Feature ID |
Alignment | Sequence Description | GO |
|---|---|---|---|---|---|---|---|
| Protein modification | |||||||
| PADG_01365 | 5′R/3′F | disulfide isomerase | protein folding | ||||
| PADG_03114 | MR | phospho-2-dehydro-3-deoxyheptonate aldolase | amino acid metabolic process | ||||
| PADG_04092 | 3′F | peptidyl-prolyl cis-trans isomerase b | protein modification process | ||||
| PADG_05011 | MF | peptidyl-prolyl cis-trans isomerase-like 3 | protein modification process | ||||
| PADG_05560 | 3′F | 26s proteasome regulatory subunit rpn-1 | small molecule metabolic process | ||||
| PADG_05731 | 3′F | hypothetical protein | amino acid metabolic process | ||||
| PADG_07241 | 5′F | dihydroxy-acid dehydratase | amino acid metabolic process | ||||
| PADG_07550 | MR | PABG_04093 | MR | microsomal signal peptidase subunit | peptidase activity | ||
| PAAG_05962 | 5′F | proteasome regulatory particle subunit | catabolic process | ||||
| Carbohydrate Metabolism | |||||||
| PADG_02145 | 5′F | PABG_06801 | 5′F | glycogen phosphorylase | carbohydrate metabolic process | ||
| PADG_03169 | MR | alpha-glucan synthase ags2 | carbohydrate metabolic process | ||||
| PADG_04432 | MF | alpha-amylase | carbohydrate metabolic process | ||||
| PADG_04922 | 3′F | cell wall glucanase | carbohydrate metabolic process | ||||
| PADG_05870 | 5′R/5′F | glucan synthesis regulatory protein | carbohydrate metabolic process | ||||
| Lipid Metabolism | |||||||
| PADG_12430 | 5′F | PABG_07295 | 3′F | amp-binding | long-chain fatty acid metabolic process | ||
| Oxidation-Reduction | |||||||
| PADG_04419 | 3′R | PABG_01064 | 3′R | proline oxidase | catabolic process | ||
| PAAG_05378 | MF | d-3-phosphoglycerate dehydrogenase | oxidoreductase activity | ||||
| PAAG_01937 | 3′F | duf887 domain-containing protein | biosynthetic process | ||||
| PAAG_11262 | mRNA/MR | hsp7-like protein | transmembrane transpor | ||||
| PADG_05080 | 5′F | pyridoxamine phosphate oxidase family protein | ion binding | ||||
| PADG_06181 | 3′F | c-5 sterol desaturase | lipid metabolic process | ||||
| PADG_07431 | 5′R/3′F/3R | chloroperoxidase-like protein | homeostatic process | ||||
| PADG_12214 | 5′R | alcohol dehydrogenase | oxidoreductase activity | ||||
| Translation | |||||||
| PADG_00995 | MF | ubiquitin-40s ribosomal protein s27a | translation | ||||
| PADG_02452 | MF | rna polymerase rpb1 c-terminal repeat domain-containing protein | |||||
| PADG_02484 | MF | valyl-trna synthetase | tRNA metabolic process | ||||
| PADG_05025 | MF | 60s ribosomal protein l26 | translation | ||||
| PADG_06082 | 3′F | pre-mrna splicing factor | translation | ||||
| PADG_06160 | MF | eukaryotic translation initiation factor 2 alpha subunit | translation | ||||
| PADG_06191 | 5′F | PABG_06964 | 3′R | trna isopentenyltransferase | tRNA metabolic process | ||
| PADG_06522 | 5′F | glycine--trna ligase | |||||
| PADG_06833 | 5′F | ATP-dependent rna helicase drs1 | ribosome biogenesis | ||||
| PADG_08605 | 5′F | 40s ribosomal protein s28 | translation | ||||
| PADG_01891 | MF | translation initiation factor rli1 | translation | ||||
| PADG_02317 | 3′R | translation machinery-associated protein 17 | |||||
| Signaling Process | |||||||
| PADG_05447 | 3′F | vacuolar membrane-associated protein iml1 | signal transduction | ||||
| PADG_06642 | 5′F | ste ste7 protein kinase | response to stress | ||||
| PADG_08337* | 3′F | gtp-binding protein rhoa | signal transduction | ||||
| Transport | |||||||
| PADG_00326 | MF | adp-ribosylation factor-like protein 1 | vesicle-mediated transport | ||||
| PADG_01303 | 3′F | abc transporter | biosynthetic process | ||||
| PADG_01567 | 3′R | sorting nexin 3 | vesicle-mediated transport | ||||
| PADG_05084 | 5′R | high affinity copper transporter | transmembrane transport | ||||
| PADG_05821 | 5′F | importin | |||||
| PADG_06982 | 3′F | ncs1 family nucleobase:cation symporter-1 | transmembrane transport | ||||
| PADG_08101 | 3′F | mrna cleavage factor complex component pcf11 | transport | ||||
| PADG_03535 | MR | PABG_01859 | MR | PAAG_01288 | MR | nucleotide binding | ion binding, ligase activity |
| PABG_11660 | 3′F | endoplasmic reticulum vesicle protein 25 | vesicle-mediated transport | ||||
| PAAG_03058 | mRNA/5′R | high-affinity methionine permease | transmembrane transport | ||||
| PAAG_03479* | 3′R | mfs multidrug | transmembrane transport | ||||
| PAAG_11682 | 3′R | duf1903-domain-containing protein | transport | ||||
| PAAG_12134 | 3′F | mfs drug transporter | transmembrane transport | ||||
| DNA Metabolism or Biogenesis | |||||||
| PADG_00916 | MR | PABG_02494 | 5′R | PAAG_07153 | 5′R | transcription factor tfiiib complex subunit brf1 | biosynthetic process |
| PADG_03251 | 3′F | c6 finger domain protein acr- | biosynthetic process | ||||
| PADG_05475 | 5′R | dna-directed rna polymerases and iii 145 kda polypeptide | biosynthetic process | ||||
| PADG_06799 | MR | camk camkl kin4 protein kinase | cell division | ||||
| PADG_07652* | MR | PABG_06307* | MR | PAAG_05737* | MR | calcium calmodulin-dependent protein kinase | cytoskeleton organization, cell division |
