Abstract
Magnesium (Mg) is the second most abundant cation in mammalian cells, and it is essential for numerous cellular processes including enzymatic reactions, ion channel functions, metabolic cycles, cellular signaling, and DNA/RNA stabilities. Because of the versatile and universal nature of Mg2+, the homeostasis of intracellular Mg2+ is physiologically linked to growth, proliferation, differentiation, energy metabolism, and death of cells. On the cellular and tissue levels, maintaining Mg2+ within optimal levels according to the biological context, such as cell types, developmental stages, extracellular environments, and pathophysiological conditions, is crucial for development, normal functions, and diseases. Hence, Mg2+ is pathologically involved in cancers, diabetes, and neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and demyelination. In the research field regarding the roles and mechanisms of Mg2+ regulation, numerous controversies caused by its versatility and complexity still exist. As Mg2+, at least, plays critical roles in neuronal development, healthy normal functions, and diseases, appropriate Mg2+ supplementation exhibits neurotrophic effects in a majority of cases. Hence, the control of Mg2+ homeostasis can be a candidate for therapeutic targets in neuronal diseases. In this review, recent results regarding the roles of intracellular Mg2+ and its regulatory system in determining the cell phenotype, fate, and diseases in the nervous system are summarized, and an overview of the comprehensive roles of Mg2+ is provided.
Keywords: magnesium, neuron, differentiation, neural network maturation, synaptogenesis, intracellular signal, neurodegenerative disease
1. Introduction
Magnesium (Mg) is the second-most abundant cation following potassium in mammalian cells, and it is essential for numerous cellular processes, including enzymatic reactions, ion channel functions, metabolic cycles, and cellular signaling, as well as the stability of biomolecules, such as RNA, DNA, and proteins [1,2,3]. Mg plays a special role in biochemistry because of its smallest ionic radius, highest charge density, and largest hydrated radius, and it coordinates six oxygen atoms in its first coordination shell [3,4]. Mg2+ links together two phosphate groups in a macromolecule, which is responsible for the folding of biomolecules, such as enzymes and DNA/RNA. The inorganic chemistry of Mg plays a key role in the first chemical processes, which lead to the origin of life, i.e., ribozymes, and the early evolution of life [5,6,7]. Because of the essential roles of Mg2+, fundamental requirements of Mg2+ for biological processes seem to pose constraints on the evolution of cells and organisms. This fundamental nature of Mg2+ in life leads to the versatility and universality of the roles of Mg2+ in living systems. The homeostasis of intracellular Mg2+ is physiologically linked to cell growth, differentiation, energy metabolism, and cell death via the control of enzymatic activities, channel openings, DNA/RNA stability, and cellular stress [1,2,3]. Hence, it is crucial to regulate the Mg2+ concentration ([Mg2+]) within optimal levels according to the cell type and environment not only for normal functions and development but also prevention of diseases. In fact, disorder of Mg2+ homeostasis is involved in cancer, diabetes, and neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and demyelination [1,2,3,8,9]. The significance of intracellular Mg2+ is universal and fundamental at the molecular level, but its multiple and complex functions lead to its cell-type-specific roles. Previous studies suggested that, especially in the nervous system, Mg2+ plays specific roles in development, brain functions, and diseases [2,3,10]. Because of the contradictory observations, e.g., Mg2+ is trophic or toxic, an activator or an inhibitor, increased or decreased in the pathology of several diseases, the roles of intracellular Mg2+ and its regulatory system are controversial. In this review, the aim is to summarize the findings regarding the roles of intracellular Mg2+ and its regulatory system for determining cell phenotypes and fates in the nervous system and to provide an overview of the comprehensive roles of Mg2+ in neuro(patho)physiology.
2. Magnesium Homeostasis in the Brain
2.1. Magnesium Homeostasis in the Nervous System
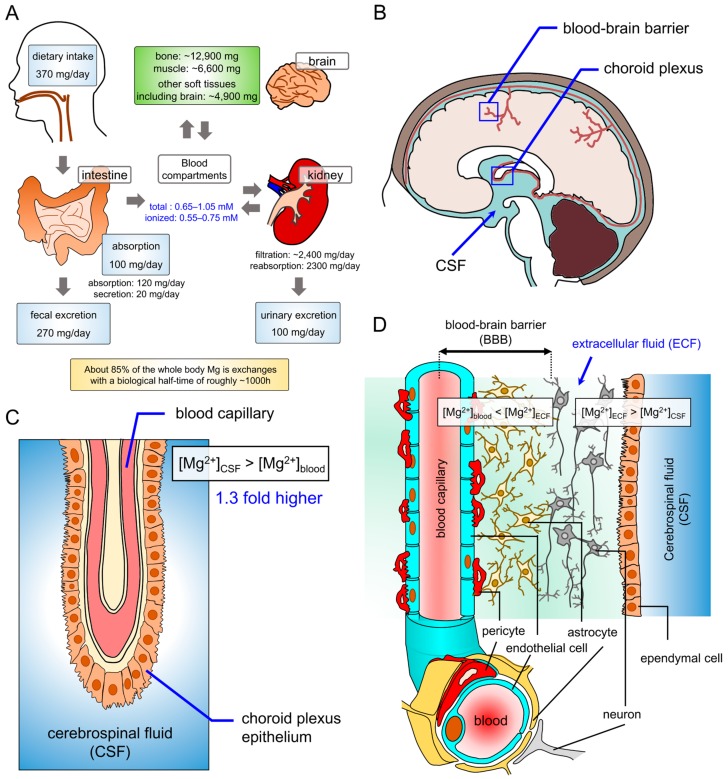
Mg2+ from daily intake is absorbed in the intestine, and it is reabsorbed by the proximal tubule (10–20%) and the thick ascending limb of Henle’s loop (50–70%) [3]. In the entire body, the majority of Mg2+ is accumulated in the bone, muscle, and soft tissues (Figure 1). The serum [Mg2+] ranges between 0.5 and 1.05 mM [11,12], the values of which reflect only 1% of the total content of Mg2+ in the body of a healthy person [3]. Even under severely Mg2+-depleted conditions, up to 80% of dietary Mg2+ can be absorbed [13], and most of Mg2+ in whole body exchanges at a very slow rate with biological half-time of 1000 hours [14]. Even in such conditions, the [Mg2+] in the serum is maintained within the normal range [3]. Extracellular fluid (ECF) in the central nervous system (CNS) is separated from the blood circulation by the blood–brain barrier (BBB). The BBB comprises endothelial cells of brain capillaries and allows passage of nutrients and electrolytes for the maintenance of ECF homeostasis. Because neuronal and glial cells are closely located with a distance of 20 to 50 nm and the volume of extracellular space is quite small in brain unlike the other organs [15,16,17], concentrations of the ECF components is greatly fluctuating. Thus, the BBB actively transport several molecules for the ECF homeostasis [17]. The [Mg2+] in ECF is maintained within a greater level compared with that of plasma or cerebrospinal fluid (CSF) [18]. The gap provides evidences for the active transport of Mg2+ in BBB. The in vitro BBB model of human brain endothelial cells, several functionally active Mg2+ transporters are expressed, such as transient receptor potential melastatin 7 (TRPM7) and MagT1 [19]. However, little has been revealed about the mechanism of Mg2+ transport in BBB. As most of the researches about Mg2+ absorption and excretion have focused on the small intestine and kidney [3,17], further investigation is required on how similar and different such organs are to CNS. In addition, the gap-junction-mediated cytosolic [Mg2+] ([Mg2+]cyto) regulates the circadian rhythm of BBB permeability in Drosophila, indicating that the intracellular [Mg2+] of BBB affects the neuronal environment in the brain [20]. The cerebrospinal fluid (CSF) fills and surrounds the brain and the spinal cord and exists at about 100 to 150 mL in the normal adult human body [21]. CSF functions as a mechanical barrier, and it is produced by the dialysis of blood and active transport of molecules, such as nutrients, hormones, metal ions and metabolites across the ependymal cells in the choroid plexus at a rate of 0.2 to 0.7 mL per minute [22]. The [Mg2+] of CSF is greater than that of blood [23,24], indicating that Mg2+ is actively transported from the blood into CSF [17]. The [Mg2+] of ventricular CSF is higher and more sensitive to changes in [Mg2+] of plasma than that of lumber CSF in cow [25]. The alteration of [Mg2+] of CSF correlates with the extracellular [Mg2+] around neurons, which affects neural activities. Thus, the [Mg2+] of CSF is closely related to various brain functions [26,27]. In particular, the [Mg2+] of CSF and cognitive functions have been reported to exhibit a positive correlation [28,29,30,31,32]. In addition, the intracellular [Mg2+] of erythrocytes significantly correlates with the [Mg2+] of CSF in the hippocampus, and further with the hippocampal synapse density and recognition and memory performance [33,34], suggesting that [Mg2+]cyto of erythrocytes is a good index of recognition and memory. These facts revealed that Mg2+ homeostasis in the human body is a key factor in brain functions, especially synaptic connectivity.
Figure 1.
Magnesium homeostasis in whole body and brain. (A) Magnesium metabolism of the human body. (B) Choroid plexus and blood–brain barrier in the human brain. (C) The enlarged image of the boxed region in panel B, i.e., choroid plexus. The enlarged image of the boxed region in the panel B, i.e., blood–brain barrier (BBB). The gradient of [Mg2+] between blood and cerebrospinal fluid (CSF). (D) The structure of BBB at cellular levels and the comparison of [Mg2+] between blood, extracellular fluid (ECF) and CSF.
2.2. Mg2+ Transport in Neurons
Mg2+ is the most abundant divalent cation in cells, and the total [Mg2+] ranges between 17 and 20 mM in mammalian cells [1,2]. The gaps between the [Mg2+]cyto and extracellular free Mg2+ concentration ([Mg2+]ex) are maintained within less than twofold (10,000- and 20,000-fold for Ca2+ and Zn2+, respectively) [1,35,36,37]. As the resting membrane potential of neurons is about −70 mV, if [Mg2+]cyto is at the electrochemical equilibrium, then its resting concentration should be 50 mM [37]. Yet, even under the Mg2+-mobilized condition, only a slight change in [Mg2+]cyto is observed, within about twofold [38,39,40]. Against the electrochemical gradient, cells exhibit several mechanisms to physiologically maintain intracellular [Mg2+] within a narrow range under resting or stimulated conditions [1,3,37]. Intracellular Mg2+ is regulated through a balance of influx, efflux, and the amount of stored intracellular Mg2+ [1,3,37], and it is fully exchanged with plasma Mg2+ within 3 to 4 hours [12]. The energy required for the transport of Mg2+ is several times greater than that required for the transport of other cations [41] because Mg2+ binds water molecules more tightly than other cations due to its highest charge density. As Mg2+ exhibits the largest hydrated radius (0.428 nm) and the smallest ionic radius (0.072 nm), the volume change between hydrated and ionic Mg2+ is almost 400-fold (Na+ and Ca2+: ∼25-fold or K+: 4-fold) [42,43]. Thus, any protein transporting Mg2+ must be capable of initially interacting with the large cation [42]. Furthermore, assuming that Mg2+ is transported in its ionic form similar to other cations, dehydrated Mg2+ passes through quite a small pore. Together, Mg2+ transporting molecules must contain both physically large initial binding sites and small pores. Because of these unique characteristics of Mg2+, the structure and mechanism of Mg2+ transporters have not been completely revealed. Although several Mg2+-transporting proteins are identified in mammalian cells [1,3,37,44,45], the association of these Mg2+-transporting systems with the neuro(patho)physiology has not been investigated much.
2.3. Mg2+ Distribution of Cells
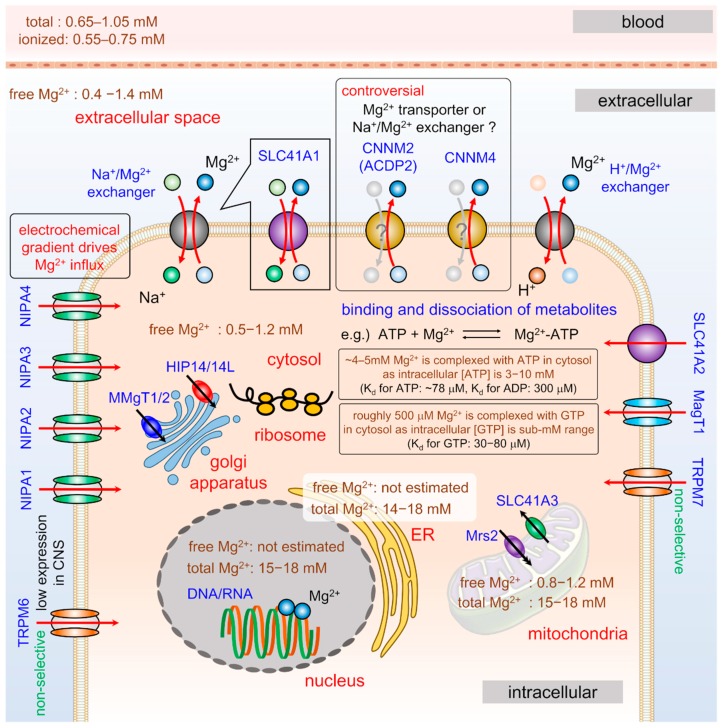
Nuclei, mitochondria, and endoplasmic or sarcoplasmic reticulum (ER/SR) compartmentalize intracellular Mg2+ with total concentrations ranging between 15 and 18 mM [1,37]. In the lumen of these organelles, only a small fraction of Mg2+ is free, and almost all of the Mg2+ is complexed with negatively charged biomolecules in each compartment because of its positive charge [2,3,37] (Figure 2).
Figure 2.
Intracellular Mg2+ distribution and machinery for Mg2+ regulation: The intracellular Mg2+ content is regulated by the balance of influx, efflux, and the intracellularly stored amount. Major storages for intracellular Mg2+ are the nuclei, mitochondria, ERs, and the ribosome. Cytosolic Mg2+ is bound to phosphometabolites, such as ATP. NIPA1, NIPA2, NIPA3, NIPA4, MagT1, TRPM6, TRPM7 and SLC41A2 contributes the uptake of extracellular Mg2+. Na+/Mg2+ and H+/Mg2+ exchangers contribute the Mg2+ efflux. Some studies support that SLC41A1, CNNM2 and CNNM4 functions as Mg2+ exchangers. MMgT1/2 and HIP14/14L is localized at golgi apparatus, and Mrs2 and SLC41A3 is localized at mitochondria. Abbreviations: ER—endoplasmic reticulum; ATP—adenosine 5’-triphosphate; TRPM6—transient receptor potential melastatin 6; TRPM7—transient receptor potential melastatin 7; CNNM2 or ACDP2—cyclin M2; CNNM4—cyclin M4.
2.3.1. Cytosol
In the cytosol, Mg2+ is complexed to a broad spectrum of biomolecules, such as phosphonucleotides, phosphometabolites, and Mg2+-binding proteins [1,37]. In particular, adenosine 5′-triphosphate (ATP) is the major intracellular pool for Mg2+ because of its abundance (on the order of millimolar concentrations) and high binding affinity (Kd of ~78 µM) [1,37,46]. The Mg2+ buffering contributes to the maintenance of free [Mg2+]cyto within a narrow range. In such intracellular environments, some biological stimuli induce slight but significant changes in [Mg2+]cyto [38,47,48,49]. Even when the changes in intracellular [Mg2+] is apparently slight, the contents and distributions of Mg-complexed biomolecules may change dramatically under the conditions in which Mg2+-buffering molecules are abundant. Thus, a slight fluctuation of intracellular [Mg2+] can impact on cellular processes more than expected.
2.3.2. Nuclei
Nuclear [Mg2+] ([Mg2+]nuc) considerably varies depending on the physiological conditions [50]. In nuclei, chromatin, nucleic acids, and free nucleotides require counterions for the neutralization of their negative charges [1,2,37]. K+ and Mg2+ are strong candidate cations for the neutralization of charges because of the low intracellular concentrations of Na+ and Ca2+. Furthermore, Mg2+ wins in the competition of K+ for binding to the negatively charged molecules, such as DNA in the nucleus, because it has more positive charges and a higher hydration energy [51]. For stabilizing the condensed state of DNA, a high concentration of counterions is required (1 to 2 M for distances between the DNA helix axes of 2 to 4 nm) [52]. Thus, in the nucleus, Mg2+ is localized at a spatially heterogeneous distribution. Recently, the genetically encoded fluorescent-protein-based Mg2+ sensor, which is named as Magnesium Ratiometric Indicator for Optical Imaging (MARIO), revealed that, after the breakdown of a nuclear envelope, [Mg2+]nuc increases and peaks during the metaphase, and it gradually decreases during cytokinesis. The condensation of chromosomes by Mg2+ is required for cell mitosis [40,53]. In yeast, the deficit of Mg2+ intake interferes with the interphase microtubule organization and mitotic spindle formation [54]. In addition, some tumor cells contain higher levels of Mg2+ in the nucleus compared with normal cells [50,55,56]. As Mg2+ is known to affect cell proliferation over several decades [57,58], nuclear Mg2+ apparently plays an important role in cell division. In addition, Mg2+ is involved in genome regulation [59]. As Mg2+ affects the solubility of chromatin, and the chromatin surrounding the DNA affects gene expression, the Mg2+-fluctuation-dependent change in chromatin folding presumably affects genome regulation [60].
2.3.3. Mitochondria
Mitochondria constitute a major cellular Mg2+ pool and contribute to the homeostatic regulation of intracellular Mg2+ [48,61,62,63]. Several physiological and pathological stimuli activate the release of mitochondrial Mg2+ into the cytosol, inducing a rapid, large decrease in the [Mg2+] in the mitochondrial matrix ([Mg2+]mito). Thus, mitochondria are a key player in the regulation of intracellular Mg2+ homeostasis [38,47,48,49,62]. As the [Mg2+]mito is typically 0.8 to 1.2 mM [1,37,64,65], mitochondrial Mg2+ predominantly combines with adenine phosphonucleotides and Mg2+-binding proteins. In mammalian cells, the mitochondrial Mg2+ influx channel mitochondrial RNA splicing 2 (Mrs2) [48,66] and mitochondrial Mg2+ exporter SLC41A3 [67] are identified. Although the disruption of the mitochondrial membrane potential (ΔΨm) triggers the release of Mg2+ from the mitochondria into the cytoplasm [47,49,61], the molecular mechanism for the release of mitochondrial Mg2+ has not been revealed.
Mitochondrial Mg2+ affects several mitochondrial functions: (1) mitochondrial energy metabolism, (2) apoptotic process, (3) mitochondrial Ca2+ homeostasis, and (4) mitochondrial DNA functions.
Mitochondrial Energy Metabolism
Mg2+ is required for a wide range of biochemical processes in mitochondrial energy metabolism. Mitochondrial Mg2+ homeostasis demonstrates the potential to regulate the rate of energy production according to energy demand. Activities of 2-oxoglutarate dehydrogenase (OGDH), which is the rate-limiting enzyme of the tricarboxylic acid (TCA) cycle, and several other enzymes are stimulated by Mg2+ [63,68]. In addition, Mg2+ contributes to the transport of ATP from the mitochondria to the cytoplasm, which is mediated by an ATP-Mg/Pi carrier [69]. The accumulation of ATP in mitochondria inhibits several enzymatic processes in the TCA cycle [70] and activities of the electron transport chain [71] in a negative feedback manner. The decrease of [Mg2+]mito by the Mrs2 knockdown affects the metabolome, especially reactions involved in the TCA cycle [48].
Apoptotic Process
Changes in [Mg2+]cyto are observed in several apoptotic cells [49,62,72]. For instance, anoxia induces the increase of [Mg2+]cyto via TRPM7 channels in hippocampal neurons [73]. Apoptotic stimuli triggers the release of Mg2+ from mitochondria in various cells, including neurons [47,49,61,62,74]. The upregulation Mrs2 levels suppress apoptosis in gastric cancer cells [75]. The downregulation of the Mrs2 level causes decreased ΔΨm and abnormal mitochondrial morphology [48]. Taken together, the accumulated mitochondrial Mg2+ through the Mrs2 channel exhibits protective effects against cellular stress. The activation of the mitochondrial ATP-sensitive potassium channel (mitoKATP), which contributes to the protective effects of an ischemic precondition [76,77], triggers the release of mitochondrial Mg2+ [47]. N-methyl-4-phenylpyridinium iodide (MPP+) is an active metabolite of PD inducer 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [78]. In MPP+-induced mitochondrial stress in the PD model, the cytosolic Mg2+ level after mobilization from the mitochondria and extracellular medium is correlated with the cell viability [39]. These studies revealed that stored and released Mg2+ in mitochondria attenuates the neurodegeneration. This is explained by the fact that Mg2+ inhibits the opening of the permeability transition pore (PTP), leading to the release of cytochrome c and consequently apoptosis [79,80]. On the other hand, the elevated [Mg2+]mito [81] and decreased [Mg2+]cyto [82] are observed in some models of the induction of apoptosis. Mg2+ stimulates the release of cytochrome c independently of the PTP [83,84]. These opposite observations in cellular apoptotic processes suggest the missing mechanism, and further work is required to associate the apoptosis process to the Mg2+ homeostasis.
Mitochondrial Ca2+ Homeostasis
Mitochondrial Ca2+ uptake plays various roles in vital signaling processes, such as bioenergetics, cell death, and sequestration of cytosolic Ca2+ transients [85]. Extramitochondrial Mg2+ suppresses the mitochondrial uptake of Ca2+ via the inhibition of the Ca2+ uniporter [85,86,87,88]. Thus, the intracellular homeostasis of Mg2+ affects Ca2+-signal-mediated mitochondrial functions, such as bioenergetics and apoptosis.
Mitochondrial DNA Functions
Mammalian mitochondria contain their own DNA (mtDNA), which encodes the essential components of the oxidative phosphorylation (OXPHOS) and RNA elements. The Mg2+ requirements for the DNA/RNA structure and protein synthesis lead to the possibility that mitochondrial Mg2+ affects mitochondrial DNA (mtDNA) functions, as well as processes of protein synthesis in mitochondria (described in detail in Chapter 5.3).
2.3.4. Endo(sarco)plasmic Reticulum
ER/SR accumulates Mg2+ (its total [Mg2+] is estimated to be between 14 and 18 mM), and Mg2+ binds to ribonuclear proteins and phospholipids [37]. The concentration of Ca2+ ([Ca2+]) is near 100 nM in the cytosol, while [Ca2+] in the ER ranges between 100 and 800 µM [89]. As high levels of Ca2+ in the ER/SR interfere with accurate measurements using fluorescent probes with a low selectivity for Mg2+, [Mg2+] in ER/SR has not been reliably determined. ER plays central roles in Ca2+ signaling in cells, including neurons [90,91]. Ca2+ is released from the intracellular Ca2+ storage in the ER/SR via inositol-1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR). Mg2+ functions as an intracellular inhibitor of IP3R [92] and RyR [93,94,95]. As IP3R or RyR-mediated Ca2+ signaling plays several roles in neuronal processes including neuronal development and plasticity [90,91,96], the interaction of Mg2+ with Ca2+ signaling can significantly affect neuro(patho)physiology [95]. The decrease of [Mg2+]cyto by caffeine as a RyR activator through an as yet unidentified mechanism in myocytes [97] suggested the possibility that SR/ER is involved in intracellular Mg2+ signals. The accurate determination of [Mg2+] in the ER/SR without Ca2+ interference has been eagerly awaited.
2.3.5. Ribosome
The ribosome is a complex molecular machine that serves as the site of protein synthesis, i.e., translation. Ribosomes comprise ribosomal RNA (rRNA) molecules and ribosomal proteins. A single 70S ribosome of Escherichia coli comprises more than 170 Mg2+ atoms [98], meaning that an entire pool of 70,000 ribosomes chelates at least 12 mM Mg2+ in a single cell [99,100]. Since Mg2+ plays crucial roles in the structural stability and/or catalytic activity of the ribosome, which cannot be replaced by the other cations [101,102,103,104], [Mg2+]cyto is closely associated with its ribosome content [105,106]. Since the majority of the ribosomes are typically translating at the maximum capacity, the overall rate of protein synthesis is often determined by the rate of ribosome synthesis [100]. In brief, [Mg2+]cyto is highly correlated with the rate of protein synthesis [107]. Furthermore, in ribosomes, Mg2+ deficits cause the loss of peptidyl transferase activity and subsequent ribosome disassembly [108] and lead to the reduction of translation [109]. Studies with prokaryotic cells reveal that low [Mg2+]cyto promotes protein expressions for Mg2+ uptake and ATP reduction, consequently making Mg2+ available for translation to satisfy the cellular demands of Mg2+ [110,111,112,113]. The homeostatic systems of intracellular [Mg2+] are suggested to be evolutionarily conserved in eukaryotic cells [112]. In mammalian cells, the mechanistic target of rapamycin (mTOR) signaling regulates several growth-related processes, including ribosome biogenesis [114]. Intracellular Mg2+ physiologically enhances mTOR signaling activities [38,115]. Thus, Mg2+ apparently regulates protein synthesis through the effect on ribosomal functions via the mTOR pathway [115,116]. In neurons, ribosomes perform protein synthesis, and their functions are essential for dendritic growth and maintenance [117,118]. Therefore, deficits of protein synthesis in ribosome disturb neurodevelopment via the reduction of neuronal connectivity [117]. The deficits of protein synthesis related to various neurodegenerative diseases, including AD [119,120] and PD [121,122,123]. Hence, [Mg2+]cyto affects the formation of a neural network via ribosome biogenesis.