| PADG_11268 | 3′F | tyrosine recombinase -like | DNA metabolic process | ||||
| PADG_11500 | MF | fungal specific transcription | biosynthetic process | ||||
| PADG_12343 | 3′F | serine threonine protein kinase | regulation of transcription | ||||
| PABG_00984 | 5′F | PAAG_03968 | 5′F | c6 transcription | biosynthetic process | ||
| PABG_03356 | 3′R | homeobox transcription | biosynthetic process | ||||
| Other/Unknown Function | |||||||
| PADG_00069 | 3′R | hypothetical protein | |||||
| PADG_00138 | 5′R | hypothetical protein | |||||
| PADG_00639 | 5′R/5′F | hypothetical protein | |||||
| PADG_01127 | 5′R | rna-binding protein | ion binding | ||||
| PADG_01198 | 3′F | vps9 domain | |||||
| PADG_01219 | MF/MR | hypothetical protein | |||||
| PADG_01476 | 3′F | PAAG_06944 | 3′F | c2h2 finger domain | ion binding | ||
| PADG_01739 | 3′R | ||||||
| PADG_01808 | MF | hypothetical protein | |||||
| PADG_01880 | 3′F | u-box domain-containing protein | |||||
| PADG_01892 | 3′R | formin binding protein | |||||
| PADG_02119 | MR | PABG_03545 | MR | protein | |||
| PADG_02181 | 5′R | had superfamily | isomerase activity | ||||
| PADG_02764 | 5′F | disulfide bond formation protein d | |||||
| PADG_02871 | 3′R | cfem domain-containing protein | |||||
| PADG_02926 | 3′R | tam domain methyltransferase | |||||
| PADG_03103 | 3′R | phytase | phosphatase activity | ||||
| PADG_03162 | 3′R | domain protein | |||||
| PADG_03436 | MF | 3 exoribonuclease family protein | |||||
| PADG_03788 | MF | polyadenylation factor subunit 64 | |||||
| PADG_04049 | 5′R/5′F | hypothetical protein | |||||
| PADG_04157 | 5′R | cellobiose dehydrogenase | |||||
| PADG_04417 | 3′F | hypothetical protein | |||||
| PADG_04448 | 5′F | polarized growth protein | |||||
| PADG_04473 | MR | duf647 domain-containing protein | |||||
| PADG_04629 | 3′R | protein | |||||
| PADG_04760 | 5′F | multiple myeloma tumor-associated protein 2 like | |||||
| PADG_04828 | MF/MR | adenylosuccinate lyase | biosynthetic process | ||||
| PADG_05226 | MF | protein | |||||
| PADG_05352 | 5′R | ubiquitin carboxyl-terminal hydrolase 19 | |||||
| PADG_05378 | 5′F | protein | |||||
| PADG_05589 | MR | protein | |||||
| PADG_05603 | 3′R | increased rdna silencing protein 4 | ion binding | ||||
| PADG_06044 | 3′F | ankyrin repeat containing protein | |||||
| PADG_06240 | 3′F | hypothetical protein | |||||
| PADG_06449 | 3′R | phosphotransferase enzyme family protein | |||||
| PADG_07205 | 5′F | protein | |||||
| PADG_07675 | MF/MR | cellular morphogenesis protein | |||||
| PADG_07897 | 5′R/5′F/5′NS | hypothetical protein | |||||
| PADG_07988 | 3′F/3′R | conserved hypothetical portein | |||||
| PADG_07990 | MF | tam domain methyltransferase | |||||
| PADG_08617 | 3′F/3′ R | PABG_07734 | 3′R | hypothetical protein | |||
| PADG_11034 | 5′R/5′F | PABG_11827 | 5′R/5′F | protein | |||
| PADG_11035 | 3′F | protein | |||||
| PADG_11277 | 5′R | protein | |||||
| PADG_11439 | 5′R | PABG_00126 | 5′R | PAAG_11926 | 5′R | hypothetical protein | |
| PADG_11473 | 5′R | hypothetical protein | |||||
| PADG_11562 | MF | hypothetical protein | |||||
| PADG_11613 | 5′R | hypothetical protein | |||||
| PADG_11652 | 3′R | PABG_01675 | 3′R | kh domain rna binding protein | RNA binding | ||
| PADG_11758 | 3′R/3′F/3′NS | hypothetical protein | |||||
| PADG_11762 | MF | hypothetical protein | |||||
| PADG_12001 | 3′F | hypothetical protein | |||||
| PADG_12385 | 5′R/5′F | PABG_06506 | 3′R | ser thr protein phosphatase family protein | |||
| PABG_06943 | 3′R | hypothetical protein | |||||
| PABG_12403 | MR/MF/MNS | hypothetical protein | |||||
| PAAG_01376 | 5′R | hypothetical protein | |||||
| PAAG_01424 | 5′F/MF | iron-sulfur cluster assembly accessory protein | biosynthetic process | ||||
| PAAG_01967 | MR | hypothetical protein | |||||
| PAAG_03361 | MF | predicted protein | |||||
| PAAG_04613 | MR | vacuolar protein sorting-associated protein 51 | |||||
| PAAG_05089 | 3′F | ||||||
| PAAG_07600 | 3′F | ||||||
| PAAG_07877 | 3′F | protein | |||||
| PAAG_11750 | 5′F/5′R | hypothetical protein | |||||
| PAAG_12291 | 3′R | camp-dependent protein kinase pathway protein | kinase activity | ||||
| PAAG_12405 | MR | hypothetical protein | |||||
| PAAG_12534 | 5′R | mitochondrial 37s ribosomal protein nam9 | rRNA binding | ||||
| PAAG_12681 | MR | hypothetical protein | ion binding | ||||
Four EV exonic sRNA sequences mapped to a common transcript in all Paracoccidioides isolates: calcium calmodulin-dependent protein kinase (PADG_07652), nucleotide binding (PADG_03535), transcription factor tfiiib complex subunit brf1 (PADG_00916), and a hypothetical protein (PADG_11439). As seen in Table 3, most of the exonic sRNAs were specific to EVs from Pb18 (89) and Pb01 (21), whereas only four were exclusive to Pb3 EVs. Ten fragments mapped to homologous transcripts in Pb18 and Pb3 EVs. Most of the target sequences have unknown functions, while the others are related to carbohydrate/protein/DNA metabolism, translation, oxidation-reduction, and the signaling process (Table 3).