3. Physiological Roles of Cellular Mg2+
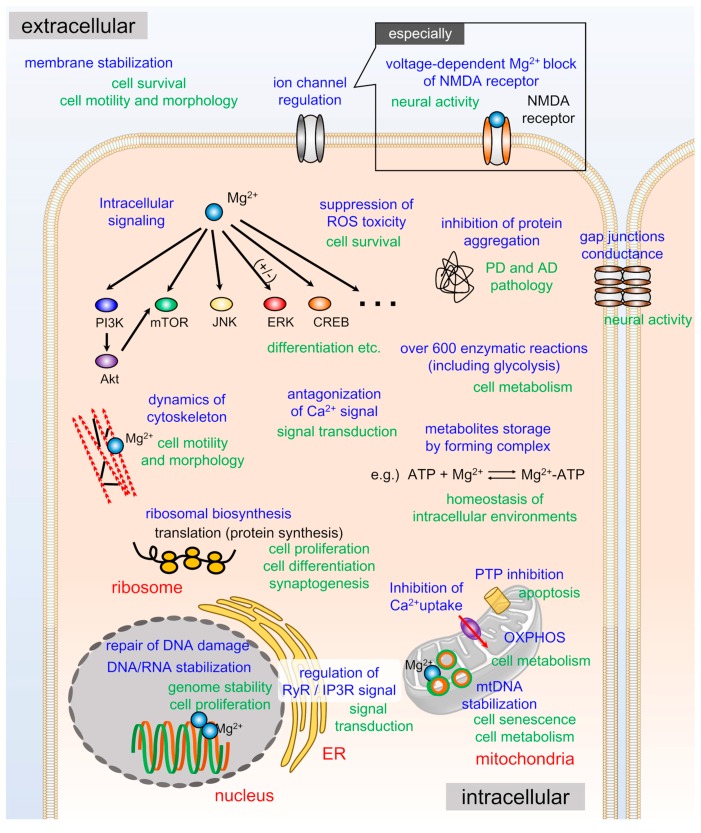
In living cells, negative charges of biomolecules are in excess of the positive ones. Thus, inorganic metal ions, such as K+, Na+, Ca2+, and Mg2+, exceed the number of anions [52]. Although Ca2+ is a better competitor for binding to negative charges of biomolecules, the intracellular Ca2+ level is maintained at low levels under normal and resting conditions [35,36]. Hence, Mg2+ is the main candidate as a counterion for neutralizing negatively charged biomolecules, e.g., RNA/DNA, reactive oxygen species (ROS), and ATP, because of its abundance and multivalence [3,4]. The properties of Mg2+ for interacting with numerous biomolecules render versatile roles, e.g., a modulator of enzymatic activities, cell protection against cellular stress, channel regulation, and DNA/RNA stabilization (Figure 3). In addition, the interaction of Mg2+ with various biomolecules serves as an intracellular buffer and a storage system for maintaining the homeostasis of intracellular molecules. Hence, it is not surprising that dysregulation of Mg2+ homeostasis is tightly connected with several disease conditions, such as neurodegenerative disease, diabetes mellitus, and metabolic syndrome [2,3].
Figure 3.
Overview of cell physiology of Mg2+. The intracellular Mg2+ plays versatile roles in neurons. Abbreviations: PI3K—phosphatidylinositol-3 kinase; mTOR—mechanistic target of rapamycin; JNK—c-Jun NH2-terminal kinase; ERK—extracellular signal-regulated kinase; CREB—cAMP response element binding; ROS—reactive oxygen species; NMDA—N-methyl-D-aspartate; AD—Alzheimer’s disease; PD—Parkinson’s disease; ATP—adenosine 5’-triphosphate; PTP—permeability transition pore; RyR—ryanodine receptor; IP3R—inositol-1,4,5-trisphosphate receptor; OXPHOS—oxidative phosphorylation; mtDNA—mitochondrial DNA.
3.1. Biochemical Reactions in Cells
Mg2+ affects more than 600 enzymatic reactions, including energy metabolism, protein synthesis, and signal transduction [2,3,37,124]. In particular, the dependence of Mg2+ in cellular energy production has been documented over several decades. The requirement of Mg2+ in various enzymatic activities of glycolysis has been discussed in the textbook of biochemistry (pp. 228, [125]). All chemical reactions are governed by the laws of thermodynamics, that is, the Gibbs free-energy change (ΔG). The ΔG for the ATP hydrolysis varies from −28 to −34 kJ/mol, depending on the [Mg2+], because positively charged Mg2+ stabilizes ATP (Chapter 3 in [126], [127]). This fact implies that fluctuations of intracellular [Mg2+] affect all ATP-related biochemical reactions in cells; intracellular Mg2+ can function as a comprehensive regulator. Furthermore, intracellular Mg2+ competes not only with Ca2+ but also with protons or amines (–NH2+). Protons (H+) are typically present at concentrations of less than 10−7 M at pH 7 and bind to phosphate groups with a pKa of 6.5. Mg2+ is removed from ATP when the pH decreases to 6.0 [51], leading to significant effects on Mg2+-dependent reactions:
| Mg · ATP + H+ ⇋ H · ATP + Mg2+ |
Each intracellular organelle has a characteristic concentration of protons ([H+]), indicating that Mg2+ impacts cellular biochemical reactions in an organelle-specific manner. Indeed, intracellular [Mg2+] rhythms dynamically tune cellular biochemistry in response to the metabolic demands throughout the daily cycle [128,129].
3.2. Intracellular Signaling
The free energies for the binding of ATP to protein kinase in the presence of Mg2+ are less than those in the absence of Mg2+ [130]. Mg2+ potentially enhances reactions of all protein kinases, that is, intracellular signal transduction. The function of intracellular Mg2+ as the second messenger has been controversial over decades [131,132]. In living cells, the electrochemical gradient of Mg2+ across the plasma membrane serves as a reservoir for signal generation. When Mg2+-permeable channels open in response to biological cues, Mg2+ influx should be initiated. In fact, although some biological stimuli induce intracellular Mg2+ mobilization [37,39,47,49,73], the regulatory mechanism of Mg2+ channels has not been revealed yet. The concentrations of Ca2+ and other cations fluctuate within several orders of magnitudes (from 10 nM to 100 µM in the case of Ca2+) in response to cellular events [35,36]. In contrast, intracellular [Mg2+] is maintained within the narrow and sub-millimolar ranges, which is considerably greater than that in the case of [Ca2+] [36]. Hence, Mg2+ is believed to function physiologically not as a biological switch but as a modulator. In 2011, the role of Mg2+ as the second messenger in immune cells was demonstrated by its three fundamental features as a second messenger: (1) Its levels increase rapidly in response to a biological stimulus. (2) It alters the rate of one or more cellular processes. (3) It exerts cell-type specific roles because it affects different complements of enzymes in a cell-type dependent manner [35,133]. This study revealed that the coupling of the Mg2+ influx with the activation of the cell-surface receptor is required for healthy functions of its downstream cellular responses. However, whether Mg2+ amplifies extracellular biological information has not been revealed. In developing neurons, gamma-aminobutyric acid (GABA)-induced Mg2+ mobilization enhances cAMP response element binding (CREB) and mTOR activities in a [Mg2+]cyto-dependent manner within the physiological dynamic range [38]. CREB signaling is essential in the several transcriptional events and the control of neuronal plasticity [134,135]. mTOR functions as an intracellular energy balance and metabolism regulator that controls protein synthesis, cell growth, and differentiation. Intracellular Mg2+ mobilization simultaneously activates CREB and mTOR signaling, and such signaling cooperatively enhances the maturation of neural networks. Notably, the [Mg2+]cyto regulation of mTOR activities exhibits sigmoidal curves, indicating that Mg2+ functions as a cooperative signal amplifier [38,136]. In the case of the Ca2+ signal, the conformational changes of a Ca2+-binding protein, such as calmodulin, are evolutionarily conserved and play central roles in the switch-like regulation of biochemical reactions. Yet, with respect to Mg2+ signals, such a protein has not been identified. After all, the answer to the question of whether Mg2+ is a second messenger or not depends on the definition of the “second messenger.” At least, Mg2+ integrates and coordinates extracellular information and affects various cellular processes in probably all types of cells. [Mg2+]-dependent properties of such intracellular signaling are apparently more primitive and fundamental than are other intracellular signals considering the involvement of Mg2+ in the early emergence of life.
3.3. ROS Toxicity
Oxygen atoms function as electron acceptors in several metabolic processes. The majority of the oxygen consumed by biological systems is reduced to water, and it is converted to ROS. In this process, intermediate substances, such as hydroxy radicals (·OH) or superoxide anions (O2−), are toxic to the living body [137,138]. Free radical production increases in Mg2+-deficient animals [63,139,140]. Since O2− may react with Mg2+ via an electron-transfer reaction, leading to the production of magnesium–oxygen species [4], Mg2+ suppresses the production of ROS in the various tissues, including the brain [39,140,141,142]. Hence, Mg2+ is considered to protect the living body from radicals because of its physicochemical properties.
3.4. Channel Regulation
Mg2+ regulates several ion channels [143]. Especially, in neurons, extracellular Mg2+ contributes to the activity control of one of the glutamate receptors, N-methyl-D-aspartate (NMDA) receptor, which plays crucial roles in neuronal functions [144,145]. In neurons at the resting membrane potential (−70 mV), Mg2+ blocks the NMDA receptor. When the membrane potential is increased to −30 mV via another glutamate receptor, AMPA receptor, activation and cation influx, Mg2+ block is relaxed, and the NMDA receptor is activated. With the decrease in extracellular [Mg2+], the membrane potential of neurons is weakly depolarized because of the relaxation of the Mg2+ block of the NMDA receptor, leading to hyperexcitability. As the NMDA receptor is involved in excitatory neurotransmission, neuroplasticity, and neuroexcitotoxicity, it plays an important role in developmental plasticity [146,147], learning and memory [32], and circadian clock rhythm [148].
3.5. DNA Protection and Genome Stability
Mg2+ is considerably required to maintain genomic stability. Mg2+ contributes to the maintenance of genome stability via two mechanisms: Its role as a cofactor in the DNA-repair-mechanism-related enzyme and as a competitive inhibitor of the DNA-damaging factor due to the binding of Mg2+ to DNA [59]. The positive charges of Mg2+ interact with the negative charges of the phosphate group of DNA, and it plays key roles in stabilizing the secondary and tertiary structures of DNA [149]. Mg(H2O)62+, in which six water molecules are coordinated with Mg2+, forms a hydrogen bond with DNA. In the coordinated state, the denaturing agent cannot attack this site in DNA. Thus, intracellular Mg2+ protects the DNA from ROS [4]. In addition to stabilizing the DNA and chromatin structure, Mg2+ is an essential cofactor in almost all enzymatic processes involved in DNA [59]. The genetic information of DNA is replicated with high fidelity. Mg2+ plays a key role in DNA replication and repair [150]. DNA templates are copied by enzymatic processes involving DNA polymerases. In these processes, Mg2+ is required for replication with high fidelity [59,151]. DNA is continuously damaged by environmental and endogenous mutagens [152]. To maintain low mutation frequencies, cells have DNA repair systems, which require optimal [Mg2+] in multiple steps. Mg2+ contributes to the accurate transfer of genome information and resultant translation of functional protein.
4. Effects of Mg2+ on the Cellular Fate and Phenotype
4.1. Formation of Neural Networks and Synaptic Activities
Mg2+ is crucial for the growth and differentiation at the cellular and tissue levels [10]. In developing neurons, the neurotransmitter-induced increase in [Mg2+]cyto mobilized from mitochondria stimulates mTOR activities in a [Mg2+]-dependent manner and facilitates the maturation of neural networks [38]. Typically, mTOR plays a central role in the regulation of the cellular metabolic state and protein synthesis in response to the high demand for growth and proliferation. In neurogenesis, the mTOR activation leads to dendritic arborization via the regulation of protein synthesis [153].
Another key player for Mg2+ mobilization in neuronal developments is the TRPM7 channel. The TRPM7 channel is composed of a non-selective divalent cation channel and protein kinase domain, which is called “chanzyme”. It is ubiquitously expressed and plays key roles in intracellular Mg2+ homeostasis [55,154,155,156,157,158,159], and the TRPM7 channel is regulated by intracellular signals and also its kinase domain [160,161,162]. Although TRPM7 is essential for early embryonic development [10,163,164,165], the contribution of Mg2+ influx through TRPM7 on the embryonic development remains controversial because TRPM7 acts as both channel and enzyme [10,164]. Several studies support the roles of kinase, which is responsible for the requirement of TRPM7 during embryogenesis [163,164,166]. The depletion of TRPM7 leads to disruption of the embryonic developments without affecting the uptake of Mg2+ in thymocytes [164]. These studies suggest a possibility that the contribution of TRPM7 in Mg2+ homeostasis is low in vivo or that disturbed Mg2+ homeostasis is compensated by other Mg2+ channels. Actually, cells possess several Mg2+ transporting systems [37,167], and Mg2+ homeostasis is robustly maintained through the compensation mechanism in vertebrates. In fact, the expressions of several Mg2+ transporters are simultaneously and dynamically changed in response to the cellular environments [168]. The properties of TRPM7 as the channel and kinase and compensatory maintenance of Mg2+ homeostasis causes the difficulties for revealing the roles of Mg2+ in animal developments. Recently, some groups have shown that inactivation of the TRPM7 channel in living mice results in impaired Mg2+ transport, supporting the notion that the TRPM7 channel indeed can function as important Mg2+ channel in vivo [165,169]. Moreover, careful analysis using TRPM7 mutants lacking kinase domain support that TRPM7-mediated Mg2+ influx is essential for embryonic developments in vertebrate [10,170].