In conclusion, although the number of putative exonic sRNA was 4 to 5-fold higher in Pb18 EVs, 10 target sequences seemed to be characteristic of P. brasiliensis, whereas 21 were exclusive to P. lutzii. These results reveal a previously unknown diversity in the composition of fungal EVs at the genus level.
3.3. Comparison of EV ncRNA Classes in Paracoccidioides EVs
The different classes of ncRNA contained in Pb18, Pb3, and Pb01 EVs were analyzed by aligning the <200-nt reads with the ncRNA database from the Saccharomyces Genome Database. The results revealed the presence of 71 different sequences of ncRNA in Paracoccidioides EVs, from which 15 were common to all isolates and 17 were shared by two of them (Figure 1B). The most abundant class of ncRNA found in Paracoccidiodes EVs was the small nucleolar (sno)RNAs (33), followed by tRNAs (16), rRNAs (10), long ncRNAs (7), and small nuclear (sn)RNAs (4), as seen in Figure 1A. The snoRNA and tRNA were also the most abundant ncRNA populations described in our previous work not only for P. brasiliensis, but also for C. albicans, C. neoformans, and S. cerevisiae [21].
The number of total ncRNA detected in EVs varied with the isolate, being slightly more abundant in P. lutzii Pb01 (45) than in P. brasiliensis Pb3 (39) or Pb18 (35). The class profiles also differed between species. For P. brasiliensis EVs, snoRNA represented about 55% of the total, followed by rRNA (23% in Pb18 and 15% in Pb3), and 11 to 15% tRNA and snRNA (Figure 1A). For P. lutzii EVs, we found similar proportions (20 to 31%) of snoRNA, rRNA, and tRNA, followed by 9 to 13% of snRNA and other ncRNAs (Figure 1A).
3.4. Secondary Structure in the EVs.
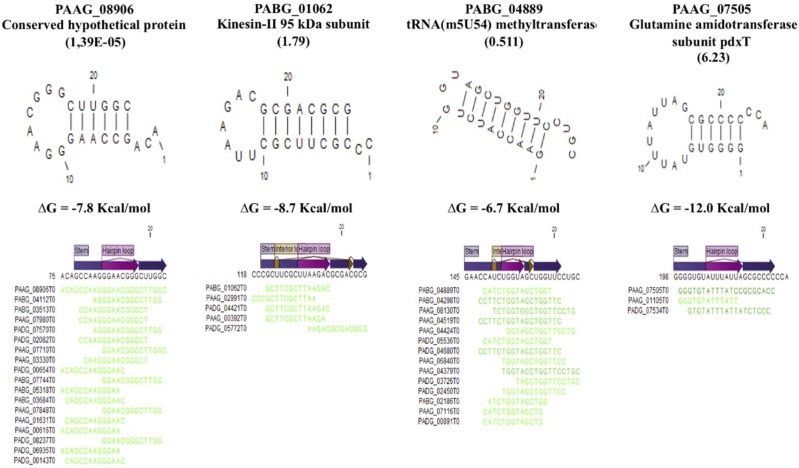
In our previous publication [21], we identified putative miRNA-like (milRNA) sequences in fungal EVs that aligned with mature miRNA following a search in the miRNA database of all organisms (http://www.mirbase.org). In the present work, we searched for sRNA sequences (up to 50 nt) bearing secondary structure in order to have a more precise view of the presence of milRNA in Paracoccidioides EVs. We applied the secondary structure analysis to RNA fragments detected in EVs at high abundance (RPKM > 5000) and for further characterization we selected 42 RNA sequences bearing secondary structures with free energy values below −3.0 Kcal/mol. The sequences with the most negative values are shown in Figure 2. We performed an alignment with these 42 sequences to infer their function and identify in which strand they would align (Supplemental Table S1). Two were present in all EV samples, while 23 were found in both Pb3 and Pb01 EVs. Interestingly, 50% of the structured RNAs aligned at the complementary strand of the RNA, thus suggesting that they could act as miRNA or the transcripts (Supplemental Table S1). Most of the structured RNAs localize to transcripts that code for hypothetical proteins, however we could also identify RNAs that align to transcripts of kinesin-II, glutamine amidotransferase and lysine methyltransferase (Figure 2).
Figure 2.
Secondary structures with the lowest deltaG among 42 sRNA sequences with values below −3 Kcal/mol. The Blast search result for these sequences is shown.