TRPM7 is highly expressed in the tips of the growth cone [171]. The TRPM7-mediated Mg2+ influx in fibroblasts is required for lamellipodia formation, cell polarization, and directed cell migration [172]. In lymphocytes, the TRPM7-mediated Mg2+ influx is apparently associated with phosphoinositide 3-kinase (PI3K)/Akt/mTOR signaling [173,174]. Neurite outgrowth is dependent on the dynamic changes of the cytoskeleton within the growth cone, and it is energy-consuming and spatiotemporally controlled process. Thus, the entry of TRPM7-dependent Mg2+ presumably enhances the activation of mTOR to meet the energy demand for the neuronal network organization. In contrast, the TRPM7-mediated Ca2+ influx causes the generation of ROS and suppresses the polarization of hippocampal neurons. Physiological ROS production is required for the polymerization of the cytoskeleton [175]. ROS physiologically regulates cytoskeletal changes via the modification of cytoskeletal and cytoskeleton-regulated molecules for the appropriate formation of the neural network [176]. These facts suggest that the TRPM7 channel is crucial for the growth cone pathfinding via the prevention of the axonal overgrowth and connection with unwanted targets. In addition, TRPM7 responds to the membrane stretch and fluid shear force [177,178]. It seems the opposite roles of TRPM7-mediated Mg2+ and Ca2+ influxes cooperatively regulate the proper growth cone pathfinding in response to the extracellular mechanical stimuli.
In nervous systems, chemical synapse and electrical synapse functionally connect neuronal cells for the formation of neural networks. Electrical synapses are physically connected by channel proteins, forming gap junctions. [Mg2+]cyto controls the strength of the electrical gap junctions [179,180]. Mathematical simulations revealed [Mg2+]cyto-dependent long-term plasticity through the regulation of electrical gap junctions [181]. Furthermore, chemical-synapse-mediated neural activities physiologically trigger Mg2+ influx in neurons [182]. The action-potential-triggered Mg2+ signal apparently coordinates chemical and electrical synaptic activities and contributes to synaptic plasticity and formation of neural network.
4.2. Neural Cell Fate Determination
Magnesium-L-threonate (MgT), which elevates the levels of Mg in the CSF of the brain, increases the numbers of neural stem cells (NSCs) in the hippocampus [29,183]. Mg2+ promotes the differentiation of NSCs to neurons instead of glial cells in vivo, while it promotes the differentiation to glial cells, not to neurons, in vitro [184,185]. In the rat brain, the Mg levels of all regions decline after postnatal day 5 [186]. Consistently, during the development of the mammalian nervous system, NSCs differentiate into neurons and glia in that sequence [187]. In addition, the activation of NMDA receptors increases the rate of oligodendrocyte differentiation via PKC activation [188]. In early development, changes in [Mg2+] around NSCs affect differentiation to oligodendrocytes via the Mg2+ modulation of NMDA receptor activities. Furthermore, TRPM7 channels may play a critical role in the proliferation and migration of astrocytes via the extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) signaling pathways [189]. Mg2+ influx is required for ERK activation in developing neuronal cells [38]. Therefore, TRPM7-mediated Mg2+ influx plays crucial roles in the proliferation and differentiation of neuronal cells.
5. Neuropathology of Mg2+ Homeostasis
5.1. Parkinson’s Disease
PD is a neurodegenerative disease characterized by clinical symptoms, including tremors and rigidity. As almost 85 to 90% of the patients are sporadic, and 10 to 15% are familial, PD is believed to be caused by genetic and environmental factors [190]. PD pathologically shows the selective loss of dopaminergic neurons and the formation of Lewy bodies in the substantia nigra of the brain [190,191]. In cellular pathology, dopamine metabolism, mitochondrial oxidative stress, impaired protein degradation systems, and neuroinflammation are widely believed to be attributed to the selective death of dopaminergic neurons [191,192]. The brains of PD patients exhibit low concentrations of Mg in CSF [193]. Epidemiological studies revealed that the high incidence of PD is attributed to nutritional deficiencies of Mg2+ [194,195,196]. Continuous low Mg intake over generations damages mitochondria, ER, ribosomes, and nuclear DNA, as well as induces the loss of the dopaminergic neurons in the substantia nigra [8]. In some familial PD patients, a mutation in Mg2+-transporting proteins, e.g., TRPM7 [197,198] and SLC41A1 [199,200], has been reported. During the development of zebrafish, TRPM7 is essential for the production or release of dopamine in dopaminergic neurons [201]. Dietary Mg2+-deficit mice are susceptible to the toxicity of MPTP, which is a chemical inducer of PD [202]. The administration of Mg2+ inhibits the MPP+ neurotoxicity to dopaminergic neurons [203]. In the PD model of pheochromocytoma (PC12) cells, MPP+ induces the release of Mg2+ from mitochondria and the influx of Mg2+ across the cell membrane [39]. The suppression of Mg2+ influx decreases the viability of MPP+-exposed cells, and cell viability is highly correlated with [Mg2+]cyto [39]. Moreover, the MPP+-induced inhibition of mitochondria itself altered the expression levels of cellular Mg2+-transporting proteins [168]. A 6-hydroxydopamine (6-OHDA)-induced PD animal model revealed lower levels of the SLC41A1 expression [204] and Mg2+ [205] compared with control rats.
α-Synuclein is a presynaptic neuronal protein that is pathologically linked to PD [191,192]. The aggregation of α-synuclein is considered to exert deleterious effects on the mitochondrial function [192,206]. Mg2+ at physiological levels directly inhibits the aggregation of α-synuclein, which is strongly promoted by other metal ions [207,208], suggesting that the interaction of Mg2+ and α-synuclein suppresses aggregation, and hence, neurotoxicity [209]. In addition, Mg2+ may inhibit the aggregation of α-synuclein by an indirect mechanism. Autophagy is a mechanism that transports misfolded protein aggregation and damaged organelles to the lysosome for degradation. The activation of autophagy promotes the clearance of cytoplasmic aggregated protein, including α-synuclein [210]. Thus, the impairment of basal autophagy causes abnormal accumulation and protein aggregation [211,212], and consequently, pathological features of PD in dopaminergic neurons [213]. mTOR signaling negatively modulates autophagy [214] and balances anabolism and catabolism in response to environmental conditions [215]. Thus, mTOR signaling affects the pathology of PD [216]. Although it is still controversial whether mTOR activity is neuroprotective or neurotoxic, the regulation of the mTOR signal is tightly connected to PD pathology via autophagy regulation [217]. Since intracellular Mg2+ is a regulator of mTOR signaling [38,116,128], the dependence of mTOR signaling on Mg2+ provides one explanation for the relationship between Mg2+ and the PD pathology. Such Mg2+ roles are expected to contribute to the protection of dopaminergic neurons in the substantia nigra from degeneration in concert with the other physiological roles of Mg2+, such as the suppression of ROS activities and the regulation of energy metabolism (described above).
5.2. Alzheimer’s Disease and Cognitive Functions
AD is the most common form of dementia in the population over 65 years old. AD is characterized by pathological features, such as hyperphosphorylated tau and extracellular senile plaques [218,219]. Senile plaques primarily comprise amyloid β (Aβ), and the accumulation of Aβ leads to the degeneration of neurons and resultant brain atrophy. Compared with healthy people, AD patients exhibit lower [Mg2+] in the CSF [198,220] and brain [220,221,222]. AD patients with lower [Mg2+] in the serum are likely to show more severe symptoms [223]. Mg2+ deficiency causes emotional memory dysfunction [224,225]. Mg2+ administration improves learning and memory in dementia patients [222] and in healthy animals [30,31,226] and promotes the recovery of cognitive function after brain injury [227,228]. In pathology, Aβ is sequentially cleaved from amyloid β precursor protein (APP) by β-secretase and γ-secretase. In contrast, α-secretase then cleaves APP into the C terminal fragment α (CTFα) and soluble APPα (sAPPα), which is neurotrophic. Since CTFα and sAPPα are elevated under high extracellular [Mg2+] conditions, the accumulation of the C terminal fragment β (CTFβ) and Aβ occur under low extracellular [Mg2+] conditions [229]. The elevation of extracellular [Mg2+] prevents the Aβ-induced reduction of the synaptic NMDA receptors via the suppression of the calcineurin overactivation in hippocampal slices [29]. MgT treatment reduces soluble APPβ and CTFβ, leading to Aβ aggregation and neuronal toxicity, which in turn prevent cognitive deficits and synaptic loss in the AD model of transgenic mice [29]. Extracellular Mg2+ in BBB reduces the influx of Aβ from blood to ECF and promote clearance of Aβ [19]. Furthermore, the treatment of MgSO4 attenuates impairments in long-term potentiation (LTP), dendritic abnormalities, and the impaired recruitment of synaptic proteins via the inhibition of glycogen synthase kinase-3β (GSK-3β) and activation of the PI3K/Akt signaling in sporadic AD model rats [230]. The inflammation is triggered by Aβ oligomers at the early stage. MgT decreases the TNF-α expression, which is a key mediator of inflammation, in glial cells and the expression of presenilin enhancer 2 and nicastrin, which are potential promoters of the Aβ synthesis, in neurons via a PI3K/Akt and nuclear factor-kappa B (NF-κB)-dependent mechanism [231]. Taken together, Mg2+ influx can suppress the proinflammatory mechanisms and protect neuronal functions in AD pathology.
5.3. Demyelination
In demyelination mutant (dmy) rats, the loss-of-function mutation of mitochondrial Mg2+ uptake gene, Mrs2, is identified. In dmy rats, an increased number of mitochondria and abnormal content of metabolites are observed [232,233]. These observations revealed an association between mitochondrial Mg2+ homeostasis and demyelination. Myelin increases the conduction velocity of the action potential and energy efficiency. Although the mechanisms of axonal pathology and demyelination are not yet completely understood, the mitochondrial dysfunction is considered to play a central role [234,235]. The dysregulation of mitochondrial Mg2+ homeostasis disrupts the ATP production via the shift of mitochondrial energy metabolism and morphology. The Mrs2 knockdown sensitizes cellular tolerance against cellular stress [48]. In addition, Mg2+-exposed oligodendrocytes exhibit more resistance to a hypoxic-ischemic injury [236]. In dmy rats, metabolic abnormality appears to lead to the downregulation of aspartoacylase, which cleaves the acetate moiety for use in the syntheses of fatty acids and steroids for myelination [237]. This report is consistent with the observation by metabolomics that decreased Mrs2 expression leads to the abnormal metabolism of fatty acids [48]. In myelinating oligodendrocytes, double-strand breaks of mtDNA cause mitochondrial dysfunctions, consequently triggering demyelination and irreversible neurological deficit [238]. Mitochondrial Mg2+ affects mtDNA function and processes of mitochondrial central dogma independent of the exterior of the mitochondria. mtDNA lacks histones responsible for the formation of nucleosomes, and its absence causes the high rate of mtDNA mutagenesis (∼10-fold greater than in nuclear DNA) [239,240,241]. Thus, the roles of Mg2+ in DNA stabilization in mitochondria seem to be more dominant than that in the nucleus. Because the interaction of Mg2+ with DNA also contributes to the DNA/RNA stabilities [59], a low [Mg2+]mito level should increase the risk of mtDNA damage. In addition, according to the endosymbiosis theory, mitochondria are endosymbiotic bacteria [242,243]. In bacteria, Mg2+ plays a key role in the regulation of protein synthesis [100]. Thus, mitochondrial Mg2+ may regulate protein synthesis in mitochondria. In fact, the separated regulation of mitochondrial and cytosolic protein synthesis in the process of the central dogma plays a central role in adaptation in the cellular nutrient environment [244].
6. Conclusions and Perspectives
Mg2+ is a versatile divalent cation because of its unique physicochemical properties. In the brain, the extracellular and intracellular Mg2+ levels dynamically change according to the biological context. Mg2+ plays crucial roles in cell proliferation, differentiation, survival, and neural network formation via the regulation of cellular metabolism, intracellular signaling, channel opening, protein synthesis, and ROS toxicity. Typically, the mobilization of intracellular Mg2+ stimulates catabolism and protein synthesis, consequently activating the cellular processes that determine the fate and phenotype. Although Mg2+ usually protects neuronal cells against cellular stress, excess levels of Mg2+ are sometimes deleterious to healthy neuronal functions. Therefore, the appropriate regulation of cellular Mg2+ homeostasis is essential for neuronal functions in the brain, and the dysregulation of Mg2+ homeostasis potentially causes and aggravates neurodegenerative diseases, such as Parkinson’s diseases, Alzheimer’s disease, and demyelination. The recovery of healthy Mg2+ homeostasis through chemotherapy targeting Mg2+-transporting system can improve cellular functions under pathological conditions. In summary, the regulation of Mg2+ homeostasis can be a candidate for a therapeutic target in neurodegenerative diseases. However, the roles of Mg2+ and its regulatory mechanisms have not been investigated much. Thus, further study is crucial for developing future therapies and deepening the understanding of its neuro(patho)physiology.
Abbreviations
| [Ca2+] | Ca2+ concentration |
| [H+] | H+ concentration |
| [Mg2+] | Mg2+ concentration |
| [Mg2+]cyto | cytosolic free Mg2+ concentration |
| [Mg2+]ex | extracellular free Mg2+ concentration |
| [Mg2+]mito | free [Mg2+] in the mitochondrial matrix |
| [Mg2+]nuc | nuclear free [Mg2+] |
| ACDP2 | cyclin M2 |
| AD | Alzheimer’s disease |
| APP | Aβ precursor protein |
| APPβ | β-amyloid precursor protein β |
| ATP | adenosine 5’-triphosphate |
| Aβ | amyloid β |
| BBB | blood–brain barrier |
| CNNM2 | cyclin M2 |
| CNS | central nervous system |
| CREB | cAMP response element binding |
| CSF | cerebrospinal fluid |
| CTFα | C terminal fragment α |
| CTFβ | C terminal fragment β |
| ECF | extracellular cellular fluid |
| ERK | extracellular signal-regulated kinase |
| GABA | gamma-aminobutyric acid |
| GSK-3β | glycogen synthase kinase-3β |
| IP3R | inositol-1,4,5-trisphosphate receptor |
| JNK | c-Jun N-terminal kinase |
| LTP | long-term potentiation |
| Mg | magnesium |
| Mg2+ | magnesium ion |
| MgT | magnesium-L-threonate |
| mitoKATP | mitochondrial ATP-sensitive potassium channel |
| MPP+ | N-methyl-4-phenylpyridinium iodide |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| Mrs2 | mitochondrial RNA splicing 2 |
| mtDNA | mitochondrial DNA |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor-kappa B |
| NMDA | N-methyl-D-aspartate |
| NSCs | neural stem cells |
| OGDH | 2-oxoglutarate dehydrogenase |
| OXPHOS | oxidative phosphorylation |
| PC12 cell | pheochromocytoma cell |
| PD | Parkinson’s disease |
| PI3K | phosphatidylinositol-3 kinase |
| PKC | protein kinase C |
| PTP | permeability transition pore |
| ROS | reactive oxygen species |
| RyR | ryanodine receptor |
| sAPPα | soluble APPα |
| TCA | tricarboxylic acid |
| TRPM6 | transient receptor potential melastatin 6 |
| TRPM7 | transient receptor potential melastatin 7 |
| ΔG | Gibbs free energy change |
| ΔΨm | mitochondrial membrane potential |
Author Contributions
R.Y., Y.S., and K.O. wrote the paper. K.O. supervised the project.
Funding
This research was supported by the Grant in Aid for Scientific Research, KAKENHI (16H01751, 17K15108, and 17K13268); the Strategic Research Foundation Grant Aided Project for Private Universities from Ministry of Education, Culture, Sport, Science and Technology, Japan (MEXT), 2014−2018 (S1411003); and the Salt Science Research Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Romani A.M.P. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf F.I., Trapani V. Cell (patho)physiology of magnesium. Clin. Sci. (Lond.) 2008;114:27–35. doi: 10.1042/CS20070129. [DOI] [PubMed] [Google Scholar]
- 3.De Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 4.Anastassopoulou J., Theophanides T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002;42:79–91. doi: 10.1016/S1040-8428(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 5.Holm N.G. The significance of Mg in prebiotic geochemistry. Geobiology. 2012;10:269–279. doi: 10.1111/j.1472-4669.2012.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert W. Origin of life: The RNA world. Nature. 1986;319:618. doi: 10.1038/319618a0. [DOI] [Google Scholar]
- 7.Doudna J.A., Cech T.R. The chemical repertoire of natural ribozymes. Nature. 2002;418:222–228. doi: 10.1038/418222a. [DOI] [PubMed] [Google Scholar]
- 8.Oyanagi K., Kawakami E., Kikuchi-Horie K., Ohara K., Ogata K., Takahama S., Wada M., Kihira T., Yasui M. Magnesium deficiency over generations in rats with special references to the pathogenesis of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology. 2006;26:115–128. doi: 10.1111/j.1440-1789.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 9.Barbagallo M., Dominguez L.J. Trace Elements and Minerals in Health and Longevity. Springer; Cham, Switzerland: 2018. Magnesium Role in Health and Longevity; pp. 235–264. [Google Scholar]
- 10.Komiya Y., Su L.-T., Chen H.-C., Habas R., Runnels L.W. Magnesium and embryonic development. Magnes. Res. 2014;27:1–8. doi: 10.1684/mrh.2014.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenstein F.W., Stanton M.F. Serum magnesium levels in the United States, 1971-1974. J. Am. Coll. Nutr. 1986;5:399–414. doi: 10.1080/07315724.1986.10720143. [DOI] [PubMed] [Google Scholar]
- 12.Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5:i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham L.A., Caesar J.J., Burgen A.S. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism. 1960;9:646–659. [PubMed] [Google Scholar]
- 14.Avioli L.V., Berman M. Mg28 kinetics in man. J. Appl. Physiol. 1966;21:1688–1694. doi: 10.1152/jappl.1966.21.6.1688. [DOI] [PubMed] [Google Scholar]
- 15.Rusakov D.A., Kullmann D.M. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J. Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Harreveld A., Malhotra S.K. Extracellular space in the cerebral cortex of the mouse. J. Anat. 1967;101:197–207. [PMC free article] [PubMed] [Google Scholar]
- 17.Ghabriel M.N., Vink R. In: Magnesium Transport across the Blood-brain Barriers. Vink R., Nechifor M., editors. University of Adelaide Press; Adelaide, Australia: 2011. [PubMed] [Google Scholar]
- 18.Bito L.Z. Blood-Brain Barrier: Evidence for Active Cation Transport between Blood and the Extraceliular Fluid of Brain. Science. 1969;165:81–83. doi: 10.1126/science.165.3888.81. [DOI] [PubMed] [Google Scholar]
- 19.Zhu D., Su Y., Fu B., Xu H. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-beta Transcytosis. Mol. Neurobiol. 2018;55:7118–7131. doi: 10.1007/s12035-018-0896-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S.L., Yue Z., Arnold D.M., Artiushin G., Sehgal A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell. 2018;173:130–139.e10. doi: 10.1016/j.cell.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hladky S.B., Barrand M.A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Praetorius J., Damkier H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 2017;312:C673–C686. doi: 10.1152/ajpcell.00041.2017. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury M.W., Sarna G.S. Homeostasis of the ionic composition of the cerebrospinal fluid. Exp. Eye Res. 1977;25(Suppl. 1):249–257. doi: 10.1016/S0014-4835(77)80022-5. [DOI] [PubMed] [Google Scholar]
- 24.Morris M.E. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes. Res. 1992;5:303–313. [PubMed] [Google Scholar]
- 25.Allsop T.F., Pauli J.V. Magnesium concentrations in the ventricular and lumbar cerebrospinal fluid of hypomagnesaemic cows. Res. Vet. Sci. 1985;38:61–64. doi: 10.1016/S0034-5288(18)31848-4. [DOI] [PubMed] [Google Scholar]
- 26.Mori K., Yamamoto T., Miyazaki M., Hara Y., Koike N., Nakao Y. Potential risk of artificial cerebrospinal fluid solution without magnesium ion for cerebral irrigation and perfusion in neurosurgical practice. Neurol. Med. Chir. (Tokyo) 2013;53:596–600. doi: 10.2176/nmc.oa2012-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong G.K.C., Lam C.W.K., Chan M.T.V., Gin T., Poon W.S. The effect of hypermagnesemic treatment on cerebrospinal fluid magnesium level in patients with aneurysmal subarachnoid hemorrhage. Magnes. Res. 2009;22:60–65. [PubMed] [Google Scholar]
- 28.Sun Q., Weinger J.G., Mao F., Liu G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology. 2016;108:426–439. doi: 10.1016/j.neuropharm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Li W., Yu J., Liu Y., Huang X., Abumaria N., Zhu Y., Huang X., Xiong W., Ren C., Liu X.-G., et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain. 2014;7:65. doi: 10.1186/s13041-014-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slutsky I., Abumaria N., Wu L.-J., Huang C., Zhang L., Li B., Zhao X., Govindarajan A., Zhao M.-G., Zhuo M., et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165–177. doi: 10.1016/j.neuron.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Bush A.I. Kalzium ist nicht alles. Neuron. 2010;65:143–144. doi: 10.1016/j.neuron.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita T., Oda Y., Horiuchi J., Yin J.C.P., Morimoto T., Saitoe M. Mg2+ block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron. 2012;74:887–898. doi: 10.1016/j.neuron.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong W., Liang Y., Li X., Liu G., Wang Z. Erythrocyte intracellular Mg2+ concentration as an index of recognition and memory. Sci. Rep. 2016;6:26975. doi: 10.1038/srep26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong W., Liang Y., Li X., Liu G., Wang Z. A Direct Quantitative Analysis of Erythrocyte Intracellular Ionized Magnesium in Physiological and Pathological Conditions. Biol. Pharm. Bull. 2019;42:357–364. doi: 10.1248/bpb.b18-00406. [DOI] [PubMed] [Google Scholar]
- 35.Chaigne-Delalande B., Lenardo M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014;35:332–344. doi: 10.1016/j.it.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapani V., Farruggia G., Marraccini C., Iotti S., Cittadini A., Wolf F.I. Intracellular magnesium detection: imaging a brighter future. Analyst. 2010;135:1855–1866. doi: 10.1039/c0an00087f. [DOI] [PubMed] [Google Scholar]
- 37.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka R., Shindo Y., Hotta K., Suzuki K., Oka K. GABA-Induced Intracellular Mg2+ Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr. Biol. 2018;28:3984–3991.e5. doi: 10.1016/j.cub.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 39.Shindo Y., Yamanaka R., Suzuki K., Hotta K., Oka K. Intracellular magnesium level determines cell viability in the MPP+ model of Parkinson’s disease. Biochim. Biophys. Acta. 2015;1853:3182–3191. doi: 10.1016/j.bbamcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Maeshima K., Matsuda T., Shindo Y., Imamura H., Tamura S., Imai R., Kawakami S., Nagashima R., Soga T., Noji H., et al. A Transient Rise in Free Mg2+ Ions Released from ATP-Mg Hydrolysis Contributes to Mitotic Chromosome Condensation. Curr. Biol. 2018;28:444–451.e6. doi: 10.1016/j.cub.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Yatsimirskii K.B. Electronic structure, energy of hydration, and stability of metal aquo ions. Theor. Exp. Chem. 1994;30:1–9. doi: 10.1007/BF00535915. [DOI] [Google Scholar]
- 42.Winkelmann G. Microbial Transport Systems. John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 43.Maret W., Wedd A. Binding, Transport and Storage of Metal Ions in Biological Cells. Volume 2. Royal Society of Chemistry; Cambridge, UK: 2014. [Google Scholar]
- 44.Politi H.C., Preston R.R. Is it time to rethink the role of Mg2+ in membrane excitability? Neuroreport. 2003;14:659–668. doi: 10.1097/00001756-200304150-00001. [DOI] [PubMed] [Google Scholar]
- 45.Quamme G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010;298:C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 46.Gout E., Rébeillé F., Douce R., Bligny R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. USA. 2014;111:E4560–E4567. doi: 10.1073/pnas.1406251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamanaka R., Shindo Y., Hotta K., Suzuki K., Oka K. NO/cGMP/PKG signaling pathway induces magnesium release mediated by mitoKATP channel opening in rat hippocampal neurons. FEBS Lett. 2013;587:2643–2648. doi: 10.1016/j.febslet.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka R., Tabata S., Shindo Y., Hotta K., Suzuki K., Soga T., Oka K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016;6:30027. doi: 10.1038/srep30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shindo Y., Fujimoto A., Hotta K., Suzuki K., Oka K. Glutamate-induced calcium increase mediates magnesium release from mitochondria in rat hippocampal neurons. J. Neurosci. Res. 2010;88:3125–3132. doi: 10.1002/jnr.22467. [DOI] [PubMed] [Google Scholar]
- 50.Kroeger H., Trösch W. Influence of the explantation milieu on intranuclear [Na], [K] and [Mg] of Chironomus thummi salivary gland cells. J. Cell. Physiol. 1974;83:19–25. doi: 10.1002/jcp.1040830104. [DOI] [PubMed] [Google Scholar]
- 51.Pasternak K., Kocot J., Horecka A. Biochemistry of magnesium. J. Elem. 2010;15:601–616. doi: 10.5601/jelem.2010.15.3.601-616. [DOI] [Google Scholar]
- 52.Korolev N., Lyubartsev A.P., Rupprecht A., Nordenskiold L. Competitive binding of Mg2+, Ca2+, Na+, and K+ ions to DNA in oriented DNA fibers: experimental and Monte Carlo simulation results. Biophys. J. 1999;77:2736–2749. doi: 10.1016/S0006-3495(99)77107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bloom K. Cell Division: Single-Cell Physiology Reveals Secrets of Chromosome Condensation. Curr. Biol. 2018;28:R117–R119. doi: 10.1016/j.cub.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uz G., Sarikaya A.T. The effect of magnesium on mitotic spindle formation in Schizosaccharomyces pombe. Genet. Mol. Biol. 2016;39:459–464. doi: 10.1590/1678-4685-GMB-2015-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandra S., Parker D.J., Barth R.F., Pannullo S.C. Quantitative imaging of magnesium distribution at single-cell resolution in brain tumors and infiltrating tumor cells with secondary ion mass spectrometry (SIMS) J. Neurooncol. 2016;127:33–41. doi: 10.1007/s11060-015-2022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukacs G.L., Zs-Nagy I., Steiber J., Gyori F., Balazs G. Relative intranuclear magnesium and phosphorus contents in normal and tumor cells of the human thyroid gland as revealed by energy-dispersive X-ray microanalysis. Scanning Microsc. 1996;10:1191–1200. [PubMed] [Google Scholar]