3.5. Paracoccidioides EVs Might Modulate the Transcriptome of Dendritic Cells
Dendritic cells make the bridge between the innate and adaptative immune responses, which ultimately define the course of the fungal infections [27]. The disease progression in paracoccidioidomycosis depends on dominant Th1 or Th2 types of immune response. The Th1-driven inflammatory immune response is responsible for protection against the disease and the IL-12 expressed by dendritic cells is detrimental to stimulate this type of response [28]. In this context, we investigated if extracellular fractions containing EVs produced by P. brasiliensis Pb18 would affect gene expression in recipient dendritic cells. In order to do that, we characterized the transcriptome of murine monocyte-derived CD11c+ (MoDC) cells co-cultivated for 48 h with the Pb18 yeast cells in a transwell system. We compared the sequences with those of the controls cultivated in the absence of fungal cells. In the transwell system, we had the fungal (upper compartment) and MoDC cell (bottom compartment) cultures communicating by a porous membrane that allowed the transit to the bottom well of soluble molecules and EVs up to 0.4 µm in size.
We observed that the indirect co-culture with Pb18 led to a slight alteration in the levels of mRNA expressed by MoDC cells when compared to the control (Figure 3 and Table S2). We detected 20 upregulated and 28 downregulated mRNAs (Figure 3). We chose to follow highly stringent criteria in this analysis (similar levels of expression in both replicates, FDR ≤ 5% and log FC ≥ 2), considering we had duplicates, but not triplicates. Among the upregulated transcripts, three code for membrane proteins (Ankar, Unc13c, and Smim7) and two code for mitochondrial proteins associated to translation elongation (mt-Te and mt-Tv) (Table S2). Among the downregulated transcripts, there was 25% enrichment for transcripts related to gene expression regulation (transcription and translation). When we applied the hypergeometric test to the annotated transcripts for the gene set enrichment analysis, the p-value of the gene expression group was 2.40 × 105, thus reinforcing the significance of this enrichment (Table S2). It was interesting to notice that three transcripts that code for transcription factors (Gabpb2, Pknox1, and Zfp575) are among the downregulated MoDC mRNAs in indirect co-cultures with Pb18 (Table S2), suggesting that sRNAs from the fungal EVs were potentially able to modulate the gene expression of the recipient cell. It is important to note that we have preliminary cytometry data showing that Pb18 yeast cells can actively release EVs under our transwell experimental conditions and that MoDC cells can internalize fungal EVs, as depicted in Figure S1.
Figure 3.
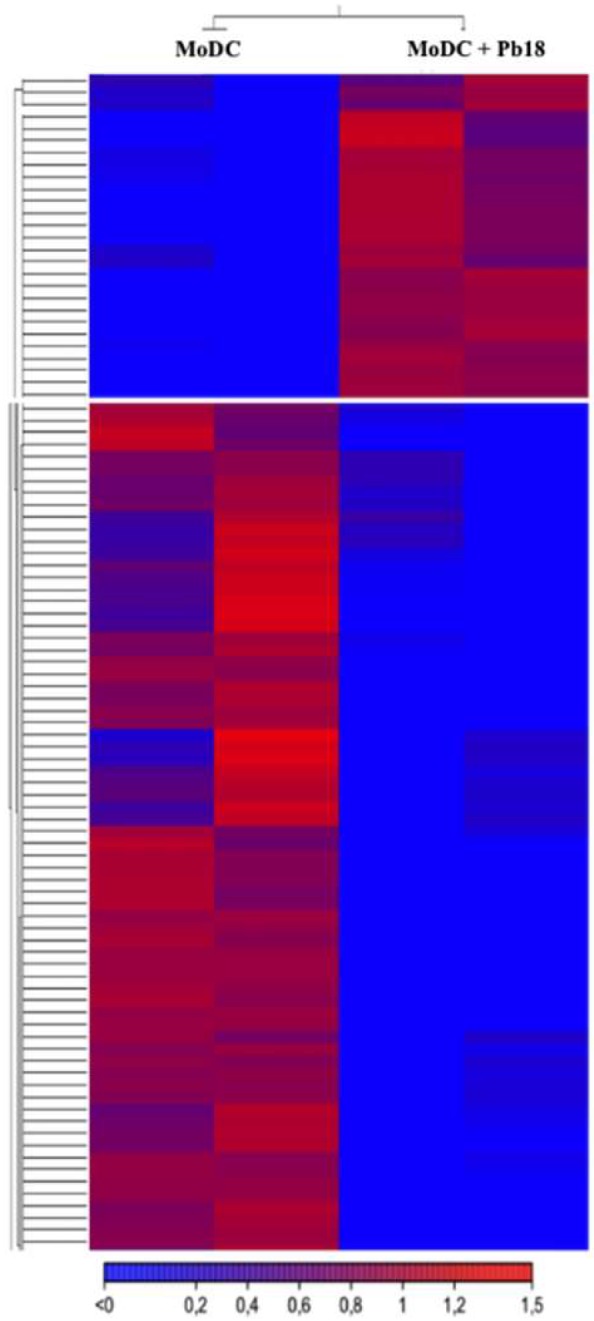
Heat map of transcripts from MoDC cells that were modulated over 2-fold and had a p-value < 0.05 when co-cultivated indirectly with Pb18 in a transwell system.
4. Discussion
In the present work we have shown that the features of mRNA and sRNA sub-populations contained in EVs from P. brasiliensis Pb18, Pb3, and P. lutzii Pb01 differ considerably between species and also between representative isolates of the same species. The results suggest that Paracoccicioides extracellular fractions containing EVs can modulate the transcription profile of dendritic cells. By using in vitro translation, we also reported for the first time in pathogenic fungi that the EV mRNAs are active and can be translated.