- 57.Rubin H. Central roles of Mg2+ and MgATP2- in the regulation of protein synthesis and cell proliferation: significance for neoplastic transformation. Adv. Cancer Res. 2005;93:1–58. doi: 10.1016/S0065-230X(05)93001-7. [DOI] [PubMed] [Google Scholar]
- 58.Wolf F.I., Trapani V., Cittadini A. Magnesium and the control of cell proliferation: Looking for a needle in a haystack. Magnes. Res. 2008;21:83–91. [PubMed] [Google Scholar]
- 59.Hartwig A. Role of magnesium in genomic stability. Mutat. Res. 2001;475:113–121. doi: 10.1016/S0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 60.Wright R.H.G., Le Dily F., Beato M. ATP, Mg2+, Nuclear Phase Separation, and Genome Accessibility. Trends Biochem. Sci. 2019;44:565–574. doi: 10.1016/j.tibs.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Kubota T., Shindo Y., Tokuno K., Komatsu H., Ogawa H., Kudo S., Kitamura Y., Suzuki K., Oka K. Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim. Biophys. Acta. 2005;1744:19–28. doi: 10.1016/j.bbamcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Shindo Y., Fujii T., Komatsu H., Citterio D., Hotta K., Suzuki K., Oka K. Newly developed Mg2+-selective fluorescent probe enables visualization of Mg2+ dynamics in mitochondria. PLoS ONE. 2011;6:e23684. doi: 10.1371/journal.pone.0023684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pilchova I., Klacanova K., Tatarkova Z., Kaplan P., Racay P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell. Longev. 2017;2017:1–8. doi: 10.1155/2017/6797460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung D.W., Brierley G.P. Magnesium transport by mitochondria. J. Bioenerg. Biomembr. 1994;26:527–535. doi: 10.1007/BF00762737. [DOI] [PubMed] [Google Scholar]
- 65.Rutter G.A., Osbaldeston N.J., Mccormackt J.G., Denton R.M. Measurement of matrix free Mg2+ concentration in rat heart mitochondria by using entrapped fluorescent probes. Biochem. J. 1990;271:627–634. doi: 10.1042/bj2710627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolisek M., Zsurka G., Samaj J., Weghuber J., Schweyen R.J., Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mastrototaro L., Smorodchenko A., Aschenbach J.R., Kolisek M., Sponder G. Solute carrier 41A3 encodes for a mitochondrial Mg2+ efflux system. Sci. Rep. 2016;6:27999. doi: 10.1038/srep27999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panov A., Scarpa A. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+ Biochemistry. 1996;35:427–432. doi: 10.1021/bi952101t. [DOI] [PubMed] [Google Scholar]
- 69.Aprille J.R. Mechanism and regulation of the mitochondrial ATP-Mg/Pi carrier. J. Bioenerg. Biomembr. 1993;25:473–481. doi: 10.1007/BF01108404. [DOI] [PubMed] [Google Scholar]
- 70.LaNoue K.F., Bryla J., Williamson J.R. Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J. Biol. Chem. 1972;247:667–679. [PubMed] [Google Scholar]
- 71.Piskacek M., Zotova L., Zsurka G., Schweyen R.J. Conditional knockdown of hMRS2 results in loss of mitochondrial Mg2+ uptake and cell death. J. Cell. Mol. Med. 2009;13:693–700. doi: 10.1111/j.1582-4934.2008.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsui Y., Funato Y., Imamura H., Miki H., Mizukami S., Kikuchi K. Visualization of long-term Mg2+ dynamics in apoptotic cells using a novel targetable fluorescent probe. Chem. Sci. 2017;8:8255–8264. doi: 10.1039/C7SC03954A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J., Zhao F., Zhao Y., Wang J., Pei L., Sun N., Shi J. Hypoxia induces an increase in intracellular magnesium via transient receptor potential melastatin 7 (TRPM7) channels in rat hippocampal neurons in vitro. J. Biol. Chem. 2011;286:20194–20207. doi: 10.1074/jbc.M110.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y., Dong Y., Xu Z., Xie Z. Propofol and magnesium attenuate isoflurane-induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med. Gas Res. 2012;2:20. doi: 10.1186/2045-9912-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y., Wei X., Yan P., Han Y., Sun S., Wu K., Fan D. Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol. Ther. 2009;8:607–614. doi: 10.4161/cbt.8.7.7920. [DOI] [PubMed] [Google Scholar]
- 76.Testai L., Rapposelli S., Martelli A., Breschi M.C., Calderone V. Mitochondrial potassium channels as pharmacological target for cardioprotective drugs. Med. Res. Rev. 2015;35:520–553. doi: 10.1002/med.21332. [DOI] [PubMed] [Google Scholar]
- 77.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langston J.W., Irwin I., Langston E.B., Forno L.S. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci. Lett. 1984;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- 79.Zoratti M., Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-A. [DOI] [PubMed] [Google Scholar]
- 80.Seo Y.-W., Shin J.N., Ko K.H., Cha J.H., Park J.Y., Lee B.R., Yun C.-W., Kim Y.M., Seol D., Kim D., et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J. Biol. Chem. 2003;278:48292–48299. doi: 10.1074/jbc.M308785200. [DOI] [PubMed] [Google Scholar]
- 81.Zhang G., Gruskos J.J., Afzal M.S., Buccella D. Visualizing changes in mitochondrial Mg2+ during apoptosis with organelle-targeted triazole-based ratiometric fluorescent sensors. Chem. Sci. 2015;6:6841–6846. doi: 10.1039/C5SC02442K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cappadone C., Merolle L., Marraccini C., Farruggia G., Sargenti A., Locatelli A., Morigi R., Iotti S. Intracellular magnesium content decreases during mitochondria-mediated apoptosis induced by a new indole-derivative in human colon cancer cells. Magnes. Res. 2012;25:104–111. doi: 10.1684/mrh.2012.0319. [DOI] [PubMed] [Google Scholar]
- 83.Eskes R., Antonsson B., Osen-Sand A., Montessuit S., Richter C., Sadoul R., Mazzei G., Nichols A., Martinou J.C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim T.H., Zhao Y., Barber M.J., Kuharsky D.K., Yin X.M. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J. Biol. Chem. 2000;275:39474–39481. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- 85.Lee S.K., Shanmughapriya S., Mok M.C.Y., Dong Z., Tomar D., Carvalho E., Rajan S., Junop M.S., Madesh M., Stathopulos P.B. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem. Biol. 2016;23:1157–1169. doi: 10.1016/j.chembiol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szanda G., Rajki A., Gallego-Sandín S., Garcia-Sancho J., Spät A. Effect of cytosolic Mg2+ on mitochondrial Ca2+ signaling. Pflugers Arch. 2009;457:941–954. doi: 10.1007/s00424-008-0551-0. [DOI] [PubMed] [Google Scholar]
- 87.Pradhan R.K., Qi F., Beard D.A., Dash R.K. Characterization of Mg2+ inhibition of mitochondrial Ca2+ uptake by a mechanistic model of mitochondrial Ca2+ uniporter. Biophys. J. 2011;101:2071–2081. doi: 10.1016/j.bpj.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boelens A.D., Pradhan R.K., Blomeyer C.A., Camara A.K.S., Dash R.K., Stowe D.F. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake-independent respiration and redox state in cardiac isolated mitochondria. J. Bioenerg. Biomembr. 2013;45:203–218. doi: 10.1007/s10863-013-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carreras-Sureda A., Pihan P., Hetz C. Calcium signaling at the endoplasmic reticulum: fine-tuning stress responses. Cell Calcium. 2018;70:24–31. doi: 10.1016/j.ceca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Luarte A., Cornejo V.H., Bertin F., Gallardo J., Couve A. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 2018;78:181–208. doi: 10.1002/dneu.22560. [DOI] [PubMed] [Google Scholar]
- 91.Kwon S.-K., Hirabayashi Y., Polleux F. Organelle-Specific Sensors for Monitoring Ca2+ Dynamics in Neurons. Front. Synaptic Neurosci. 2016;8:29. doi: 10.3389/fnsyn.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volpe P., Alderson-Lang B.H., Nickols G.A. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. I. Effect of Mg2+ Am. J. Physiol. 1990;258:C1077–C1085. doi: 10.1152/ajpcell.1990.258.6.C1077. [DOI] [PubMed] [Google Scholar]
- 93.Gusev K., Niggli E. Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J. Gen. Physiol. 2008;132:721–730. doi: 10.1085/jgp.200810119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bull R., Finkelstein J.P., Humeres A., Behrens M.I., Hidalgo C. Effects of ATP, Mg2+, and redox agents on the Ca2+ dependence of RyR channels from rat brain cortex. Am. J. Physiol. Cell Physiol. 2007;293:C162–C171. doi: 10.1152/ajpcell.00518.2006. [DOI] [PubMed] [Google Scholar]
- 95.Laver D.R. Regulation of the RyR channel gating by Ca2+ and Mg2+ Biophys. Rev. 2018;10:1087–1095. doi: 10.1007/s12551-018-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Juan-Sanz J., Holt G.T., Schreiter E.R., de Juan F., Kim D.S., Ryan T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron. 2017;93:867–881.e6. doi: 10.1016/j.neuron.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H.Y., Quamme G.A. Caffeine decreases intracellular free Mg2+ in isolated adult rat ventricular myocytes. Biochim. Biophys. Acta. 1997;1355:61–68. doi: 10.1016/S0167-4889(96)00117-6. [DOI] [PubMed] [Google Scholar]
- 98.Schuwirth B.S., Borovinskaya M.A., Hau C.W., Zhang W., Vila-Sanjurjo A., Holton J.M., Cate J.H.D. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 99.Pontes M.H., Sevostyanova A., Groisman E.A. When Too Much ATP Is Bad for Protein Synthesis. J. Mol. Biol. 2015;427:2586–2594. doi: 10.1016/j.jmb.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nierhaus K.H. Mg2+, K+, and the ribosome. J. Bacteriol. 2014;196:3817–3819. doi: 10.1128/JB.02297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiss R.L., Morris D.R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973;12:435–441. doi: 10.1021/bi00727a012. [DOI] [PubMed] [Google Scholar]
- 102.Weiss R.L., Kimes B.W., Morris D.R. Cations and ribosome structure. 3. Effects on the 30S and 50S subunits of replacing bound Mg2+ by inorganic cations. Biochemistry. 1973;12:450–456. doi: 10.1021/bi00727a014. [DOI] [PubMed] [Google Scholar]
- 103.Fagerbakke K.M., Norland S., Heldal M. The inorganic ion content of native aquatic bacteria. Can. J. Microbiol. 1999;45:304–311. doi: 10.1139/w99-013. [DOI] [PubMed] [Google Scholar]
- 104.Lee D.-Y.D., Galera-Laporta L., Bialecka-Fornal M., Moon E.C., Shen Z., Briggs S.P., Garcia-Ojalvo J., Suel G.M. Magnesium Flux Modulates Ribosomes to Increase Bacterial Survival. Cell. 2019;177:352–360.e13. doi: 10.1016/j.cell.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akanuma G., Yamazaki K., Yagishi Y., Iizuka Y., Ishizuka M., Kawamura F., Kato-Yamada Y. Magnesium Suppresses Defects in the Formation of 70S Ribosomes as Well as in Sporulation Caused by Lack of Several Individual Ribosomal Proteins. J. Bacteriol. 2018;200 doi: 10.1128/JB.00212-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akanuma G., Kobayashi A., Suzuki S., Kawamura F., Shiwa Y., Watanabe S., Yoshikawa H., Hanai R., Ishizuka M. Defect in the formation of 70S ribosomes caused by lack of ribosomal protein L34 can be suppressed by magnesium. J. Bacteriol. 2014;196:3820–3830. doi: 10.1128/JB.01896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pontes M.H., Groisman E.A. Protein synthesis controls phosphate homeostasis. Genes Dev. 2018;32:79–92. doi: 10.1101/gad.309245.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gesteland R.F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J. Mol. Biol. 1966;18:356–371. doi: 10.1016/S0022-2836(66)80253-X. [DOI] [PubMed] [Google Scholar]
- 109.Terasaki M., Rubin H. Evidence that intracellular magnesium is present in cells at a regulatory concentration for protein synthesis. Proc. Natl. Acad. Sci. USA. 1985;82:7324–7326. doi: 10.1073/pnas.82.21.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pontes M.H., Yeom J., Groisman E.A. Reducing Ribosome Biosynthesis Promotes Translation during Low Mg2+ Stress. Mol. Cell. 2016;64:480–492. doi: 10.1016/j.molcel.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gall A.R., Datsenko K.A., Figueroa-Bossi N., Bossi L., Masuda I., Hou Y.-M., Csonka L.N. Mg2+ regulates transcription of mgtA in Salmonella Typhimurium via translation of proline codons during synthesis of the MgtL peptide. Proc. Natl. Acad. Sci. USA. 2016;113:15096–15101. doi: 10.1073/pnas.1612268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hughes K.T. Mg2+-dependent translational speed bump acts to regulate gene transcription. Proc. Natl. Acad. Sci. USA. 2016;113:14881–14883. doi: 10.1073/pnas.1618222114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park M., Nam D., Kweon D.-H., Shin D. ATP reduction by MgtC and Mg2+ homeostasis by MgtA and MgtB enables Salmonella to accumulate RpoS upon low cytoplasmic Mg2+ stress. Mol. Microbiol. 2018;110:283–295. doi: 10.1111/mmi.14105. [DOI] [PubMed] [Google Scholar]
- 114.Mayer C., Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–6391. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 115.Vidair C., Rubin H. Mg2+ as activator of uridine phosphorylation in coordination with other cellular responses to growth factors. Proc. Natl. Acad. Sci. USA. 2005;102:662–666. doi: 10.1073/pnas.0409082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubin H. The membrane, magnesium, mitosis (MMM) model of cell proliferation control. Magnes. Res. 2005;18:268–274. [PubMed] [Google Scholar]
- 117.Slomnicki L.P., Pietrzak M., Vashishta A., Jones J., Lynch N., Elliot S., Poulos E., Malicote D., Morris B.E., Hallgren J., et al. Requirement of Neuronal Ribosome Synthesis for Growth and Maintenance of the Dendritic Tree. J. Biol. Chem. 2016;291:5721–5739. doi: 10.1074/jbc.M115.682161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koley S., Rozenbaum M., Fainzilber M., Terenzio M. Translating regeneration: Local protein synthesis in the neuronal injury response. Neurosci. Res. 2019;139:26–36. doi: 10.1016/j.neures.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 119.Honda K., Smith M.A., Zhu X., Baus D., Merrick W.C., Tartakoff A.M., Hattier T., Harris P.L., Siedlak S.L., Fujioka H., et al. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J. Biol. Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- 120.Ding Q., Markesbery W.R., Chen Q., Li F., Keller J.N. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rieker C., Engblom D., Kreiner G., Domanskyi A., Schober A., Stotz S., Neumann M., Yuan X., Grummt I., Schutz G., et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J. Neurosci. 2011;31:453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vilotti S., Codrich M., Dal Ferro M., Pinto M., Ferrer I., Collavin L., Gustincich S., Zucchelli S. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS One. 2012;7:e35051. doi: 10.1371/journal.pone.0035051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang H., Shin J.-H. Repression of rRNA transcription by PARIS contributes to Parkinson’s disease. Neurobiol. Dis. 2015;73:220–228. doi: 10.1016/j.nbd.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Rubin H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch. Biochem. Biophys. 2007;458:16–23. doi: 10.1016/j.abb.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 125.Bhagavan N.V. Medical Biochemistry. Academic Press; Cambridge, MA, USA: 2002. [Google Scholar]
- 126.Milo R., Phillips R. Cell Biology by the Numbers. Garland Science; Boca Raton, FL, USA: 2015. [Google Scholar]
- 127.Berg J.M., Tymoczko J.L., Stryer L. Biochemistry. 5th ed. W. H. Freeman; New York, NY, USA: 2002. [Google Scholar]
- 128.Feeney K.A., Hansen L.L., Putker M., Olivares-Yanez C., Day J., Eades L.J., Larrondo L.F., Hoyle N.P., O’Neill J.S., van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Van Ooijen G., O’Neill J.S. Intracellular magnesium and the rhythms of life. Cell Cycle. 2016;15:2997–2998. doi: 10.1080/15384101.2016.1214030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu L., Xu L., Xu M., Wan B., Yu L., Huang Q. Role of Mg2+ ions in protein kinase phosphorylation: insights from molecular dynamics simulations of ATP-kinase complexes. Mol. Simul. 2011;37:1143–1150. doi: 10.1080/08927022.2011.561430. [DOI] [Google Scholar]
- 131.Takaya J., Higashino H., Kobayashi Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes. Res. 2000;13:139–146. [PubMed] [Google Scholar]
- 132.Grubbs R.D., Maguire M.E. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6:113–127. [PubMed] [Google Scholar]
- 133.Li F.-Y., Chaigne-Delalande B., Kanellopoulou C., Davis J.C., Matthews H.F., Douek D.C., Cohen J.I., Uzel G., Su H.C., Lenardo M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomas G.M., Huganir R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 135.Zhai S., Ark E.D., Parra-Bueno P., Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stangherlin A., O’Neill J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018;28:R1403–R1405. doi: 10.1016/j.cub.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 137.Tarafdar A., Pula G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cid-Castro C., Hernandez-Espinosa D.R., Moran J. ROS as Regulators of Mitochondrial Dynamics in Neurons. Cell. Mol. Neurobiol. 2018;38:995–1007. doi: 10.1007/s10571-018-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Davies L.R., Martin S.M., Bawaney I.M., Buettner G.R., Kerber R.E. Magnesium reduces free radical concentration and preserves left ventricular function after direct current shocks. Resuscitation. 2003;56:199–206. doi: 10.1016/S0300-9572(02)00353-2. [DOI] [PubMed] [Google Scholar]
- 140.Zheltova A.A., Kharitonova M.V., Iezhitsa I.N., Spasov A.A. Magnesium deficiency and oxidative stress: an update. BioMedicine (Taipei) 2016;6:20. doi: 10.7603/s40681-016-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barbagallo M., Belvedere M., Dominguez L.J. Magnesium homeostasis and aging. Magnes. Res. 2009;22:235–246. doi: 10.1684/mrh.2009.0187. [DOI] [PubMed] [Google Scholar]
- 142.Nielsen F.H. Magnesium deficiency and increased inflammation: current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guiet-Bara A., Durlach J., Bara M. Magnesium ions and ionic channels: activation, inhibition or block - a hypothesis. Magnes. Res. 2007;20:100–106. [PubMed] [Google Scholar]
- 144.Kumar A. NMDA Receptor Function During Senescence: Implication on Cognitive Performance. Front. Neurosci. 2015;9:473. doi: 10.3389/fnins.2015.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iacobucci G.J., Popescu G.K. NMDA receptors: linking physiological output to biophysical operation. Nat. Rev. Neurosci. 2017;18:236–249. doi: 10.1038/nrn.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cull-Candy S., Brickley S., Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 147.Nechifor M. Magnesium in addiction - a general view. Magnes. Res. 2018;31:90–98. doi: 10.1684/mrh.2018.0443. [DOI] [PubMed] [Google Scholar]
- 148.Song Q., Feng G., Zhang J., Xia X., Ji M., Lv L., Ping Y. NMDA Receptor-mediated Ca2+ Influx in the Absence of Mg2+ Block Disrupts Rest: Activity Rhythms in Drosophila. Sleep. 2017;40 doi: 10.1093/sleep/zsx166. [DOI] [PubMed] [Google Scholar]
- 149.Pelletier H., Sawaya M.R., Kumar A., Wilson S.H., Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. doi: 10.1126/science.7516580. [DOI] [PubMed] [Google Scholar]
- 150.Blaszczyk U., Duda-Chodak A. Magnesium: its role in nutrition and carcinogenesis. Rocz. Panstw. Zakl. Hig. 2013;64:165–171. [PubMed] [Google Scholar]
- 151.Gao Y., Yang W. Capture of a third Mg2+ is essential for catalyzing DNA synthesis. Science. 2016;352:1334–1337. doi: 10.1126/science.aad9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cadet J., Davies K.J.A. Oxidative DNA damage & repair: an introduction. Free Radic. Biol. Med. 2017;107:2–12. doi: 10.1016/j.freeradbiomed.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.LiCausi F., Hartman N. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018;19:1544. doi: 10.3390/ijms19051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schlingmann K.P., Waldegger S., Konrad M., Chubanov V., Gudermann T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 155.Paravicini T.M., Chubanov V., Gudermann T. TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012;44:1381–1384. doi: 10.1016/j.biocel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 156.Schmitz C., Deason F., Perraud A. Molecular components of vertebrate Mg2+ homeostasis regulation. Magnes. Res. 2007;20:6–18. [PubMed] [Google Scholar]
- 157.Schmitz C., Perraud A., Johnson C.O., Inabe K., Smith M.K., Penner R., Kurosaki T., Fleig A., Scharenberg A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/S0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 158.Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A.G., Figdor C.G., Moolenaar W.H., Jalink K., van Leeuwen F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ryazanova L.V., Rondon L.J., Zierler S., Hu Z., Galli J., Yamaguchi T.P., Mazur A., Fleig A., Ryazanov A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Runnels L.W., Yue L., Clapham D.E. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 161.Demeuse P., Penner R., Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takezawa R., Schmitz C., Demeuse P., Scharenberg A.M., Penner R., Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jin J., Wu L.-J., Jun J., Cheng X., Xu H., Andrews N.C., Clapham D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA. 2012;109:E225–E233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jin J., Desai B.N., Navarro B., Donovan A., Andrews N.C., Clapham D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mittermeier L., Demirkhanyan L., Stadlbauer B., Breit A., Recordati C., Hilgendorff A., Matsushita M., Braun A., Simmons D.G., Zakharian E., et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA. 2019;116 doi: 10.1073/pnas.1810633116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ogata K., Tsumuraya T., Oka K., Shin M., Okamoto F., Kajiya H., Katagiri C., Ozaki M., Matsushita M., Okabe K. The crucial role of the TRPM7 kinase domain in the early stage of amelogenesis. Sci. Rep. 2017;7:18099. doi: 10.1038/s41598-017-18291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dribben W.H., Eisenman L.N., Mennerick S. Magnesium induces neuronal apoptosis by suppressing excitability. Cell Death Dis. 2010;1:e63. doi: 10.1038/cddis.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shindo Y., Yamanaka R., Suzuki K., Hotta K., Oka K. Altered expression of Mg2+ transport proteins during Parkinson’s disease-like dopaminergic cell degeneration in PC12 cells. Biochim. Biophys. Acta. 2016;1863:1979–1984. doi: 10.1016/j.bbamcr.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Huang J., Furuya H., Faouzi M., Zhang Z., Monteilh-Zoller M., Kawabata K.G., Horgen F.D., Kawamori T., Penner R., Fleig A. Inhibition of TRPM7 suppresses cell proliferation of colon adenocarcinoma in vitro and induces hypomagnesemia in vivo without affecting azoxymethane-induced early colon cancer in mice. Cell Commun. Signal. 2017;15:30. doi: 10.1186/s12964-017-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Komiya Y., Runnels L.W. TRPM channels and magnesium in early embryonic development. Int. J. Dev. Biol. 2015;59:281–288. doi: 10.1387/ijdb.150196lr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Turlova E., Bae C.Y.J., Deurloo M., Chen W., Barszczyk A., Horgen F.D., Fleig A., Feng Z.-P., Sun H.-S. TRPM7 Regulates Axonal Outgrowth and Maturation of Primary Hippocampal Neurons. Mol. Neurobiol. 2016;53:595–610. doi: 10.1007/s12035-014-9032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Su L.-T., Liu W., Chen H.-C., Gonzalez-Pagan O., Habas R., Runnels L.W. TRPM7 regulates polarized cell movements. Biochem. J. 2011;434:513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sahni J., Tamura R., Sweet I.R., Scharenberg A.M. TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes. Cell Cycle. 2010;9:3565–3574. doi: 10.4161/cc.9.17.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sahni J., Scharenberg A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008;8:84–93. doi: 10.1016/j.cmet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wilson C., González-Billault C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015;9:381. doi: 10.3389/fncel.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oswald M.C.W., Garnham N., Sweeney S.T., Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–691. doi: 10.1002/1873-3468.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oancea E., Wolfe J.T., Clapham D.E. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 178.Visser D., Middelbeek J., van Leeuwen F.N., Jalink K. Function and regulation of the channel-kinase TRPM7 in health and disease. Eur. J. Cell Biol. 2014;93:455–465. doi: 10.1016/j.ejcb.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 179.Palacios-Prado N., Chapuis S., Panjkovich A., Fregeac J., Nagy J.I., Bukauskas F.F. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat. Commun. 2014;5:4667. doi: 10.1038/ncomms5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palacios-Prado N., Hoge G., Marandykina A., Rimkute L., Chapuis S., Paulauskas N., Skeberdis V.A., O’Brien J., Pereda A.E., Bennett M.V.L., et al. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J. Neurosci. 2013;33:4741–4753. doi: 10.1523/JNEUROSCI.2825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Snipas M., Rimkute L., Kraujalis T., Maciunas K., Bukauskas F.F. Functional asymmetry and plasticity of electrical synapses interconnecting neurons through a 36-state model of gap junction channel gating. PLoS Comput. Biol. 2017;13:e1005464. doi: 10.1371/journal.pcbi.1005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yamanaka R., Shindo Y., Karube T., Hotta K., Suzuki K., Oka K. Neural depolarization triggers Mg2+ influx in rat hippocampal neurons. Neuroscience. 2015;310:731–741. doi: 10.1016/j.neuroscience.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 183.Jia S., Liu Y., Shi Y., Ma Y., Hu Y., Wang M., Li X. Elevation of Brain Magnesium Potentiates Neural Stem Cell Proliferation in the Hippocampus of Young and Aged Mice. J. Cell. Physiol. 2016;231:1903–1912. doi: 10.1002/jcp.25306. [DOI] [PubMed] [Google Scholar]
- 184.Wu C., Xue L.-D., Su L.-W., Xie J.-L., Jiang H., Yu X.-J., Liu H.-M. Magnesium promotes the viability and induces differentiation of neural stem cells both in vitro and in vivo. Neurol. Res. 2019;41:208–215. doi: 10.1080/01616412.2018.1544400. [DOI] [PubMed] [Google Scholar]
- 185.Liao W., Jiang M., Li M., Jin C., Xiao S., Fan S., Fang W., Zheng Y., Liu J. Magnesium Elevation Promotes Neuronal Differentiation While Suppressing Glial Differentiation of Primary Cultured Adult Mouse Neural Progenitor Cells through ERK/CREB Activation. Front. Neurosci. 2017;11:87. doi: 10.3389/fnins.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chan A.W., Minski M.J., Lim L., Lai J.C. Changes in brain regional manganese and magnesium levels during postnatal development: modulations by chronic manganese administration. Metab. Brain Dis. 1992;7:21–33. doi: 10.1007/BF01000438. [DOI] [PubMed] [Google Scholar]