The mRNA sequences presently detected in the >200-nt RNA fraction of Paracoccidoides EVs varied in number with the isolate and only five orthologs were common to EVs from more than one isolate. We have recently compared the characteristics of mRNA sequences from EVs exported by two H. capsulatum isolates, specifically G186AR and G217B [29]. The latter isolate lacks cell wall alpha-1,3-glucan, which is a virulence factor [30]. The number of EV mRNA sequences varied from 93 in G186AR (mostly related to metabolic processes) to 31 in G217B (related to transport pathways possibly requiring vesicles, oxidation-reduction, and translation mechanisms). In the present analysis for P. brasiliensis, which is genetically related to Histoplasma [31], transcripts associated with DNA metabolism/biogenesis and protein modification were enriched in all samples, while those related with translation and transport prevailed, respectively, in Pb18 and Pb01.
While there are several pieces of evidence suggesting the role of fungal EVs on host cells, specially by interfering with the course of the immune response [32,33,34,35,36], it has recently been demonstrated that EV-like liposomal particles can cross the fungal cell wall inwards [37], thus opening the possibility that EVs can be uptaken and signal fungal cells. It has recently been demonstrated that EV-associated plant sRNA can silence virulence genes in a fungal pathogen, which agrees with the hypothesis that EVs mediate trans-kingdom regulation of gene expression [38]. On the other hand, Bielska et al. (2018) demonstrated that EVs produced by virulent Cryptococcus gattii were essential to signal quiescent strains within phagolysosomes located in distant body sites, which then became virulent; that phenomenon was apparently mediated by EV protein and RNA [39]. We have here detected active mRNA within Paracoccidioides EVs and we can envision that they might be uptaken by other neighboring or distant fungal cells and play a role in the host-fungal relationship by delivering virulence transcripts. Table 1 includes, for e.g., heat shock proteins like Hsp 70 (PAAG_08003, in Pb01 EVs) and Hsp 90-like (PADG_07715, in Pb18 EVs) that have a role in virulence of dimorphic fungi [40].
On the other hand, among the siRNA described in fungi, the exonic ex-siRNAs promote gene silencing in Mucor circinelloides and Fusarium graminearum [26,41,42]. In these species, the ex-siRNAs correspond to sense and antisense siRNAs converted into double-stranded RNA (dsRNA) via RNA-dependent RNA polymerase and Dicer. They regulate translation of the same mRNA that originated it. The presence of sense and antisense exonic sRNA-like in Paracoccidioides EVs suggests that these molecules could be involved in gene silencing via dsRNA. In the present work, we can point out the finding of exonic sRNA-like sequences, in Pb18 EVs, that map to α-amylase (PADG_04422), which is essential to the synthesis of the virulence factor α-glucan [30,43], and β-glucanase (PADG_04922), that cleaves dectin-1 ligand β-glucan present in the cell wall. We propose that they could have a role in regulating the expression of virulence genes in other fungal cells.
The comparison of the small ncRNA subtypes in Paracoccidioides EVs showed clear inter-species diversity. In P. lutzii, the percentage of snoRNA was about half that in P. brasiliensis, whereas for tRNA and other ncRNA it was, respectively, 2- and 4-fold higher. Consequently, 20 sequences were exclusive of P. lutzii EVs (mostly tRNA) versus six for Pb18 and thirteen for Pb3. In our experimental conditions, H. capsulatum EVs transport almost exclusively rRNA and tRNA [29]. The tRNA sequences prevailed in the EVs from isolate G186AR and most of them were not detected in EVs from the G217R strain. The fragments of tRNAs (the tRFs) are implicated in diverse processes in the cell, from regulation of cell viability, protein synthesis, apoptosis, to RNA metabolism, including turnover and stability [44]. The tRFs present in the EVs form Trypanosoma cruzi, the causing agent of Chagas disease, can be transferred to other parasites and promote cell communication and/or to host cells to modulate gene expression or facilitate infection [45].
The gene-silencing mechanism known as RNA interference (RNAi) is prompted by small noncoding (s)RNAs averaging 25 nt that act at either a post-transcriptional or post-translational level. The RNAi-related sRNAs are short interfering RNAs (siRNA), microRNAs (miRNA), and piwi-interacting RNAs (piRNA). In fungi, the conventional miRNA pathway has only been demonstrated in C. neoformans [46], while alternative miRNA-like (mil-RNA) pathways have been reported in, for ex., Fusarium oxysporum [47], Penicillium marneffei [48], and Neurospora crassa [49].
We have previously detected 145 sequences in EVs from P. brasiliensis Pb18 that matched those of mature miRNA deposited in the miRNA database (mirbase). More recently, Curcio et al. [50] searched for matches of mil-RNAs already described in fungi, discarded those located in genes and looked for pre-miRNA secondary structure in the genome by considering their flanking nucleotides. They found that 11 mil-RNAs previously reported in fungal EVs matched the criteria for mil-RNA in the P. brasiliensis Pb18 genome. Besides, the authors found that in Pb18 the paralogous genes for Argonaute (1 and 2) and Dicer (1 and 2) (PADG_00716; PADG_03108; PADG_11946; PADG_07189) seem to be induced in the pathogenic yeast phase of the fungus, suggesting that mechanisms involving RNAi can be functional in this species [50]. We have presently shown partial secondary RNA structures in sRNA sequences from EVs that was differentially represented in the Paracoccidioides isolates. These sequences aligned to exons, notably in the reverse position. We found similar results for H. capsulatum [29], but the role of these molecules has to be investigated further. It was interesting to find, in Histoplasma EVs, a series of RNA-binding proteins and one of them, Snd1, is a component of the RNA-induced silencing complex (RISC) that is part of the RNAi machinery [51]. In Pb18 EVs [52] we have not found RNA-binding proteins that matched those found in Histoplasma EVs (data not shown).
Modulation of the host immune system by EV components has been described in C. neoformans, C. albicans, Malassezia sympodialis, P. brasiliensis and Sporothrix brasiliensis. The fungal EVs activate immune cells in vitro inducing the release of pro-inflammatory mediators, suggesting a role of fungal EVs in activating the immune system to respond to the fungal infection [32,33,34,35,36]. Importantly, in all studies performed so far, it was uncertain if the density of EVs used to stimulate host cells corresponded to that observed during physiologic and/or pathogenic conditions. Therefore, we opted for a transwell-based experimental system where the amount of fungal EVs corresponded to that physiologically produced by P. brasiliensis, even with the limitation that molecules not related to EVs may participate in the interaction with host cells. We observed that the Pknox1 and Gbpb2 transcription factors were highly downregulated (65-fold and 30-fold, respectively) in MoDC cells upon indirect co-coculture with P. brasiliensis in the transwell experiment. Pknox1 (Pbx/knotted 1 homeobox) belongs to the HOX family of transcription factors and is critical for the immune system homeostasis as it regulates the expression of IL-10 in macrophages and dendritic cells [53]. Gbpb2 (GA-binding protein subunit beta 2) is critical for T cell development and the expression regulation of IL-7R alpha [54]. IL-7 is associated with improved immune response against bacterial and viral infections, and also in fungal sepsis [55] and Pneumocystis infection [56]; however, a direct correlation of IL-7 and paracoccidioidomycosis has not been evaluated yet. On the other hand, IL-10 is a potent anti-inflammatory cytokine that is generally associated with increased infection in paracoccidioidomycosis [57]. Our experimental design using a transwell system did not allow distinguishing between the biological effects of EVs from other secreted molecules. However, considering our data suggesting that Paracoccidioides Pb18 produce EVs that are uptaken by MoDC in a transwell system (Figure S1), we feel entitled to assume that EVs from P. brasiliensis Pb18 might help to modulate the MoDC response, possibly favoring the infection at the early stages of interaction with cells of the innate immune system. This modulation could be related to regulatory sRNA carried by the fungal EVs or even by surface ligands like DC-SIGN mannose ligands exposed in the EV surface [58].
Considering that sRNA classes are involved in transcription/translation modulation in a variety of systems and potentially also in Paracoccidioides, our general results may indicate that differences in virulence among fungal isolates could be related to their distinct EV RNA content. That hypothesis will hopefully be experimentally tested.
Abbreviations
| EVs | Extracellular vesicles |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| miRNA | Micro RNA |
| ncRNA | Non-coding RNA |
| PCM | paracoccidioidomycosis |
| sRNA | Small RNA |
| snoRNAs | Small nucleolar RNA |
| tRNA | Transporter RNA |
Supplementary Materials
The following files are available online, Figure S1: Uptake of EVs from Pb18 by MoDC cells in a transwell system, Table S1: List of genes from Pb18, Pb3, and Pb01 matching partially structured sRNA, as indicated. Separated lists contain those found exclusively in one isolate or shared by two or three of them, Table S2: MoDC genes differentially expressed (upregulated in green and downregulated in pink) after incubation with Pb18 cells in a transwell system, as analyzed using the RNA-seq strategy.
Author Contributions
Conceptualization, R.P.d.S., M.L.R., S.G., L.R.A. and R.P.; Data curation, R.P.D.S., L.R.A., L.G.V.L., J.P.C.D.C., T.J.P.S.; Formal analysis, R.P.d.S., J.P.C.D.C., L.R.A. and R.P.; Funding acquisition, R.P., M.L.R. and S.G.; Methodology, R.P.d.S., J.P.C.D.C., H.F., S.G. and L.R.A; Supervision, R.P.; Writing—original draft, R.P.d.S., L.R.A., and R.P.; Writing—review & editing, R.P.d.S., L.G.V.L., M.L.R., S.G., L.R.A. and R.P.
Funding
This work was supported by Fundação de Amparo à Pesquisa (grant 13/25950-1 and scholarship 10/19410-9). Sequencing was carried out in the plataforms of Universidade Federal do Paraná (through INCT). MLR was supported by grants from the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grants 405520/2018-2, 440015/2018-9, and 301304/2017-3), and Fiocruz (grants VPPCB-007-FIO-18-2-57 and VPPIS-001-FIO-18-66).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Martinez R. New trends in paracoccidioidomycosis epidemiology. J. Fungi. 2017;3:1. doi: 10.3390/jof3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocca A.L., Amaral A.C., Teixeira M.M., Sato P.K., Shikanai-Yasuda M.A., Soares Felipe M.S. Paracoccidioidomycosis: Eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013;8:1177–1191. doi: 10.2217/fmb.13.68. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira M.M., Theodoro R.C., Nino-Vega G., Bagagli E., Felipe M.S. Paracoccidioides species complex: Ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathog. 2014;10:e1004397. doi: 10.1371/journal.ppat.1004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turissini D.A., Gomez O.M., Teixeira M.M., McEwen J.G., Matute D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017;106:9–25. doi: 10.1016/j.fgb.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matute D.R., Sepulveda V.E., Quesada L.M., Goldman G.H., Taylor J.W., Restrepo A., McEwen J.G. Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. J. Clin. Microbiol. 2006;44:2153–2157. doi: 10.1128/JCM.02540-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho K.C., Ganiko L., Batista W.L., Morais F.V., Marques E.R., Goldman G.H., Franco M.F., Puccia R. Virulence of Paracoccidioides brasiliensis and gp43 expression in isolates bearing known pbgp43 genotype. Microbes Infect. 2005;7:55–65. doi: 10.1016/j.micinf.