- 187.Miller F.D., Gauthier A.S. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 188.Cavaliere F., Benito-Muñoz M., Panicker M., Matute C. NMDA modulates oligodendrocyte differentiation of subventricular zone cells through PKC activation. Front. Cell. Neurosci. 2013;7:261. doi: 10.3389/fncel.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zeng Z., Leng T., Feng X., Sun H., Inoue K., Zhu L., Xiong Z.-G. Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways. PLoS ONE. 2015;10:e0119912. doi: 10.1371/journal.pone.0119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Verstraeten A., Theuns J., Van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet. 2015;31:140–149. doi: 10.1016/j.tig.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 191.Nguyen M., Wong Y.C., Ysselstein D., Severino A., Krainc D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci. 2019;42:140–149. doi: 10.1016/j.tins.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zeng X.-S., Geng W.-S., Jia J.-J., Chen L., Zhang P.-P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yasui M., Kihira T., Ota K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology. 1992;13:593–600. [PubMed] [Google Scholar]
- 194.Oyanagi K. The nature of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam and magnesium deficiency. Parkinsonism Relat. Disord. 2005;11(Suppl. 1):S17–S23. doi: 10.1016/j.parkreldis.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 195.Aden E., Carlsson M., Poortvliet E., Stenlund H., Linder J., Edstrom M., Forsgren L., Haglin L. Dietary intake and olfactory function in patients with newly diagnosed Parkinson’s disease: a case-control study. Nutr. Neurosci. 2011;14:25–31. doi: 10.1179/174313211X12966635733312. [DOI] [PubMed] [Google Scholar]
- 196.Miyake Y., Tanaka K., Fukushima W., Sasaki S., Kiyohara C., Tsuboi Y., Yamada T., Oeda T., Miki T., Kawamura N., et al. Dietary intake of metals and risk of Parkinson’s disease: a case-control study in Japan. J. Neurol. Sci. 2011;306:98–102. doi: 10.1016/j.jns.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 197.Hermosura M.C., Garruto R.M. TRPM7 and TRPM2 - Candidate susceptibility genes for Western Pacific ALS and PD? Biochim. Biophys. Acta. 2007;1772:822–835. doi: 10.1016/j.bbadis.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hermosura M.C., Nayakanti H., Dorovkov M.V., Calderon F.R., Ryazanov A.G., Haymer D.S., Garruto R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kolisek M., Sponder G., Mastrototaro L., Smorodchenko A., Launay P., Vormann J., Schweigel-Röntgen M. Substitution p.A350V in Na+/Mg2+ exchanger SLC41A1, potentially associated with Parkinson’s disease, is a gain-of-function mutation. PLoS One. 2013;8:e71096. doi: 10.1371/journal.pone.0071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin C.-H., Wu Y.-R., Chen W.-L., Wang H.-C., Lee C.-M., Lee-Chen G.-J., Chen C.-M. Variant R244H in Na+/Mg2+ exchanger SLC41A1 in Taiwanese Parkinson’s disease is associated with loss of Mg2+ efflux function. Parkinsonism Relat. Disord. 2014;20:600–603. doi: 10.1016/j.parkreldis.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 201.Decker A.R., McNeill M.S., Lambert A.M., Overton J.D., Chen Y.-C., Lorca R.A., Johnson N.A., Brockerhoff S.E., Mohapatra D.P., MacArthur H., et al. Abnormal differentiation of dopaminergic neurons in zebrafish trpm7 mutant larvae impairs development of the motor pattern. Dev. Biol. 2014;386:428–439. doi: 10.1016/j.ydbio.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Muroyama A., Inaka M., Matsushima H., Sugino H., Marunaka Y., Mitsumoto Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci. Res. 2009;63:72–75. doi: 10.1016/j.neures.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 203.Hashimoto T., Nishi K., Nagasao J., Tsuji S., Oyanagi K. Magnesium exerts both preventive and ameliorating effects in an in vitro rat Parkinson disease model involving 1-methyl-4-phenylpyridinium (MPP+) toxicity in dopaminergic neurons. Brain Res. 2008;1197:143–151. doi: 10.1016/j.brainres.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 204.Lin L., Yan M., Wu B., Lin R., Zheng Z. Expression of magnesium transporter SLC41A1 in the striatum of 6-hydroxydopamine-induced parkinsonian rats. Brain Res. Bull. 2018;142:338–343. doi: 10.1016/j.brainresbull.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 205.Xu R., Zhou Y., Fang X., Lu Y., Li J., Zhang J., Deng X., Li S. The possible mechanism of Parkinson’s disease progressive damage and the preventive effect of GM1 in the rat model induced by 6-hydroxydopamine. Brain Res. 2014;1592:73–81. doi: 10.1016/j.brainres.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 206.Faustini G., Bono F., Valerio A., Pizzi M., Spano P., Bellucci A. Mitochondria and α-Synuclein: Friends or Foes in the Pathogenesis of Parkinson’s Disease? Genes (Basel) 2017;8:377. doi: 10.3390/genes8120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Golts N., Snyder H., Frasier M., Theisler C., Choi P., Wolozin B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J. Biol. Chem. 2002;277:16116–16123. doi: 10.1074/jbc.M107866200. [DOI] [PubMed] [Google Scholar]
- 208.Andre C., Truong T.T., Robert J.F., Guillaume Y.C. Effect of metals on herbicides-alpha-synuclein association: a possible factor in neurodegenerative disease studied by capillary electrophoresis. Electrophoresis. 2005;26:3256–3264. doi: 10.1002/elps.200500169. [DOI] [PubMed] [Google Scholar]
- 209.Breydo L., Wu J.W., Uversky V.N. Alpha-synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta. 2012;1822:261–285. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 210.Menzies F.M., Fleming A., Rubinsztein D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 211.Wong E., Cuervo A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wills J., Credle J., Oaks A.W., Duka V., Lee J.-H., Jones J., Sidhu A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE. 2012;7:e30745. doi: 10.1371/annotation/6c09a04c-e565-4a34-b24e-90f084463e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sato S., Uchihara T., Fukuda T., Noda S., Kondo H., Saiki S., Komatsu M., Uchiyama Y., Tanaka K., Hattori N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018;8:2813. doi: 10.1038/s41598-018-21325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu Z., Yang C., Iyaswamy A., Krishnamoorthi S., Sreenivasmurthy S.G., Liu J., Wang Z., Tong B.C.-K., Song J., Lu J., et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019;20:728. doi: 10.3390/ijms20030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bockaert J., Marin P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- 217.Lan A.-P., Chen J., Zhao Y., Chai Z., Hu Y. mTOR Signaling in Parkinson’s Disease. Neuromolecular Med. 2017;19:1–10. doi: 10.1007/s12017-016-8417-7. [DOI] [PubMed] [Google Scholar]
- 218.Kametani F., Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018;12:25. doi: 10.3389/fnins.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reddy P.H., Oliver D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells. 2019;8 doi: 10.3390/cells8050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Veronese N., Zurlo A., Solmi M., Luchini C., Trevisan C., Bano G., Manzato E., Sergi G., Rylander R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers. Dis. Other Demen. 2016;31:208–213. doi: 10.1177/1533317515602674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Andrasi E., Igaz S., Molnar Z., Mako S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000;13:189–196. [PubMed] [Google Scholar]
- 222.Glick J.L. Dementias: the role of magnesium deficiency and an hypothesis concerning the pathogenesis of Alzheimer’s disease. Med. Hypotheses. 1990;31:211–225. doi: 10.1016/0306-9877(90)90095-V. [DOI] [PubMed] [Google Scholar]
- 223.Cilliler A.E., Ozturk S., Ozbakir S. Serum magnesium level and clinical deterioration in Alzheimer’s disease. Gerontology. 2007;53:419–422. doi: 10.1159/000110873. [DOI] [PubMed] [Google Scholar]
- 224.Bardgett M.E., Schultheis P.J., McGill D.L., Richmond R.E., Wagge J.R. Magnesium deficiency impairs fear conditioning in mice. Brain Res. 2005;1038:100–106. doi: 10.1016/j.brainres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 225.Bardgett M.E., Schultheis P.J., Muzny A., Riddle M.D., Wagge J.R. Magnesium deficiency reduces fear-induced conditional lick suppression in mice. Magnes. Res. 2007;20:58–65. [PubMed] [Google Scholar]
- 226.Landfield P.W., Morgan G.A. Chronically elevating plasma Mg2+ improves hippocampal frequency potentiation and reversal learning in aged and young rats. Brain Res. 1984;322:167–171. doi: 10.1016/0006-8993(84)91199-5. [DOI] [PubMed] [Google Scholar]
- 227.Enomoto T., Osugi T., Satoh H., McIntosh T.K., Nabeshima T. Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J. Neurotrauma. 2005;22:783–792. doi: 10.1089/neu.2005.22.783. [DOI] [PubMed] [Google Scholar]
- 228.Hoane M.R. Treatment with magnesium improves reference memory but not working memory while reducing GFAP expression following traumatic brain injury. Restor. Neurol. Neurosci. 2005;23:67–77. [PubMed] [Google Scholar]
- 229.Yu J., Sun M., Chen Z., Lu J., Liu Y., Zhou L., Xu X., Fan D., Chui D. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J. Alzheimers. Dis. 2010;20:1091–1106. doi: 10.3233/JAD-2010-091444. [DOI] [PubMed] [Google Scholar]
- 230.Xu Z.-P., Li L., Bao J., Wang Z.-H., Zeng J., Liu E.-J., Li X.-G., Huang R.-X., Gao D., Li M.-Z., et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS One. 2014;9:e108645. doi: 10.1371/journal.pone.0108645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yu X., Guan P.-P., Zhu D., Liang Y.-Y., Wang T., Wang Z.-Y., Wang P. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor alpha and the Activity of gamma-Secretase in a beta-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018;11:172. doi: 10.3389/fnmol.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kuramoto T., Kuwamura M., Tokuda S., Izawa T., Nakane Y., Kitada K., Akao M., Guénet J.-L., Serikawa T. A mutation in the gene encoding mitochondrial Mg2+ channel MRS2 results in demyelination in the rat. PLoS Genet. 2011;7:e1001262. doi: 10.1371/journal.pgen.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kuwamura M., Inumaki K., Tanaka M., Shirai M., Izawa T. Oligodendroglial pathology in the development of myelin breakdown in the dmy mutant rat. Brain Res. 2011;1389:161–168. doi: 10.1016/j.brainres.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 234.Kozin M.S., Kulakova O.G., Favorova O.O. Involvement of Mitochondria in Neurodegeneration in Multiple Sclerosis. Biochemistry. (Mosc.) 2018;83:813–830. doi: 10.1134/S0006297918070052. [DOI] [PubMed] [Google Scholar]
- 235.Ohno N., Ikenaka K. Axonal and neuronal degeneration in myelin diseases. Neurosci. Res. 2019;139:48–57. doi: 10.1016/j.neures.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 236.Itoh K., Maki T., Shindo A., Egawa N., Liang A.C., Itoh N., Lo E.H., Lok J., Arai K. Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic-ischemic injury. Neurosci. Res. 2016;106:66–69. doi: 10.1016/j.neures.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kuwamura M., Tanimura S., Hasegawa Y., Hoshiai R., Moriyama Y., Tanaka M., Takenaka S., Nagayoshi H., Izawa T., Yamate J., et al. Downregulation of aspartoacylase during the progression of myelin breakdown in the dmy mutant rat with mitochondrial magnesium channel MRS2 defect. Brain Res. 2019;1718:169–175. doi: 10.1016/j.brainres.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 238.Madsen P.M., Pinto M., Patel S., McCarthy S., Gao H., Taherian M., Karmally S., Pereira C.V., Dvoriantchikova G., Ivanov D., et al. Mitochondrial DNA Double-Strand Breaks in Oligodendrocytes Cause Demyelination, Axonal Injury, and CNS Inflammation. J. Neurosci. 2017;37:10185–10199. doi: 10.1523/JNEUROSCI.1378-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Alexeyev M., Shokolenko I., Wilson G., LeDoux S. The Maintenance of Mitochondrial DNA Integrity-Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013;5:a012641. doi: 10.1101/cshperspect.a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Brown W.M., George M.J., Wilson A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tatarenkov A., Avise J.C. Rapid concerted evolution in animal mitochondrial DNA. Proc. Biol. Sci. 2007;274:1795–1798. doi: 10.1098/rspb.2007.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McCutcheon J.P. From microbiology to cell biology: when an intracellular bacterium becomes part of its host cell. Curr. Opin. Cell Biol. 2016;41:132–136. doi: 10.1016/j.ceb.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Roger A.J., Munoz-Gomez S.A., Kamikawa R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017;27:R1177–R1192. doi: 10.1016/j.cub.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 244.Johnson M.A., Vidoni S., Durigon R., Pearce S.F., Rorbach J., He J., Brea-Calvo G., Minczuk M., Reyes A., Holt I.J., et al. Amino acid starvation has opposite effects on mitochondrial and cytosolic protein synthesis. PLoS ONE. 2014;9:e93597. doi: 10.1371/journal.pone.0093597. [DOI] [PMC free article] [PubMed] [Google Scholar]