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Munoz J.F., Farrer R.A., Desjardins C.A., Gallo J.E., Sykes S., Sakthikumar S., Misas E., Whiston E.A., Bagagli E., Soares C.M., et al. Genome diversity, recombination, and virulence across the major lineages of paracoccidioides. mSphere. 2016;1:e00213-16. doi: 10.1128/mSphere.00213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kalra H., Drummen G.P., Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues M.L., Franzen A.J., Nimrichter L., Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr. Opin. Microbiol. 2013;16:414–420. doi: 10.1016/j.mib.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schorey J.S., Harding C.V. Extracellular vesicles and infectious diseases: New complexity to an old story. J. Clin. Investig. 2016;126:1181–1189. doi: 10.1172/JCI81132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini-Beheshti E., Grau G.E.R. Extracellular vesicles as mediators of immunopathology in infectious diseases. Immunol. Cell Biol. 2018 doi: 10.1111/imcb.12044. [DOI] [PubMed] [Google Scholar]
- 15.Volgers C., Benedikter B.J., Grauls G.E., Hellebrand P.H.M., Savelkoul P.H.M., Stassen F.R.M. Effects of n-acetyl-l-cysteine on the membrane vesicle release and growth of respiratory pathogens. FEMS Microbiol. Lett. 2017:364. doi: 10.1093/femsle/fnx087. [DOI] [PubMed] [Google Scholar]
- 16.Mateescu B., Kowal E.J., van Balkom B.W., Bartel S., Bhattacharyya S.N., Buzas E.I., Buck A.H., de Candia P., Chow F.W., Das S., et al. Obstacles and opportunities in the functional analysis of extracellular vesicle rna - an isev position paper. J. Extracell. Vesicles. 2017;6:1286095. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 18.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Wurdinger T., Middeldorp J.M. Functional delivery of viral mirnas via exosomes. Proc. Natl. Acad. Sci. USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayraktar R., Van Roosbroeck K., Calin G.A. Cell-to-cell communication: Micrornas as hormones. Mol. Oncol. 2017;11:1673–1686. doi: 10.1002/1878-0261.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatima F., Nawaz M. Vesiculated long non-coding rnas: Offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Non-Coding RNA. 2017;3:10. doi: 10.3390/ncrna3010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peres da Silva R., Puccia R., Rodrigues M.L., Oliveira D.L., Joffe L.S., Cesar G.V., Nimrichter L., Goldenberg S., Alves L.R. Extracellular vesicle-mediated export of fungal rna. Sci. Rep. 2015;5:7763. doi: 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallejo M.C., Matsuo A.L., Ganiko L., Medeiros L.C., Miranda K., Silva L.S., Freymuller-Haapalainen E., Sinigaglia-Coimbra R., Almeida I.C., Puccia R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-galactosyl epitopes. Eukaryot. Cell. 2011;10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 24.Sükösd Z., Knudsen B., Kjems J., Pedersen C.N.S. Ppfold 3.0: Fast rna secondary structure prediction using phylogeny and auxiliary data. Bioinformatics. 2012;28:2691–2692. doi: 10.1093/bioinformatics/bts488. [DOI] [PubMed] [Google Scholar]
- 25.Williams L.M., Fu Z., Dulloor P., Yen T., Barron-Casella E., Savage W., Van Eyk J.E., Casella J.F., Everett A. Hemoglobin depletion from plasma: Considerations for proteomic discovery in sickle cell disease and other hemolytic processes. Proteom. Clin. Appl. 2010;4:926–930. doi: 10.1002/prca.201000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolas F.E., Moxon S., de Haro J.P., Calo S., Grigoriev I.V., Torres-Martinez S., Moulton V., Ruiz-Vazquez R.M., Dalmay T. Endogenous short rnas generated by dicer 2 and rna-dependent rna polymerase 1 regulate mrnas in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010;38:5535–5541. doi: 10.1093/nar/gkq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gow N.A., Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012;15:406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Thind S.K., Taborda C.P., Nosanchuk J.D. Dendritic cell interactions with Histoplasma and Paracoccidioides. Virulence. 2015;6:424–432. doi: 10.4161/21505594.2014.965586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves L.R., Peres da Silva R., Sanchez D.A., Zamith-Miranda D., Rodrigues M.L., Goldenberg S., Puccia R., Nosanchuk J.D. Extracellular vesicle-mediated rna release in Histoplasma capsulatum. mSphere. 2019;4:e00176-19. doi: 10.1128/mSphere.00176-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rappleye C.A., Eissenberg L.G., Goldman W.E. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc. Natl. Acad. Sci. USA. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y., Dukik K., Muñoz J.F., Sigler L., Schwartz I.S., Govender N.P., Kenyon C., Feng P., van den Ende B.G., Stielow J.B., et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018;90:245–291. doi: 10.1007/s13225-018-0403-y. [DOI] [Google Scholar]
- 32.da Silva T.A., Roque-Barreira M.C., Casadevall A., Almeida F. Extracellular vesicles from Paracoccidioides brasiliensis induced m1 polarization in vitro. Sci. Rep. 2016;6:35867. doi: 10.1038/srep35867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gehrmann U., Qazi K.R., Johansson C., Hultenby K., Karlsson M., Lundeberg L., Gabrielsson S., Scheynius A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PLoS ONE. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira D.L., Freire-de-Lima C.G., Nosanchuk J.D., Casadevall A., Rodrigues M.L., Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 2010;78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas G., Rocha J.D., Oliveira D.L., Albuquerque P.C., Frases S., Santos S.S., Nosanchuk J.D., Gomes A.M., Medeiros L.C., Miranda K., et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2014 doi: 10.1111/cmi.12374. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda M.A.K., de Almeida J.R.F., Jannuzzi G.P., Cronemberger-Andrade A., Torrecilhas A.C.T., Moretti N.S., da Cunha J.P.C., de Almeida S.R., Ferreira K.S. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front. Microbiol. 2018;9:2286. doi: 10.3389/fmicb.2018.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker L., Sood P., Lenardon M.D. The viscoelastic properties of the fungal cell wall allow traffic of ambisome as intact liposome vesicles. MBio. 2018:9. doi: 10.1128/mBio.02383-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai Q., Qiao L., Wang M., He B., Lin F.M., Palmquist J., Huang S.D., Jin H. Plants send small rnas in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielska E., Sisquella M.A., Aldeieg M., Birch C., O’Donoghue E.J., May R.C. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus Gattii. Nat. Commun. 2018;9:1556. doi: 10.1038/s41467-018-03991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleare L.G., Zamith-Miranda D., Nosanchuk J.D. Heat shock proteins in Histoplasma and Paracoccidioides. Clin. Vaccine Immunol. 2017;24:e00221-17. doi: 10.1128/CVI.00221-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolas F.E., Ruiz-Vazquez R.M. Functional diversity of rnai-associated srnas in fungi. Int. J. Mol. Sci. 2013;14:15348–15360. doi: 10.3390/ijms140815348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son H., Park A.R., Lim J.Y., Shin C., Lee Y.W. Genome-wide exonic small interference rna-mediated gene silencing regulates sexual reproduction in the homothallic fungus Fusarium graminearum. PLoS Genet. 2017;13:e1006595. doi: 10.1371/journal.pgen.1006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camacho E., Sepulveda V.E., Goldman W.E., San-Blas G., Nino-Vega G.A. Expression of paracoccidioides brasiliensis amy1 in a Histoplasma capsulatum amy1 mutant, relates an alpha-(1,4)-amylase to cell wall alpha-(1,3)-glucan synthesis. PLoS ONE. 2012;7:e50201. doi: 10.1371/journal.pone.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P., Kuscu C., Dutta A. Biogenesis and function of transfer rna-related fragments (trfs) Trends Biochem. Sci. 2016;41:679–689. doi: 10.1016/j.tibs.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Silva M.R., Cabrera-Cabrera F., das Neves R.F., Souto-Padron T., de Souza W., Cayota A. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: Relevance of trna-derived halves. Biomed Res. Int. 2014;2014:305239. doi: 10.1155/2014/305239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang N., Yang Y., Janbon G., Pan J., Zhu X. Identification and functional demonstration of mirnas in the fungus Cryptococcus neoformans. PLoS ONE. 2012;7:e52734. doi: 10.1371/journal.pone.0052734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R., Jiang N., Jiang Q., Sun X., Wang Y., Zhang H., Hu Z. Exploring microrna-like small rnas in the filamentous fungus Fusarium oxysporum. PLoS ONE. 2014;9:e104956. doi: 10.1371/journal.pone.0104956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau S.K., Chow W.N., Wong A.Y., Yeung J.M., Bao J., Zhang N., Lok S., Woo P.C., Yuen K.Y. Identification of microrna-like rnas in mycelial and yeast phases of the thermal dimorphic fungus Penicillium marneffei. PLoS Negl. Trop. Dis. 2013;7:e2398. doi: 10.1371/journal.pntd.0002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H.C., Li L., Gu W., Xue Z., Crosthwaite S.K., Pertsemlidis A., Lewis Z.A., Freitag M., Selker E.U., Mello C.C., et al. Diverse pathways generate microrna-like rnas and dicer-independent small interfering rnas in fungi. Mol. Cell. 2010;38:803–814. doi: 10.1016/j.molcel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curcio J.S., Batista M.P., Paccez J.D., Novaes E., Soares C.M.A. In silico characterization of micrornas-like sequences in the genome of Paracoccidioides brasiliensis. Genet. Mol. Biol. 2019;42:95–107. doi: 10.1590/1678-4685-gmb-2018-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santhekadur P.K., Das S.K., Gredler R., Chen D., Srivastava J., Robertson C., Baldwin A.S., Jr., Fisher P.B., Sarkar D. Multifunction protein staphylococcal nuclease domain containing 1 (snd1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor kappab and mir-221. J. Biol. Chem. 2012;287:13952–13958. doi: 10.1074/jbc.M111.321646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallejo M.C., Nakayasu E.S., Matsuo A.L., Sobreira T.J., Longo L.V., Ganiko L., Almeida I.C., Puccia R. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: Comparative analysis with other pathogenic fungi. J. Proteome Res. 2012;11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung E.Y., Liu J., Homma Y., Zhang Y., Brendolan A., Saggese M., Han J., Silverstein R., Selleri L., Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins pbx1 and prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu S., Zhao D.M., Jothi R., Xue H.H. Critical requirement of gabpalpha for normal T cell development. J. Biol. Chem. 2010;285:10179–10188. doi: 10.1074/jbc.M109.088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unsinger J., Burnham C.A., McDonough J., Morre M., Prakash P.S., Caldwell C.C., Dunne W.M., Jr., Hotchkiss R.S. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012;206:606–616. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruan S., Samuelson D.R., Assouline B., Morre M., Shellito J.E. Treatment with interleukin-7 restores host defense against Pneumocystis in cd4+ t-lymphocyte-depleted mice. Infect. Immun. 2016;84:108–119. doi: 10.1128/IAI.01189-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calich V.L., da Costa T.A., Felonato M., Arruda C., Bernardino S., Loures F.V., Ribeiro L.R., de Cassia Valente-Ferreira R., Pina A. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia. 2008;165:223–236. doi: 10.1007/s11046-007-9048-1. [DOI] [PubMed] [Google Scholar]
- 58.Peres da Silva R., Heiss C., Black I., Azadi P., Gerlach J.Q., Travassos L.R., Joshi L., Kilcoyne M., Puccia R. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for dc-sign receptors. Sci. Rep. 2015;5:14213. doi: 10.1038/srep14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.